Abstract
Context
The mechanism of oligo-anovulation in polycystic ovary syndrome (PCOS) is unknown.
Objectives
To evaluate follicular and endocrine characteristics of anovulatory and sporadic ovulatory cycles in women with PCOS.
Design
Prospective, longitudinal study.
Setting
Academic clinical research unit.
Participants
26 reproductive-aged women (18-38 years) with PCOS, observed during natural anovulatory (PCOS-Anov; n = 12) and sporadic ovulatory cycles (PCOS-Ov; n = 14), and 12 controls.
Interventions
Transvaginal ultrasonography and venipuncture were performed every other day for 4 to 6 weeks in women with PCOS or at 1 interovulatory interval in control subjects.
Main Outcome Measures
Follicle number and diameter (ie, ≥2 mm) were quantified at each visit. Individual growth profiles were assessed for all follicles that grew to ≥7 mm. Blood samples were assayed for follicle-stimulating hormone, luteinizing hormone, estradiol, and progesterone.
Results
Follicular excess, or heightened follicle number versus controls, was observed across anovulatory and sporadic ovulatory cycles in PCOS. In PCOS-Anov, follicles emerged cyclically in some women (6/12; 50%) and continuously in others (6/12; 50%), then grew to a mean maximum diameter of 7.2 mm and regressed within 4.7 days. In PCOS-Ov, follicles mostly emerged cyclically as part of a cohort and dominant follicles showed normal growth to ovulation—albeit mean and maximum luteal progesterone concentrations were significantly lower versus controls.
Conclusions
Follicle growth and regression were detected on ultrasonography amidst perpetual follicular excess in PCOS. Documentation of continuous follicle recruitment and turnover, the absence of persistence, and altered luteal progesterone following sporadic ovulation, provide formative data on antral follicle development in PCOS.
Keywords: polycystic ovary syndrome, anovulation, ovarian follicle, ultrasonography
Polycystic ovary syndrome (PCOS) is the main cause of anovulatory infertility in women, but the mechanism of oligo-anovulation is unknown. Most insight into disordered folliculogenesis in PCOS has been garnered from histologic studies; authors have described an “excess” of pre- and small antral follicles (1-3), and suggested that the premature “arrest” (4, 5) and “persistence” of larger antral follicles culminates in a failure of selection and ovulation (6, 7). Few studies have focused on the advanced stages of follicular development (ie, ≥2 mm), and consequently, actual disruptions in recruitment, selection, and ovulation have remained unexplored.
Antral follicle recruitment occurs in a cyclic manner during the normal menstrual cycle (8). Two or more cohorts of follicles grow and regress synchronously until 1 dominant follicle (≥10 mm) is selected for preferential growth in the early to mid-follicular phase. Increases in antral follicle count (AFC) ≥5 mm and follicle-stimulating hormone (FSH) define recruitment, whereas dominant follicle expression of luteinizing hormone (LH) receptors and production of estradiol (E2) are associated with selection (9, 10). Adequate E2 production from the dominant follicle provides the necessary systemic signals to induce an LH surge, ovulation, and progression to the luteal phase (10). Ultimately, in vivo investigations are needed to understand how recruitment, selection, and ovulation are disrupted in PCOS and, thereby, inform treatment strategies for women with oligo-anovulation.
Polycystic ovarian morphology (PCOM) reflects aspects of disordered folliculogenesis in PCOS (11). Follicular “excess,” or an increased number of follicles from the primary (0.06 mm) to small antral stage (2-5 mm), is thought to arise from increased initiation of follicular growth from the primordial pool (3), slower maturation of primary follicles (2), and/or decreased follicle loss by atresia (12). The simultaneous paucity of larger follicles (ie, >5 mm) is posited to result from both follicular and endocrine abnormalities (11, 13). Elevated LH and steroidogenic enzyme activity can augment intra-ovarian androgen production (14, 15). Androgens may then stimulate excessive follicular growth to the recruitable stage (2-5 mm) (11), where reduced concentrations of FSH may inhibit subsequent growth (11, 13). Granulosa cells from polycystic ovaries (PCO) also exhibit premature responsiveness to LH in follicles ≤4 mm, implying an early acquisition of LH receptors (4, 10). These functional abnormalities presumably terminate follicular growth within the 6- to 9-mm stage; the premature cessation of follicular growth has been termed follicular “arrest” (11, 13). Androgens may also prevent atresia and contribute to a state wherein some follicles remain at their maximum diameters for an extended period of time; an absence of timely follicular regression has been termed follicular “persistence” (6, 7).
There is still a great deal to learn about disordered antral follicle development in PCOS. First, it is unknown whether follicle populations change over time or whether follicular excess is a constant phenomenon in PCOS. Second, it is unknown whether the paucity of larger follicles (>5 mm) reflects follicular arrest or some other defect. Follicular arrest has never been observed in vivo, which is notable, as natural ovulation can occasionally occur and follicular growth can be stimulated by pharmacologic therapy (11, 13). Although histologic studies have revealed that some follicles in PCO have normal function (4), follicle growth kinetics and luteal function during sporadic ovulatory cycles have never been described in PCOS. Finally, it is unknown whether follicles are protected from atresia, and therefore, persist indefinitely, in PCO (6, 7). A more thorough understanding of the defects leading to oligo-anovulation is important for optimizing management strategies for infertility, as women with PCOS have an increased risk for ovarian hyperstimulation syndrome (OHSS) and often show resistance to first-line ovulation induction protocols (16).
High-resolution transvaginal ultrasonography provides a noninvasive means to answer these questions, as it allows for the reliable detection of follicles ≥2 mm (17). Follicular growth, atresia, and ovulation have been serially evaluated in women with regular menstrual cycles (8, 9, 18) and the techniques can be applied to PCOS. Early observations in our laboratory revealed substantial changes in follicle diameter categories on ultrasound scans performed 1 month apart in women with PCOS (19). These findings led to the notion that PCOM may not solely represent a condition of follicular excess, arrest, and persistence. The primary objective of this study was to characterize follicular and endocrine features of anovulatory cycles in women with PCOS. However, while completing these investigations, we serendipitously observed a series of natural ovulations in a subset of oligomenorrheic participants. Therefore, a secondary, post hoc objective of this study was to evaluate follicular and endocrine features of sporadic ovulatory cycles in women with PCOS. We hypothesized that: (i) follicular excess and arrest, but not persistence, would be detected during anovulatory cycles; and (ii) abnormal ovulatory follicle growth and luteal endocrine dynamics would be observed during sporadic ovulatory cycles, in women with PCOS.
Methods
Ethical considerations
Data from 2 prospective ongoing trials were evaluated in the present analysis. Both research protocols were approved by the Institutional Review Board at Cornell University and registered at ClinicalTrials.gov (Identifiers: NCT01927432, NCT01785719). Written, informed consent was obtained from all participants before study procedures were initiated.
Study participants
Women of reproductive age (18-38 years) were recruited from the general population from May 2009 to October 2017. Women who had consistent and optimal visualization of both ovaries on ultrasonography were eligible to participate. Exclusion criteria were the use of medications known or suspected to interfere with reproductive function in the 2 months prior to the study; pregnancy or lactation in the 6 months prior to the study; and history of premature ovarian failure or confounding medical conditions. Women with untreated thyroid abnormalities or hyperprolactinemia were excluded (20). One woman was taking metformin during the study, but sensitivity analyses confirmed that her inclusion in the dataset did not impact the endpoints of interest.
Women who completed the study protocols were retrospectively evaluated for inclusion herein (n = 80). Groups of interest included: (i) women with PCOS and (ii) controls. PCOS was identified by the National Institutes of Health (NIH) criteria (21), with attention to the recommendations set forth by the 2018 International Evidence-based Guideline (22). Oligo- or anovulation was judged by self-reported history of irregular menstrual cycles (ie, >35 days apart in the year prior to enrollment). Hyperandrogenism was defined as a modified hirsutism score ≥8, free androgen index (FAI) ≥6, free testosterone (T) concentration ≥0.815 ng/dL, or bioavailable T concentration ≥19.06 ng/dL (22). Thresholds to define androgen status were derived using an internal reference population of reproductive-aged women with regular menstrual cycles. Women with PCOS were further stratified based on ultrasonographic evidence of anovulation (PCOS-Anov) or sporadic ovulation (PCOS-Ov) during the study. In women with PCOS-Anov, follicular growth, but not ovulation, was observed. Nonobese women (body mass index [BMI] 18.5-30.0 kg/m2) with regular ovulatory cycles (ie, every 21-35 days) and normal androgen status were identified as controls.
Ultrasonographic measurements
Serial transvaginal ultrasonography was used to evaluate antral follicle development (8, 23, 24). In women with PCOS, scans began at a random time and were performed every other day for 4 to 6 weeks. In controls, scans were initiated in the mid-follicular phase and continued every other day for 1 interovulatory interval (IOI). An IOI was defined as the time between consecutive ovulations and represented the luteal phase of one cycle followed by the follicular phase of the next cycle (8, 24). If a large follicle (ie, ≥14 mm) was detected in either group, then ultrasound examinations were performed daily until its regression or ovulation. Ovulation was identified by the observation of a corpus luteum (25, 26) and confirmed with a subsequent rise in serum progesterone (P4) (26).
Scans were performed using a GE Voluson E8 Expert System and 6-12 MHz endovaginal transducer (GE Healthcare, Milwaukee, WI). During each examination, ovaries were imaged from their inner to outer margins in the longitudinal plane using the automated volume modality. Two-dimensional cineloops were then extracted from the 3-dimensional datasets, archived, and evaluated manually using customized software (Sante DICOM Editor, Santesoft LTD, Nicosia, Cyprus). Ninety percent of the images were reviewed by a single investigator (B.Y.J.); a second investigator (A.L.O.) with strong inter-rater agreement in assessments of ovarian morphology evaluated the remaining images. Follicle number and diameter were assessed for each ovary and visit of the scanning interval. Reliable follicle counts were achieved using the grid system approach (27). Diameter measurements were obtained in the largest cross-sectional view of the follicle and calculated as the average of its 2 orthogonal dimensions (ie, length × width). If a large follicle (≥10 mm) was detected, then these measurements were repeated in a second plane and the 4 dimensions were averaged. Mean follicle diameter was rounded to the nearest whole number (24). Mean ovarian volume was determined offline using an approximation of the formula for a prolate ellipsoid (28).
Growth and regression profiles of individual follicles that grew to ≥7 mm were assessed using the Identity Method (8, 23, 24). Briefly, the diameter and location of follicles that grew to ≥4 mm were sketched on paper for each ovary and visit of the scanning interval. Locations of individual follicles were designated by anatomical landmarks and positions relative to other follicles within the ovary and cineloop. Each follicle ≥7 mm was alphabetized (ie, uniquely identified), and changes in its diameter were tracked over time from day of first detection (ie, at 4-5 mm) to last detection (ie, at 4-5 mm) (8, 23, 24). Of note, the Non-Identity Method, which involves tabulating and sorting all follicles ≥4 mm in a hierarchical order by size (8, 23, 24), was not feasible in women with PCOS; the increased number of follicles 4 to 6 mm in size made it impossible to resolve and characterize individual growth trajectories in this way (data not shown).
As a result, growth and regression rates were determined based solely on data collected with the Identity Method. Sonographic presence was defined as the time between the first and last day of detection of a follicle (29). The growth phase of a follicle began on the day of first detection and ended on the day of maximal diameter (18). The regression phase of a follicle began on the day of maximal diameter and ended on the day of last detection (18, 29). Static phases were identified when a follicle was detected within 1 mm of its maximal diameter for at least 3 days (ie, 2 visits) (18, 29). Data from the first and last day of a static phase coincided with the end of the growth phase and beginning of the regression phase, respectively, and were included in calculations of growth and regression rates (29). Additional assessments of growth phase and growth rate were made for ovulatory dominant follicles between selection and ovulation.
Follicle number and diameter data were combined for both ovaries based on convention in women with regular menstrual cycles (8, 24). Further, in the present analysis, no differences were detected in the number of uniquely identified follicles between the left and right ovaries across groups (data not shown). The total number and proportion of follicles detected across diameter categories were graphed for each woman over the scanning interval. Diameter categories of interest (ie, AFCs) included: ≥2 mm, 2-9 mm (for the diagnostic feature of mean follicle number per ovary), 2-5 mm, ≥5 mm, 6-9 mm, and ≥10 mm. Growth profiles of uniquely identified follicles were also graphed for each woman.
Overall, “disordered follicle development” was characterized by observation of abnormal follicular and/or endocrine events in women with PCOS. Follicular “excess” was identified by an elevated number of follicles in any diameter category in women with PCOS. “Arrest” was defined by attainment of smaller mean maximum follicle diameters, whereas “persistence” was judged by longer sonographic presences and static phases, in women with PCOS compared to controls.
Cyclic recruitment was defined morphologically by an increase and subsequent decrease in AFC ≥5 mm, in association with the growth of ≥1 uniquely identified follicle to ≥7 mm (8, 24). Development of a dominant follicle was identified when 1 follicle grew to ≥10 mm and exceeded the diameter of the next largest follicle by ≥2 mm. Selection was judged as the day on which the future dominant follicle became, and remained, larger than all other follicles simultaneously detected in the ovary (9).
Biochemical and clinical measurements
Nonfasted blood samples were drawn every other day during the scanning interval to assess reproductive hormones. Blood was collected into a clot-activated tube and allowed to sit at room temperature for 30 to 60 minutes. Serum was isolated by centrifugation and stored at −80 °C until the time of analysis. Chemiluminescence enzyme immunoassays (Immulite 2000, Siemens Medical Solutions Diagnostics, Deerfield, IL) were performed to measure serum concentrations of FSH, LH, E2, and P4. Inter- and intra-assay coefficients of variation (CV) were as follows: FSH (5.4%, 3.4%), LH (5.4%, 3.4%), E2 (9.7%, 6.7%), and P4 (12.2%, 9.7%).
Fasted blood samples were drawn on a single morning of the study to assess androgen and metabolic status. Timepoints were standardized in both groups such that no dominant follicles or active corpora lutea were present at the time of venipuncture. Sex hormone–binding globulin (SHBG) and fasting insulin were evaluated by immunoassay, as indicated above. Inter- and intra-assay CVs were as follows: SHBG (5.0%, 3.1%) and insulin (6.2%, 4.8%). Total T was measured by liquid chromatography–tandem mass spectrometry (inter-assay CV: 6.4%) (30, 31). FAI was calculated as: (total T [nmol/L] / SHBG [nmol/L]) × 100 (32), and free and bioavailable T were determined based on a standard concentration of albumin (4.3 g/dL) and using an online calculator (www.issam.ch/freetesto.htm). Glucose was quantified with a standard glucometer (Accu-Check Aviva, Roche Diabetes Care, Inc., Indianapolis, IN), and the homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as a surrogate marker of insulin sensitivity as: (fasting glucose [mmol/L] × fasting insulin [mIU/mL]) ÷ 22.5 (33).
Anthropometry was performed on a single day of the study. Participants were weighed with lightweight clothes and no shoes on a standard digital scale, and BMI was calculated as weight (kilograms) divided by squared height (meters). Waist circumference was measured with soft tape at the midpoint between the lowest rib and iliac crest. Dual x-ray absorptiometry was also conducted (Discovery-A, Hologic, Inc., Bedford, MA). Total adiposity was determined through measurement of fat versus lean mass in soft tissue, and abdominal adiposity was distinguished as the region between the ribs and iliac crests.
Statistical analysis
All statistical analyses were performed with JMP Pro 12.0.1. (SAS Institute, Cary, NC). Skewed data were log-transformed prior to analysis, and the threshold for statistical significance was set at P < 0.05.
Follicular and endocrine data were centralized to the first study visit (PCOS) or ovulation (control) and evaluated in 2 ways: (i) normalized across the mean scanning interval, and (ii) averaged across the follicular and luteal phases (34). Due to the variable and unexpected timing of sporadic ovulations, we did not capture complete follicular phases (ie, from ovulatory follicle emergence to ovulation) and/or luteal phases (ie, from ovulation to menses) in all women; therefore, follicular and endocrine data were only assessed at distinct time points or averaged across menstrual cycle phases in women with PCOS-Ov.
Mixed-effects models were used to evaluate serial follicular and endocrine data in women with PCOS-Anov and controls. Combined models, including data for women with PCOS-Anov and controls, were performed to determine between-group differences in follicle number, diameter categories, growth parameters, and reproductive hormones (Main Effect: PCOS). Group-specific models, including data for 1 group at a time, were conducted to assess within-group changes in follicle number, diameter categories, and reproductive hormones (Main Effect: Day). Models were fitted separately to determine whether changes occurred in a linear, quadratic, cubic, or quartic pattern (34). Participant number, as well as participant number crossed with day, were included as random effects in all cases. The restricted maximum likelihood approach was used to identify the degree of intra-cycle versus inter-individual variation in follicular and endocrine endpoints.
Data obtained at a prespecified time (ie, on a single day or averaged across the follicular or luteal phases) were compared between groups using 2-sample t tests or 1-way analysis of covariance models, with post hoc Tukey honest significance difference tests, as appropriate (34). Metabolic endpoint comparisons were adjusted for BMI.
Results
Study participant characteristics
Thirty-eight women were eligible for inclusion in the present analysis (PCOS-Anov: n = 12; PCOS-Ov: n = 14; Controls: n = 12); demographic, diagnostic, and metabolic features are compared across groups in Table 1. Women with PCOS-Anov and PCOS-Ov were of similar ages (Both: P ≥ 0.10) but had higher BMIs (Both: P < 0.01) compared with controls. Women with PCOS-Anov differed from controls across all diagnostic features (All: P < 0.01), while women with PCOS-Ov had longer menstrual cycles (P < 0.01) and higher androgen concentrations (All: P < 0.01), but similar hirsutism scores (P = 0.15), to controls. Mean follicle number per ovary (2-9 mm) tended to be higher in women with PCOS-Ov (P = 0.06) relative to controls. When compared with their anovulatory counterparts, women with PCOS-Ov tended to have shorter menstrual cycles and lower FAI (All: P = 0.07), as well as lower free and bioavailable T concentrations and smaller mean ovarian volumes (All: P = 0.02). Women with PCOS-Anov and PCOS-Ov also had similar metabolic features to each other; however, any differences versus controls (Table 1) were lost after adjustment for BMI (All: P ≥ 0.10).
Table 1.
Demographic, Diagnostic, and Metabolic Characteristics of Study Participants
| Parameter | PCOS-Anov (n = 12) | PCOS-Ov (n = 14) | Control (n = 12) |
|---|---|---|---|
| Age, y | 26 ± 4 (21, 34)a | 27 ± 5 (20, 34)a | 30 ± 6 (19, 38)a |
| Body mass index, kg/m2 | 33.5 ± 9.1 (21.4, 48.4)a | 31.6 ± 7.2 (21.8, 46.2)a | 23.7 ± 1.5 (21.4, 26.6)b |
| Menstrual cycle length, d | 158 ± 125 (45, 365†)a | 80 ± 46 (39, 180)a | 29 ± 2 (24, 31)b |
| Modified hirsutism score | 9 ± 4 (2, 15)a | 6 ± 4 (1, 13)a, b | 3 ± 3 (0, 7ǂ)b |
| Free androgen index | 10 ± 6 (4, 23)a | 7 ± 4 (3, 16)a | 2 ± 1 (1, 3)b |
| Free testosterone, ng/dL | 1.5 ± 0.6 (0.7, 3.0)a | 1.0 ± 0.3 (0.5, 1.8)b | 0.4 ± 0.1 (0.2, 0.6)c |
| Bioavailable testosterone, ng/dL | 34.1 ± 13.5 (16.5, 69.8)a | 22.8 ± 7.4 (12.8, 41.2)b | 8.3 ± 2.7 (4.0, 13.9)c |
| Total testosterone, ng/dL | 76.0 ± 26.8 (37.2, 118.0)a | 57.1 ± 21.0 (24.4, 98.9)a | 30.2 ± 9.0 (17.6, 43.6)b |
| Mean follicle number per ovary | 53 ± 21 (14, 80)a | 46 ± 37 (14, 152)a, b | 23 ± 7 (15, 36)b |
| Mean ovarian volume, mL | 15 ± 4 (9, 20)a | 11 ± 3 (7, 18)b | 8 ± 3 (4, 13)c |
| Waist circumference, cm | 102 ± 26 (63, 157)a | 92 ± 18 (64, 127)a | 82 ± 6 (73, 92)a |
| Total adiposity, % | 37.9 ± 7.6 (27.1, 47.9)a | 37.6 ± 6.8 (27.3, 46.3)a | 27.7 ± 4.8 (18.8, 37.3)b |
| Abdominal adiposity, % | 38.3 ± 9.3 (21.3, 51.8)a | 35.9 ± 9.5 (21.7, 50.3)a | 23.9 ± 5.3 (15.3, 34.7)b |
| Fasting insulin, mmol/L | 20.2 ± 18.6 (2.2, 56.1)a | 10.8 ± 5.7 (2.2, 22.0)a | 4.6 ± 2.5 (2.0, 9.9)b |
| HOMA-IR | 5.1 ± 4.8 (0.5, 14.7)a | 2.6 ± 1.4 (0.5, 5.4)a | 1.1 ± 0.6 (0.5, 2.2)b |
Data are presented as mean ± standard deviation (minimum, maximum). Within rows, different superscript letters denote significant differences between groups, P < 0.05. Diagnostic, endocrine, and metabolic endpoints were evaluated on a single day of the scanning interval and with respect to phase of the menstrual cycle.
Abbreviations: HOMA-IR, homeostatic model assessment of insulin resistance.
†Data related to menstrual cycle length were censored to the year prior to enrollment (ie, 365 days). ǂTwo of 12 women in the control group had modified hirsutism scores of 7, while the remainder had scores ≤6.
Women with PCOS were evaluated for an average of 33 days (standard deviation [SD]: 8 days) (PCOS-Anov: 32 ± 4 days; PCOS-Ov: 34 ± 10 days). Of the 12 women with PCOS-Anov, most reported amenorrhea (n = 7; 58%), with 3 having an absence of natural menstruation for ≥1 year prior to study enrollment. The remaining 5 women reported oligomenorrhea (42%), with last menses occurring from 4 to 60 days before the first study visit (mean ± SD, 27 ± 24 days). None of the women with PCOS-Anov showed ultrasonographic or endocrinologic evidence of ovulation during the scanning interval. By contrast, of the 14 women with PCOS-Ov, most reported oligomenorrhea (n = 11; 79%), while the remaining 3 had a mean menstrual cycle length of 90 to 180 days in the year prior to enrollment. Last menses occurred approximately 1 month prior to the first study visit (mean ± SD, 30 ± 25 days; range, 8-83). All women with PCOS-Ov had ultrasonographic or endocrinologic evidence of at least 1 ovulation during the scanning interval, with 9 exhibiting 1 sporadic ovulation and 5 exhibiting either a partial (n = 2) or complete IOI (n = 3). As a result, women in this group were observed in the follicular phase, preceding an ovulation, for an average of 23 days (SD: 8 days; range, 13-39). A complete luteal phase was documented in 8 of 14 women (mean ± SD, 13 ± 2 days; range, 7-16); mean luteal phase lengths did not differ from those in controls (P = 0.57). Controls demonstrated normal IOI (mean ± SD, 29 ± 4 days), follicular phase (mean ± SD, 16 ± 4 days), and luteal phase lengths (mean ± SD, 13 ± 1 days) (8, 35). The selection and ovulation of a dominant follicle was detected at least twice in each woman in this group (ie, at the beginning and end of the IOI).
Evaluation of follicular excess
PCOS effect during anovulatory cycles.
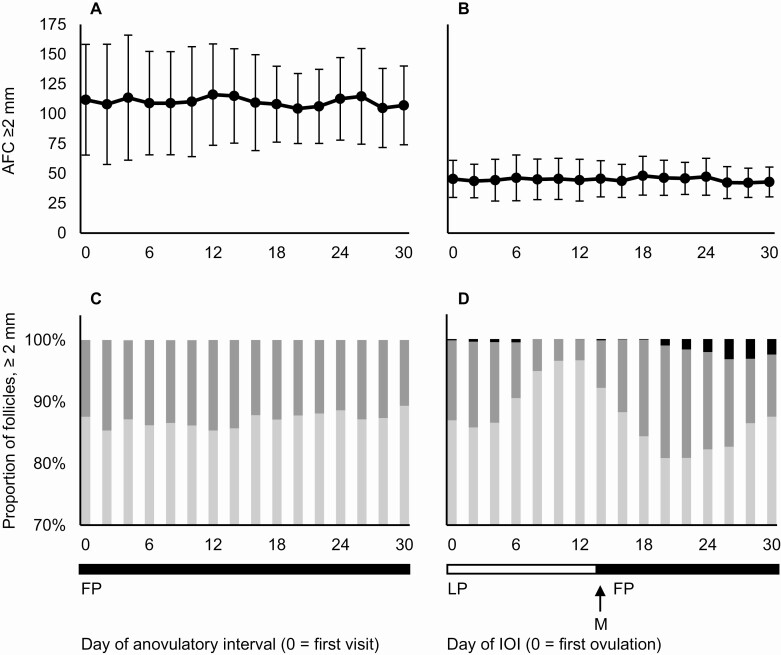
Mean profiles of AFC ≥2 mm are depicted for women with PCOS-Anov and controls in Fig. 1A and B, respectively. On any given day, AFC ≥2 mm was increased in women with PCOS-Anov compared with controls (least-squares mean difference ± standard error, 33 ± 6 follicles; PPCOS < 0.01). These differences between groups were consistent across other follicle diameter categories, including AFC 2-5 mm (29 ± 6 follicles; PPCOS < 0.01) and 6-9 mm (4 ± 1 follicles; PPCOS < 0.01).
Figure 1.
Longitudinal profiles of follicle number and follicle diameter category in women with PCOS-Anov (n = 12) and controls (n = 12). AFC ≥2 mm (mean ± standard deviation) is shown during an anovulatory interval in women with PCOS-Anov (A) and interovulatory interval in controls (B). Mean follicle number per diameter category, expressed as a proportion of AFC ≥2 mm, is also shown over time for both groups (C and D, respectively). Proportions of 2-5 mm (light gray), 6-9 mm (dark gray), and ≥10 mm (black) follicles are presented, with y-axes truncated for better visibility of changes. Abbreviations: AFC, antral follicle count; IOI, interovulatory interval; FP, follicular phase; LP, luteal phase; M, menses.
Day effect during anovulatory cycles.
To illustrate changes in follicle diameter, mean profiles of follicle diameter categories were expressed as a proportion of AFC ≥2 mm in women with PCOS-Anov and controls (Fig. 1C and D, respectively). The number of follicles in each diameter category remained constant across the anovulatory cycle in women with PCOS-Anov, with no effect of day for AFC ≥2 mm (PDay = 0.87), 2-5 mm (PDay = 0.79), or 6-9 mm (PDay = 0.16). Most of the variation in follicle diameter categories was attributed to inter-individual differences (2-5 mm: 92%; 6-9 mm: 78%), rather than intra-cycle fluctuation (2-5 mm: 8%; 6-9 mm: 22%). AFC ≥2 mm also remained constant over time in controls (PDay = 0.87). However, a significant effect of day was detected in the number of 2-5 mm (PDay = 0.02) and 6-9 mm follicles (PDay = 0.03). Variation in follicle diameter categories was attributed to inter-individual differences (2-5 mm: 89%; 6-9 mm: 43%), as well as intra-cycle fluctuation particularly in the 6- to 9-mm category (2-5 mm: 11%; 6-9 mm: 57%). Mixed-model evaluations of a day effect could not be performed in women with PCOS-Ov, owing to variability in the timing of the sporadic ovulations observed; instead, follicular data were averaged over menstrual cycle phases for comparisons to controls and women with PCOS-Anov.
By menstrual cycle phase during sporadic ovulatory and anovulatory cycles.
Mean follicle numbers per diameter category are presented by menstrual cycle phase in Table 2. Compared with controls, AFC ≥2 mm was elevated in the follicular phase (P = 0.02), and tended to be higher during the luteal phase (P = 0.06), of sporadic ovulatory cycles in women with PCOS-Ov. The number of 2-5 mm follicles also tended to be higher during both the follicular (P = 0.07) and luteal phases (P = 0.08), while the number of 6-9 mm follicles tended to be higher only during the luteal phase (P = 0.08), in women with PCOS-Ov. Significant differences in mean follicle numbers were not detected between women with PCOS-Anov and PCOS-Ov (All: P ≥ 0.10); yet, subjectively, AFCs ≥2 mm, 2-5 mm, and 6-9 mm in women with PCOS-Ov appeared to be intermediate to those in women with PCOS-Anov and controls.
Table 2.
Follicle Number per Diameter Category During Anovulatory (PCOS-Anov) and Sporadic Ovulatory Cycles (PCOS-Ov) in Women with PCOS
| Parameter | PCOS-Anov (n = 12) | PCOS-Ov (n = 14) | Control (n = 12) |
|---|---|---|---|
| Follicular phase† | |||
| AFC ≥2 mm | 110 ± 38 (56, 169)a | 92 ± 74 (31, 293)ǂ, a | 45 ± 14 (26, 68)b |
| AFC 2-5 mm | 88 ± 39 (26, 151)a | 82 ± 75 (22, 287)ǂ, a, b | 39 ± 14 (20, 66)b |
| AFC 6-9 mm | 11 ± 6 (0, 22)a | 10 ± 5 (2, 22)ǂ, a, b | 6 ± 3 (1, 13)b |
| Luteal phase | |||
| AFC ≥2 mm | NA | 78 ± 50 (37, 187)$, a | 45 ± 16 (20, 78)a |
| AFC 2-5 mm | NA | 71 ± 49 (32, 181)$, a | 41 ± 16 (18, 77)a |
| AFC 6-9 mm | NA | 6 ± 4 (2, 16)$, a | 4 ± 2 (0, 7)a |
Data are presented as mean ± standard deviation (minimum, maximum). Within rows, different superscript letters denote significant differences between groups, P < 0.05.
Abbreviations: AFC, antral follicle count; NA, not available or estimable.
†Data were obtained from an anovulatory interval in women with PCOS-Anov. ǂComplete follicular phase data, from at or before ovulatory follicle emergence to ovulation, were available for 12 of 14 women with PCOS-Ov. $Complete luteal phase data, from ovulation to menses, were available for 8 of 14 women with PCOS-Ov.
Evaluation of follicular arrest and persistence
Follicles that grew to ≥7 mm were uniquely identified and tracked over time across groups (n = 1001); growth characteristics of anovulatory follicles (n = 983) and ovulatory follicles (n = 15 in women with PCOS-Ov vs n = 13 in controls) were considered separately. Of those tracked, complete growth and regression profiles were available for 235 anovulatory follicles in women with PCOS-Anov, 143 anovulatory follicles in women with PCOS-Ov, and 70 anovulatory follicles in controls (Table 3). Overall, in women with PCOS-Anov, follicles tended to be detected for shorter intervals (ie, sonographic presence; P = 0.05) than in controls and tended to grow to smaller maximum diameters than in women with PCOS-Ov (P = 0.09). However, no other differences in growth phases/rates, static phases, or regression phases/rates were detected in anovulatory follicles across groups (All: P ≥ 0.10) (Table 3).
Table 3.
Growth Parameters of Uniquely Identified Anovulatory Follicles During Anovulatory (PCOS-Anov) and Sporadic Ovulatory Cycles (PCOS-Ov) in Women with PCOS
| Parameter | PCOS-Anov (n = 12) | PCOS-Ov (n = 14) | Control (n = 12) |
|---|---|---|---|
| Sonographic presence, d | 9.7 ± 0.4 (8.8, 10.5)a | 10.1 ± 0.4 (9.3, 11.0)a | 11.0 ± 0.5 (10.0, 12.0)a |
| Growth phase, d | 3.9 ± 0.1 (3.6, 4.2)a | 4.0 ± 0.2 (3.7, 4.3)a | 4.2 ± 0.2 (3.9, 4.6)a |
| Growth rate, mm/d | 0.60 ± 0.02 (0.56, 0.64)a | 0.63 ± 0.02 (0.59, 0.67)a | 0.63 ± 0.02 (0.58, 0.67)a |
| Static phase, d† | 4.7 ± 0.2 (4.3, 5.1)a | 4.7 ± 0.2 (4.3, 5.1)a | 4.8 ± 0.3 (4.2, 5.3)a |
| Maximum diameter, mm | 7.2 ± 0.0 (7.1, 7.3)a | 7.3 ± 0.1 (7.2, 7.4)a | 7.4 ± 0.1 (7.2, 7.5)a |
| Regression phase, d | 4.1 ± 0.2 (3.7, 4.5)a | 4.5 ± 0.2 (4.1, 5.0)a | 4.6 ± 0.2 (4.2, 5.1)a |
| Regression rate, mm/d | –0.58 ± 0.02 (–0.63, –0.53)a | –0.62 ± 0.03 (–0.67, –0.57)a | –0.56 ± 0.03 (–0.62, –0.50)a |
Data are presented as least-squares mean ± standard error (95% confidence interval). Within rows, different superscript letters denote significant differences between groups, P < 0.05. Models included data from 235 anovulatory follicles in women with PCOS-Anov, 143 anovulatory follicles in women with PCOS-Ov, and 70 anovulatory follicles in controls. †Static phases were detected in 74% of follicles (175 / 235) in women with PCOS-Anov, 73% of follicles (104 / 143) in women with PCOS-Ov, and 77% of follicles (54 / 70) in controls.
Evaluation of disordered follicle development
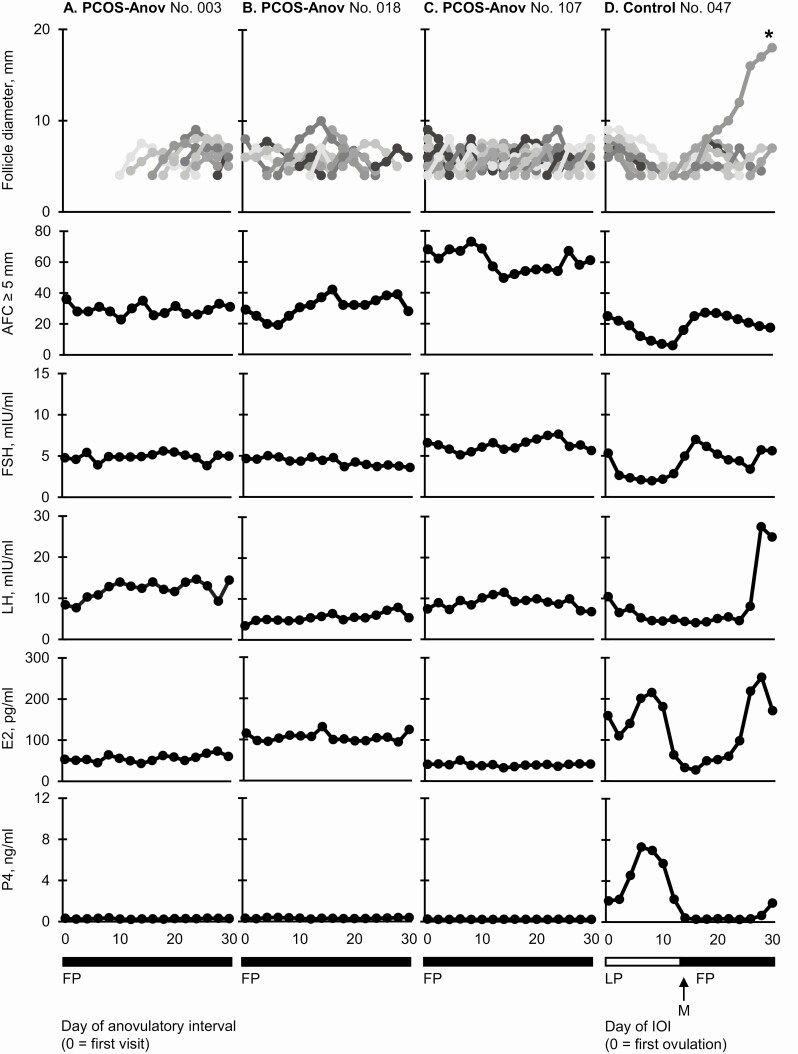
Recruitment.
Representative follicular and endocrine data are provided for 3 women with PCOS-Anov and 1 control in Fig. 2. A spectrum of disordered follicle development was observed in women with PCOS-Anov. At the mild end of the spectrum, <20% of follicles grew to ≥7 mm; follicles emerged as part of either 1 (n = 1) or 3 cohorts (n = 3), in association with changes in AFC ≥5 mm, across the anovulatory interval (ie, evidence of cyclic recruitment; n = 4; 33%) (Fig. 2A). In the middle of the spectrum, 20% to 40% of follicles grew to ≥7 mm (n = 4; 33%), and cyclic recruitment was detected 3 times (n = 2) (Fig. 2B). However, unlike in the mild group, 2 women exhibited continuous recruitment, wherein follicles emerged seemingly independent of a cohort and changes in AFC ≥5 mm (n = 2). Last, at the severe end of the spectrum, >40% of follicles grew to ≥7 mm, and evidence of cyclic recruitment could not be identified. Follicles appeared to emerge continuously throughout the anovulatory interval, with all growth profiles overlapping and any synchronous follicular growth impossible to resolve (n = 4; 33%) (Fig. 2C). On an individual level, reproductive hormones did not appear to fluctuate over time (Fig. 2A-C). By contrast, recruitment was detected 1 (n = 2; 17%), 2 (n = 8; 67%) (Fig. 2D), or 3 (n = 2; 17%) times during the IOI in all controls evaluated, in line with previous reports in regularly menstruating women (8, 24).
Figure 2.
Representative profiles of follicle growth and reproductive hormone concentrations during an anovulatory interval in women with PCOS-Anov (n = 3) and an interovulatory interval in controls (n = 1). Profiles represent women at the mild (A), moderate (B), and severe ends (C) of the spectrum of antral follicle development in PCOS-Anov, alongside a typical control (D). Each uniquely identified follicle is represented by a different gray-scale line. All follicles that grew to ≥7 mm could be individually tracked over time using the Identity Method. Asterisks indicate ovulatory follicles. Abbreviations: E2, estradiol; FP, follicular phase; FSH, follicle-stimulating hormone; IOI, interovulatory interval; LH, luteinizing hormone; LP, luteal phase; M, menses; P4, progesterone.
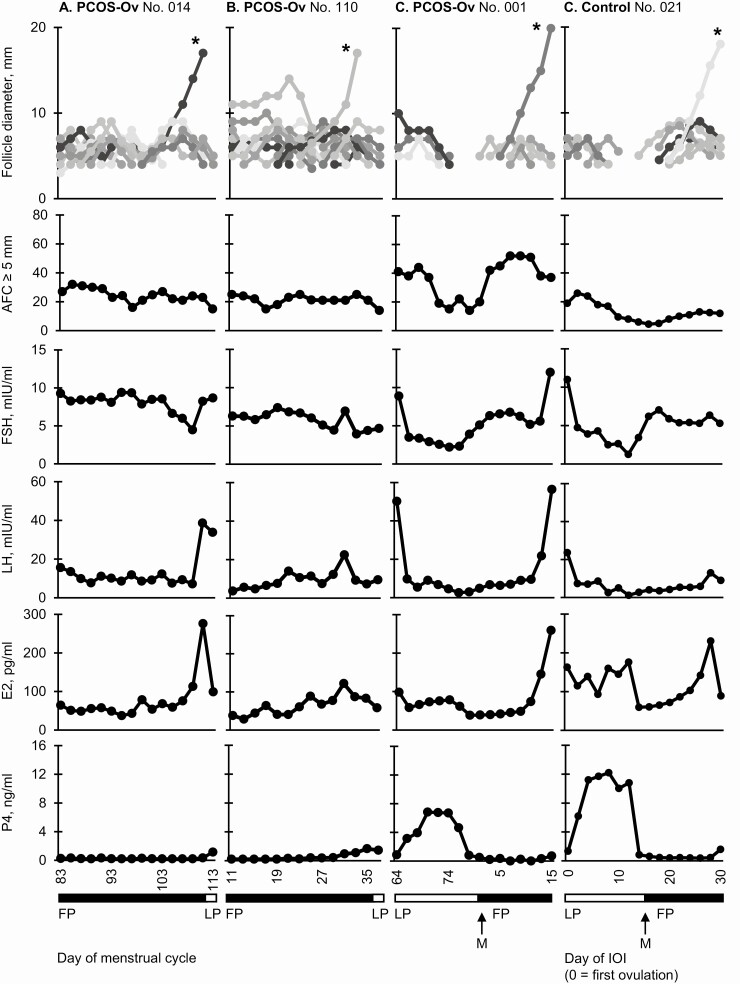
In addition, representative data are provided for 3 women with PCOS-Ov in Fig. 3. Cyclic recruitment was detected 1 (n = 2), 2 (n = 4), 3 (n = 4), or ≥4 times (n = 2) across the sporadic ovulatory cycle (n = 12; 86%). Although some anovulatory follicles appeared to emerge continuously, most could be linked to a cohort and aligned to changes in AFC ≥5 mm, similar to observations in women in the middle of the spectrum of PCOS-Anov (Fig. 3A). In 2 women with PCOS-Ov, evidence of cyclic recruitment could not be identified (n = 2; 14%); instead, a single ovulatory follicle emerged and became selected, seemingly by chance, amidst a pool of follicles undergoing continuous growth and regression (Fig. 3B). Ovulatory follicles emerged at variable times during the scanning interval, including at the end (Fig. 3A), beginning (not pictured), or in line with an IOI (Fig. 3C). On an individual level, reproductive hormones appeared to fluctuate similarly to controls, particularly during the periovulatory and luteal phases (Fig. 3D).
Figure 3.
Representative profiles of follicle growth and reproductive hormone concentrations during a sporadic ovulatory cycle in women with PCOS-Ov (n = 3) and an interovulatory interval (IOI) in controls (n = 1). Profiles exemplify cyclic (A) and continuous (B) recruitment in PCOS-Ov and compare an IOI in 1 woman with PCOS-Ov (C) to an IOI in 1 control (D). Each uniquely identified follicle is represented by a different gray-scale line. All follicles that grew to ≥7 mm could be individually tracked over time using the Identity Method. Asterisks indicate ovulatory follicles. Abbreviations: E2, estradiol; FP, follicular phase; FSH, follicle-stimulating hormone; LH, luteinizing hormone; LP, luteal phase; M, menses; P4, progesterone.
Selection and ovulation.
Complete growth profiles were available for 12 of 15 sporadic ovulatory follicles in women with PCOS-Ov and 13 of 13 ovulatory follicles in controls (Table 4); double ovulation was detected at the end of an IOI in 1 woman with PCOS-Ov and 1 control. Overall, ovulatory follicles exhibited similar growth phases and growth rates from emergence to ovulation, and from selection to ovulation, in women with PCOS-Ov compared to controls (All: P ≥ 0.10). No significant differences were detected between groups in maximum pre-ovulatory diameter (Table 4).
Table 4.
Evaluation of Selection and Sporadic Ovulation in Women with PCOS
| Parameter | PCOS-Anov (n = 12) | PCOS-Ov (n = 14) | Control (n = 12) | P Value |
|---|---|---|---|---|
| Characteristics of anovulatory DFs | ||||
| Incidence, n (%)† | 3 / 12 (25.0) | 1 / 14 (7.1) | 1 / 12 (8.3) | NA |
| Diameter on day of selection, mm | 9 ± 1 (9, 10) | NA | 11 | NA |
| Maximum diameter, mm | 10 ± 0 (10) | 14 | 11 | NA |
| Characteristics of ovulatory DFs | ||||
| Incidence, n (%)† | 0 / 12 (0) | 14 / 14 (100) | 12 / 12 (100) | NA |
| Diameter on day of selection, mm | NA | 11 ± 2 (9, 15)$ | 12 ± 2 (9, 15) | 0.54 |
| Maximum diameter, mm | NA | 19 ± 2 (16, 23)ǂ | 19 ± 2 (17, 23) | 0.83 |
| Emergence to ovulation | ||||
| Growth phase, d | NA | 14 ± 3 (11, 20)ǂ | 14 ± 2 (12, 17) | 0.77 |
| Growth rate, mm/d | NA | 1.08 ± 0.26 (0.76, 1.64)ǂ | 1.08 ± 0.16 (0.82, 1.25) | 0.98 |
| Selection to ovulation | ||||
| Growth phase, d | NA | 7 ± 3 (3, 15)$ | 6 ± 2 (4, 9) | 0.66 |
| Growth rate, mm/d | NA | 1.25 ± 0.42 (0.67, 2.00)$ | 1.26 ± 0.20 (1.00, 1.71) | 0.94 |
| Endocrine characteristics | ||||
| Follicular phase | ||||
| Mean LH, mIU/mL | NR | 13.1 ± 3.8 (7.2, 19.3) | 9.8 ± 2.5 (6.0, 13.9) | 0.02 |
| Mean FSH, mIU/mL | NR | 6.0 ± 1.5 (2.8, 8.1) | 7.8 ± 2.5 (4.3, 12.8) | 0.05 |
| Mean E2, pg/mL | NR | 101.2 ± 23.9 (64.1, 142.5) | 112.6 ± 45.0 (53.5, 204.2) | 0.45 |
| Maximum E2, pg/mL | NR | 255.6 ± 96.7 (121.0, 500.0) | 276.5 ± 88.7 (150.0, 458.0) | 0.50 |
| Luteal phase | ||||
| Mean LH, mIU/mL | NR | 6.2 ± 3.3 (2.8, 12.1) | 6.1 ± 2.8 (2.6, 12.8) | 0.95 |
| Mean FSH, mIU/mL | NR | 2.8 ± 1.2 (1.9, 4.9) | 4.9 ± 2.0 (2.6, 9.1) | <0.01 |
| Mean E2, pg/mL | NR | 122.5 ± 49.7 (67.6, 200.3) | 131.0 ± 25.3 (92.9, 177.7) | 0.67 |
| Mean P4, ng/mL | NR | 4.8 ± 1.4 (3.3, 7.8) | 8.6 ± 3.9 (4.2, 17.9) | <0.01 |
| Maximum P4, ng/mL | NR | 7.8 ± 2.4 (5.2, 13.1) | 15.2 ± 6.5 (7.3, 28.5) | <0.01 |
Data are presented as mean ± standard deviation (minimum, maximum).
Abbreviations: DF, dominant follicle; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; NA, not available or estimable; NR, reported; P4, progesterone.
†This category includes data obtained during the anovulatory interval or follicular phase in women with PCOS-Anov and PCOS-Ov, respectively. $Data were available for 13 / 15 ovulatory follicles. ǂData were available for 12 / 15 ovulatory follicles.
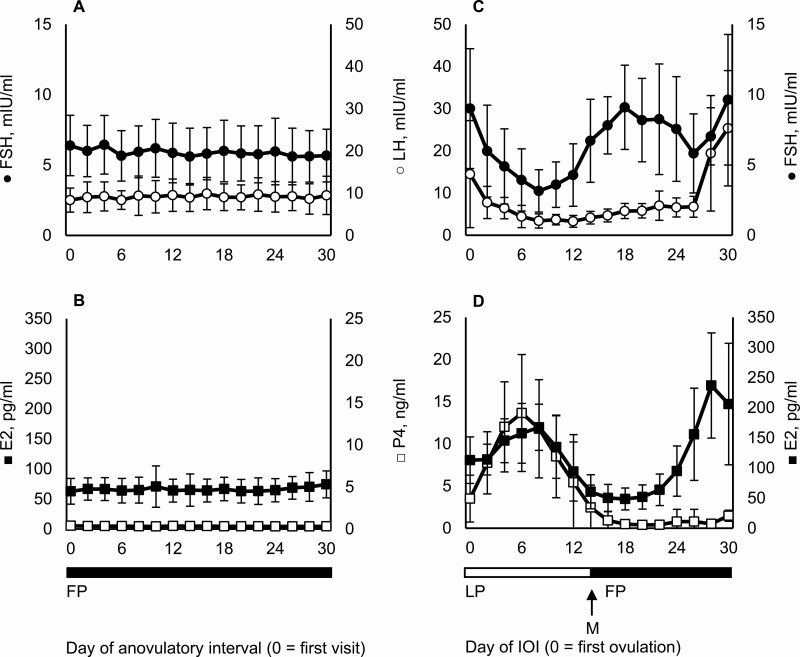
Reproductive hormones.
Mean profiles of reproductive hormones are shown for women with PCOS-Anov and controls in Fig. 4 (Figs 4A-D, respectively). On any given day, LH was higher (least-squares mean ± standard error, 9.1 ± 0.8 mIU/mL vs 8.1 ± 0.8 mIU/mL; PPCOS = 0.01), and E2 (66.1 ± 7.4 ng/mL vs 118.3 ± 7.4 ng/mL; PPCOS < 0.01) and P4 (0.3 ± 0.5 pg/mL vs 4.4 ± 0.5 pg/mL; PPCOS < 0.01) were lower, in women with PCOS-Anov compared with controls. FSH was similar between groups (5.9 ± 0.6 mIU/mL vs 6.6 ± 0.6 mIU/mL; PPCOS = 0.91) (Fig. 4).
Figure 4.
Profiles (mean ± standard deviation) of reproductive hormone concentrations during an anovulatory interval in women with PCOS-Anov (n = 12; A–B) and an interovulatory interval in controls (n = 12; C–D). Abbreviations: E2, estradiol; FP, follicular phase; FSH, follicle-stimulating hormone; LH, luteinizing hormone; LP, luteal phase; M, menses; P4, progesterone.
Reproductive hormones also remained constant over time in women with PCOS-Anov, with no effect of day on FSH (PDay = 0.76), LH (PDay = 0.90), E2 (PDay = 0.29), or P4 (PDay = 0.84). Most of the variation in reproductive hormone concentrations was attributed to inter-individual differences (FSH: 90%; LH: 87%; E2: 80%; P4: 87%), rather than intra-cycle fluctuation (FSH: 10%; LH: 13%; E2: 20%; P4: 13%) (Fig. 4A and B). By contrast, reproductive hormone concentrations changed over time in controls (All: PDay < 0.01). Most of the variation was attributed to intra-cycle fluctuation (FSH: 48%; LH: 81%; E2: 66%; P4: 60%) and not inter-individual differences (FSH: 52%; LH: 19%; E2: 33%; P4: 40%) (Fig. 4C and D).
As mixed-model evaluations of a day effect could not be performed in women with PCOS-Ov, endocrine data were averaged over menstrual cycle phases for comparisons to controls (Table 4). During the follicular phase, LH was higher (P = 0.02), while FSH tended to be lower (P = 0.05), in women with PCOS-Ov versus controls. During the luteal phase, LH was similar (P = 0.95), and FSH was lower (P < 0.01), in women with PCOS-Ov. Women with PCOS-Ov also exhibited significantly lower mean and maximum luteal concentrations of P4 (All: P < 0.01). No differences were detected between groups in follicular or luteal phase concentrations of E2 (All: P ≥ 0.10) (Table 4).
Discussion
Our results provide formative evidence of disordered antral follicle development in PCOS. During anovulatory cycles, follicles became arrested at the mid-antral stage (ie, at 7.2 mm), but regressed in an average of 4.7 days. Follicular recruitment occurred cyclically in some (6/12; 50%) and continuously in others (6/12: 50%), providing the basis for a spectrum of disordered follicle development in PCOS. In addition, the growth kinetics of ovulatory follicles did not differ among controls and women with PCOS-Ov. However, luteal phase concentrations of P4 were significantly lower during sporadic ovulatory cycles. Together, this new knowledge challenges the traditional theory of follicular persistence, and suggests that the primary defects, leading to oligo-anovulation in PCOS are follicular excess and failure of normal recruitment.
An increased number of growing follicles has long been considered a salient feature of PCO (1-3); herein, we extended these cross-sectional observations and showed that follicular excess was constant across an anovulatory interval in women with PCOS. Moreover, follicle number tended to be higher during both the follicular and luteal phases of sporadic ovulatory cycles. Follicular excess was also apparent across diameter categories in women with PCOS-Anov and PCOS-Ov. These data have important implications for the ultrasound diagnosis of PCOS. Evaluations of PCO are currently restricted to the early follicular phase of a natural cycle or following a pharmacologically induced withdrawal bleed (28, 36). This approach limits the time frame to confirm a diagnosis of PCOS, and places burden on patients when menstrual cycle status is uncertain and medications are needed to control symptomology. Our findings suggest that it could be appropriate to assess PCOM at random times; analyses are currently ongoing in our laboratory to confirm this theory.
Our findings provide the first in vivo evidence of follicular arrest in women with PCOS. Previous histologic studies showed that follicles in PCO prematurely acquire LH receptors, halting antral follicle development by the 6- to 9-mm stage (4). Consistent with these data, most uniquely identified follicles stopped growing at a mean diameter of 7.2 mm in women with PCOS-Anov. Although several follicles reached the 10-mm stage, these cases were rare (n = 3) and did not progress to ovulation (9). Growth phases and rates of anovulatory follicles to their maximum diameters did not differ between the PCOS-Anov and control groups. We interpreted these findings to mean that follicles grew at a normal rate until the time of their arrest in women with PCOS-Anov.
Follicular persistence was not documented among uniquely identified follicles in women with PCOS. Anovulatory follicles ≥7 mm tended to have shorter sonographic presences in women with PCOS-Anov, as well as similar static phases in women with PCOS-Anov and PCOS-Ov, compared to anovulatory follicles in controls. These data provide rationale for regular follicular turnover in PCOS and suggest that reduced atresia is not involved in the mechanism of follicular excess (12). However, we cannot exclude the possibility that pre- or small antral follicles are protected from atresia by androgens (7, 12). Although there were no changes over time in the number of small follicles (2-5 mm) in women with PCOS-Anov, the abundance of follicles made it challenging to characterize the growth of individual follicles in the 2- to 5-mm range. Given that assessments of follicle diameter categories are nonspecific in nature, it is possible that we did not capture the complete picture of antral follicle development in PCOS.
A spectrum of disordered follicle development was observed in women with PCOS, with some women exhibiting cyclic recruitment of 1 to 4 follicular cohorts, and others showing continuous recruitment across the scanning interval. In women with PCOS-Anov, evidence of continuous recruitment was further supported by an absence of intra-cycle variation in AFC and day effect in FSH. The biological effects, of cyclic versus continuous recruitment, remain to determined. Pharmacologic ovulation induction therapies, which stimulate release of FSH, are believed to widen the “window” of gonadotropin-dependent recruitment and promote multiple follicle selection (10, 16). Although data on antral follicle development <10 mm during stimulation cycles are sparse, it has been suggested that clomiphene citrate and/or exogenous gonadotropins can “rescue” follicles from arrest in women with PCOS (11). If true, we postulate that ovarian hyperstimulation syndrome is more likely in women with a greater proportion of growing follicles at baseline. We appreciate that our findings related to recruitment should be interpreted with caution, owing to the lack of centralization to a specific physiologic event in women with PCOS (eg, ovulation, or a distinct increase in AFC or FSH). Refinement of automated follicle-tracking software (37) may enable more efficient evaluation of follicle growth in the 2- to 9-mm range, and help to clarify disruptions in recruitment, as well as the impact of baseline follicle development on responses to ovulation induction in PCOS.
By chance, we captured ultrasonographic evidence of sporadic ovulation in a significant proportion of women with PCOS (ie, PCOS-Ov). These serendipitous findings provided a rare opportunity to gain insight into the mechanism(s) of natural ovulation in PCOS. Contrary to our hypothesis, we observed similar growth phases, growth rates, and preovulatory diameters for ovulatory follicles in women with PCOS-Ov versus controls. Ovulatory follicles in normal ovaries are believed to have early morphologic and functional advances over subordinate follicles (ie, which regress at the time of selection) (10). It may be speculated that follicles that progress to ovulation in PCO have more granulosa cells and FSH receptors than follicles destined for arrest. These differences may result in an increased ability to produce E2 (38) and/or escape the detrimental effects of excessive and untimely exposure to LH. That being said, these endocrine abnormalities have been linked to impaired oocyte development, failure of implantation, and miscarriage in PCOS (39, 40). Rescuing a follicle from arrest may, therefore, have negative implications for oogenesis, luteal function, and endometrial receptivity.
The main feature, that distinguished the sporadic ovulatory cycles of women with PCOS-Ov from the regular ovulatory cycles of controls, was the serum concentration of P4 during the luteal phase. Women with PCOS-Ov had lower mean and maximum circulating concentrations of P4 compared with controls. Reduced luteal phase concentrations of P4 have been documented in eumenorrheic women with PCO (41) and obesity (42). It is plausible that our findings reflected a dual role of ovarian and metabolic factors, as most women with PCOS-Ov had PCO and obesity; however, our study was not designed to elucidate this interplay.
We appreciate that additional studies must be performed in larger, more diverse cohorts before universal conclusions can be drawn about antral follicle development in PCOS. PCOS is a heterogeneous condition, wherein reproductive and metabolic features can vary substantially between patients (43) and worsen with obesity (44). Intermediate reproductive and metabolic features were observed in women with PCOS-Ov. Specifically, women with PCOS-Ov had smaller mean ovarian volumes and lower free androgen concentrations than their anovulatory counterparts. The observation that women with anovulatory intervals were more hyperandrogenic supports the notion that androgens are a main driver of abnormal follicular development in PCOS (11). Our previous report showed that the development of dominant follicles was associated with improved metabolic status (ie, lower fasting insulin concentrations and HOMA-IR) (19). We suspect that these findings were not repeated in the present analysis, because women with PCOS-Ov still represented a severe phenotype of the condition, based on the NIH criteria. Sporadic ovulation was a chance finding and not indicative of normal ovulatory status. Moreover, our sample size was likely insufficient to detect smaller endocrine or metabolic differences between subgroups of women with PCOS, when most had comorbid overweight or obesity. Evaluations are, therefore, ongoing in our laboratory to clarify the role of androgens and adiposity in antral follicle development in PCOS. These studies will help us to understand the endocrine or metabolic factors regulating ovulatory potential across phenotypes.
Documentation of follicular arrest, but not persistence, provides a new model for antral follicle development in women with PCOS. We anticipate that the knowledge of a spectrum of disordered follicle growth will have profound implications for the design of tailored contraceptive regimens, treatment of infertility, and the understanding of reproductive senescence in PCOS. Evidence of continuous follicular recruitment, and the potential for sporadic ovulation, may help to better understand differences in the ovulatory response to pharmacologic interventions (16). Consideration of these findings may also enable identification of better predictive markers of ovulatory response, which are necessary to guide treatment strategies and offer patients realistic expectations for treatment outcomes.
Acknowledgments
The authors would like to thank the research participants, whose contributions were invaluable to the completion of this project. They would also like to thank Annie W. Lin, PhD, MS, RD, Anna Sear, RDMS, Rene Hellwitz-Black, RDMS, Erica Bender, CNM, NP-Ob/Gyn, and Tara Bailey for their assistance in facilitating data collection in the present study.
Financial Support: The present study was funded by Cornell University, President’s Council of Cornell Women, United States Department of Agriculture, and National Institutes of Health (Grant No.: R01-HD0937848). BYJ and HVB were supported by doctoral training awards from the National Institutes of Health (T32-DK007158) and Canadian Institutes of Health Research (Grant No. 146182), respectively.
Glossary
Abbreviations
- AFC
antral follicle count
- BMI
body mass index
- CV
coefficient of variation
- E2
estradiol
- FAI
free androgen index
- FSH
follicle-stimulating hormone
- HOMA-IR
homeostatic model assessment of insulin resistance
- IOI
interovulatory interval
- LH
luteinizing hormone
- NIH
National Institutes of Health
- P4
progesterone
- PCO
polycystic ovaries
- PCOM
polycystic ovarian morphology
- PCOS
polycystic ovary syndrome
- PCOS-Anov
PCOS with an anovulatory interval
- PCOS-Ov
PCOS with a sporadic ovulation
- SD
standard deviation
- SHBG
sex hormone–binding globulin
- T
testosterone
Additional Information
Disclosure Summary: Portions of these data were presented at the 15th Annual Meeting of the Androgen Excess and PCOS Society, San Antonio, TX in October 2017 and the 100th Annual Meeting of the Endocrine Society, Chicago, IL in March 2018. The authors have no conflicts of interest to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Hughesdon P. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis.” Obstet Gynecol Surv. 1982;37(2):59-77. [DOI] [PubMed] [Google Scholar]
- 2. Maciel GA, Baracat EC, Benda JA, et al. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(11):5321-5327. [DOI] [PubMed] [Google Scholar]
- 3. Webber LJ, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017-1021. [DOI] [PubMed] [Google Scholar]
- 4. Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83(11):3984-3991. [DOI] [PubMed] [Google Scholar]
- 5. Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81(1):302-309. [DOI] [PubMed] [Google Scholar]
- 6. Chávez-Ross A, Franks S, Mason HD, Hardy K, Stark J. Modelling the control of ovulation and polycystic ovary syndrome. J Math Biol. 1997;36(1):95-118. [DOI] [PubMed] [Google Scholar]
- 7. Homburg R, Amsterdam A. Polysystic ovary syndrome–loss of the apoptotic mechanism in the ovarian follicles? J Endocrinol Invest. 1998;21(9):552-557. [DOI] [PubMed] [Google Scholar]
- 8. Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003;80(1):116-122. [DOI] [PubMed] [Google Scholar]
- 9. Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod. 2003;69(3):1023-1031. [DOI] [PubMed] [Google Scholar]
- 10. Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18(1):73-91. [DOI] [PubMed] [Google Scholar]
- 11. Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2):107-117. [DOI] [PubMed] [Google Scholar]
- 12. Webber LJ, Stubbs SA, Stark J, et al. Prolonged survival in culture of preantral follicles from polycystic ovaries. J Clin Endocrinol Metab. 2007;92(5):1975-1978. [DOI] [PubMed] [Google Scholar]
- 13. Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367-378. [DOI] [PubMed] [Google Scholar]
- 14. Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79(4):1158-1165. [DOI] [PubMed] [Google Scholar]
- 15. Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995;16(3):322-353. [DOI] [PubMed] [Google Scholar]
- 16. Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687-708. [DOI] [PubMed] [Google Scholar]
- 17. Pierson R, Adams G. Computer-assisted image analysis, diagnostic ultrasonography and ovulation induction: strange bedfellows. Theriogenology 1995;43:105-112. [Google Scholar]
- 18. Baerwald AR, Walker RA, Pierson RA. Growth rates of ovarian follicles during natural menstrual cycles, oral contraception cycles, and ovarian stimulation cycles. Fertil Steril. 2009;91(2):440-449. [DOI] [PubMed] [Google Scholar]
- 19. Lujan ME, Kepley AL, Chizen DR, Lehotay DC, Pierson RA. Development of morphologically dominant follicles is associated with fewer metabolic disturbances in amenorrheic women with polycystic ovary syndrome: a pilot study. Ultrasound Obstet Gynecol. 2010;36(6):759-766. [DOI] [PubMed] [Google Scholar]
- 20. Legro R, Arslanian S, Ehrmann D, Hoeger K, Murad M, Pasquali R, Welt C. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013;98(12):4565-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens J, Haseltine F, eds. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992:377-384. [Google Scholar]
- 22. Teede HJ, Misso ML, Costello MF, et al. ; International PCOS Network . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rouleau D, Case A, Gamelin A, Lim H, Baerwald A. A practical method for ultrasonographically monitoring the day-to-day growth of individual ovarian follicles in women undergoing assisted reproduction. Ultrasound Med Biol. 2012;38(6):1004-1010. [DOI] [PubMed] [Google Scholar]
- 24. Vanden Brink H, Chizen D, Hale G, Baerwald A. Age-related changes in major ovarian follicular wave dynamics during the human menstrual cycle. Menopause. 2013;20(12):1243-1254. [DOI] [PubMed] [Google Scholar]
- 25. Hanna MD, Chizen DR, Pierson RA. Characteristics of follicular evacuation during human ovulation. Ultrasound Obstet Gynecol. 1994;4(6):488-493. [DOI] [PubMed] [Google Scholar]
- 26. Baerwald AR, Adams GP, Pierson RA. Form and function of the corpus luteum during the human menstrual cycle. Ultrasound Obstet Gynecol. 2005;25(5):498-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lujan ME, Brooks ED, Kepley AL, Chizen DR, Pierson RA, Peppin AK. Grid analysis improves reliability in follicle counts made by ultrasonography in women with polycystic ovary syndrome. Ultrasound Med Biol. 2010;36(5):712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9(6):505-514. [DOI] [PubMed] [Google Scholar]
- 29. Noble KM, Tebble JE, Harvey D, Dobson H. Ultrasonography and hormone profiles of persistent ovarian follicles (cysts) induced with low doses of progesterone in cattle. J Reprod Fertil. 2000;120(2):361-366. [PubMed] [Google Scholar]
- 30. Snyder PJ, Bhasin S, Cunningham GR, et al. ; Testosterone Trials Investigators . Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vanden Brink H, Willis AD, Jarrett BY, et al. Sonographic markers of ovarian morphology, but not hirsutism indices, predict serum total testosterone in women with regular menstrual cycles. Fertil Steril. 2016;105(5):1322-1329.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666-3672. [DOI] [PubMed] [Google Scholar]
- 33. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487-1495. [DOI] [PubMed] [Google Scholar]
- 34. Vanden Brink H, Robertson DM, Lim H, et al. Associations between antral ovarian follicle dynamics and hormone production throughout the menstrual cycle as women age. J Clin Endocrinol Metab. 2015;100(12):4553-4562. [DOI] [PubMed] [Google Scholar]
- 35. Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35(3):376-384. [DOI] [PubMed] [Google Scholar]
- 36. Dewailly D, Lujan ME, Carmina E, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20(3):334-352. [DOI] [PubMed] [Google Scholar]
- 37. Raine-Fenning N, Deb S, Jayaprakasan K, Clewes J, Hopkisson J, Campbell B. Timing of oocyte maturation and egg collection during controlled ovarian stimulation: a randomized controlled trial evaluating manual and automated measurements of follicle diameter. Fertil Steril. 2010;94(1):184-188. [DOI] [PubMed] [Google Scholar]
- 38. Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J Clin Endocrinol Metab. 1994;79(5): 1355-1360. [DOI] [PubMed] [Google Scholar]
- 39. Dumesic DA, Abbott DH. Implications of polycystic ovary syndrome on oocyte development. Semin Reprod Med. 2008;26(1):53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(6):673-683. [DOI] [PubMed] [Google Scholar]
- 41. Joseph-Horne R, Mason H, Batty S, et al. Luteal phase progesterone excretion in ovulatory women with polycystic ovaries. Hum Reprod. 2002;17(6):1459-1463. [DOI] [PubMed] [Google Scholar]
- 42. Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473. [DOI] [PubMed] [Google Scholar]
- 43. Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update. 2009;15(4):477-488. [DOI] [PubMed] [Google Scholar]
- 44. Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95-109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.