Abstract
Purpose
To develop two nomograms predicting disease-free survival (DFS) and cancer-specific survival (CSS) and to externally validate them in multiple series.
Methods
Prospectively collected data from a single-centre series of 818 consecutive patients who underwent RC and PLND were used to build the nomogram. External validation was performed in 3,173 patients from 7 centres worldwide. Time to recurrence and to cancer-specific death were addressed with univariable and multivariable analyses. Nomograms were built to predict 2-, 5- and 8-year DFS and CSS probabilities. Predictive accuracy was quantified using the concordance index.
Results
Age, pathologic T stage, lymph-node density and extent of PLND were independent predictors of DFS and CSS (p < 0.05). Discrimination accuracies for DFS and CSS at 2, 5 and 8 years were 0.81, 0.8, 0.79 and 0.82, 0.81, 0.8, respectively, with a slight overestimation at calibration plots beyond 24 months. In the external series, predictive accuracies for DFS and CSS at 2, 5 and 8 years were 0.83, 0.82, 0.82 and 0.85, 0.85, 0.83 for European centres; 0.73, 0.72, 0.71 and 0.80, 0.74, 0.68 for African series; 0.76, 0.74, 0.71 and 0.79, 0.76, 0.73 for American series.
Conclusions
These nomograms developed from a contemporary series are simple clinical tools and provide optimal oncologic outcome prediction in all external cohorts.
Keywords: Nomogram, Prediction, Radical cystectomy, Survival, Urothelial carcinoma
Introduction
The outcome of patients with muscle-invasive urothelial carcinoma of the bladder (UCB) treated with radical cystectomy (RC) and pelvic lymph-node dissection (PLND) mainly depends on pathologic staging. Bochner et al. [1] introduced the first nomogram predicting survival of patients after RC and PLND and demonstrated its superiority over the standard American Joint Committee on Cancer (AJCC) and the tumour-node-metastasis (TNM) staging systems or standard pathologic subgroupings.
In the last decade, clinicians have become more familiar with the use of nomograms in daily clinical practice and nomograms have proven to offer the most accurate prediction of outcomes compared with other prognostic tools [2].
However, today only two nomograms predicting survival after RC are available, and only the one from International Bladder Cancer Nomogram Consortium (IBCNC) was externally validated [1, 3].
The main limitation to the applicability of this nomogram in contemporary settings is the difference between the staging system used (1997 AJCC) and the actual pathologic report (2009 TNM).
Furthermore, none of the nomograms are derived from series with prospective data acquisition.
The goal of this study was to build two nomograms based on a contemporary single-centre series with prospective data acquisition and to perform multiple external validations in series from different continents.
Methods
Study population
Data from 980 consecutive RC carried out at “Regina Elena” National Cancer Institute (Rome, Italy) between January 2000 and December 2009 were collected in a prospectively maintained database. A written informed consent was obtained from all patients before the treatment.
The study was conducted according to the Declaration of Helsinki and was approved by a local ethics committee.
A total of 162 patients were excluded for the following reasons: histology other than pure UCB (54 patients), low grade UCB (six patients), neoadjuvant treatments (18 patients) and RC without curative intent (84 patients); 818 were selected for analysis.
All patients underwent RC; standard (six nodal packages: obturator, internal and external iliac, bilaterally) and extended PLND (nine nodal packages: obturator, internal, external and common iliac bilaterally, presacral) was performed in 518 and 300 patients, respectively [4].
Pathologic stage and 2004 World Health Organization (WHO) tumour grade was assigned by a single genitourinary pathologist according to the 2002 TNM staging system. Between 2001 and 2007, 92 patients were randomly assigned to adjuvant chemotherapy or observation and treatment on relapse according to a prospective randomized trial [5].
External validation was performed in 3,173 patients who met inclusion criteria from seven centres worldwide, divided as follows: 1,793 treated at University of Southern California (USC), Los Angeles (USA) between 1976 and 2007 (American series); 796 treated between 1996 and 2008 at different European Institutions, 256 from “San Giovanni Bosco”, Turin (Italy), 161 from Padua University (Italy), 176 from Humanitas-Gavazzeni, Bergamo (Italy), 245 from Vita-Salute University, San Raffaele, Milan (Italy) and 203 from University Medical Center Hamburg-Eppendorf (Germany); 279 treated between 1995 and 2003 at Mansoura University (Egypt), (African series).
Follow-up regimen
Follow-up was performed according to institutional protocols. Generally, the follow-up schedule included physical examination and routine blood work up, at 3, 6, 12, 18 and 24 months postoperatively, alternatively abdominal ultrasonography and chest X-ray or computed tomography at 6-month intervals for the first 2 years and computed tomography (CT) yearly thereafter.
Urine cytology, urethroscopy, bone scan and positron emission tomography (PET)-CT were performed at the discretion of the treating physician. Any evidence of tumour relapse (pelvic, nodal or visceral, except upper urinary tract) was coded as disease recurrence. Cancer-related death was determined by the treating physicians or by death certificate.
Statistical analysis
Univariable and multivariable Cox regression models addressed time to recurrence and time to cancer-specific death after RC. Predictors included age, gender, pathologic tumour (pT) and pathologic node (pN) stages, lymphovascular invasion (LVI), associated carcinoma in situ, soft tissue surgical margin (STSM) status, lymph-node density (LN-d) and extent of PLND.
Multivariable Cox regression coefficients were then used to generate two nomograms predicting disease-free survival (DFS) and cancer-specific survival (CSS) probabilities, respectively. Predictive accuracy of these nomograms was quantified using Harrell’s concordance indexes (CIs), which was used in this analysis. Calibration plots were generated to explore nomogram performance. The Mann–Whitney U test and the Chi-square tests were used to evaluate differences in continuous and categorical variables, respectively. For all tests, the statistical significance was set at 0.05. Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) v.19.0 and the R statistical package v.3.1.0.
Results
Internal cohort
Clinical and pathologic characteristics of the internal series are shown in Table 1. All patients had evidence of high-grade muscle-invasive UCB at trans-urethral resection of bladder (TURB) or at final pathology except 26 (3.17 %) who underwent RC after Bacillus Calmette-Guerin (BCG) failure or for unresectable non-muscle-invasive disease. Pelvic lymph-node metastases were found in 208 patients (25.4 %). At a median follow-up of 36 months (IQR 18–65), 270 patients (33 %) died, 211 (25.6 %) of whom died of disease.
Table 1.
Clinical and pathologic features of internal cohort
| Characteristics | |
|---|---|
| Age (year) | |
| Mean ± SD (range) | 66.7 ± 9.46 (36–88) |
| Median (IQR) | 67 (60–74) |
| Gender (%) | |
| Male | 700 (85.6) |
| Female | 118 (14.4) |
| Follow-up length (month) | |
| Mean ± SD (range) | 40 ± 33 (0–150) |
| Median (IQR) | 31 (14–56) |
| pT stage (%) | |
| 0-a-is-1-2a | 311 (38) |
| 2b | 110 (13.4) |
| 3a | 123 (15) |
| 3b | 183 (22.4) |
| 4a | 91 (11.1) |
| PLND (%) | |
| Extended | 300 (36.7) |
| Standard | 518 (63.3) |
| pN stage (%) | |
| 0 | 610 (74.6) |
| 1 | 52 (6.4) |
| 2 | 156 (19) |
| Number of nodes removed | |
| Mean ± SD (range) | 26.4 ± 14 (10–90) |
| Median (IQR) | 22 (16–33) |
| LN-d | |
| Mean ± SD | 6.5 % ± 17 |
| Median (IQR) | 0 (0–2) |
| LVI (%) | 262 (32) |
| Associated CIS (%) | 229 (28) |
| Positive soft tissue surgical margins (%) | 16 (1.9) |
| Adjuvant chemotherapy (%) | 86 (10.5) |
At a median time to event of 38 months (IQR 17–61), 290 patients (35.4 %) experienced disease recurrence.
Nomogram development and calibration
DFS and CSS of the internal cohort were reported in Fig. 1. Univariable and multivariable Cox analyses were performed to identify independent predictors of DFS and CSS. At multivariable analysis age, pT stage, LN-d and extent of PLND were independent predictors of DFS (Table 2) and CSS (Table 3).
Fig. 1.
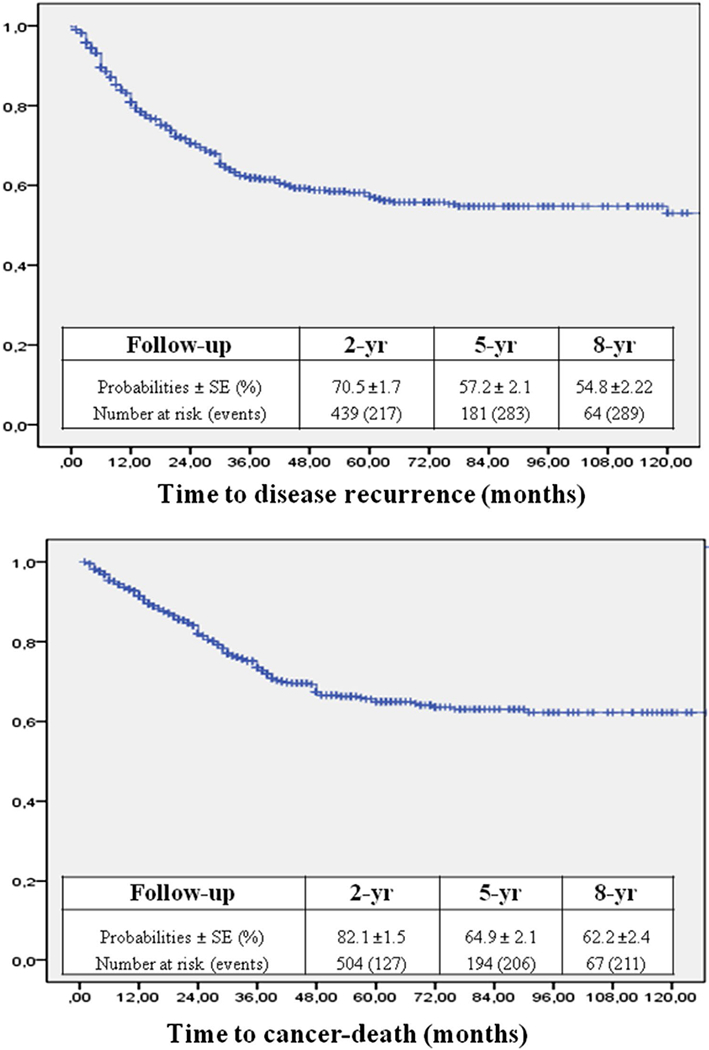
Kaplan–Meier estimates of DFS and CSS of the internal series
Table 2.
Univariable and multivariable Cox regression analyses of internal cohort for prediction of disease-free survival
| Variable | Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p | HR | 95 % CI | p | |
| Age (continuous) | 1.02 | 1.01–1.04 | <0.001 | 1.013 | 1.00–1.03 | 0.047 |
| Gender | ||||||
| Male | Reference category | Reference category | ||||
| Female | 0.9 | 0.65–1.24 | 0.521 | – | ||
| Pathologic tumour stage | ||||||
| 0-a-is-1-2a | Reference category | Reference category | ||||
| 2b | 1.93 | 1.19–3.14 | 0.008 | 2.11 | 1.281–3.476 | 0.003 |
| 3a | 5.06 | 3.41–7.51 | <0.001 | 4.823 | 3.198–7.274 | <0.001 |
| 3b | 6.79 | 4.7–9.81 | <0.001 | 5.789 | 3.894–8.606 | <0.001 |
| 4a | 9.29 | 6.2–13.92 | <0.001 | 7.524 | 4.848–11.679 | <0.001 |
| Pathologic nodal stage (according to 2002 TNM) | ||||||
| 0 | Reference category | Reference category | ||||
| 1 | 2.47 | 1.68–3.64 | <0.001 | Excluded for colinearity with LN-d | ||
| 2 | 4.26 | 3.26–5.58 | <0.001 | |||
| 3 | 7.48 | 4.38–12.77 | <0.001 | |||
| LN-d (continuous) | 1.03 | 1.02–1.033 | <0.001 | 1.02 | 1.014–1.024 | <0.001 |
| LN-d (categorical) | ||||||
| 0 % | Reference category | Reference category | ||||
| 1–11 % | 2.22 | 1.56-3.16 | <0.001 | Included in the model as continuous variable | ||
| 12–30 % | 3.97 | 2.86–5.5 | <0.001 | |||
| 31–100 % | 9.57 | 6.83–13.1 | <0.001 | |||
| Extent of PLND | ||||||
| Standard | Reference category | Reference category | ||||
| Extended | 0.52 | 0.4–0.68 | <0.001 | 0.605 | 0.45–0.80 | 0.001 |
| Associated pTis | 1.30 | 1.02–1.67 | 0.033 | 0.803 | 0.753–1.246 | 0.968 |
| Presence of LVI | 1.34 | 1.05–1.70 | 0.016 | 0.857 | 0.672–1.094 | 0.215 |
| Positive soft tissue surgical margins | 1.50 | 0.71–3.2 | 0.288 | – | ||
| Adjuvant chemotherapy | 1.1 | 0.75–1.2 | 0.9 | – | ||
Table 3.
Univariable and multivariable Cox regression analyses of internal cohort for prediction of cancer-specific survival
| Variable | Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p | HR | 95 % CI | p | |
| Age (continuous) | 1.04 | 1.02–1.05 | <0.001 | 1.021 | 1.01–1.04 | 0.01 |
| Gender | ||||||
| Male | Reference category | Reference category | ||||
| Female | 0.79 | 0.54–1.13 | 0.2 | – | ||
| Pathologic tumour stage | ||||||
| 0-a-is-1-2a | Reference category | Reference category | ||||
| 2b | 2.601 | 1.43–4.74 | 0.002 | 3.04 | 1.61–5.71 | 0.001 |
| 3a | 5.52 | 3.31–9.17 | <0.001 | 5.626 | 3.26–9.72 | <0.001 |
| 3b | 9.31 | 5.81–14.9 | <0.001 | 8.394 | 4.98–14.14 | <0.001 |
| 4a | 14.7 | 8.92–24.2 | <0.001 | 13.04 | 7.48–22.74 | <0.001 |
| Pathologic nodal stage (according to 2002 TNM) | ||||||
| 0 | Reference category | Reference category | ||||
| 1 | 3.09 | 2.01–4.75 | <0.001 | Excluded for colinearity with LN-d | ||
| 2 | 4.88 | 3.56–6.70 | <0.001 | |||
| 3 | 7.82 | 4.38–13.94 | <0.001 | |||
| LN-d (continuous) | 1.03 | 1.02–1.035 | <0.001 | 1.02 | 1.014–1.025 | <0.001 |
| LN-d (categorical) | ||||||
| 0 % | Reference category | Reference category | ||||
| 1–11 % | 2.9 | 1.96–4.29 | <0.001 | Included in the model as continuous variable | ||
| 12–30 % | 4.3 | 2.92–6.32 | <0.001 | |||
| 31–100 % | 10.6 | 7.22–15.49 | <0.001 | |||
| Extent of PLND | ||||||
| Standard | Reference category | Reference category | ||||
| Extended | 0.55 | 0.4–0.75 | <0.001 | 0.656 | 0.47–0.92 | 0.014 |
| Associated pTis | 1.34 | 1.01–1.78 | 0.043 | 1.016 | 0.773–1.363 | 0.916 |
| Presence of LVI | 1.30 | 0.98–1.73 | 0.066 | 1.04 | 0.776–1.385 | 0.81 |
| Positive soft tissue surgical margins | 1.50 | 0.71–3.2 | 0.288 | – | ||
These variables were included in the nomograms to predict 2-, 5- and 8-year DFS and CSS (Fig. 2a, b, respectively). The discrimination accuracies of the nomograms for DFS and CSS at 2, 5 and 8 year were 0.81, 0.81, 0.79 and 0.82, 0.79, 0.78, respectively. The 2-year calibration plots revealed only a slight overestimation of DFS (Fig. 3) and CSS (Fig. 4) risks.
Fig. 2.
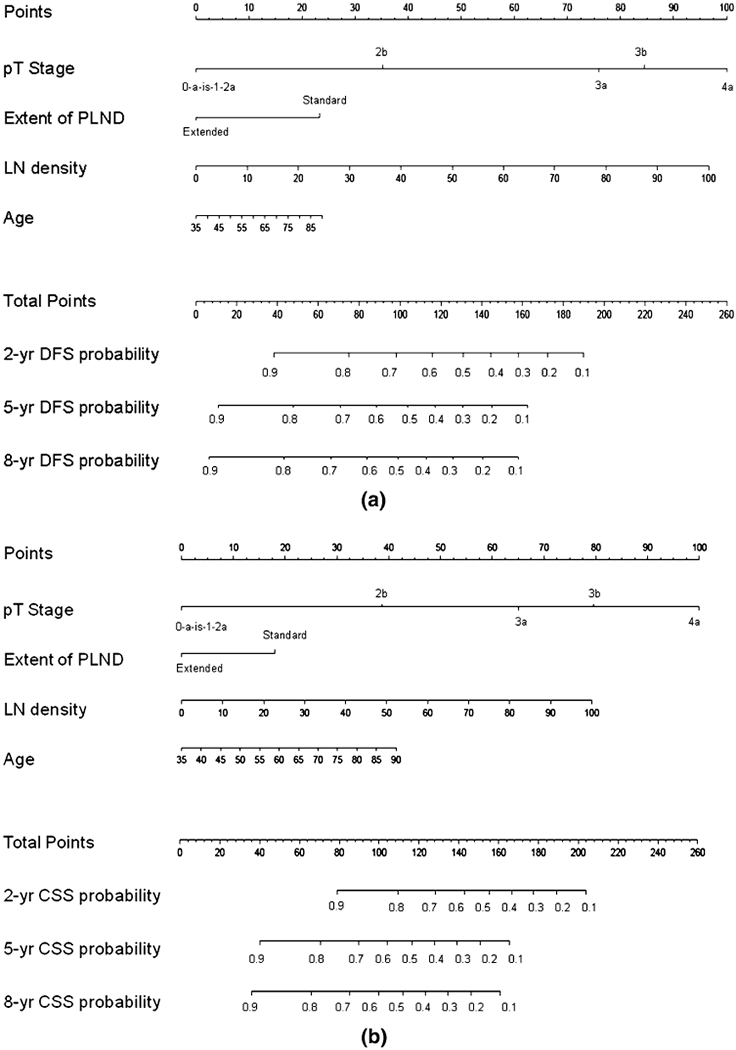
A nomogram for prediction of DFS. b Nomogram for prediction of CSS
Fig. 3.
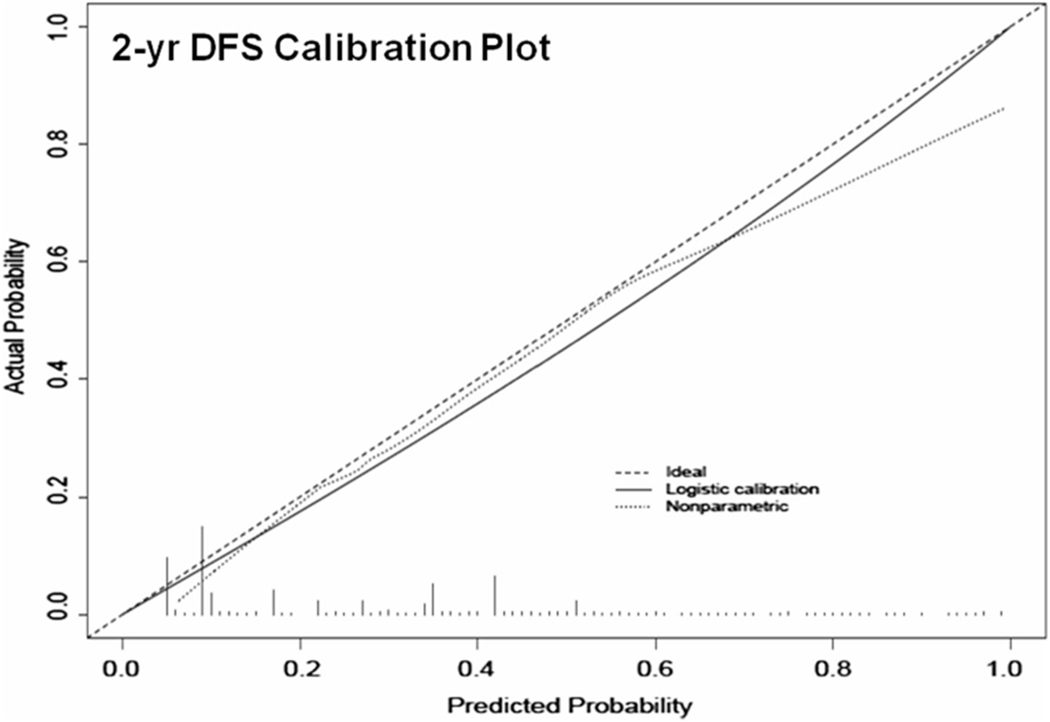
Calibration plot for prediction of 2-year disease-free survival
Fig. 4.
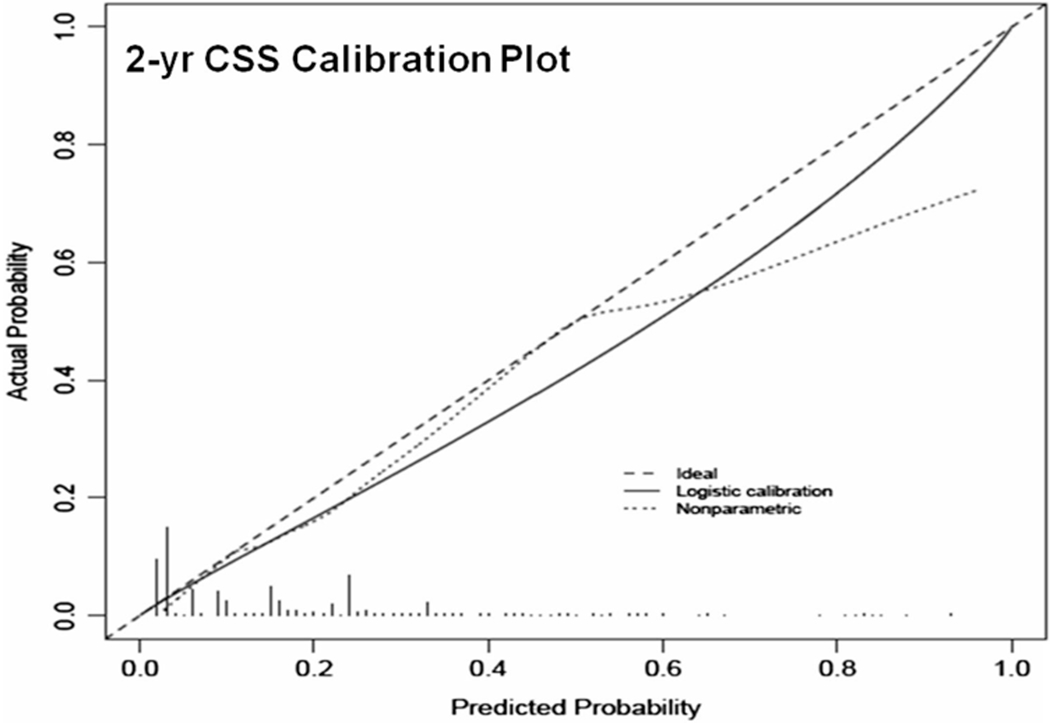
Calibration plot for prediction of 2-year cancer-specific survival
External cohort and nomogram validation
Distribution of variables included in the nomogram among series is shown in Table 4.
Table 4.
Distribution of variables included in the model among series
| Internal | European | European versus internal | American | American versus internal | African | African versus internal | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Mean (range) | 66.7 (36–88) | 65.7 (37–90) | p = 0.027 | 66.3 (23–93) | p = 0.265 | 54.3 (20–75) | p < 0.001 |
| pT stage | |||||||
| pT0-a-is-1-2a | 311 (38 %) | 438 (55 %) | p < 0.001 | 921 (51.4 %) | p < 0.001 | 112 (40.1 %) | p < 0.001 |
| pT2b | 110 (13.4 %) | 86 (10.8 %) | 228 (12.7 %) | 95 (34 %) | |||
| pT3a | 123 (15 %) | 76 (9.3 %) | 167 (9.3 %) | 34 (12.2 %) | |||
| pT3b | 183 (22.4 %) | 110 (15.1 %) | 315 (17.6 %) | 21 (7.5 %) | |||
| pT4a | 91 (11.1 %) | 78 (9.8 %) | 162 (9 %) | 17 (6.1 %) | |||
| PLND | |||||||
| Standard | 518 (63.3 %) | 485 (60.9 %) | p = 0.32 | 391 (21.8 %) | p < 0.001 | 588 (74.7 %) | p < 0.001 |
| Extended | 300 (36.7 %) | 311 (39.1 %) | 1,402 (78.2 %) | 199 (25.3 %) | |||
| LN density | |||||||
| Mean | 6.5 % | 3.3 % | p < 0.001 | 4.0 % | p < 0.001 | 5.3 % | p = 0.141 |
Patients of European series had a higher proportion of organ confined UCB (65.8 vs. 51.4 %, p < 0.001) and a significantly lower mean LN-d (3.3 vs. 6.5 %, p < 0.001) than those of the internal cohort. Patients of American series had a higher proportion of organ confined UCB (64.1 vs. 51.4 %, p < 0.001), a significantly lower LN-d (4 vs. 6.5 %, p < 0.001), a significantly longer follow-up (mean follow-up 87.4 vs. 40 months, p < 0.001) and were more likely to have undergone extended PLND (78.2 vs. 36.7 %, p < 0.001). All these patients had high-grade muscle-invasive UCB, did not undergo neoadjuvant chemotherapy, and had median age comparable with internal series.
Patients of African series were significantly younger (median age 51.7 vs. 66.7, p < 0.001) and had a significantly longer follow-up (mean follow-up 57.3 vs. 40 months, p < 0.001).
In the external series, predictive accuracies for DFS and CSS at 2, 5 and 8 years were 0.83, 0.82, 0.82 and 0.85, 0.85, 0.83 for European centres; 0.73, 0.72, 0.71 and 0.80, 0.74, 0.68 for African series; 0.76, 0.74, 0.71 and 0.79, 0.76, 0.73 for American series (Table 5).
Table 5.
Concordance indexes of both nomograms for each series
| CSS (year) | DFS (year) | |||
|---|---|---|---|---|
| Internal | 2 | 0.83 | 2 | 0.81 |
| 5 | 0.80 | 5 | 0.80 | |
| 8 | 0.79 | 8 | 0.79 | |
| European | 2 | 0.85 | 2 | 0.83 |
| 5 | 0.85 | 5 | 0.82 | |
| 8 | 0.83 | 8 | 0.82 | |
| African | 2 | 0.80 | 2 | 0.74 |
| 5 | 0.72 | 5 | 0.73 | |
| 8 | 0.70 | 8 | 0.72 | |
| American | 2 | 0.79 | 2 | 0.76 |
| 5 | 0.76 | 5 | 0.74 | |
| 8 | 0.73 | 8 | 0.71 |
Discussion
The “ideal” nomogram should combine high discrimination, ease of use and proven efficacy in external validation cohorts. The advantages of nomogram use are not only the individual estimation of prognosis but also a risk-adapted follow-up. Prognosis of patients with muscle-invasive UCB undergoing RC and PLND mainly depends on pT and pN stages. The AJCC staging system, which includes both pT and pN stages, has been considered a standard prognostic tool, however, different nomograms have demonstrated improved survival prediction accuracy [1, 6, 7].
The independent role of pT stage as predictor of CSS was confirmed by several studies, and this variable was integral part of both available nomograms predicting DFS and CSS after RC [1, 3].
In the IBCNC nomogram which was based on 1997 AJCC staging system, patients were further risk stratified for pT0, pTis, pTa and pT1 stages, while in the nomogram by Karakievicz et al. [3] based on 2002 TNM staging system, DFS risk of pT2 patients was not significantly different by that of pT1 patients (p = 0.087) In fact, at Kaplan–Meyer analysis, the DFS of 94 pT1 patients was lower than that of 163 pT2 patients, most likely due to the presence of higher stage disease at time of TURB and the small number of patients. In line with these findings, in our development cohort (818 cases), DFS and CSS of patients with pT stage ≤2a were not significantly different, probably because 96.8 % of patients (792/818) had T2 UC at TURB.
Recently, two studies from an international cohort of 4,431 patients addressed the significant impact of pT substaging into pT2a and pT2b and into pT3a and pT3b, respectively, on oncologic outcomes after RC [8, 9]. With regard to pT substaging, both IBCNC or Karakievicz nomograms grouped patients into four categories (pT1, 2, 3 and 4, respectively), while in our development series, significant differences were observed in terms of DFS and CSS between pT2b, pT3a, pT3b and pT4a compared to the reference category (pT0-a-is-1-2a). Finally, in our series, all patients underwent RC with “intent to cure”; consequently, there was no patient with pT4b UC in the internal series.
Probably, the more significant difference between our nomogram and both the available ones consists of the use of LN-d instead of pN stage. LN-d was first introduced by Herr [10].
Since then, many authors have demonstrated its superiority over pN stage, although with different cut-off points ranging from 4 to 25 % [11–13].
In our nomogram development series, at multivariable analysis, LN-d, together with pT stage, remained the strongest predictor of DFS and CSS, while pN stage was excluded by the model for colinearity. In order to provide the most informative individual risk assessment, LN-d was included in the model as continuous variable, a unique feature of this nomogram. In the IBCNC nomogram cohort, specific LN data, such as the number of removed and positive nodes, were available only for a limited number of patients. As a consequence, and as acknowledged by authors, LN-d failed to improve the prediction accuracy compared with the simple lymph-node status [1]. Similarly, in the nomogram by Karakiewicz et al. pN stage failed to discriminate prognosis between pN1 and pN2 categories. Potential reasons for such differences with available nomograms cited above could be the prospective data collection in a single centre, with all patients receiving a standard or extended PLND and all pathologic reports reviewed by a single uropathologist.
Concerning the anatomical boundaries of PLND, in a prospective series by Abol-Enein et al. [14], patients with pathological node metastases who underwent RC and standard PLND (up to iliac bifurcation) were more likely to experience disease recurrence compared with those who underwent RC and super-extended PLND (up to inferior mesenteric artery). In a recent study aimed at assessing the therapeutic role of an extended PLND (up to iliac bifurcation) versus a standard PLND in a series of 933 patients, the benefit of an extended PLND was significant across all pT stages but pT < 2 and across all pN stages [4]. However, in a retrospective comparison of two series from USC and from Bern University, super-extended PLND failed to provide any benefit in terms of cancer control outcomes compared with standard PLND [15]. Hopefully, two ongoing prospective randomized trials (the SWOG trial S1011 [16] and the German multicenter study LEA [17]) will answer the question regarding the optimal anatomical template in order to standardize PLND during RC.
This nomogram is the first to include the extent of the PLND as a variable, however, given the lack of difference reported between extended versus super-extended dissections, patients were not further stratified. The last variable included in both nomograms was the age of patients. In a recent paper by Fairey et al. [18], patients older than 80 were more likely to experience disease recurrence after RC (HR 2.06, 95 % CI 1.57–2.70) and had a significant increased risk of cancer-related death compared to the reference group of patients younger than 60 (HR 1.56, 95 % CI 1.09–2.24).
In a series of 1,545 patients who experienced recurrence after RC, Rink et al. [19] found advanced age and female gender significantly associated with CSS. However, in our nomogram development cohort, female gender was not associated with DFS or CSS.
Other pathologic features, such as LVI and positive STSM, were advocated as prognosticators of recurrence after RC and PLND. LVI was defined as the unequivocal presence of tumour cells within an endothelium-lined space with no underlying muscular walls [20, 21]. Effectively, this suggests that each equivocal focus, which is a common finding, should be clarified through immunohistochemistry to distinguish artefacts from involvement of either the lymphatic or vascular lumen, which was not performed in published series “in keeping with the pathologist’s practice” [22]. In the internal series, LVI was not an independent predictor of DFS and CSS. Novara et al. [23] provided evidence supporting STSM status as a powerful predictor of DFS and CSS. In a multicentre series of 4,410 patients, positive STSM was an independent predictor of both DFS and CSS (HR 1.52, 1.51; p < 0.001, respectively).
In the internal series, the incidence of positive surgical margins was significantly lower than that reported by Novara et al. (1.9 vs. 6.3 %). A possible explanation for this difference can be the exclusion of patients who underwent salvage RC and consequently the lack of patients with pT4b disease at final pathology in the nomogram development cohort. As a consequence, positive STSM was not a predictor of oncologic outcomes in the internal series and was not included in the model.
We recognize that the patients who formed the internal cohort were treated at a tertiary referral centre, and therefore the outcomes outside this setting can be significantly different. In fact, intrinsic limitations of the nomograms built in this study are the need of a standardized PLND, coded as standard, extended or super extended. The accuracy of these nomograms could be significantly impaired in patients undergoing “salvage RC”, or cystectomy without PLND, as well as in patients undergoing limited PLND or PLND with a template missing one or more nodal packages among obturator, hypogastric and external iliac nodes. A “separate package” PLND was also supported by a single genitor-urinary pathologist who performed a meticulous lymph-node count, a variable potentially affecting the prognostic powerful of LN-d. In addition, the nomogram development was based on a series of patients with UCB, thus the prediction accuracy for histologies other than pure UC requires further validation.
Another limitation to the use of this nomogram in contemporary settings come from the increasing use of neoadjuvant chemotherapy, based on a level 1 evidence of a 5 % overall survival increase in patients receiving three cycles of methotrexate, vinblastine, doxorubicin and cisplatin.
This survival benefit was evident in a 38 % of patients who had no residual disease at final pathology (pT0) [24]. However, recently, Reardon et al. [25] retrospectively analysed the trend in the use of perioperative chemotherapy in a cohort of 5,692 patients from the National Cancer Database. Interestingly, despite a significant increase in the use of neoadjuvant chemotherapy for muscle-invasive UCB from 10.1 % in 2006 to 20.8 % in 2010 (p = 0.005), a lot of variables, including advanced age, increasing comorbidity, lack of insurance, increased travel distance, geographic location outside the north-eastern USA and lower income were negatively associated with perioperative chemotherapy receipt.
With regard to adjuvant chemotherapy, univariable analyses failed to demonstrate any survival benefit in the internal cohort, a finding supported by a previous prospectively randomized trial [5].
Finally, these nomograms were built on a single-centre prospective series of patients treated with RC and “separate package” PLND.
Ease of use, accessibility of variables available in a contemporary pathological report, together with highly accurate discrimination of 2-, 5- and 8-year DFS and CSS on multiple series from different continents and from centres with different case-loads make these nomograms prediction tools widely applicable in daily clinical practice.
Contributor Information
Giuseppe Simone, Department of Urology, “Regina Elena” National Cancer Institute, Via Elio Chianesi 53, 00144 Rome, Italy; Department of Urology, “San Giovanni Bosco” Hospital, Turin, Italy.
Marco Bianchi, Department of Urology, Vita-Salute University, Milan, Italy.
Diana Giannarelli, Department of Urology, “Regina Elena” National Cancer Institute, Via Elio Chianesi 53, 00144 Rome, Italy.
Siamak Daneshmand, Institute of Urology, University of Southern California/Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Rocco Papalia, Department of Urology, “Regina Elena” National Cancer Institute, Via Elio Chianesi 53, 00144 Rome, Italy.
Mariaconsiglia Ferriero, Department of Urology, “Regina Elena” National Cancer Institute, Via Elio Chianesi 53, 00144 Rome, Italy.
Salvatore Guaglianone, Department of Urology, “Regina Elena” National Cancer Institute, Via Elio Chianesi 53, 00144 Rome, Italy.
Steno Sentinelli, Department of Pathology, “Regina Elena” National Cancer Institute, Rome, Italy.
Renzo Colombo, Department of Urology, Vita-Salute University, Milan, Italy.
Francesco Montorsi, Department of Urology, Vita-Salute University, Milan, Italy.
Devis Collura, Department of Urology, “San Giovanni Bosco” Hospital, Turin, Italy.
Giovanni Muto, Department of Urology, “San Giovanni Bosco” Hospital, Turin, Italy.
Giacomo Novara, Department of Surgical, Oncological and Gastroenterologic Sciences, Urology Clinic, University of Padua, Padua, Italy.
Rodolfo Hurle, Department of Urology, Humanitas-Gavazzeni, Bergamo, Italy.
Michael Rink, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Margit Fisch, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Hassan Abol-Enein, Department of Urology, Mansoura University, Mansoura, Egypt.
Gus Miranda, Institute of Urology, University of Southern California/Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Mihir Desai, Institute of Urology, University of Southern California/Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Inderbir Gill, Institute of Urology, University of Southern California/Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Michele Gallucci, Department of Urology, “Regina Elena” National Cancer Institute, Via Elio Chianesi 53, 00144 Rome, Italy.
References
- 1.Bochner BH, Kattan MW, Vora KC et al. (2006) Nomogram for predicting disease recurrence after radical cystectomy for bladder cancer. J Clin Oncol 24:3967–3972 [DOI] [PubMed] [Google Scholar]
- 2.Shariat SF, Karakiewicz PI, Suardi N, Kattan MW (2008) Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res 14:4400–4407 [DOI] [PubMed] [Google Scholar]
- 3.Karakiewicz PI, Shariat SF, Palapattu GS et al. (2006) Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol 176:1354–1361 [DOI] [PubMed] [Google Scholar]
- 4.Simone G, Papalia R, Ferriero M et al. (2013) Stage-specific impact of extended versus standard pelvic lymph node dissection in radical cystectomy. Int J Urol 20:390–397 [DOI] [PubMed] [Google Scholar]
- 5.Cognetti F, Ruggeri EM, Felici A et al. (2012) Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian, multicenter, randomized phase III trial. Ann Oncol 23:695–700 [DOI] [PubMed] [Google Scholar]
- 6.Shariat SF, Karakiewicz PI, Palapattu GS et al. (2006) Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res 12:6663–6676 [DOI] [PubMed] [Google Scholar]
- 7.Nuhn P, May M, Sun M et al. (2012) External validation of postoperative nomograms for prediction of all-cause mortality, cancer-specific mortality, and recurrence in patients with urothelial carcinoma of the bladder. Eur Urol 61:58–64 [DOI] [PubMed] [Google Scholar]
- 8.Tilky D, Reich O, Karakiewicz PI et al. (2010) Validation of the AJCC TNM substaging of pT2 bladder cancer: deep muscle invasion is associated with significantly worse outcome. Eur Urol 58:112–117 [DOI] [PubMed] [Google Scholar]
- 9.Sonpavde G, Khan MM, Svatek RS et al. (2011) Prognostic risk stratification of pathological stage T3N0 bladder cancer after radical cystectomy. J Urol 185:1216–1221 [DOI] [PubMed] [Google Scholar]
- 10.Herr HW (2003) Superiority of ratio based lymph node staging for bladder cancer. J Urol 169:943–945 [DOI] [PubMed] [Google Scholar]
- 11.Simone G, Papalia R, Ferriero M et al. (2012) Development and external validation of lymph node density cut-off points in prospective series of radical cystectomy and pelvic lymph node dissection. Int J Urol 19:1068–1074 [DOI] [PubMed] [Google Scholar]
- 12.Bruins HM, Huang GJ, Cai J, Skinner DG, Stein JP, Penson DF (2009) Clinical outcomes and recurrence predictors of lymph node positive urothelial cancer after cystectomy. J Urol 182:2182–2187 [DOI] [PubMed] [Google Scholar]
- 13.Quek M, Flanigan R (2009) The role of lymph node density in bladder cancer prognostication. World J Urol 27:27–32 [DOI] [PubMed] [Google Scholar]
- 14.Abol-Enein H, Tilki D, Mosbah A et al. (2011) Does the extent of lymphadenectomy in radical cystectomy for bladder cancer influence disease-free survival? A prospective single-center study. Eur Urol 60:572–577 [DOI] [PubMed] [Google Scholar]
- 15.Zehnder P, Studer UE, Skinner EC et al. (2011) Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol 186:1261–1268 [DOI] [PubMed] [Google Scholar]
- 16.swog.org (homepage on the Internet). SWOG S1011 bladder cancer trial: patient information. http://swog.org/patients/s1011/ [Google Scholar]
- 17.Association of Urogenital Oncology (AUO). Eingeschränkte vs Ausgedehnte lymphadenektomie LEA (Limited vs Extended lymphadenectomy LEA). In: ClinicalTrials.gov (website on the Internet). Bethesda, MD: US National Library of Medicine; 2011. Updated 7 Sept 2011 http://clinicaltrials.gov/ct2/show/NCT01215071 [Google Scholar]
- 18.Fairey AS, Kassouf W, Aprikian AG et al. (2012) Age _ 80 years is independently associated with survival outcomes after radical cystectomy: results from the Canadian Bladder Cancer Network Database. Urol Oncol 30:825–832 [DOI] [PubMed] [Google Scholar]
- 19.Rink M, Lee DJ, Kent M (2013) Predictors of cancer-specific mortality after disease recurrence following radical cystectomy. BJU Int 111(3 Pt B):E30–E36. doi: 10.1111/j.1464-410X.2012.11433.x [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi E, Horiguchi Y, Nakashima J et al. (2005) Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol 174:2120–2123 [DOI] [PubMed] [Google Scholar]
- 21.Saito K, Kawakami S, Fujii Y, Sakura M, Masuda H, Kihara K (2007) Lymphovascular invasion is independently associated with poor prognosis in patients with localized upper urinary tract urothelial carcinoma treated surgically. J Urol 178:2291–2296 [DOI] [PubMed] [Google Scholar]
- 22.Simone G, Papalia R, Loreto A, Leonardo C, Sentinelli S, Gallucci M (2008) Independent prognostic value of tumour diameter and tumour necrosis in upper urinary tract urothelial carcinoma. BJU Int 103:1052–1057 [DOI] [PubMed] [Google Scholar]
- 23.Novara G, Svatek RS, Karakiewicz PI et al. (2010) Soft tissue surgical margin status is a powerful predictor of outcomes after radical cystectomy: a multicenter study of more than 4,400 patients. J Urol 183:2165–2170 [DOI] [PubMed] [Google Scholar]
- 24.Grossman HB, Natale RB, Tangen CM et al. (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859–866 [DOI] [PubMed] [Google Scholar]
- 25.Reardon ZD, Patel SG, Zaid HB et al. (2014) Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol. doi: 10.1016/j.eururo.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


