Abstract
Esophageal endoscopic submucosal dissection (ESD) can be a curative treatment for superficial esophageal squamous cell carcinoma (SESCC). However, it is unclear whether the development of metachronous recurrence after ESD may be explained based on several risk factors. This study aimed to assess the incidence and the risk factors of metachronous recurrence of SESCC after ESD. This retrospective analysis was conducted at Samsung Medical Center, Seoul, Korea, from April 2007 to May 2018. Two hundred and fifty-three SESCC patients treated with ESD were followed using surveillance endoscopy after the procedure. Risk factors for metachronous esophageal SCC were analyzed using the Kaplan-Meier method and Cox’s proportional hazards model. Metachronous esophageal SCCs were found in 21 (8.3%) of the 253 patients. Six patients (2.4%) with extraesophageal recurrence such as lymph node metastasis confirmed by imaging were excluded from patients with metachronous recurrence and data were censored from the recurrence date. Univariate analysis revealed that the presence of many (>10) irregularly shaped multiform Lugol-voiding lesions (LVLs) around the main lesion, margin of the main LVL, and tumor differentiation were risk factors for the development of metachronous cancer. Multivariate analysis also revealed that many (>10) LVLs (hazard ratio [HR], 6.32; 95% confidence interval [CI], 1.62–24.72; p = 0.047) and unclear or spiculated margin of the main LVL (HR, 6.51; 95% CI, 1.44–29.42; p = 0.029) were associated with the risk of metachronous recurrence. Metachronous esophageal SCC develops in patients treated with ESD for SESCC. A risk assessment is important for surveillance before and after ESD for SESCC. Number of LVLs and tumor edge type are associated with an increased risk of metachronous cancer in SESCC. Patients will benefit from careful endoscopic surveillance when endoscopists pay attention to these tumor characteristics.
Introduction
Esophageal cancer is the sixth most common cause of cancer-related deaths worldwide [1]. Squamous cell carcinoma (SCC) is the major type of esophageal cancer in parts of Asia. Early stage superficial esophageal squamous cell carcinoma (SESCC) is more frequently detected with the development of techniques for endoscopic diagnosis [2, 3].
Endoscopic resection is a potentially curative treatment with minimal invasiveness for SESCC, and many studies demonstrated its favorable long-term outcomes [4]. However, it leaves a larger area of esophageal mucosa than does surgery, and metachronous SCC can occur in the preserved esophageal mucosa after endoscopic resection for SESCC [5]. To determine the possibility of a recurrence of metachronous SCCs after initial endoscopic resection, careful surveillance is needed.
A few Japanese studies investigated the incidence of and risk factors for metachronous SCC following endoscopic treatment for SESCC. The reported incidence is 12–35% [5, 6]. Male sex, alcohol consumption, smoking, multiple Lugol-voiding lesions (LVLs), and single nucleotide polymorphisms in aldehyde dehydrogenase-2 and alcohol dehydrogenase 1B were associated with metachronous recurrence [7, 8]. However, the involved sample sizes were rather small and the associations between endoscopic findings and recurrence risk have not been well investigated. Thus, this study aimed to assess the incidence of metachronous recurrence following endoscopic submucosal dissection (ESD) for SESCC and identify novel related endoscopic features using a large cohort.
Methods
Patients
Three hundred fifty-seven consecutive patients who underwent ESD for esophageal cancer at Samsung Medical Center, Seoul, Korea, were enrolled in this retrospective cohort study from April 2007 to May 2018. We excluded 33 patients diagnosed with non-SCC based on ESD specimens, 62 patients with further treatment after endoscopic resection (esophageal surgery, radiation therapy, chemoradiotherapy), and 9 patients without follow-up. Therefore, a total of 253 patients with ESCC were enrolled (Fig 1). We defined curative resection as when cancer was confined to the mucosal layer, had no lymphovascular invasion, and no resection margin positivity. Among the 253 paients of the study, 41 had noncurative resection due to lymphovascular invasion (n = 11), SM invasion (n = 40), and both (n = 10). 212 (84%) achieved curative resection, however, en-bloc and complete resection was achieved in all patients (n = 253).
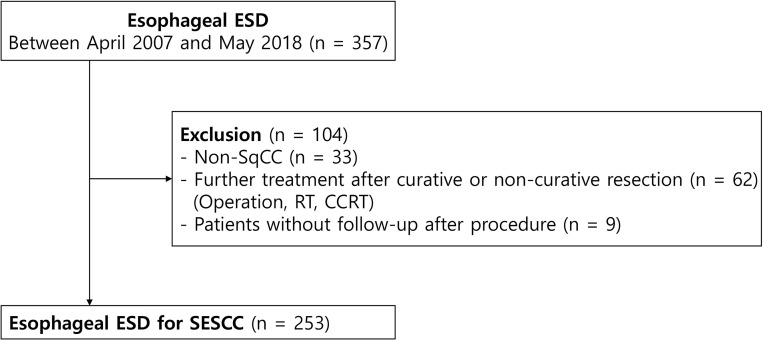
Fig 1. Flow diagram of the study.
ESD, endoscopic submucosal dissection; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; SESCC, superficial esophageal squamous cell carcinoma; SCC, squamous cell carcinoma
Endoscopic procedure and follow-up
Patients enrolled in this study have no history of previous esophageal ESD. Prior to the 1st ESD, all patients underwent an endoscopic evaluation including chromoendoscopy with narrow-band imaging (NBI) and the Lugol’s dye spray method. NBI can avoid discomfort in patients, such as pain caused by esophageal mucosal damage or severe allergic reactions that may occur after iodine staining. In addition, it is possible to observe the detailed vascular structure of the tumor surface with magnifying endoscopy with NBI, and through this, the intraepithelial papillary capillary loop pattern classification can be used to predict the invasion depth of superficial esophageal cancer [9]. In Lugol’s dye spray method, approximately 10mL of a 1% Lugol iodine solution was sprayed over the entire esophageal mucosa with a catheter after a conventional endoscopic examination. As this iodine-based absorptive staining has an affinity for glycogen in non-keratinized squamous epithelium, it is useful for the identification of squamous neoplasms [10]. Iodine staining is a useful method for diagnosing early esophageal cancer in the high-risk group with esophageal cancer and microscopic mucosal changes. It also has the advantage of being able to determine the exact border of the lesion, which is a great help in determining the extent of resection. In addition, a computed tomography (CT) scan of the chest and/or positron emission tomography-CT (PET-CT) were performed to identify possible distant or lymph node metastases. Esophageal ESD was performed using a standard technique as described elsewhere [11, 12]. In the beginning, the endoscopist marked 2–3 mm away from the edge of the cancer, which is well determined by Lugol’s iodine chromoendoscopy. After the submucosal injection, circumferential mucosal pre-cutting was performed. The submucosal layer under the lesion was dissected using various types of ESD knives after elevation of the lesion by the submucosal injection.
For follow-up, upper endoscopy was performed at 2 months after ESD to exclude the presence of any residual tumor. Endoscopy and chest and abdomen CT scans were then performed every 6 months for the first 3 years and then annually until the fifth year after ESD.
Data collection and definitions
We used a prospectively collected database of patients who underwent esophageal ESD. These data included demographic parameters (e.g. patient age, sex, body mass index, smoking and alcohol status, and past medical history), tumor characteristics (e.g. endoscopic tumor morphology; LVLs; margin of main LVL; tumor location, circumference, size, and pathology; differentiation, depth and lymphovascular invasion). The study was approved and the need for informed consent was waived by the institutional review board of Samsung Medical Center (IRB #: SMC 2020-05-114-001).
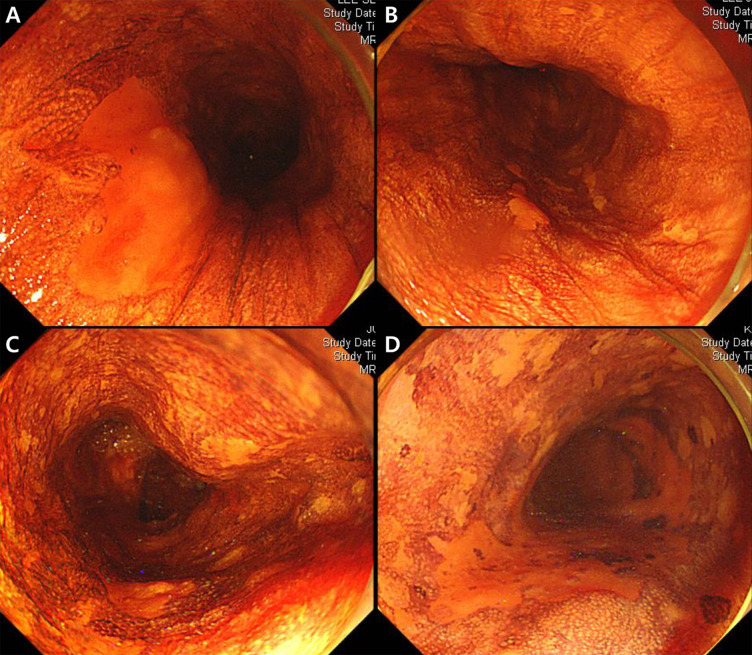
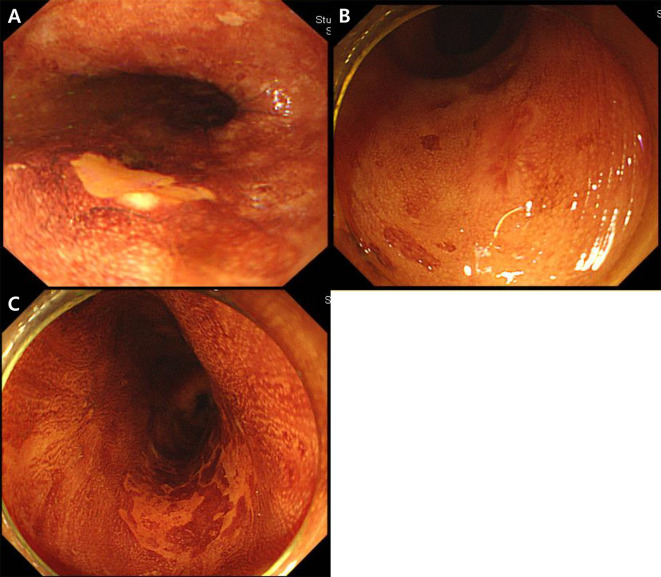
Metachronous recurrence was defined as histologically proven SESCC at another site of the ESD scar after ESD. Patients were divided into 4 groups based on the number and multiform patterns of LVLs in the background esophageal mucosa as follows: A, no LVL; B, several (≤10) small LVLs; C, many (>10) LVLs; D, multiple (>10) irregularly shaped LVLs [13, 14]. Based on the margin of the main LVL, patients were also divided into 3 groups as follows: a, clear; b, unclear; and c, spiculated (Figs 2 and 3).
Fig 2. Endoscopic images of Lugol chromoendoscopy.
(A) No Lugol voiding lesions (LVLs); (B) several (≤10) small LVLs; (C) many (>10) LVLs; (D) many (>10) irregularly shaped multiform LVLs around the main lesion.
Fig 3. Margin of main Lugol-voiding lesion (LVL).
(A) clear margin of the main LVL; (B) unclear margin of the main LVL; (C) spiculated margin of the main LVL.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) or number (%). We performed univariate analysis using the Wald chi-squared test, Cox’s proportional hazards model, and the Kaplan-Meier method for metachronous recurrence. Multivariate analysis was performed with selection of variables with at least p < 0.2 on univariate analysis. p values less than 5% were considered statistically significant. All statistical analyses were executed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Clinicopathologic characteristics
The clinicopathological characteristics of the patients with versus without metachronous recurrence of SESCC after ESD are shown in Table 1. A total of 21 (8.3%) patients experienced metachronous recurrence. Among these patients, the mean age was 66.2 ± 8.2 years; 19 (90.5%) patients were men. Endoscopic findings of 4 (19.0%), 9 (42.9%), 3 (14.3%), and 5 (23.8%) patients with metachronous recurrence represented no LVLs, several (≤10) small LVLs, many (>10) LVLs, and many (>10) irregularly shaped multiform LVLs around the main lesion, respectively. The mean tumor size of the resected specimen was 1.5 ± 0.8 cm. Most tumors (61.9%) had moderately differentiated tumors and negative lymphovascular invasion (90.5%). Regarding the margin of the main LVL, 2 (9.5%), 5 (23.7%), and 14 (66.7%) lesions were classified as clear, unclear, and spiculated, respectively.
Table 1. Baseline characteristics of patients with versus without metachronous recurrence.
| Characteristics | Metachronous recurrence | p-value | |
|---|---|---|---|
| No (n = 232) | Yes (n = 21) | ||
| Age (years) | 65.0 ± 8.0 | 66.2 ± 8.2 | 0.28 |
| Sex (male) | 219 (94.4) | 19 (90.5) | 0.66 |
| BMI (kg/m2) | 23.6 ± 2.9 | 23.5 ± 3.9 | 0.69 |
| Smoking | 0.89 | ||
| Current smoker | 36 (15.5) | 4 (19.0) | |
| Ex-smoker | 136 (58.6) | 10 (47.6) | |
| Never-smoker | 60 (25.9) | 7 (33.3) | |
| Quit smoking after procedure | 161 (69.4) | 13 (61.9) | 0.87 |
| Heavy alcohol intake (>4 days/week) | 29 (12.5) | 4 (19.0) | 0.27 |
| Alcohol intake after procedure | 0.67 | ||
| None | 165 (71.1) | 16 (76.2) | |
| Decreased before procedure | 34 (14.7) | 2 (9.5) | |
| Did not decrease before procedure | 33 (14.2) | 3 (14.3) | |
| DM | 45 (19.4) | 3 (14.3) | 0.81 |
| HTN | 81 (34.9) | 5 (23.8) | 0.72 |
|
Lugol-voiding lesion (around the main lesion) |
0.04 | ||
| No LVLs | 92 (39.7) | 4 (19.0) | |
| Several (≤10) small LVLs | 82 (35.3) | 9 (42.9) | |
| Many (>10) LVLs | 38 (16.4) | 3 (14.3) | |
| Many (>10) irregularly-shaped multiform LVLs |
20 (8.6) | 5 (23.8) | |
| Tumor location | 0.95 | ||
| Upper thoracic | 24 (10.3) | 1 (4.8) | |
| Middle thoracic | 62 (26.7) | 7 (33.3) | |
| Lower thoracic | 143 (61.6) | 13 (61.9) | |
| EG junction | 3 (1.3) | 0 (0.0) | |
| Tumor size | 1.7 ± 1.1 | 1.5 ± 0.8 | 0.86 |
| Tumor depth | 0.77 | ||
| Intraepithelial, M1 | 40 (17.2) | 4 (19.0) | |
| LP, M2 | 97 (41.8) | 7 (33.3) | |
| MM, M3 | 58 (25.0) | 7 (33.3) | |
| SM1 (<200 μm) | 5 (2.2) | 0 (0.0) | |
| SM2, 3 | 32 (13.8) | 3 (14.3) | |
| Tumor differentiation | <0.0001 | ||
| Well | 47 (20.3) | 6 (28.6) | |
| Moderate | 185 (79.7) | 13 (61.9) | |
| Poor | 0 (0.0) | 2 (9.5) | |
| Lymphovascular invasion | 9 (3.9) | 2 (9.5) | 0.16 |
| Margin of main Lugol-voiding lesion | 0.04 | ||
| Clear | 100 (43.1) | 2 (9.5) | |
| Unclear | 32 (13.79) | 5 (23.8) | |
| Spiculated | 100 (43.1) | 14 (66.7) | |
| Tumor circumference | 0.67 | ||
| <1/4 | 67 (28.9) | 5 (23.8) | |
| 1/4-2/4 | 137 (59.1) | 16 (79.2) | |
| 2/4-3/4 | 24 (10.3) | 0 (0.0) | |
| ≥3/4 | 4 (1.7) | 0 (0.0) | |
Univariate and multivariate analysis of metachronous recurrence
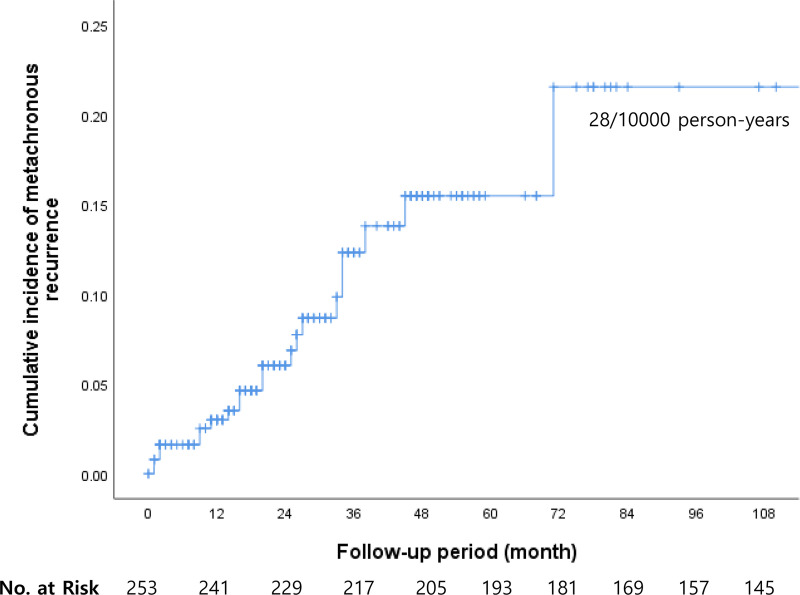
The cumulative incidence of metachronous recurrence was 8.3% of the patients allocated for surveillance. The incidence was 28/10000 person-years (Fig 4).
Fig 4. Metachronous recurrence after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma occurred in 8.3% of the patients allocated to surveillance (incidence, 28/10000 person-years).
The univariate analysis of the risk factors of metachronous recurrence after endoscopic resection is shown in Table 2. The metachronous recurrence rate was higher in patients with many (>10) irregularly shaped multiform LVLs around the main lesion (p = 0.04), poor tumor differentiation (p < 0.0001), and a spiculated margin of the main LVL (p = 0.04).
Table 2. Univariate analysis of the risk factors for metachronous recurrence after endoscopic resection.
| Variable | Univariate analysis | |
|---|---|---|
| Hazard Ratio (95% CI) | p value | |
| Age (years) | 1.03 (0.98–1.09) | 0.28 |
| Male sex | 1.39 (0.32–6.02) | 0.66 |
| BMI (kg/m2) | 0.97 (0.83–1.13) | 0.69 |
| Smoking | 0.89 | |
| Current smoker | 1 | |
| Ex-smoker | 0.80 (0.25–2.60) | |
| Never smoker | 1.00 (0.29–3.44) | |
| Quit smoking after procedure | 0.84 (0.11–6.46) | 0.87 |
| Heavy alcohol intake (>4 days/week) | 1.75 (0.64–4.79) | 0.27 |
| Alcohol intake after procedure | 0.67 | |
| None | 1 | |
| Decreased before the procedure | 0.53 (0.12–2.30) | |
| Did not decrease before procedure | 0.79 (0.23–2.72) | |
| DM | 0.86 (0.25–2.94) | 0.81 |
| HTN | 0.83 (0.30–2.30) | 0.72 |
| Lugol-voiding lesion (around the main lesion) | 0.04 | |
| No LVLs | 1 | |
| Several (≤10) small LVLs | 3.46 (1.06–11.29) | |
| Many (>10) LVLs | 3.19 (0.70–14.44) | |
| Many (>10) irregular-shaped multiform LVLs | 6.76 (1.80–25.48) | |
| Tumor location | 0.95 | |
| Upper thoracic | 1 | |
| Middle thoracic | 1.78 (0.28–11.19) | |
| Lower thoracic | 1.49 (0.25–8.72) | |
| EG junction | 2.20 (0.08–64.25) | |
| Tumor size | 0.96 (0.59–1.56) | 0.86 |
| Tumor depth | 0.77 | |
| Intraepithelial, M1 | 1 | |
| LP, M2 | 0.72 (0.21–2.50) | |
| MM, M3 | 1.25 (0.36–4.31) | |
| SM1 (<200 μm) | 1.04 (0.05–23.27) | |
| SM2, 3 | 1.69 (0.37–7.71) | |
| Tumor differentiation | <0.0001 | |
| Well | 1 | |
| Moderate | 0.60 (0.23–1.60) | |
| Poor | 19.19 (3.61–102.01) | |
| Lymphovascular invasion | 2.85 (0.66–12.31) | 0.16 |
| Margin of main Lugol-voiding lesion | 0.04 | |
| Clear | 1 | |
| Unclear | 6.73 (1.31–34.71) | |
| Spiculated | 6.62 (1.50–29.17) | |
| Tumor circumference | 0.67 | |
| <1/4 | 1 | |
| 1/4-2/4 | 1.30 (0.47–3.58) | |
| 2/4-3/4 | 0.37 (0.02–7.90) | |
| ≥3/4 | 4.24 (0.18–100.52) | |
The multivariate analysis was performed for variables with values of P less than 0.2, resulting in an increased metachronous recurrence rate for patients with many (>10) LVLs and an unclear or spiculated margin of the main LVL (hazard ratio [HR], 6.32; 95% confidence interval [CI], 1.62–24.72; p = 0.047 and HR, 6.51; 95% CI, 1.44–29.42; p = 0.032, respectively; Table 3 and Fig 5).
Table 3. Multivariable analysis of risk factors for metachronous recurrence after endoscopic resection.
| Variable | Multivariate analysis | |
|---|---|---|
| Hazard ratio (95% CI) | p value | |
| Lugol-voiding lesion (around the main lesion) | 0.047 | |
| No LVLs | 1 | |
| Several (≤10) small LVLs | 4.39(1.31–14.75) | |
| Many (>10) LVLs | 6.32(1.62–24.72) | |
| Margin of main Lugol-voiding lesion | 0.032 | |
| Clear | 1 | |
| Unclear + spiculated | 6.51(1.44–29.42) | |
| Lymphovascular invasion | 4.33(0.94–19.89) | 0.059 |
Fig 5.
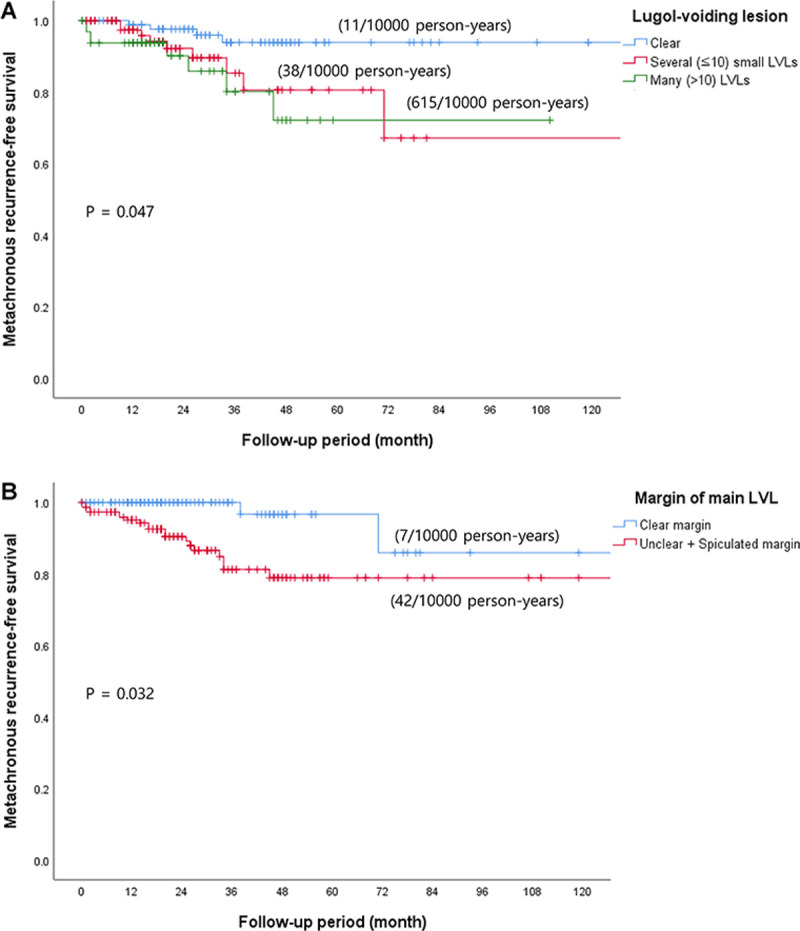
Metachronous recurrence-free survival in patients who underwent endoscopic resection for (A) Lugol-voiding lesion (LVL) and (B) Margin of the main LVL.
Discussion
Esophageal ESD has become an accepted minimally invasive treatment for SESCC. However, it leaves a larger area of esophageal mucosa than does surgery, and metachronous SCCs can occur in the preserved esophageal mucosa after endoscopic resection for SESCC [5]. Metachronous recurrence of SESCC after esophageal ESD occurred in 8.3% of the patients in our study versus 2–14% of patients in previous studies [5, 15]. There are several independent risk factors associated with recurrence, including male sex, low BMI, alcohol consumption, smoking, multiple LVLs, single nucleotide polymorphisms in aldehyde dehydrogenase-2 and alcohol dehydrogenase 1B, and treatment history of (sub)circumferential ESD [7, 8, 16]. This study also showed that morphological features of tumors such as number of LVLs and tumor edge type are associated with an increased risk of metachronous cancer in SESCC. Therefore, to determine the possibility of metachronous SCC recurrence after the initial endoscopic resection, careful surveillance is needed, especially in patients with the above mentioned risk factors.
The endoscopic findings by Lugol chromoendoscopy are useful for the detection of early-stage esophageal cancer and the risk estimation of metachronous recurrence. Previous studies demonstrated that the presence of many irregularly shaped multiform LVLs is associated with both synchronous and metachronous SESCC in patients with head and neck squamous cell carcinoma [17]. In this background, Lugol chromoendoscopy was performed before esophageal ESD to measure the procedure extent and determine the lesion appearance. In this study, we reviewed and confirmed the tumor edge type and number of LVLs around the main lesion using Lugol chromoendoscopy. Our findings demonstrated that the risk can be predicted not only by the number of LVLs but also by tumor edge type, so it is necessary to further distinguish the morphological features of the main lesion.
Shimizu et al. [5] reported that the metachronous recurrence rate of esophageal cancer was higher in patients in whom the Lugol voiding pattern of the background mucosa was scattered with a large number of patterns as opposed to the uniform type. The areas unstained by Lugol solution are histologically inflammatory lesions or various degrees of epithelial dysplastic lesions [18, 19]. Previous studies have suggested a correlation between dysplasia and carcinoma and that dysplasia is a precursor lesion of carcinoma [20]. This study assumed that these atypical epithelia were involved in the development of carcinoma during the course of field cancerization. Therefore, we assessed the number of LVL around the cancer as a risk factor for metachronous recurrence in the present study. As a result, we noticed that the number of LVL is an independent risk factor for recurrence in a multivariable analysis.
This study has some limitations. First, it was a single-center retrospective study. Because of its small cohort, it was difficult to perform a detailed subgroup analysis and test small differences with sufficient power. Second, smoking and alcohol habits did not correlate with the recurrence of SESCC in this study, although previous studies reported smoking, alcohol consumption, and dietary habits predicted the development of metachronous SESCC after endoscopic resection [21]. This may have been difficult to verify objectively because the patients were asked directly. Finally, in reviewing endoscopic feature such as LVLs, intra-observer variation may exist. Despite these limitations, this study distinguished the morphological features of tumors that can be identified by endoscopists.
In conclusion, risk assessment is important for the surveillance of the development of metachronous SESCC before and after endoscopic resection of SESCC. This study suggests that the number of LVLs and tumor edge type are associated with an increased risk of metachronous cancer in SESCC. Patients will benefit from careful endoscopic surveillance when endoscopists pay attention to these tumor characteristics.
Supporting information
(XLSX)
Data Availability
All relevant data are within the Supporting Information files.
Funding Statement
This paper was supported by a grant from The National R & D Program for Cancer Control, Ministry of Health & Welfare, Korea (1720180).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Shimizu Y, Takahashi M, Yoshida T, Ono S, Mabe K, Kato M, et al. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for superficial esophageal squamous cell carcinoma: current status of various techniques. Dig Endosc. 2013; 25 Suppl 1:13–9. 10.1111/j.1443-1661.2012.01408.x [DOI] [PubMed] [Google Scholar]
- 3.Fujiyoshi T, Tajika M, Tanaka T, Ishihara M, Mizuno N, Hara K, et al. Comparative evaluation of new and conventional classifications of magnifying endoscopy with narrow band imaging for invasion depth of superficial esophageal squamous cell carcinoma. Dis Esophagus. 2017; 30:1–8. 10.1093/dote/dox037 [DOI] [PubMed] [Google Scholar]
- 4.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009; 70:860–6. 10.1016/j.gie.2009.04.044 [DOI] [PubMed] [Google Scholar]
- 5.Shimizu Y, Tukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointest Endosc. 2001; 54:190–4. 10.1067/mge.2001.116877 [DOI] [PubMed] [Google Scholar]
- 6.Katada C, Muto M, Tanabe S, Higuchi K, Sasaki T, Azuma M, et al. Surveillance after endoscopic mucosal resection or endoscopic submucosal dissection for esophageal squamous cell carcinoma. Dig Endosc. 2013; 25 Suppl 1:39–43. 10.1111/j.1443-1661.2012.01407.x [DOI] [PubMed] [Google Scholar]
- 7.Sawada G, Niida A, Uchi R, Hirata H, Shimamura T, Suzuki Y, et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology. 2016; 150:1171–82. 10.1053/j.gastro.2016.01.035 [DOI] [PubMed] [Google Scholar]
- 8.Urabe Y, Kagemoto K, Nakamura K, Mizumoto T, Sanomura Y, Oka S, et al. Construction of a risk model for the development of metachronous squamous cell carcinoma after endoscopic resection of esopahageal squamous cell carcinoma. Esophagus. 2019; 16:141–6. 10.1007/s10388-018-0643-7 [DOI] [PubMed] [Google Scholar]
- 9.Ebi M, Shimura T, Yamada T, Mizushima T, Itoh K, Tsukamoto H et al. Multicenter, prospective trial of white-light imaging alone versus white-light imaging followed by magnifying endoscopy with narrow-band imaging for the real-time imaging and diagnosis of invasion depth in superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2015;81:1355–61. 10.1016/j.gie.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 10.ASGE Technology Committee, Wong Kee Song LM, Adler DG, Chand B, Conway JD, Croffie JMB, et al. Chromoendoscopy. Gastrointest Endosc. 2007;66:639–49. 10.1016/j.gie.2007.05.029 [DOI] [PubMed] [Google Scholar]
- 11.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006; 4:688–94. 10.1016/j.cgh.2006.03.024 [DOI] [PubMed] [Google Scholar]
- 12.Fujishiro M, Kodashima S, Goto O, Ono S, Niimi K, Yamamichi N, et al. Endoscopic submucosal dissection for esophageal squamous cell neoplasms. Dig Endosc. 2009; 21:109–15. 10.1111/j.1443-1661.2009.00837.x [DOI] [PubMed] [Google Scholar]
- 13.Muto M, Hironaka S, Nakane M, Boku N, Ohtsu A, Yoshida S. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002; 56:517–21. 10.1067/mge.2002.128104 [DOI] [PubMed] [Google Scholar]
- 14.Uno K, Koike T, Kusaka G, Takahashi Y, Ara N, Shimosegawa T. Risk of metachronous recurrence after endoscopic submucosal dissection of esophageal squamous cell carcinoma. Dis Esophagus. 2017; 30:1–8. 10.1093/dote/dox005 [DOI] [PubMed] [Google Scholar]
- 15.Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol. 2013; 19:1424–37. 10.3748/wjg.v19.i9.1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahmann PH, Pandeya N, Webb PM, Green AC, Whiteman DC, Australian Cancer S. Body mass index, long-term weight change, and esophageal squamous cell carcinoma: is the inverse association modified by smoking status? Cancer. 2012; 118:1901–9. 10.1002/cncr.26455 [DOI] [PubMed] [Google Scholar]
- 17.Muto M, Hitomi Y, Ohtsu A, Ebihara S, Yoshida S, Esumi H. Association of aldehyde dehydrogenase 2 gene polymorphism with multiple oesophageal dysplasia in head and neck cancer patients. Gut. 2000; 47:256–61. 10.1136/gut.47.2.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitamura K, Kuwano H, Yasuda M, Sonoda K, Sumiyoshi K, Tsutsui S, et al. What is the earliest malignant lesion in the esophagus? Cancer. 1996; 77:1614–9. [DOI] [PubMed] [Google Scholar]
- 19.Ohmori T, Makuuchi H, Kumagai Y. Natural course of iodine unstained area in the esophagus in mass-screening programs [in Japanese with English abstract]. Stomach Intestine. 1994; 29:911–9. [Google Scholar]
- 20.Sugimachi K, Sumiyoshi K, Nozoe T, Yasuda M, Watanabe M, Kitamura K, et al. Carcinogenesis and histogenesis of esophageal carcinoma. Cancer. 1995; 75:1440–5. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama A, Katada C, Yokoyama T, Yano T, Kaneko K, Oda I, et al. Alcohol abstinence and risk assessment for second esophageal cancer in Japanese men after mucosectomy for early esophageal cancer. PLoS One. 2017; 12:e0175182 10.1371/journal.pone.0175182 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the Supporting Information files.