Abstract
INTRODUCTION:
We investigated the potential hepatotoxicity of lopinavir/ritonavir recently used in the treatment of Severe Acute Respiratory Syndrome Coronavirus.
METHODS:
This is a retrospective cohort of critical patients in a teaching hospital: 12 treated with lopinavir/ritonavir and 30 in the standard-of-care group.
RESULTS:
Elevation occurred more frequently in patients treated with lopinavir/ritonavir (33% vs 6.7%).
DISCUSSION:
Caution is advised regarding the use of lopinavir/ritonavir in the most severe cases of Severe Acute Respiratory Syndrome Coronavirus.
INTRODUCTION
The pandemia of COVID-19, a new disease due Severe Acute Respiratory Syndrome -Coronavirus, is spreading worldwide with huge public health impact. There is an unmet and urgent need to get effective treatment, which is able to reduce morbimortality of this viral infection. Among the drugs identified as candidates, lopinavir, a human immunodeficiency virus (human immunodeficiency virus) type 1 aspartate protease inhibitor combined with ritonavir—a cytochrome P450 inhibitor—has been selected by the World Health Organization to be evaluated in clinical trials (https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/). This choice was based on the previous clinical reports on Severe Acute Respiratory Syndrome -Coronavirus infections (1), experimental data in animal models (2), and in-vitro evaluation against Middle East Respiratory Syndrome - Coronavirus (3). For SARS CoV 2, its efficacy remains unsettled. The recent randomized controlled trial comparing lopinavir/ritonavir to standard of care in COVID-19 infection did not show significant difference in clinical outcome and virological clearance (4). However, in patients with life-threatening COVID-19 infections, physicians can propose compassionate use of lopinavir/ritonavir, and multicentric ongoing trials are currently comparing lopinavir/ritonavir to standard of care or other drugs such as hydroxychloroquine or remdesivir to get more evidence for the treatment of the most severe forms.
We report 4 total bilirubin elevations with 3 jaundices occurring in 12 severely ill patients admitted in the intensive care unit (ICU) who were prospectively treated with lopinavir/ritonavir. This frequent occurrence of liver event has not been described in COVID-19 patients and should caution the use of lopinavir/ritonavir in patients with severe forms.
METHODS
Population
Forty-two patients with COVID-19 infection proven by Reverse transcription polymerase chain reaction were admitted in ICU of the CHRU de Lille from February 27 to March 21, 2020, in France. A compassionate treatment with lopinavir/ritonavir (400 lopinavir mg/100 mg ritonavir administered every 12 hours for a duration of 14 days) was proposed to the first 12 consecutive patients admitted to our center. This group of 12 patients has been compared with a second group of 30 patients consecutively admitted in our center and treated with standard of care without lopinavir/ritonavir.
Statistical analysis
Clinical characteristics and laboratory data were summarized as follows: categorical variables were presented as proportions and frequencies and continuous variables were presented as median with confidence interval (CI 95%). Comparisons between groups were performed using χ2 tests, Student t tests, and Mann-Whitney/Wilcoxon signed-rank tests as appropriate.
RESULTS
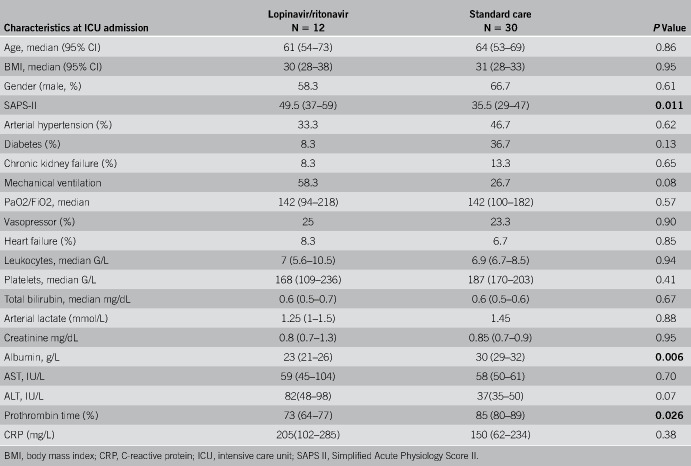
Patients were mainly male, aged of 61 years, overweight (body mass index 31 kg/m2), and had severe respiratory illness at admission with a ratio between partial pressure of oxygen and fraction of inspired oxygen (PaO2/FiO2) of 142 mm Hg. The group of patients treated with lopinavir/ritonavir was more severe at admission with Simplified Acute Physiology Score II of 49.5 (36–60) vs 35.5 (24–53.5), P = 0.011. Albumin (23 g/L [21–26] vs 30 g/L [25–35] P = 0.006) and prothrombin time (73% [62–79] vs 85% [69–92] P = 0.026) were also significantly lower. C-reactive protein (205 [102–285] vs 150 [62–234] P = 0.38), leukocytes (7 G/L [5.6–10.5] vs 6.9 G/L [6.7–8.5], P = 0.94), and total bilirubin (0.6 [0.4–0.8] vs 0.6 [0.4–0.9] P = 0.67) were similar at admission between the groups. None of the patient underwent renal replacement therapy (RRT) at admission or had a history of cirrhosis in the 2 groups. All characteristics at baseline are detailed in Table 1.
Table 1.
Characteristics at ICU admissions of lopinavir/ritonavir and standard of care patients
During the follow-up, 33% of patients in the lopinavir/ritonavir group had an increase of bilirubin or jaundice vs 6.7% (P = 0.046) in the standard of care group. Total bilirubin increased in the lopinavir/ritonavir group from the first to the seventh day and was significantly higher during this period as compared to the standard of care group in which the total bilirubin level remains stable (Figure 1.). Transaminases levels over time were not significantly different between the 2 groups.
Figure 1.
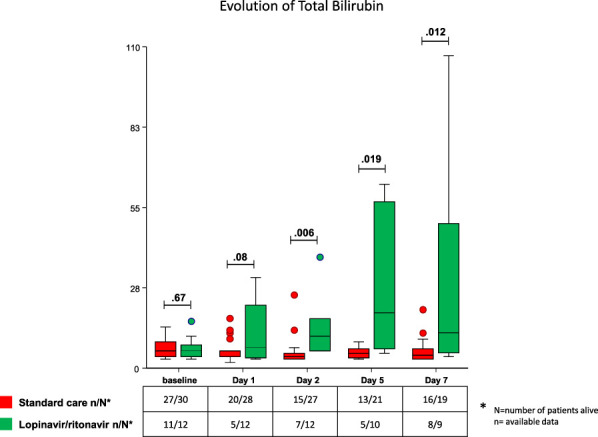
Evolution of total bilirubin from baseline to day 7 in lopinavir/ritonavir and standard of care groups.
Of our 12 patients treated with lopinavir/ritonavir, 6 died in the lopinavir/ritonavir group (3 multiorgan failure, 1 cardiac arrest, and 2 withdrawal of care) and 4 died in the standard care group (3 withdrawal of care and 1 cardiac arrest). There was no case of acute liver failure.
Kidney function and RRT recourse did not differ between the 2 groups. Extraorporeal membrane oxygenation (ECMO) was used in 2 patients in the lopinavir/ritonavir group vs 1 in the standard care group.
Finally, in the lopinavir/ritonavir group, liver adverse events were classified as serious in 2 cases with grade 3–4 and not severe in 2 cases with grade 1–2. Among these 4 patients, 3 returned to baseline bilirubin between 2 and 10 days after treatment withdrawal. The last patient did not recover from jaundice, required ECMO, and died of multiorgan failure. Death was not related to acute liver failure.
DISCUSSION
Before the use of lopinavir/ritonavir in the setting of COVID-19, liver toxicity, mostly elevated liver enzymes without jaundice, nor acute liver failure have been described in the human immunodeficiency virus population. This liver toxicity is preferentially observed in patients coinfected with hepatitis C with pre-existing abnormal liver test at the introduction of lopinavir/ritonavir (5,6). We now report elevated total bilirubin during lopinavir/ritonavir treatment.
Jaundice or elevation of total bilirubin occurred more frequently (33%) in our COVID-19 patients treated with lopinavir/ritonavir, whereas a recent trial did not show difference in bilirubin between the lopinavir/ritonavir and standard care groups. The accountability of lopinavir/ritonavir in the elevation of bilirubin has also been reported in a cohort of 417 COVID-19 patients (7). These discrepancies in liver adverse events between our experience and the results from randomized controlled trial may be related at least in part to differences in baseline disease severity and host factors. The frequency of elevated bilirubin in our standard group (6.7%) was higher than that in the study of Cao et al. (3.2% in the lopinavir/ritonavir group and 3% in the control group). Only one patient requiring mechanical ventilation was included in this trial that recruited patients suffering from mild disease. Our patients treated with lopinavir/ritonavir were more severe: all were admitted to intensive care, 58% required mechanical ventilation, 33% RRT, and 16% ECMO. Hypoalbuminemia, frequently observed (71%) in our patients, might have altered pharmacokinetics of lopinavir/ritonavir favoring overexposure, whereas less than 1% of patients from the RCTs had hypoalbuminemia. In the same way, the major inflammatory syndrome, as suggested by the levels of C-reactive protein, and potential mitochondrial dysfunction (8) could also have impaired the drug metabolism. Underlying liver steatosis might also be a contributing factor of liver toxicity of lopinavir/ritonavir because 60% of our patients were obese (9).
Thus, close monitoring, dosage of lopinavir/ritonavir, and dose adjustment should be proposed in clinical trials including critically ill patients in whom lopinavir/ritonavir could be less tolerated than in noncritically ill patients. Caution is advised regarding the widespread use of lopinavir/ritonavir in severely ill patients in ICUs, in whom liver function should be closely monitored.
CONFLICTS OF INTEREST
Guarantor of the article: Clementine Levy, MD.
Specific author contributions: C.L.: conducted the study, drafted the manuscript, and collected the data. G.L.: conducted the study, drafted the manuscript, and interpreted the data. E.P.: collected and interpreted the data. T.D.: collected and interpreted the data and drafted the manuscript. P.M.: drafted the manuscript. J.P.: conducted the study and drafted the manuscript
Financial support: None to report.
Potential competing interests: None to report.
REFERENCES
- 1.Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004;59(3):252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong JF-W, Yao Y, Yeung M-L, et al. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 2015;212(12):1904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020;11(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med 2020;382(19):1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canta F, Marrone R, Bonora S, et al. Pharmacokinetics and hepatotoxicity of lopinavir/ritonavir in non-cirrhotic HIV and hepatitis C virus (HCV) co-infected patients. J Antimicrob Chemother 2005;55(2):280–1. [DOI] [PubMed] [Google Scholar]
- 6.Palacios R, Vergara S, Rivero A, et al. Low incidence of severe liver events in HIV patients with and without hepatitis C or B coinfection receiving lopinavir/ritonavir. HIV Clin Trials 2006;7(6):319–23. [DOI] [PubMed] [Google Scholar]
- 7.Cai Q, Huang D, Yu H, et al. COVID-19: Abnormal liver function tests. J Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi CS, Nabar NR, Huang NN, et al. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov 2019;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allard J, Le Guillou D, Fromenty B, et al. Drug-induced liver injury in obesity and nonalcoholic fatty liver disease. Adv Pharmacol 2019;85:75–107. [DOI] [PubMed] [Google Scholar]