Abstract
Breast tissue can be the host of not only many benign and malignant tumors but can also be a metastatic site for various tumors such as leukemia, lung cancer, and melanoma. This report describes an unusual case of a 43-year-old female who presented with a new palpable breast lump and several similar extramammary lumps on her skin. A melanoma panel, consisting of S100, HMB45, and Melan-A stains, was included in the pathology evaluation due to diagnostic suspicion of the radiologist and revealed metastatic melanoma. This case highlights the importance of detailed history and relevant physical exam as well as clinical and imaging correlation. It serves as a reminder to radiologists to include metastatic melanoma in the differential of suspicious subcutaneous breast masses, especially in patients with multiple subcutaneous lumps in the body or abnormal skin findings.
Introduction
Cutaneous malignant melanoma can have various presentations within the breast: primary melanoma of the breast skin, melanoma metastasis (MM) to the breast, in-transit metastases to breast tissue (indicating tumor spreading through a lymph vessel away from the primary tumor before it reaches the nearest lymph node) and breast skin and finally primary breast melanoma [1]. MM may simulate a broad spectrum of primary breast malignancies and can pose a diagnostic dilemma. Although the application of a simple panel of antibodies (S100, HMB45, and Melan-A stains) assists in rendering the correct interpretation, it is reserved for selected breast tumors featuring poorly differentiated histologic appearance, which may closely mimic a triple-negative invasive carcinoma [2]. A thorough clinical history is paramount for high pretest suspicion, so that the critical diagnostic step of melanoma panel can be applied. However, as in our case, a radiologist may be the only physician the patient encounters, highlighting the need for the radiologist to also be a vigilant clinician.
Case description
A 43-year-old female presented with a 3-month onset of new palpable left breast lump and similar lumps on the chest and the back. She had a history of a biopsy-proven fibroadenoma in the left breast 3 years ago, which had been stable on imaging.
The patient had imaging at an outside facility when she noticed a new left breast lump. The results were consistent with a stable biopsy proven fibroadenoma at 2-o'clock and a benign appearing new mass most consistent with fibroadenoma at 10-o'clock in the left breast, corresponding to the area of the palpable abnormality (Fig. 1a and b), with a final Breast Imaging Reporting and Data System (BIRADS) category 2: benign.
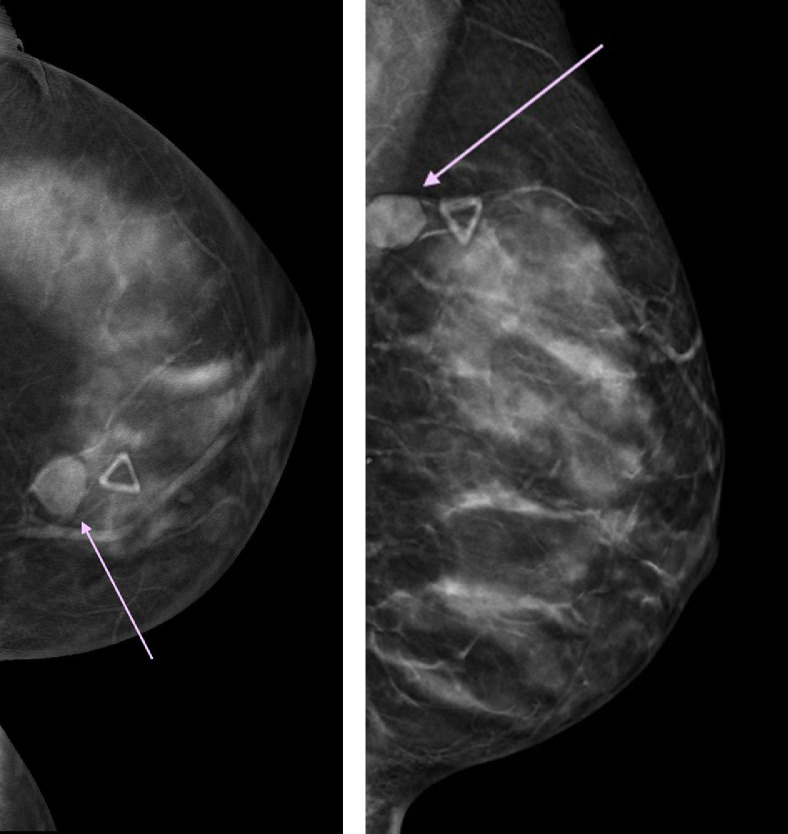
Fig 1.
(a, b) Initial diagnostic mammogram of left breast at symptom onset (a) (craniocaudal) CC tomosynthesis slice, (b) (mediolateral oblique) MLO tomosynthesis slice – demonstrated a mass at 10-o’ clock ( pink arrow), corresponding to the area of the palpable abnormality (
pink arrow), corresponding to the area of the palpable abnormality ( skin marker).
skin marker).
The patient presented to our facility for a follow up due to concern of enlargement of the palpable lump and a targeted left breast ultrasound (US) was performed. It showed an oval circumscribed hypoechoic clip containing 2.5 cm mass at 2-o'clock, consistent with the previously biopsied fibroadenoma (Fig. 2). The palpable area corresponded with a 2 × 1.2 × 0.8 cm slightly irregular hypoechoic subcutaneous mass with indistinct and microlobulated margins at 10 o'clock, 6 cm from the nipple (Fig. 3a and b). This was given a BIRADS 4: suspicious, due to the suspicious sonographic features. Additionally, 2 oval circumscribed hypoechoic masses were also noted at 10-o'clock, 7 and 8 cm from the nipple; with appearances suggestive of fibroadenomas. A biopsy was performed for the suspicious BIRADS 4 mass seen at 10-o’ clock, corresponding to the palpable lump. On further inquiry, the patient also revealed that she had similar new lumps in different parts of her body and that they all started as small bruises. Due to the unique presentation of a breast lump along with similar lumps in other parts of the body, the radiologist suggested the possibility of melanoma to the pathologist. US-guided biopsy of the suspicious left breast 10-o’ clock mass revealed that the tumor cells were ER-, PR-, GATA3-. Further testing with melanoma panel was performed, due to concern raised by the radiologist and came back positive for S100, HMB45, and Melan-A (Figs. 4 and 5), consistent with MM. Subsequently, PET-CT (Fig. 6) showed over 200 lesions including nodal disease above and below diaphragm and skeletal metastases. The 2 left breast masses, previously identified on ultrasound at 10-o'clock, besides the BIRADS 4 mass, were also FDG avid (Fig. 7), consistent with melanoma metastases. Treatment has been initiated for this patient with immunotherapy as well as with radiation for spinal lesions (Figs. 8 and 9).
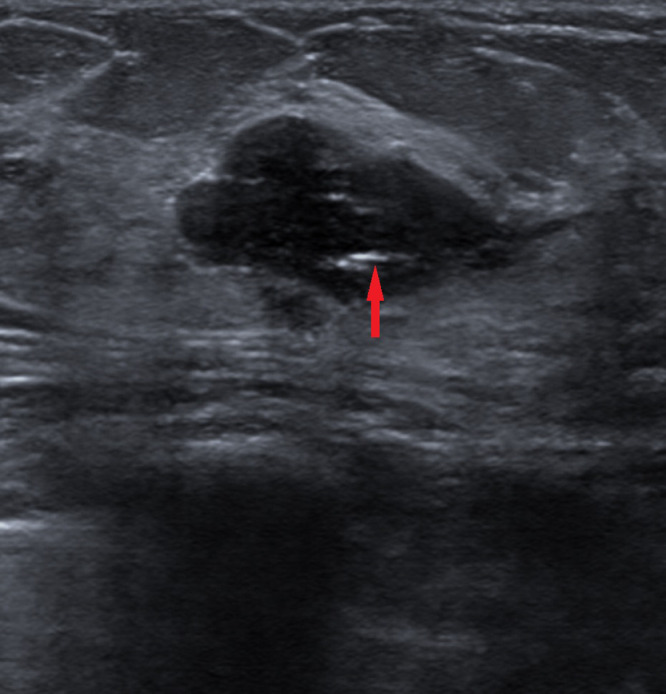
Fig 2.
Transverse ultrasound image of the left breast mass at 2-o’ clock showed stable findings of an oval circumscribed hypoechoic mass with echogenic biopsy clip ( red arrow) consistent with biopsy proven fibroadenoma. (Color version of figure is available online.)
red arrow) consistent with biopsy proven fibroadenoma. (Color version of figure is available online.)
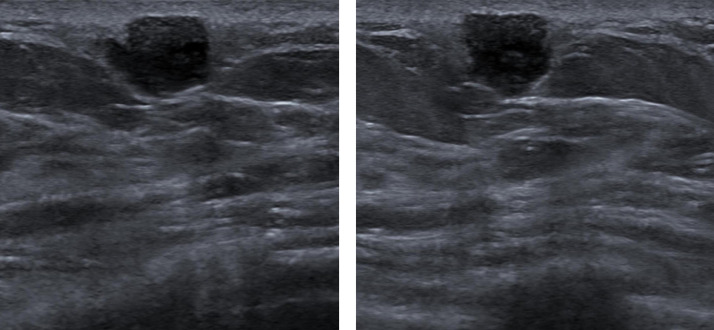
Fig 3.
(a, b) Ultrasound images (a transverse image and b longitudinal image) of the palpable abnormality in the left breast showed a slightly irregular hypoechoic subcutaneous mass with indistinct and microlobulated margins at 10-o’ clock, 6 cm from the nipple.
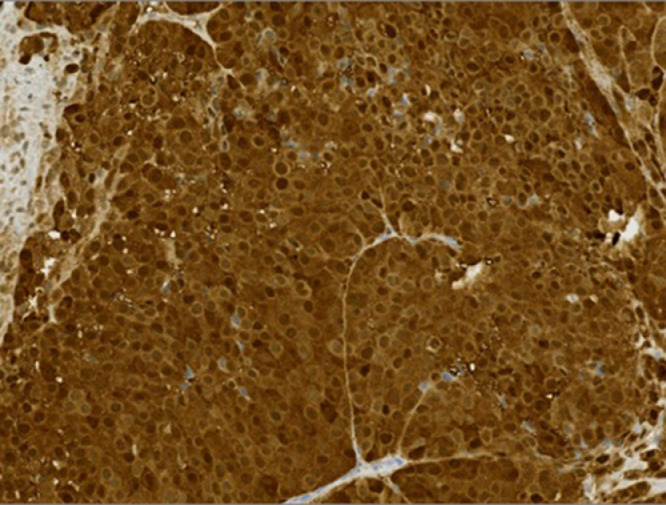
Fig 4.
S100 stain with ×20 magnification showed strongly positive nuclear and cytoplasmic staining.
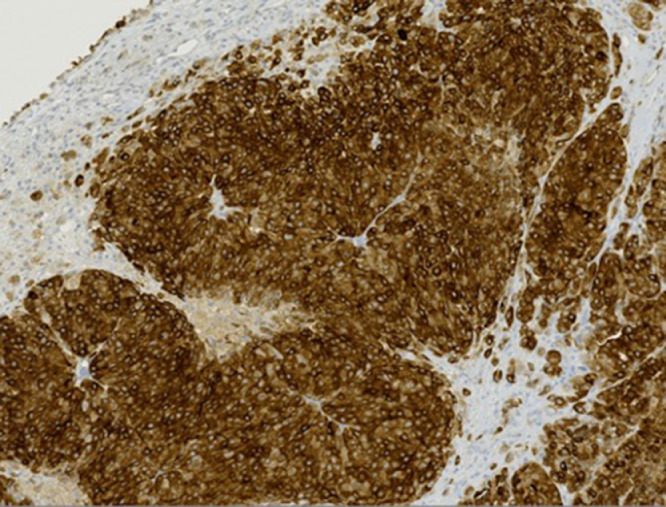
Fig 5.
Mel A stain with ×10 magnification demonstrated positive cytoplasmic staining supporting a melanocytic origin of the tumor cells.
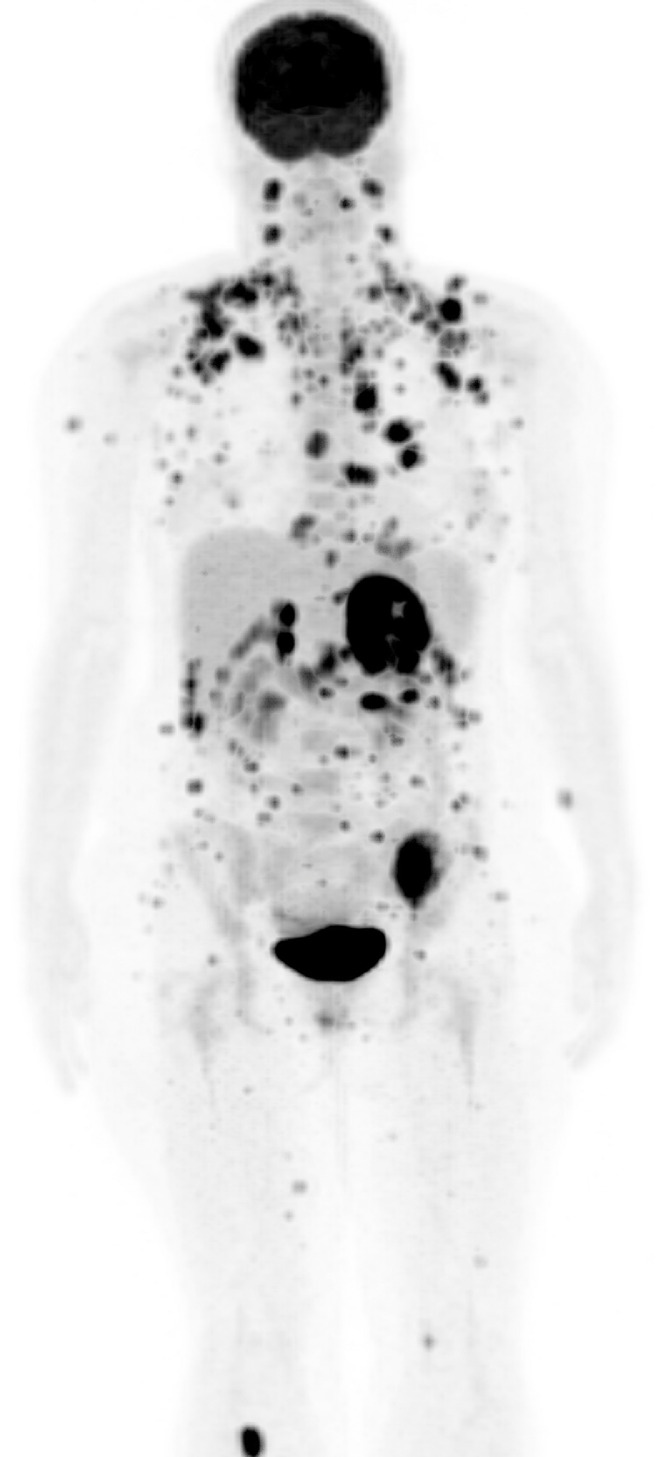
Fig 6.
Maximum intensity projection coronal image from PET-CT of the whole body revealed over 200 FDG-avid (Fluorine 18 fluorodeoxyglucose) metastatic lesions.
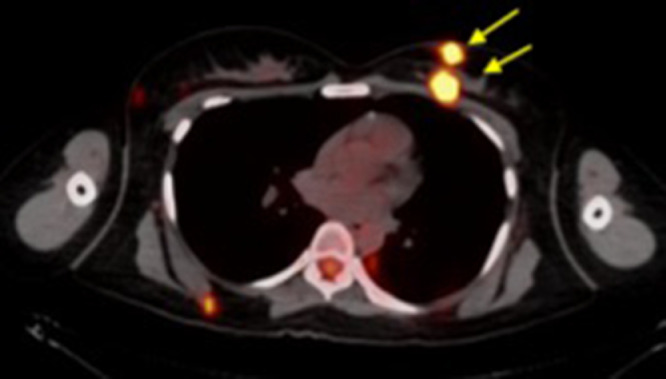
Fig 7.
PET-CT axial image through mid-chest demonstrated that besides the BIRADS 4 mass located at 10 o’ clock, the two left breast masses ( yellow arrows), previously identified on ultrasound at 10-o'clock were also FDG avid. (Color version of figure is available online.)
yellow arrows), previously identified on ultrasound at 10-o'clock were also FDG avid. (Color version of figure is available online.)
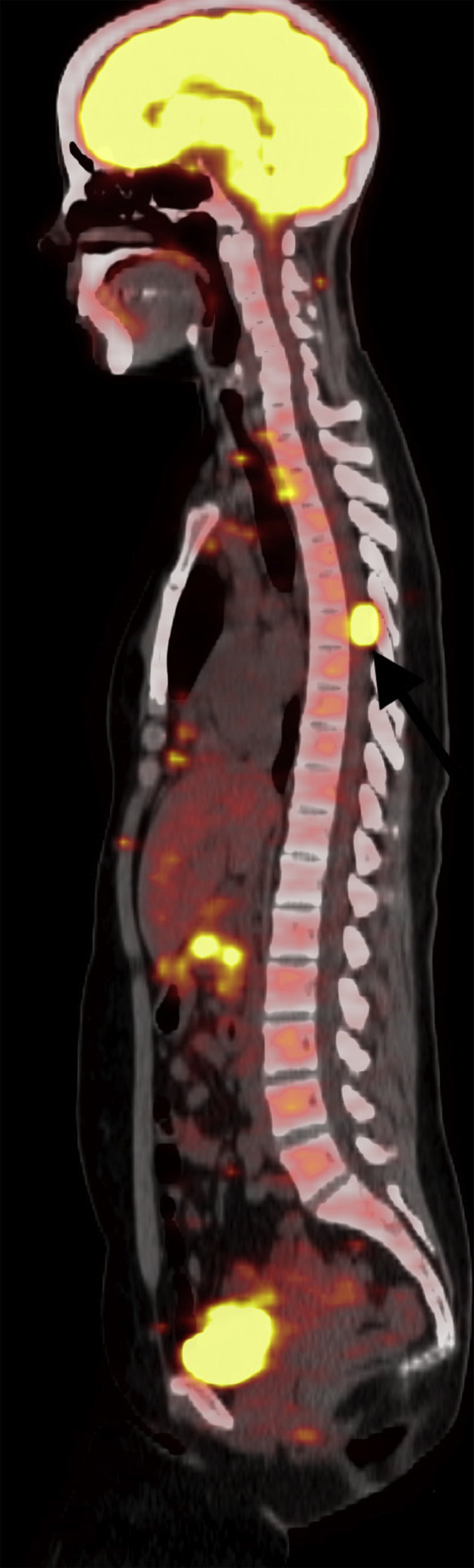
Fig 8.
PET-CT sagittal image showed an extradural spinal lesion at the level of T6 vertebra ( black arrow).
black arrow).
Fig 9.
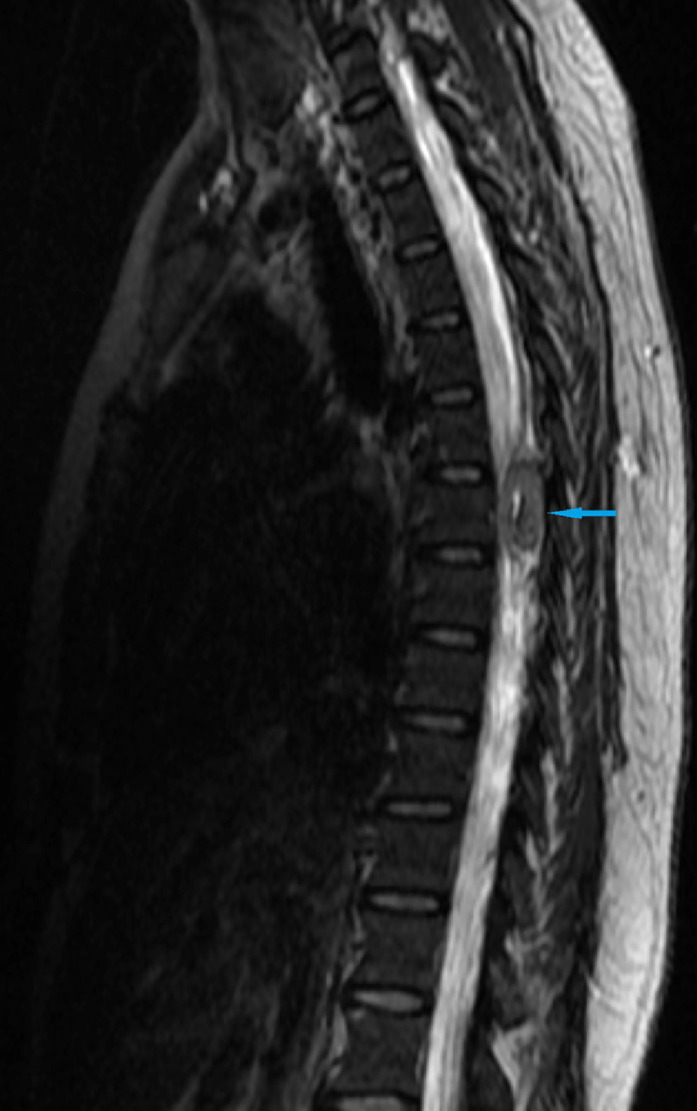
MRI spine sagittal image showed an extradural spinal lesion at the level of T6 vertebra ( blue arrow). (Color version of figure is available online.)
blue arrow). (Color version of figure is available online.)
Discussion
Malignant melanoma of the skin is one of the most rapidly increasing cancers in the world. Melanoma metastases occurs in 20% of cases, spreading via hematogenous or lymphatic routes to distant organs such as liver, lung, brain, and secondary sites in the skin [2]. Breast metastases from melanoma are uncommon and indicate widespread disease. The average age is 38-40 years, occurring more frequently in younger premenopausal women because of greater vascularity and more glandular tissue [3]. Breast metastases represent 1.3%-2.7% of all malignant breast tumors. Melanomas and lymphomas are the most common metastatic lesions in breast [4]. The most common primary sites for melanoma in premenopausal women with melanoma metastases in the breast, are the trunk and the upper limb. Melanoma can be in the breast skin or in the breast tissue and most often occurs in the outer half of the breast [5]. MM does not have specific imaging features and can simulate a broad variety of primary breast tumors. Although a simple antibody panel, consisting of S100, HMB45, and Melan-A stains, helps to render correct diagnosis, melanoma lesions presenting as primary breast tumors can present a diagnostic challenge; especially when there is inadequate patient history [6].
Lesions that arise within the subcutaneous fat or the hypodermal layer are usually benign and originate from the structures found there; namely, epidermal inclusion cysts and sebaceous cysts of cutaneous origin, fat necrosis, and lipomas from fat, lesions of lymphatic origin, vascular lesions and neurogenic lesions. The terminal duct lobular units, the functional unit of the breast, also may be located superficially, within anterior Cooper ligament extensions into the subcutaneous fat. Lesions that originate in the terminal duct lobular unit, such as, adenosis, fibroadenomas, peripheral papillomas, and superficial breast cancers can be subcutaneous in location [7]. Melanoma metastases to the breast, in-transit metastases to breast tissue (indicating tumor spreading through a lymph vessel away from the primary tumor before it reaches the nearest lymph node) and breast skin can present as subcutaneous breast masses. These should be considered in the differential diagnosis of suspicious subcutaneous breast masses. Because of its excellent spatial resolution, US is the optimal modality for localizing superficial palpable or nonpalpable breast masses.
As illustrated by this case, there are no unique imaging features to melanoma in breast. The index of suspicion should be high in patients with a present or past history of melanoma, in patients with breast masses accompanied by breast skin pigmentation or patients presenting with subcutaneous breast masses with suspicious features. This case highlights how combining medical knowledge with clinical skills helps improve diagnostic accuracy, especially in cases with MM.
Conclusions
There should be a greater degree of concern for MM in triple negative poorly differentiated carcinoma, especially in the setting of a subcutaneous breast mass with suspicious features or a breast mass presenting concurrently with other skin or subcutaneous lesions in the body. Radiologists can play an important role by suggesting the possibility of melanoma to the pathologist in such cases, and melanoma panel, consisting of S100, HMB45, and Melan-A stains, can be applied, thereby preventing diagnostic delays and avoiding unnecessary surgeries.
Patient consent
Statement of consent is not taken as no identifiers were stated or used in the article. Additionaly, no photographs were taken. Identifiers have been removed from radiologic images as well.
References
- 1.Kurul S, Tas F, Buyukbabani N, Mudun A, Baykal C, Camlica H. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35(4):202–206. doi: 10.1093/jjco/hyi068. [DOI] [PubMed] [Google Scholar]
- 2.Samaraee AA, Khout H, Barakat T, Fasih T. Breast metastasis from a melanoma. Ochsner J. 2012;12(2):149–151. [PMC free article] [PubMed] [Google Scholar]
- 3.Bassi F, Gatti G, Mauri E, Ballardini B, De Pas T, Luini A. Breast metastases from cutaneous malignant melanoma. Breast. 2004;13(6):533–535. doi: 10.1016/j.breast.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Moschetta M, Telegrafo M, Lucarelli NM, Martino G, Rella L, Stabile Ianora AA. Metastatic breast disease from cutaneous malignant melanoma. Int J Surg Case Rep. 2014;5(1):34–36. doi: 10.1016/j.ijscr.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravdel L, Robinson WA, Lewis K, Gonzalez R. Metastatic melanoma in the breast: a report of 27 cases. J Surg Oncol. 2006;94(2):101–104. doi: 10.1002/jso.20592. [DOI] [PubMed] [Google Scholar]
- 6.Bacchi CE, Wlodarski SC, Ambaye AB, Lamovec J, Salviato T, Falconieri G. Metastatic melanoma presenting as an isolated breast tumor: a study of 20 cases with emphasis on several primary mimickers. Arch Pathol Lab Med. 2013;137(1):41–49. doi: 10.5858/arpa.2011-0552-OA. [DOI] [PubMed] [Google Scholar]
- 7.Giess CS, Raza S, Birdwell RL. Distinguishing breast skin lesions from superficial breast parenchymal lesions: diagnostic criteria, imaging characteristics, and pitfalls. RadioGraphics. 2011;31(7):1959–1972. doi: 10.1148/rg.317115116. [DOI] [PubMed] [Google Scholar]