Abstract
Objective:
The objective of this study was to provide an overview of opioid-induced constipation (OIC) and its influence on disease burden and quality of life (QOL).
Methods:
This is a narrative review.
Results:
For many patients, opioid-related side effects, the most common being OIC, have the potential to significantly impair patients’ QOL. Patients with OIC often experience substantial overall burden (ie, increases in anxiety and depression, impairments in activities of daily living, low self-esteem, feelings of embarrassment) and economic burden (ie, higher health care costs, more frequent doctor visits, increased out-of-pocket medication costs), which often causes patients to modify or discontinue opioid treatment despite the analgesic benefits. OIC occurs when opioids bind to peripheral μ-opioid receptors in the gastrointestinal tract. Currently, 4 Food and Drug Administration (FDA)-approved medications are available for OIC, 3 of which are peripherally acting µ-opioid receptor antagonists (PAMORAs). PAMORAs block µ-opioid receptors in the gastrointestinal tract without affecting the central analgesic effects of the opioid and thus provide a targeted approach to OIC management. Two PAMORAs, naldemedine and methylnaltrexone, have shown significant improvements in QOL based on the Patient Assessment of Constipation Symptoms questionnaire relative to placebo. Along with pharmacologic management for OIC, health care providers should institute comprehensive communication strategies with patients to ensure OIC is effectively recognized and managed.
Discussion:
OIC has both physical and psychological impacts on patients. PAMORAs provide effective relief of OIC while also improving QOL. To augment the pharmacologic management of OIC, proactive counseling approaches between physicians and patients may help relieve some of the patient burden associated with OIC and lead to improved outcomes.
Key Words: opioid-induced constipation, quality of life, patient burden
Opioid prescription rates peaked in 2012 with over 255 million prescriptions dispensed in that year alone.1 Although prescribing rates have steadily declined over time to ∼191 million prescriptions in 2017,1 opioid analgesics are still frequently prescribed for the management of chronic cancer- and noncancer-related pain, especially when nonopioid alternatives have not been effective. Opioids may be the only treatment option able to restore a normal health-related quality of life (HR-QOL) for some patients with chronic pain; however, opioids can be associated with several side effects that can cause patients to discontinue opioid use even if significant analgesic benefits have been achieved.2–4
One of the most common side effects of opioid therapy is opioid-induced constipation (OIC). Prevalence data on OIC vary among studies due to differences in study types, definitions of OIC, data reporting and collection methods, treatment sites, patient inclusion criteria, and types of opioids used.5 Although few prospective studies have included gastrointestinal side effects such as OIC as study endpoints,6 overall estimates of OIC prevalence range from ∼ 40% to 80%.2,7,8
The presence of OIC negatively impacts patients’ HR-QOL, ability to perform daily activities,9,10 and work productivity,11 and results in significant patient burden.12–14 The distressing effects of OIC may also be coupled with related symptoms such as dyspepsia, reflux, bloating, spasm, cramping, fecal impaction, and urinary obstruction or infection, many of which could lead to increased hospitalizations and morbidities.15 Although appropriate assessment tools are available to recognize these symptoms, OIC remains under-diagnosed.16 As a consequence, OIC may go untreated even though effective treatment options are available.
This review describes the burden experienced by patients with OIC, presents a broad overview of current treatment options with a focus on peripherally acting μ-opioid receptor antagonists (PAMORAs), and provides practical clinical strategies pertinent to counseling patients with or at risk for OIC.
OVERVIEW OF OIC
Pathophysiology
OIC occurs as the result of an opioid agonist binding to peripheral μ-opioid receptors in the gastrointestinal tract.3 This binding leads to reduced bowel tone, slowed peristaltic activity, and decreased mucosal secretions, which can delay gastric emptying, slow intestinal transit, and increase fluid absorption in the gastrointestinal tract.17 These opioid-induced gastrointestinal effects can lead to difficulties in evacuating feces, excessive straining, hard stools, abdominal discomfort, and bloating.9,18–20 Difficulty in rectal evacuation may also be related to increased anal sphincter tone and decreased reflex relaxation.9 The mechanisms associated with OIC are different than those associated with other forms of constipation, which do not involve μ-opioid receptors. As a result, treatment strategies that address opioid binding to peripheral μ-opioid receptors are of particular value.21
Defining and Assessing OIC
Multiple definitions of OIC have been described, making it difficult for physicians to recognize.16 As a result, OIC is often under-diagnosed and under-treated.16 To address this problem, a consensus panel organized by the American Academy of Pain Medicine Foundation met in 2015 and reviewed multiple OIC assessment and diagnostic tools to determine what criteria should be used to define OIC. They ultimately defined OIC as a change from baseline in bowel habits after initiation of opioids that includes any of the following symptoms: reduced bowel movement frequency, development or worsening of straining to pass stool, a sense of incomplete rectal evacuation, and harder stool consistency.20
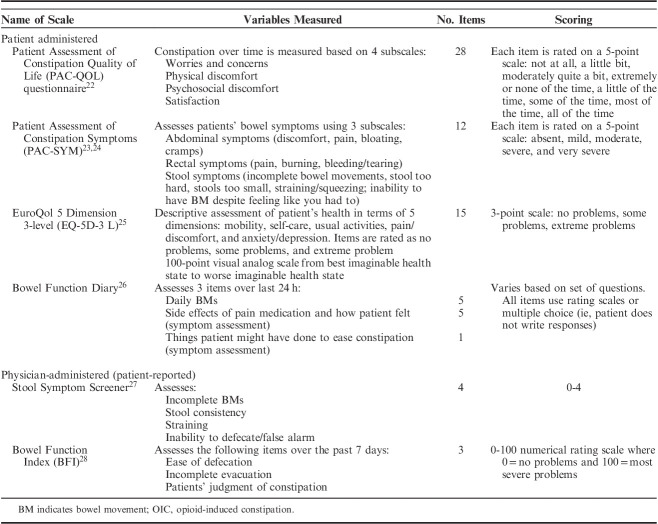
After appropriate diagnosis of OIC, it is important to be able to assess OIC during treatment to determine if the treatment is effectively providing relief from gastrointestinal symptoms. Patient-reported outcomes are essential to accomplishing this task. Table 1 lists validated measurement scales for assessing constipation. Items related to OIC that are assessed in the measurement scales include constipation intensity/severity, ease/difficulty of defecation, incomplete evacuation, straining, discomfort, constipation distress, and satisfaction.29 These tools allow patients to directly report how they feel and function while experiencing OIC,29 and provide physicians with insight into the impact OIC has on patients. It is important to realize, however, that these tools are more commonly used in clinical trials and may be more difficult to use during regular patient visits due to the number of items on each scale, the time needed to complete the scales, and the time needed to interpret the findings. In addition, although improvement in these outcomes is considered important to patients with OIC, the amount of change that is viewed as a meaningful improvement varies among patients.29
TABLE 1.
Subjective Scales Used to Assess OIC
Patient Burden Associated With OIC
A multitude of factors contribute to the overall burden and reduction in HR-QOL experienced by patients with OIC (Fig. 1). This overall burden can become so great as to cause patients to sacrifice effective management of their chronic pain in an attempt to alleviate their constipation. In a set of patient surveys assessing the impact of OIC on opioid users, >30% reported difficulties in balancing pain relief and the incidence of constipation, and were unhappy about the need to do so.13 Another survey designed to assess OIC-related issues in patients using opioids for chronic, acute, or cancer-related pain revealed that 57% of patients stopped opioid treatment due to side effects.12 According to results from the 2012 National Health and Wellness Survey, a self-administered, cross-sectional, Internet-based questionnaire survey assessing health outcomes among noncancer patients taking opioids, ∼50% of patients with OIC modified their opioid therapy because of constipation in the past 6 months and 20% discontinued opioid treatment due to constipation.4
FIGURE 1.
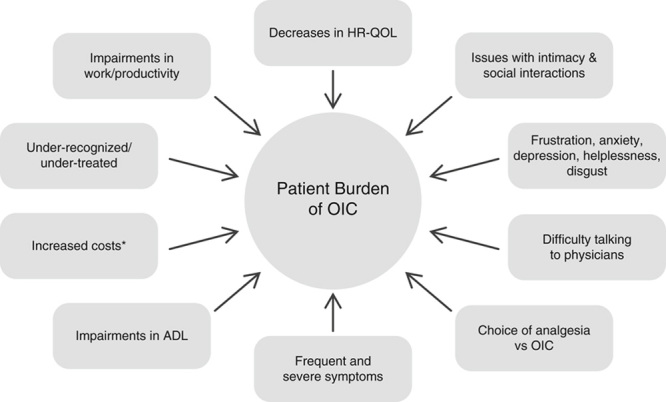
Factors associated with patient burden of OIC. ADL indicates activities of daily living; ED, emergency department; OIC, opioid-induced constipation; HR-QOL, health-related quality of life. *ED visits, office visits, nursing home visits, home health care, other outpatient care, inpatient services, laboratory services, and pharmacy use.
Abrupt modifications to opioid therapy can have several negative repercussions. For example, patients who modify their opioid therapy to relieve symptoms of OIC often experience insufficient pain management.4,30 Possibly due to increased pain, patients whose opioid regimen has been revised were significantly more likely to have pain-related surgery, emergency department visits, hospitalizations, and nontraditional health care provider visits. They also experienced worsening HR-QOL, overall impairments in work, and, ironically, greater severity of OIC symptoms.4 Moreover, patients who modified their opioid regimens due to OIC have significantly greater out-of-pocket costs for pain medications and health care provider visits compared with patients who did not modify their therapy.4
Even without modifications in opioid therapy, OIC has been shown to have a negative impact on HR-QOL. In the Internet-based survey Patient Reports of Opioid-Related Bothersome Effects (PROBE) 1, most patients who were taking opioids for chronic cancer-related or noncancer-related pain reported at least a moderately negative impact on their HR-QOL or overall well-being and on their activities of daily living as a result of OIC.2 Similar results were demonstrated in a 6-month, prospective cohort study assessing HR-QOL in patients taking opioids for chronic noncancer pain.30 Patients with OIC at baseline had higher pain interference scores in several activities, including general activities, walking, socializing, working, sleeping, and overall satisfaction with life than patients without OIC.30 These scores significantly worsened at 6 months within the group of patients with OIC, and similar findings were seen in other HR-QOL measurements throughout the study. In an ongoing international longitudinal study, which combined data from patient and physician surveys and retrospective data reviews of medical records in patients with OIC, OIC had a significant impact on daily living.14
Other burdens of OIC include low self-esteem, social isolation, and feelings of embarrassment, anger, frustration, irritation, dependence, anxiety, depression, helplessness, obsession, and disgust.12,13 Rauck and colleagues reported results from an 11-question US patient survey administered from 2014 to 2015 that asked patients questions that covered both HR-QOL issues as well as other burdens of OIC. A total of 489 patients responded to the survey.12 Almost 40% of patients indicated that OIC interfered with their work, >45% responded that OIC interfered with daily activities and their sex lives, and >43% reported that OIC interfered with social interactions and their ability to leave the house.12 In the 2012 National Health and Wellness Survey assessing noncancer patients taking opioids, patients with OIC reported significantly higher percentages of time missed from work, impairment while working, overall work impairment, and activity impairment compared with those without OIC.6 These patients also showed worse scores on the physical and mental portions of the Short Form-8 Health Survey.6 Similar findings were seen in a European quantitative, questionnaire-based international survey.13 In that survey, 951 patients with OIC reported spending too much time in the bathroom, problems maintaining their normal routines, difficulties with intimacy, trouble pursing hobbies, difficulties completing household chores, and concerns about socializing.13 In addition, data from a 2014 international cross-sectional health care survey demonstrated that OIC is associated with more frequent physician and alternative care provider visits.6 Individuals with OIC also more commonly experienced side effects, including sleepiness, nausea, mood changes, dizziness, bloating, and abdominal pain or discomfort.6
In patients taking opioids for cancer-related pain, additional concerns regarding their underlying condition may contribute to the burden of OIC. Dhingra et al31 performed a semistructured interview survey of patients with advanced cancer to better understand the psychological distress and burden associated with OIC. Patients expressed beliefs that OIC was a dangerous condition that led to declining health, and that the presence of OIC indicated that their cancer would metastasize or that there was a problem with their body’s functional capabilities. They also described multiple factors associated with the psychological distress of OIC, including lack of effective nonpharmacologic treatments, knowledge that they could not control their own bodies, fear of health risks, and increased anxiety.31
Economic Burden
In addition to reductions in HR-QOL and other patient burden, patients with OIC also have higher health care costs than other patients on opioid therapy without OIC. Figure 2 summarizes the annual health care costs for patients with and without OIC.
FIGURE 2.
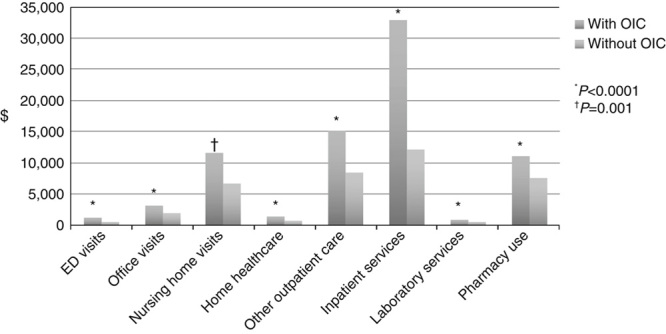
Costs associated with patients with and without OIC. Data from Iyer et al.32 ED indicates emergency department; OIC, opioid-induced constipation.
In a retrospective, observational, matched cohort study comparing costs and resource use between patients on opioid therapy with and without OIC, patients with OIC had significantly higher health care costs due to more frequent and longer emergency department visits, home visits, nursing home visits, other outpatient care, inpatient care, laboratory services, and pharmacy services.32 A retrospective claims analysis also evaluated health care costs and resource utilization for patients with and without OIC from 2007 to 2011 among 3 different subsets of patients: nonelderly, elderly, and those in long-term care facilities. In all 3 cohorts, patients with OIC had significantly higher total health care costs than patients without OIC.15 In terms of resource utilization, nonelderly patients with OIC had more physician office visits compared with those without OIC, and elderly patients with OIC had more emergency department visits than those without OIC. Compared with patients without OIC, there were no significant differences in health care resource use in patients with OIC living in long-term care facilities.15 Similar results were observed in a retrospective, observational, matched-cohort study utilizing database health care records from 81,780 patients who received opioids after total hip or total knee arthroplasty. Both mean length of hospital stay and costs of hospital stays were longer among patients who experienced OIC than in patients who did not experience OIC.5 Severity of OIC symptoms also impacts patient costs. In a cost analysis of Swedish patients with OIC who were receiving strong opioids, higher total costs (direct and indirect) were observed in patients experiencing severe OIC (EUR 1525) compared with patients experiencing moderate (EUR 1196) or mild (EUR 1088) OIC.7
In addition to increased costs associated with OIC itself, the 2012 National Health and Wellness Survey showed that patients who modified their therapy due to OIC had significantly greater costs associated with multiple other factors, including resources for treating pain, surgery, emergency department visits, out-of-pocket medication costs, and visits to both traditional and nontraditional health care providers.4 Overall, these data demonstrate the substantial, multifaceted burden experienced by patients with OIC and underscore the importance of effective treatments.
Treatment Options for OIC
Available data suggest that among patients utilizing chronic opioid therapy, tolerance to opioid-induced gastrointestinal side effects rarely develops.33 As previously mentioned, patients often modify their treatment regimens or discontinue opioid therapy completely due to side effects, which often results in uncontrolled pain.2–4 Therefore, treatments for OIC must aim to reduce constipation symptoms while allowing patients to continue to receive adequate pain relief. First-line treatments often include nonpharmacologic options such as dietary and lifestyle changes, including increased intake of fluid and fiber and increased exercise. In addition, over-the-counter remedies such as stool softeners, laxatives, and dietary supplements may be used.18,29 In patients with OIC, the American Gastroenterological Association recommends use of laxatives as first-line agents.19 However, over-the-counter agents do not target the μ-opioid receptor, a key factor in the pathophysiology of OIC,29 and are often ineffective.34
There are 4 prescription medications currently approved by the United States Food and Drug Administration (FDA) for OIC: lubiprostone, methylnaltrexone, naldemedine, and naloxegol. Lubiprostone is an orally administered chloride channel-2 agonist that increases fluid in the gastrointestinal tract without interacting with the opioid receptors.35 It is indicated for chronic idiopathic constipation in adults, OIC in adult patients with chronic noncancer pain, and irritable bowel syndrome with constipation. The other 3 approved prescription medications have a different mechanism of action. Methylnaltrexone, naldemedine, and naloxegol are PAMORAs that block the µ-opioid receptors in the gastrointestinal tract and thus decrease the constipation associated with opioid therapy without affecting the central analgesic effects of the opioid.36 Naldemedine and naloxegol are oral medications administered once daily, and methylnaltrexone is available as both a tablet administered once daily or as a solution injected subcutaneously once daily. All 3 PAMORAs (including both the oral and subcutaneous formulations of methylnaltrexone) are indicated for the treatment of OIC in adults with chronic noncancer pain.37–39 Subcutaneous methylnaltrexone is the only PAMORA indicated for the treatment of OIC in adults with advanced illness or pain caused by active cancer.37 In a recent systematic review of 14 randomized controlled trials that assessed PAMORA therapy in patients with OIC, Schwenk et al40 concluded that the PAMORAs as a drug class are effective for the treatment of OIC. Likewise, Pergolizzi et al41 conducted a comprehensive review of PAMORAs approved for OIC, which concluded that the PAMORAs were safe and effective for the treatment of OIC. The authors tabulated safety variables and determined that adverse events commonly reported were often mild to moderate abdominal pain, flatulence, nausea, and diarrhea, for example, which are generally gastrointestinal-related, and an expected consequence of effective laxation.41
Clinical Trials of Treatments for OIC That Assess HR-QOL
Each of the 3 PAMORAs indicated for the treatment of OIC have published analyses that include HR-QOL and patient-reported and/or cost effectiveness outcomes. Common patient-reported outcome assessment tools include the Patient Assessment of Constipation Symptoms (PAC-SYM) and PAC-Quality of Life (PAC-QOL) questionnaires (Table 1).22,23 The PAC-SYM is a 12-item questionnaire that measures constipation-related stool, rectal and abdominal symptoms.23,24 The PAC-QOL questionnaire assesses patient burden of constipation on patients’ functioning and well-being.22 It consists of 28 items that encompass constipation-related HR-QOL measures, including worries and concerns, physical discomfort, psychological discomfort, and satisfaction. The PAC-SYM and PAC-QOL are often used together to assess the magnitude and degree of interference associated with constipation.
Katakami and colleagues conducted a multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 study to assess the safety, efficacy, and HR-QOL of naldemedine 0.2 mg daily in patients with cancer and OIC. A follow-up, 12-week, open-label study further examined the effects of naldemedine on HR-QOL.42 Compared with placebo, naldemedine demonstrated significantly greater improvements on all measures of bowel function, including spontaneous bowel movements and complete spontaneous bowel movement. In the open-label extension study, patients with OIC treated with naldemedine reported significant improvements relative to baseline in all domains of the PAC-SYM and PAC-QOL questionnaires and significantly greater improvements in their HR-QOL on the PAC-SYM questionnaire stool domain and the PAC-QOL questionnaire dissatisfaction domain compared with patients receiving placebo.42
In the COMPOSE-3 randomized, double-blind, phase 3 trial, long-term safety and HR-QOL were evaluated among patients with chronic noncancer pain who received oral naldemedine 0.2 mg daily or placebo.43 HR-QOL outcomes were measured using the PAC-SYM and PAC-QOL questionnaires, which comprised scores from 4 domains including physical discomfort, psychosocial discomfort, satisfaction, and worries or concerns. At weeks 2, 12, 24, 36, and 52, patients who received naldemedine experienced significant (P<0.0001) improvements relative to baseline on the Patient Assessment of Quality of Life questionnaires.
In a placebo-controlled, double-blind analysis of subcutaneous methylnaltrexone 12 mg (once daily or once every other day) for OIC in patients with advanced illnesses, more patients who were administered methylnaltrexone reported rescue-free bowel movements within the first 4 hours of the first dose (34.2% vs. 9.9%, P<0.001).44 At the end of the 28-day double-blind period, significantly greater improvements in PAC-QOL total scores were observed in the methylnaltrexone groups relative to placebo (methylnaltrexone 12 mg daily, P<0.001 and methylnaltrexone 12 mg every other day, P<0.05 both vs. placebo).
In a cost-effectiveness analysis of methylnaltrexone plus standard care or standard care alone for patients with advanced illness and OIC, methylnaltrexone plus standard of care reduced the time patients spent being constipated by 38 days. Despite higher drug costs, these improvements were accompanied by reductions in constipation-related costs (eg, nursing time for enemas or disimpaction).45 Patients receiving methylnaltrexone plus standard care also reported improved quality adjusted life-days compared with standard care alone (76.1 vs. 67.4 d, difference=8.8 d).45 These results show that effective treatment of OIC can improve HR-QOL while remaining cost-effective.
HR-QOL outcomes were also assessed in noncancer patients who received naloxegol (12.5 or 25.0 mg daily) or placebo in the KODIAC-04 study. PAC-SYM and PAC-QOL total and domain scores were maintained throughout the main 12-week study as well as the 12-week extension study.46
Addressing Gaps in Communication
Another aspect of OIC that adds additional burden to the patient is the difference in OIC perceptions between physicians and patients. A prospective, longitudinal, observational cohort study showed that only 65% of physicians knew their patients met the definition of OIC, and 40% of physicians indicated they were not aware of the impact of OIC on their patients.47 Although health care providers and patients were in agreement regarding the severity of pain the patient was experiencing, providers under-rated the extent of OIC symptoms, the impact of OIC on the management of pain, and how OIC affected patients’ HR-QOL.47
Ineffective communication between physicians and patients may be at least partially to blame for these discrepancies in OIC perception. Nearly 70% of patients indicated they were embarrassed to discuss OIC with their physician.12 Both Andresen et al13 and Coyne et al14 reported that only ∼40% to 60% of patients indicated that their physician had discussed the potential for OIC. Nearly 14% of patients expressed concern that their pain medication or dose would be changed, and 9% reporting being embarrassed.14 Rauck et al12 noted that patients frequently reported that “my doctor doesn’t care” or “my doctor is more embarrassed about talking about OIC than me.”
Ineffective communication is also most likely a factor for poor patient education about their OIC. Half of the patients in the survey reported by Andresen et al13 indicated they would have liked their physician to provide additional information about OIC. Although 64% of patients claimed that they got most of their information from their physicians, other sources included search engines (45%), health forums (28%), leaflets in their health care provider’s workplace (21%), television (20%), newspapers/magazines (19%), blogs (12%), other online resources (8%), and partners, friends, and family members (16%, 16%, and 14%, respectively).
Better communication between health care providers and patients may help remove some of the barriers to the effective management of OIC.12 Some of these improvements may be as simple as asking more direct and proactive questions. Table 2 lists potential questions health care providers can ask patients to start the conversation about OIC. Physicians as well as nurses and physician assistants can address patients’ reluctance to talk about bowel movements,13,14 ask patients about any side effects they are experiencing due to opioid use (including constipation), determine if patients are getting sufficient pain relief, and address the impact that OIC has on a patient’s life.
TABLE 2.
Potential Questions to Improve Communication Between Physicians and Patients Regarding OIC

Providing patients with reassurance that they are not alone, that other patients have described a similar effect, or just acknowledging that you understand they are embarrassed can help gain a patient’s trust and open the lines of communication. Stress to them that open communication is essential to effective management of OIC. Asking these questions may go a long way toward individualizing advice to patients and addressing specific questions that need answers.
CONCLUSIONS
Opioid analgesics are frequently prescribed for chronic cancer-related and noncancer-related pain, especially when nonopioid alternatives have not been effective. However, opioid use frequently results in unwanted side effects, such as OIC, that place an additional disease burden on patients who are already experiencing chronic pain. OIC has both physical and psychological impacts that can be debilitating to patients. Thus, effective and targeted treatment strategies are needed. When nonpharmacologic and over-the-counter treatments are ineffective, PAMORAs such as naldemedine and methylnaltrexone can be utilized for effective management of OIC while also improving ratings of HR-QOL and reducing health care costs without compromising the central analgesic effects of opioids.12–14 A more proactive approach to effective communication between physicians and patients may improve outcomes relative to OIC treatment, help patients feel more empowered, and minimize concerns patients and physicians have regarding the use of opioids and OIC.12
ACKNOWLEDGMENT
The authors acknowledge the technical, editorial, and medical writing assistance provided under the direction of the author by Dana A. Franznick, PharmD, of Echelon Brand Communications, LLC, an OPEN Health company, Parsippany, NJ, USA. Funding for this assistance was provided by Salix Pharmaceuticals, a division of Bausch Health US, LLC, Bridgewater, NJ, USA.
Footnotes
C.E.A. has served as a consultant to Salix, AstraZeneca, Shionogi, BDSI, Vertex, Lilly, Teva, Novartis, US WorldMeds, Pfizer, and Flowonix, and as a speaker to US WorldMeds, DSI, Amgen, Novartis, Scilex, Lilly, and Teva.
REFERENCES
- 1. US Opioid Prescribing Rate Maps. 2018. Available at: www.cdc.gov/drugoverdose/maps/rxrate-maps.html Accessed January 31, 2019.
- 2. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 2009;10:35–42. [DOI] [PubMed] [Google Scholar]
- 3. Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;328:2332–2343. [DOI] [PubMed] [Google Scholar]
- 4. Gupta S, Patel H, Scopel J, et al. Impact of constipation on opioid therapy management among long-term opioid users, based on a patient survey. J Opioid Manag. 2015;11:325–338. [DOI] [PubMed] [Google Scholar]
- 5. Wittbrodt ET, Gan TJ, Datto C, et al. Resource use and costs associated with opioid-induced constipation following total hip or total knee replacement surgery. J Pain Res. 2018;11:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5:137–144. [DOI] [PubMed] [Google Scholar]
- 7. Hjalte F, Berggren AC, Bergendahl H, et al. The direct and indirect costs of opioid-induced constipation. J Pain Symptom Manage. 2010;40:696–703. [DOI] [PubMed] [Google Scholar]
- 8. Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. [DOI] [PubMed] [Google Scholar]
- 9. Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract. 2014;2014:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coyne KS, Margolis MK, Yeomans K, et al. Opioid-induced constipation among patients with chronic noncancer pain in the United States, Canada, Germany, and the United Kingdom: laxative use, response, and symptom burden over time. Pain Med. 2015;16:1551–1565. [DOI] [PubMed] [Google Scholar]
- 11. Siemens W, Becker G. Methylnaltrexone for opioid-induced constipation: review and meta-analyses for objective plus subjective efficacy and safety outcomes. Ther Clin Risk Manag. 2016;12:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rauck RL, Hong KJ, North J. Opioid-induced constipation survey in patients with chronic noncancer pain. Pain Pract. 2017;17:329–335. [DOI] [PubMed] [Google Scholar]
- 13. Andresen V, Banerji V, Hall G, et al. The patient burden of opioid-induced constipation: new insights from a large, multinational survey in five European countries. United European Gastroenterol J. 2018;6:1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coyne KS, LoCasale RJ, Datto CJ, et al. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res. 2014;6:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wan Y, Corman S, Gao X, et al. Economic burden of opioid-induced constipation among long-term opioid users with noncancer pain. Am Health Drug Benefits. 2015;8:93–102. [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta A. Improving the recognition and diagnosis of opioid-induced constipation in clinical practice. J Fam Pract. 2015;64 (10 suppl 1):jfp_6410l. [PubMed] [Google Scholar]
- 17. Farmer AD, Holt CB, Downes TJ, et al. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol Hepatol. 2018;3:203–212. [DOI] [PubMed] [Google Scholar]
- 18. Hanson B, Siddique SM, Scarlett Y, et al. American Gastroenterological Association Institute Technical review on the medical management of opioid-induced constipation. Gastroenterology. 2019;156:229–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019;156:218–226. [DOI] [PubMed] [Google Scholar]
- 20. Webster LR. Opioid-induced constipation. Pain Med. 2015;16 (suppl 1):S16–S21. [DOI] [PubMed] [Google Scholar]
- 21. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014;26:1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marquis P, De La Loge C, Dubois D, et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40:540–551. [DOI] [PubMed] [Google Scholar]
- 23. Frank L, Kleinman L, Farup C, et al. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34:870–877. [DOI] [PubMed] [Google Scholar]
- 24. Mapi Research Trust. PROQOLID database: patient assessment of constipation—symptoms (PAC-SYM) [Web Page]. 1999. Available at: https://eprovide.mapi-trust.org/instruments/patient-assessment-of-constipation-symptoms#basic_description Accessed November 21, 2018.
- 25. Van Reenen M, Oppe M. EQ-5D-3L User Guide. Rotterdam, The Netherlands: EuroQol Research Foundation; 2015. [Google Scholar]
- 26. Mapi Research Trust. Bowel Function Diary (BF Diary) [Web Page]. 2011. Available at: https://eprovide.mapi-trust.org/instruments/bowel-function-diary Accessed November 26, 2018.
- 27. Coyne KS, Currie BM, Holmes WC, et al. Assessment of a stool symptom screener and understanding the opioid-;induced constipation symptom experience. Patient. 2015;8:317–327. [DOI] [PubMed] [Google Scholar]
- 28. Rentz AM, Yu R, Muller-Lissner S, et al. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12:371–383. [DOI] [PubMed] [Google Scholar]
- 29. Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16:2324–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veiga DR, Mendonca L, Sampaio R, et al. Incidence and health related quality of life of opioid-induced constipation in chronic noncancer pain patients: a prospective multicentre cohort study. Pain Res Treat. 2018;2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dhingra L, Shuk E, Grossman B, et al. A qualitative study to explore psychological distress and illness burden associated with opioid-induced constipation in cancer patients with advanced disease. Palliat Med. 2013;27:447–456. [DOI] [PubMed] [Google Scholar]
- 32. Iyer S, Davis KL, Candrilli S. Opioid use patterns and health care resource utilization in patients prescribed opioid therapy with and without constipation. Manag Care. 2010;19:44–51. [PubMed] [Google Scholar]
- 33. Panchal SJ, Muller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182(suppl 5A):11S–18S. [DOI] [PubMed] [Google Scholar]
- 35. Spierings EL, Brewer RP, Rauck RL, et al. Lubiprostone for opioid-induced constipation does not interfere with opioid analgesia in patients with chronic noncancer pain. Pain Pract. 2017;17:312–319. [DOI] [PubMed] [Google Scholar]
- 36. Webster LR, Israel RJ. Oral methylnaltrexone does not negatively impact analgesia in patients with opioid-induced constipation and chronic noncancer pain. J Pain Res. 2018;11:1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Relistor [package insert]. Bridgewater, NJ: Salix Pharmaceuticals; 2018.
- 38. Symproic [package insert]. Florham Park, NJ: Shionogi Inc.; 2018.
- 39. Movantik [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2018.
- 40. Schwenk ES, Grant AE, Torjman MC, et al. The efficacy of peripheral opioid antagonists in opioid-induced constipation and postoperative ileus: a systematic teview of the literature. Reg Anesth Pain Med. 2017;42:767–777. [DOI] [PubMed] [Google Scholar]
- 41. Pergolizzi JV, Jr, Christo PJ, LeQuang JA, et al. The use of peripheral μ-opioid receptor antagonists (PAMORA) in the management of opioid-induced constipation: an update on their efficacy and safety. Drug Des Devel Ther. 2020;14:1009–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katakami N, Harada T, Murata T, et al. Randomized phase 3 and extension studies: efficacy and impacts on quality of life of naldemedine in subjects with opioid-induced constipation and cancer. Ann Oncol. 2018;29:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Webster LR, Nalamachu S, Morlion B, et al. Long-term use of naldemedine in the treatment of opioid-induced constipation in patients with chronic non-cancer pain: a randomized, double-blind, placebo-controlled phase 3 study. Pain. 2018;159:987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain. 2011;12:554–562. [DOI] [PubMed] [Google Scholar]
- 45. Earnshaw SR, Klok RM, Iyer S, et al. Methylnaltrexone bromide for the treatment of opioid-induced constipation in patients with advanced illness—a cost-effectiveness analysis. Aliment Pharmacol Ther. 2010;31:911–921. [DOI] [PubMed] [Google Scholar]
- 46. Webster L, Tummala R, Diva U, et al. A 12-week extension study to assess the safety and tolerability of naloxegol in patients with noncancer pain and opioid-induced constipation. J Opioid Manag. 2016;12:405–419. [DOI] [PubMed] [Google Scholar]
- 47. LoCasale RJ, Datto C, Wilson H, et al. The burden of opioid-induced constipation: discordance between patient and health care provider reports. J Manag Care Spec Pharm. 2016;22:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]