Abstract
Objective
To update our previous analysis of the clinical and pathological impact of the change in the submission of lymphadenectomy specimens from en bloc to 13 separate anatomically defined packets, which took place at the University of Southern California in May 2002, and to determine whether lymph node (LN) packeting resulted in any change in oncological outcomes.
Patients and Methods
A total of 846 patients who underwent radical cystectomy (RC) with super-extended LN dissection for cTxN0M0 bladder cancer between January 1996 and December 2007 were identified. Specimens of 376 patients were sent en bloc (group 1), and specimens of 470 patients were sent in 13 separate anatomical packets (group 2).
Results
The pathological tumour stage distribution and the proportion of LN-positive patients (group 1: 82 patients [22%] versus group 2: 99 patients [21%]; P = 0.80) were similar between the two groups: the median [range] number of total LNs identified increased significantly (group 1: 32 [10–97] versus group 2: 65 [10–179]; P < 0.001). LN density decreased (group 1, 11% versus group 2, 4%; P = 0.005). The median [range] number of positive LNs removed was similar (group 1: 0 [0–30] versus group 2: 0 [0–97]; P = 0.87). No nodal stage shift was observed. The 5-year overall survival (group 1: 58% versus group 2: 59%; P = 0.65) and recurrence-free survival rates (group 1: 68% versus group 2: 70%; P = 0.57) were similar.
Conclusions
The incidence of patients with positive LNs remained unchanged, regardless of how the LN specimen was submitted. Submitting 13 separate nodal packets significantly increased the total LN yield, but did not result in a significant increase in the number of positive LNs or a consecutive nodal stage shift and did not affect oncological outcomes. Based on these results LN density is not an accurate prognosticator.
Keywords: cystectomy, lymphadenectomy, outcome, separate packets, lymph node density
Introduction
Ten years ago several reports showed improved survival when a higher number of lymph nodes (LNs) were removed during radical cystectomy (RC) for bladder cancer [1,2]. At about the same time, LN density (defined as the ratio of positive LNs to the total number of removed LNs) was reported to be a prognostic marker for disease-specific survival [3,4]. With the aim of verifying these findings and to provide information for future LN mapping studies, the method of lymphadenectomy specimen submission to the pathologist during RC at the University of Southern California changed in May 2002 from en bloc to 13 separate, anatomically defined nodal packets. Our analysis in 2007 showed that submitting lymphadenectomy specimens in separate packets significantly increased the number of total LNs identified per patient and consequently decreased LN density. At the time, the results suggested that when separate lymph node packets were sent, there was a slightly but not significantly higher yield [5]. In the present study, we update our previous findings, evaluating whether the change in submission methodology had an impact on histopathological results and whether alterations in LN yield translated into better survival.
Materials and Methods
Patients
The present retrospective analysis was based on our internal review board-approved prospectively collected institutional cystectomy database, which includes data on patients treated since 1971. Included were patients who underwent RC and super-extended pelvic LN dissection (LND) with intent to cure between January 1996 and December 2007 for clinically non-metastatic (cN0M0: no palpable mass in bimanual examination under anaesthesia, no CT evidence of extravesical disease) urothelial bladder cancer. Patients were excluded if they had received neoadjuvant therapy (n = 55), had positive LNs on staging CT scans (n = 34) or had positive surgical margins (n = 10). The change to submission methodology occurred in May 2002. All patients who underwent en bloc RC were operated on before May 2002 and were analysed as group 1. Patients who underwent RC and extended LND with LN submission in separate nodal packets had surgery between May 2002 and December 2007 and were combined in group 2, without any overlap between the groups. Of the 846 patients, 376 underwent en bloc lymphadenectomy (group 1) and 470 (group 2) underwent LN submission in 13 separate nodal packets. Both groups were compared with respect to patient characteristics, rate of peri-operative complications and mortality (within 30 days of surgery), postoperative pathological staging, use of adjuvant therapies as well as oncological outcomes. None of the patients was lost to follow-up.
Surgical Technique
The surgical approach involving super-extended pelvic LND and specimen submission has been described previously [5]. Briefly, template boundaries for super-extended LND were the inferior mesenteric artery proximally, the genitofemoral nerve laterally, the circumflex iliac vein and Cloquet’s node distally, the hypogastric vessels posteriorly, including the obturator fossa, the presciatic (fossa of Marcille) nodes bilaterally and the presacral area (medial to the internal iliac artery and caudal to the common iliac artery). The 13 nodal packets included left paraaortic, right paracaval, bilateral common iliac, external iliac, obturator fossa, presciatic and presacral packet, as well as Cloquet’s node.
Pathological Findings
All specimens were examined according to the same protocol applied by three dedicated genitourinary pathologists of the department of pathology, including a gross and microscopic examination. Multiple sections and histological evaluation were performed on the primary bladder tumour, bladder wall (adjacent and distant bladder mucosa from the primary tumour) and all LNs. LNs were identified visually and by palpation without clearing techniques, solvents or special stains. No distinction was made regarding the amount of LN tumour involvement (microscopic versus macroscopic) or extracapsular nodal extension. Histological grading was performed according to the method used by Bergkvist et al. [6]. The postoperative TNM staging was based on the 6th edition (2002) of the American Joint Committee on Cancer staging manual. Pathological characteristics of the bladder tumour that were evaluated included tumour multifocality, carcinoma in situ, lymphovascular invasion and surgical margin status. The only difference was the method by which LNs were submitted to the pathologist at RC, that is en bloc (group 1) or in separate packets (group 2).
Statistical Analysis
The statistical software package SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used. Pearson’s chi-squared and Fisher’s exact test were applied to examine the association between categorical demographic and clinical variables, and the Wilcoxon’s rank-sum test was used to analyse differences in not normally distributed continuous variables. Kaplan–Meier plots were used to estimate the probabilities of overall (OS) and recurrence-free survival (RFS) for every year since RC. Log-rank tests were used to compare the survival differences according to pathological subgroups. All P values reported are two-sided. A P value <0.05 was taken to indicate statistical significance. In addition to the global group comparison, inter-surgeon variability in overall LN counts and LN positivity was assessed, evaluating the three urologists with the highest RC volumes.
Results
The median (range) follow-up for patients with the en bloc submission technique (group 1) was 9.6 (0.3–13.4) years compared with 3.9 (0.3–7.9) years for those with the submission of 13 separate packets after May 2002 (group 2; P < 0.001). Patients from both groups were of similar ages at the time of surgery (P = 0.81) and similar gender distribution (P = 0.38), with predominantly males affected. In both groups, an equal proportion of patients (21%) was found to have LN-positive disease (group 1, n = 82; group 2, n= 99; P = 0.79 (Table 1)).
Table 1.
Patient characteristics.
| Group 1 | P | Group 2 | |||||
|---|---|---|---|---|---|---|---|
| Number of patients | 376 | 0.81 | 470 | ||||
| Median (range) age, years | 68 (33–93) | 0.38 | 69 (36–92) | ||||
| Gender: male/female, % | 77/23 | 79/21 | |||||
| Pathological subgroups, n (%) | LN− | LN+ | 0.86 | LN− | LN+ | ||
| pT0 | 27 (7) | 27 (100) | 0 | – | 34 (7) | 34 (100) | 0 |
| pTa | 14 (4) | 14 (100) | 0 | – | 16 (3) | 16 (100) | 0 |
| pTis | 48 (13) | 45 (94) | 3 (6) | 0.16 | 69 (15) | 68 (99) | 1 (1) |
| pT1 | 68 (18) | 62 (91) | 6 (9) | 0.8 | 78 (16) | 72 (92) | 6 (8) |
| pT2 | 85 (22) | 69 (81) | 16 (19) | 0.6 | 101 (22) | 85 (84) | 16 (16) |
| pT3 | 105 (28) | 58 (55) | 47 (45) | 0.84 | 138 (30) | 78 (57) | 60 (43) |
| pT4 | 29 (8) | 19 (66) | 10 (34) | 0.31 | 34 (7) | 18 (53) | 16 (47) |
| Overall LN positive patients, n (%) | 82 (21) | 0.79 | 99 (21) | ||||
| pN1 | 23 (6) | 0.46 | 24 (5) | ||||
| pN2 | 56 (15) | 71 (15) | |||||
| pN3 | 0 | 4 (1) | |||||
| Median (range) total no. LNs identified | 32 (10–97) | <0.001 | 65 (10–179) | ||||
| Median (range) positive LNs identified | 0 (0–30) | 0.87 | 0 (0–97) | ||||
| Median (range) LN density, % | 11 (1–73) | 0.0046 | 4 (1–88) | ||||
| Peri-operative mortality, n (%) | 6 (1.6) | 0.32 | 4 (0.9) | ||||
| Adjuvant chemotherapy, n (%) | 98 (26) | 0.006 | 85 (18) | ||||
| Median (range) OS, years | 8.3 (0.3–13.4) | 0.65 | 7.9 (0.3–7.9) | ||||
| Median (range) RFS, years | 13.4 (0.3–13.4) | 0.57 | 7.9 (0.3–7.9) |
LN, lymph node; OS, overall survival; RFS, recurrence-free survival.
Mortality
The peri-operative mortality rate (during hospital stay) was 1.6% (six patients) in group 1 compared with 0.9% (four patients) in group 2 (P = 0.32).
Pathological Findings
No difference was found between the two cohorts when assessing pathological stages of the primary bladder tumour (P = 0.86). The detailed comparison on a stage-by-stage level and stratified according to LN status showed no difference either. In group 1 organ-confined but LN-positive disease was detected in 25 patients (10%), while 57 patients (42%) had extravesical and LN-positive disease. Similarly, in group 2, 23 patients (7.8%) had organ-confined but LN-positive disease, while 76 (44%) had extravesical and LN-positive disease (Table 1).
Total Number of LNs Identified and Positive LNs
The median (range) number of LNs identified in group 1 was 32 (10–97), which was significantly lower than in group 2, in which the median (range) was 65 (10–179) LNs (P < 0.001). The overall number of positive LNs detected did not differ between the two groups (P = 0.87; Table 1). Likewise, similar impacts on overall LN counts and LN positivity were found when individually assessed for the three surgeons with the highest intra-institutional cystectomy volumes, who together performed 88% of all analysed RCs (Table 2A,B; P = 0.46). Analysis of the number of positive LNs identified per patient (group 1: median 3, range 1–30 versus group 2: median 3, range 1–97) showed no difference between the groups. Of the 82 patients with LN metastases in group 1, 23 (6%) had nodal stage pN1, 56 (15%) had stage pN2, while no patient was found to have stage pN3 disease. Similarly, pN stage in group 2 was pN1 in 24 patients (5%), pN2 in 71 patients (15%) and pN3 in four patients (1%; P = 0.46 (Table 1)).
Table 2.
Lymph node counts for (A) all three surgeons and (B) each of the three surgeons: data from the three surgeons with the highest volumes (n = 744).
| (A) |
All three surgeons |
P | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LN submission | En bloc | 13 packets | |||||||||||
| No. of RCs performed January 1996 to December 2007 | 330 | 414 | |||||||||||
| Total LNs identified, median (range) | 34 (10–97) | 68 (10–179) | <0.001 | ||||||||||
| Total positive LNs identified, median (range) | 0 (0–30) | 0 (0–97) | 0.46 | ||||||||||
| (B) | Surgeon 1 | Surgeon 1 | P | Surgeon 2 | Surgeon 2 | P | Surgeon 3 | Surgeon 3 | P | ||||
| En bloc | 13 packets | En bloc | 13 packets | En bloc | 13 packets | ||||||||
| No. of RCs performed January 1996 to December 2007 | 224 | 186 | 49 | 159 | 57 | 69 | |||||||
| Total LNs identified, median (range) | 37 (10–97) | 67 (10–179) | <0.001 | 31 (10–80) | 71 (13–162) | <0.001 | 26 (10–63) | 63 (10–132) | <0.001 | ||||
| Total positive LNs identified, median (range) | 0 (0–30) | 0 (0–53) | 0.43 | 0 (0–23) | 0 (0–97) | 0.36 | 0 (0–17) | 0 (0–81) | 0.6 | ||||
LN, lymph node; RC, radical cystectomy.
Lymph Node Density
Among patients who were LN-positive, there was a significantly higher median LN density in group 1 than in group 2 (group 1: 11% versus group 2: 4%; P = 0.0046 (Table 1)).
Adjuvant Chemotherapy
Overall, fewer patients received adjuvant chemotherapy after 2002 (group 1: 26% versus group 2: 18%; P = 0.006 (Table 1)).
Patient Outcome
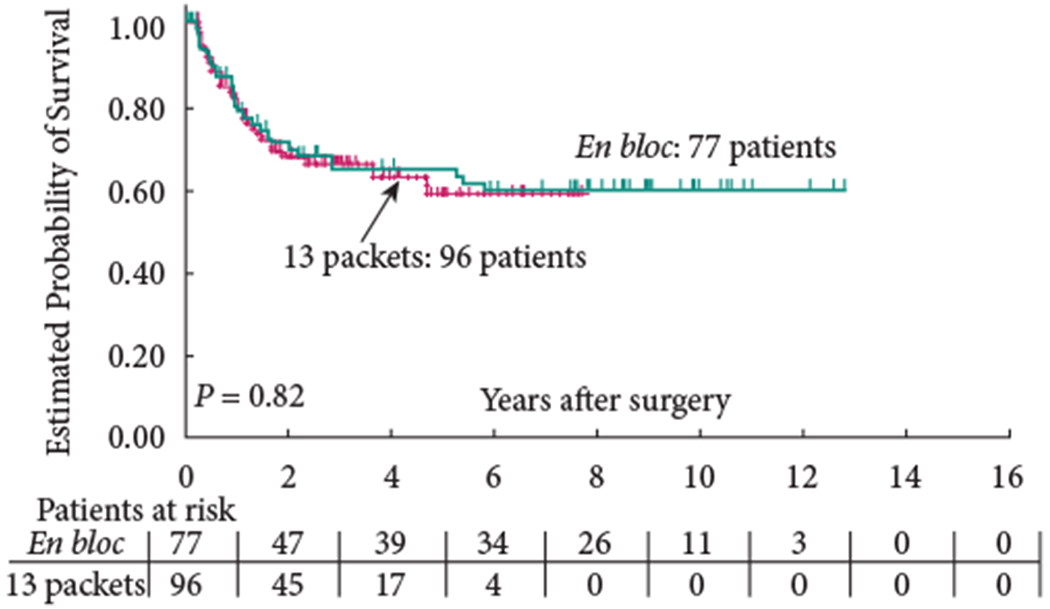
The 5-year OS (group 1: 58% versus group 2: 59%; P = 0.65) and 5-year RFS (group 1: 68% versus group 2: 70%; P = 0.57) were similar between the two groups (Figs 1,2). In addition, RFS remained unaffected when stratified according to pathological subgroups (organ-confined LN-negative disease, extravesical LN-negative disease, LN-positive disease; Figs 3–5).
Fig. 1.
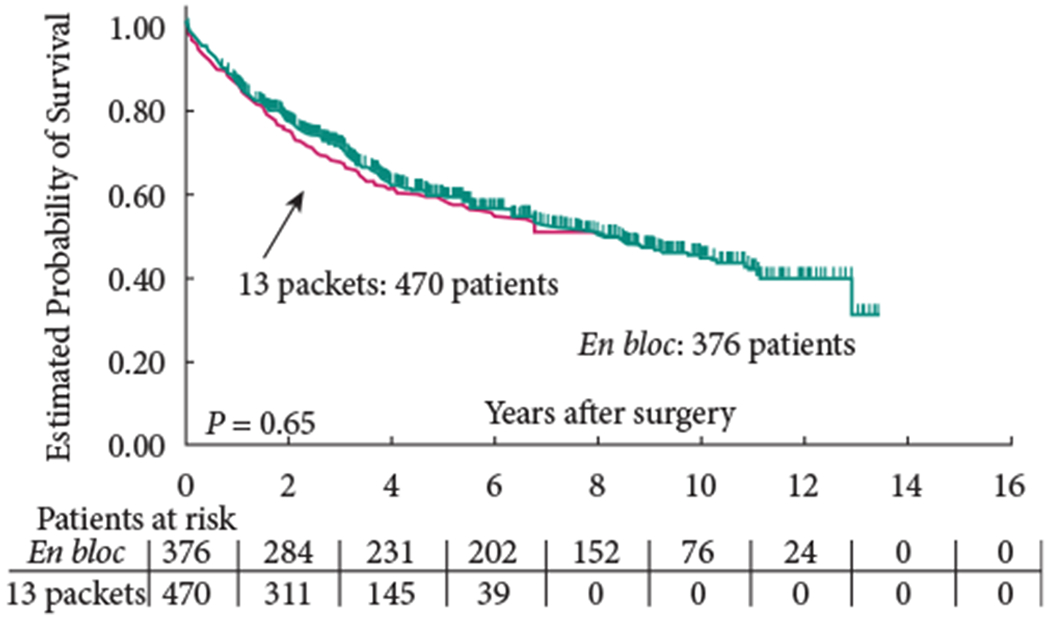
Overall survival.
Fig. 2.
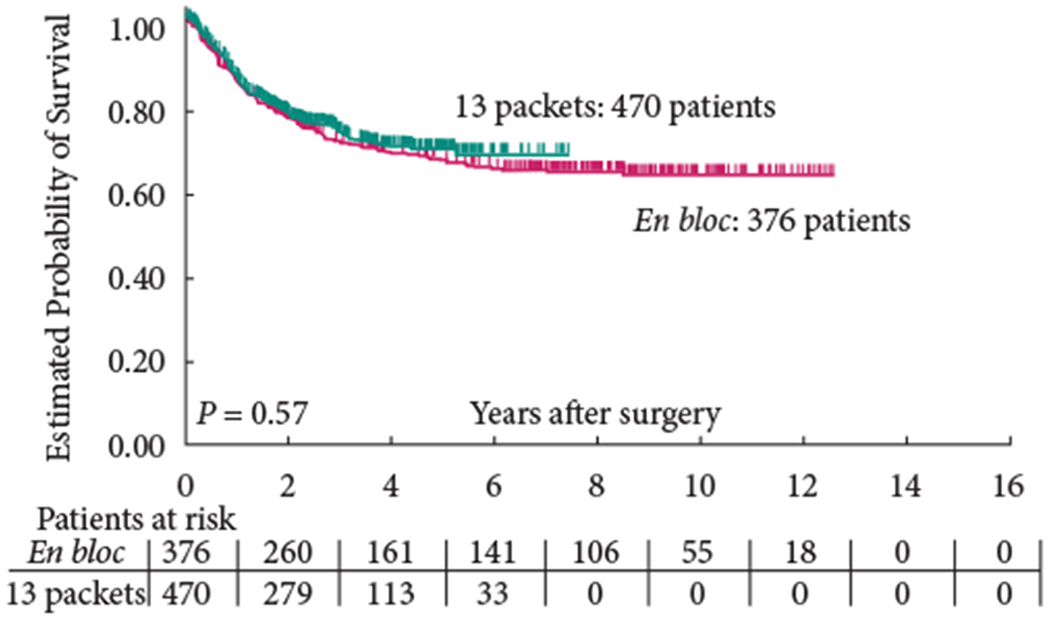
Recurrence-free survival.
Fig. 3.
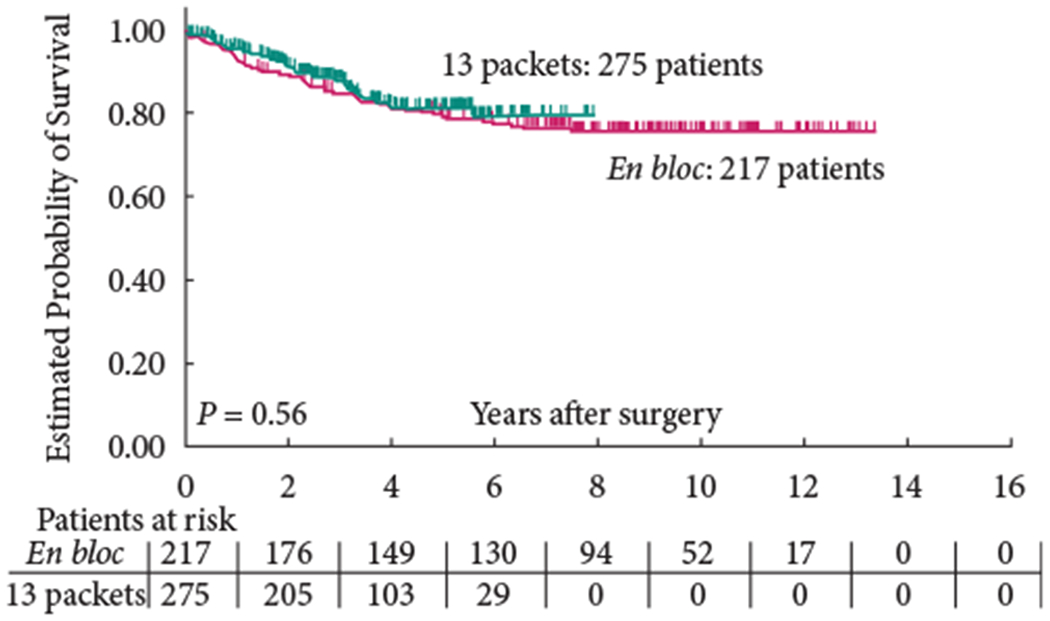
Recurrence-free survival of patients with organ-confined, lymph node-negative disease.
Fig. 5.
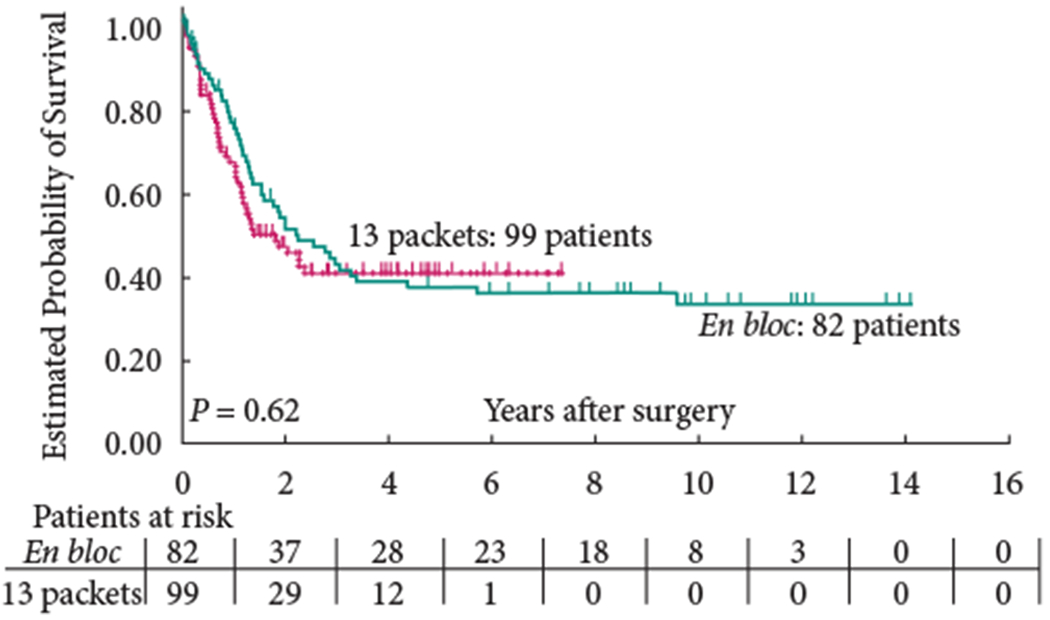
Recurrence-free survival of patients with extravesical, lymph node-positive disease.
Discussion
The standard of care for patients with carcinoma invading the bladder muscle is RC with extended pelvic LND, as it provides excellent local control, accurate staging and can offer long-term survival to patients with few LN metastases [7,8]. The removal of a greater number of LNs has been associated with improved survival [1,7], although the extent of the LND remains a topic of controversy and is the subject of two ongoing randomized trials [9,10]. According to the University of Southern California institutional philosophy, a super-extended LND has always been performed during RC since the early 1980s. In May 2002 we changed our submission methodology for the lymphadenectomy specimens from en bloc to separate packets to better identify the total number of LNs removed during RC and to allocate eventual LN metastases.
Analysing the data in 2007, we observed that the change in methodology significantly increased the number of total LNs identified per patient without any difference in LN positivity or postoperative LN staging. In the present study, we evaluated the long-term clinical and pathological impact of the change in lymphadenectomy specimen submission methodology and determined whether LN packeting resulted in any change in oncological outcomes.
In the present patient cohort the lymphadenectomy tissue submission with 13 separate packets instead of the en bloc technique significantly increased the total number of LNs identified per patient. Various authors have tried to define minimum LN numbers as a surrogate for LND quality and to establish general standards [1,11], but multiple factors influence the final LN yield [12]: the individual LN count of a patient [13]; the elected LND template [14]; the surgeon’s thoroughness of dissection [5]; and the pathological evaluation, including institutional policies and histological definition of a LN applied [15]. Postoperative LN counts are therefore highly variable and inter-institutional comparisons impractical. By contrast, large consecutive template comparison studies with well-defined patient groups and long follow-up have clearly shown an outcome benefit in relation to the extent of LND. In comparison with a limited or unilateral LND, both the extended as well as super-extended (up to the inferior mesentery artery take-off) LND are equally superior in terms of postoperative staging and provide optimum local and systemic oncological control [8,16]. Consequently, the adherence to a meticulous LND technique within a clearly defined extended [8] template is more important than the total number of LNs identified [17].
To overcome the drawback of highly variable institutional LN counts, LN density has been identified as a significant prognostic variable [3,18]. LN density is thought to be helpful for risk stratification of LN-positive patients as it takes two important prognostic factors – the LN tumour burden (number of nodes involved) as well as the meticulousness of the LND (number of nodes removed) – into account. The rationale of evaluating the ratio of positive to total LNs is thought to be less influenced by the above-mentioned factors. In the present study the number of LNs identified changed according to the change in submission technique, while the number of positive LNs did not alter, validating the diligence of the pathologists in evaluation of the LNs, whether sent en bloc or separately. Accordingly, LN density dropped from 11 to 4% as more overall LNs were identified after 2002. Fundamentally, the change of a single factor affecting the overall LN count results in a change in LN density. Analysing the differences between the three high-volume surgeons at our institutions confirmed this speculation, as different numbers of total but not positive LNs per surgeon were noted. In summary, our findings of a larger LN yield without an increase in positive nodes resulted in a decreased LN density. This outcome therefore leads us to question the strength of LN density as an accurate prognosticator for long-term oncological outcomes.
Survival data remained almost identical, with a 5-year OS of ~60% and a 5-year RFS close to 70%. This is not surprising because the extirpative part of the surgery did not change. Moreover, especially for patients with pT4 tumours who have a poor prognosis, it is unlikely that the LND and, in particular, also the technique of tissue submission should play a substantial role in the survival probability. Including patients with pathological stage pT0-pT1 disease, who have a <10% probability of showing positive LNs could make it more likely not to find a difference between the two patient cohorts, but detailed analyses showed no differences within pathological subgroups.
It is important to note that the surgical technique and limits of LND remained identical during the study period. Apparently, despite using a ‘thinner grid’ to identify LNs within smaller packets the pathologists do not detect more positive LNs. Routinely, microscopic haematoxylin and eosin-stained analysis is performed, which may miss micrometastasis (defined as the presence of cancer cells in LN circulation in patients with no demonstrable evidence of disease spread according to conventional radiographical and pathological staging). Recent data using quantitative real-time PCR for expression of cytokeratines (CK-19/-20) and uroplakin as markers for normal and malignant epithelial/urothelial cells, showed positive expression results in former histologically LN-negative staged tumours [19–21]. It could be that tissue processing rather than the submission methodology limits the findings. It also explains why LN-negative patients have been found to benefit from a more extended LND [1,11,22–24]. Overall, 5 years later, the change of submission methodology did not result in a nodal stage shift, as a similar overall incidence of LN-positive patients as well as similar numbers of positive LNs within LN-positive patients were observed.
At our institution, investigating multiple packets increased the time for gross examination by up to 40 min, while time for microscopic examination did not significantly differ. The analysis of an increased number of pathological specimens also increased costs; however, the detailed impact on costs is not shown because this depends too much on local billing and reimbursement policies.
The present study has several limitations. The first is inherent in its retrospective nature. Second, inter-pathologist variability can influence LN counts, although specimens were examined by three dedicated genitourinary pathologists during the study period, limiting this variability. Third, there was a significant difference in the median follow-up time for the two cohorts (9.6 compared with 3.9 years), which could have affected the presented outcome data; however, the majority of recurrences typically occurred within 2–4 years, so the follow-up time is thought to be long enough.
Ultimately, it is the meticulous removal of all fibroadipose tissue with complete skeletonization of pelvic structures within the boundaries of an extended template [8] that is more important than the LN submission technique or the total number of LNs identified. This is likely to result in both the most accurate postoperative staging based on a representative number of total LNs and the most effective removal of metastatic LNs.
In conclusion, the submission of 13 separate nodal packets after a super-extended LND instead of the en bloc technique significantly increased the total number of LNs identified; however, the modified lymphadenectomy specimen submission did not increase the number of positive LNs detected nor result in a postoperative nodal stage shift or change in oncological outcomes. The significant decrease in LN density as the result of increasing LN numbers calls into question its value as a uniquely applicable risk stratification tool.
Fig. 4.
Recurrence-free survival of patients with extravesical, lymph node-negative disease.
Abbreviations
- LN
lymph node
- RC
radical cystectomy
- LND
lymph node dissection
- OS
overall survival
- RFS
recurrence-free survival
Footnotes
Conflict of Interest
None declared.
References
- 1.Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol 2002; 167: 1295–8 [PubMed] [Google Scholar]
- 2.Herr HW, Faulkner JR, Grossman HB et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol 2004; 22:2781–9 [DOI] [PubMed] [Google Scholar]
- 3.Kassouf W, Leibovici D, Munsell MF, Dinney CP, Grossman HB, Kamat AM. Evaluation of the relevance of lymph node density in a contemporary series of patients undergoing radical cystectomy. J Urol 2006; 176: 53–7; discussion 7 [DOI] [PubMed] [Google Scholar]
- 4.Stein JP, Cai J, Groshen S, Skinner DG. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol 2003; 170: 35–41 [DOI] [PubMed] [Google Scholar]
- 5.Stein JP, Penson DF, Cai J et al. Radical cystectomy with extended lymphadenectomy: evaluating separate package versus en bloc submission for node positive bladder cancer. J Urol 2007; 177: 876–81; discussion 81-2 [DOI] [PubMed] [Google Scholar]
- 6.Bergkvist A, Ljungqvist A, Moberger G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir Scand 1965; 130: 371–8 [PubMed] [Google Scholar]
- 7.Stenzl A, Cowan NC, De Santis M et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 2011; 59: 1009–18 [DOI] [PubMed] [Google Scholar]
- 8.Zehnder P, Studer UE, Skinner EC et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: a comparative study. J Urol 2011; 186: 1261–8 [DOI] [PubMed] [Google Scholar]
- 9.LEA. Available at: http://dgu.clinicalsite.org/de/cat/423/trial/1149. Accessed November 2014
- 10.SWOG S. [Accessed November 2014]; Available at: http://www.swog.org/Visitors/newsletters/2011/08/index.asp?a=s1011.
- 11.Leissner J, Hohenfellner R, Thuroff JW, Wolf HK. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int 2000; 85: 817–23 [DOI] [PubMed] [Google Scholar]
- 12.Seiler R, Thalmann GN, Zehnder P. Pelvic lymph node dissection in the context of radical cystectomy: a thorough insight into the connection between patient, surgeon, pathologist and treating institution. Res Rep Urol 2013; 5: 121–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weingartner K, Ramaswamy A, Bittinger A, Gerharz EW, Voge D, Riedmiller H. Anatomical basis for pelvic lymphadenectomy in prostate cancer: results of an autopsy study and implications for the clinic. J Urol 1996; 156: 1969–71 [DOI] [PubMed] [Google Scholar]
- 14.Bochner BH, Cho D, Herr HW, Donat M, Kattan MW, Dalbagni G. Prospectively packaged lymph node dissections with radical cystectomy: evaluation of node count variability and node mapping. J Urol 2004; 172: 1286–90 [DOI] [PubMed] [Google Scholar]
- 15.Svatek R, Zehnder P. Role and extent of lymphadenectomy during radical cystectomy for invasive bladder cancer. Curr Urol Rep 2012; 13: 115–21 [DOI] [PubMed] [Google Scholar]
- 16.Dhar NB, Klein EA, Reuther AM, Thalmann GN, Madersbacher S, Studer UE. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol 2008; 179: 873–8; discussion 8 [DOI] [PubMed] [Google Scholar]
- 17.Dorin RP, Daneshmand S, Eisenberg MS et al. Lymph node dissection technique is more important than lymph node count in identifying nodal metastases in radical cystectomy patients: a comparative mapping study. Eur Urol 2011; 60: 946–52 [DOI] [PubMed] [Google Scholar]
- 18.Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol 2003; 169: 943–5 [DOI] [PubMed] [Google Scholar]
- 19.Kurahashi T, Hara I, Oka N, Kamidono S, Eto H, Miyake H. Detection of micrometastases in pelvic lymph nodes in patients undergoing radical cystectomy for locally invasive bladder cancer by real-time reverse transcriptase-PCR for cytokeratin 19 and uroplakin II. Clin Cancer Res 2005; 11: 3773–7 [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Kakehi Y, Zeng Y, Taoka R, Tsunemori H, Inui M. Uroplakin II as a promising marker for molecular diagnosis of nodal metastases from bladder cancer: comparison with cytokeratin 20. J Urol 2005; 174: 2138–42; discussion 42-3 [DOI] [PubMed] [Google Scholar]
- 21.Autenrieth M, Nawroth R, Semmlack S, Gschwend JE, Retz M. [Muscle-invasive urothelial carcinoma of the bladder. Detection and topography of micrometastases in lymph nodes]. Urologe A 2008; 47: 1157–61 [DOI] [PubMed] [Google Scholar]
- 22.Rink M, Shariat SF, Xylinas E et al. Does increasing the nodal yield improve outcomes in patients without nodal metastasis at radical cystectomy? World J Urol 2012;30: 807–14 [DOI] [PubMed] [Google Scholar]
- 23.Poulsen AL, Horn T, Steven K. Radical cystectomy: extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol 1998; 160:2015–9; discussion 20 [PubMed] [Google Scholar]
- 24.Shirotake S, Kikuchi E, Matsumoto K et al. Role of pelvic lymph node dissection in lymph node-negative patients with invasive bladder cancer. Jpn J Clin Oncol 2010; 40: 247–51 [DOI] [PubMed] [Google Scholar]


