Abstract
African Americans are subject to health disparities in smoking and chronic pain. Given that nicotine has analgesic properties, increases in acute pain may be an expression of the tobacco abstinence syndrome, particularly among African American smokers with chronic pain. This report is a secondary analysis of data from an ongoing study of individual differences in laboratory-derived tobacco abstinence phenotypes in African American smokers. We tested whether overnight smoking abstinence increased acute pain and whether abstinence-induced changes in acute pain were correlated with other expressions of tobacco abstinence and amplified among smokers with chronic pain. African American smokers (N = 214; 10+ cig/day) attended a baseline visit (when chronic pain was reported), and two counterbalanced experimental sessions (ad libitum smoking vs. 16-hr smoking abstinence). At both experimental sessions, measures of self-reported acute pain and other tobacco abstinence symptoms were administered. Smoking abstinence significantly increased acute pain (d = .17, p = .01). Correlations between abstinence-induced changes in acute pain and abstinence-induced changes in negative affect, r = .15, p = .02, smoking urges, r = .13, p = .05, and composite nicotine withdrawal symptoms, r = .13, p = .06, were small and nonsignificant after correction for multiple tests, indicating that phenotypic variation in abstinence-provoked changes in acute pain and other tobacco abstinence expressions were largely independent. Baseline levels of chronic pain predicted greater abstinence-induced pain amplification at experimental sessions (βs = .29 =.31; ps < .001). Acute pain is greater following overnight tobacco abstinence (vs. satiation) among African American smokers, predominantly among those with chronic pain. Addressing pain in tobacco addiction science, treatment, and health equity programming warrants consideration.
Keywords: pain, chronic pain, tobacco abstinence, health disparities, African Americans
General Scientific Summary
This study is the first controlled experimental investigation of the intersection of pain, smoking, and the tobacco abstinence syndrome in African American smokers. Our findings suggest that acute pain may be a distinct phenotypic expression of tobacco abstinence that is disproportionately expressed among African American smokers with chronic pain.
The Comorbidity of Pain and Tobacco Addiction
The prevalence of tobacco cigarette smoking among persons with chronic pain has been reported to be up to two times greater than observed in the general population (Zvolensky, McMillan, Gonzalez, & Asmundson, 2009). Smokers with chronic pain endorse higher levels of pain intensity and functional impairment (vs. nonsmokers with chronic pain; Weingarten et al., 2008), less confidence in their ability to quit smoking, and greater expectations for difficulty quitting during future cessation attempts (Ditre, Kosiba, Zale, Zvolensky, & Maisto, 2016).
Disparities in Pain and Tobacco Addiction Facing African Americans
African Americans are subject to disparities in tobacco-related disease and chronic pain. Risk of lung cancer and other chronic diseases due to smoking are higher for African Americans than other racial/ethnic groups (Haiman et al., 2006). African American smokers are less likely to initiate a quit attempt and are more likely to relapse after quitting, relative to White smokers (Choi, Okuyemi, Kaur, & Ahluwalia, 2004). In comparison to other racial/ethnic groups, African Americans report greater clinical pain and pain-related disability (Edwards, Doleys, Fillingim, & Lowery, 2001; Hooten, Knight-Brown, Townsend, & Laures, 2012) and poorer pain treatment outcomes (Hooten et al., 2012). Given that pain and smoking may be related and concomitantly contribute to health disparities among African Americans, understanding mechanisms linking pain and smoking among African Americans is valuable.
Indirect Evidence to Support the Hypothesis that Pain Could Be Increased by Smoking Abstinence, Particularly in African American Smokers
Although sustained abstinence or significant reductions in smoking over extended periods may improve pain in chronic pain patients (Behrend et al., 2014; Kaye, Prabhakar, Fitzmaurice, & Kaye, 2012; Nakajima & Al’Absi, 2014; cf., Shi, Hooten, & Warner, 2011), it is plausible that short-term tobacco abstinence may increase immediate pain. Extant studies have found that acute pain can be a potent immediate motivator of smoking (Ditre & Brandon, 2008; Ditre, Brandon, Zale, & Meagher, 2011; Ditre, Heckman, Butts, & Brandon, 2010), and that nicotine can produce acute analgesic effects (Ditre, Heckman, Zale, Kosiba, & Maisto, 2016). In rodent models, removal of nicotine following periods of chronic nicotine exposure increases pain sensitivity (Grabus, Martin, & Imad Damaj, 2005). In a human PET brain imaging study, increased availability of nicotinic acetylcholine receptors during smoking abstinence was associated with increased pain sensitivity (Cosgrove et al., 2010). Furthermore, one study found that smokers who were abstinent for 48 hrs had lower pain tolerance to a cold pressor stimulus relative to nonsmokers (Nakajima & Al’Absi, 2014).
Although none of the aforementioned evidence on the intersection of pain and smoking has been reported among predominately African American populations, there is reason to suspect that an acute effect of tobacco administration or abstinence on acute pain could be particularly pronounced in African American smokers. African Americans have been shown to report higher levels of pain unpleasantness and emotional response to pain (Riley et al., 2002) and greater sensitivity to experimental pain-induction using a modified submaximal tourniquet procedure (Edwards et al., 2001), raising the possibility that an acute pharmacological stimulus, such as tobacco deprivation, may generate robust effects on acute pain in African American smokers.
Conceptual Model of Chronic Pain, Acute Pain, and Tobacco Use and Abstinence
In this research, we test aspects of a conceptual model of the mechanisms linking pain and smoking, which proposes pain-smoking connections at both momentary and protracted levels of analysis as follows (see Figure 1). Nicotine has known momentary analgesic effects due in part to the activation of acetylcholinergic and endogenous opioid systems (Damaj et al., 2007; Marubio et al., 1999), thus, smoking behavior may be reinforced by alleviating pain. With long-term smoking, it is possible that repeated exposure to nicotine and habitual experience of smoking-induced analgesia may cause protracted neuroadaptations in brain pathways underlying pain processing, tissue inflammatory responses, as well as promote learned associations between pain and smoking. Once such behavioral and biological changes have sufficiently accumulated, nicotine deprivation may cause a disruption of biological and behavioral homeostasis that provokes compensatory increases in pain among smokers. The experience of increased pain as an expression of tobacco abstinence could motivate smoking and recapitulate the cycle of pain and smoking as part of a positive feedback loop (Ditre & Brandon, 2008; Ditre et al., 2011). The model further hypothesizes that individuals with a concomitant chronic pain problem may be particularly vulnerable to the aforementioned momentary interactions between acute pain, smoking, and abstinence for two reasons. First, the neurobiological dysregulation in pain processing pathways that occur as a consequence of chronic pain (Ditre et al., 2011) may also synergize with nicotine’s effect on those pathways and thus, accelerate the development of nicotine-induced neuroadaptations during abstinence or increase sensitivity to acute nicotine analgesic effects. Second, the frequency and intensity of pain episodes among people with chronic pain may provide greater opportunity for pain-induced cueing of smoking and hence, stronger learned pain-smoking associations.
Figure 1.
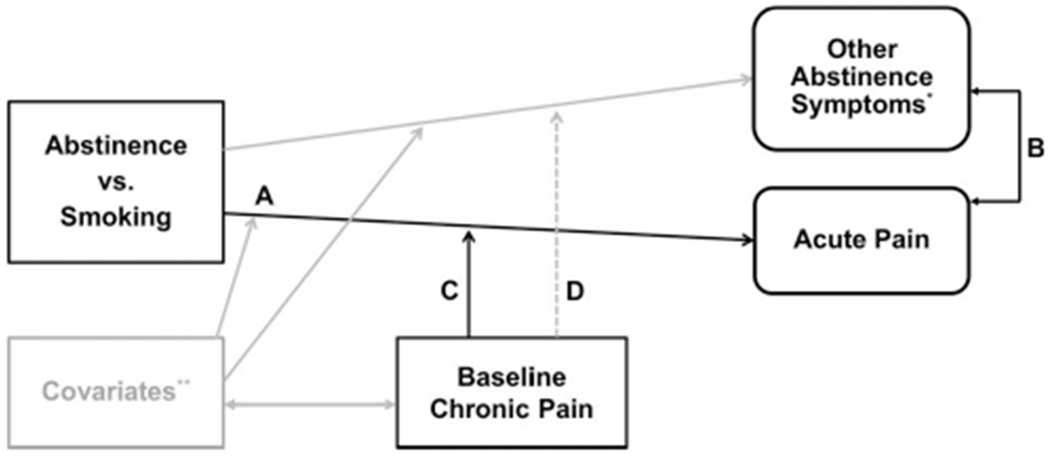
Conceptual model of chronic pain, acute pain, and tobacco use and abstinence. Solid black lines = hypothesized associations of model; double-sided arrow = correlations; broken line = hypothesized to have small effect to show discriminant validity of the model; gray lines = additional associations distal to the model; letters = represents pathways for primary hypotheses of the study. * Other abstinence symptoms = urges to smoke, composite withdrawal symptoms, and negative affect. ** Covariates that may be associated with both chronic pain and an amplification of the effect of abstinence status on acute pain and other symptoms = sex, age, cigarettes per day, menthol preference, emotional disorder symptomatology, drug abuse, alcohol use problems, and stress reaction.
The Current Study
In this secondary analysis of data from an ongoing study of individual differences in laboratory-derived tobacco abstinence phenotypes in African American smokers, we tested elements of the conceptual model of the intersection of pain, smoking, and abstinence effects proposed in Figure 1. We hypothesized that self-reported acute pain would be increased by overnight smoking abstinence (vs. ad libitum smoking; see Figure 1, Path A). We then tested whether abstinence-induced changes in self-reported acute pain were correlated with other tobacco abstinence effects to determine whether phenotypic expressions of acute pain in response to abstinence (vs. ad libitium smoking) were empirically overlapping or independent from known manifestations of the tobacco abstinence syndrome (i.e., smoking urges, nicotine withdrawal symptoms, and negative affect; Figure 1, Path B). We also tested the hypothesis that baseline chronic pain would predict the extent of abstinence-induced increases in acute pain (Figure 1, Path C), over and above correlates of chronic pain and tobacco abstinence effects. Finally, to test the specificity of the putative predictive influence of chronic pain on changes in acute pain, per se, we examined the extent to which baseline chronic pain predicted abstinence-induced changes in other tobacco abstinence effects, with the expectation that these associations would be small in size (Figure 1, Path D).
Method
Participants
This report is a secondary analysis of data from the Southern California Tobacco Addiction Phenotype Project, which is an ongoing study that investigates individual differences in laboratory-derived tobacco abstinence phenotypes among smokers of African American ancestry (Bello, Pang, Chasson, Ray, & Leventhal, 2017). Participants were 214 nontreatment-seeking daily smokers (M age = 47.7 years, SD = 11.04) recruited from the Los Angeles area via newspaper, online advertisements, or word-of-mouth. Eligible participants were regular cigarette smokers for 2+ years, currently smoking at least 10 cigarettes/day, fluent in English, and reported having non-Hispanic African American ancestry. Exclusion criteria were (a) current DSM–IV non-nicotine substance dependence (including marijuana dependence) to minimize alcohol and drug withdrawal symptoms during study conditions; (b) breath carbon monoxide (CO) levels <10 ppm (ppm) at intake; (c) daily use of any other tobacco products or marijuana (nondaily use permitted); (d) current use of nicotine replacement therapy or psychiatric medications; (e) currently pregnant or breastfeeding; and (f) planning to cut down or quit smoking in the next 30 days. Participants who met criteria for current DSM–IV substance abuse and/or DSM–IV-related psychiatric disorders were permitted to participate in the study to enhance generalizability to the greater population of smokers who may have comorbid behavioral conditions (Kalman, Morissette, & George, 2005). This study was approved by the University of Southern California Institutional Review Board (HS-13–00225).
Procedure
After an initial phone screen, participants attended a baseline visit involving informed consent, an in-person intensive eligibility screen involving breath alcohol and carbon monoxide (CO) levels analysis, structured clinical psychiatric interviews, and administration of chronic pain assessments and individual differences measures described below.
Participants then attended two counterbalanced experimental sessions both beginning approximately at 12 p.m.—one nonabstinent and one following 16-hr abstinence. Experimental procedures at both sessions were identical with the exception that participants were instructed not to smoke after 8 p.m. the night before their abstinent session, whereas for the nonabstinent session, participants were instructed to smoke as they normally would prior to their visit and then were instructed to smoke a cigarette of their preferred brand in the laboratory at the beginning of the session (to standardize for smoking recency). Participants were also instructed to avoid alcohol and use of any other drugs, tobacco products, or marijuana prior to their sessions. To confirm compliance with instructions, breath alcohol content (BrAC = 0.000 required for session continuation) and CO levels were measured at the start of both sessions. Participants were allowed two attempts to meet CO criteria for abstinence. Participants exceeding the maximum CO reading of 9 ppm at the abstinent session were considered nonabstinent and were rescheduled to complete their session on a different day (n = 23). Participants who failed to meet CO criteria for abstinence (<9 ppm) at their second attempt were discontinued from the study n = 2). Participants who missed their sessions were allowed to reschedule each session twice. Participants who were lost to follow up or exceeded the number of allotted reschedules were discontinued from the study (n = 27). Of these 27 participants, 19 participants dropped out prior to a session that was scheduled to be abstinent and 8 participants dropped out prior to a session that was scheduled to be nonabstinent. After breath alcohol and CO assessment, participants completed self-report measures of acute pain, smoking urges, nicotine withdrawal symptomatology, and negative affect at a single time-point that served as the primary tobacco abstinence-related outcomes for this report. Participants were compensated approximately $200 for the completing all three sessions.
Baseline Session Measures
The Graded Chronic Pain Scale.
The Graded Chronic Pain Scale (GCPS; von Korff, Ormel, Keefe, & Dworkin, 1992) is an eight-item self-report measure of chronic pain. Per GCPS scoring guidelines (von Korff et al., 1992), we computed several distinct chronic pain indices as follows. The persistence classification score was based on one item which instructed participants to report the number of days they experienced pain during the past 180 days. Responses ranging 90–180 days were classified as persistent pain (89 days or less = nonpersistent pain). The characteristic pain severity score summed responses to three questions for which participants rated their pain “right now,” their “worst” pain in the past 3 months, and their pain “on average” in the past 3 months on an 11-point scale, ranging from 0 (no pain) to 10 (pain as bad as it could be), with a total score range from 0 to 30 (α = .88 in this sample). The disability score summed responses to three items assessing interference with “daily activities,” “recreational/social activities,” and “ability to work (including housework)” in the past 3 months on an 11-point scale, ranging from 0 (no interference) to 10 (unable to carry on any activities) and one item measuring the number of days pain interfered with “usual activities like work, school, or housework” on an 11-point scale, ranging from 0 (none) to 10 (76–90 days), with a total score range of 0–40 (α = .96 in this sample). Chronic pain grade was computed by taking the sum of the characteristic pain severity and disability scores, yielding five grades with clinical descriptors (0 = no pain problem; Grade I = low intensity, low interference; Grade II = high intensity; Grade III = moderate interference; Grade IV = severe interference). Consistent with prior work (Ozdemir-Karatas, Peker, Balik, Uysal, & Tuncer, 2013), these pain grades were used to classify participants into three, distinct groups based on the clinical significance of their self-reported chronic pain levels: no pain (GCPS Grade 0), clinically nonsignificant pain (GCPS Grades I–II), and clinically significant pain (GCPS Grades III-IV). The GCPS has been shown to have sufficient clinical utility and excellent reliability and validity (von Korff et al., 1992).
Demographic and smoking characteristics.
Participants reported demographic information and smoking history (e.g., cigarettes smoked per day) using an author-constructed questionnaire.
The Fagerström Test for Nicotine Dependence.
The Fagerstrom Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) is a well-validated six-item measure of the severity of nicotine dependence.
The Inventory of Depression and Anxiety Symptoms.
The Inventory of Depression and Anxiety Symptoms (IDAS; Watson et al., 2007) assesses symptom dimensions from emotional syndromes experienced during the past 2 weeks on a 5-point scale, ranging from 1 (not at all) to 5 (extremely). The 20-item General Depression (α = .89), five-item Social Anxiety (α = .78), 8-item Panic (α = .79), and four-item Traumatic Intrusions (α = .84) subscales were included and the average response per item within each subscale was utilized. The IDAS has demonstrated strong internal consistency, convergent validity, and good discriminant validity in prior work (Watson et al., 2007).
The Drug Abuse Screening Test.
The Drug Abuse Screening Test (Bohn, Babor, & Kranzler, 1991) assesses past-year drug use and drug-related consequences, excluding tobacco and alcohol, based on the sum of affirmative responses to 10 different types of drug problems (α = .77) with strong validity (Villalobos Gallegos, Pérez-López, Mendoza-Hassey, Graue-Moreno, & Marín-Navarrete, 2015).
The Alcohol Use Disorders Identification Test.
The Alcohol Use Disorders Identification Test (AUDIT; Saunders, Aasland, Babor, de la Fuente, & Grant, 1993) instructs respondents to rate the frequency of 10 different items assessing frequency of alcohol use and alcohol-related problems each on 4-point Likert scales and yields a sum score (α = .81 in this sample). The AUDIT has shown good psychometric properties in prior work (de Meneses-Gaya, Zuardi, Loureiro, & Crippa, 2009).
The Multidimensional Personality Questionnaire-Brief Form Stress Reaction Scale.
Given the robust link between neuroticism and chronic pain (Cvijetic et al., 2014), we included the Multidimensional Personality Questionnaire-Brief Form Stress Reaction Scale (MPQ-BF; Patrick, Curtin, & Tellegen, 2002) 12-item stress reaction scale. This scale assesses personality-based tendencies toward negative emotionality and neuroticism by instructing participants to respond to true/false self-statements (e.g., “I sometimes get very upset and tense as I think of the day’s events”). The MPQ-BF Stress Reaction Scale yields a sum composite (α = .85).
Experimental Sessions Measures
Acute pain.
The Pain Numeric Rating Scale (PNRS; Jones, Vojir, Hutt, & Fink, 2007) was used to assess acute pain on a 0–10 numeric rating scale of pain felt “right now,” ranging from 0 (no pain) to 10 (pain as bad as it could be)—the standard measure of pain in ambulatory and inpatient settings (Krebs, Carey, & Weinberger, 2007)—in conjunction with descriptors from the Verbal Descriptor Scale and the Faces Pain Scale (Jones et al., 2007). The PNRS with descriptors from the Verbal Descriptor Scale and Faces Pain Scale has been demonstrated to have sufficient clinical utility and validity for assessment of pain intensity levels (Jones et al., 2007).
Each of the following measures has demonstrated adequate psychometric properties and sufficient sensitivity to overnight abstinence manipulations (Cox, Tiffany, & Christen, 2001; Hughes, 2007b).
The Brief Questionnaire of Smoking Urges.
Participants rated the extent to which they agreed with 10 statements indicative of smoking urges (e.g., “All I want is a cigarette”) on a 6-point scale, ranging from 0 (strongly disagree) to 5 (strongly agree) based on how they were feeling “right now,” which was averaged to generate a mean composite.
Modified Version of the Minnesota Nicotine Withdrawal Scale.
An 11-item modified version of the Modified Version of the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986) instructed participants to rate the intensity of DSM–IV-based nicotine withdrawal symptoms experienced “so far today” (i.e., craving, irritability, anxiety, concentration problems, restlessness, impatience, hunger, cardiovascular and autonomic activation, increased eating, drowsiness, and headaches) on a 6-point scale ranging from 0 (none) to 5 (severe).1 A mean score across items was computed.
The Profile of Mood States Negative Affect Scale.
The Profile of Mood States Negative Affect Scale (POMS; McNair, Lorr, & Droppleman, 1971 instructs respondents to indicate how they were feeling “right now” on a 5-point scale, ranging from 0 (not at all) to 4 (extremely) in response to adjectives of different mood states (e.g., restless, lonely, blue). As in prior work (Leventhal, Ameringer, Osborn, Zvolensky, & Langdon, 2013), a negative affect composite composed of the means of each negative affect subscale (Anger, Anxiety, Confusion, Depression, and Fatigue) was computed.
Data Analysis
Descriptive analyses.
We reported descriptive statistics and tested correlations between baseline characteristics and the chronic pain indices (i.e., GCPS Grade, Pain Severity, Disability, and Persistence classification; see Table 1). We used paired-sample t tests as a manipulation check to assess whether the smoking abstinence condition affected each tobacco abstinence effect (i.e., urges to smoke, composite withdrawal symptoms, and negative affect) and report internal consistency estimates of each study outcome by abstinence condition (Cronbach’s alpha; see Table 2). We also conducted analysis of variance (ANOVA) to determine whether there were order effects on abstinence-induced changes in acute pain, smoking urges, composite withdrawal symptoms, and negative affect as a result of the counterbalanced experimental design.
Table 1.
Sample Descriptives and Correlations Between Baseline Characteristics and Variables of Primary Interest
| Correlations (r) |
|||||
|---|---|---|---|---|---|
| Variable | M (SD) or n (%) | GCPS Gradea | GCPS Pain Severityb | GCPS Disabilityc | GCPS Persistenced |
| Sex (female) | 94(44.1%) | −.01 | −.08 | .03 | −.02 |
| Age | 47.72 (11.04) | .15* | .21** | .17* | .13 |
| Cigarettes per day | 14.44 (6.67) | .07 | .10 | .08 | .02 |
| Menthol preference | 127 (59.6%) | .02 | .03 | −.03 | −.05 |
| FTND | 5.28 (1.95) | .02 | .04 | −.02 | −.06 |
| IDAS | |||||
| Social anxiety | 1.41 (.63) | .25*** | .18** | .29**** | .13 |
| Panic | 1.27 (.45) | .36**** | .37**** | .36**** | .29**** |
| Traumatic intrusions | 1.34 (.63) | .31**** | .21** | .32**** | .19** |
| General depression | 1.80 (.59) | .36**** | .29**** | .38**** | .30**** |
| DAST-10 | 1.93 (2.28) | .19** | .23** | .18** | .17* |
| AUDIT | 3.57 (4.90) | .05 | .04 | .12 | .02 |
| MPQ-BF Stress reaction | 3.36 (3.24) | .32**** | .27**** | .30**** | .22** |
| GCPS | |||||
| Gradea | 1.35 (1.31) | — | |||
| Pain Severityb | 8.26 (8.26) | .83**** | — | ||
| Disabilityc | 8.36(11.23) | .90**** | .73**** | — | |
| Persistenced | 38 (19.2%) | .56**** | .59**** | .50**** | — |
Note. N = 212–214. Persistence (n = 198 because of nonresponse for this outcome). GCPS = Graded Chronic Pain Scale; FTND = Fagerstrom Test for Nicotine Dependence (range 0–10); IDAS = Inventory of Depression and Anxiety Symptoms (range 1–4); DAST-10 = Drug Abuse Screening Test (range 0 –10); AUDIT = Alcohol Use Disorders Identification Test (range 0 –30); MPQ-BF = Multidimensional Personality Questionnaire-Brief Form (range O –12).
GCPS Chronic Pain Grade (range 0–4).
GCPS Characteristic Pain Severity (range 0–30).
GCPS Disability Score (range 0–40).
GCPS Persistence Classification (at least half of prior 180 days experienced pain).
p < .05.
p < .01.
p < .001.
p < .0001.
Table 2.
Effects of Smoking Abstinence on Acute Pain and Other Tobacco Abstinence Outcomes
| Nonabstinent |
Abstinent |
Abstinence-induced change score |
Effect of abstinence condition |
||||
|---|---|---|---|---|---|---|---|
| Measure | M (SD) | α | M (SD) | α | M (SD) | t | d |
| PNRS | 1.39 (2.11) | — | 1.72 (2.34) | — | .33 (1.89) | 2.57 | .17* |
| QSU | 1.09 (1.21) | .95 | 3.01 (1.29) | .92 | 1.91 (1.44) | 19.36 | 1.33**** |
| MNWS | .78 (.75) | .83 | 1.52 (.98) | .87 | .75 (.92) | 11.89 | .82**** |
| POMS-NA | .52 (.49) | .95 | .67 (.58) | .97 | .15 (.45) | 4.93 | .33**** |
Note. N = 210–214 because of nonuniform patterns of missing data across outcomes. PNRS = Pain Numeric Rating Scale (range 0–10); QSU = Questionnaire of Smoking Urges (range 0–5); MNWS = Minnesota Nicotine Withdrawal Scale (range 0–5); POMS = Profile of Mood States (range 0–5). NA = Negative Affect. Abstinence-induced change score = score in abstinent condition-score in nonabstinent condition.
p < .05.
p < .0001.
Primary analysis.
We reported descriptive statistics and distribution of scores for ratings of acute pain in nonabstinent and abstinent conditions (see Figure 2A and 2B). To determine whether acute pain was altered by tobacco abstinence, paired sample t tests were used to test the effect on abstinence condition on PNRS scores (see Table 2). To investigate to what extent the overall increase in mean PNRS ratings reflected worsening of existing pain versus transition from no pain to some pain, we conducted ANOVAs to test whether the magnitude of change in acute pain from nonabstinent to abstinent conditions differed between those who reported no acute pain (PNRS rating of 0) at the nonabstinent condition versus those who reported a rating of 1 or higher on the PNRS at nonabstinent. To examine whether acute pain cohered with other tobacco abstinence indicators, we tested Pearson correlations of abstinence-induced change scores (difference score in abstinent condition–score in nonabstinent condition) in acute pain with abstinence-induced changes in MNWS, The Brief Questionnaire of Smoking Urges (QSU; Cox et al., 2001), and POMS-Negative Affect (bolded values in Table 3).
Figure 2.
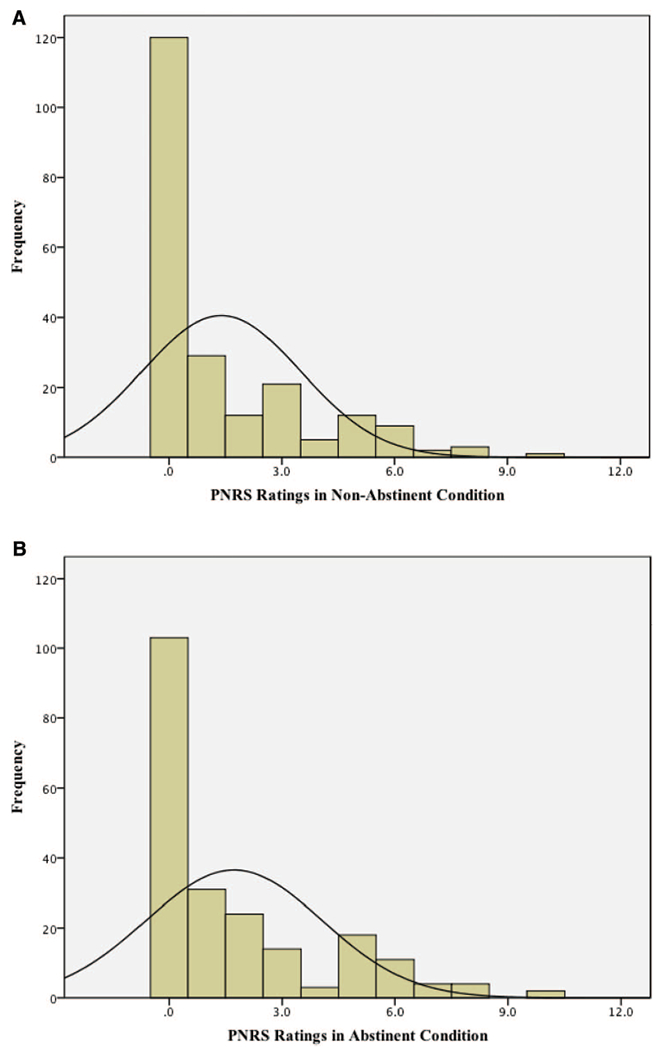
A: Histogram plot for acute pain ratings in nonabstinent condition. Distribution of scores of PNRS ratings in nonabstinent condition (N = 214). PNRS = Pain Numeric Rating Scale (range 0–10). B: Histogram plot for acute pain ratings in abstinent condition. Distribution of scores of PNRS ratings in abstinent condition (N = 214). PNRS = Pain Numeric Rating Scale (range 0–10). See the online article for the color version of this figure.
Table 3.
Correlations Among Nonabstinent Scores, Abstinent Scores, and Abstinence-Induced Change Scores of Acute Pain and Other Tobacco Abstinence Measures
| Correlations (r) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Nonabstinent scores | ||||||||||||
| 1. PNRS | — | |||||||||||
| 2. QSU | .08 | — | ||||||||||
| 3. MNWS | .33**** | .35**** | — | |||||||||
| 4. POMS-NA | .37**** | .13† | .56**** | — | ||||||||
| Abstinent scores | ||||||||||||
| 5. PNRS | .64**** | .04 | .29**** | .35**** | — | |||||||
| 6. QSU | .14* | .34**** | .25*** | .13† | .22** | — | ||||||
| 7. MNWS | .31**** | .27**** | .46**** | .37**** | .38**** | .61**** | — | |||||
| 8. POMS-NA | .34**** | .14* | .47**** | .65**** | .42**** | .35**** | .61**** | — | ||||
| Abstinence-induced change scores | ||||||||||||
| 9. PNRS | −.32**** | −.04 | .003 | .03 | .52**** | .11**** | .12 | .14**** | — | |||
| 10. QSU | .07 | −.54**** | −.07 | .01 | .17* | .61**** | .32**** | .20** | .13†a | — | ||
| 11. MNWS | .08 | .01 | −.32**** | −.06 | .18** | .44**** | .69**** | .27**** | .13†a | .39**** | — | |
| 12. POMS-NA | .03 | .04 | −.02 | −.26*** | .15* | .30**** | .37**** | .56**** | .15*a | .24*** | .41**** | — |
Note. N = 210–214 due to nonuniform patterns of missing data across outcomes. PNRS = Pain Numeric Rating Scale; QSU = Questionnaire of Smoking Urges; MNWS = Minnesota Nicotine Withdrawal Scale; POMS = Profile of Mood States; NA = Negative Affect; Abstinence-induced change score = score in abstinent condition–score in nonabstinent condition (bolded values illustrate correlations between abstinence-induced change scores).
Nonsignificant after Bonferroni correction (p < .017).
p < .10.
p < .05.
p < .01.
p < .001.
p < .0001.
Linear regression models were used to test whether baseline chronic pain predicted the magnitude of pain response to tobacco abstinence (see Table 4). One GCPS chronic pain index was entered as the regressor and the abstinence-induced change in acute pain (PNRS) was entered as the outcome variable. Separate models were used to test each of the four quantitative GCPS chronic pain indices as stand-alone predictors. We then examined the specificity of this association by conducting regression models for the four GCPS indices in the same fashion using the other tobacco abstinence symptoms as outcomes (i.e., QSU, MNWS, POMS-Negative Affect). All regressions were first tested in unadjusted models, which included only the outcome variable’s respective nonabstinent score as a covariate to partial out the impact of baseline levels that may be associated with chronic pain as in prior work on linkages between behavioral traits and abstinence-induced changes in tobacco withdrawal (Bello et al., 2017; Leventhal et al., 2013). We then retested these analyses in adjusted models, which included sex, age, cigarettes per day, menthol preference, emotional disorder symptomatology (IDAS), drug abuse (DAST), alcohol use problem (AUDIT), and MPQ-BF stress reaction as additional covariates. Lastly, we ran one-way analyses of covariance (ANCOVAs) to evaluate differences between groups with no pain, clinically nonsignificant pain, and clinically significant pain on abstinence-induced change scores of acute pain (PNRS), while controlling for nonabstinent acute pain scores for descriptive purposes and report the least squares mean in Figure 3.
Table 4.
Association of Baseline Chronic Pain With Abstinence-Induced Changes in Acute Pain and Other Tobacco Abstinence Outcomes at the Experimental Sessions
| GCPS regressor |
||||||||
|---|---|---|---|---|---|---|---|---|
| Grade (β) |
Pain severity (β) |
Disability (β) |
Persistence (β) |
|||||
| Outcome | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| PNRS | .32† | .31† | .30† | .31† | .30† | .30† | .30† | .29† |
| QSU-Total | .04 | .07 | .06 | .11 | .04 | .07 | .07 | .09 |
| MNWS | .08 | .10 | .13 | .15 | .06 | .09 | .08 | .09 |
| POMS-NA | .09 | .05 | .16 | .16 | .08 | .04 | .04 | −.0003 |
Note. N = 210–214 because of nonuniform patterns of missing data across outcomes. N = 198 for Persistence subscale due to additional non-responses from participants for this outcome. GCPS = Graded Chronic Pain Scale; PNRS = Pain Numeric Rating Scale; QSU = Questionnaire of Smoking Urges; MNWS = Minnesota Nicotine Withdrawal Scale; POMS = Profile of Mood States (NA = Negative Affect). Outcome = abstinence-induced change score (score while abstinent-nonabstinent). Unadjusted model included only a single GCPS regressor and the respective nonabstinent score as a covariate. Adjusted models additionally included sex, age, cigarettes per day, menthol preference, nicotine dependence, emotional disorder symptomatology (IDAS), drug abuse (DAST), alcohol use problem (AUDIT), and MPQ-BF stress reaction.
Statistically significant after Bonferroni correction (p < .003).
Figure 3.

Abstinence-induced changes in acute pain by clinical pain status. Least-squares means (±standard error) of PNRS abstinence-induced change scores for GCPS Chronic Pain Grades grouped by clinical significance of pain, adjusted for nonabstinent PNRS scores. PNRS = Pain Numeric Rating Scale (range 0–10); no pain = GCPS Grade 0 (n = 60); clinically nonsignificant pain = GCPS Grades I-II (nN = 108); clinically significant pain = GCPS Grades III-IV (N = 44). † Significantly greater than “no pain” (p < .0001). * Significantly greater than “clinically nonsignificant pain” (p < .001).
Abstinence condition effects are reported as Cohen’s d effect size estimates and regression results are reported as standardized coefficients (β). Significance was set atp < .05 two-tailed. Type-I error was controlled using the Bonferroni correction for multiple testing, resulting in thresholds of the 3 tests in the correlational analysis (.05/3; p < .017) and for the 16 tests of associations between chronic pain and tobacco abstinence effects (.05/16; p < .003). Data were analyzed using IBM SPSS Version 22. Additional supplementary analyses were conducted to examine the robustness and generalizability of the results and are detailed below.
Results
Preliminary Analyses
As reported in Table 1, the overall sample was, on average, middle-aged and moderate-to-heavy smokers with medium levels of nicotine dependence. Substance use and emotional symptomatology were low, on average. Based on chronic pain grade classifications, we found that 60 participants reported no pain (GCPS Grade 0), 108 participants reported clinically nonsignificant pain (GCPS Grades I–II), and 44 participants endorsed clinically significant chronic pain (GCPS Grades III–IV). Sex, cigarettes per day, menthol preference, nicotine dependence, and alcohol use disorders were not significantly associated with any of the chronic pain indices. Age and baseline levels of social anxiety were significantly associated with Chronic Pain Grade, Pain Severity, and Disability (rs = .15–.29, ps < .05). Baseline levels of Panic, Traumatic Intrusions, General Depression, drug abuse, and stress reaction significantly correlated with all four chronic pain indices (rs = .17-.38, ps < .05). In addition, there were strong correlations between all chronic pain indices (rs = .50-.90, ps < .0001; see Table 1). Smoking urge (QSU), composite nicotine withdrawal symptoms (MNWS), and negative affect (POMS) were found to have high internal consistency and significantly greater scores in abstinent versus nonabstinent sessions, with abstinence effect sizes ranging from small to large (see Cronbach’s alpha and Cohen’s ds in Table 2). We found no significant order effects on abstinence-provoked changes in acute pain and other abstinence outcomes (ps ≥ .09).
Primary Analyses
Effects of smoking abstinence on acute pain.
Acute pain (PNRS) ratings were significantly greater, on average, following abstinent (M = 1.72, SD = 2.34) versus nonabstinent (M = 1.39, SD = 2.11) sessions in the overall sample, paired sample t(213) = 2.57, p = .01, with a small-sized abstinence effect d = .17; see Table 2). Examination of the distribution of scores show that 56.1% of the sample (n = 120) reported “no [acute] pain” (PNRS rating of 0) in the nonabstinent condition and 48.1% (n = 103) reported “no [acute] pain” in the abstinent condition. The distribution of acute pain scores was positively skewed for both conditions, although there was a modest shifting of the overall distribution to higher pain ratings in the abstinent condition relative to the nonabstinent condition (see histogram plots, Figure 2A and 2B).
The increase in mean pain scores from nonabstinent to abstinent was significantly greater among those who reported no pain (PNRS rating = 0) versus those who reported some pain (PNRS rating >1) at their nonabstinent session; difference between groups, F(1, 212) = 8.037, p = .005. For those without pain during their nonabstinent session (n = 120), the mean abstinence-induced change score was significantly greater from zero (M = 0.65, SD = 1.43; Cohen’s d = 0.45; one sample t(119) = 4.98, p < .0001). For those with some pain during their nonabstinent session (n = 94), there was no significant change from nonabstinent to abstinent conditions (mean abstinent-induced change score: M = 0.07, SD = 2.29; Cohen’s d = 0.03; p = .75). Thus, the increase in mean pain scores produced by abstinence in the overall sample was driven by smokers who experienced a transition from no pain while nonabstinent to some pain while abstinent.
Correlations between abstinence-induced changes in acute pain and other tobacco abstinence symptoms.
As illustrated in Table 3, individual differences in acute pain were associated with other tobacco abstinence symptoms both in the abstinent and nonabstinent states. Importantly, Table 3 also illustrates that abstinence-induced increases in acute pain exhibited small correlations with abstinence-induced changes in negative affect, smoking urges, and MNWS scores (rs = .13-.15, ps = .03–.059), which were not significant after the Bonferroni correction.2
Association of chronic pain with abstinence-induced changes in acute pain and other tobacco abstinence indices.
As depicted in Table 4, all four GCPS chronic pain indices were positively associated with abstinence-induced changes in acute pain both in the linear regression models that controlled for only baseline (nonabstinent) acute pain and the adjusted models, which controlled for demographics, smoking, and emotional and substance use variables (ps < .001). The effect sizes of these associations were of medium magnitude (βs = .29–.31). Tests of the specificity of these associations to pain-related abstinence by examining GCPS indices as predictors of other tobacco abstinence outcomes demonstrated smaller-sized associations that were not significant after the Bonferroni correction (βs = .0003–.16; ps = .04–.99; see Table 4).
Differences in abstinence-induced changes in acute pain by clinical pain status.
As categorical pain status is often used to operationalize pain in clinical settings, we conducted one-way ANCOVAs covarying nonabstinent acute pain scores to examine differences in abstinence-induced changes in acute pain by clinical pain status, which showed significant group differences, F(2, 208) = 10.84, p < .0001 (see Figure 3). Pairwise contrasts revealed that participants with clinically significant pain exhibited greater abstinence-induced increases in acute pain than participants with clinically nonsignificant pain and participants with no pain (see Figure 3). Abstinence-induced changes in acute pain did not significantly differ in pairwise contrasts of clinically nonsignificant pain and no pain groups.
Supplementary Analyses
Main and interactive effects of baseline chronic pain and nonabstinent acute pain score with abstinent acute pain ratings.
Given that using abstinence-induced change scores for study outcomes may reduce reliability, we retested the linear regression models of the effects of chronic pain using the acute pain score during smoking abstinence as the outcome after adjusting for the nonabstinence acute pain score to determine the robustness of the findings across different outcomes (see Supplementary Table S1 in the online supplementary material). We found that all GCPS chronic pain indices and nonabstinent acute pain scores significantly predicted increases in acute pain at the abstinent session with the Bonferroni correction for all outcomes (βs = .24-.54; ps < .002). In addition, there were no significant interactions between the chronic pain indices and the nonabstinent acute pain score in predicting acute pain during the abstinence condition, suggesting that the association between baseline chronic pain and acute pain while abstinent did not significantly differ depending on the levels of acute pain while nonabstinent.
Interactive effects of baseline chronic pain and age with abstinence-induced change scores in acute pain and other tobacco withdrawal outcomes.
Because of the high variability in age in our sample, we tested the generalizability of the findings and found a significant interaction effect between GCPS chronic pain grade and age predicting abstinence-induced changes in acute pain (β = −.21; p = .002), indicating the association between chronic pain grade and abstinence-induced changes in acute pain was weaker for those who were older relative to younger participants. We found no significant interactions between the other three chronic pain indices and age predicting abstinence-induced changes in acute pain and other tobacco abstinence symptoms.
Discussion
In this laboratory study of African American smokers, we found that acute pain was significantly greater in abstinent versus nonabstinent states in the overall study sample. The magnitude of the abstinence effect on acute pain was small (d = .17) relative to effect magnitudes reported for well-known indicators of the acute tobacco abstinence syndrome. Effect sizes for composite withdrawal symptom indices and urges to smoke were relatively large within this sample, which is consistent with previous reports (e.g., ds > 0.8; Hughes, 2007a; Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010). The abstinence effect for acute pain may have been small in the overall sample because the effect was being driven by particular subsections of the sample, including smokers with more severe chronic pain and participants who reported having experienced no acute pain after ad libitum smoking. Although the current data do not indicate that increased pain is a universal response to tobacco abstinence in African American smokers, the results from this study provide some support that pain may be an abstinence phenotype in segments of the African American smoker population.
Associations between abstinence-induced increases in acute pain and other expressions of the tobacco abstinence syndrome were small and not statistically significant after correction for Type I error. In the syndrome concept in psychopathology (American Psychiatric Association, 2013), candidate symptoms for a common syndrome should exhibit moderate shared variance with other syndrome features, thus, associations that are large suggest two (redundant) measures of a common facet of the syndrome, whereas null associations suggest that the two indices are not part of a common syndrome. Our findings suggest that the acute pain phenotype during overnight abstinence may not be cohesive with other tobacco abstinence syndrome phenotypes for the typical African American smoker. In concert with the evidence described above that pain was affected by abstinence predominately in subsections of the sample, pain may be a rarer phenotype that does not cohere with other common elements of the tobacco abstinence syndrome in the general population of African American smokers. Whether certain subgroups of the African American smoker population evince greater coherence between pain and other manifestations of the tobacco withdrawal syndrome warrants further inquiry.
Differences in acute pain during abstinent and nonabstinent states were more pronounced in smokers who had more severe, persistent, and disabling chronic pain. Findings were pronounced for the distinction between clinically significant and clinically nonsignificant pain3 and were specific to acute pain, but not other symptoms of the tobacco abstinence syndrome. The acute analgesic effects of nicotine (Ditre et al., 2016) may be mediated by activation of nicotinic acetylcholine receptors (i.e., α4β2 subtype; Damaj et al., 2007), endogenous opioid systems (Marubio et al., 1999), or the release of beta-endorphins (Pomerleau, 1992). It is possible that, for individuals with chronic pain, repeated simulation of these pain processing pathways via habitual tobacco use may result in neuroadaptations that change their homeostatic setpoints or sensitizes smokers to acute analgesic effects of nicotine administration. However, because our study design contrasted overnight abstinence with recent smoking (i.e., the ad libitum smoking condition), the extent to which differences between the two conditions are driven by susceptibility to withdrawal-related disruption of homeostatic set point versus the dissipation of nicotine-induced analgesia is unknown.
Although African American smokers with chronic pain may be particularly sensitive to a jump in acute pain ratings from nonabstinent to abstinent states, primary analyses also showed that increases in acute pain while abstinent were disproportionately larger for those who reported no acute pain after immediately smoking a cigarette following a period of ad libitum smoking. The reason for why these seemingly disparate subsets of the African American smoker population were more sensitive to abstinence effects on acute pain is unclear. An important consideration is that our abstinence manipulation was designed to maximize the difference in tobacco exposure across the two conditions, with the nonabstinent acute pain measurement occurring not only after 16 hr of ad libitum smoking in the natural environment, but also having immediately smoked a cigarette in the laboratory. One possibility is that people who experience complete amelioration of acute pain immediately following tobacco self-administration tend to be those who express more pain during acute abstinence, which is not necessarily mutually exclusive from having a chronic pain problem. To tease apart the relative influence of acute intoxication from nicotine in the pain-abstinence association, an ideal design would involve inclusion of an additional experimental condition following a very brief period of abstinence that is short enough to precede an experience of feeling deprived but long enough after the acute experience of smoking (and bolus of nicotine) subsides. This warrants further investigation.
Because this study included only African Americans, we could not empirically test whether these findings generalize to other race/ethnicities. Although we presume that pain may be a predictor and consequence of tobacco abstinence effects across ethnic groups, it is possible that these processes may be particularly robust in African Americans. Relative to Whites, African Americans have greater difficulty with quitting smoking and poorer cessation success (Choi et al., 2004), are reported to experience more severe symptoms in some domains of withdrawal (Bello et al., 2016), and experience higher levels of clinical pain and pain-related disability (Edwards et al., 2001; Hooten et al., 2012; Riley et al., 2002). African Americans also receive lower quality pain care than Whites (i.e., inadequate assessment and treatment of pain, receiving significantly smaller doses of opioid analgesics; Anderson, Green, & Payne, 2009). One speculative consequence of this disparity is that African American smokers with chronic pain (vs. other ethnic groups) may be more apt to use smoking to cope with pain due to the greater accessibility of cigarettes (vs.medical treatment of pain conditions). Future work examining pain-abstinence associations among various ethnic groups is needed.
Several limitations in the study are worth noting. First, we used self-report measures to assess acute pain and preexisting chronic pain, which may have been vulnerable to response biases. As such, the inclusion of more objective measures (e.g., physiological, behavioral, and brain imaging methods) and pain thresholds (including perceptual thresholds) in addition to self-reported data may aid in a more precise and accurate assessment of chronic pain and other pain-related outcomes. Second, smoking abstinence was experimentally manipulated over a short (but important) duration of time and not part of a self-motivated quit attempt. Whether the results generalize to those who are attempting to quit smoking or to longer periods of abstinence during which nicotine offset effects can be distinguished from long-term abstinence phenomena is unknown. In addition, the current study used a nonclinical pain sample of African American smokers, thus, continued exploration of these effects utilizing clinical pain samples or those seeking treatment for chronic pain is warranted to observe whether these effects still hold. Lastly, our sample had high variability in age, and we found that one of the four chronic pain measures exhibited more robust associations with abstinence-induced changes in pain in younger smokers. Further study of moderators of the intersection of chronic pain, tobacco abstinence, and acute pain may be warranted.
In summary, the current study provides initial evidence that increased acute pain may represent a tobacco abstinence effect that is distinct from other expressions of the tobacco abstinence syndrome and is disproportionately expressed in African American smokers with chronic pain. Given that acute pain has been shown to be a potent motivator of smoking (Ditre & Brandon, 2008; Ditre et al., 2010), and that treatment-seeking pain patients reliably endorse smoking in response to pain (Patterson et al., 2012), one clinical implication of this work is that increased pain during the early stages of a quit attempt could precipitate relapse. If extended to clinical samples, these results raise the possibility that tailoring of smoking cessation interventions to account for tobacco abstinence-induced amplification or onset of acute pain may be warranted, especially among African American smokers suffering with chronic pain. Pending replication and extension of the current findings, another potential clinical implication of the study is that it may behoove clinicians to consider assessment of both baseline chronic pain and acute pain to inform conceptualization and treatment of tobacco withdrawal symptomatology in African American smokers. More research examining individual differences in abstinence-induced acute pain within other settings (e.g., treatment centers) or among other populations (e.g., clinical pain samples) are warranted to further inform the development and tailoring of smoking cessation interventions for African American smokers. Moreover, additional research of the psychobiological and psychosocial substrates underlying the changes in acute pain that result from acute tobacco abstinence and administration may be fruitful to elucidate the basis of pain-smoking comorbidity and open up new avenues for the treatment of pain and tobacco addiction among smokers. Such research may also be important to provide a scientific agenda for health equity promotion by addressing two disabling conditions—tobacco addiction and chronic pain—that have disproportionate public health consequences for the African American population.
Supplementary Material
Acknowledgments
This research was supported by funds from the American Cancer Society (grant RSG-13-163-01), National Institute on Drug Abuse (grant K08-DA025041), and the National Science Foundation Graduate Research Fellowship (grant DGE-1418060). Funding sources had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. Hypotheses and results of this article were presented at the 78th Annual Scientific Meeting of the College on Problems of Drug Dependence in Palm Springs, California.
Footnotes
We used an 11-item modified version of the MNWS, which included the following items: (a) craving; (b) irritability; (c) anxiety; (d) concentration problems; (e) restlessness; (f) impatience; (g) hunger; (h) cardiovascular and autonomic activation (i.e., tremor, heart racing, sweating, dizzy, stomach or bowel problems); (i) increased eating; (j) drowsiness; and (k) headaches. The 11-item modified version of the MNWS included “headaches” as an additional item and did not include items for “depressed mood,” “insomnia,” and other possible symptoms (i.e., coughing, decreased pleasure from events, and impulsivity).
Given that the MNWS composite index includes items assessing individual symptoms (e.g., anxiety, irritability, hunger), we conducted additional analyses of abstinence-induced change scores on the MNWS individual symptom items. Results revealed a range of negligible to small-sized correlations with abstinence-induced changes in acute pain (rs = .003–.15, ps = .04–.97).
We also explored whether there were nonlinear relations between each chronic pain index (GCPS Chronic Pain Grade, GCPS Pain Severity, GCPS Disability Score, and GCPS Persistence Classification) and acute pain following abstinence and found no evidence supporting nonlinear effects of chronic pain indices on abstinence-induced changes in acute pain.
Supplemental materials: http://dx.doi.org/10.1037/abn0000367.supp
Contributor Information
Mariel S. Bello, Department of Psychology, University of Southern California
Julia F. McBeth, Department of Preventive Medicine, Keck School of Medicine, University of Southern California
Matthew G. Kirkpatrick, Department of Preventive Medicine, Keck School of Medicine, University of Southern California
Kelly E. Dunn, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Joseph W. Ditre, Department of Psychology, Syracuse University
Lara A. Ray, Department of Psychology, University of California, Los Angeles
Adam M. Leventhal, Departments of Preventive Medicine and Psychology, University of Southern California
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Anderson KO, Green CR, & Payne R (2009). Racial and ethnic disparities in pain: Causes and consequences of unequal care. The Journal of Pain, 10, 1187–1204. 10.1016/j.jpain.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Behrend C, Schonbach E, Coombs A, Coyne E, Prasarn M, & Rechtine G (2014). Smoking cessation related to improved patient-reported pain scores following spinal care in geriatric patients. Geriatric Orthopaedic Surgery & Rehabilitation, 5, 191–194. 10.1177/2151458514550479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello MS, Pang RD, Chasson GS, Ray LA, & Leventhal AM (2017). Obsessive-compulsive symptoms and negative affect during tobacco withdrawal in a non-clinical sample of African American smokers. Journal of Anxiety Disorders, 48, 78–86. 10.1016/j.janxdis.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello MS, Pang RD, Cropsey KL, Zvolensky MJ, Reitzel LR, Huh J, & Leventhal AM (2016). Tobacco withdrawal amongst African American, Hispanic, and White smokers. Nicotine & Tobacco Research, 18, 1479–1487. 10.1093/ntr/ntv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, & Kranzler HR (1991). Validity of the drug abuse screening test (DAST-10) in inpatient substance abusers: Problems of drug dependence In Harris LS (Ed.), Problems of drug dependence, 1991: Proceedings of the 53 rd Annual Scientific Meeting, The Committee on Problems of Drug Dependence, Inc; (DHHS Publication No. ADM 92–1888. NIDA Research Monograph, Vol. 119, p. 233). Rockville, MD: Department of Health and Human Services. [Google Scholar]
- Choi WS, Okuyemi KS, Kaur H, & Ahluwalia JS (2004). Comparison of smoking relapse curves among African-American smokers. Addictive Behaviors, 29, 1679–1683. 10.1016/j.addbeh.2004.02.060 [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, McKee S, Bois F, Alagille D, Tamagnan GD, … Staley JK (2010). Beta2* nicotinic acetylcholine receptors modulate pain sensitivity in acutely abstinent tobacco smokers. Nicotine & Tobacco Research, 12, 535–539. 10.1093/ntr/ntq040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3, 7–16. 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- Cvijetic S, Bobic J, Grazio S, Uremovic M, Nemcic T, & Krapac L (2014). Quality of life, personality and use of pain medication in patients with chronic back pain. Applied Research in Quality ofLife, 9, 401–411. 10.1007/s11482-013-9219-9 [DOI] [Google Scholar]
- Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, … Martin BR. (2007). Genetic approaches identify differential roles for alpha4beta2* nicotinic receptors in acute models of antinociception in mice. The Journal of Pharmacology and Experimental Therapeutics, 321, 1161–1169. 10.1124/jpet.106.112649 [DOI] [PubMed] [Google Scholar]
- de Meneses-Gaya C, Zuardi AW, Loureiro SR, & Crippa JAS (2009). Alcohol Use Disorders Identification Test (AUDIT): An updated systematic review of psychometric properties. Psychology & Neuroscience, 2, 83–97. 10.3922/j.psns.2009.1.12 [DOI] [Google Scholar]
- Ditre JW, & Brandon TH (2008). Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology, 117, 467–472. 10.1037/0021-843X.117.2.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, & Meagher MM (2011). Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychological Bulletin, 137, 1065–1093. 10.1037/a0025544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, & Brandon TH (2010). Effects of expectancies and coping on pain-induced motivation to smoke. Journal of Abnormal Psychology, 119, 524–533. 10.1037/a0019568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Zale EL, Kosiba JD, & Maisto SA (2016). Acute analgesic effects of nicotine and tobacco in humans. Pain, 157, 1373–1381. 10.1097/j.pain.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Kosiba JD, Zale EL, Zvolensky MJ, & Maisto SA (2016). Chronic pain status, nicotine withdrawal, and expectancies for smoking cessation among lighter smokers. Annals of Behavioral Medicine, 50, 427–435. 10.1007/s12160-016-9769-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Doleys DM, Fillingim RB, & Lowery D (2001). Ethnic differences in pain tolerance: Clinical implications in a chronic pain population. Psychosomatic Medicine, 63, 316–323. 10.1097/00006842-200103000-00018 [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, & Imad Damaj M (2005). Nicotine physical dependence in the mouse: Involvement of the alpha7 nicotinic receptor subtype. European Journal of Pharmacology, 515, 90–93. 10.1016/j.ejphar.2005.03.044 [DOI] [PubMed] [Google Scholar]
- Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, & Le Marchand L (2006). Ethnic and racial differences in the smoking-related risk of lung cancer. The New England Journal of Medicine, 354, 333–342. 10.1056/NEJMoa033250 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hooten WM, Knight-Brown M, Townsend CO, & Laures HJ (2012). Clinical outcomes of multidisciplinary pain rehabilitation among African American compared with Caucasian patients with chronic pain. Pain Medicine, 13, 1499–1508. 10.1111/j.1526-4637.2012.01489.x [DOI] [PubMed] [Google Scholar]
- Hughes JR (2007a). Effects of abstinence fromtobacco: Valid symptoms and time course. Nicotine & Tobacco Research, 9, 315–327. 10.1080/14622200701188919 [DOI] [PubMed] [Google Scholar]
- Hughes JR (2007b). Measurement of the effects of abstinence from tobacco: A qualitative review. Psychology of Addictive Behaviors, 21, 127–137. 10.1037/0893-164X.21.2.127 [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43, 289 –294. 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Jones KR, Vojir CP, Hutt E, & Fink R (2007). Determining mild, moderate, and severe pain equivalency across pain-intensity tools in nursing home residents. Journal of Rehabilitation Research and Development, 44, 305–314. 10.1682/JRRD.2006.05.0051 [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, & George TP (2005). Co-morbidity of smoking in patients with psychiatric and substance use disorders. The American Journal on Addictions, 14, 106–123. 10.1080/10550490590924728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye AD, Prabhakar AP, Fitzmaurice ME, & Kaye RJ (2012). Smoking cessation in pain patients. The Ochsner Journal, 12, 17–20. [PMC free article] [PubMed] [Google Scholar]
- Krebs EE, Carey TS., & Weinberger M (2007). Accuracy of the pain numeric rating scale as a screening test in primary care. Journal of General Internal Medicine, 22, 1453–1458. 10.1007/s11606-007-0321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, & Langdon KJ (2013). Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug and Alcohol Dependence, 133, 324–329. 10.1016/j.drugalcdep.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, & Pickworth WB (2010). A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors, 35, 1120–1130. 10.1016/j.addbeh.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d’Exaerde A,… Changeux JP. (1999). Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature, 398, 805–810. 10.1038/19756 [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, & Droppleman LF (1971). Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services. [Google Scholar]
- Nakajima M, & Al’Absi M (2014). Nicotine withdrawal and stress-induced changes in pain sensitivity: A cross-sectional investigation between abstinent smokers and nonsmokers. Psychophysiology, 51, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir-Karatas M, Peker K, Balik A, Uysal O, & Tuncer EB (2013). Identifying potential predictors of pain-related disability in Turkish patients with chronic temporomandibular disorder pain. The Journal of Headache and Pain, 14, 17 10.1186/1129-2377-14-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, & Tellegen A (2002). Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment, 14, 150–163. 10.1037/1040-3590.14.2.150 [DOI] [PubMed] [Google Scholar]
- Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, & Morasco BJ (2012). Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. The Journal of Pain, 13, 285–292. 10.1016/j.jpain.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF (1992). Nicotine and the central nervous system: Biobe-havioral effects of cigarette smoking. The American Journal of Medicine, 93(Suppl. 1), S2–S7. 10.1016/0002-9343(92)90619-M [DOI] [PubMed] [Google Scholar]
- Riley JL III, Wade JB, Myers CD, Sheffield D, Papas RK, & Price DD (2002). Racial/ethnic differences in the experience of chronicpain. Pain, 100, 291–298. 10.1016/S0304-3959(02)00306-8 [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction, 88, 791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Shi Y, Hooten WM, & Warner DO (2011). Effects of smoking cessation on pain in older adults. Nicotine & Tobacco Research, 13, 919–925. 10.1093/ntr/ntr097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos Gallegos L, Pérez-López A, Mendoza-Hassey R, Graue-Moreno J, & Marín-Navarrete R (2015). Psychometric and diagnostic properties of the Drug Abuse Screening Test (DAST): Comparing the DAST-20 vs. the DAST-10. Salud Mental, 38, 89–94. 10.17711/SM.0185-3325.2015.012 [DOI] [Google Scholar]
- von Korff M, Ormel J, Keefe FJ, & Dworkin SF (1992). Grading the severity of chronic pain. Pain, 50, 133–149. 10.1016/0304-3959(92)90154-4 [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, … Stuart S. (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological Assessment, 19, 253–268. 10.1037/1040-3590.19.3.253 [DOI] [PubMed] [Google Scholar]
- Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, & Warner DO (2008). An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician, 11, 643–653. [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan K, Gonzalez A, & Asmundson GJ (2009). Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine & Tobacco Research, 11, 1407–1414. 10.1093/ntr/ntp153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


