Abstract
In humans, all but 1% of monosomy 45.X embryos die in utero and those who reach term suffer from congenital abnormalities and infertility termed Turner’s syndrome (TS). By contrast, XO female mice on various genetic backgrounds show much milder physical defects and normal fertility, diminishing their value as an animal model for studying the infertility of TS patients. In this article, we report that XO mice on the C57BL/6J (B6) genetic background showed early oocyte loss, infertility or subfertility and high embryonic lethality, suggesting that the effect of monosomy X in the female germline may be shared between mice and humans. First, we generated XO mice on either a mixed N2(C3H.B6) or B6 genetic background and compared the number of oocytes in neonatal ovaries; N2.XO females retained 45% of the number of oocytes in N2.XX females, whereas B6.XO females retained only 15% of that in B6.XX females. Second, while N2.XO females were as fertile as N2.XX females, both the frequency of delivery and the total number of pups delivered by B6.XO females were significantly lower than those by B6.XX females. Third, after mating with B6 males, both N2.XO and B6.XO females rarely produced XO pups carrying paternal X chromosomes, although a larger percentage of embryos was found to be XO before implantation. Furthermore, B6.XO females delivered 20% XO pups among female progeny after mating with C3H males. We conclude that the impact of monosomy X on female mouse fertility depends on the genetic background.
Keywords: Turner’s syndrome, monosomy X, XO female mouse, oocyte reserve, female infertility, embryo development, Patchy fur mutation
Introduction
The sex of an individual is determined by the combination of sex chromosomes, XX or XY, at conception in most mammalian species. To adjust the X-linked gene dosage, one of the two X chromosomes in the XX female is subject to random inactivation. Nonetheless, the absence of one X chromosome in the female leads to severe defects in humans; less than 1% of monosomy 45.X (XO) embryos survive in utero, and those who do reach term suffer from congenital abnormalities, such as short stature and webbed neck, collectively termed Turner’s syndrome (TS) (Turner, 1938; Singh and Carr, 1966; Ogata and Matsuo, 1995; Hook and Warburton, 2014). The 45.X genotype accounts for 3% of all females conceived, 15% of spontaneous abortions and 1 in 1500–2500 female live births (Saenger, 1996; Sybert and McCauley, 2004). The severity of TS syndrome has a wide spectrum since 45.X/46.XX mosaicism is common, and only 18% of the cases detected in live births are putative non-mosaic 45.X (Jacobs et al., 1997). The cause for the early death of the 45.X conceptus can be largely attributed to vascular abnormalities leading to impaired fetal-placental circulation (Urbach and Benvenisty, 2009). By contrast, XO female mice show much milder physical defects. Most XO embryos survive to term and show no gross anomalies except for lower body weights than their XX littermates (Cattanach, 1962; Burgoyne et al., 1983). These striking somatic differences between the two species can be attributed to the greater number of genes that escape from X chromosome inactivation in humans. The number of ‘escapees’ has been estimated to be about 15% of X-linked genes in humans and 3% in mice (Fisher et al., 1990; Carrel and Willard, 2005; Berletch et al., 2010; Yang et al., 2010; Berletch et al., 2015; Tukiainen et al., 2017). Human embryos may not tolerate the haplodeficiency of X-linked genes that are normally expressed from two copies.
Non-mosaic TS patients are infertile due to a lack of oocytes in streak gonads (Modi et al., 2003; Reynaud et al., 2004; Peek et al., 2019). The oocytes may have been present but are lost during the early oogenesis in fetal life (Carr et al., 1968; Speed, 1988). Elucidating the mechanism by which XO oocytes are eliminated is critical for understanding the cause of infertility and for counseling TS patients (Oktay and Bedoschi, 2019). Animal models are desirable for such studies. However, XO female mice are usually fertile, diminishing their relevance to studying infertility of TS patients. In XO mice, unlike humans, the majority of germ cells go through the meiotic prophase I (MPI) and contribute to follicle formation at or soon after birth (Speed, 1986). It has been reported that the oocyte population in the XO mouse declines to half of that in the XX mouse perinatally, and this smaller ovarian reserve reflects in a shorter reproductive life span as oocytes are consumed with age (Burgoyne and Baker, 1981). Nonetheless, XO female mice can be fertile beyond 6 months of age, which corresponds to 30 years in women (Burgoyne and Baker, 1981, our unpublished data). This difference in fertility between the two species cannot be explained by X-inactivation escapees because the inactive X chromosome becomes reactivated in the XX female germline prior to the onset of meiosis, and the two X chromosomes remain active until the end of oocyte growth in both humans and mice (Mangia et al., 1975; Sugimoto and Abe, 2007; Chuva De Sousa Lopes et al., 2008). Expression of the large non-coding RNA X inactive specific transcript (Xist) in cis is essential for the initiation of X inactivation in XX somatic cells as well as primordial germ cells while it is repressed in oocytes (McCarrey and Dilworth, 1992; Fukuda et al., 2015). Therefore, the half dosage of X-linked gene products should exert similar effects on oocyte development in humans and mice.
Several genetic mouse models are available for generating XO females (Cattanach, 1962; Burgoyne et al., 1983; Lane and Davisson, 1990; Ashworth et al., 1991; Eicher et al., 1991; Burgoyne and Evans, 2000). A mutation or chromosomal rearrangement on the X chromosome causes XY non-disjunction during spermatogenesis, and the sperm lacking sex chromosomes sire XO daughters carrying maternal X chromosomes. Once generated, the XO female produces the oocytes lacking X chromosomes and, consequently, XO daughters carrying paternal X chromosomes. In this article, we chose the Patchy fur (Paf) mutation on the X chromosome for generating XO females because of simplicity of maintenance and breeding scheme (Fig. 1). The Paf mutation, possibly involving inversion, occurs near the boundary of pseudoautosomal region (PAR) and causes a high incidence of XY non-disjunction (Lane and Davisson, 1990; Korobova et al., 1998; Burgoyne and Evans, 2000). This mutation was originally identified in the C3H/HeSnJ (C3H) strain and maintained on this genetic background in the Jackson Laboratory. To make the XO mouse comparable with various mutant mice, which are often on the C57BL/6J (B6) genetic background, we backcrossed the Paf mutation onto B6. While XPafY males maintained the hair-loss phenotype, XXPaf females lost the Patchy fur phenotype and became indistinguishable from XO females on B6. Our preliminary results indicated that the fourth backcross was sufficient to render the ovarian reserve significantly smaller than that in the first backcross (N2) generation. The objective of our current study was to compare the reproductive performance of XO females in the N2 and advanced backcross generations and assess whether the XO female mouse on the B6 genetic background shares some features of infertility in human TS patients.
Figure 1.
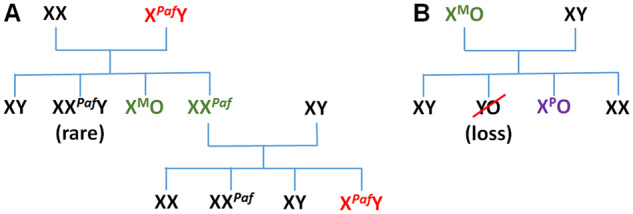
Mouse breeding scheme. (A) Cross between XX females and XPafY males generates XMO females carrying maternal X chromosomes and XXPaf females. Cross between XXPaf females and XY males produces XPafY males. By using B6 XX and XY mice for crosses, the genetic background gets closer to B6 at every generation (backcross). (B) Cross between XMO females and XY males generates XPO females carrying paternal X chromosomes. YO embryos die before implantation.
Materials and methods
Animals
All animal experiments were conducted in accordance with the Guide to the Care and Use of Experimental Animal issued by the Canadian Council on Animal Care and with the approval from the Animal Research Committee of McGill University. Paf breeding pairs on the C3H background were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The original C3H.XPafY males were crossed with B6 females (the Jackson Laboratory) to produce (B6.C3H)F1.XXPaf and XO females, which were identified by the presence or absence of the Xist transcript by RT-PCR (Fig. 1). F1.XXPaf females were crossed with B6 males to produce N2-XPafY males, which were identified by the hair-loss phenotype. The original C3H.XXPaf females were crossed with B6 males to produce (C3H.B6)F1.XPafY males, which were crossed with B6 females to produce N2-XXPaf and XO females. This manner of alternative backcross was continued to produce XPafY males, XXPaf females and XO females of up to the N8 generation. Although N12 is generally required before the genetic background is fully replaced, the frequency of XO female production by XPafY males diminished with backcross beyond N8.
Xist RT-PCR
Total RNA was extracted from each tail biopsy using TRIzol (Invitrogen, ThermoFisher Scientific, Saint-Laurent, QC, Canada) according to the manufacturer’s protocol, and subject to cDNA synthesis and PCR amplification of the Xist transcript, using the conditions and primers as previously given (Kay et al., 1994). Xist transcripts were detectable in XX females but not in XO females or XY males.
Immunofluorescence staining of wholemount ovaries
Ovaries were isolated from females at 4 days after birth, and fixed in a mixture of cold methanol:dimethylsulphoxide (4:1) and stored at −20°C overnight or longer. Meanwhile, a piece of tail was taken from each female for genotyping. Once identified, XXPaf and XO ovaries were subject to wholemount immunofluorescence (IF) staining as detailed by Faire et al. (2015) with modifications (Liu et al., 2019). In brief, ovaries were rehydrated in 1:1 methanol:phosphate buffered saline (PBS), washed in Holding Buffer (PBS containing 1% TritonX-100, 3% bovine serum albumin, 1% goat serum), and incubated with rat TRA98 antibody (1:100, B-Bridge #73-003, Tokyo, Japan) and rabbit anti-TAp63α antibody (1:100, Cell Signaling Technologies #4892, Danvers, MA, USA) overnight. Ovaries were then washed and incubated with goat anti-rat IgG conjugated with Alexa Fluor 488 (1:1000, Pierce, ThermoFisher Scientific), goat anti-rabbit IgG conjugated with rhodamine red X (1:1000, Jackson Immunoresearch Laboratories, West Grove, PA, USA) and 0.4 µg/ml DAPI (Roche Diagnostics, Mannheim, Germany) in the dark overnight. After washing, dehydrating in gradient concentrations of methanol, and clearing in 1:2 benzyl alcohol:benzyl benzoate overnight, the ovaries were stored at 4°C until imaging with a Zeiss 780 confocal microscope (Molecular Imaging Centre, MUHC). Representative z-stacks were acquired with the resolution parameters including 1024 × 1024, 16-bit, 16 line averaging and bidirectional acquisition. Numbers of TRA98-positive cells (green), TAp63α-positive cells (red) and the cells positive for both (yellow) were counted using the ‘Surfaces’ algorithm in IMARIS 8.2, and the total number of oocytes was estimated by subtracting the number of both-positive cells from the sum of either green or red cells.
Fertility test
XXPaf and XO females of N2, N7 and N8 generations at 2 months of age were individually caged with B6 males until pregnancy was confirmed. B6 males were replaced when females did not get pregnant within 2 weeks. The live pups were counted on the delivery day and left with their mothers for lactation up to 25 days. From XXPaf mothers, the numbers of wild-type male, XPafY male (based on the hair-loss phenotype), and female pups were recorded. From XO mothers, the genotype (XX or XO) of female pups was determined by Xist RT-PCR. After weaning, the females were caged with B6 males to repeat the above procedure until they reached 180 days of age. The females were euthanized at least 7 days after the last weaning, and their ovaries were fixed in 2% paraformaldehyde in the microtubule-stabilizing buffer (Messinger and Albertini, 1991) and embedded in paraffin. Histological sections from the central region of ovary were deparaffinized, subject to antigen retrieval as previously described (Taketo et al., 2005), IF-stained with rabbit anti-MSY2 antibody (1:500, AbCam #ab33164, Toronto, ON, Canada), followed by goat anti-rabbit IgG conjugated with FITC (1:1000, Jackson Immunoresearch Laboratories), and mounted in Prolong Antifade Mounting Medium containing DAPI (Molecular Probe, ThermoFisher Scientific). Fluorescent signals were examined and recorded under an epifluorescence microscope system (Leica DM6000B, Germany).
Collection and culture of embryos after natural mating
N6-XXPaf and XO females at 2 months of age were mated with B6 or C3H males and separated in the morning when copulation plugs were found. The contents of oviducts were flushed out with M2 medium on post-fertilization D1.5 and observed under a stereo microscope. The two-cell stage embryos were further cultured in droplets of synthetic oviductal medium enriched with potassium medium under mineral oil (both from Sigma-Aldrich Canada, Oakville, ON, Canada), and observed daily for 3 days.
FISH analysis of blastocyst-stage embryos
Blastocyst-stage embryos on the third day in culture (D1.5 + 3d) were rinsed in a washing buffer (PBS containing 3% bovine serum albumin) twice, transferred into 5 µl droplets of lysis buffer (0.01N HCl and 0.1% Tween-20 in water), and left to dry. After rinsing in PBS, followed by 70%, 95% and 100% ethanol, the slides were air-dried and stored at −20°C. The slides were processed for fluorescence in situ hybridization with the probes for chromosome X (XMPX) and Y (XMPY) (MetaSystems, Germany) according to the manufacturer’s protocol, and mounted with DAPI and examined as described above. The genotype of each embryo was determined by the presence of XX, XO or XY paints in at least 80% of blastomeres.
Statistical analysis
The numbers of oocytes in the neonatal ovaries of different genotypes and generations (n = 4∼5 females, at least two litters, each) were compared by Sidak’s multiple comparisons test and presented as means ± SEM. The age of conception was estimated from the delivery day by assuming 19.5 days in gestation, and grouped into three ranges, presented as means ± SD. Total numbers of pups delivered by XX and XO females of the same generation were compared by Student’s t-test. Pregnancy rates and numbers of pups/litter per female (n = 7∼12 each) were compared by Fisher’s exact test and two-way ANOVA (genotype and age) followed by Tukey’s multiple comparisons test, respectively. The ratios of progeny (live pups or embryos) with the two phenotypic sexes and/or genotypes (XX, XO) were compared by χ2-test. P-value <0.05 was considered to indicate statistical significance.
Results
Greater oocyte loss in the neonatal XO ovary with further backcross onto B6
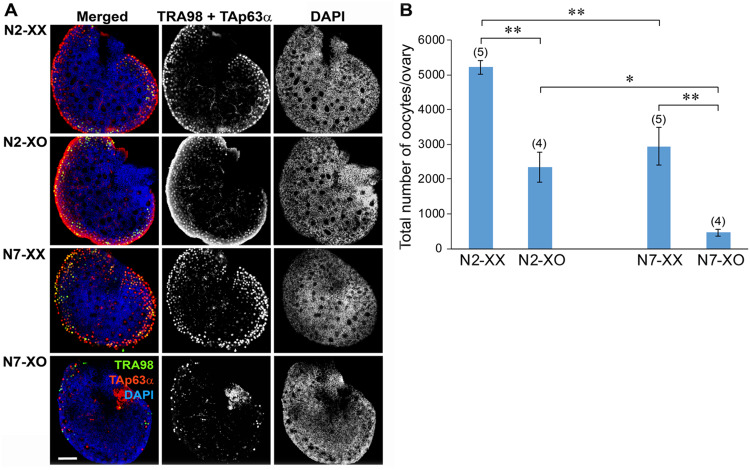
To examine the effects of genetic background on the ovarian reserve, which is largely established by 4 days after birth, we crossed wild-type B6.XX females with XPafY males in (C3H.B6)F1 and N6 generations to produce XX and XO females in N2 and N7 generations, respectively. We identified the oocytes in the wholemount ovaries by IF staining with TRA98 and anti-TAp63α antibodies (Fig. 2A). TRA98 is expressed in the oocyte nucleus through the MPI, but it becomes downregulated while TAp63α is upregulated when the oocyte reached the early-to-mid diplotene stage (Enders and May, 1994; Suh et al., 2006; Livera et al., 2008; Deutsch et al., 2011; Carmell et al., 2016). Therefore, IF staining of both markers was necessary to capture the entire oocyte population in neonatal ovaries (Liu et al., 2019). The ovaries of both XX and XO females in the N2 generation (N2-XX and N2-XO ovaries, respectively) and XX females in the N7 generation (N7-XX ovaries) appeared morphologically similar. TRA98-positive oocytes were concentrated in the peripheral region while TAp63α-positive oocytes were distributed over the ovary and formed follicles in the central region, in agreement with the wave of MPI progression from the central to peripheral region. In comparison, the ovaries of XO females in the N7 generation (N7-XO ovaries) were devoid of oocytes/follicles in the central region and harbored sparse oocytes with either TRA98 or TAp63α IF staining in the peripheral region.
Figure 2.
Number and distribution of oocytes in the XX and XO ovaries of N2 and N7 generations at 4 days after birth. (A) Immunofluorescence staining of wholemount ovaries. Merged images are followed by staining of germ cell markers (TRA98 and TAp63α) or nuclear staining with DAPI alone. Note the absence of oocytes in the central region and fewer oocytes in the peripheral region in the N7-XO ovary compared to other types of ovaries. (B) Total number of oocytes per ovary. Mean ± SEM. The number of ovaries examined is shown in parentheses above each column. * and ** indicate significant differences at P < 0.05 and 0.01, respectively, by Sidak’s multiple comparison test.
We estimated the total number of oocytes in each wholemount ovary (Fig. 2B) as described in Materials and methods. The number of oocytes in the N2-XO ovary was on average 45.0% of that in the N2-XX ovary, whereas the number of oocytes in the N7-XO ovary was only 15.6% of that in the N7-XX ovary. Since the number of oocytes in the N7-XX ovary was significantly smaller than that in the N2-XX ovary, we compared the oocyte populations in N2-XO versus N7-XO ovaries by Sidak’s multiple comparisons test. Significant differences were found in all comparisons, most importantly between N2-XO and N7-XO ovaries at P < 0.05. We conclude that the XO female on the B6 genetic background retains fewer oocytes, particularly in the central region, compared to the XO female on a mixed genetic background, by the time when the ovarian reserve is established.
Reduced fertility in the XO female on the B6 genetic background
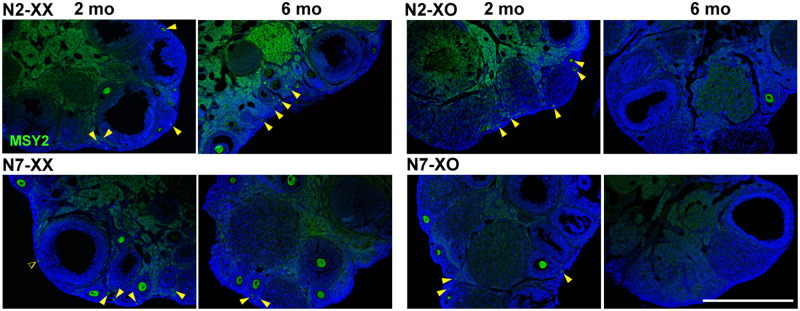
We next asked whether the smaller ovarian reserve in the N7-XO female was reflected in reproductive performance at puberty. We identified the oocytes in histological sections of ovaries at 2 months of age by IF staining of MSY2, which is detectable at the diplotene stage and beyond (Gu et al., 1998; Medvedev et al., 2008). We found that all XX and XO ovaries in N2 and N7 generations showed a similar distribution of primordial and growing follicles (Fig. 3).
Figure 3.
Oocytes in the XX and XO ovaries of N2 and N7 generations at 2 and 6 months (mo) of age. Oocytes in histological ovarian sections were immunofluorescence-stained for MSY2 (green) with DAPI counterstaining (blue). Arrowheads indicate oocytes in primordial follicles. Note the absence of oocytes in the N7-XO ovary at 6 months of age. The dull staining in the central region was caused by non-specific binding of the FITC-conjugated secondary antibody. Scale bar 600 µm.
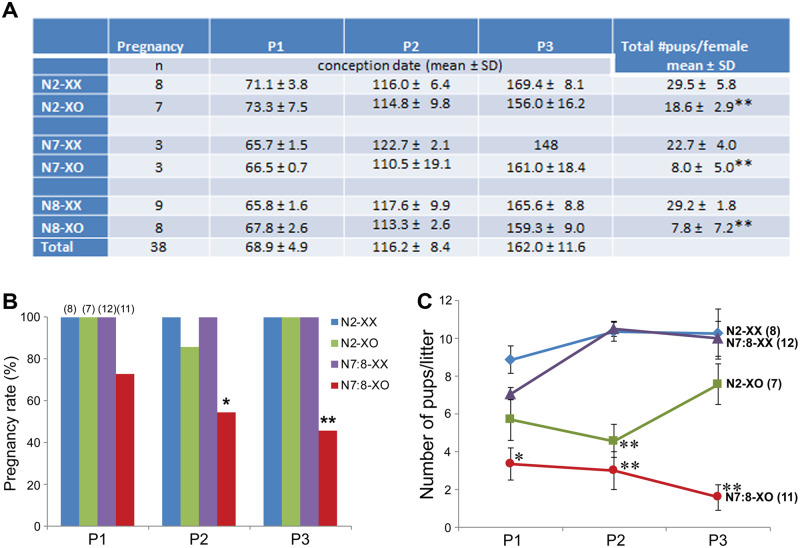
We then crossed XX and XO females in N2, N7 and N8 generations from 60 to 180 days of ages with wild-type B6 males, and examined pregnancy, delivery and sex/genotype of live pups. All XX females delivered three litters during this period. Because of the breeding scheme (see Materials and methods) and highly successful fertilization, the presumed conception dates fit into three narrow age ranges as given in the table (Fig. 4A), which were designated as P1, P2 and P3 for simplicity. All but one N2-XO females also delivered three litters each, comparable to N2-XX females. The exceptional N2-XO female apparently failed to get pregnant or carry pups during the second age range. By contrast, all but three of a total 11 N7- and N8-XO females delivered fewer than three litters and two delivered none. Therefore, we assigned their presumable conception dates into the closest age ranges defined by other females.
Figure 4.
Fertility of adult XX and XO females in N2 and N7-N8 generations. (A) Pregnancy age ranges and the total number of pups delivered by each female. **Significant difference from XX females of the same generation at P < 0.001 by Student’s t-test. (B) Percentage of females which became pregnant and delivered pups in three reproductive age ranges. * and ** indicate significant differences in N7:8-XO females compared to N7:8-XX females at P < 0.05 and 0.01, respectively, by Fisher’s exact test. (C) Number of pups per litter. Mean ± SEM. * and ** indicate significant differences from other females at P < 0.05 and 0.01, respectively, by two-way ANOVA (genotype and ages) followed by Tukey’s multiple comparisons test.
We compared the total number of live pups delivered by each female during the tested period as given in Fig. 4A. The numbers of pups (mean ± SD) delivered by N2-, N7- and N8-XX females were 29.5 ± 5.8 (n = 8), 22.7 ± 4.0 (n = 3) and 29.2 ± 1.8 (n = 9), respectively, comparable to each other. The number of pups delivered by each N2-XO female was 18.6 ± 2.9 (n = 7), significantly lower than that by an N2-XX female at P < 0.01 by t-test. The numbers of pups delivered by N7- and N8-XO females were 8.0 ± 5.0 and 7.8 ± 7.2, respectively, similar to each other and significantly lower than those by N7- and N8-XX females at P < 0.01. Since no difference was found between N7- and N8-XO females, the results were combined as N7:8-XO females for further analyses. As summarized in Fig. 4B, the pregnancy rates of N7:8-XO females were significantly lower than those by other types of females at P2 and P3 (P < 0.05 and 0.01, respectively, by Fisher’s exact test). There was a tendency toward declining pregnancy rate in N7:8-XO females with age, but the regression analysis did not indicate significant correlation.
We next compared the number of pups per litter delivered by each female within three age ranges (Fig. 4C). The numbers of pups delivered by N7:8-XX females were comparable with those by N2-XX females in all age ranges, suggesting that the shift of the genetic background to B6 had little impact on the fecundity of XX females. The numbers of pups delivered by N2-XO females were significantly lower than those delivered by N2-XX females at P2 (P < 0.01 by two-way ANOVA followed by Tukey’s multiple comparisons test). This was expected as all YO embryos die during preimplantation development although no significant difference was found at P1 or P3. The numbers of pups delivered by N7:8-XO females were the lowest and were significantly different from those delivered by N7:8-XX females at P1, P2 and P3 (P < 0.05, 0.01 and 0.01, respectively), or those by N2-XO females at P3 (P < 0.05).
After the fertility test was completed at 6 months of age, ovaries were examined histologically (Fig. 3). Oocytes in primordial and growing follicles were seen in N2-XX, N2-XO and N7-XX ovaries, but rarely in N7-XO ovaries. These results suggest that the smaller ovarian reserve in the XO female on the B6 genetic background reflected in infertility, premature fertility loss or subfertility.
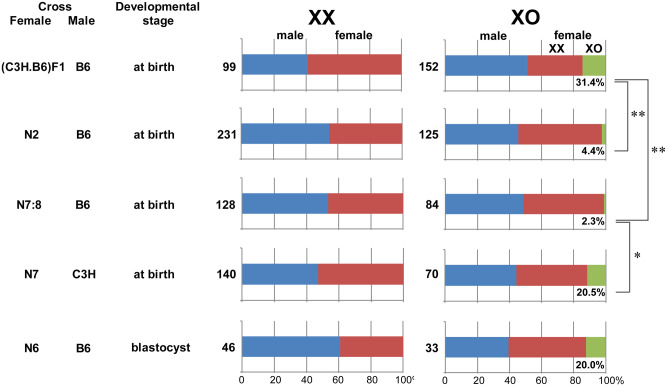
Deficit of XpO progeny from the XO female on the B6 genetic background
XO females were anticipated to produce nullisomy X oocytes and consequently XO daughters carrying single paternal X chromosomes (XpO) after mating with wild-type XY males (Fig. 1). However, XpO daughters were rarely delivered by either N2- or N7:8-XO females (4.4% and 2.3% of female pups, respectively) (Fig. 5). It has been reported that the survival of XpO embryos decreases on the B6 genetic background (Hunt, 1991). To confirm the contribution of the B6 genetic background to the deficit of XPO pups, we crossed wild-type C3H females with N7-XPafY males to produce semi-(C3H.B6)F1-XO females, which delivered a significantly larger population of XO daughters (31.4% of female pups, P < 0.01 by χ2-test) than N2- or N7:8-XO females. To determine whether the deficit of XPO pups delivered by N7:8-XO females can be attributed to the B6 genetic background of mothers or the B6 genetic background of XPO embryos, we crossed N7-XO females with C3H males. In total, 20.5% of female pups delivered were of the XO genotype. This ratio of XO female production was significantly larger than that delivered by N7:8-XO females after crossing with B6 males (P < 0.05 by χ2-test) and comparable to that delivered by (C3H.B6)F1 females. These results indicate that N7-XO females produced nullisomy X oocytes, which were fertilized and developed into healthy XpO pups on the (B6.C3H)F1 genetic background but not on the B6 genetic background. The sex ratios of pups delivered by XXPaf littermates are shown on the left for comparison in Fig. 5.
Figure 5.
Ratio of phenotypical (or XY) male, XX female and XO female progeny produced by XX and XO females crossed with B6 or C3H males. The number under each green bar indicates the percentage of XO females among female progeny. The number on the left of each bar indicates the total number of pups examined. * and ** indicate significant differences at P < 0.05 and 0.01, respectively, by χ2-test.
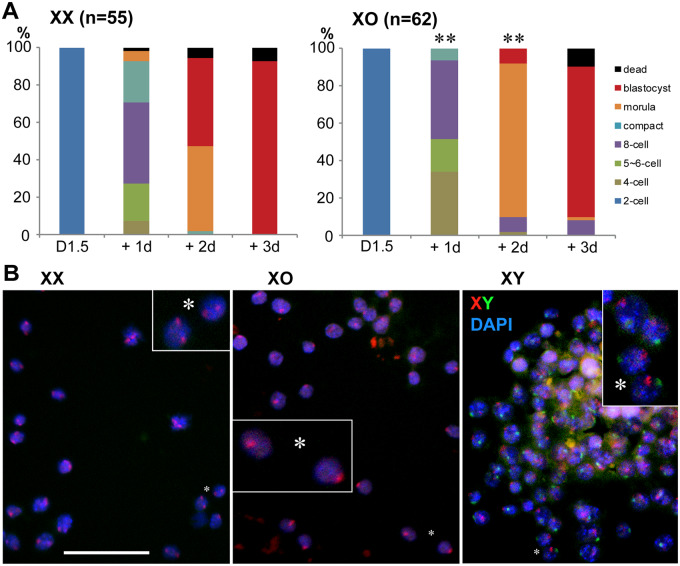
To delineate when XpO embryos on the B6 genetic background were lost during pregnancy, we collected two-cell-stage embryos from N6-XO and XX females after natural mating with B6 males and examined their preimplantation development in culture. The numbers of two-cell-stage embryos recovered at post-fertilization D1.5 were comparable between XX (7.0 ± 0.5, n = 12) and XO (6.0 ± 0.7, n = 12) mothers. As shown in Fig. 6A, embryos from XX females (n = 55) developed progressively in culture, and 93% reached the blastocyst stage at D1.5 + 3d. In comparison, development of embryos from XmO females (n = 62) was sluggish. After 1 day in culture, a larger percentage was found at the four-cell stage, while very few had reached the eight-cell stage with compaction. After 2 days in culture, 82% were still at the morula stage, while very few had reached the blastocyst stage. Both were significantly different at P < 0.01 by χ2-test. After 3 days in culture, however, 81% of embryos had reached the blastocyst stage, and no significant difference was found from XX mothers.
Figure 6.
Preimplantation development in culture of naturally fertilized oocytes from N6-XX and XO females. (A) Total numbers of zygotes at the two-cell stage, as shown in parentheses, were collected at D1.5 and cultured for 3 days. Development of embryos from XO females was delayed at D1.5 + 1 and +2 days in culture compared to those from XX females (P < 0.01 by χ2-test), but no difference was found at D1.5 + 3 days. (B) Genotyping of blastocyst embryos by FISH with X- and Y-chromosome-paint (red and green, respectively) with DAPI nuclear staining. * indicates the same position in the insert at a higher magnification (×4). Bar 50 µm.
We identified the sex chromosomes carried by the blastocyst-stage embryos after 3 days in culture using X and Y chromosome paint (Fig. 6B). Of 46 blastocyst-stage embryos recovered from XX mothers, 28 were XY and 18 were XX, not significantly different from the expected sex ratio (Fig. 5). Of 33 embryos recovered from XO mothers, 13 were XY, 16 were XX and 4 were XO (20% of female embryos) (Fig. 5). This ratio of XO embryos among female progeny was comparable to the live pups delivered by N7-XO females after mating with C3H males. We speculate that XpO embryos on the B6 genetic background were lost at or after implantation in utero.
Discussion
XO female mice on various genetic backgrounds are fertile, and therefore not considered to be a good animal model for understanding the infertility of human TS patients. Our current results demonstrate that the XO female mouse in advanced backcross generations to B6 (B6.XO) exhibits the features which may be relevant to human TS, i.e. early oocyte loss, subfertility and high lethality of XO embryos carrying paternal X chromosomes in utero. Further study in the B6.XO mouse would contribute to elucidating the influence of monosomy X in the female germline in mammalian species.
Our results showed that the B6.XO female retained fewer oocytes than the B6.XX female or the N2-XO female at 4 days after birth, when the ovarian reserve was largely established. The initial oocyte population at the onset of meiosis in the XX ovary declines during fetal and neonatal development in both humans and mice (Baker, 1963; McClellan et al., 2003; Malki et al., 2014; Tharp et al., 2020). In the mouse, the XO female loses additional oocytes during perinatal development to retain a half as many oocytes as the XX female in largely Schofield albino outbred strain (Burgoyne and Baker, 1985). Our current results showed that while the oocyte loss in the XO ovary (to 45% of the XX ovary) in the N2(C3H.B6) generation was comparable to the published data, the oocyte loss in the B6.XO ovary (to 16% of the XX ovary) in the N7 generation was much greater. One possible explanation for the greater loss of XO oocytes is the haplodeficiency of X-linked gene products. It has been reported that the ratio of X-linked versus autosomal gene transcript levels is lower in XO oocytes than in XX oocytes from the onset of meiosis (Sangrithi et al., 2017; Hamada et al., 2020). However, no difference was observed in the number of oocytes between B6.XO and XX ovaries until birth (Alton et al., 2008; Vaz and Taketo, unpublished data), suggesting that while the haplodeficiency of X-linked gene products has little effects on the oocyte survival in fetal life, the B6 genetic background facilitates the loss of XO oocytes during a short period after birth. This is the period when the oocytes complete homologous chromosome synapsis, enter the MPI arrest and form follicles. It has been postulated that the single X chromosome in the XO oocyte lacks homologous synapsis and triggers ‘meiotic silencing of unsynapsed chromatin’ (MSUC) (Burgoyne and Baker, 1981; Speed, 1986; Turner et al., 2005; Cloutier et al., 2015; Cloutier et al., 2016). Furthermore, silencing of X-linked genes is variable, and the XO oocyte may survive or die depending on the repertoire of silenced genes (Cloutier et al., 2016). We have previously reported that around 80% of prenatal B6.XO oocytes exhibit the single X chromosome marked by phosphorylation of histone variant H2AFX (γH2AFX), which is essential for MSUC (Alton et al., 2008; Cloutier et al., 2015). We speculate that the B6 genetic background enhanced MSUC, resulting in a greater loss of XO oocytes. However, it remains puzzling why the oocytes in the central region were preferentially eliminated in the B6.XO ovary. Alternatively, γH2AFX in the XO oocyte may have been recognized by the TAp63α-CHEK2 surveillance mechanism which eliminates the oocytes with DNA damage or persistent double strand breaks (Rinaldi et al., 2017). It is intriguing that TAp63α began to be expressed in the oocytes of the central region during the MPI progression. There may be a delicate timing of TAp63α expression and availability of the CHEK2 surveillance. Further study is needed to delineate the molecular mechanism of XO oocyte elimination in the neonatal ovary.
Our results also showed that very few B6.XO females had normal levels of fertility. Two out of 11 N7:8-XO females never delivered pups and two delivered only once while all XX females and all but one N2-XO females delivered three litters from 2 to 6 months of age. Furthermore, we found a tendency toward declining fertility in the B6.XO female with age and, in fact, very few oocytes remained in the B6.XO ovaries at 6 months of age. These results suggest that the smaller ovarian reserve in the neonatal B6.XO female led to infertility or premature fertility loss. However, we cannot exclude the possibility that the B6 genetic background facilitated the exhaustion of XO oocytes/follicles during postnatal ovarian development. Since the numbers of naturally ovulated oocytes were comparable between B6.XO and XX females at young ages, we do not anticipate problems in follicular recruitment or growth to contribute to the subfertility of B6.XO females.
The total number of live pups delivered by each B6.XO female was significantly lower than that delivered by a B6.XX or N2-XO female. The number delivered by an N7:8-XO female was 33% of that delivered by an N7:8-XX female, while the number delivered by an N2-XO female was 63% of that by delivered an N2-XX female. Litter sizes delivered in three age ranges, on average 2.3, 3.9 and 5.4 months of ages, were 48%, 29% and 17% by the N7:8-XO female and 64%, 51% and 74% by the N2-XO female, respectively, compared to those delivered by their corresponding XX females. These results are consistent with the tendency toward declining fertility in the B6.XO female with age. The number of oocytes recruited into ovulation is independent of the ovarian reserve until near the reproductive senescence (Brook et al., 1984). Therefore, the subfertility of the B6.XO female at young ages and decline in fertility with age is reminiscent of premature ovarian insufficiency (POI) in humans. POI may result from defects at many levels. Our current model, the B6.XO female, suggests that the number of oocytes/follicles in the ovarian reserve is the major contributing factor. Nevertheless, effects of other factors in the incidence of POI remain to be elucidated. In humans, some X-linked genes, such as BMP15 and FMR1, have been linked to POI; however, no single X chromosome gene plays a causative role, and the POI phenotype is presumed to derive from the additive effect of X-linked and non-X-linked factors (Toniolo, 2006; Qin et al., 2015). The distinct effects of the B6 genetic background on fertility loss in the XO mouse may provide an opportunity to address the non-X-linked factors responsible for POI.
The smaller litter size delivered by an XO female can be attributed to the loss of all YO and some XO embryos. The shortage of XO pups has been well documented since the first discovery of XO female mice (Cattanach, 1962; Luthardt, 1976; Hunt, 1991; Banzai et al., 1995). Nonetheless, around 20% of female pups are of XO genotype in various breeding schemes, in agreement with our results when the (C3H.B6)F1-XO female was crossed with a B6 male or the N7-XO female was crossed with a C3H male. For comparison, only 4.4% and 2.8% of female pups were of XO genotype when N2-XO and N7-XO females were crossed with B6 males. We confirmed that the numbers of two-cell-stage embryos recovered from N6-XO and N6-XX females were comparable. There appears to be a strong B6 genetic background effect on the survival of XO embryos carrying paternal X chromosomes. In our breeding scheme, where XPafY males of (B6.C3H)F1, N6 and N7 were crossed with B6 females, the ratios of female pups were 50.3% (n = 171), 54.5% (n = 246) and 44.7% (n = 219), respectively, suggesting that B6.XO embryos carrying maternal X chromosomes were fully viable in B6.XX mothers.
If the single X chromosome is randomly segregated into oocytes at the first meiotic division in XO females, equal ratios of XX, XY, XO and YO progeny are anticipated after fertilization. However, it has been demonstrated that segregation of the X chromosome in the XO oocyte is not random; the X chromosome is preferentially retained in 60% of oocytes on either the C3H or B6 genetic background (Kaufman, 1972; Sakurada et al., 1994; LeMaire-Adkins and Hunt, 2000). Consequently, 40% of oocytes would produce XO and YO embryos after fertilization and their complete loss would result in a smaller litter size by 40%. This is close to our observation in the N2-XO female. The average litter size delivered by each N7:8-XO female was much smaller, partly due to the inclusion of pregnancy failures. When the litter sizes of only successful deliveries were taken, their averages were 65.3% (n = 8), 52.4% (n = 6) and 30.5% (n = 5) of those delivered by N7:8-XX females (n = 12) in the three age ranges examined, P1, P2 and P3, respectively. The litter sizes at P1 and P2 were not far off the expectation. The litter size at P3 appeared to be much smaller, but the difference did not reach statistical significance probably due to small sample sizes. It remains possible that the small ovarian reserve affected the quality of oocytes near the reproductive senescence in the B6.XO female.
Another contributing factor to the loss of XpO embryos in the B6.XO female would be the paternal origin of the X chromosome, which is epigenetically distinct from the maternal X chromosome (Hunt, 1991; Thornhill and Burgoyne, 1993; Latham and Rambhatla, 1995). It has been shown that XpO embryos (on a mixed genetic background) display developmental retardation at gastrulation and early organogenesis while XmO embryos do not, although no selective loss of the retarded embryos during gestation was observed (Jamieson et al., 1998). It is plausible that the B6 genetic background exacerbated the developmental delay in XpO embryos and resulted in their loss. Alternatively, the X chromosome from B6 males may be inferior to support XpO embryonic development than that from C3H males since the genetic background affects DNA methylation (Engler et al., 1991; Weichman and Chaillet, 1997). However, this explanation is unlikely because of the high rate of XO female production by the (C3H.B6)F1.XO female after mating with B6 males. It appears that the survival of XpO embryos depended on their own genetic background, at least 87.5% B6, but not on the genetic background of mothers or fathers alone.
The cause for the loss of B6.XpO embryo remains to be identified. We exclude the contribution of maternal factors such as RNAs and metabolites which were stored in the oocytes during growth and meiotic progression prior to fertilization, because the oocytes of B6.XO females produced XpO live pups after mating with C3H males but failed to do so with B6 males. In humans, the rare survivor of XO conceptus is not suitable for studying the cause of miscarriage in XO embryos, and so human embryonic stem cells that had spontaneously lost one of two X chromosomes were used to analyze gene expression profiles in the cells upon differentiation into fundamental cell lineages (Urbach and Benvenisty, 2009). Their results suggested that the placenta was the only plausible tissue contributing to the XO embryonic death, and that the haplodeficiency of X inactivation escapees including those in the PAR was responsible. In our model, defects in the B6.XpO placenta are likely because of the embryonic loss at or post-implantation. However, fewer genes escape X inactivation in the mouse and only Sts has been conserved in PAR between the two species. Further study warrants clarifying the molecular mechanism of B6.XpO embryo loss.
The subfertility in the XO female mouse on the B6 genetic background is still milder than that of human TS patients. However, the early oocyte loss in the human 45.X conceptus can be at least partly attributed to the severe defects in somatic cells. The B6.XO female mouse provides an animal model for investigating the consequence of X haplodeficiency in the female germline, independent of somatic defects.
Acknowledgements
We are grateful to Dr. Diana Laird for helping us to optimize the whole-mount-IF staining protocol and Min Fu (Molecular Imaging Platform, McGill University Health Centre) for assisting us to use confocal microscopy. We also thank Dr. Anna Naumova (RI-MUHC) for helpful discussions.
Authors’ roles
B.V. and T.T. contributed to conception and study design, execution, analyses and interpretation of data. F.E.M. and X.L. contributed to experimental designs, acquisition of data and analyses. T.T. contributed to manuscript drafting. B.V., F.E.M. and X.L. provided editing and approval of the version to be published.
Funding
This work was partially supported by the grants from the Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN-2018-04464) and Canadian Institutes of Health Research (CIHR, MOP-137028) to T.T. B.V. was supported by McGill Claude Gagnon Urology Research Studentship. X.L. was supported by the McGill Center for Research in Reproduction and Development (CRRD) and Fonds de la recherche en sante du Quebec (FRSQ) Postdoctoral Fellowships.
Conflict of interest
None to declare.
References
- Alton M, Lau MP, Villemure M, Taketo T.. The behavior of the X- and Y-chromosomes in the oocyte during meiotic prophase in the B6.YTIR sex-reversed mouse ovary. Reproduction 2008;135:241–252. [DOI] [PubMed] [Google Scholar]
- Ashworth A, Rastan S, Lovell-Badge R, Kay G.. X-chromosome inactivation may explain the difference in viability of XO humans and mice. Nature 1991;351:406–408. [DOI] [PubMed] [Google Scholar]
- Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond 1963;B158:417–433. [DOI] [PubMed] [Google Scholar]
- Banzai M, Omoe K, Ishikawa H, Endo A.. Viability, development and incidence of chromosome anomalies of preimplantation embryos from XO mice. Cytogenet Cell Genet 1995;70:273–277. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Ma W, Yang F, Shendure J, Noble WS, Disteche CM, Deng X.. Escape from X inactivation varies in mouse tissues. PLoS Genet 2015;11:e1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Disteche CM.. Escape from X inactivation in mice and humans. Genome Biol 2010;11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JD, Gosden RG, Chandley AC.. Maternal ageing and aneuploid embryos—evidence from the mouse that biological and not chronological age is the important influence. Hum Genet 1984;66:41–46. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Baker TG.. Oocytes depletion in XO mice and their XX sibs from 12 to 200 days post partum. J Reprod Fertil 1981;61:207–212. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Baker TG.. Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J Reprod Fertil 1985;75:633–645. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Evans EP. A high frequency of XO offspring from XPafY* male mice: evidence that the Paf mutation involves an inversion spanning the X PAR boundary. Cytogenet Cell Genet 2000;91:57–61. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Tam PPL, Evans EP.. Retarded development of XO conceptuses during early pregnancy in the mouse. J Reprod Fertil 1983;68:387–393. [DOI] [PubMed] [Google Scholar]
- Carmell MA, Dokshin GA, Skaletsky H, Hu Y-C, van Wolfswinkel JC, Igarashi KJ, Bellott DW, Nefedov M, Reddien PW, Enders GC.. A widely employed germ cell marker is an ancient disordered protein with reproductive functions in diverse eukaryotes. eLife 2016;5:e19993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D, Haggar R, Hart A.. Germ cells in the ovaries of XO female infants. Am J Clin Pathol 1968;49:521–526. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF.. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005;434:400–404. [DOI] [PubMed] [Google Scholar]
- Cattanach BM. XO mice. Genet Res Camb 1962;3:487–490. [Google Scholar]
- Chuva De Sousa Lopes SM, Hayashi K, Shovlin TC, Mifsud W, Surani MA, McLaren A.. X chromosome activity in mouse XX primordial germ cells. PLoS Genet 2008;3:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier JM, Mahadevaiah SK, ElInati E, Nussenzweig A, Tóth A, Turner JM.. Histone H2AFX links meiotic chromosome asynapsis to prophase I oocyte loss in mammals. PLoS Genet 2015;11:e1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier JM, Mahadevaiah SK, ElInati E, Tóth A, Turner J.. Mammalian meiotic silencing exhibits sexually dimorphic features. Chromosoma 2016;125:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch GB, Zielonka EM, Coutandin D, Weber TA, Schäfer B, Hannewald J, Luh LM, Durst FG, Ibrahim M, Hoffmann J.. DNA damage in oocytes induces a switch of the quality control factor TAp63α from dimer to tetramer. Cell 2011;144:566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, Eppig JJ, Washburn LL.. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet 1991;57:221–230. [DOI] [PubMed] [Google Scholar]
- Enders GC, May JJI.. Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic Day 11 to adult in male and female mice. Dev Biol 1994;163:331–340. [DOI] [PubMed] [Google Scholar]
- Engler P, Haasch D, Pinkert CA, Doglio L, Glymour M, Brinster R, Storb U.. A strain-specific modifier on mouse chromosome 4 controls the methylation of independent transgene loci. Cell 1991;65:939–947. [DOI] [PubMed] [Google Scholar]
- Faire M,, Skillern A, Arora R, Nguyen DH, Wang J,, Chamberlain C, German MS, Fung JC, Laird DJ.. Follicle dynamics and global organization in the intact mouse ovary. Dev Biol 2015;403:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher EMC, Beer-Romero P, Brown LG, Ridley A, McNeil JA, Lawrence JB, Willard HF, Bieber FR, Page DC.. Homologous ribosomal protein genes on the human X and Y chromosomes: Escape from X inactivation and possible implications for Turner syndrome. Cell 1990;63:1205–1218. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Mitani A, Miyashita T, Umezawa A, Akutsu H.. Chromatin condensation of Xist genomic loci during oogenesis in mice. Development 2015;142:4049–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Tekur S, Reinbold R, Eppig JJ, Choi Y-C, Zheng JZ, Myrray MT, Hecht NB.. Mammalian male and female germ cells express a germ cell-specific Y-box protein, MSY2. Biol Reprod 1998;59:1266–1274. [DOI] [PubMed] [Google Scholar]
- Hamada N, Hamazaki N, Shimamoto S, Hikabe O, Nagamatsu G, Takada Y, Kato K, Hayashi K.. Germ cell-intrinsic effects of sex chromosomes on early oocyte differentiation in mice. PLoS Genet 2020;16:e1008676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EB, Warburton D.. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45, X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum Genet 2014;133:417–424. [DOI] [PubMed] [Google Scholar]
- Hunt PA. Survival of XO mouse fetuses: effect of parental origin of the X chromosome or uterin environment? Development 1991;111:1137–1141. [DOI] [PubMed] [Google Scholar]
- Jacobs P, Dalton P, James R, Mosse K, Power M, Rovinson D, Skuse D.. Turner syndrome: a cytogenetic and molecular study. Ann Hum Genet 1997;61:471–483. [DOI] [PubMed] [Google Scholar]
- Jamieson RV., Tan S-S, Tam PPL. Retarded postimplantation development of XO mouse embryos: impact of the parental origin of the monosomic X chromosome. Dev Biol 1998;201:13–25. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. Non-random segregation during mammalian oogenesis. Nature 1972;238:465–466. [DOI] [PubMed] [Google Scholar]
- Kay GF, Barton SC, Surani MA, Rastan S.. Imprinting and X chromosome counting mechanisms determine Xist expression in early mouse development. Cell 1994;77:639–650. [DOI] [PubMed] [Google Scholar]
- Korobova O, Lane PW, Perry J, Palmer S, Ashworth A, Davisson MT, Arnheim N.. Patchy fur, a mouse coat mutation associated with X–Y nondisjunction, maps to the pseudoautosomal boundary region. Genomics 1998;54:556–559. [DOI] [PubMed] [Google Scholar]
- Lane PW, Davisson MT.. Patchy Fur (Paf), a semidominant X-linked gene associated with a high level of X-Y nondisjunction in male mice. J Hered 1990;81:43–50. [DOI] [PubMed] [Google Scholar]
- Latham KE, Rambhatla L.. Expression of X-linked genes in androgenetic, gynogenetic, and normal mouse preimplantation embryos. Dev Genet 1995;17:212–222. [DOI] [PubMed] [Google Scholar]
- LeMaire-Adkins R, Hunt PA.. Nonrandom segregation of the mouse univalent X chromosome: evidence of spindle-mediated meiotic drive. Genetics 2000;156:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Castle V, Taketo T.. Interplay between Caspase 9 and X-linked Inhibitor of Apoptosis Protein (XIAP) in the oocyte elimination during fetal mouse development. Cell Death Dis 2019;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livera G, Pertre-Lazar B, Guerquin M-J, Trautmann E, Coffigny H, Habert R.. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction 2008;135:3–12. [DOI] [PubMed] [Google Scholar]
- Luthardt FW. Cytogenetic analysis of oocytes and early preimplantation embryos from XO mice. Dev Biol 1976;54:73–81. [DOI] [PubMed] [Google Scholar]
- Malki S, van der Heijden GW, O’Donnell KA, Martin SL, Bortvin A. A role for retrotransposon LINE-1 in fetal oocyte attrition in mice. Dev Cell 2014;29:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia F, Abbo Halbasch G, Epstein CJ.. X chromosome expression during oogenesis in the mouse. Dev Biol 1975;45:366–368. [DOI] [PubMed] [Google Scholar]
- McCarrey JR, Dilworth DD.. Expression of Xist in mouse germ cells correlates with X-chromosome inactivation. Nat Genet 1992;2:200–203. [DOI] [PubMed] [Google Scholar]
- McClellan KA, Gosden R, Taketo T.. Continuous loss of oocytes throughout meiotic prophase in the normal mouse ovary. Dev Biol 2003;258:334–348. [DOI] [PubMed] [Google Scholar]
- Medvedev S, Yang J, Hecht NB, Schultz RM.. CDC2A (CDK1)-mediated phosphorylation of MSY2 triggers maternal mRNA degradation during mouse oocyte maturation. Dev Biol 2008;321:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger SM, Albertini DF.. Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J Cell Sci 1991;100:289–298. [DOI] [PubMed] [Google Scholar]
- Modi DN, Sane S, Bhartiya D.. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod 2003;9:219–225. [DOI] [PubMed] [Google Scholar]
- Ogata T, Matsuo N.. Turner syndrome and female sex chromosome aberrations: deduction of the principal factors involved in the development of clinical features. Hum Genet 1995;95:607–629. [DOI] [PubMed] [Google Scholar]
- Oktay K, Bedoschi G.. Fertility preservation in girls with Turner syndrome: limitations, current success and future prospects. Fertil Steril 2019;111:1124–1126. [DOI] [PubMed] [Google Scholar]
- Peek R, Schleedoorn M, Smeets D, van de Zande G, Groenman F, Braat D, van der Velden J, Fleischer K.. Ovarian follicles of young patients with Turner’s syndrome contain normal oocytes but monosomic 45, X granulosa cells. Hum Reprod 2019;34:1686–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Jiao X, Simpson JL, Chen Z-J.. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update 2015;21:787–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud K, Cortvrindt R, Verlinde F, De Schepper J, Bourgain C, Smitz J.. Number of ovarian follicles in human fetuses with the 45,X karyotype. Fertil Steril 2004;81:1112–1119. [DOI] [PubMed] [Google Scholar]
- Rinaldi V, Bolcun-Filas E, Kogo H, Kurahashi H, Schimenti J.. The DNA damage checkpoint eliminates mouse oocytes with chromosome synapsis failure. Mol Cell 2017;67:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenger P. Turner's syndrome. N Engl J Med 1996;335:1749–1754. [DOI] [PubMed] [Google Scholar]
- Sakurada K, Omoe K, Endo A.. Increased incidence of unpartnered single chromatids in metaphase II oocytes in 39,X(XO) mice. Experientia 1994;50:502–505. [DOI] [PubMed] [Google Scholar]
- Sangrithi MN, Royo H, Mahadevaiah SK, Ojarikre O, Bhaw L, Sesay A, Peters AH, Stadler M, Turner JM.. Non-canonical and sexually dimorphic X dosage compensation states in the mouse and human germline. Dev Cell 2017;40:289–301.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP, Carr DH.. The anatomy and histology of XO human embryos and fetuses. Anat Rec 1966;155:369–384. [DOI] [PubMed] [Google Scholar]
- Speed RM. Oocyte development in XO foetuses of man and mouse: the posssible role of heterologous X-chromosome pairing in germ cell survival. Chromosoma 1986;94:115–124. [DOI] [PubMed] [Google Scholar]
- Speed RM. The possible role of meiotic pairing anomalies in the atresia of human fetal oocytes. Hum Genet 1988;78:260–266. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Abe K.. X chromosome reactivation initiates in nascent primordial germ cells in mice. PLoS Genet 2007;3:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E-K, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F.. p63 protects the female germ line during meiotic arrest. Nature 2006;444:624–628. [DOI] [PubMed] [Google Scholar]
- Sybert VP, McCauley E.. Turner's syndrome. New Engl J Med 2004;351:1227–1238. [DOI] [PubMed] [Google Scholar]
- Taketo T, Lee C-H, Zhang J, Li Y, Lee C-YG, Lau Y-FC.. Expression of SRY proteins in both normal and sex-reversed XY fetal mouse gonads. Dev Dyn 2005;233:612–622. [DOI] [PubMed] [Google Scholar]
- Tharp ME, Malki S, Bortvin A.. Maximizing the ovarian reserve in mice by evading LINE-1 genotoxicity. Nat Commun 2020;11:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill AR, Burgoyne PS.. A paternally imprinted X chromosome retards the development of the early mouse embryo. Development 1993;118:171–174. [DOI] [PubMed] [Google Scholar]
- Toniolo D. X-linked premature ovarian failure: a complex disease. Curr Opin Genet Dev 2006;16:293–300. [DOI] [PubMed] [Google Scholar]
- Tukiainen T, Villani A-C, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A.. Landscape of X chromosome inactivation across human tissues. Nature 2017;550:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner HH. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinology 1938;23:566–574. [PubMed] [Google Scholar]
- Turner JMA, Mahadevaiah SK, Fernandes-Capetillo O, Nussenzweig A, Xu X, Deng C-X, Burgoyne PS.. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 2005;37:41–47. [DOI] [PubMed] [Google Scholar]
- Urbach A, Benvenisty N.. Studying early lethality of 45,XO (Turner’s Syndrome) embryos using human embryonic stem cells. PLoS One 2009;4:e4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichman K, Chaillet JR.. Phenotypic variation in a genetically identical population of mice. Mol Cell Biol 1997;17:5269–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Babak T, Shendure J, Disteche CM.. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res 2010;20:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]