Abstract
Objectives
This study estimates the prevalence of chronic kidney disease (CKD) among Chilean adults and examines its associations with sociodemographic characteristics, health behaviours and comorbidities.
Design
Analysis of cross-sectional data from the two most recent large nationally representative Chilean Health Surveys (Encuesta Nacional de Salud, ENS) 2009–2010 and 2016–2017.
Participants
Adults aged 18+ years with serum creatine data (ENS 2009–2010: n=4583; ENS 2016–2017: n=5084).
Primary and secondary outcome measures
Reduced kidney function (CKD stages 3a–5) based on the estimated glomerular filtration rate (eGFR <60 mL/min/1.73 m2) was the primary outcome measure. Using the urine albumin-to-creatinine ratio (ACR ≥30 mg/g), increased albuminuria was ascertained among adults aged 40+ years with diabetes and/or hypertension. Both outcomes were analysed using logistic regression with results summarised using OR. CKD prevalence (stages 1–5) among adults aged 40+ years was estimated including participants with an eGFR of >60 mL/min/1.73 m2 but with increased albuminuria (stages 1–2).
Results
Overall, 3.2% (95% CI: 2.4% to 3.8%) of adults aged 18+ in ENS 2016–2017 had reduced kidney function. After full adjustment, participants with hypertension (OR: 2.37; 95% CI: 1.19 to 4.74) and those with diabetes (OR: 1.66; 95% CI: 1.03 to 2.66) had significantly higher odds of reduced kidney function. In ENS 2016–2017, 15.5% (13.5% to 17.8%) of adults aged 40+ years with diabetes and/or hypertension had increased albuminuria. Being obese versus normal-weight (OR: 1.66; 95% CI: 1.08 to 2.54) and having both diabetes and hypertension versus having diabetes alone (OR: 2.30; 95% CI: 1.34 to 3.95) were significantly associated with higher odds of increased albuminuria in fully-adjusted analyses. At least 15.4% of adults aged 40+ years in ENS 2016–2017 had CKD (stages 1–5), including the 9.6% of adults at CKD stages 1–2.
Conclusions
Prevention strategies and Chilean guidelines should consider the high percentage of adults aged 40 years and older at CKD stages 1–2.
Keywords: chronic renal failure, epidemiology, public health, nephrology
Strengths and limitations of this study.
Data were from large and nationally representative Chilean Health Surveys.
Chronic kidney disease was ascertained using both the estimated glomerular filtration rate and albuminuria in people aged 40+ years to include all stages of the disease.
Albuminuria data were available from only a subsample of participants (those with diabetes and/or hypertension).
Relying on single-point-in-time measurements of serum creatinine and/or albuminuria might induce some bias to the results.
The observational nature of this study means that only associations between variables can be assessed.
Introduction
Chronic kidney disease (CKD) is a leading global public health problem,1–3 with a substantial burden on healthcare systems, decreased quality of life4 and poor prognosis for patients. CKD is defined as reduced kidney function shown by glomerular filtration rate (GFR) of <60 mL/min/1.73 m² (based on measured serum creatinine values) and/or markers of kidney damage (eg, albuminuria as indicated by increased albumin-to-creatinine ratio (ACR), that measures excess albumin excretion in the urine), of at least 3 months duration, regardless of the underlying cause.2 5 Evidence recently published suggests that the prevalence of CKD in the general population is increasing worldwide,6 in part due to population ageing and increases in the prevalence of comorbid conditions for CKD such as hypertension, diabetes mellitus and obesity.4 However, other studies in high-income countries such as the UK have shown stagnation and even falling prevalence over time.7
The natural history of CKD is worsening of kidney function with time. Among the most important complications of CKD are the development of acute kidney failure, progression to end-stage kidney disease (ESKD), and onset of cardiovascular disease (CVD).1 8 While CKD is a precursor to ESKD, CKD patients are between five and 10 times more likely to die prematurely than to progress to ESKD:2 this is largely attributable to death from CVD.2 4 9
In Chile, much attention has been paid to patients with ESKD, who are in need of renal replacement treatment (RRT) such as dialysis or renal transplant, with well-documented registries of the population being treated under these regimes.10–12 These registers have shown a significant increase in the use of dialysis, with more than 20 000 individuals having dialysis in 2017.10 However, there is insufficient evidence in Chile on individuals at the earlier stages of the disease.
International studies suggest that given the trends and natural history of CKD,13 there is a significantly higher prevalence of CKD at the earlier stages, affecting around 35% of individuals aged 70 years and over,4 with a high burden for healthcare systems. Moreover, there is evidence of increases in the comorbidities for CKD in the Chilean population, such as hypertension, diabetes mellitus and obesity,14-16 therefore suggesting a probable increase in the current and/or future prevalence of CKD. Although most studies in Chile to date have estimated the economic burden of RRT for ESKD, the increase in healthcare resource utilisation for the earlier stages of CKD is also significant,17 namely an increase in the use of emergency departments and outpatient visits, hospitalisation, medical expenditure and pharmacy costs,17 18 with increasing costs as the disease progresses.18
The limited data on CKD prevalence and its distribution across population subgroups is an important gap in the evidence, that impedes effective decision-making in the healthcare sector. Therefore, it is important to study both the early and end-stages of CKD in the general population (ie, not just those patients who are known to the Chilean healthcare system), in order to have accurate information to help guide strategies for prevention, diagnosis and treatment of CKD in Chile. Using data from the two most recent Chilean National Health Surveys (Encuesta Nacional de Salud, ENS) 2009–2010 and 2016–2017, this study estimates the prevalence of CKD and examines its associations with sociodemographic characteristics, health behaviours and comorbidities.
Methods
Study population and data collection
The sampling design and methods of data collection of the ENS 2009–2010 and 2016–2017 have been reported elsewhere, in detail in Spanish14 15 and in summary in English.19 Both surveys were cross-sectional study designs, with a new sample selected each time representative of the adult Chilean population at national, regional and rural/urban levels. Both were complex random samples, using multistage, stratified cluster probability sampling of households, based on the 2002 Chilean National Census.19 Participants aged 17 years and older completed a face-to-face interview to provide information on self-reported health, household characteristics, socioeconomic position (SEP) including years spent in full-time education, health behaviours and living conditions (ENS 2009–2010: n=5293; ENS 2016–2017: n=6233).
In the second stage, anthropometric measurements (including height and weight), reported information on diagnosed conditions, measured blood pressure and biological samples (blood and urine) were collected by trained nurses (ENS 2009–2010: n=4956; ENS 2016–2017: n=5451). Kidney function was evaluated by measuring blood creatinine using the Jaffé kinetic method traceable to isotope dilution-mass spectrometry (IDMS) to calculate the estimated glomerular filtration rate (eGFR). Valid data on the eGFR was available for the majority of participants aged 18+ assessed at the second stage by trained nurses (ENS 2009–2010: n=4583; ENS 2016–2017: n=5084). Although urine samples were collected from all consenting participants, urinary analyses to determine albuminuria were performed only in the subsample of individuals classed as having diabetes mellitus (hereafter referred to as diabetes) and/or hypertension (both variables self-reported doctor-diagnosed or identified from the survey measurements) (ENS 2009–2010: n=2523; ENS 2016–2017: n=3907).
The interview response rates from the eligible population were 85% (ENS 2009–2010) and 67% (ENS 2016–2017).14 15 Participants gave written consent prior to data collection, measurements and biological sampling.19
Definition of CKD
Given the cross-sectional nature of both health surveys, repeated laboratory values for the same participant were not possible, so for this study, kidney function (based on serum-creatinine eGFR), and a marker of kidney damage (based on albuminuria), were estimated relying on single-point-in-time measurements of serum creatinine and, where available, the urine ACR.
Presence of reduced kidney function using eGFR
Based on the serum creatinine values, the eGFR was calculated for adults aged 18+ years using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, as this has shown better accuracy compared with the true GFR than the Modification of Diet in Renal Disease (MDRD) equation.20 The continuous values of eGFR (mL/min/1.73 m2) were grouped into six categories based on the Kidney Disease Improving Global Outcomes (KDIGO)5 2012 classification recommendations as follows:
G1:≥ 90 mL/min/1.73 m2 (normal or high).
G2: 60–89 mL/min/1.73 m2 (mildly decreased).
G3a: 45–59 mL/min/1.73 m2 (mildly to moderately decreased).
G3b: 30–44 mL/min/1.73 m2 (moderately to severely decreased).
G4: 15–29 mL/min/1.73 m2 (severely decreased).
G5: < 15 mL/min/1.73 m2 (kidney failure).
As in similar studies, individuals with eGFR <60 mL/min/1.73 m2 were classed as having reduced kidney function (or CKD stages G3a–G5).
Presence of increased albuminuria using ACR
Based on the KDIGO classification recommendations,5 three albuminuria categories were based on the urine ACR as follows:
A1:<30 mg/g (normal to mildly increased).
A2: 30–300 mg/g (moderately increased).
A3:>300 mg/g (severely increased).
Increased albuminuria was defined as an ACR ≥30 mg/g. Due to orthostatic albuminuria in adolescents and young adults,21 and no information on whether women were currently menstruating (which could lead to protein contamination of the urine), only participants aged 40 years and over with survey-defined diabetes and/or hypertension were considered for the analysis of albuminuria.
Presence of CKD using eGFR and/or ACR
The presence of CKD was ascertained using both measures of kidney disease, to include CKD stages 1 and 2 into the analysis.13 Adopting both measures was complicated in the present study, however, due to ACR data being available only for participants with survey-defined diabetes and/or hypertension and to the reduced validity of ACR as indicating CKD in participants below the age of 40 years. Hence, we limited this analysis to participants aged 40 years and older.
For participants with no ACR data available, the presence of CKD was ascertained using eGFR data alone (CKD stages G3a–G5).5 For participants with diabetes and/or hypertension, the presence of CKD was ascertained using eGFR data (CKD stages G3a–G5) and/or ACR data (increased albuminuria). Participants with increased albuminuria but having ‘mildly decreased’, ‘normal’, or ‘high’ kidney function (G1–G2) are classified in the KDIGO guidelines as being in CKD stages 1 or 2 (corresponding to A2 and A3, respectively). For the purposes of clarity, the KDIGO recommendations used in this study to estimate the prevalence of CKD by eGFR and albuminuria categories are set out in online supplementary table S1 of the online supplementary appendix.
bmjopen-2020-037720supp001.pdf (66.7KB, pdf)
Demographics, SEP, health behaviours and comorbid conditions
Age of participants was grouped into three categories: 18–44, 45–64 and 65+ years for the analysis of reduced kidney function, and in two categories: 40–64, and 65+ years for the analysis of increased albuminuria and the presence of CKD using eGFR and/or ACR. Years spent in formal education was our chosen measure of SEP, grouped as <8, 8–12 and >12 years.14 Smoking status at time of interview was categorised as current smoker, ex-smoker and non-smoker. Participants were classed as living in an urban or rural area.15 Survey-defined diabetes was classed as fasting blood glucose ≥126 mg/dL (≥7.0 mmol/L) and/or self-report of medical diagnosis. Similarly, survey-defined hypertension was classed as systolic blood pressure ≥140 mm Hg or diastolic ≥90 mm Hg and/or self-report of medical diagnosis. Body mass index was calculated as weight in kilogrammes (kg) divided by height in metres squared (m2), classifying participants into four groups: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2).22 23
Statistical analysis
Using descriptive analysis, we examined the characteristics of the analytical samples (means and SD for continuous variables, percentages for categorical variables).
Reduced kidney function
Using the eGFR data only, the distribution of the participants across the six eGFR categories and the prevalence of reduced kidney function were estimated for all adults (aged 18 years and over) in both survey years. The pattern of reduced kidney function by age was explored by calculating the mean age of the participants by eGFR category and survey year. In addition, the prevalence of reduced kidney function in each year was estimated by demographic factors, SEP, health behaviours and comorbidities. Underweight participants were excluded from the statistical modelling due to small numbers and potential confounding with ill-health. In multivariate analysis on participants with complete data on all variables, gender-adjusted and age-adjusted logistic regression models were used to examine the relationships between the odds of reduced kidney function and demographics, SEP, health behaviours and comorbidities. As the associations did not change over time (data not shown), the results reported in this present study are taken from the multivariate analysis conducted on data pooled across the two survey years to increase precision (a binary indicator for survey year was included in the model). Only those variables that were statistically significant (p<0.05) in the univariate models were retained in the multivariate analyses.
Increased albuminuria
The same analytical strategies as described above were repeated to examine albuminuria. A logistic regression model was used to explore the relationships between increased albuminuria and demographics, SEP, health behaviours and comorbidities. For the reasons discussed earlier, this analysis was conducted only on participants aged 40+ years old with diabetes, hypertension or both. An additional three-category variable capturing comorbidity (diabetes only, hypertension only, diabetes and hypertension) was included in the regression model.
CKD stages 1–5
In a final analysis, we examined the distribution of the six eGFR and three albuminuria categories among participants aged 40 years and older.3 Using this cross-classification, the presence of CKD stages 1 and 2 was estimated using both measures of kidney disease which identified participants with diabetes and/or hypertension who had an eGFR of >60 mL/min/1.73 m2 but with increased albuminuria.
All analyses were adjusted for the complex survey design of the ENS and were performed using Stata V.15.1. Statistical significance was set at p<0.05 for two-tailed tests, with no adjustment for multiple comparisons.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research (which involves secondary analysis of existing data).
Results
The distribution of the analytical sample across the key variables is shown in online supplementary table S2 of the online supplementary appendix. The key variables showed little change over time, with the exception of a decrease in current smoking and increasing obesity, diabetes and hypertension.
Reduced kidney function (CKD stages G3a–G5)
Table 1 shows the distribution of the participants aged 18+ years across the six eGFR categories by survey year. The prevalence of reduced kidney function based on eGFR data alone was 3.2% (95% CI: 2.6% to 4.0%) in ENS 2016–2017. There was no statistically significant difference (p=0.12) from the 2.5% (1.9% to 3.2%) prevalence in ENS 2009–2010 (table 1).
Table 1.
Prevalence of reduced kidney function (based on eGFR values only) in the Chilean population aged 18 years and older
| CKD stage* | eGFR† (mL/min/1.73 m2) |
Prevalence | |||
| ENS 2009–2010 | ENS 2016–2017 | ||||
| N | % (95% CI) | N | % (95% CI) | ||
| Stage G1‡ | ≥90 | 3293 | 77.6.0 (75.5 to 79.6) | 3511 | 77.9 (75.9 to 79.8) |
| Stage G2‡ | 60–89 | 1104 | 19.9 (18.0 to 21.9) | 1301 | 18.8 (17.1 to 20.8) |
| Stage G3a | 45–59 | 122 | 1.6 (1.2 to 2.3) | 176 | 2.1 (1.6 to 2.8) |
| Stage G3b | 30–44 | 47 | 0.5 (0.3 to 0.8) | 65 | 0.6 (0.4 to 0.8) |
| Stage G4 | 15–29 | 13 | 0.2 (0.1 to 0.4) | 20 | 0.4 (0.2 to 0.8) |
| Stage G5 | <15 | 4 | 0.1 (0.0 to 0.4) | 11 | 0.1 (0.1 to 0.4) |
| CKD stages G3a–G5 | 186 | 2.5 (1.9 to 3.2) | 272 | 3.2 (2.6 to 4.0) | |
*Presence of reduced kidney function or CKD (stages G3a–G5) indicated by eGFR <60 mL/min/1.73 m2 in accordance with KDIGO guidelines:5 shown by cells in dark grey shading. G2: mildly decreased eGFR; G3a: mildly to moderately decreased; G3b: moderately to severely decreased; G4: severely decreased and G5: kidney failure.
†eGFR (measured in mL/min/1.73 m2) determined by CKD-EPI equation.
‡Stages G1 and G2 CKD are diagnosed by the presence of raised albuminuria in the presence of normal to high eGFR. Thus, for the purposes of this table, using eGFR values only, these individuals have been classified together with the majority with no CKD.
CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; ENS, Encuesta Nacional de Salud; KDIGO, Kidney Disease Improving Global Outcomes.
The prevalence of reduced kidney function by demographics, SEP, health behaviours and presence of comorbidities in each survey year is shown in table 2. Patterns of association were similar in each survey. Reduced kidney function prevalence, as expected, increased with age, with prevalence among those aged 65+ years reaching 15.0% (95% CI: 11.5% to 19.2%) and 19.1% (15.3 to 23.6%) in 2009–2010 and 2016–2017, respectively. For males and females, the prevalence of stages G3a–G5 was higher in ENS 2016–2017 than in ENS 2009–2010, but the 95% CIs overlapped. Stages G3a–G5 prevalence was higher among participants with less than 8 years of formal education and was higher among those with diabetes and among those with hypertension.
Table 2.
Prevalence of reduced kidney function by demographics, socioeconomic position, health behaviours and comorbidities in Chilean adults 18 years or over
| ENS 2009–2010 | ENS 2016–2017 | |
| CKD* | CKD* | |
| % (95% CI) | % (95% CI) | |
| Age | ||
| 18–44 | 0.1 (0.0 to 0.3) | 0.3 (0.1 to 0.8) |
| 45–64 | 1.9 (1.0 to 3.4) | 1.3 (0.7 to 2.3) |
| 65+ | 15.0 (11.5 to 19.2) | 19.1 (15.3 to 23.6) |
| Gender | ||
| Male | 2.0 (1.3 to 3.1) | 3.3 (2.3 to 4.6) |
| Female | 2.9 (2.1 to 3.9) | 3.2 (2.4 to 4.3) |
| Education | ||
| <8 years | 5.4 (4.1 to 7.3) | 10.8 (8.1 to 14.3) |
| 8–12 years | 1.9 (1.3 to 2.9) | 1.9 (1.3 to 2.6) |
| >12 years | 1.5 (0.6 to 3.4) | 1.3 (0.6 to 2.7) |
| Residence | ||
| Urban | 2.4 (1.8 to 3.2) | 3.0 (2.4 to 3.9) |
| Rural | 3.0 (1.7 to 5.2) | 4.8 (3.2 to 7.1) |
| Smoking | ||
| Current | 1.1 (0.6 to 2.2) | 0.6 (0.2 to 1.4) |
| Ex-smoker | 3.2 (2.1 to 5.0) | 4.9 (3.5 to 6.8) |
| Never | 3.4 (2.3 to 5.0) | 4.4 (3.2 to 5.9) |
| BMI† | ||
| Underweight | 9.1 (2.3 to 29.9) | 11.7 (3.3 to 34.1) |
| Normal | 2.0 (1.2 to 3.2) | 2.7 (1.7 to 4.1) |
| Overweight | 2.4 (1.5 to 3.9) | 2.9 (1.9 to 4.4) |
| Obese | 2.6 (1.6 to 4.1) | 3.5 (2.5 to 4.8) |
| Diabetes‡ | ||
| No | 1.8 (1.4 to 2.5) | 2.5 (1.9 to 3.4) |
| Yes | 7.6 (4.4 to 12.8) | 8.1 (5.7 to 11.4) |
| Hypertension§ | ||
| No | 1.0 (0.6 to 1.7) | 0.6 (0.4 to 1.0) |
| Yes | 6.3 (4.7 to 8.4) | 9.6 (7.6 to 12.1) |
*eGFR (measured in mL/min/1.73 m2) determined by CKD-EPI equation. Presence of reduced kidney function or CKD (stages G3a–G5) considered when eGFR <60 mL/min/1.73 m2.
†Underweight: BMI <18.5 kg/m2; normal weight: BMI 18.5–24.9 kg/m2; overweight: BMI 25–29.9 kg/m2; obese: BMI ≥30 kg/m2.
‡Diabetes: fasting blood glucose ≥126 mg/dL (≥7.0 mmol/L) and/or self-report of medical diagnosis.
§Hypertension: SBP ≥140 mm Hg and/or DBP ≥90 mm Hg, and/or self-report of medical diagnosis.
BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ENS, Encuesta Nacional de Salud; SBP, systolic blood pressure.
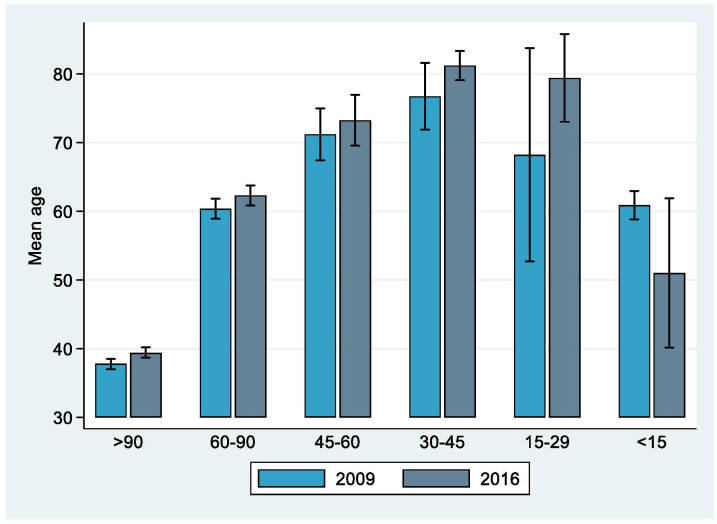
The mean age of participants aged 18 years and older across the six eGFR categories in both ENS followed an inverted U shape, as shown in figure 1. Mean age increased as kidney function decreased until eGFR 30–45 mL/min/1.73 m2, but was then lower at stages G4 (severely decreased kidney function) and G5 (kidney failure) (online supplementary table S3 showing the mean and 95% CI values).
Figure 1.
Mean age (95% CIs) by eGFR values for participants aged 18 years and over of ENS 2009–2010 and ENS 2016–2017. eGFR (measured in mL/min/1.73 m2) determined by CKD-EPI equation. Presence of reduced kidney function (CKD stages G3a–G5) considered as eGFR <60 mL/min/1.73 m2. Categories based on definition by KDIGO.5 CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; ENS, Encuesta Nacional de Salud; KDIGO, Kidney Disease Improving Global Outcomes.
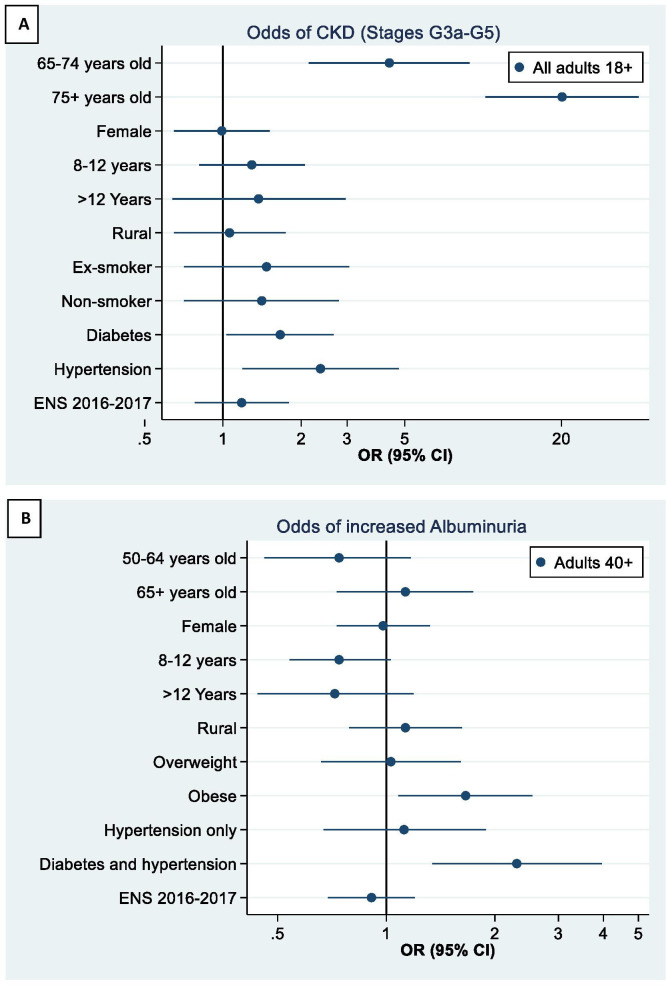
Figure 2A shows the results from the multivariable logistic regression model of reduced kidney function (stages G3a–G5) among all participants aged 18+ years. After adjustment for age and gender, participants with hypertension (OR: 2.37; 95% CI: 1.19 to 4.74) and participants with diabetes (OR: 1.66; 1.03 to 2.66) had significantly higher odds of reduced kidney function. Educational level and living in rural areas (vs urban) did not show any association with reduced kidney function in fully-adjusted analyses.
Figure 2.
(A) Association between demographics, health behaviours, comorbid conditions and survey year and reduced kidney function (CKD stages G3a–G5). Reference categories: age: 55–64 years; gender: male; educational level:<8 years; living in urban area; current smoker; survey year: 2009–2010. Estimate not shown for persons 18–54 due to the very low prevalence of reduced kidney function at younger ages. (B) Association between demographics, health behaviours, comorbid conditions and survey year and increased albuminuria (A2–A3). Reference categories: age: 40–49 years old; gender: male; educational level:<8 years; living in urban area; BMI category: normal (18.5–25 kg/m2); survey-defined diabetes only; survey year: 2009–2010. Variables not significant at 5% level in individual models were dropped from the final model. BMI, body mass index; CKD, chronic kidney disease; ENS, Encuesta Nacional de Salud.
Increased albuminuria (ACR ≥30mg/g)
The estimates and accompanying 95% CIs for the presence of increased albuminuria among adults aged 40 years and over in whom it was measured are shown in online supplementary table S4 of the online supplementary appendix. Among participants aged 40+ years with measured ACR (and so had diabetes and/or hypertension), the prevalence of increased albuminuria was 18.3% (15.8% to 21.2%) and 15.5% (13.5% to 17.8%) in ENS 2009–2010 and ENS 2016–2017, respectively. The pattern by gender showed some difference over time, being higher for men in ENS 2009–2010 (20.0% in men vs 16.8% among women) but lower in ENS 2016–2017 (12.3% in men, 18.4% among women) (table 3).
Table 3.
Prevalence of increased albuminuria by demographics, socioeconomic position, health behaviours and comorbidities
| ENS 2009–2010 | ENS 2016–2017 | |
| Increased albuminuria* | Increased albuminuria* | |
| % (95% CI) | % (95% CI) | |
| Age | ||
| 40–64 | 17.0 (13.1 to 21.7) | 11.2 (8.9 to 14.0) |
| 65+ | 21.9 (17.9 to 26.5) | 26.4 (22.2 to 31.1) |
| Gender | ||
| Male | 20.0 (16.0 to 24.7) | 12.3 (9.9 to 15.2) |
| Female | 16.8 (13.7 to 20.5) | 18.4 (15.4 to 21.9) |
| Education | ||
| <8 years | 24.1 (19.8 to 29.1) | 21.3 (17.9 to 25.3) |
| 8–12 years | 14.7 (11.6 to 18.4) | 14.1 (11.0 to 17.8) |
| >12 years | 17.2 (10.8 to 26.2) | 10.1 (6.3 to 15.7) |
| Residence | ||
| Urban | 17.7 (15.0 to 20.8) | 15.3 (13.1 to 17.8) |
| Rural | 22.0 (15.5 to 30.2) | 17.0 (13.0 to 22.0) |
| Smoking | ||
| Current | 17.5 (13.0 to 23.0) | 14.2 (10.0 to 19.8) |
| Ex-smoker | 21.4 (16.1 to 28.0) | 15.5 (12.0 to 19.8) |
| Never | 17.2 (13.7 to 21.4) | 16.4 (13.3 to 20.0) |
| BMI† | ||
| Underweight | 17.7 (4.5 to 49.3) | 15.9 (3.5 to 49.3) |
| Normal | 14.6 (9.7 to 21.5) | 14.8 (10.3 to 20.7) |
| Overweight | 15.6 (12.2 to 19.6) | 11.1 (8.6 to 14.1) |
| Obese | 22.7 (18.0 to 28.1) | 19.4 (15.8 to 23.7) |
| Diabetes‡ | ||
| No | 15.9 (13.2 to 19.1) | 11.3 (9.2 to 13.8) |
| Yes | 27.7 (21.2 to 35.2) | 29.2 (23.6 to 35.6) |
| Hypertension§ | ||
| No | 9.4 (6.8 to 12.8) | 6.2 (4.3 to 8.9) |
| Yes | 23.0 (19.5 to 26.9) | 22.5 (19.4 to 25.9) |
*Albuminuria results limited to participants aged 40+ with diabetes and/or hypertension (diagnosed or survey-detected). Albuminuria determined by the urine albumin-creatinine ratio (ACR, measured in mg/g). Increased albuminuria (A2–A3) considered when ACR ≥30 mg/g.
†Underweight: BMI <18.5 kg/m2; normal weight: BMI 18.5–24.9 kg/m2; overweight: BMI 25–29.9 kg/m2; obese: BMI ≥30 kg/m2.
‡Diabetes: fasting blood glucose ≥126 mg/dL (≥7.0 mmol/L) and/or self-report of medical diagnosis.
§Hypertension: SBP ≥140 mm Hg and/or DBP ≥90 mm Hg, and/or self-report of medical diagnosis.
BMI, body mass index; DBP, diastolic blood pressure; ENS, Encuesta Nacional de Salud; SBP, systolic blood pressure.
Figure 2B shows the results from the multivariable logistic regression model of increased albuminuria. After adjusting for age and gender, being obese versus normal weight (OR: 1.66; 95% CI: 1.08 to 2.54) and having diabetes and hypertension versus having diabetes alone (OR: 2.30; 95% CI: 1.34 to 3.95) were significantly associated with higher odds of increased albuminuria. Participants with higher levels of formal education (compared with <8 years) had lower odds of increased albuminuria, although the results did not attain statistical significance (8–12 years: OR: 0.74; 95% CI: 0.54 to 1.03; >12 years: OR: 0.72; 95% CI: 0.44 to 1.19).
CKD (stages 1–5)
Table 4 shows the distribution of the six eGFR and three albuminuria categories among ENS participants aged 40+ years. Prevalence of CKD based on eGFR data (stages G3a–G5) was 4.4% in ENS 2009–2010 and 5.8% in ENS 2016–2017 (shown in table 4 by the row percentages). Prevalence of increased albuminuria based on ACR data among those with diabetes and/or hypertension was 12.0% in ENS 2009–2010 and 11.7% in ENS 2016–2017 (shown in table 4 by the column percentages).
Table 4.
Distribution of CKD by eGFR and ACR among participants aged 40+
| eGFR category* | Albuminuria category† | |||||||||
| Not measured‡ | A1 <30 mg/g | A2 30–300 mg/g | A3 >300 mg/g | Row | ||||||
| N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | |
| 2009–2010 | ||||||||||
| G1 (≥90) | 645 | 25.5 (22.6 to 28.6) | 869 | 30.6 (27.7 to 33.6) | 149 | 4.7 (3.7 to 6.0) | 24 | 0.9 (0.4 to 1.8) | 1687 | 61.7 (58.4 to 64.9) |
| G2 (60–89) | 217 | 8.6 (6.5 to 11.1) | 671 | 20.5 (18.1 to 23.1) | 142 | 4.1 (3.1 to 5.4) | 23 | 0.7 (0.4 to 1.2) | 1053 | 33.9 (30.7 to 37.2) |
| G3a (45–59) | 15 | 0.3 (0.1 to 0.8) | 66 | 1.6 (1.0 to 2.6) | 31 | 0.9 (0.5 to 1.6) | 8 | 0.1 (0.0 to 0.3) | 120 | 2.9 (2.1 to 4.1) |
| G3b (30–44) | 8 | 0.2 (0.1 to 0.6) | 23 | 0.5 (0.3 to 0.9) | 11 | 0.2 (0.1 to 0.5) | 5 | 0.1 (0.0 to 0.1) | 47 | 1.0 (0.6 to 1.5) |
| G4 (15–29) | 2 | 0.0 (0.0 to 0.1) | 2 | 0.0 (0.0 to 0.2) | 3 | 0.1 (0.0 to 0.5) | 5 | 0.2 (0.0 to 0.5) | 12 | 0.3 (0.1 to 0.8) |
| G5 (<15) | 2 | 0.2 (0.0 to 0.9) | 0 | – | 0 | – | 2 | 0.0 (0.0 to 0.1) | 4 | 0.2 (0.0 to 0.8) |
| Column N, % (95% CI) | 889 | 34.8 (31.6 to 38.0) | 1631 | 53.3 (50.0 to 56.5) | 336 | 10.1 (8.5 to 11.9) | 67 | 1.9 (1.3 to 2.8) | 2923 | 100 (N/A) |
| 2016–2017 | ||||||||||
| G1 (≥90) | 484 | 17.9 (15.5 to 20.5) | 1294 | 40.2 (37.4 to 43.1) | 167 | 4.8 (3.6 to 6.4) | 16 | 0.4 (0.2 to 0.8) | 1961 | 63.3 (60.3 to 66.1) |
| G2 (60–89) | 196 | 5.2 (4.0 to 6.8) | 866 | 21.3 (19.0 to 23.8) | 164 | 3.8 (3.0 to 4.8) | 26 | 0.6 (0.4 to 1.0) | 1252 | 31.0 (28.3 to 33.9) |
| G3a (45–59) | 19 | 0.4 (0.2 to 0.9) | 107 | 2.3 (1.6 to 3.4) | 36 | 0.8 (0.5 to 1.4) | 14 | 0.3 (0.1 to 0.6) | 176 | 3.8 (2.9 to 4.9) |
| G3b (30–44) | 6 | 0.0 (0.0 to 0.1) | 33 | 0.5 (0.3 to 0.8) | 18 | 0.4 (0.2 to 0.7) | 8 | 0.1 (0.0 to 0.2) | 65 | 1.0 (0.7 to 1.4) |
| G4 (15–29) | 2 | 0.1 (0.0 to 0.7) | 4 | 0.2 (0.1 to 0.7) | 8 | 0.3 (0.1 to 0.8) | 6 | 0.1 (0.0 to 0.6) | 20 | 0.7 (0.4 to 1.4) |
| G5 (<15) | 5 | 0.2 (0.0 to 0.8) | 0 | – | 0 | – | 5 | 0.1 (0.0 to 0.2) | 10 | 0.2 (0.1 to 0.7) |
| Column N, % (95% CI) | 712 | 23.8 (21.1 to 26.7) | 2304 | 64.5 (61.4 to 67.5) | 393 | 10.1 (8.6 to 11.9) | 75 | 1.6 (1.1 to 2.2) | 3484 | 100 (N/A) |
*eGFR (measured in mL/min/1.73 m2) determined by CKD-EPI equation. G1: normal to high eGFR; G2: mildly decreased eGFR; G3a: mildly to moderately decreased; G3b: moderately to severely decreased; G4: severely decreased and G5: kidney failure.
†Albuminuria determined by the urine albumin-creatinine ratio (ACR, measured in mg/g). A1 (normal): <30 mg/g; A2 (moderately increased): 30–300 mg/g; A3 (severely increased): >300 mg/g.
‡Albuminuria not measured as these participants had no diabetes or hypertension.
ACR, albumin-creatinine ratio; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; N/A, not applicable.
The prevalence of CKD in adults aged 40 years and older, based on eGFR and albuminuria criteria (CKD stages 1–5), was 14.8% in ENS 2009–2010 and 15.4% in ENS 2016–2017. Based on the ENS 2009–2010, those with CKD comprised the 10.4% with increased albuminuria but mildly decreased, normal or high eGFR (5.6% at CKD stage 1; 4.8% at CKD stage 2) and the 4.4% at CKD stages 3a–5. Similarly, based on the ENS 2016–2017, those with CKD comprised the 9.6% with increased albuminuria but mildly decreased, normal or high eGFR (5.2% at CKD stage 1; 4.4% at CKD stage 2) and the 5.8% at CKD stages 3a–5.
Discussion
In this representative sample of the Chilean population, the prevalence of reduced kidney function estimated by eGFR <60 mL/min/1.73 m² (CKD stages G3a–G5) in adults 18 years and older was 3.2% in the most recent survey (ENS 2016–2017). There is some difference from the prevalence reported in other developing and developed countries.1 2 4 6 8 24 25 Chile, as a developing country, has a younger population structure compared with developed countries such as the UK, therefore we would expect a lower crude prevalence of CKD.26 CKD prevalence in England (among adults aged 16+ years) using the same definition was 5.2% based on Health Survey for England 2009–2010 data.22 Additionally, there is high heterogeneity between countries in the prevalence of comorbid conditions for CKD such as diabetes and hypertension, and other demographic and socioeconomic factors such as age, diet, educational level, geography, pollution and climate,6 26 so differences in prevalence should be expected. Evidence on gender differences in CKD prevalence is inconclusive, with some studies showing higher prevalence in women—as women tend to develop reduced kidney function at an earlier age than men4 6 while others show higher prevalence in men.27 28 Our analyses suggest similar levels of CKD among men and women in Chile (p=0.52).
Both hypertension and diabetes were significantly associated with higher odds of CKD in multivariable regression models, supporting the evidence that these are important comorbidities for reduced kidney function. Diabetes can lead to several micro-vascular and macro-vascular diseases, such as CVD and nephropathy, which contribute significantly to the higher mortality of this group of individuals,29 as well as having a higher risk of developing CKD.28 Moreover, there are several studies showing that diabetes is associated with the development of increased albuminuria and faster progression of CKD.28 30–32 Evidence from other Latin American countries30–32 suggests that diabetes and worse glycaemic control are significant predictors for increased albuminuria, faster progression of CKD and need for RRT. On the other hand, a meta-analysis which analysed the risk factors for development and progression of CKD, showed that diabetes was marginally predictive of progression from late-stage CKD to ESKD (HR: 1.16, 95% CI: 0.98 to 1.38; p = 0.08).28
Socioeconomic factors may influence both direct and indirect effects on CKD and its complications.33 34 Although our analyses showed a socioeconomic gradient in the crude prevalence of reduced kidney function, with higher prevalence among those with fewer years spent in formal education, the educational differences did not attain significance in the fully-adjusted models. Given the marked social and economic inequalities in Chile,35 36 and the evidence that social environment and economic conditions are important elements in the pathway of CKD, from the higher prevalence of risk factors to the development and complications of CKD and ESKD,2 33 34 our findings suggest that the comorbid conditions that we adjusted for in our regression analysis are possible mediators of the SEP and CKD relationship. The social gradient, as captured in the Chilean health surveys by years spent in formal education, is marked in many of the comorbidities for CKD such as diabetes, hypertension and obesity.14 15 36 Further research is needed using cohort studies in the Chilean population to determine if education or other indicators of SEP are significant predictors of CKD, progression to ESKD and premature mortality33 and what the mechanisms are.
Although the prevalence of CKD based on eGFR data was low compared with other countries, our results using both estimates of eGFR and albuminuria showed that at least 9.6% of adults aged 40 years and over in ENS 2016–2017 had normal kidney function but increased albuminuria, thus considered as CKD stage 1 or 2 by the KDIGO definition.5 As albuminuria was not measured in participants without diabetes or hypertension, this estimate must be treated with caution due to the potential underestimation of actual prevalence.
The multivariate analyses showed that being obese (vs normal weight) and having both hypertension and diabetes (compared with diabetes alone) were significantly and independently associated with increased albuminuria. Although these results should be treated with caution for the reason described above, it is important to take our findings into consideration and explore them further, given the high prevalence of diabetes, hypertension and obesity in the Chilean population, as these conditions are associated with a higher risk of increased albuminuria, with increased albuminuria being an independent risk factor for the progression of CKD and premature mortality.8 9 28
The inverted-U shape for the age pattern of reduced kidney function (ascertained using eGFR) suggests increased mortality rate in individuals with CKD as the condition progresses to the more advanced stages. This result can probably be explained by the increased all-cause and cardiovascular-mortality of individuals with CKD as their eGFR decreases and levels of albuminuria increase, shown in several studies.2 9 29 37 Cardiovascular mortality rates can be more than 50% higher in CKD patients, and this risk increases further in those with increased albuminuria.2 In addition to the higher mortality rates at the more advanced stages of CKD, mortality is higher in the older population compared with younger individuals.9 This could explain why in Chile, long-term survivors to the more advanced CKD stages are younger compared with individuals at earlier stages. To further investigate this hypothesis, a cohort study of Chilean patients must be conducted to fill the gap in evidence on the incidence and progression of CKD, including follow-up to death.
Our study has several limitations. The use of estimated instead of true GFR may have introduced bias due to the variations in levels of serum creatinine by differences in muscle mass, diet and other environmental factors not related to kidney disease, or through confounding by interactions with variables such as age or weight that are included in the CKD-EPI equation used to ascertain eGFR.6 26 Although the introduction of IDMS calibration for serum creatinine assays has improved the variability of serum creatinine readings, and the use of CKD-EPI instead of MDRD22 26 has improved precision, there are still issues with regard to using eGFR to assess CKD prevalence. Moreover, there is still an ongoing debate as to whether eGFR precisely estimates true GFR for persons with diabetes,38 39 obesity6 40 and in other populations with different racial, ethnic and regional variations in muscle mass and diet outside North America, Europe and Australia.20 Given the high prevalence of diabetes and obesity in Chile, and due to the racial and ethnic differences, the results from this study should be treated with caution. Additionally, relying on single-point-in-time measurements to measure eGFR may have introduced bias to the results, with possible underestimation and overestimation of CKD in younger and older populations, respectively,6 41 and an underestimation of the differences between CKD stages.41 Future studies looking to obtain more precise estimates may need to consider including repeated laboratory measurements of serum creatinine, urine albumin and creatinine to confirm chronicity of the disease and to measure albuminuria among all adults.
Conclusion and policy implications
Our results show that based on the KDIGO definition for CKD, the prevalence of stages 1–5 in Chilean adults 40 years and older is 15.4%. Our study provides the distribution by CKD stage in this population, showing that 9.6% have increased albuminuria but mildly decreased, normal or high eGFR (stages 1 and 2) and that 5.8% have CKD stages 3a–5. Although the prevalence of reduced kidney function has not increased significantly between 2009–2010 and 2016–2017, there is a concerning high percentage of adults 40 years and over with CKD stages 1 and 2 that should be considered in prevention strategies and Chilean guidelines. The information from our study may be useful to clinicians, entities focused on planning prevention strategies and healthcare management and decision and policy-makers.
Supplementary Material
Footnotes
Twitter: @j_mindell
Contributors: MW, SS and JSM contributed to the study design, were involved in analysis and interpretation of data and preparation of the manuscript. EP and MP were involved in the preparation of the manuscript.
Funding: This work was supported by Chilean Scholarship 'Becas Chile, CONICYT'. EP was funded by the United Kingdom National Institute for Health Research (NIHR) Applied Research Collaboration North Thame (ARC North Thames) at Barts Health NHS Trust.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Both Health Surveys were approved by the Ethics Research Committee of the Faculty of Medicine at the Pontificia Universidad Católica de Chile.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The full data sets can be accessed in through the Ministry of Health of Chile website found at: http://epi.minsal.cl/encuestas-poblacionales/
References
- 1.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–22. 10.1016/S0140-6736(12)60033-6 [DOI] [PubMed] [Google Scholar]
- 2.Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 3.Bailey RA, Wang Y, Zhu V, et al. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on kidney disease: improving global outcomes (KDIGO) staging. BMC Res Notes 2014;7:415. 10.1186/1756-0500-7-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 2016;11:e0158765. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Guideline C KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol 2017;13:104–14. 10.1038/nrneph.2016.163 [DOI] [PubMed] [Google Scholar]
- 7.Aitken GR, Roderick PJ, Fraser S, et al. Change in prevalence of chronic kidney disease in England over time: comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open 2014;4:e005480. 10.1136/bmjopen-2014-005480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser SDS, Roderick PJ, Aitken G, et al. Chronic kidney disease, albuminuria and socioeconomic status in the health surveys for England 2009 and 2010. J Public Health 2014;36:577–86. 10.1093/pubmed/fdt117 [DOI] [PubMed] [Google Scholar]
- 9.Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015;26:2504–11. 10.1681/ASN.2014070714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poblete Badal H. XXXVII cuenta de hemodiálisis Crónica (HDC) en Chile, 2017. Available: www.nefro.cl
- 11.United States Renal Data System Volume 2 - end-stage renal disease (ESRD) in the United States, 2017. Available: https://www.usrds.org/2017/view/Default.aspx [Accessed January 2018].
- 12.Instituto de Salud Pública Registro nacional de trasplante, 2017. Available: http://www.ispch.cl/sites/default/files/Registro%20Nacional%202017.pdf
- 13.Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 2016;165:473–81. 10.7326/M16-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MINSAL ENS - Encuesta nacional de salud, 2019. Available: http://epi.minsal.cl/encuesta-ens-descargable/
- 15.MINSAL Encuesta Nacional de Salud ENS Chile 2009-2010, 2016. Available: http://web.minsal.cl/portal/url/item/bcb03d7bc28b64dfe040010165012d23.pdf
- 16.MINSAL Enfoque de riesgo para la prevención de enfermedades cardiovasculares. Subsecretaría de Salud Pública DdEnT, 2014. www.redcrónicas.cl [Google Scholar]
- 17.McQueen RB, Farahbakhshian S, Bell KF, et al. Economic burden of comorbid chronic kidney disease and diabetes. J Med Econ 2017;20:585–91. 10.1080/13696998.2017.1288127 [DOI] [PubMed] [Google Scholar]
- 18.Vupputuri S, Kimes TM, Calloway MO, et al. The economic burden of progressive chronic kidney disease among patients with type 2 diabetes. J Diabetes Complications 2014;28:10–16. 10.1016/j.jdiacomp.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 19.Mindell JS, Moody A, Vecino-Ortiz AI, et al. Comparison of health examination survey methods in Brazil, Chile, Colombia, Mexico, England, Scotland, and the United States. Am J Epidemiol 2017;186:648–58. 10.1093/aje/kwx045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis 2014;63:820–34. 10.1053/j.ajkd.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uehara K, Tominaga N, Shibagaki Y. Adult orthostatic proteinuria. Clin Kidney J 2014;7:327–8. 10.1093/ckj/sfu040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser SDS, Aitken G, Taal MW, et al. Exploration of chronic kidney disease prevalence estimates using new measures of kidney function in the health survey for England. PLoS One 2015;10:e0118676–16. 10.1371/journal.pone.0118676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weir CB, Jan A. BMI classification Percentile and cut off points. StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 24.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- 25.Ramirez-Rubio O, McClean MD, Amador JJ, et al. An epidemic of chronic kidney disease in central America: an overview. J Epidemiol Community Health 2013;67:1–3. 10.1136/jech-2012-201141 [DOI] [PubMed] [Google Scholar]
- 26.Hu J-R, Coresh J. The public health dimension of chronic kidney disease: what we have learnt over the past decade. Nephrol Dial Transplant 2017;32:ii113–20. 10.1093/ndt/gfw416 [DOI] [PubMed] [Google Scholar]
- 27.Qin X, Wang Y, Li Y, et al. Risk factors for renal function decline in adults with normal kidney function: a 7-year cohort study. J Epidemiol Community Health 2015;69:782–8. 10.1136/jech-2014-204962 [DOI] [PubMed] [Google Scholar]
- 28.Tsai W-C, Wu H-Y, Peng Y-S, et al. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine 2016;95:e3013. 10.1097/MD.0000000000003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–73. 10.1016/S0140-6736(12)61350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chini LSN, Assis LIS, Lugon JR. Relationship between uric acid levels and risk of chronic kidney disease in a retrospective cohort of Brazilian workers. Braz J Med Biol Res 2017;50:e6048. 10.1590/1414-431x20176048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yepes Delgado CE, Pérez Dávila S, Montoya Jaramillo M, et al. Stage progression and need for renal replacement therapy in a renal protection programme in Colombia. A cohort study. Nefrologia 2017;37:330–7. 10.1016/j.nefroe.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 32.Cardoso CRL, Leite NC, Salles GC, et al. Aortic stiffness and ambulatory blood pressure as predictors of diabetic kidney disease: a competing risks analysis from the Rio de Janeiro type 2 diabetes cohort study. Diabetologia 2018;61:455–65. 10.1007/s00125-017-4484-z [DOI] [PubMed] [Google Scholar]
- 33.Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis 2015;22:6–15. 10.1053/j.ackd.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vart P, Gansevoort RT, Joosten MM, et al. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med 2015;48:580–92. 10.1016/j.amepre.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 35.The World Bank GINI index (world bank estimate), 2017. Available: https://data.worldbank.org/indicator/SI.POV.GINI?end=2015&locations=CL&start=1987&view=chart
- 36.Riumallo-Herl CJ, Kawachi I, Avendano M. Social capital, mental health and biomarkers in Chile: assessing the effects of social capital in a middle-income country. Soc Sci Med 2014;105:47–58. 10.1016/j.socscimed.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luis-Lima S, Porrini E. An overview of errors and flaws of estimated GFR versus true GFR in patients with diabetes mellitus. Nephron 2017;136:287–91. 10.1159/000453531 [DOI] [PubMed] [Google Scholar]
- 39.Porrini E, Ruggenenti P, Luis-Lima S, et al. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol 2019;15:177–90. 10.1038/s41581-018-0080-9 [DOI] [PubMed] [Google Scholar]
- 40.Lemoine S, Guebre-Egziabher F, Sens F, et al. Accuracy of GFR estimation in obese patients. Clin J Am Soc Nephrol 2014;9:720–7. 10.2215/CJN.03610413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Broe ME, Gharbi MB, Zamd M, et al. Why overestimate or underestimate chronic kidney disease when correct estimation is possible? Nephrol Dial Transplant 2017;32:ii136–41. 10.1093/ndt/gfw267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037720supp001.pdf (66.7KB, pdf)