ABSTRACT
Central nervous system (CNS) blood vessels contain a functional blood-brain barrier (BBB) that is necessary for neuronal survival and activity. Although Wnt/β-catenin signaling is essential for BBB development, its downstream targets within the neurovasculature remain poorly understood. To identify targets of Wnt/β-catenin signaling underlying BBB maturation, we performed a microarray analysis that identified Fgfbp1 as a novel Wnt/β-catenin-regulated gene in mouse brain endothelial cells (mBECs). Fgfbp1 is expressed in the CNS endothelium and secreted into the vascular basement membrane during BBB formation. Endothelial genetic ablation of Fgfbp1 results in transient hypervascularization but delays BBB maturation in specific CNS regions, as evidenced by both upregulation of Plvap and increased tracer leakage across the neurovasculature due to reduced Wnt/β-catenin activity. In addition, collagen IV deposition in the vascular basement membrane is reduced in mutant mice, leading to defective endothelial cell-pericyte interactions. Fgfbp1 is required cell-autonomously in mBECs to concentrate Wnt ligands near cell junctions and promote maturation of their barrier properties in vitro. Thus, Fgfbp1 is a crucial extracellular matrix protein during BBB maturation that regulates cell-cell interactions and Wnt/β-catenin activity.
KEY WORDS: Blood-brain barrier, Fgfbp1, Wnt/β-catenin signaling, Basement membrane, Collagen IV
Summary: Fgfbp1 is necessary for collagen IV deposition in the CNS endothelium basement membrane and may facilitate presentation of Wnt ligands to their receptors on EC membranes by concentrating them close to cell junctions.
INTRODUCTION
Central nervous system (CNS) blood vessels form a unique structure termed the blood-brain barrier (BBB) that is crucial to maintain neuronal and glial function by supplying nutrients and ions to the brain parenchyma while blocking toxins and pathogenic insults (Liebner et al., 2018). During CNS development, endothelial cells (ECs) tightly coordinate angiogenesis with formation of a functional barrier. They express high levels of tight junction (TJ) proteins that limit paracellular permeability from the blood into the brain, as well as specialized transporters to facilitate transport of nutrients across the endothelium; concomitantly, expression of proteins required for bulk transcytosis and formation of fenestrae (transcellular permeability) is suppressed (Biswas et al., 2020). BBB integrity is impaired in various neurological diseases such as ischemic stroke, multiple sclerosis and neurodegenerative diseases (Jiang et al., 2018; Spencer et al., 2018; Sweeney et al., 2018). Thus, understanding the mechanisms of neurovascular barrier formation is essential for developing interventions for these CNS diseases.
Acquisition of BBB properties is achieved by close interactions among vascular, neuronal and glial compartments of the neurovascular unit (NVU) via the vascular basement membrane (BM) (Biswas et al., 2020). Laminins (heterotrimeric glycoproteins containing α-, β- and γ-chains) and collagen IV are two crucial components of the BM secreted by ECs, astrocytes and pericytes (Thomsen et al., 2017). Neurovascular barrier function is impaired under genetic or pathological perturbations of the vascular BM composition. Genetic ablation of either pro-collagen type IV alpha 1 (Col4a1) (Gould et al., 2005) or laminin-α2 chain (Lama2) (Menezes et al., 2014; Sixt et al., 2001) in mice leads to cerebral hemorrhage, junctional disorganization and BBB leakage. The BBB permeability abnormalities in Lama2−/− mice are also associated with changes in mural cell coverage and astrocyte endfeet polarization around blood vessels (Menezes et al., 2014). Astrocyte-specific deletion of laminin γ1 also causes hemorrhagic stroke (Chen et al., 2013), defects in pericyte differentiation, loss of astrocyte endfeet polarization and BBB leakage (Yao et al., 2014). Similarly, global deletion of integrin αV or β8, an extracellular matrix (ECM) receptor (Bader et al., 1998; Mobley et al., 2009), neuroepithelium-specific deletion of integrin β8 (Arnold et al., 2014), or endothelial-specific ablation of TGF-β receptor 2 (Tgfbr2) (Nguyen et al., 2011) lead to defects in CNS angiogenesis, BBB formation and perivascular astrogliosis due to diminished integrin-mediated TGF-β1 activation. Pericyte deficiency also increases BBB permeability owing to aberrant localization of astrocyte endfeet around blood vessels due to defects in BM proteins (Armulik et al., 2010; Daneman et al., 2010). Thus, perturbations in vascular BM proteins trigger abnormal mural cell coverage or astrocyte endfeet polarization and affect BBB maturation.
In addition to promoting NVU assembly, the vascular BM also plays an important role in signaling. Wnt/β-catenin activity, essential for CNS angiogenesis and BBB development (Liebner et al., 2018), is regulated by heparan sulfate proteoglycans (HSPGs) in the ECM (Lin, 2004; Pataki et al., 2015; Ren et al., 2018). Wnt ligands induce angiogenesis, proliferation, survival and barrier formation through stabilization and nuclear translocation of β-catenin (Dejana and Kühl, 2010). Mice deficient for Wnt7a/b, Frizzled 4 (Fzd4), Lrp5/6 and β-catenin (Ctnnb1) have defects in both CNS angiogenesis and BBB formation (Cho et al., 2017a; Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008; Zhou et al., 2014). Several transcription factors downstream of Wnt/β-catenin signaling are also crucial for BBB development. We have shown that Sox17, a transcription factor downstream of Wnt/β-catenin signaling in ECs, regulates both arterial differentiation and BBB formation (Corada et al., 2019; Corada et al., 2013). Endothelial-specific inactivation of Sox17 prevents BBB maturation, suggesting that Sox17 is a positive regulator that functions together with Wnt signaling to induce BBB properties (Corada et al., 2019). Foxf2, a transcription factor identified recently to function downstream of the Wnt/β-catenin pathway in brain ECs (Hupe et al., 2017; Sabbagh et al., 2018) also regulates BBB function in vivo (Reyahi et al., 2015) and induces BBB properties in vitro when overexpressed in HUVEC cells together with Zic3 (Hupe et al., 2017).
How do transcription factors that function downstream of Wnt/β-catenin signaling regulate BBB properties? This is still not completely understood, because most downstream secreted or membrane-bound effector proteins essential for BBB development have not been identified. To address this issue, we performed a microarray analysis and identified Fgfbp1 (encoding fibroblast growth factor binding protein 1) as a novel gene regulated by Wnt/β-catenin signaling in primary mouse brain endothelial cells (mBECs). Fgfbp1 is highly expressed in the CNS endothelium during BBB formation, and is secreted in the vascular BM. Genetic ablation of Fgfbp1 in the CNS endothelium causes a transient hypervascularization, but delays BBB maturation as measured by upregulation of Plvap in ECs and increased neurovascular leakage due to low levels of Wnt/β-catenin activity. Moreover, collagen IV deposition in the vascular BM is reduced in mutant mice, leading to defects in EC-pericyte interactions. Fgfbp1 is required cell-autonomously in mBECs to promote both paracellular and transcellular barrier properties in vitro and concentrate Wnt ligands near cell junctions. Thus, Fgfbp1 is a secreted ECM protein crucial for mediating NVU cell interactions and maintaining Wnt/β-catenin activity in ECs, two processes essential for BBB maturation.
RESULTS
Fgfbp1 expression is regulated by Wnt/β-catenin signaling both in vitro and in vivo
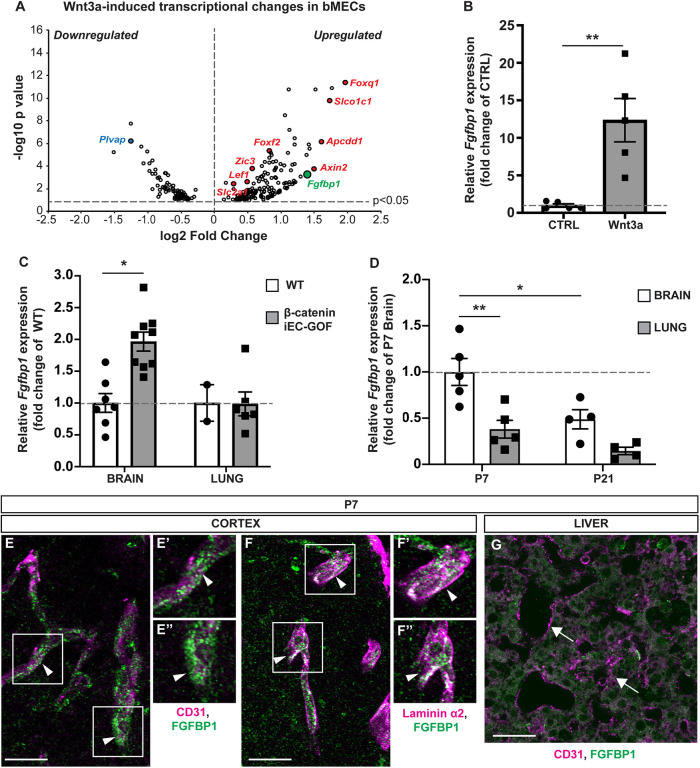
To identify novel downstream effectors of Wnt/β-catenin signaling that regulate BBB development, we performed a gene chip analysis of transcripts that are differentially expressed in mBECs in the absence or presence of recombinant Wnt3a. In addition to several established Wnt/β-catenin targets such as Axin2, Apcdd1, Slco1c1, Foxf2, Foxq1, Zic3 and Plvap (Daneman et al., 2009; Hupe et al., 2017; Liebner et al., 2008; Mazzoni et al., 2017; Sabbagh et al., 2018; Stenman et al., 2008; Zhou et al., 2014), we identified Fgfbp1 as a gene that is highly upregulated in Wnt3a-treated mBECs (Fig. 1A). We confirmed by RT-PCR that Fgfbp1 mRNA expression increases 13-fold in Wnt3a-treated compared with untreated mBECs (Fig. 1B). To validate these findings in vivo, we isolated brain and lung microvascular fragments from either wild-type (WT) or inducible endothelial-specific β-catenin gain-of-function [Cdh5(PAC)–CreERT2 X β-catlox(ex3)/lox(ex3) (β-cateniniEC-GOF)] (Liebner et al., 2008) postnatal day (P) 7 pups, a week after inducing recombination with tamoxifen. RT-PCR analysis showed that Fgfbp1 mRNA is significantly enriched in brain, but not lung, microvessels of β-cateniniEC-GOF compared with WT mice (Fig. 1C). Moreover, Fgfbp1 mRNA levels are higher in brain compared with lung microvessels throughout postnatal development (P7 and P21); however, expression abates by P21 (Fig. 1D). Thus, Fgfbp1 is induced by Wnt/β-catenin signaling in brain ECs, but its expression declines during development in parallel with reduced Wnt activity and gradual BBB maturation.
Fig. 1.
Fgfbp1 is induced by Wnt/β-catenin activation in brain ECs. (A) Volcano plot representing transcriptional changes in Wnt3a-treated versus untreated primary mBECs. Each dot represents a single gene. Only genes with P<0.05 were plotted. Red dots correspond to upregulated genes whereas blue dots correspond to downregulated genes. Fgfbp1 is shown in green. (B) Dotted bar graph of the fold change in Fgfbp1 mRNA expression for Wnt3a-treated versus untreated mBECs. Each dot represents an independent experiment. Data are mean±s.e.m.; n=5; **P<0.005; Student's t-test. (C) Dotted bar graph of the fold change in Fgfbp1 mRNA expression for brain or lung microvessels isolated from P8 WT (n=7 brain; n=2 lungs) and β-cateniniEC-GOF (n=9 brain; n=6 lung) pups. Each dot represents a single animal. Data are mean±s.e.m. *P<0.05; Student's t-test. (D) Dotted bar graph of the fold change in Fgfbp1 mRNA expression in brain and lung microvessels isolated from WT P7 (n=5) and P21 (n=4) pups. Each dot represents a single animal. Data are mean±s.e.m.; *P<0.05, **P<0.005; one-way ANOVA. (E-G) Immunofluorescence for Fgfbp1 in brain (E-F″) and liver (G) vasculature of P7 WT pups. Vessels are marked with CD31 (red), vascular BM with laminin α2 (red) and Fgfbp1 (green). Fgfbp1 levels are very low in liver (G, arrows), but the protein levels are high in brain blood vessels (E-E″, arrowheads), where it colocalizes with laminin α2 (F-F″, arrowheads). E′,E″ and F′,F″ show magnifications of boxed areas in E and F, respectively. Scale bars: 10 µm.
To determine the localization of Fgfbp1 protein, we performed immunofluorescence in both brain (cortex) and liver of WT P7 pups. Fgfbp1 is found predominantly in the vascular BM of developing CD31+ (Pecam1+) cortical vessels, and colocalizes with the BM protein laminin α2, but not the EC junctional protein CD31 (Fig. 1E-F″). Thus, Fgfbp1 is likely secreted from ECs and accumulates in the vascular BM. Consistent with our RT-PCR results, we detected very low Fgfbp1 protein levels in liver blood vessels (Fig. 1G).
Endothelial-specific ablation of Fgfbp1 causes hypervascularization during postnatal CNS development
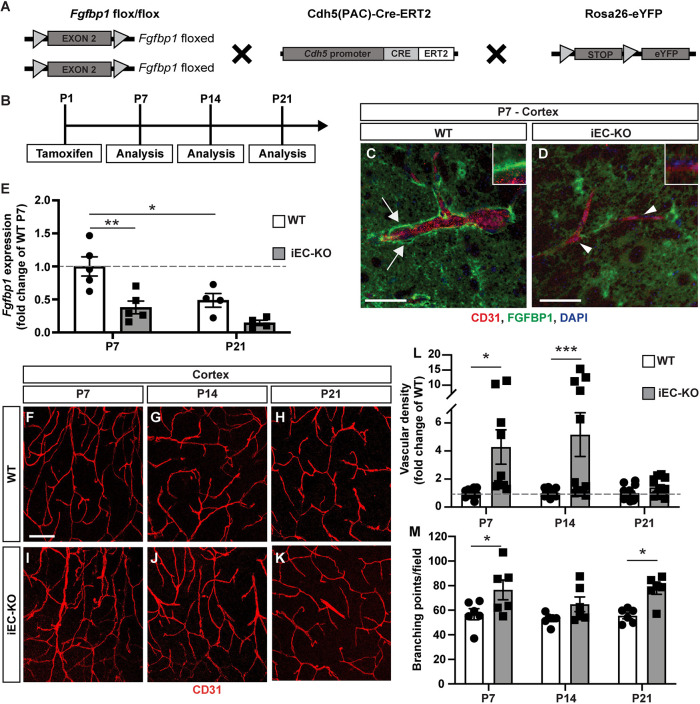
To investigate the role of Fgfbp1 in CNS vascular development, we generated an inducible endothelial-specific Fgfbp1 knockout (Fgfbp1iEC-KO) mouse strain by crossing Fgfbp1fl/fl mice with a Cdh5(PAC)-CreERT2 strain (Pitulescu et al., 2010) (Fig. 2A). We induced Fgfbp1 deletion in ECs by injecting tamoxifen at P1 and analyzed their vascular phenotype at P7, P14 or P21 (Fig. 2B). We verified by immunostaining that Fgfbp1 expression is reduced around CD31+ vessels in the cortex of Fgfbp1iEC-KO compared with WT P7 mice (Fig. 2C,D, white arrows and arrowheads, insets). We also confirmed that the Cdh5(PAC)-CreERT2 strain induced homogeneous recombination in all cortical CD31+ vessels of Fgfbp1iEC-KO mice by uniform eGFP expression arising from the ROSA26-eYFP reporter. However, there is some residual Fgfbp1 protein in Fgfbp1iEC-KO cortices likely because of expression by neurons or astrocytes. RT-PCR analysis of Fgfbp1 mRNA expression in microvascular fragments isolated from either P7 or P21 WT or Fgfbp1iEC-KO pups also showed a persistent reduction, rather than complete loss, in Fgfbp1 mRNA levels (Fig. 2E), likely due to the presence of pericytes and astrocyte endfeet.
Fig. 2.
Vascular abnormalities in cortices of Fgfbp1iEC-KO mice during postnatal development. (A,B) Diagrams showing the breeding strategy (A) and experimental setup (B) to produce and analyze Fgfbp1iEC-KO mice. (C,D) Immunofluorescence for Fgfbp1 (green), CD31 (red) and DAPI (blue) in 100 µm thick vibratome sections of P7 WT (C) and Fgfbp1iEC-KO (D) brains. Fgfbp1 is expressed at high levels in the vascular BM (C, arrows) in WT brains, but is reduced in Fgfbp1iEC-KO brains (D, arrowheads and insets). Expression of Fgfbp1 protein by neurons or astrocytes is still detected in Fgfbp1iEC-KO mice. (E) Bar graph of fold change in Fgfbp1 mRNA expression for ECs freshly isolated from brains of WT and Fgfbp1iEC-KO P7 and P21 pups. Each dot represents a single animal. Data are mean±s.e.m.; *P<0.05, **P<0.005; one-way ANOVA. Fgfbp1 is significantly reduced in P7 mutant mice. (F-K) Analysis of vascular morphology in the cortex of WT (F-H) and Fgfbp1iEC-KO (I-K) mice at P7 (F,I), P14 (G,J) and P21 (H,K). The vasculature is visualized by immunofluorescence for CD31 (red). (L,M) Quantification of vascular density (L) and branching points (M) in cortices of WT and Fgfbp1iEC-KO mice at P7, P14 and P21. Each dot represents the average of at least three confocal acquisitions from a single animal. The cortical vascular density is increased in Fgfbp1iEC-KO pups at P7 and P14. Data are mean±s.e.m. *P<0.05, ***P<0.0005; Student's t-test. Scale bars: 50 µm in C,D; 100 µm in F-K.
We next analyzed vascular density and branching points for CD31+ cortical blood vessels in WT and Fgfbp1iEC-KO pups (Fig. 2F-K). The cortical vascular density was significantly increased in Fgfbp1iEC-KO mutant mice at both P7 and P14, however it was similar to WT controls by P21 (Fig. 2L). The vasculature from Fgfbp1iEC-KO mutant pups also displayed more branching points at P7 and P21 (Fig. 2M). This hypervascularization phenotype was also observed in the retinal vasculature of mutant mice at P7, although less prominently than in the cortex (Fig. S1). The retinal vascular network of Fgfbp1iEC-KO pups showed a significant increase in radial growth from the optic nerve towards the periphery during the first week of development, and the number of tip cell protrusions was higher compared with WT littermates (Fig. S1A-F′,G,K). However, neither vascular density nor the number of branching points or tip cells was significantly different between the two genotypes (Fig. S1H,I,J). Overall, these data show that Fgfbp1 is important for CNS angiogenesis.
Fgfbp1 alters BBB maturation by perturbing deposition of collagen IV and endothelial cell-pericyte interactions
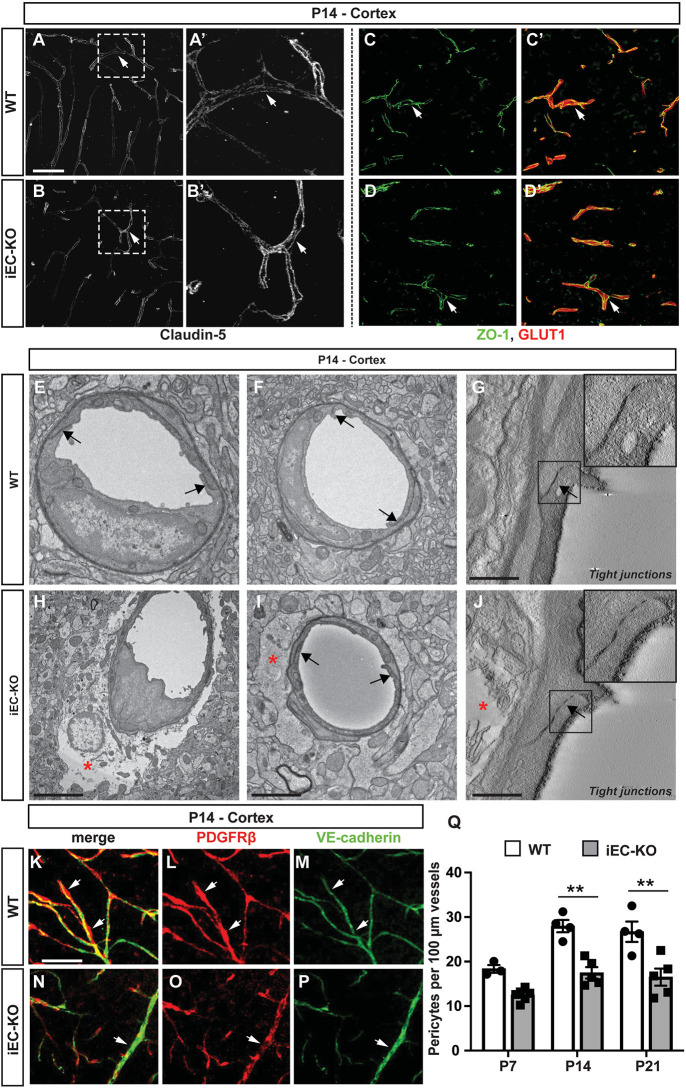
To examine the role of Fgfbp1 in BBB development, we analyzed expression and subcellular localization of two crucial BBB TJ proteins, claudin 5 (Nitta et al., 2003), and ZO-1 (Tjp1) in the cortex of WT and Fgfbp1iEC-KO mice at P14; however, we did not detect any difference between the two genotypes (Fig. 3A-D′). We then analyzed TJ morphology at the ultrastructural level in P14 cortical capillaries from both genotypes using transmission electron microscopy (TEM) (Fig. 3E-J). Brain ECs appear morphologically normal with properly organized junctional strands in both WT and Fgfbp1iEC-KO pups at P14 (Fig. 3E-J, black arrows), suggesting no obvious structural defects in TJs in the absence of Fgfbp1. In addition, ECs had a low number of caveolae regardless of the phenotype (Fig. 3E,F,H,I). However, some capillaries showed abnormal swelling of astrocyte endfeet in Fgfbp1iEC-KO mice at P14 (Fig. 3H-J, red asterisks).
Fig. 3.
Ultrastructural integrity of tight junctions is maintained in Fgfbp1iEC-KO mice. (A-D′) Immunofluorescence for claudin 5 (A-B′), ZO-1 (green) and Glut1 (red) (C-D′) in the cortex of WT (A,A′,C,C′) or Fgfbp1iEC-KO (B,B′,D,D′) P14 pups. The expression and localization of claudin 5 and ZO-1 is similar between the two genotypes. (E-J) TEM analysis of the cortical capillaries from WT (E-G) and Fgfbp1iEC-KO (H-J) mice at P14 (60 nm thick sections, n=3 animals/group). There is no difference in tight junction morphology (E-J, black arrows and insets in G and J) between the two genotypes. However, a subset of capillaries in Fgfbp1iEC-KO show abnormal astrocyte endfeet swelling (E-J, red asterisk). (K-P) Analysis of pericytes in the cortex of WT (K-M) and Fgfbp1iEC-KO (N-P) P14 pups. Brain sections (100 µm thick) were stained for PDGFRβ (red) and VE-cadherin (green). (Q) Quantification of the pericyte number in WT and Fgfbp1iEC-KO at P7, P14 and P21 cortices. Each dot represents the average of at least three confocal acquisitions from a single animal. There is reduced pericyte number in Fgfbp1iEC-KO P14 and P21 pups compared with WT pups. Data are mean±s.e.m. **P<0.01; Student's t-test. Scale bars: 50 µm in A-D′,K-P; 1 µm in E,F,H,I; 250 nm in G,J.
To gain insights into the role of Fgfbp1 in the NVU organization, we performed immunofluorescence for PDGFRβ (a pericyte marker), glial fibrillary acidic protein (GFAP, astrocyte marker) and aquaporin IV (astrocyte endfeet marker), and quantified the number of vessel-associated pericytes and vessel coverage by astrocyte endfeet in WT and Fgfbp1iEC-KO cortices. Fgfbp1iEC-KO mice showed a moderate but statistically significant decrease in pericyte number at P14 and P21 (Fig. 3K-Q); however, GFAP expression and aquaporin IV vessel coverage were not affected in mutant mice compared with WT littermates (Fig. S2A-M). Therefore, Fgfbp1 is essential to maintain the association of ECs with pericytes at the NVU.
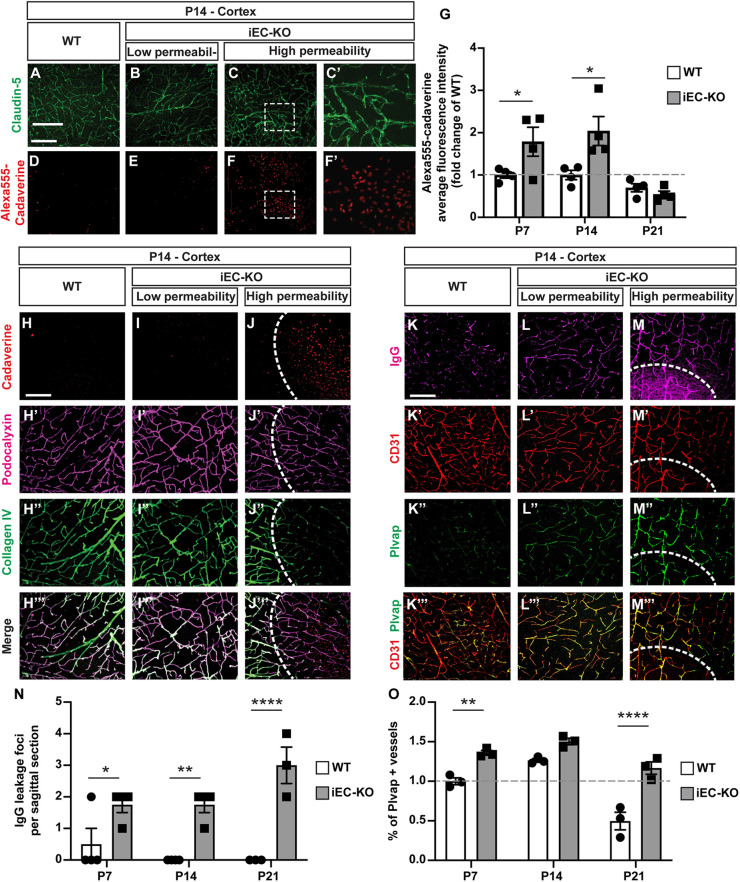
To assess whether blood vessels from Fgfbp1iEC-KO mice have a mature functional barrier, we analyzed diffusion of a small molecular weight (MW) tracer, Cadaverine-AlexaFluor 555 (950 Da), across the CNS vasculature 2 h after its intraperitoneal injection in both genotypes from P7 to P21 (Fig. 4A-F′) (Armulik et al., 2010; Corada et al., 2019). We detected multiple scattered focal areas of high Cadaverine-Alexa555 leakage from the vasculature in the cortex of Fgfbp1iEC-KO at P7 and P14, but very few in WT mice (there were multiple focal areas with low Cadaverine-Alexa555 leakage in both genotypes at P7 and P14), although claudin 5 localization appears normal in both genotypes even at sites of high BBB leakage (Fig. 4A-F′, white arrows). There was a twofold increase in the average fluorescence tracer intensity within the cortex of Fgfbp1iEC-KO compared with WT animals at P7 and P14 (Fig. 4G). However, BBB function was restored by P21 in Fgfbp1iEC-KO mice, suggesting that EC-derived Fgfbp1 is crucial for the timing of barrier maturation.
Fig. 4.
Fgfbp1iEC-KO mice show delayed BBB maturation. (A-F′) Immunofluorescence analysis of BBB permeability to Cadaverine-AlexaFluor 555 in the cortex of WT (A,D) and Fgfbp1iEC-KO (B,C,E,F) P14 pups. Leakage of Cadaverine-Alexa555 into the cortical parenchyma was assessed 2 h after intraperitoneal injection of tracer (red; D-F′). Sections (100 µm thick) were also stained for claudin 5 (green; A-C′) to correlate tracer leakage with TJ integrity. Fgfbp1iEC-KO pups have increased BBB permeability in the cortex. C′ and F′ show magnifications of the boxed area in C and F, respectively. (G) Quantification of BBB permeability to Cadaverine-Alexa555 in the cortex of WT and Fgfbp1iEC-KO P7, P14 and P21 pups. Each dot represents the average fluorescence intensity of the tracer from confocal stacks of 4-9 cortical regions with either low or high BBB permeability per animal. Data are mean±s.e.m. and fold change compared with wild-type mice. *P<0.05; Student's t-test. Fgfbp1iEC-KO pups have increased BBB permeability at P7 and P14. (H-J‴) Analysis of BBB permeability and collagen IV deposition in the BM of cortical vessels in WT (H-H‴) and Fgfbp1iEC-KO (I-J‴) P14 pups. Leakage of Cadaverine-Alexa555 into the brain parenchyma was assessed 2 h after injection of the tracer in the peritoneum (H-J, red; dashed line in J). Sections (100 µm thick) were stained for podocalyxin (blood vessels, magenta; H′,I′,J′) and collagen IV (green; H″,I″,J″). Fgfbp1iEC-KO pups have increased BBB permeability in areas lacking collagen IV in the BM (dashed white line). (K-M‴) Analysis of BBB permeability, serum IgG leakage or Plvap expression in the cortex of WT (K) and Fgfbp1iEC-KO (L,M) P14 pups. Sections (100 µm thick) were stained for serum IgG to identify BBB leakage (magenta; K-M; dashed line in M), CD31 (red; K′-M′), Plvap (green; K″-M″) or merge (K‴-M‴). Plvap expression is higher in regions with leaky BBB and serum IgG extravasation. Dashed line indicates the area of BBB leakage. (N,O) Quantification of the number of IgG leakage foci (N) and Plvap expression (O) in the cortex of WT and Fgfbp1iEC-KO P7, P14 and P21 pups. Each dot represents a single animal. The number of foci with IgG leakage (i.e. high BBB permeability) is increased in Fgfbp1iEC-KO pups. Data are mean±s.e.m. *P<0.05, **P<0.005, ****P<0.00005; Student's t-test. Scale bars: 100 µm.
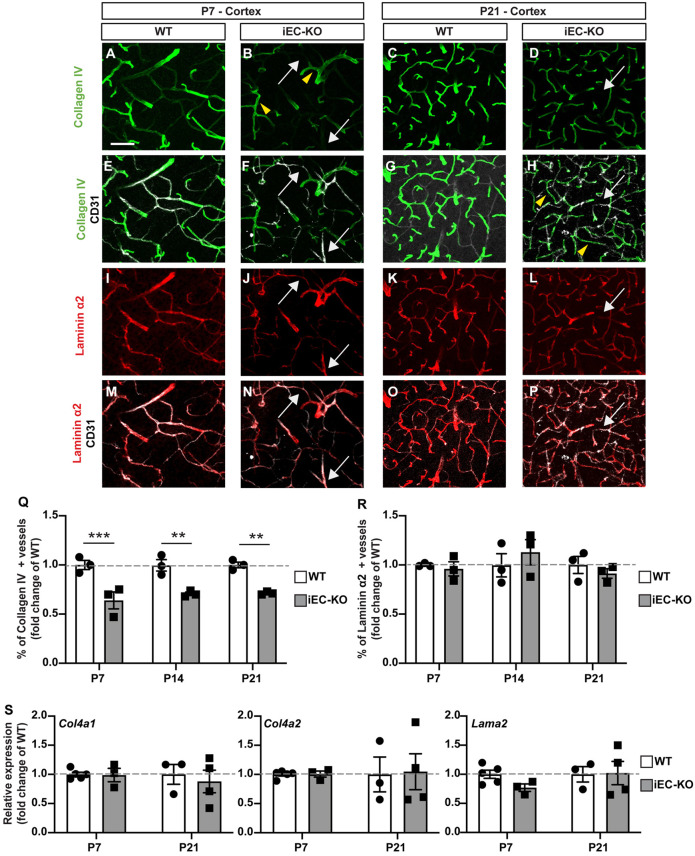
As the ultrastructural TJ morphology appears normal and the number of caveolae is low in Fgfbp1iEC-KO CNS endothelium (Fig. 3E-J), we analyzed the composition of vascular BM as a crucial regulator of BBB function. Collagen IV staining was reduced significantly in the perivascular space of some Fgfbp1iEC-KO podocalyxin-positive blood vessels throughout the cortex (Fig. 4H-J‴). This strongly correlates with focal regions of high vascular permeability to Cadaverine-Alexa555 into the parenchyma (Fig. 4J-J‴). In contrast, cortical areas without or with low vascular leakage in Fgfbp1iEC-KO mice showed normal collagen IV perivascular staining comparable with WT animals (Fig. 4H-I‴). Fgfbp1iEC-KO mice also had a significant higher number of cortical regions with increased permeability to serum IgG compared with WT controls [Fig. 4K-M,N (M, dashed line)]. Finally, expression of Plvap, a marker of an immature BBB, was significantly higher in cortical areas of mutant mice with either low or high BBB permeability compared with WT mice (Fig. 4K″-M‴,O). Thus, Fgfbp1 regulates barrier maturation by controlling expression of Plvap in ECs and deposition of collagen IV into the vascular BM.
Fgfbp1 specifically disrupts collagen IV deposition to the vascular basement membrane
Because Fgfbp1iEC-KO mice show a strong correlation between increased vascular leakage and decreased collagen IV deposition in the ECM at P14 (Fig. 4J-J‴), we investigated more thoroughly the vascular BM composition by immunostaining for collagen IV, laminin α2, α4 and α5, fibronectin and perlecan (Hspg2), and the vascular markers CD31 and Glut1 (Slc2a1) from P7 to P21. Although collagen IV deposition is reduced from P7 to P21 in the vascular BM of Fgfbp1iEC-KO mice, laminin α2, α4 and α5 levels were similar in both genotypes at all developmental stages (Fig. 5A-R, Fig. S3A-N). We detected multiple vessels positive for laminin α2, but not collagen IV, in P7 and P21 Fgfbp1iEC-KO cortices (Fig. 5F,H,N,P). In contrast, fibronectin and perlecan deposition in the vascular BM were increased by P7, potentially to compensate for collagen IV loss (Fig. S4A-R). However, the vascular coverage for perlecan was similar at P14 and P21 between the two genotypes. In contrast, the vascular coverage for fibronectin was reduced during development, and by P21 Fgfbp1iEC-KO mice showed reduced coverage compared with WT controls (Fig. S4A-R).
Fig. 5.
Fgfbp1 is essential for collagen IV deposition to the vascular basement membrane. (A-P) Analysis of BM composition in cortices of WT and Fgfbp1iEC-KO pups at P7 (A,B,E,F,I,J,M,N) and P21 (C,D,G,H,K,L,O,P). Sections (100 µm thick) were stained for CD31 (white; E-H,M-P) to identify blood vessels, collagen IV (green, A-H) and laminin α2 (red, I-P). Yellow arrowheads indicate vessels positive for both collagen IV and laminin α2. White arrows indicate vessels positive for laminin α2 and negative for collagen IV. (Q-R) Quantification of collagen IV (Q) and laminin α2 vessel coverage in the cortex of WT and Fgfbp1iEC-KO P7, P14 and P21 pups. Each dot represents the average of at least three confocal acquisitions from a single animal. Data are mean±s.e.m. **P<0.005, ***P<0.0005; Student's t-test. Collagen IV coverage is reduced in cortical vessels of Fgfbp1iEC-KO. (S) Dotted bar graphs of the fold change in expression of Col4a1, Col4a2 and Lama2 mRNAs in ECs freshly isolated from the brains of WT and Fgfbp1iEC-KO P7 and P21 pups. Each dot represents a single animal. Data are mean±s.e.m. There is no difference in either genotype by Student's t-test. Scale bar: 50 µm.
Collagen IV contains three α chains that assume a quaternary structure. Although six α chains are present in mammalian genomes, endothelial collagen IV primarily contains α1 and α2 chains (Gould et al., 2005; Thomsen et al., 2017; Vanlandewijck et al., 2018). To address whether altered collagen IV deposition in Fgfbp1iEC-KO mice is due to reduced expression of Col4a1 and Col4a2, we performed RT-PCR in brain-derived microvascular fragments of WT and Fgfbp1iEC-KO pups at P7 and P21. The RNA levels of Col4a1 and Col4a2 were comparable between WT and mutant mice at both developmental stages (Fig. 5S), suggesting that reduced collagen IV is not because of decreased mRNA expression by ECs, but likely impaired deposition of the mature protein into the vascular BM. Furthermore, Lama2, Lama4 and Lama5 mRNAs (genes encoding laminin α2, α4 and α5, respectively) are comparable between WT and Fgfbp1iEC-KO (Fig. 5S, Fig. S3O,P). Therefore, Fgfbp1 elimination in ECs specifically affects deposition of collagen IV into the vascular BM.
Fgfbp1 is required to maintain endothelial cell barrier properties in vitro
To examine cell biological mechanisms by which Fgfbp1 modulates endothelial barrier properties in vitro, we silenced Fgfbp1 mRNA in primary mBECs using three different targeting siRNAs (siBP1 #1, #2 and #3) and verified their efficiency by RT-PCR after inducing Fgfbp1 expression with recombinant Wnt3a (Fig. S5A). Because siBP1 #2 displayed the highest efficacy in reducing Fgfbp1 mRNA and protein levels 100 h after Wnt3a treatment (Fig. S5A,B), we selected this reagent for in vitro studies.
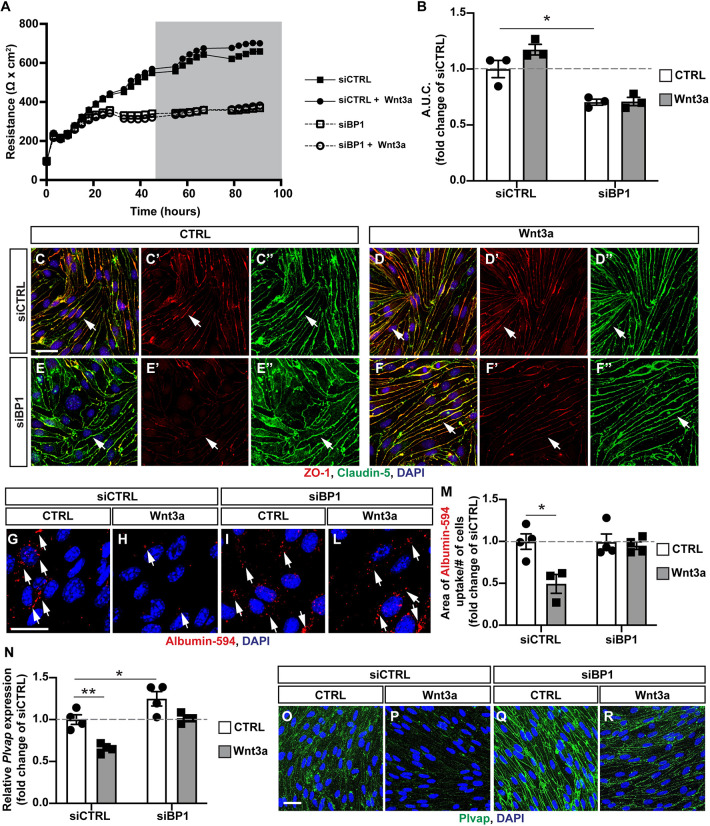
We assessed the integrity of the paracellular barrier in control and siBP1-transfected mBECs by measuring transendothelial electrical resistance (TEER) in the absence or presence of Wnt3a (Mazzoni et al., 2017). mBECs transfected with siBP1 showed a significantly reduced TEER that was not rescued by Wnt3a (Fig. 6A,B). Moreover, we did not observe abnormalities in expression or subcellular localization of claudin 5 in the absence of Fgfbp1 by immunofluorescence, consistent with our in vivo data (Fig. 3A-D′); however, ZO-1 expression is reduced in siBP1-treated compared with control mBECs (Fig. 6C-C″,E-E″). In addition, Wnt3a did not rescue ZO-1 expression in siBP1-transfected mBECs (Fig. 6D-D″,F-F″), consistent with reduced TEER (Fig. 6A,B).
Fig. 6.
Fgfbp1 knockdown impairs Wnt-induced paracellular and transcellular barrier properties in brain endothelial cells in vitro. (A) TEER of a monolayer of mBECs transfected with either an siRNA targeting Fgfbp1 (siBP1) or a scramble siRNA (siCTRL). The gray area represents the period of time during which cells were treated with either recombinant Wnt3a or vehicle (control). (B) Quantification of the area under the curve (A.U.C.) during Wnt3a treatment (gray area in A). Each dot represents an independent experiment. siBP1-transfected cells have reduced TEER compared with siCTRL-transfected cells. Data are mean±s.e.m. *P<0.05; one-way ANOVA. (C-F″) Immunofluorescence for claudin 5 (green) and ZO-1 (red) in mBECs transfected with either siBP1 or siCTRL either with or without Wnt3a. There is no difference in claudin 5 localization, but ZO-1 expression is reduced in siBP1-transfected compared with siCTRL-transfected mBECs. (G-L) Immunofluorescence for the uptake of Albumin-Alexa 594 (red dots, white arrows) in siCTRL or siBP1-transfected mBECs with or without Wnt3a. DAPI (blue) labels nuclei. (M) Quantification of Albumin-Alexa 594 uptake by siCTRL- or siBP1-transfected mBECs with or without Wnt3a. Each dot represents an independent experiment. Wnt3a reduces the uptake of Albumin-Alexa594 in siCTRL, but not in siBP1-transfected mBECs. Data are mean± s.e.m. *P<0.05; one-way ANOVA. (N) Fold change expression of Plvap mRNA in siCTRL or siBP1-transfected mBECs either untreated or treated with Wnt3a. Each dot represents an independent experiment. Plvap expression is reduced by Wnt3a treatment in siCTRL-transfected but not siBP1-transfected mBECs. Data are mean±s.e.m. *P<0.05, **P<0.005; one-way ANOVA. (O-R) Immunofluorescence for Plvap (green) and DAPI (blue) in siCTRL or siBP1-transfected mBECs with or without Wnt3a. Scale bars: 20 µm.
We then assessed whether Fgfbp1 affects caveolar and adsorptive-mediated endocytosis using the albumin-AlexaFluor 594 uptake assay (Mazzoni et al., 2017). Wnt3a decreased uptake of labeled albumin in siCTRL-, but not siBP1-transfected mBECs, suggesting that Fgfbp1 is necessary for Wnt-mediated maturation of the transcellular barrier in primary mBECs (Fig. 6G-M). In addition, Plvap mRNA and protein expression are reduced by Wnt3a treatment in siCTRL- but not in siBP1-transfected mBECs (Fig. 6N-R), consistent with our in vivo data showing increased Plvap expression in Fgfbp1iEC-KO mice (Fig. 4O). Thus, Fgfbp1 is required cell-autonomously in mBECs in vitro to promote maturation of both paracellular and transcellular barrier properties.
Fgfbp1 has been implicated in regulation of FGF2 availability to the ECM (Kurtz et al., 1997; Lametsch et al., 2000) by reducing FGF2 affinity for HSPGs (Aigner et al., 2001). To test the effects of Fgfbp1 on FGF2/FGFR1 signaling in mBECs, we measured phosphorylation of FGFR1 and ERK, a MAPK, 30 min after FGF2 exposure in siCTRL- and siBP1-transfected mBECs. We did not see any difference in FGFR1 and ERK phosphorylation in primary mBECs after FGF2 treatment in siCTRL- or siBP1-transfected mBECs, suggesting that it does not affect this signaling pathway (Fig. S5C).
Fgfbp1 modulates the endothelial cell response to Wnts by concentrating them near cell junctions
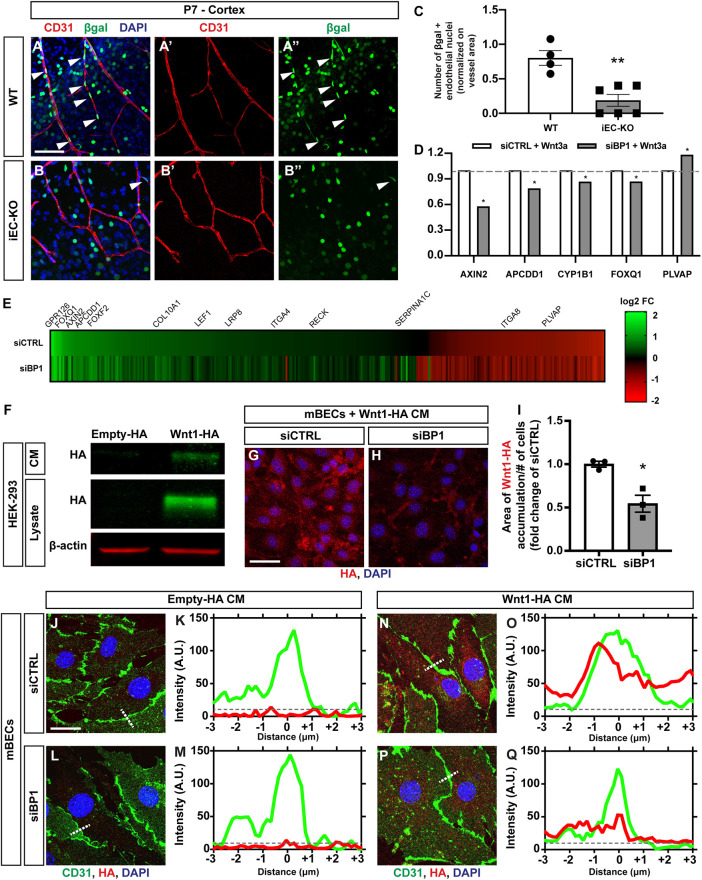
Because loss of Fgfbp1 affects Wnt-mediated regulation of mBEC barrier properties in vitro (Fig. 6), we tested whether Fgfbp1 modulates Wnt/β-catenin activation in the CNS vasculature in vivo. We crossed WT and Fgfbp1iEC-KO mice to a Wnt reporter (BAT-GAL) line, which expresses β-galactosidase in cells with β-catenin activity (Corada et al., 2019; Daneman et al., 2009; Liebner et al., 2008). Immunofluorescence for CD31 and β-galactosidase in cortices from WT and Fgfbp1iEC-KO P7 mice and quantification of β-galactosidase+ EC nuclei showed a significant reduction in Wnt/β-catenin activation in ECs from Fgfbp1iEC-KO compared with WT (Fig. 7A-C). To ascertain the effects on known Wnt/β-catenin targets and identify novel genes that are transcriptionally altered by Fgfbp1, we performed gene chip analysis from siCTRL- and siBP1-tranfected mBECs after Wnt3a treatment. siBP1-transduced mBECs showed reduced induction of four Wnt target genes Axin2, Apcdd1, Cyp1b1 and Foxq1 (Corada et al., 2019; Hupe et al., 2017; Mazzoni et al., 2017; Sabbagh et al., 2018), and upregulation of Plvap in the presence of Wnt3a when compared with siCTRL-transfected mBECs (Fig. 7D). Heatmap analysis showed that the overall response to Wnt3a is distinct between siBP1- and siCTRL-transfected cells (Fig. 7E; Table S1), suggesting that a subset of Wnt/β-catenin-induced targets is regulated by Fgfbp1 in mBECs.
Fig. 7.
Fgfbp1 regulates Wnt/β-catenin activation in brain ECs by concentrating Wnt ligands near cell junctions. (A-B″) Representative images of cortical vasculature from BAT-lacZ (A) and Fgfbp1iEC-KO/BAT-lacZ (B) reporter mice at P7. Sections (100 µm thick) were stained for CD31 to identify blood vessels (red, A′ and B′) and β-galactosidase (β-gal) to identify cells with transcriptionally active β-catenin (green, A″ and B″). Arrowheads indicate β-gal-positive EC nuclei. (C) Quantification of the number of β-gal-positive EC nuclei in the cortex of WT and Fgfbp1iEC-KO mice crossed to BAT-lacZ at P7. Each dot represents the average of at least three confocal acquisitions from a single animal. Fgfbp1iEC-KO mice show reduced Wnt/β-catenin activity in CNS ECs compared with WT mice. Data are mean±s.e.m. **P<0.005; Student's t-test. (D) Fold changes in mRNA expression of five established Wnt/β-catenin targets upon Wnt3a exposure in siCTRL- or siBP1-transfected mBECs. The data were derived from the Affymetrix analysis of Wnt3a-induced transcriptional changes in siCTRL- and siBP1-transfected mBECs (Table S1). Results are shown as fold change of siBP1 versus siCTRL. The mRNA expression of four genes (Axin2, Apcdd1, Cyp1b1 and Foxq1) is reduced and Plvap is upregulated in siBP1-transfected mBECs in the presence of Wnt3a compared with siCTRL transfected cells. *P<0.05; Student's t-test. Dashed gray line indicates the average fold change in siCTRL-transfected mBECs upon Wnt3a exposure. (E) Heatmap of Wnt3a-induced transcriptional changes in siCTRL- and siBP1-transfected mBECs (Affymetrix). Only genes that show significant (P<0.05) changes after Wnt3a treatment in either siCTRL- or siBP1-transfected cells are reported. Plotted genes are ordered based on decreasing fold-change in expression in Wnt3a-treated versus untreated siCTRL-transfected cells. Upregulated genes are in green and downregulated genes are in red (the intensity is shown in the scale). (F) Western blot analysis of HA tag levels in supernatants (CM) and lysates of HEK293 cells transfected with either empty or Wnt1-HA plasmid. (G,H) Immunofluorescence for HA tag (red) and DAPI (blue) in Wnt3a-treated siCTRL- or siBP1-transfected mBECs after incubation with Wnt1-HA CM for 24 h. (I) Quantification of Wnt1-HA distribution areas in Wnt3a-treated siCTRL- or siBP1-transfected cells after incubation with Wnt1-HA CM. Each dot represents an independent experiment. Wnt1-HA distribution is reduced in siBP1-transfected mBECs compared with siCTRL-transfected cells. Data are mean±s.e.m. *P<0.05; Student's t-test. (J-Q) Representative images (J,L,N,P) and fluorescence intensity plot profiles (K,M,O,Q) for CD31 (green), HA tag (red) and DAPI (blue) in siCTRL- (J,K,N,O) and siBP1-transfected mBECs (L,M,P,Q) after incubation with either control CM (J-M) or Wnt1-HA CM (N-Q). Histograms in K,M,O and Q represent fluorescence intensity profiles along the white dotted lines in J,L,N and P, respectively. Dashed gray line indicates background fluorescence. Scale bars: 100 µm in A,B; 20 µm in G,H; 10 µm in J,L,N,P.
Finally, we tested whether Fgfbp1 may facilitate Wnt ligand accumulation near EC membranes to modulate Wnt/β-catenin signaling. We transfected HEK293 cells with either a plasmid encoding HA-tagged Wnt1 (Wnt1-HA) or a control empty vector to generate Wnt1-HA-conditioned media (Wnt1-HA CM) and control (Empty-HA) CM and administered them to either siCTRL- or siBP1-transfected mBECs that were pre-treated with Wnt3a for 48 h to induce Fgfbp1 expression. We confirmed Wnt1-HA expression and secretion into the medium by western blot (Fig. 7F) as well as Wnt/β-catenin pathway activation in primary mBECs after exposure to either Wnt3a, Wnt1-HA or a sequential administration of both ligands by measuring LRP6 receptor phosphorylation (Fig. S5D). After incubation with Wnt1-HA CM, siBP1-transfected mBECs displayed a smaller area of Wnt1-HA binding to the EC membrane compared with siCTRL-transfected cells as quantified by HA immunofluorescence (Fig. 7G-I). Moreover, whereas no HA tag immunoreactivity was detected in either siCTRL- or siBP1-transfected cells incubated with control CM (Fig. 7J-M), siCTRL-transfected mBECs incubated with Wnt1-HA CM had higher fluorescence intensity compared with siBP1-transfected cells, with a peak largely overlapping with the CD31 fluorescence at BEC junctions (Fig. 7N-Q). siBP1-transfected, but not siCTRL-transfected, primary mBECs also failed to phosphorylate the LRP6 receptor in the presence of Wnts (Wnt3a, Wnt1-HA or a sequential exposure; Fig. S5D) suggesting that Fgfbp1 is necessary for proper pathway activation. Overall, these data suggest that Fgfbp1 may facilitate presentation of Wnt ligands to their receptors on EC membranes by concentrating them close to cell junctions.
DISCUSSION
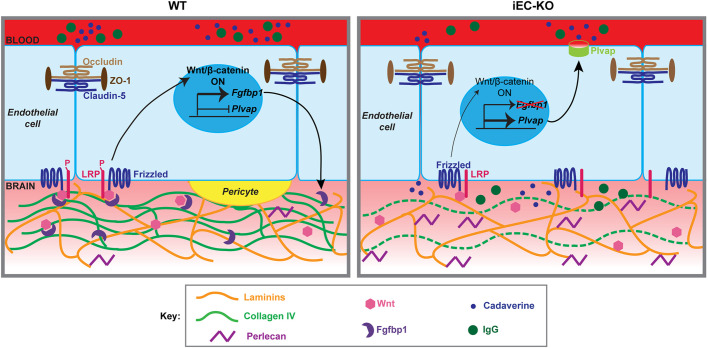
During CNS development, angiogenesis and BBB maturation require coordinated interactions among neuronal, glial and vascular cells with the BM, as well as appropriate signals that drive transcriptional responses in ECs (Biswas et al., 2020). Although the roles of many vascular BM components crucial for interactions among EC, pericytes and astrocytes have been elucidated (Thomsen et al., 2017), how these are connected to endothelial Wnt/β-catenin activity and BBB maturation is not understood. Our study clarifies this crucial step between regulation of vascular BM composition and Wnt/β-catenin activity in the NVU. We have identified Fgfbp1 as a novel secreted protein in the vascular BM regulated by Wnt/β-catenin signaling in CNS ECs during BBB formation, a finding corroborated by our inspection of additional expression databases from the developing CNS vasculature (Hupe et al., 2017; Sabbagh et al., 2018). Our functional studies demonstrate that Fgfbp1 is essential for BBB maturation, operating at two levels. First, Fgfbp1 regulates Wnt/β-catenin activity in the CNS endothelium, which in turn suppresses Plvap to enable barrier maturation (Figs 4K″-M‴,O, 6N-R, 7D,E, 8). Second, EC-derived Fgfbp1 is crucial for both collagen IV deposition in the vascular BM and EC-pericyte interactions (Figs 3K-Q, 4, 8). Thus, Fgfbp1 is a key secreted ECM protein that links interactions among NVU cells with endothelial Wnt/β-catenin activity crucial for proper BBB maturation (Fig. 8). Below, we discuss how Fgfbp1 regulates both Wnt/β-catenin activity and collagen IV deposition to promote BBB maturation, and the implications for neurological diseases.
Fig. 8.
Proposed model for the role of Fgfbp1 in BBB maturation. In WT brain ECs, the Wnt/β-catenin pathway induces transcription of the Fgfbp1 gene. Fgfbp1 protein (purple crescent) is secreted in the BM, where it mobilizes Wnt ligands (pink hexagons) and facilitates their interaction with the Frizzled/LRP receptors near cell junctions, thus maintaining Wnt/β-catenin signaling and BBB properties (e.g. suppression of Plvap). In addition, Fgfbp1 promotes secretion and/or stabilization of collagen IV (green), but not laminin (orange) in the BM, thus stabilizing EC-pericyte (yellow) interactions. In the absence of Fgfbp1, Wnt ligands, that are either immobilized or sequestered by HSPGs (purple) in the BM interact less effectively with their cognate Frizzled/LRP receptors, leading to reduced β-catenin signaling in ECs, increased expression of Plvap and increased BBB permeability.
Fgfbp1 – a novel regulator of Wnt/β-catenin activity
Fgfbp1 is a highly conserved protein that interacts with FGF2 in a dose-dependent manner by reducing its affinity for HSPGs in the ECM (Kurtz et al., 1997; Lametsch et al., 2000). Exogenous administration of Fgfbp1 stimulates FGF2-dependent EC growth and chemotaxis in vitro (Aigner et al., 2001) and enhances FGF2-mediated blood vessel growth in the chick chorioallantoic membrane; its overexpression induces lethality in chick embryos due to vascular hemorrhage (Gibby et al., 2009; Tassi et al., 2001). To date, however, Fgfbp1 has not been implicated in regulation of Wnt signaling. Our genetic data in Fgfbp1iEC-KO mice demonstrate that Fgfbp1 is crucial to sustain Wnt/β-catenin activation in the CNS endothelium in vivo (Fig. 7A-C) and induce some downstream target genes (Fig. 7D). However, Fgfbp1 does not influence other Wnt target genes in ECs, (e.g. Lef1, Sox17, Foxf2 and Zic3; Fig. 7E; Table S1). Moreover, Fgfbp1 does not affect FGF2/FGFR1 signaling in BECs in vitro (Fig. S5C). HSPGs regulate interactions among Wnts and their receptors (Lin, 2004; Pataki et al., 2015; Ren et al., 2018). We postulate that Fgfbp1 increases Wnt ligand availability in the ECM, in a manner analogous to its FGF2 function in other cell types, by interacting with HSPGs (Fig. 8). Alternatively, Fgfbp1 may regulate expression of Wnt receptors or inhibitors necessary to either tether Wnt ligands to the plasma membrane or increase their availability (Fig. 8). Our in vitro data demonstrate that Wnt1-HA is found either in close proximity to the membrane or internalized inside ECs that express Fgfbp1 (Fig. 7G-Q). As Wnts can interact with Frizzled/Lrp receptors at the plasma membrane, cell junctions or endosomes (Brunt and Scholpp, 2018) and Wnt/Norrin ligands can induce receptor internalization in ECs (Zhang et al., 2017), Fgfbp1 may increase interactions of Wnts with receptors in any of these compartments to activate the pathway, a model supported by analysis of LRP6 phosphorylation (Fig. S5D). Wnt7a/b ligands require Gpr124 (Adgra2) and Reck co-receptors to activate the pathway, and Reck tethers Wnt7a/b to the membrane (Cho et al., 2017b; Vallon et al., 2018; Vanhollebeke et al., 2015). Our gene chip data show that Fgfbp1 regulates expression of both Reck and Sfrp1 (Table S1) suggesting that Fgfbp1 may regulate Wnt availability by controlling: interactions with HSPGs, expression of Wnt inhibitors (e.g. Sfrp1) or expression of Reck to maintain Wnt/β-catenin activation during BBB maturation.
The mechanisms of BBB leakage and the origin of heterogeneity in BBB permeability observed in Fgfbp1iEC-KO mice remain unclear (Fig. 4A-G). Our in vitro studies suggest that Fgfbp1 may regulate both paracellular and transcellular BBB permeability in primary mBECs (Fig. 6), although we did not observe any ultrastructural abnormalities in CNS endothelium of Fgfbp1ECKO mice by TEM (Fig. 3). Therefore, Fgfbp1 may be required for Wnt-mediated maturation of paracellular and transcellular barrier properties in CNS ECs. We have found that Fgfbp1 protein levels vary among CNS ECs at P7, consistent with variable Wnt/β-catenin activation. Cortical ECs undergo proliferation and sprouting from P5-P10 that gradually decreases until P25 when they become quiescent (Harb et al., 2013). This second postnatal wave of angiogenesis (P5-P10) in the developing cortex may be driven by activity-dependent mechanisms to match the metabolic demands of neurons with the vascular density (Lacoste et al., 2014). Therefore, cortical regions with higher BBB permeability in Fgfbp1iEC-KO mice may correspond to angiogenic vessels with high levels of Fgfbp1 that fail to form a mature BBB in the developing postnatal cortex of mutant mice (Biswas et al., 2020). This hypothesis remains to be tested in the future.
The role of Fgfbp1 in collagen IV deposition and pericyte-EC interactions
Collagen IV is the major BM component responsible not only for vessel stabilization but also maintenance of BM integrity under mechanical stress; however, it is dispensable for BM assembly during development (Pöschl et al., 2004). Mutations in Col4a1 and Col4a2, associated with hemorrhagic events, do not affect production and trimerization of collagen IV chains, but impair its secretion into the ECM (Gould et al., 2005; Gunda et al., 2014; Jeanne et al., 2012). Although transcription of Col4a1 and Col4a2 is not altered in Fgfbp1iEC-KO mice, deposition of collagen IV into the vascular BM is impaired (Figs 5, 8). The reduced collagen IV vessel coverage may result from defects in secretion from the ER to the ECM. Many proteins are important for collagen IV trafficking and stability, including Hsp47 (SERPINH1; a collagen-specific ER chaperone), Tango1 (Mia3; ER to Golgi transport) and SPARC (extracellular chaperone required for spatial assembly of collagen IV in the vascular BM) (Chioran et al., 2017). In our gene chip array data, expression of SERPINA1C, a member of the Hsp47 family, is reduced in siBP1-transfected cells (Table S1), suggesting that Fgfbp1 may regulate trafficking of collagen IV. Although Wnt/β-catenin signaling regulates collagen IX levels (Wehner et al., 2017), collagen IV is not a target of the pathway in CNS ECs (Table S1) (Hupe et al., 2017; Sabbagh et al., 2018), excluding the possibility that defects in collagen IV are caused by aberrant Wnt/β-catenin signaling in Fgfbp1iEC-KO mice.
The reduced number of pericytes in Fgfbp1iEC-KO mice (Fig. 3K-Q) suggests that Fgfbp1 affects their recruitment to ECs. It is possible that secretion of collagen IV is altered in Fgfbp1iEC-KO mice as a consequence of pericyte reduction. Pericytes produce collagen IV and multiple laminin chains (Jeon et al., 1996) in vitro, and recent single cell RNA sequencing data from non-neural cells in mice confirm that pro-collagen type IV chains (Col4a1-a4) are expressed by mural cells [pericytes and vascular smooth muscle cells (vSMCs)] (Vanlandewijck et al., 2018). Moreover, recruitment of pericytes to vascular tubes triggers production and deposition of collagen IV, laminins, nidogens and perlecan in an EC/pericyte co-culture (Stratman and Davis, 2012; Stratman et al., 2009). However, collagen IV protein levels are not affected in mice with fewer pericytes (Armulik et al., 2010). Alternatively, changes in either collagen IV or Fgfbp1 deposition to the vascular BM may affect pericyte recruitment. Genetic ablation of Col4a1 in mice causes cerebral hemorrhage and BBB leakage due to aberrant pericyte coverage (Gould et al., 2005). Proper N-sulfation of HSPGs is also crucial for binding and activation of PDGF-BB and subsequent pericyte recruitment. Mice deficient for Ndst1, the enzyme responsible for N-sulfation of heparan sulfates, have decreased pericyte coverage (Abramsson et al., 2007) and loss of perlecan impairs BBB repair after ischemic stroke due to aberrant pericyte recruitment (Nakamura et al., 2019). It is possible that Fgfbp1 within the vascular BM influences both collagen IV deposition and N-sulfation of HSPGs that affect EC-pericyte interactions and pericyte physiology in Fgfbp1iEC-KO mice. This may establish a ‘vicious cycle’ in which BM defects affect pericytes and vice-versa.
Mutations in COL4A1 are a genetic determinant of cerebral small vessel disease (SVD), a group of multifactorial illnesses characterized by vessel tortuosity, vascular hyperbranching, BM focal disruption and increased susceptibility to cerebral hemorrhage (Chung et al., 2019; Gould et al., 2006). Given the similarities between such clinical manifestations, the phenotypes observed in Fgfbp1iEC-KO mice and many genes implicated in SVD, it will be interesting to further investigate whether Fgfbp1 is a candidate gene mutated in some SVD cases. Single nucleotide polymorphisms in FGFBP1 are associated not only with regulation of bone mineral density (Hoppman et al., 2010), but also hypertension (Tomaszewski et al., 2011), which is a major risk factor for cerebral hemorrhage and strokes (Jeanne et al., 2012). Future studies are warranted to investigate a role for Fgfbp1 in these complex neurovascular diseases.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Italian Ministry of Health or Columbia University Irving Medical Center and carried out in accordance with guidelines established under the directive 86/609/EEC or IACUC protocols regarding protection of animals used for scientific purposes. The following mouse strains were used: Fgfbp1flox/flox, generated by Taconic Artemis in a C57BL/6N background; Cdh5(PAC)-CreERT2 (VEC-PAC) (Wang et al., 2010), kindly provided by Dr R. Adams (University of Münster, Münster, Germany); ROSA26-EYFP (R26-EYFP) (Srinivas et al., 2001), kindly donated by Dr S. Casola (IFOM, Milan, Italy); β-cateninflox(ex3)/flox(ex3) (Harada et al., 1999), generously provided by Dr M. Taketo (Kyoto University, Kyoto, Japan); and Tg(BAT-lacZ) (Daneman et al., 2009; Liebner et al., 2008), kindly provided by Dr S. Piccolo (University of Padua, Padua, Italy). For mBEC preparation, C57BL/6J WT animals were purchased from Charles River Laboratories. To induce Cre-mediated recombination, tamoxifen (Sigma-Aldrich) was prepared by dissolution into 10% ethanol/corn oil, and was administered to newborns (P1) by a single intragastric injection at a 35 µg/g body weight dose, as previously described (Pitulescu et al., 2010). Both male and female mice were used for all experiments.
Mouse genotyping
Genomic DNA was isolated from tail biopsies by incubating them in buffer (0.5 mg/ml Proteinase K, 0.5% Triton X in 1× Gitschier buffer) overnight at 56°C. Proteinase K was then inactivated by incubating samples at 95°C for 10 min. Primer pairs used to amplify the desired genomic sequences are reported in Table S2.
Preparation and culture of mBECs
For ex vivo experiments, mouse brain microvascular fragments were isolated as previously described (Calabria et al., 2006; Liebner et al., 2000). For in vitro assays, brain microvascular fragments were resuspended in mBEC medium and plated on collagen I derived from rat tail (Sigma-Aldrich). ECs were selected for resistance to puromycin (Sigma-Aldrich) and grown in either media containing recombinant murine Wnt3a (100-500 ng/ml, Peprotech) or control media [1% bovine serum albumin (BSA) in PBS].
Fgfbp1 silencing in vitro
Three different Stealth RNAi duplexes (Invitrogen) were used to knock down Fgfbp1 mRNA. Sequences of the siRNAs used are listed in Table S3. Stealth RNAi negative control Med GC Duplex #3 (Invitrogen) was used as a negative control in all experiments. We transfected 25 nM of siRNA using Lipofectamine 2000 (Invitrogen), following the manufacturer's instructions.
Gene expression analysis
Total mRNA was extracted from mBECs after treatment with Wnt3a and/or siBP1#2. Gene expression was analyzed using the GeneChip ST 1.0 array (Affymetrix) covering 29,000 murine genes. For each condition, mRNA obtained from three different experiments was assayed to account for biological variability. Genes differentially expressed between the two conditions were identified as those having at least a twofold change and P<0.05.
Quantitative real-time RT-PCR (qRT-PCR)
Total mRNA from cultured mouse primary BECs or freshly isolated brain- and liver-derived microvessels was extracted using the RNeasy Micro Kit (Qiagen) following the manufacturer's instructions as previously described (Corada et al., 2019). cDNA was prepared from 500 ng of extracted RNA, using the High Capacity cDNA Archive kit (Applied Biosystems) and Random Hexamers kit (New England BioLabs). Then, 5 ng of cDNA was amplified in triplicate using specific, pre-designed TaqMan Gene Expression assays (Table S4; Applied Biosystems). Data were collected using the I/Prism 7900 HT Thermocycler. For each sample, expression levels were determined using the comparative threshold cycle (Ct) method, with Gapdh as a housekeeping standard.
Tissue preparation
For immunofluorescence analysis, brains, livers and eyes were isolated from WT and Fgfbp1iEC-KO mice and fixed in 4% paraformaldehyde (PFA) in PBS overnight at 4°C. Cadaverine-AlexaFluor 555-injected animals were perfused with PBS, then 4% PFA/PBS and post-fixed in 4% PFA overnight at 4°C. Brains and livers were embedded in 4% low melting point agarose in PBS, and 100 µm sections were cut using a vibratome (1000 Plus, The Vibratome Company). Retinas were dissected as previously described (Corada et al., 2013).
Cadaverine-AlexaFluor 555 permeability analysis
P7, P14 and P21 pups were injected intraperitoneally with lysine-fixable Cadaverine-AlexaFluor 555 (Thermo Fisher Scientific) at a 25 mg/kg dose. After 2 h, animals where anesthetized by intraperitoneal injection with avertin (Sigma-Aldrich) at a 20 mg/kg dose and perfused, as described above.
Immunofluorescence
Brain and liver sections and whole-mount retinas were incubated overnight in blocking solution (1% fish skin gelatin, 0.5% Triton X-100 and 5% normal donkey serum in PBS), then incubated overnight at 4°C with the desired primary antibodies diluted in antibody buffer (1% fish skin gelatin, 0.25% Triton X-100 in PBS). Samples were washed 3× with 0.25% Triton X-100 in PBS, then incubated for 4 h at room temperature (RT) with secondary antibodies in antibody buffer. Samples were then washed 3× with 0.25% Triton X-100 in PBS and 2× with PBS, post-fixed with 4% PFA/PBS at RT for 5 min, washed 3× in PBS and finally mounted on glass slides using Vectashield with DAPI (Vector Laboratories, for brain sections) or Prolong Gold antifade reagent (Thermo Fisher Scientific).
For the mBEC culture, cells were fixed for 30 min with ice-cold 95% ethanol, then 1 min with acetone at RT and washed 3× in ice-cold PBS. They were then incubated for 1 h in blocking buffer (10% BSA in PBS with 0.1% Triton X-100) at RT. Samples were then incubated overnight at 4°C with the required antibodies diluted in 1% BSA in PBT (PBS plus 0.1% Triton X-100), washed 3× with PBT, and incubated for 2 h in the appropriate secondary antibodies diluted in 1% BSA in PBT. After 3× PBT washes, samples were post-fixed in 4% PFA/PBS for 10 min at RT and rinsed thoroughly with PBS.
The following primary antibodies against mouse antigens were used for immunofluorescence analysis: rabbit anti-FGFBP1 (1:150, Bioss Antibodies, bs-1768R), rat anti-laminin α2 (1:200, Abcam, ab11576), rabbit anti-GFP (1:300, Invitrogen, A-21311), Armenian hamster anti-CD31 (1:200, Millipore, MAB1398Z), rat anti-CD31 (1:100, BD Biosciences, 553370), rat anti-heparan sulfate proteoglycan (1:100, Millipore, MAB1948P), rabbit anti-fibronectin (1:400, Sigma-Aldrich, F3648), mouse anti-claudin 5-AlexaFluor 488 (1:200, Invitrogen, 352588), goat anti-podocalyxin (1:200, R&D Systems, AF1556), rabbit anti-collagen IV (1:200, Serotec, 2150-1470), rat anti-PLVAP (1:100, Santa Cruz Biotechnology, sc-19603), mouse anti-ZO-1 (1:250, Thermo Fisher Scientific, 339100), chicken anti-β-galactosidase (1:500, Abcam, ab9361), rabbit anti-VE-cadherin (1:200, BD Biosciences, 550548), goat anti-GFAP (1:200, Abcam, ab53554), goat anti-PDGFRβ (1:200, R&D Systems, AF1042) and rabbit anti-aquaporin IV (1:200, Millipore, AB3594). Antibodies against laminin α4 and α5 were kindly provided by Prof. Lydia Sorokin (University of Münster, Münster, Germany). Secondary antibodies used were conjugated with Alexa 488, Alexa 594 or 647 (A-21209, A-21208, A-21432, A-11055, A-21206, A-31572 used at 1:1000; A-21113, A-21110, A-21447, A-31571, A-31573 used at 1:500; Invitrogen).
Transmission electron microscopy
Animals were anesthetized by intraperitoneal injection of Avertin (20 mg/kg, Sigma-Aldrich) and intracardially perfused with 1-2 ml of 0.025% heparin in PBS, followed by perfusion with fixative solution (2% PFA and 2% glutaraldehyde in 0.2 M sodium cacodylate) for 20 min of 5 ml/min. After perfusion, animals were kept in a plastic bag for 90 min, then brains were dissected and stored in fixative solution until processing. Samples were treated with 1% reduced osmium tetroxide, stained with 0.3% thiocarbohydrazide in 0.1 M sodium cacodylate (pH 7.0), treated with reduced osmium tetroxide for another 30 min and dehydrated in an ethanol series. Samples were then embedded in Epon resin and sectioned at 60 nm for electron microscopy observation.
Transendothelial electrical resistance
mBECs transfected with either siCTRL or siBP1 were plated on poly-D-lysine- and collagen IV-coated, gold electrode array 96-well plates (Applied Biophysics) and grown to confluency, then switched to low serum [1% fetal bovine serum (FBS)] medium for 24 h and treated with Wnt3a, as described above. Using the ECIS Z-θ system (Applied Biophysics), the electrical resistance of the cultures was recorded every 20 min for the duration of the experiment. Resistance curves for each experimental condition were generated using GraphPad Prism software, and the areas under the curve were calculated for the duration of treatment with Wnt3a, as previously described (Mazzoni et al., 2017).
Albumin uptake assay
mBECs transfected with either siCTRL or siBP1 were plated on poly-D-lysine- and collagen IV-coated, glass-bottom 24-well plates (Greiner Bio-One) and grown to confluence, then switched to low serum (1% FBS) medium for 24 h and treated with recombinant mouse Wnt3a as described (Mazzoni et al., 2017). Cells were incubated with AlexaFluor 594-albumin (50 µg/ml; Thermo Fisher Scientific) for 1 h at 37°C, washed with PBS to remove excess albumin and fixed with 4% PFA.
Wnt1-HA distribution assay
To prepare Wnt1-HA-conditioned medium (Wnt1-HA CM), the murine Wnt1 cDNA was cloned into the pCAGGS-HA plasmid and transfected into HEK293 cells using the FuGENE6 reagent (Promega) following the manufacturer's instructions. The empty pCAGGS-HA plasmid was used to produce control conditioned medium (Empty-HA CM). Supernatants from transfected cells were collected 24 h after transfection and concentrated using Centriprep centrifugal columns with a 3 kDa cutoff (Amicon). For the Wnt1-HA distribution assay, siCTRL- or siBP1-transfected mBECs were plated on gelatin-coated dishes, incubated with Wnt3a for 48 h to induce expression of Fgfbp1. After 48 h, Wnt3a was washed away and mBECs were incubated overnight with either Wnt1-HA or control CM, fixed with 4% PFA for 20 min and thoroughly washed with PBS before immunostaining for HA and CD31.
Image acquisition and quantification
Immunofluorescence images were acquired by confocal microscopy (TCS SP2, Sp5 or SP8, Leica or Zeiss LSM700) using 10×, 20× and 40× objectives. For comparison purposes, all images of the same staining were acquired under constant acquisition settings. At least three confocal z-projections from at least three animals per group were used for quantifications. All images were processed using ImageJ and quantifications were carried out blinded. To quantify vascular density, images of CD31-stained brain sections and whole-mount retinas were thresholded and binarized, and ratios of CD31+ pixels to total pixel numbers in each field were calculated. The numbers of branch points for each field, numbers of IgG leakage foci per section and numbers of PDGFRβ-positive pericytes per field were counted manually. Leakage of Cadaverine-AlexaFluor 555 was assessed by measuring the average pixel intensity of each field. To quantify the percentage of vessels covered by BM proteins (collagen IV, laminin α2, α4 and α5, fibronectin, perlecan) and by Plvap, images were thresholded and binarized, then the corresponding CD31 image was also thresholded and binarized to be used as a selection mask for the BM protein image. The number of BM-positive pixels within the selected area was then quantified and expressed as a ratio over the number of CD31-positive pixels. Albumin uptake in vitro was quantified by measuring the area covered by albumin and dividing it by the number of cells in the field, as previously described (Mazzoni et al., 2017). For analysis of the Wnt1-HA distribution assay, the lowest z-stacks were excluded from the maximum projection to avoid interference from background signal arising from the plate coating. The area of Wnt1-HA distribution was determined by measuring the area covered by anti-HA staining divided by the number of cells per field. The intensity and localization of Wnt1-HA distribution was assessed by first tracing a line perpendicular to CD31 junctional strands, then analyzing fluorescence intensity plot profiles along that line for both CD31 and HA signals.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 8.1. Data are mean±s.e.m. Normality of datasets was determined using the Shapiro–Wilk test. Datasets showing normal distribution were compared using a two-tailed Student's t-test for pairwise comparisons, or one-way ANOVA with post-hoc Tukey's correction for multiple comparisons. For non-normally distributed datasets, a non-parametric Mann–Whitney test was used. P-values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgements
We thank Veronica Sundell (Uppsala University), Emeli Hermansson (Uppsala University), Grace Prochilo (CUIMC) and Fabrizio Orsenigo (IFOM) for technical assistance with some experiments in this study. We are also grateful to Tyler Cutforth (CUIMC) for his editorial input on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.C., D.A., M.G.L., E.D.; Methodology: A.C., M.C., P.U.M., D.A., M.G.L., E.D.; Validation: A.C., M.C., P.U.M., D.A., M.G.L., E.D.; Formal analysis: A.C., M.C., P.U.M., D.A., M.G.L., E.D.; Investigation: A.C., M.C., G.V.B., A.A.M., M.A.G., S.B., H.H., A.D., P.U.M., D.A., M.G.L., E.D.; Resources: A.C., D.A., M.G.L., E.D.; Data curation: A.C., D.A., M.G.L., E.D.; Writing - original draft: A.C., D.A.; Writing - review & editing: A.C., M.C., G.V.B., A.A.M., S.B., P.U.M., D.A., M.G.L., E.D.; Visualization: A.C., D.A., M.G.L., E.D.; Supervision: A.D., P.U.M., D.A., M.G.L., E.D.; Project administration: P.U.M., D.A., M.G.L., E.D.; Funding acquisition: D.A., M.G.L., E.D.
Funding
A.C. and D.A. are supported by Fondation Leducq (15CDV-02). D.A. is also supported by grants from the National Institutes of Health/National Institute of Mental Health (R01 MH112849) and National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01 NS107344), plus unrestricted gifts from both John F. Castle and Newport Equities LLC to the Division of Cerebrovascular Diseases and Stroke, Department of Neurology, Irving Medical Center, Columbia University. E.D., P.U.M. and M.G.L. are supported by the Vetenskapsrådet (Swedish Research Council), the Knut och Alice Wallenbergs Stiftelse, Associazione Italiana per la Ricerca sul Cancro (AIRC; Investigator Grants 18683 and 21320), the AIRC 5×1000 call ‘Metastatic disease: the key unmet need in oncology’ of the MYNERVA project #21267, the European Research Council (project EC-ERC-V-EPC, grant 742922), Initial Training Networks BtRAIN (H2020 Marie Skłodowska-Curie Actions, grant 675619), the Fondazione Cariplo (2014-1038, 2016-0461) and Fondazione Telethon (GGP14149). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.185140.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.185140.reviewer-comments.pdf
References
- Abramsson A., Kurup S., Busse M., Yamada S., Lindblom P., Schallmeiner E., Stenzel D., Sauvaget D., Ledin J., Ringvall M. et al. (2007). Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes Dev. 21, 316-331. 10.1101/gad.398207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner A., Butscheid M., Kunkel P., Krause E., Lamszus K., Wellstein A. and Czubayko F. (2001). An FGF-binding protein (FGF-BP) exerts its biological function by parallel paracrine stimulation of tumor cell and endothelial cell proliferation through FGF-2 release. Int. J. Cancer J. Int. Cancer 92, 510-517. [DOI] [PubMed] [Google Scholar]
- Armulik A., Genové G., Mäe M., Nisancioglu M. H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K. et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557-561. 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- Arnold T. D., Niaudet C., Pang M.-F., Siegenthaler J., Gaengel K., Jung B., Ferrero G. M., Mukouyama Y.-S., Fuxe J., Akhurst R. et al. (2014). Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development 141, 4489-4499. 10.1242/dev.107193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader B. L., Rayburn H., Crowley D. and Hynes R. O. (1998). Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95, 507-519. 10.1016/S0092-8674(00)81618-9 [DOI] [PubMed] [Google Scholar]
- Biswas S., Cottarelli A. and Agalliu D. (2020). Neuronal and glial regulation of CNS angiogenesis and barriergenesis. Development 147, dev182279 10.1242/dev.182279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt L. and Scholpp S. (2018). The function of endocytosis in Wnt signaling. Cell. Mol. Life Sci. 75, 785-795. 10.1007/s00018-017-2654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria A. R., Weidenfeller C., Jones A. R., de Vries H. E. and Shusta E. V. (2006). Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J. Neurochem. 97, 922-933. 10.1111/j.1471-4159.2006.03793.x [DOI] [PubMed] [Google Scholar]
- Chen Z.-L., Yao Y., Norris E. H., Kruyer A., Jno-Charles O., Akhmerov A. and Strickland S. (2013). Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J. Cell Biol. 202, 381-395. 10.1083/jcb.201212032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chioran A., Duncan S., Catalano A., Brown T. J. and Ringuette M. J. (2017). Collagen IV trafficking: The inside-out and beyond story. Dev. Biol. 431, 124-133. 10.1016/j.ydbio.2017.09.037 [DOI] [PubMed] [Google Scholar]
- Cho C., Smallwood P. M. and Nathans J. (2017a). Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron 95, 1221-1225. 10.1016/j.neuron.2017.08.032 [DOI] [PubMed] [Google Scholar]
- Cho C., Smallwood P. M. and Nathans J. (2017b). Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron 95, 1056-1073.e1055. 10.1016/j.neuron.2017.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Marini S., Pera J., Norrving B., Jimenez-Conde J., Roquer J., Fernandez-Cadenas I., Tirschwell D. L., Selim M., Brown D. L. et al. (2019). Genome-wide association study of cerebral small vessel disease reveals established and novel loci. Brain 142, 3176-3189. 10.1093/brain/awz233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M., Orsenigo F., Morini M. F., Pitulescu M. E., Bhat G., Nyqvist D., Breviario F., Conti V., Briot A., Iruela-Arispe M. L. et al. (2013). Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat. Commun. 4, 2609 10.1038/ncomms3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M., Orsenigo F., Bhat G. P., Conze L. L., Breviario F., Cunha S. I., Claesson-Welsh L., Beznoussenko G. V., Mironov A. A., Bacigaluppi M. et al. (2019). Fine-tuning of Sox17 and canonical Wnt coordinates the permeability properties of the blood-brain barrier. Circ. Res. 124, 511-525. 10.1161/CIRCRESAHA.118.313316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Agalliu D., Zhou L., Kuhnert F., Kuo C. J. and Barres B. A. (2009). Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 106, 641-646. 10.1073/pnas.0805165106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A. A. and Barres B. A. (2010). Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562-566. 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. and Kühl M. (2010). The role of wnt signaling in physiological and pathological angiogenesis. Circ. Res. 107, 943-952. 10.1161/CIRCRESAHA.110.223750 [DOI] [PubMed] [Google Scholar]
- Gibby K. A., McDonnell K., Schmidt M. O. and Wellstein A. (2009). A distinct role for secreted fibroblast growth factor-binding proteins in development. Proc. Natl. Acad. Sci. USA 106, 8585-8590. 10.1073/pnas.0810952106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould D. B., Phalan F. C., Breedveld G. J., van Mil S. E., Smith R. S., Schimenti J. C., Aguglia U., van der Knaap M. S., Heutink P. and John S. W. (2005). Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 308, 1167-1171. 10.1126/science.1109418 [DOI] [PubMed] [Google Scholar]
- Gould D. B., Phalan F. C., van Mil S. E., Sundberg J. P., Vahedi K., Massin P., Bousser M. G., Heutink P., Miner J. H., Tournier-Lasserve E. et al. (2006). Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl. J. Med. 354, 1489-1496. 10.1056/NEJMoa053727 [DOI] [PubMed] [Google Scholar]
- Gunda B., Mine M., Kovács T., Hornyák C., Bereczki D., Várallyay G., Rudas G., Audrezet M.-P. and Tournier-Lasserve E. (2014). COL4A2 mutation causing adult onset recurrent intracerebral hemorrhage and leukoencephalopathy. J. Neurol. 261, 500-503. 10.1007/s00415-013-7224-4 [DOI] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M. and Taketo M. M. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931-5942. 10.1093/emboj/18.21.5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb R., Whiteus C., Freitas C. and Grutzendler J. (2013). In vivo imaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow Metab. 33, 146-156. 10.1038/jcbfm.2012.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppman N., McLenithan J. C., McBride D. J., Shen H., Bruder J., Bauer R. L., Shaffer J. R., Liu J., Streeten E. A., Shuldiner A. R. et al. (2010). A common variant in fibroblast growth factor binding protein 1 (FGFBP1) is associated with bone mineral density and influences gene expression in vitro. Bone 47, 272-280. 10.1016/j.bone.2010.04.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe M., Li M. X., Kneitz S., Davydova D., Yokota C., Kele J., Hot B., Stenman J. M. and Gessler M. (2017). Gene expression profiles of brain endothelial cells during embryonic development at bulk and single-cell levels. Sci. Signal. 10, eaag2476 10.1126/scisignal.aag2476. [DOI] [PubMed] [Google Scholar]
- Jeanne M., Labelle-Dumais C., Jorgensen J., Kauffman W. B., Mancini G. M., Favor J., Valant V., Greenberg S. M., Rosand J. and Gould D. B. (2012). COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am. J. Hum. Genet. 90, 91-101. 10.1016/j.ajhg.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H., Ono M., Kumagai C., Miki K., Morita A. and Kitagawa Y. (1996). Pericytes from microvessel fragment produce type IV collagen and multiple laminin isoforms. Biosci. Biotechnol. Biochem. 60, 856-861. 10.1271/bbb.60.856 [DOI] [PubMed] [Google Scholar]
- Jiang X., Andjelkovic A. V., Zhu L., Yang T., Bennett M. V. L., Chen J., Keep R. F. and Shi Y. (2018). Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 163-164, 144-171. 10.1016/j.pneurobio.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Wang H.-L., Darwiche N., Harris V. and Wellstein A. (1997). Expression of a binding protein for FGF is associated with epithelial development and skin carcinogenesis. Oncogene 14, 2671-2681. 10.1038/sj.onc.1201117 [DOI] [PubMed] [Google Scholar]
- Lacoste B., Comin C. H., Ben-Zvi A., Kaeser P. S., Xu X., Costa L. F. and Gu C. (2014). Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron 83, 1117-1130. 10.1016/j.neuron.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametsch R., Rasmussen J. T., Johnsen L. B., Purup S., Sejrsen K., Petersen T. E. and Heegaard C. W. (2000). Structural characterization of the fibroblast growth factor-binding protein purified from bovine prepartum mammary gland secretion. J. Biol. Chem. 275, 19469-19474. 10.1074/jbc.M002550200 [DOI] [PubMed] [Google Scholar]
- Liebner S., Kniesel U., Kalbacher H. and Wolburg H. (2000). Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur. J. Cell Biol. 79, 707-717. 10.1078/0171-9335-00101 [DOI] [PubMed] [Google Scholar]
- Liebner S., Corada M., Bangsow T., Babbage J., Taddei A., Czupalla C. J., Reis M., Felici A., Wolburg H., Fruttiger M. et al. (2008). Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409-417. 10.1083/jcb.200806024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S., Dijkhuizen R. M., Reiss Y., Plate K. H., Agalliu D. and Constantin G. (2018). Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 135, 311-336. 10.1007/s00401-018-1815-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X. (2004). Functions of heparan sulfate proteoglycans in cell signaling during development. Development 131, 6009-6021. 10.1242/dev.01522 [DOI] [PubMed] [Google Scholar]
- Mazzoni J., Smith J. R., Shahriar S., Cutforth T., Ceja B. and Agalliu D. (2017). The Wnt inhibitor Apcdd1 coordinates vascular remodeling and barrier maturation of retinal blood vessels. Neuron 96, 1055-1069.e1056. 10.1016/j.neuron.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes M. J., McClenahan F. K., Leiton C. V., Aranmolate A., Shan X. and Colognato H. (2014). The extracellular matrix protein laminin alpha2 regulates the maturation and function of the blood-brain barrier. J. Neurosci. 34, 15260-15280. 10.1523/JNEUROSCI.3678-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley A. K., Tchaicha J. H., Shin J., Hossain M. G. and McCarty J. H. (2009). Beta8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain. J. Cell Sci. 122, 1842-1851. 10.1242/jcs.043257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Ikeuchi T., Nara K., Rhodes C. S., Zhang P., Chiba Y., Kazuno S., Miura Y., Ago T., Arikawa-Hirasawa E. et al. (2019). Perlecan regulates pericyte dynamics in the maintenance and repair of the blood-brain barrier. J. Cell Biol.. 218, 3506-3525. 10.1083/jcb.201807178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.-L., Lee Y. J., Shin J., Lee E., Park S. O., McCarty J. H. and Oh S. P. (2011). TGF-β signaling in endothelial cells, but not neuroepithelial cells, is essential for cerebral vascular development. Lab. Invest. 91, 1554-1563. 10.1038/labinvest.2011.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T., Hata M., Gotoh S., Seo Y., Sasaki H., Hashimoto N., Furuse M. and Tsukita S. (2003). Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 161, 653-660. 10.1083/jcb.200302070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataki C. A., Couchman J. R. and Brábek J. (2015). Wnt signaling cascades and the roles of syndecan proteoglycans. J. Histochem. Cytochem. 63, 465-480. 10.1369/0022155415586961 [DOI] [PubMed] [Google Scholar]
- Pitulescu M. E., Schmidt I., Benedito R. and Adams R. H. (2010). Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat. Protoc. 5, 1518-1534. 10.1038/nprot.2010.113 [DOI] [PubMed] [Google Scholar]
- Pöschl E., Schlötzer-Schrehardt U., Brachvogel B., Saito K., Ninomiya Y. and Mayer U. (2004). Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131, 1619-1628. 10.1242/dev.01037 [DOI] [PubMed] [Google Scholar]
- Ren Z., van Andel H., de Lau W., Hartholt R. B., Maurice M. M., Clevers H., Kersten M. J., Spaargaren M. and Pals S. T. (2018). Syndecan-1 promotes Wnt/β-catenin signaling in multiple myeloma by presenting Wnts and R-spondins. Blood 131, 982-994. 10.1182/blood-2017-07-797050 [DOI] [PubMed] [Google Scholar]
- Reyahi A., Nik A. M., Ghiami M., Gritli-Linde A., Pontén F., Johansson B. R. and Carlsson P. (2015). Foxf2 is required for brain pericyte differentiation and development and maintenance of the blood-brain barrier. Dev. Cell 34, 19-32. 10.1016/j.devcel.2015.05.008 [DOI] [PubMed] [Google Scholar]
- Sabbagh M. F., Heng J. S., Luo C., Castanon R. G., Nery J. R., Rattner A., Goff L. A., Ecker J. R. and Nathans J. (2018). Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. eLife 7, e36187 10.7554/eLife.36187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O. and Sorokin L. M. (2001). Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 153, 933-946. 10.1083/jcb.153.5.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J. I., Bell J. S. and DeLuca G. C. (2018). Vascular pathology in multiple sclerosis: reframing pathogenesis around the blood-brain barrier. J. Neurol. Neurosurg. Psychiatry 89, 42-52. 10.1136/jnnp-2017-316011 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.-S., William C. M., Tanabe Y., Jessell T. M. and Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J. M., Rajagopal J., Carroll T. J., Ishibashi M., McMahon J. and McMahon A. P. (2008). Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247-1250. 10.1126/science.1164594 [DOI] [PubMed] [Google Scholar]
- Stratman A. N. and Davis G. E. (2012). Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc. Microanal. 18, 68-80. 10.1017/S1431927611012402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman A. N., Malotte K. M., Mahan R. D., Davis M. J. and Davis G. E. (2009). Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114, 5091-5101. 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. D., Sagare A. P. and Zlokovic B. V. (2018). Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133-150. 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi E., Al-Attar A., Aigner A., Swift M. R., McDonnell K., Karavanov A. and Wellstein A. (2001). Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J. Biol. Chem. 276, 40247-40253. 10.1074/jbc.M104933200 [DOI] [PubMed] [Google Scholar]
- Thomsen M. S., Routhe L. J. and Moos T. (2017). The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 37, 3300-3317. 10.1177/0271678X17722436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski M., Charchar F. J., Nelson C. P., Barnes T., Denniff M., Kaiser M., Debiec R., Christofidou P., Rafelt S., van der Harst P. et al. (2011). Pathway analysis shows association between FGFBP1 and hypertension. J. Am. Soc. Nephrol. 22, 947-955. 10.1681/ASN.2010080829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon M., Yuki K., Nguyen T. D., Chang J., Yuan J., Siepe D., Miao Y., Essler M., Noda M., Garcia K. C. et al. (2018). A RECK-WNT7 receptor-ligand interaction enables isoform-specific regulation of Wnt bioavailability. Cell Rep. 25, 339-349.e339. 10.1016/j.celrep.2018.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhollebeke B., Stone O. A., Bostaille N., Cho C., Zhou Y., Maquet E., Gauquier A., Cabochette P., Fukuhara S., Mochizuki N. et al. (2015). Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. eLife 4, e06489 10.7554/eLife.06489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandewijck M., He L., Mäe M. A., Andrae J., Ando K., Del Gaudio F., Nahar K., Lebouvier T., Laviña B., Gouveia L. et al. (2018). A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475-480. 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- Wang Y., Nakayama M., Pitulescu M. E., Schmidt T. S., Bochenek M. L., Sakakibara A., Adams S., Davy A., Deutsch U., Lüthi U. et al. (2010). Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483-486. 10.1038/nature09002 [DOI] [PubMed] [Google Scholar]
- Wehner D., Tsarouchas T. M., Michael A., Haase C., Weidinger G., Reimer M. M., Becker T. and Becker C. G. (2017). Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat. Commun. 8, 126 10.1038/s41467-017-00143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Chen Z.-L., Norris E. H. and Strickland S. (2014). Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 5, 3413 10.1038/ncomms4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Lai M. B., Khandan L., Lee L. A., Chen Z. and Junge H. J. (2017). Norrin-induced Frizzled4 endocytosis and endo-lysosomal trafficking control retinal angiogenesis and barrier function. Nat. Commun. 8, 16050 10.1038/ncomms16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang Y., Tischfield M., Williams J., Smallwood P. M., Rattner A., Taketo M. M. and Nathans J. (2014). Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Invest. 124, 3825-3846. 10.1172/JCI76431 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.