Abstract
Androgen deprivation therapy (ADT) is the primary systemic therapy for treating locally advanced or metastatic prostate cancer (PCa). Despite its positive effect on PCa patient survival, ADT causes various adverse effects, including increased cardiovascular risk factors and cardiotoxicity. Lifespans extension, early use of ADT, and second-line treatment with next-generation androgen receptor pathway inhibitors would further extend the duration of ADT and possibly increase the risk of ADT-induced cardiotoxicity. Meanwhile, information on the molecular mechanisms underlying ADT-induced cardiotoxicity and measures to prevent it is limited, mainly due to the lack of specifically designed preclinical studies and clinical trials. This review article compiles up-to-date evidence obtained from observational studies and clinical trials, in order to gain new insights for deciphering the association between ADT use and cardiotoxicity. In addition, potential cardioprotective strategies involving GnRH receptors and second messenger cGMP are discussed.
Keywords: Androgen deprivation therapy, cardiotoxicity, GnRH agonists, cGMP, sildenafil citrate, prostate cancer
1. Introduction
Prostate cancer (PCa) is still one of the most common cause of mortality and morbidity among men globally [1]. In 2016, 1.4 million new cases of PCa were reported and 381,000 men died because of PCa [1]. Since 2000, the number of PCa survivors are increasing and are expected to increase further due to age, early screening and better therapeutics for PCa [2]. Androgen deprivation therapy (ADT) has been the primary systemic therapy for metastatic PCa during the past 75 years. ADT is also given as a neoadjuvant, concurrent or adjuvant therapy with radiation for localized or locally advanced PCa. Since 1990, the majority of locally advanced and metastatic PCa patients received gonadotropin-releasing hormone (GnRH) agonists as the first-line ADT treatment. Despite the survival benefit, ADT is associated with significant adverse effects, including sexual dysfunction, vasomotor flushing, loss of libido, fatigue, gynecomastia, anemia, osteoporosis, insulin insensitivity, diabetes and cardiovascular disease [3, 4]. Recent observational studies and a randomized controlled trials (RCT) have suggested that ADT, particularly GnRH agonists, is associated with increased incidence of cardiovascular (CV) events.
Epidemiological studies have estimated that ~50% of PCa patients will undergo ADT at some point in their treatment course [5] and at least 20–30% of these patients will develop risk for CV events, particularly with GnRH agonists [6–8]. A recent study using the Surveillance, Epidemiology, and End Results (SEER) database and Swedish cancer registry estimated that cardiovascular disease (CVD) is the most common cause of death in PCa patients who survive longer than 10 years after cancer diagnosis [9]. Since PCa patients are surviving longer, GnRH agonists-mediated CV events is a growing concern. Although the association between GnRH agonists and cardiac risk events is well established, several clinical studies have not supported this association. A possible explanation is that clinical trials often exclude patients at highest risk of CVD. Also, the survival benefit of GnRH agonists may undermine the risk ratio associated with CV adverse events. In fact, no RCT has been designed to determine the CV risk events/death from ADT as a primary endpoint of analysis except the PRONOUNCE trail (NCT02663908). The PRONOUNCE trial is currently recruiting advanced PCa patients with predefined CVD to determine the CVD related death among the patients treated with GnRH agonist (leuprolide) and GnRH antagonist (degarelix), but the results are expected only in 2021. For these reasons, understanding of cardiac events associated with ADT is incomplete, which impedes the design of preventive or curative interventions for CV risks/toxicity. Here, we review the currently available evidence on the association between various types of ADT and CV events. We also focus on the possible underlying molecular mechanisms of ADT-associated CV events, including the role of GnRH receptor (GnRHR). Finally, we have outlined our perspectives on the role of cGMP-hydrolyzing phosphodiesterase 5 (PDE5) inhibitors in possible management of risk for CV toxicity in PCa patients treated with GnRH agonists.
2. Prostate cancer and androgen deprivation therapy
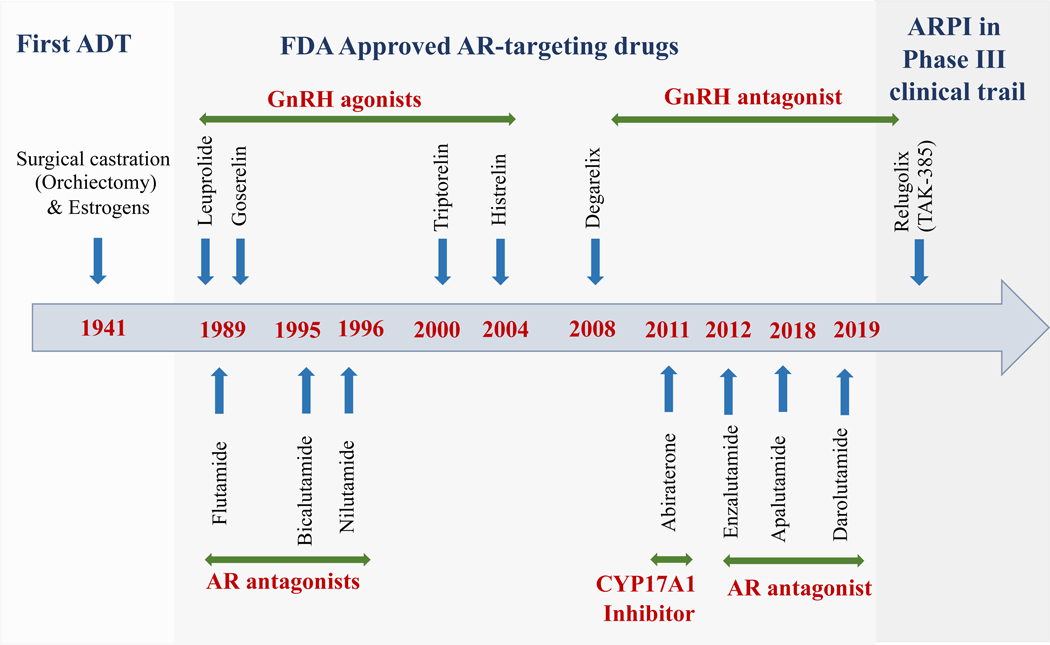
In 1941, Huggins and Hodges first showed that androgen deprivation, primarily by surgical castration or estrogen treatment reduces PCa growth [10]. Since then, various approaches have been developed to target the androgen signaling axis (Fig. 1). The principle of ADT is to reduce the physiological concentration of circulating androgens (300–1000 ng/dl) to the castrated levels (≤50 ng/dl) [11]. ADT is achieved either by the surgical removal of the testis (bilateral orchiectomy) or chemically blocking the hypothalamus/pituitary/testicular axis (using GnRH agonists or antagonists and/or antiandrogens) to reduce the effect of androgen signaling on the prostate (Fig. 2). In contemporary days, the patients who undergo bilateral orchiectomy is very few and hence mostly chemical castration is prevalent in the management of PCa.
Figure 1. Landmarks in targeted therapies of androgen signaling approved for advanced prostate cancer management.
Graphical representation of drugs targeting the AR pathway for prostate cancer, starting from surgical castration to currently approved and in-use drugs. Drugs were classified according to their targets; novel agent still in clinical trials is given at the end. Abbreviations: AR – androgen receptor, CYP17A1 – cytochrome P450 family 17 subfamily A member 1, GnRH – gonadotropin-releasing hormone.
Fig. 2. Androgen receptor pathway inhibitors and their mechanism of action.
Testosterone, the major driver of prostate cell proliferation and survival, is synthesized by the testis under the control of LH, which is regulated by the hypothalamus/pituitary axis. De novo testosterone then reaches the prostate and is converted into more potent DHT form by the enzyme 5-α reductase. Either T or DHT forms a complex with AR, which then binds with an AR-specific DNA sequence and induces transcription of genes. Since 1941 various strategies were developed to inhibit the AR signaling axis in prostate cancer. A surgical procedure to remove the testis, to eliminate the source of T. The drugs which act on various organs are indicated in red. Part of the figure icons are obtained from Servier Medical Art (http://smart.servier.com), licensed under a Creative Common Attribution 3.0. (https://creativecommons.org/licenses/by/3.0/). The male urinary tract is simplified to show only urinary bladder and prostate. Abbreviations: Apa – apalutamide, AR – androgen receptor, CYP17A1 – cytochrome P450 family 17 subfamily A member 1, Dar - darolutamide, DHEA – dehydroepiandrosterone, DHT –dihydrotestosterone, Enz – enzalutamide, GnRH – gonadotropin-releasing hormone, LH – luteinizing hormone, PSA – prostate-specific antigen, and T – testosterone.
GnRH agonists: the first-line systemic therapeutic agent for ADT
The high affinity GnRH agonists were originally developed as birth control agents. Contrary to natural pulsatile stimulation, these agonists continuously stimulate the anterior pituitary to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Chronic administration of agonists desensitizes the GnRHR and thus reduces gonadotropin secretion and testosterone synthesis [12]. The first report of the clinical benefit of GnRH agonists in PCa in 1982 suggested that GnRH agonists could serve as an alternative ADT strategy [13]. Since then, various GnRH agonists (leuprolide, goserelin, triptorelin, histrelin) have been developed and largely replaced the use of Diethylstilbestrol (DES) and surgical orchiectomy (Fig. 1).
GnRH antagonists were developed as an alternative for GnRH agonists. These antagonists bind to GnRHRs and competitively prevent the native peptide from binding and thereby preventing the release of LH. GnRH antagonists do not cause a testosterone flare in PCa patients and thus can prevent the additional use of antiandrogens [14]. Earlier generations of GnRH antagonists were not well received in the clinic because of their limited solubility and induction of histamine release [14], whereas more recent GnRH antagonists have overcome these limitations. GnRH agonists and GnRH antagonists were generally well tolerated in clinical trials, except an incidence of anaphylaxis was reported in the patients receiving GnRH antagonists [15, 16]. Further, GnRH agonists are easier to administer than antagonists due to lower dose frequency and cost, as well as simpler drug compound reconstitution.
3. Testosterone and cardiovascular system
Typically, testosterone (androgens) is known to play a vital role in the development of male reproductive system and masculine features such as increased muscle, bone mass, and the growth of body hair. In addition, though studies are scant, it has been shown that testosterone has multitude of cardioprotective effects, which may account for maintaining lipid profiles and minimizing inflammation at physiological concentrations of testosterone. These cardio protective effects are supported by androgen receptor (AR) expression in cardiovascular tissues [17, 18]. In cardiac tissues, androgen can executes its function either through its conventional AR signaling (Fig. 2) or the non-genomic signaling through MAPK, PI3K/Akt, PKC, etc [19]. Further, androgens have also been shown to induce NO-mediated cGMP production which results in vascular relaxation In general, low physiological levels of testosterone are associated with increased cardiovascular risk events such as coronary artery disease (CAD), congestive heart failure (CHF) and metabolic changes including altered lipid profile and insulin resistance, and diabetes [21, 22]. Indirectly, pre-existing risk factors such as altered lipid profiles and insulin resistance, and diabetes may increase CV risk and related mortality. On the other hand, few studies have shown that testosterone supplementation in hypogonadal men are associated with increased CV risk, which is further complicated by gender discrepancy, protective effect of estrogen, and deleterious effect of supraphysiological testosterone levels [22]. Overall, though it is not clinically proven, majority of the studies and experimental evidence show that circulating testosterone has beneficial effects on cardiovascular system [21].
In a completely different scenario such as intentional androgen deprivation for PCa, the association with CV risk events is still unclear and several hypotheses have been proposed. In the following section, we provide details of the underlying possible mechanism of various form of ADT agents induced CVD and how ADT-induced cardiotoxicity is different from physiological dysfunction.
4. ADT-related cardiovascular risk and adverse events
Despite the clinical success of ADT (GnRH agonists) in the management of PCa, there is a growing body of data suggesting that ADT is associated with increased CV adverse events [3, 6–8, 23–27]. European Society of Cardiology (ESC) classifies cancer therapy-induced CV complications into the following nine major categories: i) myocardial dysfunction and heart failure (HF), ii) coronary artery disease (CAD), iii) valvular disease, iv) arrhythmias (especially QT prolongation), v) hypertension, vi) thromboembolic disease, vii) peripheral vascular disease and stroke, viii) pulmonary hypertension, and ix) pericardial complications [28]. Long-term administration of GnRH agonists may lead to some of the CV risk events such as myocardial dysfunction, HF, CAD, arrhythmias, thromboembolic disease, and stroke, which are further discussed below.
In an observational study using the SEER database, Keating et al. [7] first showed in 2006 that GnRH agonist use is associated with significantly increased risk of developing diabetes, CAD, myocardial infarction (MI), and sudden cardiac death among patients with loco-regional PCa. Subsequent studies confirmed these associations, particularly between GnRH agonists use and CV risk events among PCa patients [6–8, 23–27, 29–32], as summarized in Table 1. For example, Tsai et al. [27] found that GnRH agonists and antiandrogens significantly increased risk of CVD-related mortality. Similarly, Van Hemelrijck and colleagues reported that ADT increased the risk of CVD, which was highest in patients treated with GnRH agonists compared to orchiectomy and combined androgen blockade, and lowest among those who received antiandrogens [24]. In addition, ADT was not only linked to cardiac dysfunction [25] but also to vascular dysfunction caused by peripheral arterial disease and venous thromboembolism [25, 26, 33].
Table 1:
Evidence from population-based observational studies for the relationship between GnRH agonists use, and CV/outcomes risk events.
| Study/Database (Study period) | Number of patients with stage and age | Type of ADT and duration | CV risk event and median follow-up | Cardiovascular risk events/mortality incidence | Adjusted HR; p-value |
|---|---|---|---|---|---|
| Keating et al. [7] SEER (1992–1999) | 73,196 Men with locoregional PCa Mean age - 72 years | GnRH agonist and/or AA vs. No ADT Median - 4.5 years | Until first CV risk event with Median follow-up of 4.55 years. | CHD | 1.16 (1.10–1.21); p=0.001 |
| MI | 1.11 (1.01–1.21); p=0.03 | ||||
| SCD | 1.16 (1.05–1.27); p=0.004 | ||||
| Tsai et al. [27] US CaPSURE (1995–2004) | 4,892 Men with localized PCa Median age - 64 yrs | GnRH agonist and/or AA vs. No ADT Median - 4.1 months | Median follow-up of 3.8 years | CV mortality with RP | 2.6 (1.4–1.7); p=0.002 |
| CV mortality with EBRT, BT or CT | 1.07 (1.02–1.1); p=0.004 | ||||
| Saigal et al. [8] SEER (1992–1996) | 22,816 Men with PCa | Any medical ADT vs. No ADT | Follow-up of 5 years after diagnosis | CV morbidity | 1.20 (1.15–1.26); ND 20% higher CV morbidity among the patients who received ADT vs. No ADT for 1 year. |
| >65 years of age | Mean - 21 months | ||||
| Alibhai et al. [36] | 19,079 Men with PCa | GnRH agonist and/or AA | Mean follow-up of 6.47 years | MI | 0.92 (0.84–1.00); NS |
| SCD | 0.96 (0.83–1.10); NS | ||||
| Ontario Cancer Registry (1995–2005) | >66 (mean 75) years of age | Orchiectomy vs. No ADT | Diabetes | 1.24 (1.15–1.35); p<0.05 | |
| Keating et al. [6] US VHA (2001–2004) | 37,443 Men with locoregional PCa until Dec 2005 or death | GnRH agonists, vs. no ADT Ever vs. never users | Mean follow-up of 2.6 years | CHD | 1.17 (1.09–1.25); p=0.001 |
| MI | 1.11 (0.95–1.30); p=0.18 | ||||
| SCD | 1.44 (1.28–1.64); p=0.01 | ||||
| Stroke | 1.17 (1.03–1.33); p=0.02 | ||||
| AA monotherapy vs. no ADT (unadjusted) | CHD | 1.27 (1.05–1.53) | |||
| Van Hemelrijck et al. [24] PcBaSE Sweden (1997–2007)a | 76,600 Men with PCa vs. matched non-cancer men | GnRH agonist use (SIR) b | Until death | MI | 1.28 (1.11–1.47) a |
| Arrhythmia | 1.27 (1.10–1.47) a | ||||
| IHD | 1.30 (1.17–1.45) a | ||||
| Heart failure | 1.46 (1.28–1.67) a | ||||
| Stroke | 1.27 (1.12–1.43) a | ||||
| GnRH agonist use (SMR) b | MI | 1.28 (1.07–1.53) a | |||
| Arrhythmia | 0.64 (0.38–1.09) a | ||||
| IHD | 1.01 (0.88–1.16) a | ||||
| Heart failure | 1.23 (0.89–1.71) a | ||||
| Stroke | 1.01 (0.77–1.34) a | ||||
| Antiandrogens (SIR) b | MI | 1.12 0.94 to 1.34 | |||
| Arrhythmia | 1.38 1.16 to 1.65 | ||||
| IHD | 1.13 0.99 to 1.30 | ||||
| Heart failure | 1.15 0.95 to 1.41 | ||||
| Stroke | 1.19 1.02 to 1.40 | ||||
| Antiandrogens (SMR) b | MI | 0.98 0.75 to 1.27 | |||
| Arrhythmia | 0.38 0.17 to 0.87 | ||||
| IHD | 0.79 0.65 to 0.96 | ||||
| Heart failure | 0.53 0.28 to 0.99 | ||||
| Stroke | 0.81 0.54 to 1.20 | ||||
| Azoulay et al. [25] UK GPRD) (1988–2008) | 15,375 men received ADT Mean age 72.3 years. | Current users of GnRH agonists CAB Oral antiandrogens | Mean follow-up of 3.9 years | ||
| Stroke/TIAs | 1.18 (1.00–1.39) | ||||
| 1.26 (0.93–1.72) | |||||
| 1.47 (1.08–2.01) | |||||
| Hu et al. [26] SEER (1992–2007) | 182,757 Men with locoregional PCa >65 yrs of age | GnRH agonist vs. No ADT | Mean follow-up of 5.1 years | PAD | 1.16 (1.12–1.21); p<0.001 |
| VTE | 1.10 (1.04–1.15); p<0.001 | ||||
| Jespersen et al. [29] | 31,571 Men with PCa Median age at PCa diagnosis was 71 years. | GnRH agonist/AA vs. No ADT adjusted | Median follow-up of 3.3 years | MI | 1.33 (1.15–1.53) |
| Stroke | 1.21 (1.05–1.39) | ||||
| Danish Cancer Registry (2002–2010) | |||||
| Gandaglia et al. [23] SEER (1995–2009) | 140,474 Men with non-metastatic PCa >65 years of age | GnRH agonist vs. No ADT | Mean (median) follow-up of 75.3 months (6.3 years) | CAD | 1.11 (1.07–1.15); p<0.001 |
| AMI | 1.09 (1.04–1.15); p<0.001 | ||||
| SCD | 1.18 (1.12–1.24); p<0.001 | ||||
| O’Farrell et al. [30] PCBaSe Sweden 2.0 | 41362 Men with Prostate cancer | GnRH agonist vs. age-matched men w/o PCa | All incidental CVD | CVD | 1.21 (1.18–1.25) |
| W/o baseline risk factors | CVD | 1.19 (1.14–1.24) | |||
| Men with a history of Statin use | CVD | 1.20 (1.12–1.28) | |||
| AA vs. age-matched men w/o PCa | All incidental CVD W/o baseline risk factors Men with a history of Statin use | CVD | 0.87 (0.82–0.91) | ||
| CVD | 0.81 (0.75–0.87) | ||||
| CVD | 0.86 (0.78–0.95) | ||||
| O’Farrell et al. 2016 [31] PCBaSe Sweden 3.0 | 42 263 men with PCa | GnRH agonists vs. No ADT | DVT | 1.67 (1.40–1.98) | |
| PE | 1.61 (1.42–1.82) | ||||
| AA vs. No ADT | DVT | 0.49 (0.33–0.74) | |||
| PE | 0.58 (0.45–0.75) | ||||
| Morgia et al., [79] Italian multicenter, | 1075 patients with PCa | GnRH agonists vs. AA alone | CV complications | OR= 3.95 (1.01–15.34) | |
| p<.05 | |||||
| cross-sectional study (2010 to 2012) | CAB (GnRH agonists +AA) vs. AA alone | CV complications | OR= 3.37(1.10–10.30) | ||
| p<.05 | |||||
Abbreviations: AA-antiandrogen; AMI - acute myocardial infarction; AS-active surveillance; BT-brachytherapy; CAD-coronary artery disease; CaPSURE - Cancer of the Prostate Strategic Urologic Research Endeavour; CHD-coronary heart disease; CT-cryotherapy; EBRT-external beam radiation therapy; CVD- Cardiovascular disease [(IHD- ischemic heart disease), arrhythmia, HF & stroke]; DVT-Deep vein thrombosis; GnRH-gonadotropin-releasing hormone; ID- Incidental diabetes; MI-myocardial infarction; PcBaSE-Prostate Cancer data Base Sweden; ND- no detail; NS- not significant; OR- Odds ratio; PAD-peripheral artery disease; PE - Pulmonary embolism; RP- radical prostatectomy; SCD-sudden cardiac death; SEER-surveillance, epidemiology, and end results; SES- socio economic status; SIR-standardized incident ratios; SMR-standardized mortality ratios; US-United States; TIAs- transient ischemic attacks; VHA-Veterans Healthcare Administration; VTE-venous thromboembolism; WW-watchful waiting.
The data were shown only to GnRH agonist use for SIRs and SMRs and were adjusted for the circulatory disease at baseline, SES, and PCa stage.
SIR and SMR is higher than AA, Orchiectomy, GnRH agonists with short-term AAs and curative treatment.
The PCa patients receiving ADT for 1 year had 20% higher CV-related morbidity compared to the ones who did not receive ADT [8]. CVD risk was also significantly higher during the first 12 months of ADT treatment, as compared with the following months. Similarly, Veterans Healthcare Administration data indicated that the current use of GnRH agonists significantly increased risk of MI, whereas increased incidence of CAD, sudden cardiac death, stroke, and diabetes were observed among both current and past users of GnRH agonists [6]. Few retrospective analyses revealed that 4-month neo-adjuvant ADT use was associated with an increased risk of all-cause mortality among low-risk PCa patients [34] and those with preexisting CV risk factors [35]. In contrary, Alibhai et al. [36] reported that continuous ADT for 6 months or more was likely to cause diabetes and fragility fracture, but not CV events. The later discrepancies could be due to the difference in the treatment period, treatment combination (GnRH agonists with or without antiandrogens) [34, 35], and pooled analyses of all form of ADT vs. non ADT [36].
Besides the observational studies, a number of meta-analyses have focused on the association between ADT and CV risk [37–41] which is summarized in Table 2. Meta-analyses of the European Organization for Research and Treatment of Cancer [EORTC] trial did not find significant CV risk associated with ADT [42]. Also, three phase III randomized clinical trials with a follow-up of at least eight years did not reveal a close association between GnRH agonist use and increased risk of CV mortality. Further, a meta-analysis of eight trials comprising 4141 PCa patients showed no increased risk of CV death following GnRH agonist therapy [39]. Similarly, in 2012 Wilcox et al. showed no increase in cardiac events following six month neoadjuvant use of GnRH agonists as compared with radiation therapy alone [40]. The disagreements between population-based studies and RCTs could be due to the fact that most of the RCTs included only the PCa patients with minimal or no risk of CVD. To the contrary, the population studies are based on more practical and inclusive representation of the real-world population. Interestingly, a pooled analysis of six phase III prospective trials in 2328 patients showed that the users of GnRH antagonists had significantly lower cardiac events (Hazard ratio = 0.44, 95% CI, 0.26–0.74; p = 0.002) than the patients who received GnRH agonists [41]. Supportively, a recent multinational randomized phase 3 trial reported that compared to leuprolide (GnRH agonist), relugolix (GnRH antagonist) treatment reduced adverse CV events by 54% in advanced PCa patients [43]. The later reports support the notion that GnRH agonist is associated with increased CV risk events.
Table 2:
Meta-analyses of randomized controlled trials for relationship between GnRH agonists use and CV/outcomes risk events.
| Study/Database (Study period) | Type of study | Type of ADT vs. control group | Cardiovascular risk bevents/mortality incidence | Adjusted HR; p-value |
|---|---|---|---|---|
| Nyguen et al. [39] 4141 men with PCa Median follow-up of 7.6 years to 13.2 y ears |
Meta-analysis of eight RCT s Cardiovascular death as the separate endpoint | GnRH agonist- based ADT versus no immediate use of ADT | CV mortality | 0.93 (0.79–1.10) p=0.41 |
| Wilcox et al. [40] 802 men with PCa | TROG 96.01 trial | Radiotherapy (RT) versus RT plus 3 or 6 months neoadiuvant ADT | Cumulative fatal cardiac events RT for RT plus 6-mo ADT |
7.5% (4.8–11.1) 6.4% (3.9–9.9) |
| Albertson et al. [41] 2328 men with PCa | Pooled analysis of six RCTs | GnRH agonists vs. antagonists | CV adverse events within 1 year of ADT in men with pre-existing CVD conditions | 0.44 (0.26–0.74) |
| Bosco et al. [37] | Meta-analysis of eight observational studies | GnRH agonists, orchiectomy and antiandrogens vs. No ADT | The relative risk of nonfatal CVD (GnRH agonists) CVD (Orchiectomy ) CVD (Antiandrogens) Non-fatal ID (Agonists) Non-fatal MI (Agonists) Fatal MI (Agonists). |
1.38 (1.29–1.48) 1.44 (1.28–1.62) 1.21 (1.07–1.36) 1.39 (1.26–1.54 1.57 (1.26–1.94) 1.51 (1.24–1.84) |
| Guo et al. [38] 170,851 ADT users vs. 256,704 non-ADT users. | Pooled meta-analysis of five retrospective population-based cohort studies | GnRH agonists, Antiandrogens (AA) GnRH agonists plus AA, & orchiectomy vs. Non-ADT | DVT (GnRH agonists) DVT (GnRH agonists plus oral AA) DVT (AA alone) DVT (Orchiectomy) PE (GnRH agonists) PE (Orchiectomy) |
1.47 (1.07– 2.03); p=0.017; I2=96.3% 2.55 (2.21–2.94); p<0.001; I2=0.0%, 1.49 (1.13–1.96); p=0.004; I2=0.0% 1.80 (0.93–3.47);p=0.079; I2=94.8% 2.26 (1.78–2.86);p< 0.001 2.12 (1.44–3.11);p< 0.001; I2=57.2%) |
Abbreviations: AA-antiandrogen; ADT-androgen deprivation therapy; CVD-cardiovascular disease; DVT-Deep venous thrombosis; GnRH-gonadotropin-releasing hormone; ID-ischemic disease; MI-myocardial infarction; PE- pulmonary embolism.
Few observational studies also suggest that antiandrogen monotherapy is associated with increased CAD, MI, arrhythmia, heart failure and stroke (Table 1). Using VA database Keating et al., showed that antiandrogen monotherapy treatment had 27% increase in CAD [6]. Similarly, Van Hemelrijck et al. using Swedish database showed that antiandrogen monotherapy is associated with slight increase in CVD events but not related deaths [24]. Further, the same group with an updated dataset showed that PCa patients receiving antiandrogen had increased risk of CVD during the first year when they had 2 or more CV events in past year [30]. The antiandrogen associated CV risk events reduced after 12 months [30]. Studies also show mixed observations on CV risk events in PCa patients who had received orchiectomy as their primary therpay when compared to PCa patients without any form of ADT [6, 32–35, 38–40, 44]. However, in contemporary days, the number of patients receiving orchiectomy is minimal [32, 39, 42] and the procedure is becoming less common due to various confounders. To maintain the focus of review on current treatment trends, we excluded any details which solely compare the orchiectomy associated CV effect to other type of ADT.
5. Potential mechanisms of ADT-induced cardiovascular adverse effects
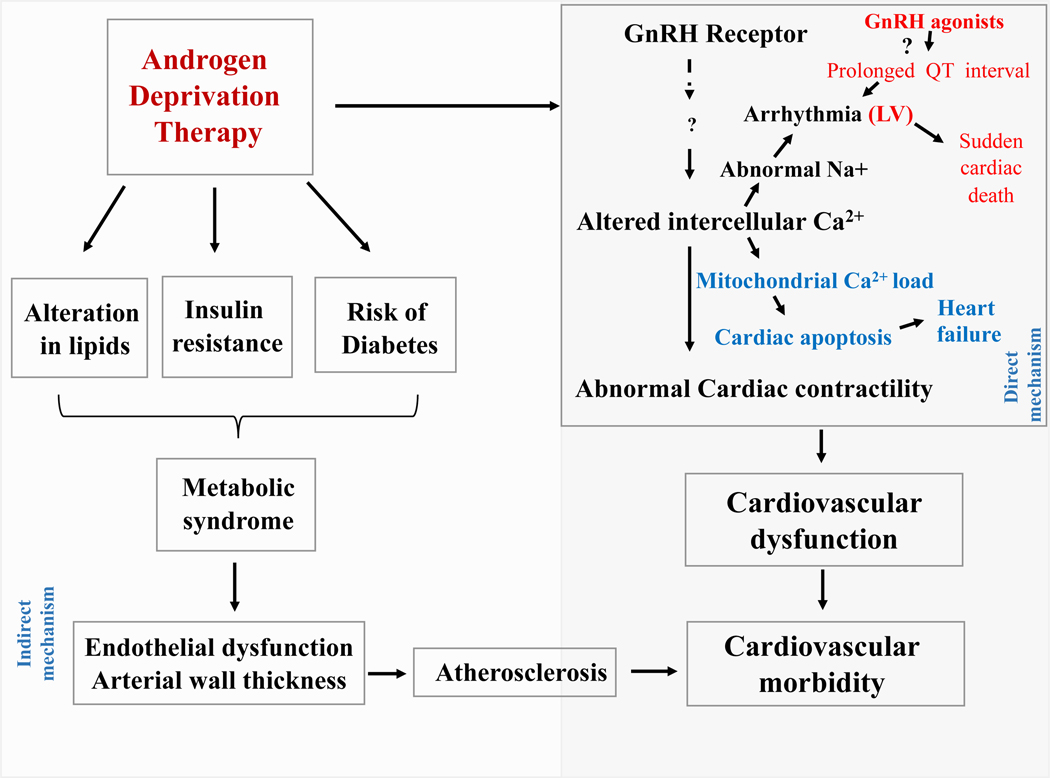
The mechanism by which GnRH agonists mediate CV risk and cardiotoxic events is still unclear, although several hypotheses have been proposed, which are summarized in Figure 3. Since testosterone maintains lean body mass, it has been suggested that ADT-induced hypogonadism is a causative factor for the development of metabolic syndrome [44]. In fact, there is a significant correlation between men with higher testosterone level and lower incidence of CVD and vice versa [21, 45]. Initial studies suggested that lower testosterone level and decreased metabolic function contribute to the increased CV risk [44, 46–48]. In 1990, Tayek et al. [49] demonstrated that 12-month GnRH agonist (buserelin) use led to an increase in body weight, cholesterol, and fat mass, which are linked to CV risk. The adverse effects on body composition occurred as early as 3–6 months after initiation of ADT [50]. A significant increase in fat mass was also reported in men who had undergone treatment with ADT for 12 months [51]. The ADT-associated alteration in body fat and mass was associated with altered lipid profiles [46–48]. Similar findings of increased fat percentage and body weight with a decline in lean body mass were also reported under androgen deprivation conditions [48]. Thus it appears that the longer the duration of ADT, the stronger the correlation with body mass increase [51]. Further, the median age of 66 years among PCa patients and predisposing factors such as diabetes, altered metabolic profiles make them generally vulnerable to CVD risk.
Fig. 3. ADT (GnRH agonist)-associated adverse events and pathogenic mechanisms.
ADT alters lipid profiles and insulin resistance. These alterations can induce the development of metabolic syndrome. Further, the accumulation of lipids can lead to endothelial dysfunction and arterial wall thickness, which can result in the development of atherosclerosis. Mechanistically, ADT can alter the intracellular calcium in cardiomyocytes through GnRH receptors. This abnormal calcium gradient can alter a plethora of normal cardiac functions such as cardiac contractility cardiac apoptosis, and heart rate.
Obesity and insulin resistance are independent and established risk factors for type 2 diabetes and CVD [52]. Evidence also linked the use of GnRH agonists to insulin resistance [53] and the progression of diabetes mellitus, supporting a potential correlation between ADT and metabolic complications in men with PCa. ADT-mediated alterations in lipid profiles could lead to the development of metabolic syndrome and CVD, which is supported by various clinical studies [6, 7, 36]. The metabolic alterations can also accelerate atherosclerosis, a major predisposing factor for CVD [54] as illustrated in Figure 3. GnRH agonists had a significantly greater effect on metabolic profile as compared with surgical orchiectomy [7]. However, ADT-mediated alterations of metabolism are apparently different from the classical metabolic profiles associated with diabetes and CVD [48, 55].
GnRH agonists may also induce cardiac injury by destabilizing established atherosclerotic plaques during the initial testosterone surge [56]. The increased testosterone may also promote angiogenesis or neutrophil migration. GnRH agonists can act on GnRHR present on T-lymphocytes to induce interferon-γ production, which in turn drives the pro-inflammatory environment and increases the risk of plaque rupture. These events can reduce atherosclerotic plaque stability, which subsequently leads to platelet activation and increased risk for clot formation [56].The destabilization of atherosclerotic plaques is thought to precede the ischemic CVD events triggered by blood clots [57, 58]. Recently, it was shown that GnRH agonist administration induced atherosclerosis in mice. Four months treatment of GnRH agonists alone induced atherosclerosis in low density lipoprotein receptor (LDLR) knockout mice with normal chow diet [59]. In addition, 4 weeks of leuprolide acetate administration destabilized established atherosclerotic plagues in apolipoprotein E (ApoE) knockout mice by recruiting macrophages and inducing necrosis [60]. Both in vitro and in vivo studies have shown that T cells of atherosclerotic plaques express GnRHR, and GnRH agonists induced proliferation and activation of these cells through GnRH receptor type I [60, 61]. Furthermore, GnRH receptors favor the generation of Th1-type cells [62], which promote atherosclerosis.
The pituitary axis contributing to the hormonal imbalance has also been proposed as an underlying mechanism but not clinically proven. GnRH agonist could potentially alter male hormones and the modulation may differ from physiological state or with other ADT treatment regimen. These GnRH agonist-mediated hormonal imbalance may affect the cardiac function outside the direct action of testosterone. For example, GnRH agonists lead to an initial surge in LH and FSH, which is in contrast to the rapid inhibition of these hormones by GnRH antagonists (e.g. degarelix) [63]. Importantly, FSH levels did not reach the level of GnRH agonists -mediated decline even after 12 months of GnRH agonist (leuprolide) treatment [43, 63]. The spike and continued presence of trace FSH levels are important in the context of low-level expression of FSH receptors in cardiac myocytes [56, 64] and human adipocytes [65]. An increase in FSH was also observed after orchiectomy along with higher LH and reduced anti-Mullerian hormone (AMH) [66], whereas GnRH agonist use decreased LH while sustaining AMH levels [67]. GnRH agonists also decrease the serum inhibin level, which causes a secondary FSH surge, creating a feedback loop under GnRH agonist treatment [67]. The altered hormonal levels during ADT (particularly with GnRH agonists) may affect cardiac function and CVD risk. In particular, FSH may promote atherosclerotic plaque formation and other metabolic changes associated with ADT [68].
The third possible mechanism by which GnRH agonists contribute to CV risk is via direct action on cardiomyocytes. GnRHR expression has been reported in various extra-gonadal tissues including heart, kidney, and immune cells [69, 70]. In addition, GnRH binding has been detected in multiple tissues and tumors, including the hypothalamus, pituitary, gonads, breast, and prostate cancers. Mechanistically, GnRH agonists trigger the release of gonadotropins in the pituitary gland through the inositol trisphosphate-protein kinase C (PKC) pathway [71] and MAPK-dependent phospholipase A2 [71]. Calcium mobilization also plays a major role in the release of sex hormone. Even though human cardiac tissues express GnRHR mRNA, there is no evidence which suggests that the GnRH agonists mediate calcium accumulation in human cardiac cells. A preclinical study by Dong et al. [72] suggested that GnRH agonists have positive effects on cardiomyocyte contractile function. As opposed to the classical GnRH/PKC-mediated intracellular calcium accumulation and gonadotropin release in the pituitary, the cardiac contractility and intracellular calcium concentration increases via a GnRHR/protein kinase A (PKA)-dependent mechanism. GnRH activates PKA, which phosphorylates several important substrates in cardiomyocytes, including phospholamban, an L-type calcium channel on the sarcolemma and components of the contractile apparatus. PKA could have an essential role in a GnRH-associated cardiac response [73]. PKA may phosphorylate the ryanodine receptor/Ca2+ release channel, which in turn regulates channel conductance in cardiomyocytes [74]. Exposure of mouse cardiomyocytes to GnRH agonists resulted in a significant change in myocardial intracellular calcium concentration, thus altering the cells contractility [72]. High doses of GnRH analogs increased intracellular Ca2+ in the myocardium under resting and electrostimulation conditions through non-conventional GnRH/PKA receptor signaling [72]. As illustrated in Figure 3, this non-conventional signaling can phosphorylate the sarcolemmal L-type calcium channel and phospholamban, which in turn modulate contractile function in cardiomyocytes. In addition, electrolytes modification could play a role in the cardiac QT prolongation observed during a six-month treatment with GnRH agonists [75]. The non-classical action of GnRH/PKA is further supported by the in vitro observation that GnRH can act through both PKA and PKC mediated signaling mechanisms in the mature gonadotrope LbetaT2 cell line [76].
The extra gonadal (adrenal gland and intraprostatic) steroidogenesis is responsible for 5–10% of circulating androgens. In clinics, this extra-gonadal androgen biosynthesis and prostate AR signaling were managed by non-steroidal antiandrogens and androgen biosynthesis inhibitor (Figs. 1 & 2). Though, the antiandrogen monotherapy is not common across the globe, still it is given in few Asian and European countries during clinical management of PCa. The androgen biosynthesis inhibitor and antiandrogens will inhibit CYP17A1-mediated androgen precursor synthesis and AR signaling, respectively (Fig. 2). The extra gonadal steroidogenesis has limited influence on physiological testosterone. In fact, antiandrogen monotherapy may slightly increase the circulating testosterone than the normal physiological level [77]. In this scenario, these elevated testosterone levels may be associated with elevated CV risk, as seen with supraphysiological testosterone administration associated with elevated hematocrit [78]. Yet, the higher peripheral testosterone can be regulated through negative feed-back hypothalamic-pituitary-adrenal [HPA] axis [21, 22]. This might be different from the supra physiological testosterone levels observed during androgen supplementation. It is currently undetermined whether the antiandrogens associated normal or supra physiological testosterone levels have any causal relationship with CVD in men with PCa. Few observational studies (Table 1) suggest that antiandrogen monotherapy is associated with increased CHD, MI, arrhythmia, heart failure and stroke (Table 1). Hemelrijck and group suggested that antiandrogen monotherapy increased the CV risk events during the first year of therapy and the risk become non-significant over the time [30]. Similarly, the thromboembolic disease events are reduced in PCa patients who received antiandrogens monotherapy compared to age-matched PCa patients [31]. Interestingly, a multicenter observational study revealed that patients who received antiandrogen monotherapy had reduced CV risk events compared to the PCa patients who received GnRH agonists as ADT [79]. The protective effect of testosterone in general may contribute to the reduced/non-significant observation between antiandrogens and CVD risk [31]. There is also a possibility that the patients who receive antiandrogens monotherapy are younger and have less aggressive PCa [30]. In addition, the patients may receive antiandrogens for a short time, and will cross over to systemic ADT such as GnRH agonists treatment which will further dilute the real risk ratio. Further, most of the observational studies describing the associations derive these conclusion from either cancer registry or institutional data base which do not mention about the testosterone levels during antiandrogen monotherapy.
6. Proposed strategies to reduce CV risk and toxicity induced by GnRH agonists: PDE5 inhibition and cGMP signaling
As summarized in Table 3, various types of anticancer drugs have been shown to induce cardiotoxic effects. However, the cellular and molecular mechanisms underlying GnRH agonist-induced cardiotoxicity may be fundamentally different than other major anticancer drugs (Table 3). Despite abundant evidence linking ADT and CVD risks and cardiotoxicity, currently, there is a paucity of studies to identify, develop or even conceptualize strategies to address ADT-induced CVD risk factors and cardiac dysfunction. In order to fill this apparent gap of knowledge, we hypothesize that a class of cGMP-specific phosphodiesterase 5 (PDE5) inhibitors may be effective in reducing CVD adverse effects. Since early 2002, we have described the powerful cardioprotective effects of PDE5 inhibitors (i.e. sildenafil, vardenafil, tadalafil) against myocardial infarction, heart failure, and doxorubicin-induced cardiomyopathy [80–87]. As of today, close to 160 clinical trials with PDE5 inhibitors (http://www.clinicaltrials.gov) have focused on potential CV benefits. However, no study has evaluated the efficacy of PDE5 inhibitors in limiting CVD events in patients with PCa receiving GnRH agonists.
Table 3:
Comparative summary of adverse effects of the commonly used systemic anticancer drugs on cardiovascular health.
| Class of Anticancer Drugs | Types of Drugs in Clinical Use | Potential Events and/or Mechanisms of Anticancer Drug- induced Adverse Effects on Cardiovascular Health |
|---|---|---|
| Androgen-deprivation therapy (ADT) |
GnRH agonists: Buserelin, Goserelin, Histrelin, Leuprolide, Triptorelin, GnRH antagonists: Abarelix, Degarelix, Androgen receptor blockers: Abiraterone acetate, Apalutamide Bicalutamide, Cyproterone, Darolutamide, Flutamide, Nilutamide, Seviteronel |
GnRH agonists increase intracellular Ca2+ and activate PKA signaling that phosphorylates sarcolemmal L-type calcium channel and phospholamban in cardiomyocytes; Prolongation of cardiac QT- interval; Hyperlipidemia; Diabetic complications; Peripheral arterial disease. |
| Alkylating agents | Cyclophosphamide, Cisplatin | Endothelial dy sfunction; Arterial vasoconstriction; Ventricular dysfunction; Microbiota dysbiosis; Renal and vascular damage |
| Anthracyclines | Doxorubicin, Daunorubicin, Epirubicin, Idarubicin | Oxidative stress, DNA damage; Necrotic and apoptotic cell death in heart and blood vessels; Fibrotic and inflammatory changes in vascular wall and myocardium; Endothelial dysfunction; Ventricular dy sfunction; Mitochondrial injury. |
| Cancer immune checkpoint blockade therapy |
CTLA-4 inhibitors: Ipilimumab PD-1 inhibitors: Nivolumab, Pembrolizumab PD-L1 inhibitors: Atezolizumab, Avelumab, BMS-946559, Durvalumab |
Myocarditis and pericarditis; Heart block; Cardiomyopathy; Myocardial fibrosis; Ventricular dysfunction. |
|
Fluoropyrimidines antimetabolites |
Capecitabine, Carmofur, Doxifluridine, 5-Fluorouracil, T egafur | Coronary vasospasm and subsequent myocardial ischemia; Endothelial and myocardial cell apoptosis; Oxidative stress; Mitochondrial dysfunction; Inflammation; Thrombi formation. |
| Tyrosin kinase inhibitors | Pazopanib, Sorabenib, Sunitinib | Endothelial dysfunction; Reduced NO bioavailability; Vascular rarefaction; Hypothyroidism |
| VEGF inhibitors | Bevacizumab, Vandetanib | Endothelial dysfunction; Reduced NO bioavailability; Increased endothelin production and arterial vasoconstriction; Ventricular dysfunction; Platelet aggregation; Myocardial inflammation. |
Abbreviations: ADT-androgen-deprivation therapy; CTLA-4-cytotoxic T-lymphocyte associated protein 4; GnRH-gonadotropin-releasing hormone; NO-nitric oxide; PD-1-programmed cell death 1; PD-L1-ligand of programmed cell death 1; PKA-protein kinase A; VEGF-vascular endothelial growth factor.
As mentioned above, the possible underlying mechanism(s) of GnRH agonist-induced cardiac dysfunction and toxicity relates to GnRHR expression in the heart. Therefore, targeting GnRHR/PKA signaling may be a therapeutic strategy to prevent these CV adverse events [72]. PDE5 inhibitors trigger a cardioprotective effect against doxorubicin-induced cardiotoxicity, by generating therapeutic levels of nitric oxide (NO) and cGMP. The activation of cGMP-dependent protein kinase G (PKG) signaling can potentially modulate the PKA-dependent mechanism that controls cardiomyocyte contractility and viability (Figure 4). In fact, cross-talk between cGMP and cAMP signaling pathways has been demonstrated in human platelets by Li et al. [88] in 2003. However, it remains largely unknown how PKG and PKA interact in the setting of co-treatment with a GnRH agonist and PDE5 inhibitor. Further in-depth studies are clearly needed to fully address this unsolved issue.
Fig. 4. Cross-talk between ADT (GnRH agonists) and cGMP-mediated signaling: potential adverse events and possible preventive mechanisms.
GnRH agonists can regulate PKA signaling in cardiac cells via GnRHRs thereby altering the intracellular calcium. The altered calcium can lead to mitochondrial influx and induce cardiomyocyte apoptosis, eventually impairing cardiac contractility or causing heart failure. PDE5 inhibitors can upregulate NO in cardiac cells through constitutively active eNOS. The increased NO can upregulate cGMP which may protect cardiomyocytes through cGMP/PKG signaling and inhibition of mitochondrial calcium uptake. The GnRH agonists mediated potential CV events are given in the left box and the our perspective are given in the right box along with posssible mechanistic relation. The mitochondrion icon is from Servier Medical Art (http://smart.servier.com), licensed under Creative Common Attribution 3.0. (https://creativecommons.org/licenses/by/3.0/). Abbreviations : cGMP – cyclic guanosine monophosphate, eNOS – endothelial nitric oxide synthase, GC – guanylate cyclase, GTP – guanosine-5′-triphosphate, H2S - hydrogen sulfide, iNOS – inducible NOS, mitoKATP - ATP-sensitive mitochondrial potassium channel, MPTP - membrane permeability transition pore, PDE5 – phosphodiesterase type 5, PKA – protein kinase A, PKG – protein kinase G, MPTP – mitochondrial permeability transition pore, NO – nitric oxide.
7. Summary and perspectives
Given the probability of long-term survival of men with PCa and prolonged treatment with ADT, the systemic and metabolic side effects of this therapy on vital organs and functions is an emerging concern. In particular, the close association between ADT and CVD leads to decreased quality of life as well as life span. This relationship was supported by the fact that CVD is the primary reason for death among men with PCa [9].
A plethora of observational studies have provided evidence that ADT results in various levels of CV risk events, and the risk of CVD is not uniform among different modes of ADT. CV risk events in PCa patients due to orchiectomy and antiandrogen have mixed observations compared to PCa patients who did not received any form of ADT [6, 23–26, 29–31, 36]. Besides, the second generation antiandrogens are not given as first line monotherapy. Nevertheless, observational studies coupled with preclinical evidence suggest that patients with a history of cardiac events may be at higher risk of death among PCa patients who receive GnRH agonists as ADT strategy. Further, ADT-induced metabolic alterations may also cause CV events [46, 48, 51]; which would likely manifest later than the observational time frame of less than a year. Intriguingly, a randomized study which reported that GnRH agonist has higher adverse CV events compared to GnRH antagonist [43]. Thus, further studies to understand the cellular and molecular mechanisms of GnRH agonist-associated cardiotoxicity are still needed, which can help in identifying novel therapeutic targets for alleviating ADT toxicity.
The direct evidence of GnRH agonists on CV predisposing events was shown in various experimental models, supported by the explanation of increased fat mass, insulin sensitivity, and altered metabolism-mediated arterial stiffness and atherosclerosis. Of interest, one preclinical study showed that GnRH agonists could induce adverse CV events through an altered GnRH/PKA axis but further validation is needed [72]. These studies have tested the hypothesis that common strategies of managing PCa patients with CV risk events may not be enough. Significant efforts have been made in the past to intervene in the ADT-induced adverse CV events, but not CVD [3]. Therefore, it is important to consider a safer pharmacological agent to use as a preventive agent irrespective of ADT type. We have built a compelling case for the potential use of PDE5 inhibitors (e.g., sildenafil citrate) in preventing and/or treating ADT (GnRH agonist)-induced cardiotoxicity in PCa patients. Because PDE5 inhibitors are FDA approved and widely prescribed drugs outside the context of PCa, these drugs hold great promise as a repurposed cardioprotectant that could be used in conjunction with ADT for local, advanced or metastatic PCa patients.
Considering the efficacy and number of patients receiving ADT, providers should discuss the increased CVD risk and monitor patients closely. Because of the recent change in the treatment landscape of ADT to ADT with enzalutamide or chemo-hormonal therapy for advanced PCa, there is an increase in the number of patients under ADT and hence, the interest in the associated CVD risk events also increased. Interestingly, a recent meta-analysis by Iacovelli et al. [89] revealed that the use of the second-generation hormonal agent abiraterone acetate also significantly increased CVD risk. The risk is higher during the early period of ADT (up to 12 months) treatment; hence the concurrent monitoring of hormones such as androgens, insulin, FSH, ACTH might be useful to track the development of metabolic disease, which is considered one of the causes of CVD. Further, a baseline cardiac risk assessment should be performed to tailor the cancer treatment and cardiac management strategy. Therefore, until pharmacological agents like the one proposed above are validated in a prospective clinical trial, physicians should consider a comprehensive effort to minimize/mitigate ADT-induced adverse events. If needed, a collaborative, multidisciplinary approach should be considered by involving oncologists and cardiologists to overcome cardiac comorbidities.
Search strategy and selection criteria
References for this review were identified through searches of PubMed with the search terms “androgen deprivation therapy”, “heart”, “toxicity”, and “prostate cancer” from 1985 until March 2020. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final list of 89 references was generated on the basis of originality and relevance to the broad scope of this review.
Highlights.
Androgen deprivation therapy (ADT) causes various adverse effects, including increased risk of cardiovascular events
Improved life span and novel androgen receptor inhibitors further extend the risk of cardiovascular disease among cancer survivors
Among various ADT agents, gonadotropin releasing hormone agonists are associated with higher cardiotoxicity
The underlying molecular mechanisms of ADT-induced cardiotoxicity is still limited
Acknowledgments
We thank Dr. Jessica Mercer for her editorial contributions. The authors are in part supported by grants from the National Institutes of Health (UO1 CA185148 to SKB; RO1 CA221813, DK120866, HL118808 to RCK; and RO1 HL134366 to RCK & AD) and the Department of Defense Idea Award (PC170891 to SKB & RCK). LX is a recipient of the Pauley Pilot Research Grant from the VCU Pauley Heart Center. The funding agencies had no involvement in the conception, design or views presented in the review.
Abbreviations
- ADT
Androgen deprivation therapy
- AMH
Anti-Müllerian hormone
- Apa
Apalutamide
- ApoE
Apolipoprotein E
- AR
Androgen receptor
- ARPI
AR pathway inhibitors
- CAD
Coronary artery disease
- cGMP
Cyclic guanosine monophosphate
- CV
Cardiovascular
- CVD
Cardiovascular disease
- CYP17A1
Cytochrome P450 family 17 subfamily A member 1
- DES
Diethylstilbestrol
- DHEA
Dehydroepiandrosterone
- DHT
Dihydrotestosterone
- eNOS
Endothelial nitric oxide synthase
- EORTC
European Organization for Research and Treatment of Cancer
- ESC
European Society of Cardiology
- FSH
Follicle-stimulating hormone
- GC
Guanylate cyclase
- GnRH
Gonadotropin-releasing hormone
- GnRH
Gonadotropin-releasing hormone
- GTP
Guanosine-5′-triphosphate
- iNOS
Inducible NOS
- LDLR
Low density lipoprotein receptor
- LH
Luteinizing hormone
- LH
Luteinizing hormone
- MPTP
Mitochondrial permeability transition pore
- NO
Nitric oxide
- PCa
Prostate cancer
- PDE5
Phosphodiesterase 5
- PKA
Protein kinase A
- PKA
Protein kinase A
- PKG
Protein kinase G
- PSA
Prostate-specific antigen
- RCT
Randomized controlled trials
- SEER
Surveillance, Epidemiology, and End Results
- T
Testosterone
Footnotes
Declaration of interests
SKB is one of the co-founders of Sanguine Diagnostics and Therapeutics, Inc.
LX is a co-founder of Xiamen Innovo Medical Technology Co. Ltd., China
BAT has served as a paid advisor/consultant to Janssen Pharmaceuticals.
Other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].C. Global Burden of Disease Cancer, C. Fitzmaurice TF Akinyemiju FH Lami Al, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, Aremu O, Artaman A, Asgedom SW, Assadi R, Atey TM, Avila-Burgos L, Awasthi A, Ba Saleem HO, Barac A, Bennett JR, Bensenor IM, Bhakta N, Brenner H, Cahuana-Hurtado L, Castaneda-Orjuela CA, Catala-Lopez F, Choi JJ, Christopher DJ, Chung SC, Curado MP, Dandona L, Dandona R, das Neves J, Dey S, Dharmaratne SD, Doku DT, Driscoll TR, Dubey M, Ebrahimi H, Edessa D, El-Khatib Z, Endries AY, Fischer F, Force LM, Foreman KJ, Gebrehiwot SW, Gopalani SV, Grosso G, Gupta R, Gyawali B, Hamadeh RR, Hamidi S, Harvey J, Hassen HY, Hay RJ, Hay SI, Heibati B, Hiluf MK, Horita N, Hosgood HD, Ilesanmi OS, Innos K, Islami F, Jakovljevic MB, Johnson SC, Jonas JB, Kasaeian A, Kassa TD, Khader YS, Khan EA, Khan G, Khang YH, Khosravi MH, Khubchandani J, Kopec JA, Kumar GA, Kutz M, Lad DP, Lafranconi A, Lan Q, Legesse Y, Leigh J, Linn S, Lunevicius R, Majeed A, Malekzadeh R, Malta DC, Mantovani LG, McMahon BJ, Meier T, Melaku YA, Melku M, Memiah P, Mendoza W, Meretoja TJ, Mezgebe HB, Miller TR, Mohammed S, Mokdad AH, Moosazadeh M, Moraga P, Mousavi SM, Nangia V, Nguyen CT, Nong VM, Ogbo FA, Olagunju AT, Pa M, Park EK, Patel T, Pereira DM, Pishgar F, Postma MJ, Pourmalek F, Qorbani M, Rafay A, Rawaf S, Rawaf DL, Roshandel G, Safiri S, Salimzadeh H, Sanabria JR, Santric Milicevic MM, Sartorius B, Satpathy M, Sepanlou SG, Shackelford KA, Shaikh MA, Sharif-Alhoseini M, She J, Shin MJ, Shiue I, Shrime MG, Sinke AH, Sisay M, Sligar A, Sufiyan MB, Sykes BL, Tabares-Seisdedos R, Tessema GA, Topor-Madry R, Tran TT, Tran BX, Ukwaja KN, Vlassov VV, Vollset SE, Weiderpass E, Williams HC, Yimer NB, Yonemoto N, Younis MZ, Murray CJL, Naghavi M, Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study, JAMA Oncol 4(11) (2018) 1553–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A, Cancer treatment and survivorship statistics, 2016, CA Cancer J Clin 66(4) (2016) 271–89. [DOI] [PubMed] [Google Scholar]
- [3].Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B, Smith MR, Adverse effects of androgen deprivation therapy and strategies to mitigate them, Eur Urol 67(5) (2015) 825–36. [DOI] [PubMed] [Google Scholar]
- [4].Gupta D, Lee Chuy K, Yang JC, Bates M, Lombardo M, Steingart RM, Cardiovascular and Metabolic Effects of Androgen-Deprivation Therapy for Prostate Cancer, J Oncol Pract 14(10) (2018) 580–587. [DOI] [PubMed] [Google Scholar]
- [5].Shahinian VB, Kuo YF, Gilbert SM, Reimbursement policy and androgen-deprivation therapy for prostate cancer, N Engl J Med 363(19) (2010) 1822–32. [DOI] [PubMed] [Google Scholar]
- [6].Keating NL, O’Malley AJ, Freedland SJ, Smith MR, Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer, J Natl Cancer Inst 102(1) (2010) 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Keating NL, O’Malley AJ, Smith MR, Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer, J Clin Oncol 24(27) (2006) 4448–56. [DOI] [PubMed] [Google Scholar]
- [8].Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS, Urologic P. Diseases in America, Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer, Cancer 110(7) (2007) 1493–500. [DOI] [PubMed] [Google Scholar]
- [9].Epstein MM, Edgren G, Rider JR, Mucci LA, Adami HO, Temporal trends in cause of death among Swedish and US men with prostate cancer, J Natl Cancer Inst 104(17) (2012) 1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huggins C, and Hodges CV, Studies on Prostatic Cancer: I. The Effect of Castration, of Estrogen and of Androgen on Serum Phosphatases in Metastatic Carcinoma of the Prostate Cancer Research 1 (1941) 293–297. [Google Scholar]
- [11].Nishiyama T, Serum testosterone levels after medical or surgical androgen deprivation: a comprehensive review of the literature, Urol Oncol 32(1) (2014) 38 e17–28. [DOI] [PubMed] [Google Scholar]
- [12].Conn PM, Crowley WF Jr., Gonadotropin-releasing hormone and its analogs, Annu Rev Med 45 (1994) 391–405. [DOI] [PubMed] [Google Scholar]
- [13].Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, Comaru-Schally AM, Schally AV, Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists, Proc Natl Acad Sci U S A 79(5) (1982) 1658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shore ND, Experience with degarelix in the treatment of prostate cancer, Ther Adv Urol 5(1) (2013) 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Klotz L, Miller K, Crawford ED, Shore N, Tombal B, Karup C, Malmberg A, Persson BE, Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists, Eur Urol 66(6) (2014) 1101–8. [DOI] [PubMed] [Google Scholar]
- [16].Salciccia S, Gentilucci A, Cattarino S, Sciarra A, GNRH-agonist or antagonist in the treatment of prostate cancer: a comparision based on oncological results, Urologia 83(4) (2016) 173–178. [DOI] [PubMed] [Google Scholar]
- [17].Lopes RA, Neves KB, Pestana CR, Queiroz AL, Zanotto CZ, Chignalia AZ, Valim YM, Silveira LR, Curti C, Tostes RC, Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement, Am J Physiol Heart Circ Physiol 306(11) (2014) H1485–94. [DOI] [PubMed] [Google Scholar]
- [18].Ruizeveld de Winter JA, Trapman J, Vermey M, Mulder E, Zegers ND, van der Kwast TH, Androgen receptor expression in human tissues: an immunohistochemical study, J Histochem Cytochem 39(7) (1991) 927–36. [DOI] [PubMed] [Google Scholar]
- [19].Ikeda Y, Aihara K, Yoshida S, Akaike M, Matsumoto T, Effects of androgens on cardiovascular remodeling, J Endocrinol 214(1) (2012) 1–10. [DOI] [PubMed] [Google Scholar]
- [20].Wynne FL, Khalil RA, Testosterone and coronary vascular tone: implications in coronary artery disease, J Endocrinol Invest 26(2) (2003) 181–6. [DOI] [PubMed] [Google Scholar]
- [21].Goodale T, Sadhu A, Petak S, Robbins R, Testosterone and the Heart, Methodist Debakey Cardiovasc J 13(2) (2017) 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morris PD, Channer KS, Testosterone and cardiovascular disease in men, Asian J Androl 14(3) (2012) 428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gandaglia G, Sun M, Popa I, Schiffmann J, Abdollah F, Trinh QD, Saad F, Graefen M, Briganti A, Montorsi F, Karakiewicz PI, The impact of androgen-deprivation therapy (ADT) on the risk of cardiovascular (CV) events in patients with non-metastatic prostate cancer: a population-based study, BJU Int 114(6b) (2014) E82–E89. [DOI] [PubMed] [Google Scholar]
- [24].Van Hemelrijck M, Garmo H, Holmberg L, Ingelsson E, Bratt O, Bill-Axelson A, Lambe M, Stattin P, Adolfsson J, Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population-Based PCBaSe Sweden, J Clin Oncol 28(21) (2010) 3448–56. [DOI] [PubMed] [Google Scholar]
- [25].Azoulay L, Yin H, Benayoun S, Renoux C, Boivin JF, Suissa S, Androgen-deprivation therapy and the risk of stroke in patients with prostate cancer, Eur Urol 60(6) (2011) 1244–50. [DOI] [PubMed] [Google Scholar]
- [26].Hu JC, Williams SB, O’Malley AJ, Smith MR, Nguyen PL, Keating NL, Androgen-deprivation therapy for nonmetastatic prostate cancer is associated with an increased risk of peripheral arterial disease and venous thromboembolism, Eur Urol 61(6) (2012) 1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR, Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality, J Natl Cancer Inst 99(20) (2007) 1516–24. [DOI] [PubMed] [Google Scholar]
- [28].Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Zamorano JL, Aboyans V, Achenbach S, Agewall S, Badimon L, Baron-Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol C, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, Lancellotti P, Lip GY, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Roffi M, Torbicki A, Vaz Carneiro A, Windecker S, Authors M./Task Force, E.S.C.C.f.P. Guidelines, R. Document, 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC), Eur J Heart Fail 19(1) (2017) 9–42. [DOI] [PubMed] [Google Scholar]
- [29].Jespersen CG, Norgaard M, Borre M, Androgen-deprivation therapy in treatment of prostate cancer and risk of myocardial infarction and stroke: a nationwide Danish population-based cohort study, Eur Urol 65(4) (2014) 704–9. [DOI] [PubMed] [Google Scholar]
- [30].O’Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M, Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer, J Clin Oncol 33(11) (2015) 1243–51. [DOI] [PubMed] [Google Scholar]
- [31].O’Farrell S, Sandstrom K, Garmo H, Stattin P, Holmberg L, Adolfsson J, Van Hemelrijck M, Risk of thromboembolic disease in men with prostate cancer undergoing androgen deprivation therapy, BJU Int 118(3) (2016) 391–8. [DOI] [PubMed] [Google Scholar]
- [32].Thomsen FB, Sandin F, Garmo H, Lissbrant IF, Ahlgren G, Van Hemelrijck M, Adolfsson J, Robinson D, Stattin P, Gonadotropin-releasing Hormone Agonists, Orchiectomy, and Risk of Cardiovascular Disease: Semi-ecologic, Nationwide, Population-based Study, Eur Urol 72(6) (2017) 920–928. [DOI] [PubMed] [Google Scholar]
- [33].Sun M, Choueiri TK, Hamnvik OP, Preston MA, De Velasco G, Jiang W, Loeb S, Nguyen PL, Trinh QD, Comparison of Gonadotropin-Releasing Hormone Agonists and Orchiectomy: Effects of Androgen-Deprivation Therapy, JAMA Oncol 2(4) (2016) 500–7. [DOI] [PubMed] [Google Scholar]
- [34].Nanda A, Chen MH, Moran BJ, Braccioforte MH, Dosoretz D, Salenius S, Katin M, Ross R, D’Amico AV, Neoadjuvant hormonal therapy use and the risk of death in men with prostate cancer treated with brachytherapy who have no or at least a single risk factor for coronary artery disease, Eur Urol 65(1) (2014) 177–85. [DOI] [PubMed] [Google Scholar]
- [35].Nanda A, Chen MH, Braccioforte MH, Moran BJ, D’Amico AV, Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction, JAMA 302(8) (2009) 866–73. [DOI] [PubMed] [Google Scholar]
- [36].Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, Paszat LF, Impact of androgen deprivation therapy on cardiovascular disease and diabetes, J Clin Oncol 27(21) (2009) 3452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M, Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis, Eur Urol 68(3) (2015) 386–96. [DOI] [PubMed] [Google Scholar]
- [38].Guo Z, Huang Y, Gong L, Gan S, Chan FL, Gu C, Xiang S, Wang S, Association of androgen deprivation therapy with thromboembolic events in patients with prostate cancer: a systematic review and meta-analysis, Prostate Cancer Prostatic Dis 21(4) (2018) 451–460. [DOI] [PubMed] [Google Scholar]
- [39].Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, Beckman JA, Choueiri TK, Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials, JAMA 306(21) (2011) 2359–66. [DOI] [PubMed] [Google Scholar]
- [40].Wilcox C, Kautto A, Steigler A, Denham JW, Androgen deprivation therapy for prostate cancer does not increase cardiovascular mortality in the long term, Oncology 82(1) (2012) 56–8. [DOI] [PubMed] [Google Scholar]
- [41].Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J, Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist, Eur Urol 65(3) (2014) 565–73. [DOI] [PubMed] [Google Scholar]
- [42].Studer UE, Whelan P, Albrecht W, Casselman J, de Reijke T, Hauri D, Loidl W, Isorna S, Sundaram SK, Debois M, Collette L, Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891, J Clin Oncol 24(12) (2006) 1868–76. [DOI] [PubMed] [Google Scholar]
- [43].Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, Akaza H, Bossi A, van Veenhuyzen DF, Selby B, Fan X, Kang V, Walling J, Tombal B, Investigators HS, Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer, N Engl J Med (2020). [DOI] [PubMed] [Google Scholar]
- [44].Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R, Hypogonadism and metabolic syndrome: implications for testosterone therapy, J Urol 174(3) (2005) 827–34. [DOI] [PubMed] [Google Scholar]
- [45].Elagizi A, Kohler TS, Lavie CJ, Testosterone and Cardiovascular Health, Mayo Clin Proc 93(1) (2018) 83–100. [DOI] [PubMed] [Google Scholar]
- [46].Basaria S, Lieb J 2nd, Tang AM, DeWeese T, Carducci M, Eisenberger M, Dobs AS, Long-term effects of androgen deprivation therapy in prostate cancer patients, Clin Endocrinol (Oxf) 56(6) (2002) 779–86. [DOI] [PubMed] [Google Scholar]
- [47].Langley RE, Cafferty FH, Alhasso AA, Rosen SD, Sundaram SK, Freeman SC, Pollock P, Jinks RC, Godsland IF, Kockelbergh R, Clarke NW, Kynaston HG, Parmar MK, Abel PD, Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09), Lancet Oncol 14(4) (2013) 306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, Kantoff PW, Changes in body composition during androgen deprivation therapy for prostate cancer, J Clin Endocrinol Metab 87(2) (2002) 599–603. [DOI] [PubMed] [Google Scholar]
- [49].Tayek JA, Heber D, Byerley LO, Steiner B, Rajfer J, Swerdloff RS, Nutritional and metabolic effects of gonadotropin-releasing hormone agonist treatment for prostate cancer, Metabolism 39(12) (1990) 1314–9. [DOI] [PubMed] [Google Scholar]
- [50].Boxer RS, Kenny AM, Dowsett R, Taxel P, The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer, Aging Male 8(3–4) (2005) 207–12. [DOI] [PubMed] [Google Scholar]
- [51].Haseen F, Murray LJ, Cardwell CR, O’Sullivan JM, Cantwell MM, The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis, J Cancer Surviv 4(2) (2010) 128–39. [DOI] [PubMed] [Google Scholar]
- [52].Paneni F, Costantino S, Cosentino F, Insulin resistance, diabetes, and cardiovascular risk, Curr Atheroscler Rep 16(7) (2014) 419. [DOI] [PubMed] [Google Scholar]
- [53].Cefalu WT, Wang ZQ, Werbel S, Bell-Farrow A, Crouse JR 3rd, Hinson WH, Terry JG, Anderson R, Contribution of visceral fat mass to the insulin resistance of aging, Metabolism 44(7) (1995) 954–9. [DOI] [PubMed] [Google Scholar]
- [54].Bhatia N, Santos M, Jones LW, Beckman JA, Penson DF, Morgans AK, Moslehi J, Cardiovascular Effects of Androgen Deprivation Therapy for the Treatment of Prostate Cancer: ABCDE Steps to Reduce Cardiovascular Disease in Patients With Prostate Cancer, Circulation 133(5) (2016) 537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Smith MR, Lee H, McGovern F, Fallon MA, Goode M, Zietman AL, Finkelstein JS, Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome, Cancer 112(10) (2008) 2188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tivesten A, Pinthus JH, Clarke N, Duivenvoorden W, Nilsson J, Cardiovascular risk with androgen deprivation therapy for prostate cancer: potential mechanisms, Urol Oncol 33(11) (2015) 464–75. [DOI] [PubMed] [Google Scholar]
- [57].Falk E, Shah PK, Fuster V, Coronary plaque disruption, Circulation 92(3) (1995) 657–71. [DOI] [PubMed] [Google Scholar]
- [58].Hansson GK, Inflammation, atherosclerosis, and coronary artery disease, N Engl J Med 352(16) (2005) 1685–95. [DOI] [PubMed] [Google Scholar]
- [59].Hopmans SN, Duivenvoorden WC, Werstuck GH, Klotz L, Pinthus JH, GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model, Urol Oncol 32(8) (2014) 1126–34. [DOI] [PubMed] [Google Scholar]
- [60].Knutsson A, Hsiung S, Celik S, Rattik S, Mattisson IY, Wigren M, Scher HI, Nilsson J, Hultgardh-Nilsson A, Treatment with a GnRH receptor agonist, but not the GnRH receptor antagonist degarelix, induces atherosclerotic plaque instability in ApoE(−/−) mice, Sci Rep 6 (2016) 26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chen HF, Jeung EB, Stephenson M, Leung PC, Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro, J Clin Endocrinol Metab 84(2) (1999) 743–50. [DOI] [PubMed] [Google Scholar]
- [62].Cavanagh PC, Dunk C, Pampillo M, Szereszewski JM, Taylor JE, Kahiri C, Han V, Lye S, Bhattacharya M, Babwah AV, Gonadotropin-releasing hormone-regulated chemokine expression in human placentation, Am J Physiol Cell Physiol 297(1) (2009) C17–27. [DOI] [PubMed] [Google Scholar]
- [63].Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, Jensen JK, Olesen TK, Schroder FH, The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel- group phase III study in patients with prostate cancer, BJU Int 102(11) (2008) 1531–8. [DOI] [PubMed] [Google Scholar]
- [64].Stilley JA, Christensen DE, Dahlem KB, Guan R, Santillan DA, England SK, Al-Hendy A, Kirby PA, Segaloff DL, FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice, Biol Reprod 91(3) (2014) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu XM, Chan HC, Ding GL, Cai J, Song Y, Wang TT, Zhang D, Chen H, Yu MK, Wu YT, Qu F, Liu Y, Lu YC, Adashi EY, Sheng JZ, Huang HF, FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway, Aging Cell 14(3) (2015) 409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Huhtaniemi IT, Dahl KD, Rannikko S, Hsueh AJ, Serum bioactive and immunoreactive follicle-stimulating hormone in prostatic cancer patients during gonadotropin-releasing hormone agonist treatment and after orchidectomy, J Clin Endocrinol Metab 66(2) (1988) 308–13. [DOI] [PubMed] [Google Scholar]
- [67].Eldar-Geva T, Liberty G, Chertin B, Fridmans A, Farkas A, Margalioth EJ, Spitz IM, Relationships between FSH, inhibin B, anti-Mullerian hormone, and testosterone during long-term treatment with the GnRH-agonist histrelin in patients with prostate cancer, Eur J Endocrinol 162(1) (2010) 177–81. [DOI] [PubMed] [Google Scholar]
- [68].Crawford ED, Schally AV, Pinthus JH, Block NL, Rick FG, Garnick MB, Eckel RH, Keane TE, Shore ND, Dahdal DN, Beveridge TJR, Marshall DC, The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy, Urol Oncol 35(5) (2017) 183–191. [DOI] [PubMed] [Google Scholar]
- [69].Skinner DC, Albertson AJ, Navratil A, Smith A, Mignot M, Talbott H, Scanlan-Blake N, Effects of gonadotrophin-releasing hormone outside the hypothalamic-pituitary-reproductive axis, J Neuroendocrinol 21(4) (2009) 282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kakar SS, Jennes L, Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues, Cancer Lett 98(1) (1995) 57–62. [PubMed] [Google Scholar]
- [71].Naor Z, Harris D, Shacham S, Mechanism of GnRH receptor signaling: combinatorial cross-talk of Ca2+ and protein kinase C, Front Neuroendocrinol 19(1) (1998) 1–19. [DOI] [PubMed] [Google Scholar]
- [72].Dong F, Skinner DC, Wu TJ, Ren J, The heart: a novel gonadotrophin-releasing hormone target, J Neuroendocrinol 23(5) (2011) 456–63. [DOI] [PubMed] [Google Scholar]
- [73].Kuo IY, Ehrlich BE, Signaling in muscle contraction, Cold Spring Harb Perspect Biol 7(2) (2015) a006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR, PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts, Cell 101(4) (2000) 365–76. [DOI] [PubMed] [Google Scholar]
- [75].Gagliano-Juca T, Travison TG, Kantoff PW, Nguyen PL, Taplin ME, Kibel AS, Huang G, Bearup R, Schram H, Manley R, Beleva YM, Edwards RR, Basaria S, Androgen Deprivation Therapy Is Associated With Prolongation of QTc Interval in Men With Prostate Cancer, J Endocr Soc 2(5) (2018) 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Grafer CM, Thomas R, Lambrakos L, Montoya I, White S, Halvorson LM, GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems, Mol Endocrinol 23(7) (2009) 1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lund F, Rasmussen F, Flutamide versus stilboestrol in the management of advanced prostatic cancer. A controlled prospective study, Br J Urol 61(2) (1988) 140–2. [DOI] [PubMed] [Google Scholar]
- [78].Olga M ABS. Calof, Lee Martin L., Kenny Anne M., Urban Randall J., Joyce L. Tenover, Shalender Bhasin, Adverse Events Associated With Testosterone Replacement in Middle-Aged and Older Men: A Meta-Analysis of Randomized, Placebo-Controlled Trials, The Journals of Gerontology: Series A 60(11) 1451–1457. [DOI] [PubMed] [Google Scholar]
- [79].Morgia G, Russo GI, Tubaro A, Bortolus R, Randone D, Gabriele P, Trippa F, Zattoni F, Porena M, Mirone V, Serni S, Del Nero A, Lay G, Ricardi U, Rocco F, Terrone C, Pagliarulo A, Ludovico G, Vespasiani G, Brausi M, Simeone C, Novella G, Carmignani G, Leonardi R, Pinnaro P, De Paula U, Corvo R, Tenaglia R, Siracusano S, Mantini G, Gontero P, Savoca G, Ficarra V, Foundation LUNA, d.U. Societa Italiana, Prevalence of Cardiovascular Disease and Osteoporosis During Androgen Deprivation Therapy Prescription Discordant to EAU Guidelines: Results From a Multicenter, Cross-sectional Analysis From the CHOsIng Treatment for Prostate canCEr (CHOICE) Study, Urology 96 (2016) 165–170. [DOI] [PubMed] [Google Scholar]
- [80].Das A, Xi L, Kukreja RC, Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling, J Biol Chem 280(13) (2005) 12944–55. [DOI] [PubMed] [Google Scholar]
- [81].Das A, Xi L, Kukreja RC, Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta, J Biol Chem 283(43) (2008) 29572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC, Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice, Am J Physiol Heart Circ Physiol 294(3) (2008) H1398–406. [DOI] [PubMed] [Google Scholar]
- [83].Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC, Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity, Circulation 111(13) (2005) 1601–10. [DOI] [PubMed] [Google Scholar]
- [84].Koka S, Das A, Zhu SG, Durrant D, Xi L, Kukreja RC, Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin- induced cardiomyopathy without interfering with chemotherapeutic effect, J Pharmacol Exp Ther 334(3) (2010) 1023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, Park MA, Qureshi I, Lee R, Dent P, Kukreja RC, Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction, Proc Natl Acad Sci U S A 107(42) (2010) 18202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Das A, Durrant D, Mitchell C, Dent P, Batra SK, Kukreja RC, Sildenafil (Viagra) sensitizes prostate cancer cells to doxorubicin- mediated apoptosis through CD95, Oncotarget 7(4) (2016) 4399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Das A, Durrant D, Salloum FN, Xi L, Kukreja RC, PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer, Pharmacol Ther 147 (2015) 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Li Z, Ajdic J, Eigenthaler M, Du X, A predominant role for cAMP-dependent protein kinase in the cGMP-induced phosphorylation of vasodilator-stimulated phosphoprotein and platelet inhibition in humans, Blood 101(11) (2003) 4423–9. [DOI] [PubMed] [Google Scholar]
- [89].Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, Muraglia A, Porcaro AB, Siracusano S, Brunelli M, Mazzarotto R, Artibani W, Tortora G, The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer, Clin Genitourin Cancer 16(3) (2018) e645–e653. [DOI] [PubMed] [Google Scholar]