Abstract
INTRODUCTION:
To compare outcomes in patients hospitalized with coronavirus (COVID-19) receiving famotidine therapy with those not receiving famotidine.
METHODS:
Retrospective, propensity-matched observational study of consecutive COVID-19–positive patients between February 24, 2020, and May 13, 2020.
RESULTS:
Of 878 patients in the analysis, 83 (9.5%) received famotidine. In comparison to patients not treated with famotidine, patients treated with famotidine were younger (63.5 ± 15.0 vs 67.5 ± 15.8 years, P = 0.021), but did not differ with respect to baseline demographics or preexisting comorbidities. Use of famotidine was associated with a decreased risk of in-hospital mortality (odds ratio 0.37, 95% confidence interval 0.16–0.86, P = 0.021) and combined death or intubation (odds ratio 0.47, 95% confidence interval 0.23–0.96, P = 0.040). Propensity score matching to adjust for age difference between groups did not alter the effect on either outcome. In addition, patients receiving famotidine displayed lower levels of serum markers for severe disease including lower median peak C-reactive protein levels (9.4 vs 12.7 mg/dL, P = 0.002), lower median procalcitonin levels (0.16 vs 0.30 ng/mL, P = 0.004), and a nonsignificant trend to lower median mean ferritin levels (797.5 vs 964.0 ng/mL, P = 0.076). Logistic regression analysis demonstrated that famotidine was an independent predictor of both lower mortality and combined death/intubation, whereas older age, body mass index >30 kg/m2, chronic kidney disease, National Early Warning Score, and higher neutrophil-lymphocyte ratio were all predictors of both adverse outcomes.
DISCUSSION:
Famotidine use in hospitalized patients with COVID-19 is associated with a lower risk of mortality, lower risk of combined outcome of mortality and intubation, and lower levels of serum markers for severe disease in hospitalized patients with COVID-19.
INTRODUCTION
Among the many therapeutic alternatives that are currently being tested for treating patients with coronavirus (COVID-19) is the use of the histamine-2 receptor antagonist, famotidine. Following an early anecdotal report from Wuhan, China, that suggested that famotidine use was commonly observed in COVID-19 survivors (1), a retrospective cohort study of 1,620 hospitalized patients with COVID-19 demonstrated that patients receiving famotidine had statistically significant reductions in both in-hospital death and a combined death and intubation (2). More recently, a small case series of nonhospitalized patients with COVID-19 treated with high-dose oral famotidine documented a marked improvement in symptoms in all cases shortly after starting the drug (3). In light of a potential beneficial therapeutic effect, the purpose of the present study was to examine the impact of famotidine on adverse clinical outcomes in a COVID-19 hospitalized cohort.
METHODS
Setting and design
This retrospective, observational study was conducted at Hartford Hospital, an 890-bed tertiary care medical center in Hartford, Connecticut. The Hartford Hospital Institutional Review Board (Assurance number: FWA000000601) approved the study and certified that it met the criteria for a waiver of the requirement to obtain informed consent. Extracted data were reviewed by J.F.M. (author) and S.T., M.S. (acknowledged).
Study population
The study group for this report was derived from an electronic database collected at Hartford Hospital encompassing consecutive patients screened for COVID-19 between February 24, 2020, and May 13, 2020. All patients who tested positive for severe acute respiratory syndrome (SARS-CoV-2) by nasopharyngeal polymerase chain reaction and who required inpatient admission were included in this study.
Famotidine use
Patients were classified as receiving famotidine if they were treated with either oral or intravenous drug, at any dose, within +/− 7 days of COVID-19 screening and/or hospital admission. Famotidine use was extracted directly from the electronic medical record.
Primary and secondary outcomes
The primary outcomes of the study included in-hospital death as recorded in the discharge status of the patient, requirement for mechanical ventilation, and the composite of death or requirement for ventilation. Secondary outcomes included serum markers of disease severity including white blood cell count, lymphocyte count, eosinophil count, neutrophil-lymphocyte ratio, platelet count, serum ferritin, C-reactive protein (CRP), high sensitivity d-dimer, National Early Warning Score (NEWS), and procalcitonin. All of these data were extracted from the electronic medical record.
Additional variables
Potential predictive variables were chosen based on prior reports of risk factors for acute outcomes in patients positive for COVID-19. Covariates included baseline demographics, preexisting comorbidities, and treatment with antiviral, antimalarial, and corticosteroid medications. Demographic variables included age, sex, race, smoking status, and body mass index (BMI). Comorbidities included history of preexisting hypertension, diabetes mellitus, obesity (BMI >30 kg/m2), coronary artery disease, heart failure, atrial fibrillation, chronic obstructive pulmonary disease, chronic kidney disease (CKD), or prior history of malignancy. In-hospital treatment medications included use of hydroxychloroquine, azithromycin, remdesivir, and corticosteroids.
Statistical approach
Continuous variables that were normally distributed were compared with a Student t test. If not normally distributed, the Mann-Whitney U test was used. Categorical variables were analyzed using the χ2 test or Fisher exact test, as appropriate. Univariate analyses were performed using mortality or combined mortality/ventilation as the dependent variables, and multivariate logistic regression was performed by entering all predictors using a P value of less than 0.15 cutoff. Propensity score matching was performed to balance the groups. Patients treated with famotidine were matched 1:9 with patients not treated with famotidine using nearest neighbor matching with a caliper of 0.2. Additional analysis included logistic regression and Cox proportional hazard regression using the propensity-matched sample. Proportional hazard assumptions were checked by using log-log survival plots. All effects were considered significant at a P value of less than 0.05. The statistical analyses were performed with SPSS v26.0 (IBM, Armonk, NY). Propensity score matching was performed using Propensity Score Matching for SPSS, version 3.0.4 (SPSS, Chicago, IL).
Sensitivity analysis
To determine whether there was any relationship between the level of patient acuity and the impact of famotidine on mortality, we performed an additional sensitivity analysis on 2 subsets of patients comparing results in patients with a mean NEWS ≤3 and a mean NEWS >3.
RESULTS
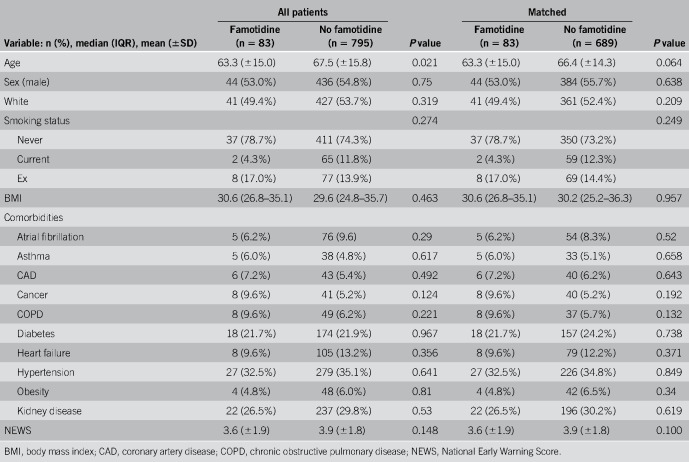
Of 878 patients in the analysis, 83 (9.5%) received famotidine. The mean age of the entire study group was 67 ± 16 years, and 480 were males (54.7%). A total of 191 (21.8%) patients died during the hospitalization, 239 (27.2%) required mechanical ventilation, and 101 (11.5%) met the criteria for combined death and intubation. Table 1 lists baseline demographics, comorbidities, and severity of illness of the famotidine and nonfamotidine study cohorts both pre- and post-matching. As shown, there were no significant differences in the propensity-matched cohorts.
Table 1.
Demographics and comorbidities: all patients and subpopulations with and without famotidine
Famotidine use
Of the 83 patients who received famotidine, 55 (66.3%) patients received the drug only as an inpatient, 24 (28.9%) were taking the drug before hospital admission and received famotidine as an inpatient, and the remaining 4 (4.8%) were taking the drug before hospital admission and did not receive famotidine as an inpatient.
Famotidine was administered orally in 83% of cases and intravenously in the remaining 17%. Dosing for oral administered famotidine was 20 mg/d in 95.2% of cases and 40 mg/d in the remaining 4.8% of cases. Intravenous famotidine was administered as a 20 mg/2 mL solution in all cases, For inpatient famotidine use, the median total dose was 80 mg (range 40–160 mg) and was received over a median of 4 days (range 2–8 days).
Hydroxychloroquine, azithromycin, remdesivir, and corticosteroid use
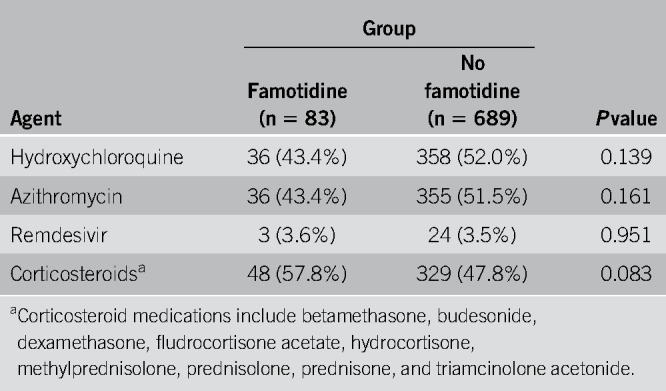
For the matched study group of 772 patients with COVID-19, a total of 394 (51.0%) received hydroxychloroquine, 391 (50.6%) received azithromycin, 27 (3.5%) received remdesivir, and 377 (48.8%) received corticosteroids. As shown in Table 2, there was no significant difference between the famotidine and nonfamotidine cohorts with respect to treatment for all 4 of these agents.
Table 2.
Treatment with hydroxychloroquine, remdesivir, and corticosteroids
Primary outcomes
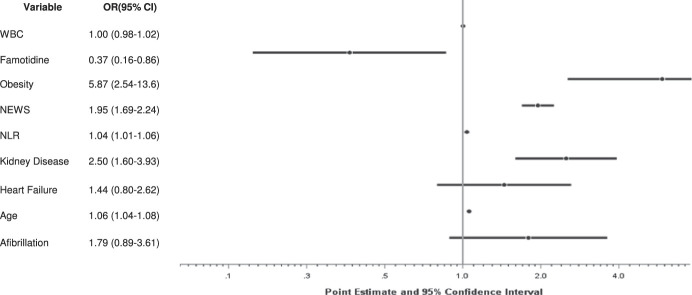
In-hospital death, intubation, and combined death/intubation occurred in 12 (14.5%), 18 (21.7%), and 6 (7.2%) patients in the famotidine group, respectively, compared with 179 (26.0%), 221 (32.1%), and 95 (13.8%) patients in the nonfamotidine group, respectively. The results of the logistic regression to assess the independent predictors of death in the matched cohort are shown in Figure 1. The analysis identified famotidine use to be associated with a significant reduction in the risk of in-hospital mortality (odds ratio 0.366, 95% confidence interval (CI) 0.155–0.862, P = 0.021). This association was replicated using the combined death or intubation endpoint (odds ratio 0.469, 95% CI 0.228–0.965, P = 0.04). Age, BMI >30 kg/m2, CKD, and NEWS were significantly associated with an increase risk in both models. A higher neutrophil-lymphocyte ratio was associated with an increased risk of death, whereas an increase white blood cell count was not associated with death and/or intubation.
Figure 1.
Adjusted ORs for the risk of death. CI, confidence interval; OR, odds ratio; NLR, neutrophil-to-lymphocyte ratio; WBC, white blood cells
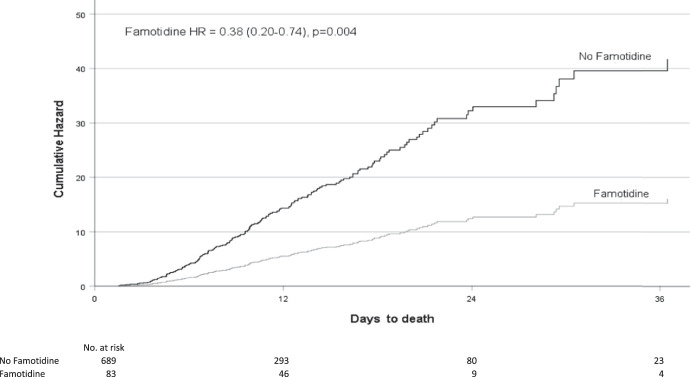
When performing a Cox proportional hazards model on the propensity-matched cohorts, the results were similar to the logistic regression model. Famotidine use was associated with a significant reduction in the risk of in-hospital mortality (hazard ratio [HR] 0.386, 95% CI 0.202–0.740, P = 0.004), Figure 2. This association was again replicated using the combined death or intubation end point (HR 0.495, 95% CI 0.310–0.789, P = 0.003).
Figure 2.
Adjusted hazard ratios (HRs) and number at risk of death for famotidine versus non-famotidine users.
Propensity score matching between famotidine and nonfamotidine patients to correct for age difference did not alter the significance of differences in either mortality or combined mortality and intubation in the analysis.
Secondary outcomes
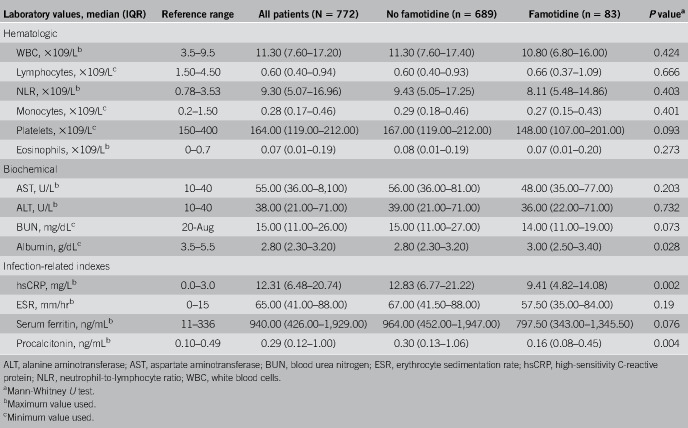
Table 3 shows laboratory test results for the famotidine and nonfamotidine groups. Patients receiving famotidine expressed lower levels of serum markers for severe disease including median peak CRP levels (9.4 vs 12.7 mg/dL, P = 0.002), median procalcitonin levels (0.16 vs 0.30 ng/mL, P = 0.004) and a nonsignificant trend to lower median mean ferritin levels (797.5 vs 964.0 ng/mL, P = 0.076).
Table 3.
Laboratory findings: all patients and in subpopulations with and without famotidine
Additional sensitivity analysis
An additional sensitivity analysis demonstrated a relationship between the level of patient acuity and the impact of famotidine on mortality. In the cohort with a mean NEWS of ≤3, after adjusting for age, history of atrial fibrillation, heart failure, CKD, obesity, neutrophil-lymphocyte ratio, white blood cell count, and asthma, the famotidine association was not significant (HR 0.86, 95% CI 0.10–7.91). In the cohort with a mean NEWS of >3 with similar adjustment, however, famotidine was significant (HR 0.43, 95% CI 0.19–0.99, P = 0.046).
DISCUSSION
As of early June 2020, there have been nearly 7 million cases of diagnosed COVID-19 and over 400,000 deaths reported worldwide. While approximately 80% of patient report mild or moderate symptoms, the remaining 20% develop severe or critical disease and require hospitalization. In-hospital mortality has been reported to range from 10% to 26%, but rises much higher in those who require admission to an intensive care unit and those who require mechanical ventilation. Despite multiple trials that are currently underway to investigate the safety and efficacy of a large number of possible therapeutic agents, no drug to date has been shown to reduce COVID-19 mortality.
The main finding of our single-center, retrospective study of hospitalized patients with COVID-19 is that use of famotidine is associated with improved clinical outcomes including lower in-hospital mortality and a lower composite end point of death and/or intubation. The impact of the drug on mortality appears to be most pronounced in patients with the highest level of acuity as measured by the mean NEWS. In addition, famotidine patients were shown to have lower levels of markers for serious disease including serum ferritin levels, CRP, and procalcitonin. The observed benefit of famotidine was unrelated to concurrent use of experimental treatments including hydroxychloroquine, azithromycin, remdesivir, and corticosteroids. Apart from the impact of famotidine, our analyses demonstrating the increased risk of death in patients with COVID-19 who are older, having comorbidities of obesity and CKD, or having higher NEWS and higher neutrophil-lymphocyte ratios are in agreement with the literature.
Our results on mortality and combined mortality/intubation corroborate the findings from the recent report by Freedberg et al. (2) that examined the effect of famotidine use on clinical outcomes in 1,620 consecutive hospitalized patients with COVID-19 infection from February 25, 2020, to April 13, 2020, at a single medical center. The authors reported that famotidine, received within 24 hours of hospital admission in 84 patients with COVID-19, was associated with reduced risk for death or intubation (adjusted HR 0.42, 95% CI 0.21–0.85) and also with reduced risk for death alone (HR 0.30, 95% CI 0.11–0.80). After baseline patient characteristics were balanced using propensity score matching, these relationships were unchanged (HR for famotidine and death or intubation: 0.43, 95% CI 0.21–0.88).
The mechanism by which famotidine might improve COVID-19 outcomes is currently unknown. One theory, based on earlier reports on the efficacy of famotidine in inhibiting human immunodeficiency virus replication (4), is that famotidine may directly inhibit the SARS-CoV-2 virus. Recent studies using a Vero E6 cell-based assay, however, have failed to demonstrate any direct inhibitory effect of famotidine on SARS-CoV-2 infection (5). A second theory, based on computational methods that identified famotidine as a potential agent capable of binding and inhibiting key SARS-CoV-2 proteases critical to viral replication (6,7), has likewise been discounted (5).
A more recent mechanism that has been postulated is that famotidine's effect is achieved via its antagonism or inverse agonism of the histamine-2 receptor, inferring that the SARS-CoV-2 infection that results in COVID-19 is at least partially mediated by pathological histamine release and perhaps dysfunctional mast cell activation (5,8). Preventing the deleterious sequelae of this histamine release has been suggested as fundamental to preventing the cytokine storm that may cause acute respiratory distress syndrome, leading to hypoxia, sepsis, organ failure, and ultimately death in the patient with COVID-19 (8). Lower levels of ferritin, CRP, and procalcitonin in famotidine-treated patients in this study are compatible with the hypothesis that the drug may limit the abnormal excessive cytokine release from an uncontrolled immune activation.
In addition, it is notable that certain unusual clinical aspects of COVID-19 could be explained by excessive histamine release and stimulation of the histamine-2 receptor. First, along with early typical nonspecific viral symptoms of fever, sore throat, cough, headache, diarrhea, and myalgia, some patients with COVID-19 may experience anosmia, ageusia, and skin rashes including pruritis and urticarial symptoms (9,10). All of these symptoms could be explained by histamine signaling. Second, seriously ill patients with COVID-19 with hypoxia and abnormal pulmonary computed tomography findings who require intubation have been found to have near-normal compliance (i.e., >50 mLcmH2O) with little response to positive end-expiratory pressure ventilation (5). In part, this observation could be related to a loss of pulmonary restriction mediated by the histamine-2 receptor on smooth muscle cells and/or pericytes. Third, it is notable that limited lung autopsy specimens have demonstrated a paucity of neutrophils and eosinophils in postmortem photomicrographs (11). The histamine-2 receptor has been documented to inhibit neutrophil effector functions including O2 release (12), platelet-activating factor–induced chemotaxis (13) and leukotriene biosynthesis (14), as well as inhibition of eosinophil peroxidase release (15) and eosinophil chemotaxis (16).
In humans, histamine is found in nearly all tissues of the body stored in the granules of tissue mast cells and serum basophils. Mast cells located in the submucosa of the respiratory tract and in the nasal cavity represent a barrier of protection against microorganisms (17). Their functions include mastocytosis by secreting histamine, leukotrienes, and proteases (18). They also play a role in inflammation development via release of multiple proinflammatory cytokines and chemokines (19). Mast cells are known to be triggered by viruses (20), and it has been documented that they have the angiotensin-converting enzyme 2 receptor used by SARS-CoV-2 to gain entry to cells and replicate (21).
The findings in this report should be interpreted with caution in light of the single-center, retrospective, and observational nature of the study. Assessment of the possible effects of additional H2 receptor antagonists (such as cimetidine, nizatidine, or ranitidine) was not possible due to limited cases. Additional studies are needed to ascertain the potential efficacy of famotidine in the patient with COVID-19, including the impact of drug dose, route of administration, and timing of therapy. In light of the need for additional trials, famotidine is currently being tested under an Investigational New Drug application waiver for treating COVID-19 in a double-blind randomized clinical trial in combination with either hydroxychloroquine or remdesivir (ClinicalTrials.gov Identifier: NCT04370262).
In summary, we found that famotidine is associated with improved clinical outcomes in hospitalized patients with COVID-19, including lower in-hospital mortality, a lower composite of death and/or intubation, and lower levels of serum markers for serious disease. Additional studies are warranted to fully evaluate the impact of famotidine in the COVID-19 population.
CONFLICTS OF INTEREST
Guarantor of the article: Jeffrey F. Mather, MS.
Specific author contributions: J.F.M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors played a part in concept and design. J.F.M. performed data acquisition. All authors had a role in interpretation and drafting the manuscript. J.F.M. and R.L.S. performed statistical analysis. R.G.M. and J.F.M. wrote the manuscript, and all authors gave critical revision of the manuscript for important intellectual content.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN?
✓ Despite multiple trials that are currently underway to investigate the safety and efficacy of a large number of possible therapeutic agents, no drug to date has been shown to reduce COVID-19 mortality.
✓ It has been postulated that famotidine's effect is achieved via its antagonism or inverse agonism of the histamine-2 receptor, inferring that the SARS-CoV-2 infection that results in COVID-19 is at least partially mediated by pathological histamine release.
✓ As a histamine-2 receptor antagonist, famotidine is a therapeutic option in COVID-10–positive patient therapy.
WHAT IS NEW HERE?
✓ We describe a propensity-matched comparison of patients with COVID-19 treated with and without famotidine.
✓ Famotidine use was significantly associated with a reduction in death and either death or intubation.
✓ Famotidine users demonstrated lower levels of serum markers for severe disease in hospitalized patients with COVID-19.
ACKNOWLEDGEMENTS
We are grateful to Stephen Thompson and Michelle Snyder for their assistance in developing and validating the data extraction process. We thank all the patients involved in this study.
REFERENCES
- 1.Borrell B. New York clinical trial quietly tests heartburn remedy against coronavirus. Science Website. https://www.sciencemag.org/news/2020/04/new-york-clinical-trial-quietly-tests-heartburn-remedy-against-coronavirus (2020). Accessed May 1, 2020.
- 2.Freedberg DE, Conigliaro J, Wang TC, et al. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: A propensity score matched retrospective cohort study. Gastroenterology 2020. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janowitz T, Gablenz E, Pattinson D, et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: A case series. Gut 2020;0:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourinbaiar AS, Fruhstorfer EC. The effect of histamine type 2 receptor antagonists on human immunodeficiency virus (HIV) replication: Identification of a new class of antiviral agents. Life Sci 1996;59(23):365–70. [DOI] [PubMed] [Google Scholar]
- 5.Malone RW, Tisdall P, Fremont-Smith P, et al. COVID-19: famotidine, histamine, mast cells, and mechanisms. Preprint Res Sq 2020;rs.3.rs-30934. doi: 10.21203/rs.3.rs-30934/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B 2020;10(5):766–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand K, Ziebuhr J, Wadhwani P, et al. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science 2003;300(5626):1763–7. [DOI] [PubMed] [Google Scholar]
- 8.Kritas SK, Ronconi G, Caraffa A, et al. Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy. J Biol Regul Homeost Agents 2020;34(1):9–14. [DOI] [PubMed] [Google Scholar]
- 9.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study. Clin Infect Dis 2020;71(15):889–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliezer M, Hautefort C, Hamel A, et al. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg 2020;146:674–75. [DOI] [PubMed] [Google Scholar]
- 11.Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020;15(5):700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burde R, Seifert R, Buschauer A, et al. Histamine inhibits activation of human neutrophils and HL-60 leukemic cells via H2-receptors. Naunyn Schmiedebergs Arch Pharmacol 1989;340(6):671–8. [DOI] [PubMed] [Google Scholar]
- 13.Rabier M, Damon M, Chanez P, et al. Inhibition by histamine of platelet-activating-factor-induced neutrophil chemotaxis in bronchial asthma. Int Arch Allergy Appl Immunol 1989;89(2-3):314–7. [DOI] [PubMed] [Google Scholar]
- 14.Flamand N, Plante H, Picard S, et al. Histamine-induced inhibition of leukotriene biosynthesis in human neutrophils: Involvement of the H2 receptor and cAMP. Br J Pharmacol 2004;141(4):552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezeamuzie CI, Philips E, Histamine H. (2) receptors mediate the inhibitory effect of histamine on human eosinophil degranulation. Br J Pharmacol 2000;131(3):482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadee AA, Anderson R, Sher R. In vitro effects of histamine on eosinophil migration. Int Arch Allergy Appl Immunol 1980;63(3):322–9. [DOI] [PubMed] [Google Scholar]
- 17.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997;77(4):1033–79. [DOI] [PubMed] [Google Scholar]
- 18.Mukai K, Tsai M, Saito H, et al. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 2018;282(1):121–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochim Biophys Acta 2012;1822(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall JS, Portales-Cervantes L, Leong E. Mast cell responses to viruses and pathogen products. Int J Mol Sci 2019;20(17):4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta 2000;1480(1-2):245–57. [DOI] [PubMed] [Google Scholar]