Abstract
Only female insects transmit diseases such as malaria, dengue and Zika; therefore, control methods that bias the sex ratio of insect offspring have long been sought. Genetic elements such as sex-chromosome drives can distort sex ratios to produce unisex populations that eventually collapse, but the underlying molecular mechanisms are unknown. We report a male-biased sex-distorter gene drive (SDGD) in the human malaria vector Anopheles gambiae. We induced super-Mendelian inheritance of the X-chromosome-shredding I-PpoI nuclease by coupling this to a CRISPR-based gene drive inserted into a conserved sequence of the doublesex (dsx) gene. In modeling of invasion dynamics, SDGD was predicted to have a quicker impact on female mosquito populations than previously developed gene drives targeting female fertility. The SDGD at the dsx locus led to a male-only population from a 2.5% starting allelic frequency in 10–14 generations, with population collapse and no selection for resistance. Our results support the use of SDGD for malaria vector control.
Subject terms: Molecular engineering, Genetics, Gene regulation
A sex-distorter gene drive causes population collapse in the malaria mosquito.
Main
Sex-chromosome drivers are genetic elements that interfere with chromosome segregation during meiosis and are over-represented in progeny1. In heterogametic sex, they cause an unbalanced male-to-female ratio among offspring, which can potentially lead to population suppression or extinction. Relatively few sex-chromosome drives have been characterized, most likely because they produce an evolutionary conflict with the rest of the genome that selects for autosomal suppressors or resistant sex chromosomes2,3.
Mathematical modeling predicts that a driving sex distorter will spread in a population and, in the absence of resistance, cause eventual collapse4,5. Population collapse using natural sex-chromosome drives has been reported in laboratory colonies of Drosophila6,7. In the field, a population crash of the species Drosophila neotestacea was detected in Washington State due to a natural X-chromosome distorter that produced a female-only population8. Therefore, sex-distorter drives could conceivably be harnessed for invasive pest or vector control9,10.
Although Y drives are less common than X drives, they have been described in Aedes aegypti and Culex pipiens mosquitoes11,12. Y drives are particularly attractive for mosquito vector control because they can progressively reduce the number of females and hence disease transmission as they spread. In addition, Y drives are likely to be more effective than X drives because they can increase at a greater rate the fraction of heterogametic driving individuals3–5. Synthetic sex distorters have been generated in A. gambiae mosquitoes by using site-specific nucleases such as I-PpoI or CRISPR–Cas9, which cleave conserved repeated sequences in the mosquito ribosomal DNA gene cluster located exclusively on the X chromosome13,14. These nucleases, when expressed during spermatozoa development, selectively cleave the X chromosome, thereby favoring the production of Y-bearing gametes and causing a 95% male bias in the progeny13,14. However, attempts to convert synthetic sex-ratio distorters into Y-chromosome drives have been unsuccessful so far. In most insect species, including A. gambiae, the sex chromosomes are transcriptionally shut down during gametogenesis, a process known as meiotic sex-chromosome inactivation15,16, which prevents the transcription of X-shredding nucleases if they are inserted into the Y chromosome (personal observation, A.C. and R.G.).
Recently, a gene drive that targeted the dsx gene reached 100% frequency in 7–11 generations and crashed a caged population of 600 mosquitoes without inducing resistance17. We hypothesized that it might be possible to circumvent meiotic sex-chromosome inactivation by developing an autosomal male-biased sex distorter and coupling sex-ratio distortion with drive. This could result in a quicker impact on disease transmission and a synergistic effect (robustness) between the sex distorter and gene-drive components. Here we report the design and validation of an SDGD to spread the X-chromosome-shredding I-PpoI endonuclease and produce a male-only insect population.
Results
Designing an SDGD
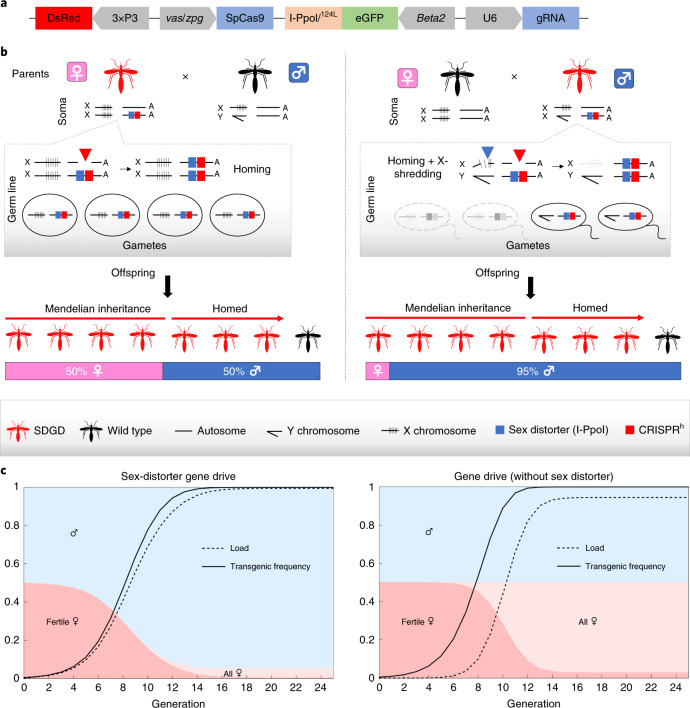
We designed an SDGD system by combining (on the same construct) a CRISPR-based gene drive that targets a haplosufficient female fertility gene with the I-PpoI endonuclease, which in turn cleaves a conserved sequence in the X-linked ribosomal gene cluster (Fig. 1a,b). We used mathematical modeling to test the likely spread of this SDGD design. Our results indicate that our SDGD could spread rapidly from a low starting frequency to produce a largely unisex male population and would also impose a fitness load by impairing female fertility, which together would eliminate the population (Fig. 1c). This SDGD design is different from the previously reported CRISPR-based gene drives that target recessive female fertility genes and impose a fitness load by the generation of homozygous sterile mutants17,18. The modeling predicted that this SDGD would quickly bias the population toward males and gradually reduce the abundance of biting females, which both reduce pathogen transmission (by females only) and suppress the population (Table 1 and Supplementary Fig. 1).
Fig. 1. Driving a sex-distorter system in the autosome.
a, Schematic overview of the construct used to build an SDGD, which contains four transcription units: the I-Ppol nuclease (variant W124L), expressed as a fusion protein with the enhanced green fluorescent protein (eGFP) visual marker, under the control of the male-specific beta2-tubulin germline promoter; the SpCas9 nuclease, regulated by a promoter that is active in the germ line of both males and females (from the vasa or zpg gene); a gRNA under the control of the ubiquitous U6 polymerase III promoter, designed for homing at previously characterized haplosufficient fertility genes; and a 3xP3::DsRed gene as a fluorescent integration marker. b, Mode of action of the autosomal SDGD. The sex-distorter (I-Ppol; blue square) and gene-drive (CRISPRh; red square) components are linked head to tail in the same construct that is integrated in the autosome within a fertility gene. In the germ line of a female transgenic mosquito (highlighted in red), the CRISPRh component is active (red arrowhead), leading to super-Mendelian inheritance of the transgene by homology-directed repair. In the germ line of a male transgenic mosquito, both the gene-drive (red arrowhead) and sex-distorter (blue arrowhead) transcription units are active, leading to homing of the construct (by action of CRISPRh) and shredding of the X chromosome (by action of I-PpoI targeting ribosomal DNA repeats; indicated by vertical lines). This results in a bias of the sex ratio toward males in the progeny and super-Mendelian inheritance of the transgene. c, Idealized predictions (discrete-generation deterministic model) of transgenic frequency for spread in a population (solid line) alongside the load on the target population (dashed line) for an SDGD construct (left; fraction of male progeny (m) = 0.95) and a gene drive (right; m = 0.50) targeting a female fertility gene. The colored shading represents the fraction of males (blue) and females (pink) in the population, with fertile females indicated by a darker color. This idealized model makes several assumptions that are likely to vary by strain, including but not limited to full fitness in males and heterozygous females (fully recessive female fertility gene); complete sterility in homozygous females; 95% SDGD transmission in male and female heterozygotes; no generation of drive-resistant mutations; no loss of function of the sex distorter; and single release of male drive heterozygotes equal to 1% of the male population.
Table 1.
Comparison of performance of gene-drive and sex-distorter genetic control approaches in terms of efficacy, spread and robustness
| Construct type | Construct name | Homing rate | Male-biased sex-ratio distortion | Spread in caged population | Population suppression | Development of resistance in cages | Impact of heterozygotes on population sizea | Component redundancy | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Gene drive | dsxFCRISPRh | 92% males; 99% females | 50% | Yes | Yes | No | No | No | 17 |
| Sex-distorter gene drive | SDGDdsx | 92% males; 99% females | 93% | Yes | Yes | No | Yes | Yes | This study |
| Autosomal sex distorter | gfp111A-2 | 0% | 95% | No | Yes (over-flooding ratio of 3×) | No | Yes | No | 13 |
| Y drive | NA | 100% males; 0% females* | 95%* | Yes* | NA | NA | Yes* | No | NA |
Homing rate is defined as the fraction of transgenic progeny above Mendelian inheritance. An asterisk denotes values based on a hypothetical X-shredder construct inserted on the Y chromosome generating 95% male offspring, all of which inherit the transgene. NA, not applicable. aAbility of the construct to have an impact on the population size (that is, the number of females) in heterozygosity (or hemizygosity for the Y drive) as compared to constructs targeting recessive female fertility loci, which impact population size when homozygote transgenic females are generated.
We generated distinct A. gambiae SDGD strains targeting three haplosufficient genes (AGAP011377, AGAP007280 and AGAP005958) with established roles in female fertility18. We assessed the activity of three SDGD constructs (SDGD011377, SDGD007280 and SDGD005958) in the progeny of crosses between SDGD-heterozygous and wild-type individuals by scoring the fraction of offspring containing the drive element and the sex ratio of the progeny. SDGD007280 had severely reduced fertility, and we did not recover enough progeny to assess drive activity. We found average inheritance rates of 79% (±0.17% (s.d.)) for SDGD011377 and 98% (±0.08% (s.d.)) for SDGD005958 (Supplementary Fig. 2 and Supplementary Table 1). Furthermore, we observed a male bias ranging from 92% to 94% in the progeny of males heterozygous for SDGD011377 and SDGD005958. Monitoring of life history traits revealed a dramatic reduction of female fertility in females heterozygous for SDGD011377 and SDGD005958 (Supplementary Fig. 3), similarly to previous findings where in the same genes were targeted with a vasa-Cas9 gene-drive construct18. We attributed this reduction in fertility to ectopic expression of the vasa promoter and subsequent conversion to a null genotype for the target gene in somatic tissues, where the gene product is required18–20. In addition, the vasa promoter is known to induce maternal deposition of Cas9 into the developing embryo, resulting in deleterious mutations of the paternally inherited gene copy, in addition to the null allele inherited from the mother, imposing additional fitness costs to heterozygous female offspring. We also observed a strong reduction in the fertility of heterozygous males, particularly in SDGD007280 and SDGD005958 (Supplementary Fig. 3). We hypothesized that male sterility in SDGD007280 and partial male sterility in SDGD005958 was due to locus-dependent high expression of the I-PpoI nuclease, which, if persisting in spermatozoa, shreds the maternally inherited X chromosome in the fertilized embryo, resulting in embryo lethality13,21. Despite high levels of drive transmission and male bias, unintended and severe fertility costs prevented the spread of SDGD011377 and SDGD005958 into caged mosquito populations when these were seeded at 12.5% allelic frequency (Supplementary Fig. 4). SDGD005958 failed to persist in the populations and disappeared after two generations. SDGD011377 was stable for eight generations, owing to a better balance of drive and fitness costs. This, in turn, generated low-level population suppression by maintaining a sex ratio of approximately 65% males (Supplementary Fig. 4).
Optimization of temporal and spatial characteristics and level of expression of Cas9 and I-PpoI
Our initial findings revealed that SDGD constructs targeting female fertility genes could bias both their own inheritance and the sex ratio of progeny. However, fitness costs, most likely associated with non-optimal spatial and temporal activity of both the Cas9 and I-PpoI genes, impaired SDGD spread into mosquito populations. To minimize the ectopic activity of Cas9, we replaced the vasa promoter with the regulatory regions of the zero population growth (zpg) gene (AGAP006241). The zpg promoter has previously been applied to regulate Cas9 expression in gene-drive constructs, increasing the fertility of heterozygous individuals as compared to those harboring constructs using vasa17,19. Previous studies have also shown that the expression levels of I-PpoI during spermatogenesis are crucial in determining whether the outcome is sex bias or sterility; high levels of activity correlate with male sterility13,21. The destabilized version of I-PpoI (W124L13) used in this study was previously found to confer the highest levels of fertility while maintaining strong male bias from at least three independent genomic loci13. However, this I-PpoI variant impaired male fertility when expressed under the transgenic beta2-tubulin promoter inserted into the AGAP011377, AGAP007280 and AGAP005958 loci. To reduce the transcriptional activity of the beta2-tubulin promoter, we generated three variants by inserting a G+C-rich sequence of 100 bp in proximity to conserved sequences at position −244, −271 or −355 with respect to the ATG start codon (Supplementary Fig. 5). Each variant was tested for expression using a dual-fluorescence reporter system in vivo (Supplementary Fig. 5). For subsequent experiments, we selected beta2-tubulin promoter variant 244 (beta2244), which showed transcriptional activity that was about 8.1% that of the wild-type promoter sequence (Supplementary Fig. 6). The initial SDGD plasmid was then modified to replace the vasa promoter with the zpg regulatory sequences (as described in ref. 17), while the beta2-tubulin promoter was replaced with beta2244.
An SDGD targeting the dsx gene
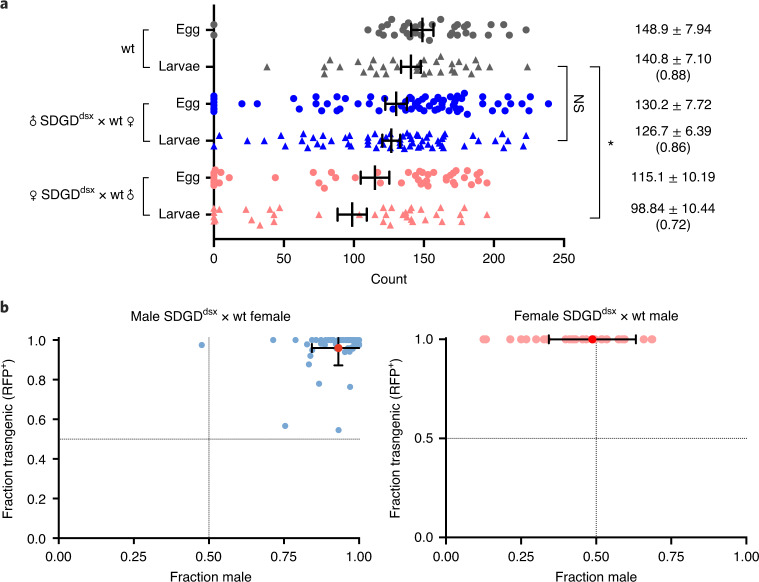
To maximize the performance of the SDGD, we developed the construct SDGDdsx, containing the zpg-Cas9 transcription unit, beta2244-I-PpoI and a guide RNA (gRNA) designed to target the intron 4–exon 5 boundary of the dsx gene (AGAP004050), because we previously reported that this site minimizes the development of resistance to a gene drive17. In addition, females that are homozygous for dsxF exhibit an ‘inter-sex’ phenotype and are viable but unable to bite17; therefore, this affects the vector competence of the population earlier than an SDGD targeting a standard female fertility locus, in which homozygous females are sterile but can bite and transmit. Unlike SDGD007280, SDGD011377 and SDGD005958, SDGDdsx had no measurable impact on the fertility of heterozygotes: the larval output of SDGDdsx males was comparable to that of controls (126.7 ± 50.7 (s.d.) and 140.8 ± 40.8 (s.d.), respectively; P = 0.39; Fig. 2a and Supplementary Table 1). The fertility of SDGDdsx heterozygous females, measured as viable offspring, was reduced as compared to controls (98.8 ± 63 (s.d.) and 140.8 ± 40.8 (s.d.), respectively; P = 0.012), although it was still sufficient to produce a large number of fertile individuals (Fig. 2a). High levels of maternal nuclease deposition can affect the fertility of the female progeny17,18,20; however, we did not observe a significant difference in fertility when comparing females inheriting the transgene from a transgenic female parent to those inheriting the trangene from a male parent (Supplementary Fig. 7). As expected, we observed a marked male bias (93.1% ± 0.08% (s.d.)) in the offspring of SDGDdsx heterozygous males (Fig. 2b). The sex-distortion phenotype was stably transmitted from male mosquitoes to their transgenic male offspring, and no differences were observed as compared to males that inherited the construct from a female or a male (Supplementary Table 3). Strong super-Mendelian inheritance of the construct of 96.0% ± 0.08% (s.d.) and 99.9% ± 0.01% (s.d.) was observed from both males and females, respectively, based on the frequency of red fluorescent protein (RFP)+ progeny from heterozygous parents (Fig. 2b), making SDGDdsx suitable for population suppression experiments.
Fig. 2. Fertility and sex and inheritance bias of an SDGD targeting the female isoform of the sex-determination dsx gene (AGAP004050).
a, Counts of eggs and hatched larvae determined in individual crosses (n ≥ 33) of SDGDdsx-heterozygous females and males to wild-type (wt) mosquitoes. While male fertility was comparable to that of wild type (male fertility 0.86; no significant difference, NS), females showed a 37% reduction in larval output as compared to wild type (female fertility of 0.627; *P = 0.0124, Kruskal–Wallis test). Values on the right indicate the mean count ± s.e.m., with larval hatching rate in parentheses. b, Scatterplots showing the fraction of SDGDdsx transgene inheritance (y axis) against sex bias (x axis) in the progeny of individual SDGDdsx trans-heterozygous males (left; n = 63) and females (right; n = 39) crossed to wild-type individuals. Individual blue and pink dots represent the progeny derived from a single female, and the red dot indicates the average of the population. Error bars correspond to s.d. Both male and female SDGDdsx-heterozygous mosquitoes showed super-Mendelian inheritance of the transgene determined by scoring the presence of the RFP marker in the progeny. Male SDGDdsx-mosquitoes showed a strong bias in sex ratio toward males (0.93 ± 0.09). Dotted lines indicate the fraction of males (x axis) and the fraction of SDGD (y axis) as expected by Mendelian inheritance.
SDGDdsx invades caged mosquito populations
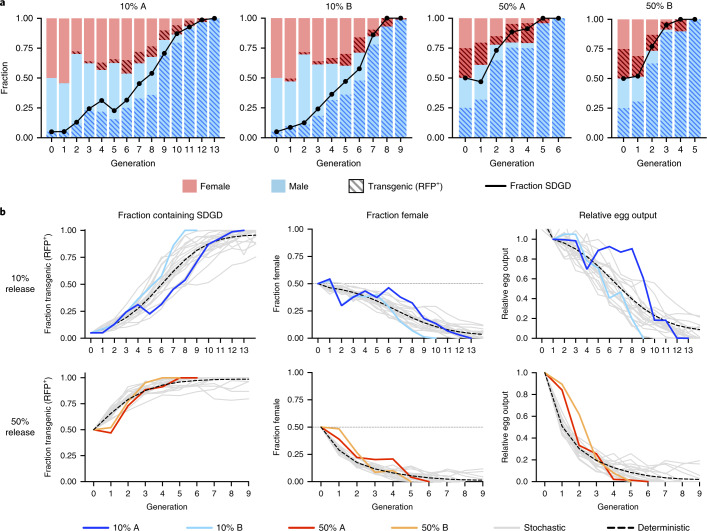
We used fertility, inheritance bias, sex-distortion data and mutant phenotype information to develop both deterministic and stochastic discrete-generation models (Methods and Supplementary Tables 2, 4 and 5) to predict the spread of SDGDdsx into mosquito populations, simulating the release of 10% and 50% SDGDdsx-heterozygous mosquitoes into caged populations of 600 individuals. The stochastic model predicted that the transgene would quickly invade the population, reaching 100% allelic frequency and leading to collapse of the population in 93% and 98% of 10,000 simulations after 30 generations from a 10% and 50% SDGD release, respectively (Fig. 3). The deterministic model, however, showed differences in outcome depending on the values for the fertility of heterozygous females and males, ranging from population elimination to suppression and to the disappearance of SDGDdsx if male fertility was below 0.5 compared to wild type (Supplementary Figs. 8 and 9). To test the model prediction, we released SDGDdsx heterozygotes at either 2.5% or 25% allelic frequency into two populations of 600 caged mosquitoes, each in two replicates. At each generation, larvae were screened for the presence of the fluorescence marker linked to the transgene, and subsequently the fraction of males and females in the population was assessed. We observed a rapid spread of SDGDdsx in all four populations, with the transgene reaching 100% allelic frequency between 4 and 12 generations. The spread of SDGDdsx induced a strong bias of the population sex ratio toward males, accompanied by a progressive reduction of egg output, which led to population elimination at generations 5 and 6 for the replica cages that started with 25% SDGDdsx allelic frequency and at generations 9 and 13 for the replica cages that started with 2.5% SDGDdsx release (Fig. 3).
Fig. 3. Kinetics of SDGDdsx spread in target mosquito populations.
The spread of SDGDdsx was investigated in two different experiments starting with an allelic frequency of 2.5% (10% male release) and 25% (50% male and female release), in replica (cage A and cage B). The 10% release cages were set up with a starting population of 300 wild-type females, 270 wild-type males and 30 SDGDdsx-heterozygous males. The 50% release cages were started with 150 wild-type females, 150 wild-type males, 150 SDGDdsx-heterozygous males and 150 SDGDdsx-heterozygous females (allelic frequency of 25%). Each consecutive generation was established by selecting 600 larvae. The frequency of the transgene (fraction of RFP+ individuals), the sex ratio (female/male) and the relative egg output (fraction of eggs produced relative to the first generation) were recorded at each generation. a, The bar plots represent the fraction of males and females (blue and pink shading, respectively) for each population, and the striped pattern shows the fraction of transgenic individuals. Black lines indicate the total fraction of individuals containing SDGDdsx (as a fraction of RFP+) individuals. b, The frequency of the transgene, the sex ratio and the relative egg output superimposed on both a deterministic model (black dashed lines) and 20 representative stochastic simulations (gray solid lines) of the dynamics of invasion of SDGDdsx based on release scenarios of 25% and 2.5% SDGDdsx allelic frequency. In 93% and 98% of the stochastic simulations (of 10,000 runs), the release of SDGD-heterozygous individuals at a starting frequency of 2.5% and 25%, respectively, is predicted to collapse the population within 30 generations. Dotted lines indicate the expected Mendelian distribution of sex. Fitness and life history parameter estimates are provided in Supplementary Table 2.
Fitness of female progeny in SDGDdsx males
SDGD-heterozygous males generated < 6% female progeny, and female offspring inherited an X chromosome from male gametes exposed to the I-PpoI nuclease during spermatogenesis. We investigated whether the inheritance of a potentially damaged X chromosome affected female fertility and the SDGD homology-directed repair rate. We crossed females that carried one ‘I-PpoI-exposed’ X chromosome from the father to wild-type males and compared their fertility parameters to those of daughters of SDGDdsx females that carried two unaffected copies of the X chromosome. We observed that females inheriting one I-PpoI-exposed X chromosome did not significantly differ in fertility (measured as the number of hatched larvae) nor in drive inheritance, suggesting that, if there is a contribution to fitness of a damaged X chromosome in females, this was not detectable in our assay. To further investigate the potential impact of I-PpoI-exposed X chromosomes, we modeled additional fitness reductions in individuals with a damaged X chromosome using deterministic discrete-generation cage simulations of a theoretical scenario of SDGDdsx release (10% males and 50% males/50% females) into a caged population (Supplementary Fig. 10). The model predicted little or no effect during the initial spread of the transgene, but a reduction in the suppression load that correlated with the cost of the damaged X chromosome was detected (Supplementary Fig. 10).
Dynamics of sex-distorter drive
Driving a sex distorter into a female fertility locus could impose a sufficiently high load on the population to the point that the population is suppressed and eliminated. However, the dynamics of an SDGD are complex and depend not only on the fertility of SDGDdsx-heterozygous individuals (Supplementary Figs. 8 and 9) but also on the rate of male bias (Supplementary Fig. 11), and in certain scenarios these dynamics are not intuitive. For example, when female (W/D, where W is the wild-type allele and D represents the SDGD allele) fertility is reduced, such as below 0.5, the load on the population increases with increasing sex distortion, whereas for higher female fertility, such as above 0.5, the load is greater when there is no sex distortion (equivalent to a gene drive without a sex distorter, m = 0.5; Supplementary Fig. 11). The sex distorter allows the SDGD construct to spread at low (or even zero) female fertility, imposing a substantial load (Supplementary Fig. 12). This is because the male bias mitigates the effect of low female fitness. Overall, increasing sex distortion makes the construct less sensitive to variation in female heterozygous fertility (Supplementary Fig. 12). At the limit of complete male bias (male progeny = 100%), the load is independent of female fertility because no SDGD females are created and only SDGD-heterozygous males can pass on the construct. Based on our experimental parameter estimates for SDGDdsx, the SDGD allele is predicted to be present in an intermediate equilibrium with wild-type and nonfunctional resistance alleles at a sufficient frequency to induce a dramatic population reduction and possibly prevent reinvasion events (Supplementary Fig. 13).
Discussion
Our results show that SDGDdsx functions as a sex-distorter autosomal gene drive. In four cage experiments, SDGDdsx progressively biased the sex ratio toward males, with eventual population collapse. Notably, we did not observe the development of functional mutations at the target dsx site that blocked the spread of the distorter. This observation further supports the notion that the dsx sequence at the intron 4–exon 5 boundary is highly functionally constrained and validates its use as a target sequence for gene-drive solutions in anopheline mosquitoes. It should also be noted that a sex distorter that simultaneously destroys the female isoform of the dsx gene while reducing the female population also decreases the opportunity of resistant mutations to arise (because they are not selected in males). In addition, targeting a sequence present in hundreds of copies on the X chromosome reduces the likelihood that nuclease-induced resistance will evolve to block the sex-distorter component.
Our SDGD solution also combines a number of features in terms of efficacy, robustness and predicted time to impact (on disease transmission), which differ from those for previously described gene drives or autosomal sex-distorter systems, making it particularly attractive for field implementation (Table 1). In two replicate caged experiments, SDGDdsx consistently induced population collapse starting from an allelic frequency of 2.5%. For field experiments, this translates into mosquito numbers to be released that are within the range of production capability; recent studies modeling the impact of hypothetical X-shredder Y-drive mosquitoes on a national scale predict that the release of as few as ten males in 1% of human settlements will achieve over 90% population suppression after 4 years22.
SDGDdsx is predicted to show a higher level of robustness than a gene drive alone, even if one of the critical components breaks down or mutates, due to the synergy of the components. Loss or inactivation of the I-PpoI sequence will result in the generation of functional dsx gene drive that will also contribute to population suppression (Supplementary Fig. 12), and loss of function of either of the two drive components (Cas9 or gRNA) will produce nonfunctional dsx alleles (R) that, in heterozygous individuals, will still contribute to the production of male-biased progeny owing to the presence of functional I-PpoI. Mutations and recombination events of the constructs involving both the drive and distorter will generate R nonfunctional dsx mutations. These R mutations are constantly generated at the target locus by the action of the nuclease17, but they are not selected because they do not restore function of the dsx gene and homozygous R females are sterile; therefore, they are continuously lost as they arise.
Modeling based on our experimental data shows that SDGDdsx offers some important advantages in short-term drive dynamics and long-term outcomes. Importantly, the number of transmission-competent (that is, biting) females is reduced more quickly by SDGDdsx than by a standard gene drive targeting the same locus (Supplementary Fig. 1), which could lead to a strong effect on disease transmission (time to impact after release). In comparing a distorting and a non-distorting gene drive, the equilibrium load imposed by SDGDdsx is less sensitive to female fitness costs, which is particularly relevant given the uncertain extrapolation of fitness effect measurements from the lab to the field.
Previous modeling of gene drive without the sex distorter showed that under certain conditions (for example, leaky expression of the drive construct) there can be an accumulation of nonfunctional cleavage-resistant sequences, which prevents the transgene from going to fixation17,23. Deterministic modeling of SDGDdsx indicates that there is also the potential for the transgene to go to an intermediate equilibrium frequency and population suppression, rather than complete fixation and elimination (Supplementary Figs. 8, 9 and 12). The lower the SDGD fertility in heterozygous individuals, the more likely an intermediate equilibrium is reached. For the observed fertility values of SDGDdsx-heterozygous females, stochastic models predicted population elimination for finite cage populations in 93–98% of the simulations, with kinetics of spread in line with observed data. Under field or semi-field conditions, the fertility estimates of heterozygous individuals could differ and tilt the balance one way or the other toward population reduction rather than population elimination. Achieving a strong population reduction may be regarded as less effective than elimination in a field scenario; however, it could help achieve long-term stable vector control via a higher tolerance to repopulation through migration as compared to a system that quickly eliminates an entire target population.
Males carrying a non-driving I-PpoI construct designed to cause dominant male sterility21 were recently released in a field location of Burkina Faso24 as part of a phased, step-by-step assessment of novel genetic approaches to malaria control, following independent guidance and recommendations25,26. This opened the way to the use of an I-PpoI-based distorter for the implementation of genetic vector control measures.
We believe that SDGDdsx outperforms other anopheline gene drives, combining efficacy, resistance management and robustness, and is well suited as an anti-malaria intervention.
Methods
Ethics statement
Generation of SDGD constructs
To create SDGD vectors p172 (vas2; GenBank accession MT270142) and p182 (zpg), the β2-eGFP(F2A)I-PpoI transcription unit from pBac[3xP3-DsRed]β2-eGFP::I-PpoI-124L13 was excised by AscI digestion and cloned into AscI-digested p165 (vas2-CRISPRh (ref. 18): GenBank accession KU189142) and p174 (zpg-CRISPRh (ref. 19); GenBank accession MH541847), respectively. SDGD vectors were further modified by BsaI-mediated Golden Gate assembly to contain gRNA spacers targeting AGAP011377 (GCAGACGTAGAAATTTTC), AGAP007280 (GGAAGAAAGTGAGGAGGA), AGAP005958 (GAGATACTGGAGCCGCGAGC)18 and AGAP004050 (GTTTAACACAGGTCAAGCGG)17. To include the beta2244 promoter modification, plasmid p182 was further modified to generate p182–244 (GenBank accession MT270141) according to the beta2244 variant described below. Additional sequences of all vectors are available as Supplementary Information.
Microinjection of embryos and selection of transformed mosquitoes
All mosquitoes were reared under standard conditions of 80% relative humidity and 28 °C. The mosquitoes were blood fed on anesthetized mice or by Hemotek, and freshly laid embryos were aligned and used for microinjections as described previously27. To generate SDGD mosquitoes, we injected respective docking line17,18 embryos with solution containing p174 or p182–244 and a plasmid-based source of PhiC31 integrase28 (at 200 ng μl–1 and 400 ng μl–1, respectively). All surviving G0 larvae were crossed to wild-type mosquitoes, and G1 positive transformants were identified using a fluorescence microscope (Nikon, Eclipse TE200) as RFP+ larvae for the recombination-mediated cassette exchange (RMCE) events.
Containment of gene-drive mosquitoes
All mosquitoes were housed at Imperial College London in an insectary that is compliant with Arthropod Containment Guidelines Level 2 (ref. 29). All genetically modified (GM) work was performed under institutionally approved biosafety and GM protocols. In particular, GM mosquitoes containing constructs with the potential to show gene drive were housed in dedicated cubicles, separated from the external environment by at least six doors requiring two levels of security card access. Moreover, because the insectary is located in a city with a northern temperate climate, A. gambiae mosquitoes are also ecologically contained. The physical and ecological containment of the insectary are compliant with guidelines set out in a recent commentary calling for safeguards in the study of synthetic gene-drive technologies30.
Mutagenesis of the beta2-tubulin promoter
Bioinformatic analysis of the regulatory region of the beta2-tubulin gene (AGAP008622) was performed using the Promoter2.0 Prediction Server31 and the Neural Network Promoter Prediction tool32 to identify the conserved region. A synthetic 100-bp DNA sequence with a G+C content of 65% (sequence reported in Supplementary Fig. 3) was designed using Geneious R11 (https://www.geneious.com/) and cloned into the beta2-tubulin promoter at position −244, −271 or −355 with respect to the ATG start codon using site-specific mutagenesis of plasmid pBac[3xP3-DsRed]β2-eGFP::I-PpoI-124L13 by nested PCR with primer pairs B2–355_r and B2–355_f, B2–271_r and B2–271_f, and B2–244_r and B2–244_f followed by Spac-fwd and Spac-rev, for the beta2355, beta2271 and beta2244 variants, respectively. A second unmodified copy of the beta2-tubulin promoter was cloned to express the mCherry gene.
| Name | Sequence |
| B2–355_r | GGCCAACTCGGGTCCGAGTCGTCTTCTTGGATGGGATGATG |
| B2–355_f | CGCCAGCACTCTCAGACTCAATACGAATTTATTTGTGGCATCG |
| B2–271_r | GGCCAACTCGGGTCCGAGTCATATGACTACTATGATCATCTTTTGC |
| B2–271_f | CGCCAGCACTCTCAGACTCAGAG CCG TAC GTG CCG G |
| B2–244_r | GGCCAACTCGGGTCCGAGTCCACGAAATGATCCGGCAC |
| B2–244_f | CGCCAGCACTCTCAGACTCACAGAACCTTCAGAGACGTTG |
| Spac-fwd | GTGAGAAGTGCGCGTCTCGTTCCCGCAGCTCGCCAGCACTCTCAGACTCA |
| Spac-rev | CATCCGCCCTAACTCCGCCCGTGGGTCGTTGGCCAACTCGGGTCCGAGTC |
Dual-fluorescence assay experiment
Three- to five-day-old adult male heterozygous mosquitoes were collected in Falcon tubes and anesthetized on ice 5 min before dissection. Testes were micro-dissected using an Olympus SZX7 optical microscope, and pictures of gonads were taken using the EVOS imaging system (Thermo-Fisher) with magnification of ×20 and the following exposure settings: bright field: gain 50%; GFP channel: gain 30%, 120 ms; RFP channel: gain 80%, 120 ms. Unmodified pictures were then analyzed using ImageJ software33. Testis areas were selected using the freeform selection tool, and integrated density and mean gray values were measured for the GFP and RFP channels independently using the same selection area. A reading for the background (same selection area) was then subtracted from the integrated density value for each testis to remove background noise. The value for fluorescence intensity was measured as the ratio between the GFP reading and the mCherry reading and normalized to the value of the unmodified beta2-tubulin control.
Phenotypic assays
Phenotypic assays designed to examine SDGD inheritance and relative fecundity in mosquitoes were carried out essentially as described before17,18. Briefly, the offspring of heterozygous individuals crossed to wild-type counterparts were screened by RFP expression. Non-fluorescent progeny were kept as controls. Groups of 50 male and 50 female blood-fed mosquitoes were mated to an equal number of wild-type mosquitoes for 5 d, and a minimum of 40 females were allowed to lay individually. The entire egg and larval progeny was counted for each lay. Females that failed to give progeny and had no evidence of sperm in their spermathecae were excluded from the analysis. To determine the inheritance and sex-ratio bias of SDGD, the entire larval progeny was screened for the presence of DsRed, which is linked to the SDGD allele, and all the progeny was sexed at the pupal stage to determine the sex ratio. Statistical differences between genotypes were assessed using the Kruskal–Wallis test.
Cage trial assays
To perform cage trials of SDGD011377 and SDGD005958, we introduced 100 heterozygous transgenic males into a population of 100 wild-type males and 200 wild-type females (transgenic allelic frequency of 12.5%) in triplicate. As a control, 100 heterozygous transgenic males from the autosomal self-limiting sex-distorter gfp124L-2 line13 were released at the same frequency in a separate population, in triplicate. In addition, a population of 200 wild-type males and 200 wild-type females served as a negative control.
For the starting generation only, age-matched male and female pupae were allowed to emerge in separate cages and were mixed only when all the pupae had emerged. Mosquitoes were left to mate for 5 d before they were blood fed on anesthetized mice. After 2 d, the mosquitoes were set to lay in a 300-ml egg bowl filled with water and lined with filter paper. The eggs produced from the cage were photographed and counted using JMicroVision v1.27. Before counting, the eggs were dispersed using gentle water spraying in the egg bowl to homogenize the population, and 450 eggs were randomly selected to seed the next generation. Larvae emerging from the 450 eggs were counted and screened for the presence of the RFP marker to score the transgenic rate of the progeny. All the pupae were sexed to determine the sex ratio of the population.
To perform cage trials of SDGDdsx, we set up two different experiments, in replicate. The 10% release cages were set up with a starting population of 300 wild-type females, 270 wild-type males and 30 SDGDdsx-heterozygous males (starting allelic frequency of 2.5%). The 50% release cages were started with 150 wild-type females, 150 wild-type males, 150 SDGDdsx-heterozygous males and 150 SDGDdsx-heterozygous females (allelic frequency of 25%). For the starting generation only, age-matched male and female pupae were allowed to emerge in separate cages and were mixed only when all the pupae had emerged. Mosquitoes were left to mate for 5 d before blood feeding. After 2 d, the mosquitoes were set to lay in a 300-ml egg bowl filled with water and lined with filter paper. All larvae were allowed to hatch, and each consecutive generation was established by randomly selecting 600 larvae, split into 3 trays of 200 larvae each. All 600 larvae were screened for the presence of the RFP marker, and the pupae from one tray were sexed to determine the sex ratio. On day 8, mosquitoes were offered a second blood meal, and all the eggs produced were photographed and counted using Egg counter software34.
Statistical analysis
Statistical analysis was performed as indicated using GraphPad Prism version 7.0.
Population genetics model
Discrete time
To model the results of the cage experiments, we use discrete-generation recursion equations for the genotype frequencies, with males and females treated separately, similarly to Kyrou et al.17. We extend the previous study17 to model the SDGD by including a sex bias and possible X-chromosome damage in the progeny of SDGD males, although here we do not include parental effects on fitness (as these effects were not strongly observed). We consider three alleles at the female fertility target site, W (wild type), D (driving sex distorter) and R (nonfunctional nuclease resistant). We also differentiate between the two possible types of X chromosome: x (wild type) and X, which denotes an X chromosome that has passed through an SDGD male and survived X-shredding but may be damaged, resulting in an additional fitness cost to the individual carrying it. and denote the genotype frequency of females and males, respecitvely, in the total population, where the first set of indices denotes alleles at the target site {WW, WD, WR, DD, DR, RR} and the second set denotes the sex chromosomes, pq = {xx, xX, XX} for females and q = {x, X} for males. There were 18 female genotypes and 12 male genotypes; six types of eggs: EW,x, ED,x, ER,x, EW,X, ED,X and ER,X, where the first index refers to the target site allele and the second index to the sex chromosome; and eight types of sperm: SW,X, SR,X (no SD,x, because we assume that SDGD males only contribute X chromosomes), SW,X, SD,X, SR,X, SW,Y, SD,Y and SR,Y.
Homing
Adults of genotype W/D at the target site produced gametes at meiosis in the ratio W:D:R as follows:
Here df and dm are the rates of transmission of the driver allele in the two sexes and uf and um are the fractions of non-drive gametes at the target site that are repaired by meiotic end-joining and are nonfunctional and resistant to the drive (R). In all other genotypes, inheritance at the target site is Mendelian.
Sex distortion
The SDGD X-shredder only affects the sex ratio of the progeny if it is in males. It destroys the X chromosome while males are making their sperm, resulting in mostly Y-bearing sperm. From male SDGD heterozygotes, progeny will therefore consist of m1 (1/2 < m1 ≤ 1) males and (1 − m1) females; from male SDGD homozygotes (D/D), the progeny will be m2 (1/2 < m2 ≤ 1) males and (1 − m2) females. For simplicity, when comparing to the experiment, we assume m1 = m2 = m. We assume no mutations cause loss of function of the sex distorter from the construct or resistance to X-shredding.
All X chromosomes contributed by SDGD males that survived X-shredding are assumed to be ‘damaged’ X chromosomes (versus wild-type X chromosomes), reflected in the reduced reproductive fitness of the individual carrying it (see ‘Fitness’). We assume a damaged X chromosome was susceptible to further shredding if it was inherited by an SDGD male and, for simplicity, that the fitness cost of carrying a damaged X chromosome is the same no matter how many times the chromosome passes through an SDGD male and survives X-shredding.
Fitness
We let represent the reproductive fitnesses of female and male genotypes relative to a fitness of 1 for wild-type homozygotes, where {ij} denotes alleles at the target site of the construct {WW, WD, WR, DD, DR, RR} and the second set of indices denotes pq = {xx, xX, XX} for females and q = {x, X} for males. While all fitness parameters are retained in the recursion equations for generality, for comparison with the experiment, we assume that the target gene is needed for female fertility; thus, females with D/D, D/R and R/R at the target site are sterile. There is no reduction in fitness in W/R females from carrying only one copy of the target gene (W/R), but W/D females have reduced fitness due to the presence of the SDGD construct, as observed experimentally (Supplementary Table 2). We assume no costs to males with no copies of the driving sex distorter (W/R, R/R), but that males with one or two copies of the SDGD (W/D, D/D, D/R) have a fitness reduction consistent with experimental observation (Supplementary Table 2).
If the individual also carries a damaged X chromosome, we assume that this imposes an additional cost that affects reproductive success. To calculate the overall fitness of the genotype, the fitness value associated with carrying the damaged X chromosome is multiplied by the fitness value associated with D (or R) alleles at the target site (Supplementary Tables 4 and 5). Reduced fitness in males with a copy of the damaged X chromosome is (1 − sX,m) and in females with two copies of the damaged X chromosome is (1 − sX,f), with sX,f, sX,m = 0 if there is no cost and 1 if the damaged X chromosome causes sterility. For females with one damaged X chromosome and one wild-type x chromosome, the reduction is (1 − hX,fsX,f), where hX,f is the dominance coefficient (0 for fully recessive; 1 for fully dominant). For baseline parameters, we assume these costs are equal to zero.
Recursion equations
We first consider the gamete contributions from each genotype. The proportions Ek,l(t) with allele k = {W, D, R} at the target site and sex chromosome l = {x, X} in eggs produced by females participating in reproduction are given in terms of the female genotype frequencies :
| 1 |
where i and j are each summed such that {1, 2, 3} corresponds to {W, D, R}. The coefficients in equation (1) correspond to the proportion of the gametes from female individuals of genotypes (ij, pq) that carry alleles (k, l), as shown in Supplementary Table 4 (rows correspond to genotypes; columns correspond to alleles).
The proportions with allele k = {W, D, R} at the target site and sex chromosome l = {x, X, Y} in sperm are given in terms of the male genotype frequencies :
| 2 |
where, again, i and j are each summed such that {1, 2, 3} corresponds to {W, D, R}. The coefficients in equation (2) correspond to the proportion of the gametes from male individuals of type (ij, qY) that carry alleles (k, l), as shown by the rows and columns, respectively, in Supplementary Table 5. Note that SD,x(t) = 0 because SDGD males only contribute damaged X chromosomes, so no entry for this is included in Supplementary Table 5.
We define the proportion of females in the population as
and the average female reproductive fitness as
Analogously, for the male proportion, we define the proportion of males in the population as
and the average male fitness as
Note that in equations (1) and (2), the normalization factor in the denominator is therefore and , respectively.
The load on the population incorporates reductions in female and male fertility and decreased frequency of females due to the SDGD spreading in the population, and at time (t) is defined as
This equals zero when only wild-type individuals are present and one if the SDGD has been established and the average female fitness, or fraction of females present, is equal to zero. We note that increases in load predicted by the cage model do not predict absolute changes in population density in the field but can be an indication of comparative potential reductions35.
To model cage experiments, we start with an equal number of males and females. For 50% release, the initial frequency for wild-type females and males is and for heterozygote drive females and males is . For 10% release of males only, and and all females are wild type, . Assuming random mating, we obtain the following recursion equations for the female genotype frequencies in generation (t + 1):
where pq = {xx, xX, XX}, and δij is the Kronecker delta. The factors account for the factor of 1/2 for homozygosity at the target site (for ij = {W/W, D/D, R/R}) and at the sex chromosomes (for pq = {xx, XX}). We obtain the following recursion equations for the male genotype frequencies:
where q = {x, X} and accounts for the factor of 1/2 for homozygosity at the target site (for ij = {W/W, D/D, R/R}).
Stochastic version
In the stochastic version of the model described above, random values for probabilistic events are taken from the appropriate multinomial distributions, with probabilities estimated from the experiment where applicable (Supplementary Table 2). To model the cage experiments, 150 female and 150 male wild-type adults (or 300 females and 270 males for 10% release of males only) along with 150 female and 150 male heterozygotes (or no females and 30 males for 10% release) are initially present. Females may fail to mate or mate once in their life with a male of a given genotype, according to its frequency in the male population, chosen randomly and with replacement such that males may mate multiple times. The number of eggs from each mated female is multiplied by the egg production of the male relative to the wild-type male, to account for experimental observations of reduced egg production from SDGD fathers. The eggs may hatch or not, with a probability that depends on the product of larval hatching values from the mother and father, relative to wild type. To start the next generation, 600 larvae are randomly selected, unless fewer than 600 larvae have hatched, in which case the smaller amount initiates the next generation, following the experiment. The probability of subsequent survival to adulthood is assumed to be equal across genotypes. Assuming very large population sizes allows results for the genotype frequencies that are indistinguishable from the deterministic model. For the deterministic egg count, we use the large population limit of the stochastic model.
Population dynamics model (continuous time)
To model changing population sizes in the field (for Supplementary Fig. 1), we use a continuous-time population dynamics model with one life stage and logistic density dependence in the recruitment rate based on models developed previously36,37. Here represents the abundance of adult individuals, f(t) and m(t) represent the total abundances of adult females and males, and and are the genotype abundances where, as above, the first set of indices denotes alleles at the target site and the second set denotes the sex chromosomes. Populations are normalized to the prerelease wild-type population size such that n(t = 0) = 1, and time is continuous and measured in generations. The dynamics of the total population size are given by the following differential equation:
The total recruitment rate of adults incorporates a density-dependent factor (the term in parentheses) based on Deredec et al.5 and depends on the total number of females, f(t), multiplied by the average female fitness, . Because SDGD males may have reduced fertility, the recruitment rate is also dependent on the average male fitness, (we assume that the number of males is not limiting and that all males participate in mating). Rm is the intrinsic growth rate of the population per generation at low density.
The equations for the individual genotype populations for females and males are
Egg and sperm proportions and are as defined in equations (1) and (2) in the discrete-generation model above, with and instead of frequencies and .
All calculations were carried out using Wolfram Mathematica38.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41587-020-0508-1.
Supplementary Information
Integrated supplementary information
Model prediction of the reduction over time in abundance of transmission competent (that is biting) females, normalised by the initial total population size, for the SDGDdsx construct compared to dsxFCRISPRh (Kyrou et al., 2018), using a continuous-time population dynamics model (see Supp. modeling Methods) for ‘field' release of 1% heterozygous transgenic males in the male population. The SDGDdsx construct is predicted to suppress the population of transmission competent females faster than the dsxFCRISPRh, mainly due to the creation of a male bias in the population by the sex distorter. Parameters used for SDGDdsx are in Table S2; dsxFCRISPRh parameters were estimated from Kyrou et al. (2018) using an average W/D female fitness of 0.4335; for both, Rm (intrinsic growth rate per generation) = 6. At long times (not shown), the SDGDdsx population rebounds to an intermediate equilibrium (suppressed) population.
Scattered plots showing the fraction of transgene inheritance (y-axis) against male bias (x-axis) in the progeny of heterozygous male SDGD011377 (left-hand panel) SDGD005958 and (right-hand panel) crossed to wild type females. Individual coloured dots represent the progeny derived from a single female and the red dots indicate the average of the population (with respective values indicated next to the plot ± s.e.m.). Error bars indicate standard deviation. SDGD at both loci showed a high transmission rate of the transgene determined by scoring in the progeny the presence of RFP marker that is linked to the SDGD allele. The progeny of SDGD/+ at both loci showed a strong sex ratio distortion towards males. Dotted lines indicate expected Mendelian inheritance.
SDGD constructs expressing the Cas9 nuclease under the control of the Vas2 promoter were generated targeting the fertility loci AGAP011377, AGAP007280 and AGAP005859 (as indicated). SDGD heterozygous male and female were crossed to wild-type counterparts. Each dot represents progeny of individual females. Fecundity was measured by counting the number of eggs per female and the hatched larvae. Values on the right represent average ± s.e.m. A strong fertility effect was observed in heterozygous SDGD females at 3 loci, while male fecundity was strongly impaired by targeting 7280 and 5859 loci. Vertical red bars indicate average count, and error bars indicate standard deviation. A minimum of 20 females were analysed for each cross.
In these experiments 100 heterozygous transgenic males were introduced into a population of 100 wild-type males and 200 wild-type females (transgenic allele frequency of 12.5%). The frequency of the transgene was monitored every generation together with the fraction of males in the population and the total number of eggs laid. Each consecutive generation was established by collecting 450 eggs. The frequency of the SDGD011377 (red lines) and SDGD005958 (blue lines) was compared to that of the autosomal self-limiting sex-distorter gfp124L-2 (grey lines) (Galizi et al., 2014) as well as to that of wild-type populations (black lines) as control. Each genotype was tested in triplicate cages. The SDGD005958 allele disappeared from the populations at generation 2 due to the strong fertility effects. The SDGD011377 alleles persisted in the populations despite the fertility effects but failed to increase over the frequency of release on subsequent generations. The fraction of males in the population was stably biased to about 65%.
(a) Schematic overview of the AGAP008622 (beta2-tubulin) locus and the three beta2 promoter variants generated by placing a 100bp GC-rich DNA sequence (blue rectangle) upstream of the start codon at position 244, 271 and 355. (b) The double-fluorescence reporter assay developed to detect the effects of the beta2 promoter modifications on its transcription level. A construct for each modification was generated harbouring the modified beta2 promoter (stars key) driving an eGFP marker, while a second, unmodified, beta2 promoter was driving a mCherry protein. The constructs were integrated within the same autosomal docking line by PhiC31-mediated integration by replacing a 3xP3::CFP cassette with a 3xP3::DsRed as integration marker. C) The sequence of 100bp GC-rich DNA region inserted at the 5’ of the beta2 promoter.
(a) Dissected testis from beta2wt, beta2271, beta2244 and beta2355 transgenic lines containing a single heterozygous insertion were microphotographed under the same exposure settings (as indicated), using mCherry as internal control. Line beta2271 showed GFP fluorescence intensity comparable to background; GFP expression was detectable at increased exposure (100% gain, 120ms, inset). Scale bar, 200 µm. (b) Quantification of fluorescence intensity as GFP/mCherry ratio, normalized to the beta2wt control (100%). Average relative intensity is indicated above the bars. *** indicates P value < 0.001 (ordinary One-way ANOVA). A minimum of 31 testes were analysed from individual expressing each promoter variant.
Male and female SDGDdsx heterozygotes that had inherited a maternal or paternal copy of the SDGDdsx allele were crossed to wild type counterparts and assessed for fecundity. The total larval output is plotted for individual females (dots). Red bars indicate the average and the mean count (± s.e.m.) is shown. Females inheriting the transgene from the mother (G1♀ → G2♀) have significantly lower larval progeny (*P = 0.0256, Kruskal-Wallis test) compared to wild-type control.
Time dynamics of the frequency of SDGDdsx transgenic individuals in the population as a function of W/D (where D represent the SDGD allele and W the wild-type allele) female fitness (wWD,xx), as predicted by the deterministic discrete-generation model at 25% initial allelic frequency. The graph shows the frequency of SDGD heterozygote males and females as a proportion of the male (or female) population, with other parameter estimates and baseline values given in Supp Table 2 (SDGD male fitness = 0.854; m (sex distortion) = 0.93). The predicted outcome at high W/D female fitness is elimination of the population, and at lower fitness, an intermediate equilibrium with W, R and D alleles. The result (black line) for the experimental estimate for female SDGD heterozygote fitness, wWD,xx = 0.627, is in a parameter region where even a small (positive) change leads to a prediction of population elimination instead of suppression.
Time dynamics of the frequency of SDGDdsx transgenic individuals in the population as a function of the fitness of SDGD males (assume wWD,xY = wDR,xY = wDD,xY) as predicted by the deterministic discrete-generation model. Initial release is 50% of SDGD heterozygote males and females as a proportion of the male (or female) population, with other parameter estimates and baseline values given in Supp Table 2 (W/D female fitness wWD,xx = 0.627; m = 0.93). For low SDGD male fitness (<≈0.5), the construct is eventually lost.
modeling the impact of fitness reduction, due to the inheritance of damaged X chromosomes passed through X-shredding in a SDGD male, on the spread of the SDGD transgenics as predicted by the deterministic discrete-generation cage model. For simplicity, we assume that the additional cost to carrying one damaged X chromosome in males is the same as the cost in females that carry two copies of the damaged X (sX,f = sX,m), and females with one damaged X and one wildtype X chromosome have only half the fitness cost of females with two copies (dominance coefficient hX,f = 1/2). Estimates used for other parameters given in Supplementary Table 2.
Effect of female W/D heterozygote fitness (D refers to the SDGDdsx allele) on the SDGD genotype frequency (that is, individuals with at least one copy of the SDGD) and load after 200 generations, as predicted by the deterministic discrete-generation model. Parameter estimates and baseline values given in Supp Table 2 (SDGD male fitness = 0.854). The possible outcomes (load = 1 and population elimination; intermediate equilibrium with W, D and R; or load = 0 and construct lost) depend upon the sex distortion m (0.5 [no sex bias] to 1 [only male progeny]) and the female W/D heterozygote fitness (). For low female fitness, adding an X-shredder (increasing sex distortion m) is predicted to beneficially increase the load on the population. For complete female heterozygous sterility (purple line), the drive construct disappears from the population if there is no sex distortion (m = 1/2), whereas sufficiently increasing the sex distortion allows the SDGDdsx to spread and impose a load on the population. The presence of the X-shredder keeps the construct predominantly in males, therefore mitigating the heterozygous female sterility effect. By contrast if female fitness is sufficiently high (lines of fitness 0.5 or greater on plot), the load decreases at high sex distortion because due to male bias, SDGDdsx males replace high-fitness W/D heterozygote females which decreases the ability of the construct to spread. For a complete male sex bias, m = 1, no W/D heterozygote females are created (and therefore no female/male SDGDdsx homozygotes), since all X chromosomes are shredded and SDGDdsx males have no female progeny; thus the load at m = 1 does not depend on the fitness of female W/D heterozygote individuals since none are present. Only SDGDdsx heterozygous males can pass on the construct, with the SDGDdsx allele present in reduced frequency in an intermediate equilibrium with wildtype and resistance alleles and a load less than one for these parameters. (In general, the amount of reduction in SDGDdsx frequency and load at m = 1 will depend on parameters such as the homing rate, here less than 100%, the rate of resistant R mutation, and the relative fertility of the R and SDGDdsx allele).
(Top panel) Comparison of the predicted equilibrium load (that is, the reduction in reproductive output by the population after 400 generations) for release of SDGDdsx or dsxFCRISPRh into a wild-type population, varying the fitness cost to heterozygote W/D females. The discrete generation model predicts that the SDGDdsx construct (blue solid line) is more robust to reductions in female heterozygote fitness compared to dsxFCRISPRh (red line), still maintaining a substantial load even at 100% reduction in female heterozygote fitness (that is females heterozygous for the drive are completely non-viable). We also consider the possibility that the X-shredder component may be lost from the SDGDdsx construct during homing (bottom panel, blue dashed line), such that out of the drive alleles transmitted from female or male W/D individuals, 0.01% will not have a functioning X-shredder component. For low to mid-fitness costs, the predicted load is similar to that of the dsxFCRISPRh drive-only construct since the fraction of drive individuals without an intact X-shredder is high; for high fitness costs, the load merges with that of intact SDGDdsx (blue solid line) since almost all drive individuals have an intact X-shredder. We use representative parameters for both constructs for comparison (drive transmission df = dm = 0.95 for both males and females; rate of resistance uf = um = 0.5; X-shredding parameter for SDGDdsx is m=0.95; no reduction in fitness for heterozygote males).
Time dynamics of the frequency of SDGDdsx transgenic individuals (solid lines) and relative eggs output (dotted lines) in the population as predicted by the deterministic discrete-generation model using experimental parameters given in Supp Table 2 and assuming two initial releases of 50% of SDGD heterozygote males and females (black line) or 10% SDGD heterozygous males only (grey line). Independently of the release scenarios, for these parameters, the frequency of transgenic individuals reaches an intermediate equilibrium while W, R and D alleles and the egg output is reduced (population suppressed).
Supplementary information
Supplementary Figs. 1–13, Tables 1–5 and Note
Acknowledgements
This work was supported by grants from the Bill & Melinda Gates Foundation and additionally by grants from the European Commission (731060 - Infravec2) to A.C.
Author contributions
A.S., A.M.H., T.N. and A.C. designed the research, and A.S., A.M.H., R.G., K.K. and C.T. performed the research. A.K.B. and A.B. performed modeling; D.M., M.G. and G.M. performed insect husbandry; A.S., A.M.H. and A.C. analyzed data; and A.S. and A.C. wrote the paper, with input from all authors.
Data availability
The full sequence of vectors is provided through the NCBI database. The GenBank accession codes for vectors p172 and p182–244 are MT270142 and MT270141, respectively. Sanger sequencing of vector p182–244 is available as Supplementary Information. Additional vector sequences are provided as Supplementary Note.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alekos Simoni, Andrew M. Hammond.
Change history
8/6/2020
A Correction to this paper has been published: 10.1038/s41587-020-0658-1
Supplementary information
Supplementary information is available for this paper at 10.1038/s41587-020-0508-1.
References
- 1.Helleu, Q., Gérard, P. R. & Montchamp-Moreau, C. Sex chromosome drive. Cold Spring Harb. Perspect. Biol. 7, a017616 (2015). [DOI] [PMC free article] [PubMed]
- 2.Taylor JE, Jaenike J. Sperm competition and the dynamics of X chromosome drive: stability and extinction. Genetics. 2002;160:1721–1731. doi: 10.1093/genetics/160.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burt, A. & Trivers, R. Genes in Conflict: the Biology of Selfish Genetic Elements (Harvard University Press, 2009).
- 4.Hamilton WD. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 5.Deredec A, Godfray HCJ, Burt A. Requirements for effective malaria control with homing endonuclease genes. Proc. Natl Acad. Sci. USA. 2011;108:E874–E880. doi: 10.1073/pnas.1110717108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyttle TW. Experimental population genetics of meiotic drive systems. I. Pseudo-Y chromosomal drive as a means of eliminating cage populations of Drosophila melanogaster. Genetics. 1977;86:413–445. doi: 10.1093/genetics/86.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price TAR, Hodgson DJ, Lewis Z, Hurst GDD, Wedell N. Selfish genetic elements promote polyandry in a fly. Science. 2008;322:1241–1243. doi: 10.1126/science.1163766. [DOI] [PubMed] [Google Scholar]
- 8.Pinzone CA, Dyer KA. Association of polyandry and sex-ratio drive prevalence in natural populations of Drosophila neotestacea. Proc. Biol. Sci. 2013;280:20131397. doi: 10.1098/rspb.2013.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hastings IM. Selfish DNA as a method of pest control. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994;344:313–324. doi: 10.1098/rstb.1994.0069. [DOI] [PubMed] [Google Scholar]
- 10.Sinkins SP, Gould F. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 2006;7:427–435. doi: 10.1038/nrg1870. [DOI] [PubMed] [Google Scholar]
- 11.Hickey WA, Craig GB. Genetic distortion of sex ratio in a mosquito, Aedes aegypti. Genetics. 1966;53:1177–1196. doi: 10.1093/genetics/53.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeny TL, Barr R. Sex ratio distortion caused by meiotic drive in a mosquito, Culex pipiens L. Genetics. 1978;88:427–446. doi: 10.1093/genetics/88.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galizi R, et al. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat. Commun. 2014;5:3977. doi: 10.1038/ncomms4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galizi R, et al. A CRISPR–Cas9 sex-ratio distortion system for genetic control. Sci. Rep. 2016;6:31139. doi: 10.1038/srep31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner JMA. Meiotic sex chromosome inactivation. Development. 2007;134:1823–1831. doi: 10.1242/dev.000018. [DOI] [PubMed] [Google Scholar]
- 16.Taxiarchi C, et al. High-resolution transcriptional profiling of Anopheles gambiae spermatogenesis reveals mechanisms of sex chromosome regulation. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-51181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyrou K, et al. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018;36:1062–1066. doi: 10.1038/nbt.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammond A, et al. A CRISPR–Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond, A. M. et al. Improved CRISPR-based suppression gene drives mitigate resistance and impose a large reproductive load on laboratory-contained mosquito populations. Preprint at bioRxiv10.1101/360339 (2018).
- 20.Gantz VM, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl Acad. Sci. USA. 2015;112:E6736–E6743. doi: 10.1073/pnas.1521077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windbichler N, Papathanos PA, Crisanti A. Targeting the X chromosome during spermatogenesis induces Y chromosome transmission ratio distortion and early dominant embryo lethality in Anopheles gambiae. PLoS Genet. 2008;4:e1000291. doi: 10.1371/journal.pgen.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.North AR, Burt A, Godfray HCJ. Modelling the potential of genetic control of malaria mosquitoes at national scale. BMC Biol. 2019;17:26. doi: 10.1186/s12915-019-0645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaghton AK, Hammond A, Nolan T, Crisanti A, Burt A. Gene drive for population genetic control: non-functional resistance and parental effects. Proc. Biol. Sci. 2019;286:20191586. doi: 10.1098/rspb.2019.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scudellari M. Self-destructing mosquitoes and sterilized rodents: the promise of gene drives. Nature. 2019;571:160–162. doi: 10.1038/d41586-019-02087-5. [DOI] [PubMed] [Google Scholar]
- 25.James S, et al. Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of malaria in sub-Saharan Africa: recommendations of a scientific working group. Am. J. Trop. Med. Hyg. 2018;98:1–49. doi: 10.4269/ajtmh.18-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct, Board on Life Sciences, Division on Earth and Life Studies, & National Academies of Sciences, Engineering, and Medicine. Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (National Academies Press, 2016). [PubMed]
- 27.Fuchs, S., Nolan, T. & Crisanti, A. Mosquito transgenic technologies to reduce Plasmodium transmission. Methods Mol. Biol.923, 601–622 (2013). [DOI] [PubMed]
- 28.Volohonsky G, et al. Tools for Anopheles gambiae transgenesis. G3. 2015;5:1151–1163. doi: 10.1534/g3.115.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott TW. Containment of arthropod disease vectors. ILAR J. 2005;46:53–61. doi: 10.1093/ilar.46.1.53. [DOI] [PubMed] [Google Scholar]
- 30.Akbari OS, et al. Safeguarding gene drive experiments in the laboratory. Science. 2015;349:927–929. doi: 10.1126/science.aac7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knudsen S. Promoter2.0: for the recognition of PolII promoter sequences. Bioinformatics. 1999;15:356–361. doi: 10.1093/bioinformatics/15.5.356. [DOI] [PubMed] [Google Scholar]
- 32.Reese, M. G., Harris, N. L. & Eeckman, F. H. Large scale sequencing specific neural networks for promoter and splice site recognition. In Proceedings of the Pacific Symposium on Biocomputing (eds. Hunter, L. & Klein, T.) 737–738 (World Scientific, 1996).
- 33.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollahosseini A, et al. A user-friendly software to easily count Anopheles egg batches. Parasit. Vectors. 2012;5:122. doi: 10.1186/1756-3305-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deredec A, Burt A, Godfray HCJ. The population genetics of using homing endonuclease genes in vector and pest management. Genetics. 2008;179:2013–2026. doi: 10.1534/genetics.108.089037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaghton A, Beaghton PJ, Burt A. Vector control with driving Y chromosomes: modeling the evolution of resistance. Malar. J. 2017;16:286. doi: 10.1186/s12936-017-1932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaghton A, et al. Requirements for driving antipathogen effector genes into populations of disease vectors by homing. Genetics. 2017;205:1587–1596. doi: 10.1534/genetics.116.197632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfram Research, Inc. Mathematica (Wolfram Research, Inc., 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model prediction of the reduction over time in abundance of transmission competent (that is biting) females, normalised by the initial total population size, for the SDGDdsx construct compared to dsxFCRISPRh (Kyrou et al., 2018), using a continuous-time population dynamics model (see Supp. modeling Methods) for ‘field' release of 1% heterozygous transgenic males in the male population. The SDGDdsx construct is predicted to suppress the population of transmission competent females faster than the dsxFCRISPRh, mainly due to the creation of a male bias in the population by the sex distorter. Parameters used for SDGDdsx are in Table S2; dsxFCRISPRh parameters were estimated from Kyrou et al. (2018) using an average W/D female fitness of 0.4335; for both, Rm (intrinsic growth rate per generation) = 6. At long times (not shown), the SDGDdsx population rebounds to an intermediate equilibrium (suppressed) population.
Scattered plots showing the fraction of transgene inheritance (y-axis) against male bias (x-axis) in the progeny of heterozygous male SDGD011377 (left-hand panel) SDGD005958 and (right-hand panel) crossed to wild type females. Individual coloured dots represent the progeny derived from a single female and the red dots indicate the average of the population (with respective values indicated next to the plot ± s.e.m.). Error bars indicate standard deviation. SDGD at both loci showed a high transmission rate of the transgene determined by scoring in the progeny the presence of RFP marker that is linked to the SDGD allele. The progeny of SDGD/+ at both loci showed a strong sex ratio distortion towards males. Dotted lines indicate expected Mendelian inheritance.
SDGD constructs expressing the Cas9 nuclease under the control of the Vas2 promoter were generated targeting the fertility loci AGAP011377, AGAP007280 and AGAP005859 (as indicated). SDGD heterozygous male and female were crossed to wild-type counterparts. Each dot represents progeny of individual females. Fecundity was measured by counting the number of eggs per female and the hatched larvae. Values on the right represent average ± s.e.m. A strong fertility effect was observed in heterozygous SDGD females at 3 loci, while male fecundity was strongly impaired by targeting 7280 and 5859 loci. Vertical red bars indicate average count, and error bars indicate standard deviation. A minimum of 20 females were analysed for each cross.
In these experiments 100 heterozygous transgenic males were introduced into a population of 100 wild-type males and 200 wild-type females (transgenic allele frequency of 12.5%). The frequency of the transgene was monitored every generation together with the fraction of males in the population and the total number of eggs laid. Each consecutive generation was established by collecting 450 eggs. The frequency of the SDGD011377 (red lines) and SDGD005958 (blue lines) was compared to that of the autosomal self-limiting sex-distorter gfp124L-2 (grey lines) (Galizi et al., 2014) as well as to that of wild-type populations (black lines) as control. Each genotype was tested in triplicate cages. The SDGD005958 allele disappeared from the populations at generation 2 due to the strong fertility effects. The SDGD011377 alleles persisted in the populations despite the fertility effects but failed to increase over the frequency of release on subsequent generations. The fraction of males in the population was stably biased to about 65%.
(a) Schematic overview of the AGAP008622 (beta2-tubulin) locus and the three beta2 promoter variants generated by placing a 100bp GC-rich DNA sequence (blue rectangle) upstream of the start codon at position 244, 271 and 355. (b) The double-fluorescence reporter assay developed to detect the effects of the beta2 promoter modifications on its transcription level. A construct for each modification was generated harbouring the modified beta2 promoter (stars key) driving an eGFP marker, while a second, unmodified, beta2 promoter was driving a mCherry protein. The constructs were integrated within the same autosomal docking line by PhiC31-mediated integration by replacing a 3xP3::CFP cassette with a 3xP3::DsRed as integration marker. C) The sequence of 100bp GC-rich DNA region inserted at the 5’ of the beta2 promoter.
(a) Dissected testis from beta2wt, beta2271, beta2244 and beta2355 transgenic lines containing a single heterozygous insertion were microphotographed under the same exposure settings (as indicated), using mCherry as internal control. Line beta2271 showed GFP fluorescence intensity comparable to background; GFP expression was detectable at increased exposure (100% gain, 120ms, inset). Scale bar, 200 µm. (b) Quantification of fluorescence intensity as GFP/mCherry ratio, normalized to the beta2wt control (100%). Average relative intensity is indicated above the bars. *** indicates P value < 0.001 (ordinary One-way ANOVA). A minimum of 31 testes were analysed from individual expressing each promoter variant.
Male and female SDGDdsx heterozygotes that had inherited a maternal or paternal copy of the SDGDdsx allele were crossed to wild type counterparts and assessed for fecundity. The total larval output is plotted for individual females (dots). Red bars indicate the average and the mean count (± s.e.m.) is shown. Females inheriting the transgene from the mother (G1♀ → G2♀) have significantly lower larval progeny (*P = 0.0256, Kruskal-Wallis test) compared to wild-type control.
Time dynamics of the frequency of SDGDdsx transgenic individuals in the population as a function of W/D (where D represent the SDGD allele and W the wild-type allele) female fitness (wWD,xx), as predicted by the deterministic discrete-generation model at 25% initial allelic frequency. The graph shows the frequency of SDGD heterozygote males and females as a proportion of the male (or female) population, with other parameter estimates and baseline values given in Supp Table 2 (SDGD male fitness = 0.854; m (sex distortion) = 0.93). The predicted outcome at high W/D female fitness is elimination of the population, and at lower fitness, an intermediate equilibrium with W, R and D alleles. The result (black line) for the experimental estimate for female SDGD heterozygote fitness, wWD,xx = 0.627, is in a parameter region where even a small (positive) change leads to a prediction of population elimination instead of suppression.
Time dynamics of the frequency of SDGDdsx transgenic individuals in the population as a function of the fitness of SDGD males (assume wWD,xY = wDR,xY = wDD,xY) as predicted by the deterministic discrete-generation model. Initial release is 50% of SDGD heterozygote males and females as a proportion of the male (or female) population, with other parameter estimates and baseline values given in Supp Table 2 (W/D female fitness wWD,xx = 0.627; m = 0.93). For low SDGD male fitness (<≈0.5), the construct is eventually lost.
modeling the impact of fitness reduction, due to the inheritance of damaged X chromosomes passed through X-shredding in a SDGD male, on the spread of the SDGD transgenics as predicted by the deterministic discrete-generation cage model. For simplicity, we assume that the additional cost to carrying one damaged X chromosome in males is the same as the cost in females that carry two copies of the damaged X (sX,f = sX,m), and females with one damaged X and one wildtype X chromosome have only half the fitness cost of females with two copies (dominance coefficient hX,f = 1/2). Estimates used for other parameters given in Supplementary Table 2.
Effect of female W/D heterozygote fitness (D refers to the SDGDdsx allele) on the SDGD genotype frequency (that is, individuals with at least one copy of the SDGD) and load after 200 generations, as predicted by the deterministic discrete-generation model. Parameter estimates and baseline values given in Supp Table 2 (SDGD male fitness = 0.854). The possible outcomes (load = 1 and population elimination; intermediate equilibrium with W, D and R; or load = 0 and construct lost) depend upon the sex distortion m (0.5 [no sex bias] to 1 [only male progeny]) and the female W/D heterozygote fitness (). For low female fitness, adding an X-shredder (increasing sex distortion m) is predicted to beneficially increase the load on the population. For complete female heterozygous sterility (purple line), the drive construct disappears from the population if there is no sex distortion (m = 1/2), whereas sufficiently increasing the sex distortion allows the SDGDdsx to spread and impose a load on the population. The presence of the X-shredder keeps the construct predominantly in males, therefore mitigating the heterozygous female sterility effect. By contrast if female fitness is sufficiently high (lines of fitness 0.5 or greater on plot), the load decreases at high sex distortion because due to male bias, SDGDdsx males replace high-fitness W/D heterozygote females which decreases the ability of the construct to spread. For a complete male sex bias, m = 1, no W/D heterozygote females are created (and therefore no female/male SDGDdsx homozygotes), since all X chromosomes are shredded and SDGDdsx males have no female progeny; thus the load at m = 1 does not depend on the fitness of female W/D heterozygote individuals since none are present. Only SDGDdsx heterozygous males can pass on the construct, with the SDGDdsx allele present in reduced frequency in an intermediate equilibrium with wildtype and resistance alleles and a load less than one for these parameters. (In general, the amount of reduction in SDGDdsx frequency and load at m = 1 will depend on parameters such as the homing rate, here less than 100%, the rate of resistant R mutation, and the relative fertility of the R and SDGDdsx allele).
(Top panel) Comparison of the predicted equilibrium load (that is, the reduction in reproductive output by the population after 400 generations) for release of SDGDdsx or dsxFCRISPRh into a wild-type population, varying the fitness cost to heterozygote W/D females. The discrete generation model predicts that the SDGDdsx construct (blue solid line) is more robust to reductions in female heterozygote fitness compared to dsxFCRISPRh (red line), still maintaining a substantial load even at 100% reduction in female heterozygote fitness (that is females heterozygous for the drive are completely non-viable). We also consider the possibility that the X-shredder component may be lost from the SDGDdsx construct during homing (bottom panel, blue dashed line), such that out of the drive alleles transmitted from female or male W/D individuals, 0.01% will not have a functioning X-shredder component. For low to mid-fitness costs, the predicted load is similar to that of the dsxFCRISPRh drive-only construct since the fraction of drive individuals without an intact X-shredder is high; for high fitness costs, the load merges with that of intact SDGDdsx (blue solid line) since almost all drive individuals have an intact X-shredder. We use representative parameters for both constructs for comparison (drive transmission df = dm = 0.95 for both males and females; rate of resistance uf = um = 0.5; X-shredding parameter for SDGDdsx is m=0.95; no reduction in fitness for heterozygote males).
Time dynamics of the frequency of SDGDdsx transgenic individuals (solid lines) and relative eggs output (dotted lines) in the population as predicted by the deterministic discrete-generation model using experimental parameters given in Supp Table 2 and assuming two initial releases of 50% of SDGD heterozygote males and females (black line) or 10% SDGD heterozygous males only (grey line). Independently of the release scenarios, for these parameters, the frequency of transgenic individuals reaches an intermediate equilibrium while W, R and D alleles and the egg output is reduced (population suppressed).
Supplementary Figs. 1–13, Tables 1–5 and Note
Data Availability Statement
The full sequence of vectors is provided through the NCBI database. The GenBank accession codes for vectors p172 and p182–244 are MT270142 and MT270141, respectively. Sanger sequencing of vector p182–244 is available as Supplementary Information. Additional vector sequences are provided as Supplementary Note.