Abstract
INTRODUCTION:
It has been hypothesized that people suffering from inflammatory bowel disease (IBD) have an increased risk of coronavirus disease (COVID-19). However, it is not known whether immunosuppressive therapies exacerbate the COVID-19 outcome.
METHODS:
We reviewed data on the prevalence and clinical outcomes of COVID-19 in patients with IBD.
RESULTS:
COVID-19 prevalence in patients with IBD was comparable with that in the general population. Therapies using antitumor necrosis factor-α agents have been associated with better clinical outcomes.
DISCUSSION:
Management and treatments provided by gastroenterologists were effective in reducing COVID-19 risk. Antitumor necrosis factor-α agents seem to mitigate the course of COVID-19.
INTRODUCTION
The coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has aroused concern in healthcare teams for patients with immune-mediated inflammatory diseases, such as inflammatory bowel disease (IBD). There are 2 major questions that are essential for managing patients with IBD. First, does COVID-19 occur more frequently in patients with IBD? Second, do therapies for IBD influence clinical course of COVID-19?
EPIDEMIOLOGY OF COVID-19 IN IBD
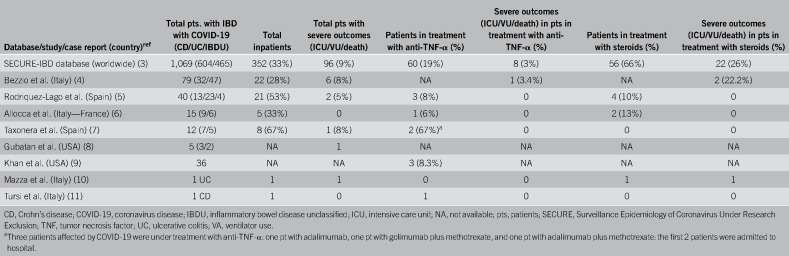
Preliminary studies did not report any COVID-19 cases in patients with IBD (1,2). However, subsequent studies including the Surveillance Epidemiology of Coronavirus Under Research Exclusion-IBD database, which includes global data of patients with IBD and COVID-19 (3), several cohort studies (4–9), and case reports (10–12), have reported 1,258 patients with IBD affected by COVID-19. Pooled data suggest that COVID-19 does not occur more frequently in patients with IBD than in the general population (6–8). Indeed, Gubatan et al. reported, among their 165 patients with IBD from California, a prevalence of 3% comparable with 2.8% in the general population (8). Taxonera et al. (7) found an incidence rate of 6.2 COVID-19 cases per 1000 patients with IBD in Madrid, whereas in the general population, it was 6.6 cases per 1,000. However, after adjusting for age, the incidence rate was 4.9 per 1000 patients with IBD with a lower standardized risk than that in the general population (odds ratio 0.74, 95% confidence interval 0.70–0.77; P < 0.001) (7). Finally, a combined Italian and French study reported a cumulative incidence of 2.5 COVID-19 cases per 1000 patients with IBD. This was comparable with that observed in the general population of 1.7 per 1,000 individuals (6). Interestingly, clinical symptoms of COVID-19 in patients with IBD seem to be milder, with a relatively lower frequency of serious and complicated cases than in the general population (1–8). Allocca et al. (6) did not report any COVID-19-associated deaths in their cohort of 15 patients with IBD compared with a mortality rate of 13% in the general population. Furthermore, they reported no significant difference in the standardized mortality ratio between patients with IBD and the general population (odds ratio 0.95, 95% confidence interval: 0.84–1.06; P = 0.36). The clinical and demographic variables associated with unfavorable COVID-19 outcomes, such as old age, presence of comorbidities, and being male, were comparable between patients with IBD and the general population (4,8). As shown by the study of the Italian Group for the Study of Inflammatory Bowel Disease, which included 79 patients with IBD, aged older than 65 years, had comorbidities, and active IBD were associated with worse COVID-19 outcomes (4). Thus, adding IBD activity at the time of COVID-19 diagnosis is a risk factor for a worse clinical outcome.
IMPACT OF IBD TREATMENTS ON COVID-19 INCIDENCE AND OUTCOME
The impact of IBD-therapeutic armamentarium on COVID-19 incidence and outcome is currently a controversial topic. A retrospective study from the United States including a large cohort of patients with IBD reported that the use of antitumor necrosis factor (TNF)-α agents or thiopurines was not associated with an increased risk of developing COVID-19 (9). In fact, the incidence rate of COVID-19 per 1000 patients with IBD was of 0.61 in patients treated with anti-TNF-α and 1 in those not in treatment with anti-TNF-α (P = 0.618).
The literature shows that the therapy's effect on COVID-19 outcome varies across patients (Table 1). In the Surveillance Epidemiology of Coronavirus Under Research Exclusion-IBD database, we found evidence of greater prevalence of milder COVID-19 cases in patients treated with anti-TNF-α than that in patients undergoing steroid treatments (3). As of May 15, 2020, 19% of patients treated with anti-TNF-α agents required hospitalization and only a minority (3%) experienced unfavorable outcomes, defined as intensive care unit admission, ventilator use, or death (3). Conversely, 66% of patients taking oral or parenteral steroids needed hospitalization, with 26% experiencing unfavorable outcomes (3). Further support to this theory comes from the results of the Italian Group for the Study of Inflammatory Bowel Disease study, which reported a 60% reduction in mortality among patients receiving anti-TNF-α antibodies (although not statistically significant); however, corticosteroid use was associated, with a trend toward statistical significance, with COVID-19-related pneumonia (P = 0.05) and death (P = 0.064) (4). Therefore, the data thus far strongly suggest that the use of anti-TNF-α antibodies as monotherapy is associated with better COVID-19 outcomes than the use of steroids. The rationale for the beneficial effect of anti-TNF-α antibodies on COVID-19 clinical course is closely linked to SARS-CoV-2 pathogenesis. The SARS-CoV-2 uses the functional receptor angiotensin-converting enzyme 2 (ACE2) for host cell entry. This causes increased production of TNF-α and TNF-α-converting enzyme-dependent shedding of the ectodomain of ACE2 that further assists viral cell entry. Wang et al. postulated that the use of anti-TNF-α antibodies could be effective in reducing both SARS-CoV viremia and the consequent organ damage (13), considering that ACE2 is overexpressed in inflamed mucosa (14). Furthermore, anti-TNF-α agents could achieve effective control of inflammatory mediators, which makeup the “cytokine storm” that occurs in severe COVID-19-related pneumonia, thereby mitigating the course of the disease. Further evidence comes from the intentional use of 2 10 mg/kg doses of infliximab, 1 week apart, in a patient with severe ulcerative colitis and COVID-19-related pneumonia (5). The patient achieved a satisfactory recovery from intestinal and pulmonary disease without complications (5). On the other hand, steroid use should be avoided, if possible, or rapid steroid tapering should be considered owing to the risk of respiratory or opportunistic infection that could complicate the course of COVID-19.
Table 1.
Outcomes of COVID-19 in patients with IBD according to treatment with anti-TNF-α antibodies or steroids
CONCLUSIONS
Although significant uncertainty remains, the data accumulated thus far have demonstrated that the clinical course of COVID-19 and its prevalence in patients with IBD are milder and lesser than those in the general population. The recommendations for reducing COVID-19 risk and the IBD treatments provided by gastroenterologists could potentially explain the reason for this. Anti-TNF-α agent use might provide double beneficial effects: first, to maintain IBD clinical remission and second, to mitigate the clinical course of COVID-19. However, the data we have reported have been obtained from retrospective studies that have not been designed to evaluate the effect of different therapies on COVID-19 outcomes. Therefore, we must exercise caution in interpreting the current presented data, and further properly designed epidemiological studies should be conducted.
CONFLICTS OF INTEREST
Guarantor of the article: Antonio Tursi, MD.
Specific author contributions: Alfredo Papa, MD, PhD, and Antonio Tursi, MD, contributed equally to the manuscript. A.P., A.G., and A.T. planned and conducted the study; A.P. and A.T. collected and interpreted data; A.T. and A.P. drafted the manuscript; A.T., A.G. and A.P. approved the final draft submitted.
Financial support: None to report.
Potential competing interests: A.P., A., G. and A.T. declare any conflict of interest.
REFERENCES
- 1.Norsa L, Indriolo A, Sansotta N, et al. Uneventful course in IBD patients during SARS-CoV-2 outbreak in Northern Italy. Gastroenterology 2020;159(1):371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An P, Ji M, Ren H, et al. Prevention of COVID-19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol Hepatol 2020;5(6):525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner EJ, Ungaro RC, Colombel JF, et al. SECURE-IBD database public data update. (www.covidibd.org). Accessed May 10, 2020.
- 4.Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: An IG-IBD study. Gut 2020;69(7):1213–7. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Lago I, Ramírez de la Piscina P, Elorza A, et al. Characteristics and prognosis of patients with ixnflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain), Gastroenterology 2020. [Epub ahead of print April 21, 2020.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allocca M, Fiorino G, Zallot C, et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol 2020;18(9):2134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taxonera C, Sagastagoitia I, Alba C, et al. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2020;52(2):276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubatan J, Levitte S, Balabanis T, et al. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology 2020. [Epub ahead of print May 7, 2020.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan N, Patel D, Xie D, et al. Impact of anti-TNF-α and thiopurines medications on the development of COVID-19 in patients with inflammatory bowel disease: A nationwide VA cohort study. Gastroenterology 2020. [Epub ahead of print May 29, 2020.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazza S, Sorce A, Peyvandi F, et al. A fatal case of COVID-19 pneumonia occurring in a patient with severe acute ulcerative colitis. Gut 2020;69(6):1148–9. [DOI] [PubMed] [Google Scholar]
- 11.Tursi A, Angarano G, Monno L, et al. Covid-19 infection in Crohn's disease under treatment with adalimumab. Gut 2020;69(7):1364–5. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs J, Clark-Snustad K, Lee S. Case report of a SARS-CoV-2 infection in a patient with ulcerative colitis on tofacitinib. Inflamm Bowel Dis 2020;26(7):e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Ye L, Ye L, et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res 2007;128:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgueño JF, Reich A, Hazime H, et al. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis 2020;26(6):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]