Cryptococcal meningitis accounts for 15% of all HIV-related deaths [1]. The overall number of cryptococcal meningitis cases has remained relatively stable in many low-to-middle income countries (LMICs) despite increasing roll-out of antiretroviral therapy (ART). Increasing numbers of patients are at risk of developing cryptococcal meningitis following ART failure or discontinuation, offsetting declines in those presenting for the first time with advanced HIV [2–4]. Over half of patients diagnosed with cryptococcal meningitis in recent studies in sub-Saharan Africa are ART-experienced (i.e. currently receiving or previously received ART) [5,6]. Although there is robust evidence from prospective randomized trials that ART initiation should be delayed until 4–6 weeks after starting antifungal therapy in ART-naïve cryptococcal meningitis patients [7,8], the approach to ART management among ART-experienced cryptococcal meningitis patients lacks adequate evidence, with a paucity of published data.
We are a group of clinicians and researchers from AMBIsome Therapy Induction OptimisatioN (AMBITION), a phase III randomized controlled trial exploring novel treatments for HIV-associated cryptococcal meningitis [9]. In this viewpoint, we aim to synthesize the existing literature on the management of ART-experienced cryptococcal meningitis patients and present the consensus that we have reached regarding the optimal management of these patients, an area where there remains considerable clinical uncertainty.
ART-experienced cryptococcal meningitis patients are a heterogenous group. They can be broadly categorized as those with recent ART initiation (within 6 months); poor/nonadherence to ART with detectable predominantly wild-type virus; treatment failure with ART resistance mutations; or any combination of these. Most studies to date have found no overall difference in acute mortality between patients developing cryptococcal meningitis prior to initiating ART compared with those who are ART-experienced [5,10–14]. One limited study from Botswana including only 26 ART-experienced participants reported 8% in-hospital mortality among ART-experienced cryptococcal meningitis patients versus 21% among ART-naïve [13]. However, a subsequent larger study at the same hospital including 81 other ART-experienced cryptococcal meningitis patients, found no difference in acute mortality between ART-experienced (28%) and ART-naïve individuals (26%). The larger study found higher 1-year mortality in the ART-experienced group, possibly indicating ongoing ART treatment failure or defaulting in this population [14]. However, no such difference was found during long-term follow-up within the antifungal combinations for treatment of cryptococcal meningitis in africa (ACTA) trial [11].
Given the marked heterogeneity in the ART-experienced population, Rhein et al. in Uganda performed a secondary analysis of an adjunctive sertraline trial comparing outcomes among patients who were ART naïve (n = 324), who had initiated, restarted or switched ART in the last 14 days (n = 51), or who had received ART for over 14 days (n = 230) [15]. Although 2-week mortality was similar between ART-naïve and ART-experienced patients overall, 2.5-fold higher mortality was observed among ART-experienced patients who had initiated ART within the previous 14 days (47% vs. 19% in patients on ART for 15 days to 6 months, P < 0.01). These patients likely had subclinical cryptococcal meningitis when ART was initiated, with early immune recovery resulting in an exaggerated central nervous system inflammatory response and fatal unmasking immune reconstitution inflammatory syndrome (IRIS) [10,15]. These findings of excess mortality with unmasking IRIS require further confirmation.
There are currently no data to guide ART management in ART-experienced cryptococcal patients. In particular, addressing whether ART should be continued or temporarily interrupted in patients presenting with suspected unmasking IRIS, optimal timing and choice of ART regimen for re-initiation if withdrawn because of nonadherence, and when failing ART regimens should be switched. In the absence of clear evidence, our pragmatic consensus-based approach, meant to provide interim guidance pending controlled trials, is as follows:
-
1.
We recommend that the balance of risks favours discontinuing ART in patients who are diagnosed with cryptococcal meningitis within 14 days following ART initiation as they are likely to have had active cryptococcal meningitis at ART initiation, and are at high risk of IRIS (which may potentially be abrogated by discontinuing ART at this early stage prior to significant immune reconstitution). The assumption in these cases is that ART was initiated in the context of active central nervous system (CNS) infections. Limited evidence to date suggests increased mortality in these individuals [15]. This includes patients who have re-initiated ART or switched regimens in the previous 14 days. Such patients should then be managed as ART-naïve individuals, with appropriate ART re-initiated at 4–6 weeks post initiation of antifungals.
-
2.
ART should be continued in patients reporting good adherence to ART for 15 days to 6 months. These patients may be presenting with possible unmasking IRIS or with CNS cryptococcal infections that have developed after ART initiation in the context of incomplete immune recovery. Factors, which favour a diagnosis of unmasking IRIS are good ART adherence, low viral load, rise in CD4+ count, and low CSF fungal burden [16]. In all of these cases, our opinion is that interrupting ART is unlikely to improve the clinical course of cryptococcal meningitis as any reversal in the established ART-induced immune restoration and associated IRIS will take days to weeks. Furthermore, stopping ART could place patients at risk for developing drug resistance (if on an non-nucleoside reverse transcriptase inhibitor [NNRTI]-based regimen [17] but much less likely if on dolutegravir-based regimen [18]) and other AIDS-related complications.
-
3.
In patients receiving ART for more than 6 months, the primary explanation for the development of cryptococcal meningitis is most likely virologic failure with an associated drop in CD4+ count resulting from either sub-optimal adherence and/or drug resistance, rather than unmasking IRIS [15]. Another possible explanation is an immunovirological discordant response to ART, although this is uncommon. A good adherence history and prompt viral load measurement could help discriminate, although accurately assessing adherence is challenging and unreliable. If point of care or rapid viral load testing is available and shows viral suppression, we recommend continuation of ART. In those with demonstrated virologic failure, or in whom rapid viral load testing cannot be performed, we advise that ART be discontinued at meningitis diagnosis. ART-experienced patients with virologic failure because of poor adherence may be at similar risk of poor outcomes with rapid ART re-initiation or improved ART adherence following cryptococcal meningitis diagnosis as was observed for ART-naïve patients in the COAT trial [7]. ART continuation in the context of established drug resistance could be futile and potentially lead to further resistance. If discontinued, appropriate ART should be re-introduced after 4–6 weeks of antifungal therapy, alongside enhanced adherence counselling. ART regimens should be chosen based on adherence assessment and genotypic resistance testing if available, and in line with national HIV guidelines (for example, switching from first to second line ART regimen if there has been virological failure).
-
4.
Patients who have defaulted their ART or are not adherent, regardless of prior duration of ART use, should be approached as ART-naïve with ART re-initiated after 4–6 weeks of antifungal treatment. The ART regimen should be selected according to ART history and in line with national HIV guidelines.
Figure 1 below is an illustration of the suggested management of antiretroviral therapy-experienced participants diagnosed with cryptococcal meningitis.
Fig. 1.
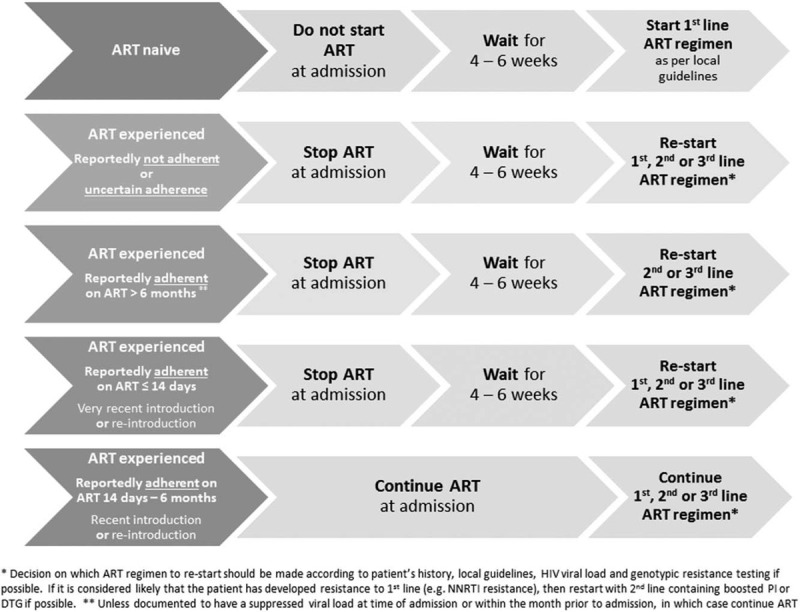
Suggested management of antiretroviral therapy-experienced participants diagnosed with cryptococcal meningitis.
This clinical approach is not all encompassing, reflecting difficulties in providing guidance covering every possible scenario. However, in the absence of detailed recommendations in most national HIV guidelines, we believe this provides a framework for management in most ART-experienced cryptococcal meningitis patients. We acknowledge that many clinicians are understandably reluctant to discontinue ART but we advocate for cryptococcal meningitis patients to be approached as unique cases where withholding ART may be in their best interest. Further research is required to rigorously evaluate ART management strategies that aim to optimize outcomes in ART-experienced cryptococcal meningitis patients and enable us to move from an approach based on expert opinion to one underpinned by a firm evidence base.
Acknowledgements
All of the named authors are co-investigators and/or contributors to the ongoing AMBITION trial, which is jointly funded through the European Developing Countries Clinical Trials Partnership (EDCTP), the Swedish International Development Cooperation Agency (SIDA), and the Wellcome Trust/Medical Research Council (UK)/UKAID Joint Global Health Trials.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenforde MW, Mokomane M, Leeme T, Patel RKK, Lekwape N, Ramodimoosi C, et al. Advanced human immunodeficiency virus disease in Botswana following successful antiretroviral therapy rollout: Incidence of and temporal trends in cryptococcal meningitis. Clin Infect Dis 2017; 65:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osler M, Hilderbrand K, Goemaere E, Ford N, Smith M, Meintjes G, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis 2018; 66:S118–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mpoza E, Rajasingham R, Tugume L, Rhein J, Nabaggala MS, Ssewanyana I, et al. Cryptococcal antigenemia in human immunodeficiency virus antiretroviral therapy–experienced Ugandans with virologic failure. Clin Infect Dis 2019; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molloy SF, Kanyama C, Heyderman RS, Loyse A, Kouanfack C, Chanda D, et al. ACTA Trial Study Team. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 2018; 378:1004–1017. [DOI] [PubMed] [Google Scholar]
- 6.Flynn AG, Meya DB, Hullsiek KH, Rhein J, Williams DA, Musubire A, et al. Evolving failures in the delivery of human immunodeficiency virus care: lessons from a Ugandan meningitis cohort 2006-2016. Open Forum Infect Dis 2017; 4: ofx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Hullsiek KH, Musubire A, et al. COAT Trial Team. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eshun-Wilson I, Okwen MP, Richardson M, Bicanic T. Early versus delayed antiretroviral treatment in HIV-positive people with cryptococcal meningitis. Cochrane Database Syst Rev 2018; 7:CD009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence DS, Youssouf N, Molloy SLF, Alanio A, Alufandika M, Boulware DR, et al. AMBIsome Therapy Induction OptimisatioN (AMBITION): high dose AmBisome for cryptococcal meningitis induction therapy in sub-Saharan Africa: study protocol for a phase 3 randomised controlled non-inferiority trial. Trials 2018; 19:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beardsley J, Wolbers M, Kibengo FM, Ggayi ABM, Kamali A, Cuc NTK, et al. CryptoDex Investigators. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 2016; 374:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanyama C, Molloy SF, Chan AK, Lupiya D, Chawinga C, Adams J, et al. One-year mortality outcomes from the advancing cryptococcal meningitis treatment for Africa Trial of cryptococcal meningitis treatment in Malawi. Clin Infect Dis 2020; 70:521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis JN, Meintjes G, Harrison TS. Outcomes of cryptococcal meningitis in antiretroviral naïve and experienced patients in South Africa. J Infect 2010; 60:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisson GP, Nthobatsong R, Thakur R, Lesetedi G, Vinekar K, Tebas P, et al. The use of HAART is associated with decreased risk of death during initial treatment of cryptococcal meningitis in adults in Botswana. J Acquir Immune Defic Syndr 2008; 49:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RKK, Leeme T, Azzo C, Tlhako N, Tsholo K, Tawanana EO, et al. High mortality in HIV-associated cryptococcal meningitis patients treated with amphotericin B-based therapy under routine care conditions in Africa. Open Forum Infect Dis 2018; 5:ofy267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhein J, Hullsiek KH, Evans EE, Tugume L, Nuwagira E, Ssebambulidde K, et al. ASTRO-CM study team. Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis 2018; 5:ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddow LJ, Colebunders R, Meintjes G, Lawn SD, Elliott JH, Manabe YC, et al. International Network for the Study of HIV-associated IRIS (INSHI). Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis 2010; 10:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geretti AM, Fox Z, Johnson JA, Booth C, Lipscomb J, Stuyver LJ, et al. INSIGHT Strategies for Management of Antiretroviral Therapy (SMART) Study Group. Sensitive Assessment of the Virologic Outcomes of Stopping and Restarting Non-Nucleoside Reverse Transcriptase Inhibitor-Based Antiretroviral Therapy. PLoS One 2013; 8:e69266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llibre JM, Pulido F, García F, García Deltoro M, Blanco JL, Delgado R. Genetic barrier to resistance for dolutegravir. AIDS Rev 2015; 17:56–64. [PubMed] [Google Scholar]


