Abstract
Epidemiological data in COVID-19 mortality indicate that men are more prone to die of SARS-CoV-2 infection than women, but biological causes for this sexual dimorphism are unknown. We discuss the prospective behavioral and biological differences between the sexes that could be attributed to this sex-based differentiation. The female sex hormones and the immune stimulatory genes, including Toll-like receptors, interleukins, and micro-RNAs present on X-chromosome, may impart lesser infectivity and mortality of the SARS-CoV-2 in females over males. The sex hormone estrogen interacts with the renin-angiotensin-aldosterone system, one of the most critical pathways in COVID-19 infectivity, and modulates the vasomotor homeostasis. Testosterone on the contrary enhances the levels of the two most critical molecules, angiotensin-converting enzyme 2 (ACE2) and the transmembrane protease serine-type 2 (TMPRSS2), transcriptionally and posttranslationally, thereby increasing viral load and delaying viral clearance in men as compared with women. We propose that modulating sex hormones, either by increasing estrogen or antiandrogen, may be a therapeutic option to reduce mortality from SARS-CoV-2.
Keywords: hormones, immunity, morbidity, SARS-CoV-2, sex bias
INTRODUCTION
Coronaviruses, which belong to the Coronaviridae genus, are enveloped RNA viruses. Of the six coronavirus species discovered, four strains, i.e., 229E, OC43, NL63, and HKU1, cause common cold symptoms only in immunocompromised individuals. However, the other two strains, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), are linked to fatal illness (42). SARS-CoV was the agent of severe acute respiratory syndrome outbreaks in China in 2002–2003, and MERS-CoV was responsible for severe respiratory disease outbreaks in 2012 in the Middle East and continues to be ongoing (42, 56). The phylogenetic analysis reveals that novel SARS-CoV-2 is closely related to two bat-derived SARS-like coronaviruses, namely bat-SL-CoVZC45 and bat-SL-CoVZXC21 (88–89% sequence similarity), but it is more distant from SARS-CoV (∼79% similarity) and MERS-CoV (∼50% similarity). SARS-CoV-2 first started spreading via human-human transmission likely in December 2019, and on January 30, 2020, the World Health Organization (WHO) declared the outbreak as a pandemic and named it COVID-19. Internationally, COVID-19 is considered as the sixth public health emergency, after H1N1 (2009), polio (2014), Ebola in West Africa (2014), Zika (2016), and Ebola in the Democratic Republic of Congo (2019). COVID-19 has primarily affected adults, and the mortality rate is highest in those with comorbidities, including cardiovascular and endocrine diseases such as hypertension and diabetes mellitus (10). Symptoms of SARS-CoV-2 infection include fever, cough, dyspnea, myalgia, headache, and diarrhea. To date, the infection has resulted in more than 535,181 deaths, with more than 11,468,979 confirmed cases globally as per the WHO (as updated on July 7, 2020).
Of note is that there is a significant sexual dimorphism with men more infected than women (22), and the available worldwide data of COVID-19 fatality rates indicate a 2.5% greater mortality in men as compared with women (Fig. 1). India presently has a low mortality rate and equal rates between men and women. Such sex bias in the present case could be due to both behavioral and biological differences between the sexes (Fig. 2). Among the behavioral differences, men have higher rates of risk factors for infection and death, including smoking and drinking, and may have lower rates of hand washing, delayed admission to a hospital, and inability to follow social distancing requirements due to essential occupations or voluntary noncompliance with social restrictions. Among the biological differences, the additional X-chromosome and absence of the Y-chromosome and sex hormone expression appear to play a pivotal role. In this review, we focus on endocrine, genetic, and immune differences between women and men that could result in meaningful differences in acquisition and consequences of SARS-CoV-2 infection.
Fig. 1.
COVID-19 fatality may be influenced by sex-based differentiation. The reported COVID-19 fatality rate is higher in men as compared with women as per the available data for 47 countries. Source: https://globalhealth5050.org/covid19. Black bars represent %COVID-19 fatality rate in males and light gray bars %COVID-19 fatality rate in females for each country. Bars are presented in decreasing order of countrywise %difference between the male and female death rates.
Fig. 2.
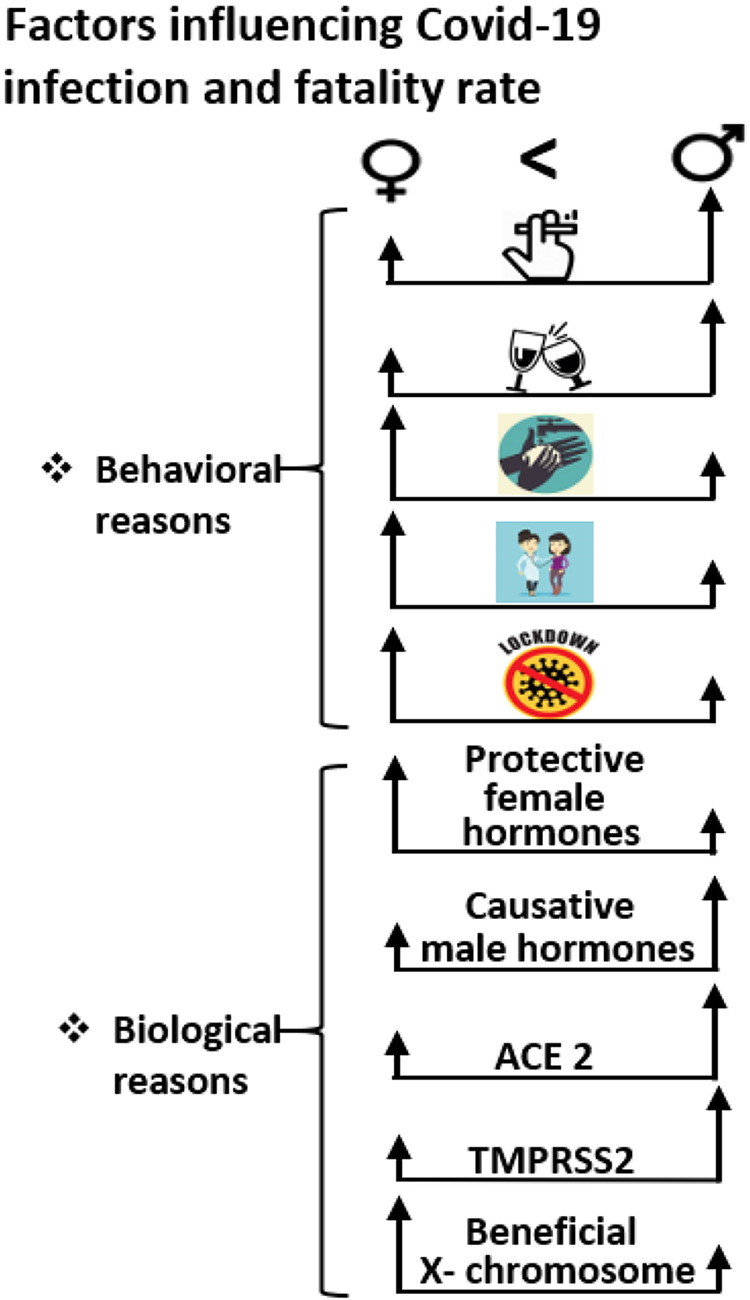
Behavioral, hormonal, and X-chromosomal influence on COVID-19 infectivity. Straight vertical black arrows with respective heights represent the relative influence of each of the behavioral and biological factors on the 2 sexes. Behavioral factors in men such as increased smoking and drinking, lower rates of hand washing, delayed admission to a hospital, and noncompliance with social restrictions could attribute to their increased susceptibility to infection and fatality. Biologically, beneficial effects of estrogen over testosterone could be linked to sex-based differentiation between sexes. Furthermore, increased expression of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine type 2 (TMPRSS2) in men may attribute to their increased viral load and decreased viral clearance capacity. Potential beneficial effects of immune-stimulatory genes encoded from the 2 X-chromosomes in women vs. 1 X-chromosome and 1 Y-chromosome in men may influence sex-based difference between sexes.
SEX-BASED DIFFERENTIATION
Influence of Sex Hormones on SARS-CoV-2 Infectivity
The endocrine system controls the release, transport, and function of hormones (12). The hypothalamic-pituitary-gonadal (HPG) axis, including the hypothalamus, pituitary gland, and the gonads, controls reproduction and sexual development by releasing gonadotropin-releasing hormone (GnRH) from the hypothalamus. GnRH in turn stimulates the pituitary gland to release luteinizing hormone and follicle-stimulating hormone, stimulating the gonads to produce sex steroid hormones, including estrogen, progesterone, testosterone, and peptide hormones such as activin, inhibin, and follistatin (12, 28).
Apart from sexual development, the HPG axis has been extensively linked with pulmonary and cardiovascular physiology. Estrogen, progesterone, and testosterone are the three most important sex steroid hormones impacting these organ systems. Humans express two types of estrogen receptors (ER), ERα and ERβ (41), and the expression of these receptors varies between organs and cell types. Estrogen acts via classical receptor-mediated, nonclassical, and non-ligand-mediated genomic (nuclear) and nongenomic (extranuclear) pathways to control mechanisms of gene expression, protein modifications and signaling to influence cellular functions (29). The endogenous estrogens produced in female mammals exists in three forms, estrone (E1), 17β-estradiol (E2), and estriol (E3); among these, E2 is the most prevalent and functional. The lipophilic nature of the hormones facilitates their diffusion through cell membranes, which enables them to directly affect the genetic material inside cells thereby to integrate into and alter membrane properties of cells (4). Women as compared with men produce higher levels of estrogen, which results in more robust innate, humoral, and cellular immune responses mediated by ER signaling (Fig. 2) (5, 7). In men, higher testosterone secretion has an inhibitory effect on the immune system through the upregulation of IL-10, an anti-inflammatory cytokine (24).
The immune system comprises innate and adaptive immunity. The innate immune response is the first line of defense against viral infections, including Toll-like receptors (TLRs), which identify viral proteins and nucleotides (13). Compared with male immune cells, female immune cells exhibit a 10-fold higher expression of TLRs (35). Adaptive immune responses provide more specific and longer-lasting protection through maintenance of memory of prior antigens mediated by lymphocytes (T and B cells) and immunoglobulins. Again, compared with men, women have higher number of regulatory T cells and immunoglobulins.
Many cellular components of the innate and adaptive immune system express ERα, including regulatory T cells, B cells, macrophages, monocytes, dendritic cells, and natural killer cells (29–31). The expression of hormone receptors on immune cells facilitates greater response to antigens, vaccines, and infections in women as compared with men. Under inflammatory conditions, ER activation induces endothelial nitric oxide synthase (eNOS/NOS3) and IFNγ expression in T cells. Estrogen can bind to estrogen response elements (EREs) on multiple target genes along with transcription factors to increase gene transcription (18). For example, ERE binding on the eNOS promoter results in increased eNOS expression, upregulated vasodilatory NO secretion, and increased blood flow to sites of inflammation (36, 39), a proinflammatory pathway that facilitates heightened immune responses, vasomotor relaxation, and reduced oxidative stress (Fig. 2). In contrast, the testosterone/androgen system is generally less effective at generating immune and inflammatory responses, which may lead to sexual dimorphism in SARS-CoV-2 viral infectivity.
X-Chromosome Contributions to Immunological Differences Between the Sexes
Gene diversity due to random transcriptional inactivation of the X-chromosome in women and a dosage difference from having two X-chromosomes mediates a heightened immune response to infectious diseases in females (44). The X-chromosome encodes several immune-regulatory genes, including the IL-2R γ-chain, IL-3R α-chain, IL-13 α-chain, IL-1R associated kinase 1, TLR7, Gata-binding protein 1, forkhead box protein 3, and cluster of differentiation ligand. TLR7 recognizes single-strand RNA viruses and promotes the production of antibodies and proinflammatory cytokines, including IL-6 and IL-1. The human X-chromosome also encodes 113 miRNAs; contrary to this, the Y-chromosome encodes only two miRNAs (Fig. 2) (33). miRNAs are short-nucleotide noncoding RNA molecules, which bind to complementary sequences within the 3′-untranslated region of target genes to silence gene translation. Estrogen activates several immune-responsive miRNAs such as miR-18a, miR-148a, miR-223, miR-451, miR-486, and miR-708 and regulates the expression of microRNAs in a cell-specific manner (29). Estrogen regulated miRNAs have been implicated in estrogen-mediated promotion of cell inflammation and autoimmune diseases (29).
Another prominent and well-studied example of sexual dimorphism in a viral disease is the response to human immunodeficiency virus: Acquired Immune Deficiency Syndrome (HIV-AIDS). Compared with males, females mount more robust activation of B and T cells following HIV-1 infection associated with higher baseline counts of immunoregulatory CD4+ T and CD8+ T cells (57). Several such clinical investigations have confirmed that female sex hormones, primarily estrogen and X-chromosome dosage, confer stronger innate and adaptive immune response to viral infections in women as compared with men (13, 32, 40). Thus, biologically, both estrogen and the number of X-chromosomes may result in protection against SARS-CoV-2 infectivity (Fig. 2).
Sex-Based Regulation of SARS-CoV-2 Infectivity Via RAAS/ACE2 Axis
Renin-angiotensin-aldosterone system (RAAS), a pathway critically implicated in SARS-CoV-2 infection and majorly regulated by estrogen (15, 16), is one of the most important hormonal mechanisms that regulates blood pressure, fluid volume, and sodium-potassium balance, thereby providing homeostatic maintenance of the vascular system (11). Renin catalyzes angiotensinogen to generate angiotensin I (Ang I). Ang I in turn is cleaved by the angiotensin-converting enzyme (ACE) to produce angiotensin II (Ang II), a potent vasoconstrictor that stimulates aldosterone production (19).
In a vascular system, the effect of ACE, a dipeptidyl carboxypeptidase I, is counterbalanced by its homolog ACE2, a carboxypeptidase (20). ACE2 cleaves angiotensin I and angiotensin II into the inactive peptide angiotensin 1–9 and the active peptide angiotensin 1–7, respectively, which have vasodilator and antiproliferative properties. Like ACE, ACE2 is also a zinc metalloenzyme, with its catalytic subunit oriented toward the extracellular space. Whereas ACE is expressed in selected tissues, including lung capillaries, small intestine, renal tubules, uterus, and male genitalia, ACE2 is expressed primarily in pulmonary, cardiac, renal, and intestinal tissues. Based on animal data, in the lung, ACE2 is expressed on the surface of alveolar epithelium, bronchiolar epithelium, endothelial cells, and smooth muscle cells of pulmonary vessels (52).
A recent gene ontology study found a strong association between the ACE2-expressing pulmonary alveolar type II (AT2) cells and the genes responsible for the SARS-CoV-2 viral internalization, replication, and transmission (55). ACE2 has been established as the most relevant protein in COVID-19 pathobiology as a binding site for the SARS-CoV-2 spike glycoprotein, mediating cellular entry of the virus. In this mechanism, the transmembrane protease serine-type 2 (TMPRSS2) also plays cardinal role (37). Of interest and relevance, however, is that bioinformatics analyses using publicly available data with bulk RNA sequencing (RNA-seq), single-cell RNA sequencing (scRNA-seq), and chromatin immunoprecipitation sequencing (ChIP-seq) have identified more ACE2-expressing AT2 cells in men compared with women (47), potentially contributing to increased viral susceptibility.
Recent reports indicate substantial amounts of ACE2 expression in human adult testis tissue, particularly in the spermatogonia, Leydig, and Sertoli cells (46). Leydig cells secrete testosterone under the influence of leutinizing hormone (LH) from the pituitary gland, whereas sertoli cells help in the spermatogenesis. ChIP-seq data identified and predicted binding of androgen receptor (AR) to 4,000 bp upstream of the ACE2 transcription start site, which may enhance ACE2 expression (47). Moreover, increased expression of ACE2 in Asian males as well as an association of the increased expression of ACE2 in testes with delayed viral clearance of SARS-CoV-2 in males aligns with this sex-biased fatality (Fig. 2) (50). The ACE2-enriched Leydig and Sertoli cells further induce the expression of genes that are involved in cell-cell junction and immunity (46). Furthermore, spermatogonia and spermatids display increased TMPRSS2 expression (25, 46). Yet another study reports that androgen-bound activated ARs in males upregulate the expression of TMPRSS2 both transcriptionally and posttranslationally (Fig. 2) (43). Despite the presence of ACE2 in the testis, it has yet to be validated whether this tissue enzyme has any role to play in the severity of COVID-19.
TMPRSS2 effectively blocks the enzymatic site of the ACE2, preventing the proteolysis of Ang II to Ang 1–7 (21). As stated above, TMPRSS2 also facilitates the binding of the SARS-CoV-2 spike glycoprotein and contributes to viral infectivity. Though TMPRSS2 expression is responsive to both androgen as well as estrogen stimulation (3), it has been observed that elderly women are at lower risk of fatal events due to COVID-19 than the elderly men (2), which could potentially be mediated by lower estrogen expression in postmenopausal females, contributing to suppressed viral entry. Several animal experiments and clinical investigations have interrogated the functional association between the RAAS and estrogen (17, 51, 53). Estrogen dramatically inhibits Ang II-induced reactive oxygen species formation, ERK phosphorylation, aldosterone secretion, endothelin-1 (ET-1) gene expression, and cell proliferation in different cell types (17). ET-1 is a potent vasoconstrictor and promotes vascular remodeling, particularly in the lungs (1, 8). Estrogen can directly promote ACE2 expression through the two-estrogen response elements (ERE) present on the ACE2 promoter (45). These data showed that a small population of ACE2-expressing AT2 is particularly prone to SARS-CoV-2 virus infection. Of the two estrogen receptor isoforms, ERα mediates the protective effect of estrogen in the prevention of hypertension-related disorders and suppression of NADPH oxidase (53, 54).
Similar to the dual function of ACE and ACE2, there are two receptors for the Ang II protein, AT1R and AT2R. Ang II binding to AT1R causes vasoconstriction, sodium reabsorption, and vascular growth, clinical hallmarks of hypertension, whereas AT2R binding by Ang II causes vasodilation and antiproliferation. Sex hormones regulate the expression of AT receptors, where testosterone enhances the expression of AT1R in males compared with estrogen, which instead enhances AT2R expression in females (54). This could be associated with increased vasoconstriction and severe consequences in men, who are already more prone to catching SARS-CoV-2 infection (34). Thus, the combinatorial role of endocrine system-mediated regulation of SARS-CoV-2-RAAS axis and X-chromosome-stimulated immune responses strongly support the observed sex-biased COVID-19 fatality. Sexual dimorphism is observed in various diseases, such as hypertension, cardiovascular, inflammatory diseases, HIV, and influenza virus pathogenesis, where the women are less infected than the men (9, 29, 39). Since the underlying mechanisms that contribute to sex-based immune cell regulation are not yet completely defined, more studies, design of experiments, and the accuracy of clinical trials are still needed to better understand the complete system.
Potential Therapeutic Strategies to Combat SARS-CoV-2
Blocking the interaction between SARS-CoV-2 and the ACE2 receptor and subsequent viral endocytosis into the cells are some of the primary targets for developing drugs and vaccines against COVID-19 (6, 26). Strategies include blocking the surface of ACE2 by anti-ACE2 antibody or peptides, slowing viral entry into cells by competitive binding of SARS-CoV-2 with soluble ACE2, or blocking the priming of the spike protein by using TMPRSS2 inhibitor. Two drugs, Arbidol, a virus-host cell fusion inhibitor, and Camostat mesylate, a clinically approved TMPRSS2 inhibitor, have entered clinical trials and may prove promising for preventing SARS-CoV-2 infection (23). The WHO’s “solidarity trial” of repurpose drugs, such as with the antiviral remdesivir, combination antivirals lopinavir/ritonavir (used in HIV) with or without interferon-β, and the malaria drug hydroxychloroquine and chloroquine are ongoing, involving more than 45 countries worldwide (48). The goal of this study is to determine which if any of these drugs reduces the fatality rate, hospitalization time, and the need for ventilators in those with COVID-19 or in healthcare staff or comorbid patients (48). Alternative therapeutics options such as bioengineered antibodies, cytokines, and nucleic acid-based therapies target virus gene expression.
Nevertheless, vaccines seem to be the ultimate answer. Several pharma companies and research institutions have worked tirelessly on it, and few pharma companies have reached phases 2 to 3 of clinical trials (49). These include a lipid nanoparticle (LNP)-encapsulated mRNA vaccine developed by Moderna/NIAID, nonreplicating viral vector vaccines such as a ChAdOx1-S, developed by the University of Oxford/AstraZeneca, and two vaccines, an inactivated virus vaccine and a DNA vaccine by India. Soon, these vaccines would be available in the market to check the virus menace. A better understanding of the sex-based host-virus interactions seems to be a crucial step in developing therapeutics against COVID-19. Furthermore, sex-specific vaccine trials would enhance the effectiveness of the therapeutic approaches, with the already known fact of females responding better postvaccination. Comorbidity-based fatality in men or otherwise has been a major concern; curtailing it with available therapeutics combined with newer approaches would provide best protective alternatives.
Estrogen, with its immune-boosting properties, is being considered as an alternative for COVID-19 treatment or in reducing its complications (14, 38). Comparative studies between men and premenopausal and postmenopausal women are underway, which will help determine the impact of hormone replacement therapy on the pathology in COVID-19. Moreover, androgen deprivation therapy, based on luteinizing hormone-releasing hormone agonist/antagonists or androgen receptors inhibitors, and which decreases TMPRSS2 levels in prostate cancer patients, may prevent recipients from SARS-CoV-2 infection (27). Overall, sex hormone-mediated regulation of SARS-CoV-2 infectivity could provide a new direction for COVID 19 treatment.
Conclusion and Hypothesis
In conclusion, the greater COVID-19 fatality rate noted in men as compared with women could be a consequence of several biological differences between the sexes. RAAS-associated comorbidity needs to be neutralized. The protective immune-stimulatory effect of estrogens and of an additional X-chromosome in women as well as immune suppression by testosterone in men are the other contributing biases. Androgen-mediated increased expression of ACE2 and TMPRSS2, the two most crucial molecules involved in SARS-CoV-2 infectivity, could be significant contributors to sex biasness. The few countries showing greater COVID-19 mortality in women as compared with men could be attributed to behavioral differences as well as genetic and/or biological differences.
The greater mortality rate of men over women may be hypothesized as a result of suppressed immune responses due to testosterone. Moreover, the binding of estrogen on the ACE2 and immune regulatory cells may have a double advantage; it may hinder with the binding of the virus and strengthen the immune system to combat the infection in females. Of note, the reversible effects of the hormones allow short-term hormone therapy treatment in COVID-19 patients, thus avoiding any long-term side effects.
GRANTS
T.P. avails UGC fellowship, ref 21/06/2015(i)EU-V; R.K. avails Career development award from American Heart Association (19CDA34730030); B.G. avails NIH Grant R01HL135872.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.C., T.P., K.S., and Q.P. analyzed data; N.C., T.P., K.S., and Q.P. interpreted results of experiments; N.C., T.P., K.S., and Q.P. prepared figures; N.C., T.P., K.S., R.K., B.B.G., and Q.P. drafted manuscript; N.C., T.P., K.S., R.K., B.B.G., and Q.P. edited and revised manuscript; N.C., T.P., K.S., R.K., B.B.G., and Q.P. approved final version of manuscript.
ACKNOWLEDGMENTS
CSIR, ICMR, New Delhi, and the CSIR-Institute of Genomics and Integrative Biology, Delhi, India, are acknowledged.
REFERENCES
- 1.Ali Z, Mishra A, Kumar R, Alam P, Pandey P, Ram R, Thinlas T, Mohammad G, Pasha MA. Interactions among vascular-tone modulators contribute to high altitude pulmonary edema and augmented vasoreactivity in highlanders. PLoS One 7: e44049, 2012. doi: 10.1371/journal.pone.0044049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 12: 10087–10098, 2020. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baena E, Shao Z, Linn DE, Glass K, Hamblen MJ, Fujiwara Y, Kim J, Nguyen M, Zhang X, Godinho FJ, Bronson RT, Mucci LA, Loda M, Yuan GC, Orkin SH, Li Z. ETV1 directs androgen metabolism and confers aggressive prostate cancer in targeted mice and patients. Genes Dev 27: 683–698, 2013. doi: 10.1101/gad.211011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia A, Sekhon HK, Kaur G. Sex hormones and immune dimorphism. ScientificWorldJournal 2014: 1–8, 2014. doi: 10.1155/2014/159150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird MD, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol 252: 57–67, 2008. doi: 10.1016/j.cellimm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brojakowska A, Narula J, Shimony R, Bander J. Clinical implications of SARS-Cov2 interaction with renin angiotensin system. J Am Coll Cardiol 75: 3085–3095, 2020. doi: 10.1016/j.jacc.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol 16: 35–50, 2016. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 8.Charu R, Stobdan T, Ram RB, Khan AP, Qadar Pasha MA, Norboo T, Afrin F. Susceptibility to high altitude pulmonary oedema: role of ACE and ET-1 polymorphisms. Thorax 61: 1011–1012, 2006. doi: 10.1136/thx.2006.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairweather D, Petri MA, Coronado MJ, Cooper LT. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol 8: 269–284, 2012. doi: 10.1586/eci.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 8: e21, 2020. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 98: 121–128, 2006. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Fuentes N, Silveyra P. Endocrine regulation of lung disease and inflammation. Exp Biol Med (Maywood) 243: 1313–1322, 2018. doi: 10.1177/1535370218816653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S, Klein RS. Sex drives dimorphic immune responses to viral infections. J Immunol 198: 1782–1790, 2017. doi: 10.4049/jimmunol.1601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandi G, Facchinetti F, Bitzer J. The gendered impact of coronavirus disease (COVID-19): do estrogens play a role? Eur J Contracept Reprod Health Care 25: 233–234, 2020. doi: 10.1080/13625187.2020.1766017. [DOI] [PubMed] [Google Scholar]
- 15.Harvey PJ, Morris BL, Miller JA, Floras JS. Estradiol induces discordant angiotensin and blood pressure responses to orthostasis in healthy postmenopausal women. Hypertension 45: 399–405, 2005. doi: 10.1161/01.HYP.0000157161.78721.5c. [DOI] [PubMed] [Google Scholar]
- 16.Hilliard LM, Sampson AK, Brown RD, Denton KM. The “his and hers” of the renin-angiotensin system. Curr Hypertens Rep 15: 71–79, 2013. doi: 10.1007/s11906-012-0319-y. [DOI] [PubMed] [Google Scholar]
- 17.Hong HJ, Liu JC, Chan P, Juan SH, Loh SH, Lin JG, Cheng TH. 17β-estradiol downregulates angiotensin-II-induced endothelin-1 gene expression in rat aortic smooth muscle cells. J Biomed Sci 11: 27–36, 2004. doi: 10.1007/BF02256546. [DOI] [PubMed] [Google Scholar]
- 18.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29: 2905–2919, 2001. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohli S, Sharma K, Qadar Pasha MA. Basic Interplay between genes of vascular homeostasis modulates hypertension. Biol Med (Aligarh) 8: 1–18, 2015. [Google Scholar]
- 20.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE. Sex-specific SARS-CoV-2 mortality: among hormone-modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D. Int J Mol Sci 21: 2948, 2020. doi: 10.3390/ijms21082948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 55: 105924, 2020. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, Smoot J, Gregg AC, Daniels AD, Jervey S, Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci 6: 315–331, 2020. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol 167: 2060–2067, 2001. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study (Preprint) medRxiv 2020. doi: 10.1101/2020.03.21.20037267. [DOI]
- 26.Macova M, Armando I, Zhou J, Baiardi G, Tyurmin D, Larrayoz-Roldan IM, Saavedra JM. Estrogen reduces aldosterone, upregulates adrenal angiotensin II AT2 receptors and normalizes adrenomedullary Fra-2 in ovariectomized rats. Neuroendocrinology 88: 276–286, 2008. doi: 10.1159/000150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Carbone GM, Cavalli A, Pagano F, Ragazzi E, Prayer-Galetti T, Alimonti A. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 31: 1040–1045, 2020. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosselman S, Polman J, Dijkema R. ER β: identification and characterization of a novel human estrogen receptor. FEBS Lett 392: 49–53, 1996. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- 29.Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol 9: 2279–2289, 2018. doi: 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett 97: 107–113, 2005. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Pierdominici M, Maselli A, Colasanti T, Giammarioli AM, Delunardo F, Vacirca D, Sanchez M, Giovannetti A, Malorni W, Ortona E. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett 132: 79–85, 2010. doi: 10.1016/j.imlet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol 88: 4711–4720, 2014. doi: 10.1128/JVI.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai R, Ahmed SA. Sexual dimorphism of miRNA expression: a new perspective in understanding the sex bias of autoimmune diseases. Ther Clin Risk Manag 10: 151–163, 2014. doi: 10.2147/TCRM.S33517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampson AK, Moritz KM, Denton KM. Postnatal ontogeny of angiotensin receptors and ACE2 in male and female rats. Gend Med 9: 21–32, 2012. doi: 10.1016/j.genm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex-differences in resident immune cell phenotype underlies more efficient. Blood 118: 5918–5927, 2011. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaul PW. Rapid activation of endothelial nitric oxide synthase by estrogen. Steroids 64: 28–34, 1999. doi: 10.1016/S0039-128X(98)00105-6. [DOI] [PubMed] [Google Scholar]
- 37.Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: Serendipity or opportunity for intervention? Cancer Discov 10: 779–782, 2020. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suba Z. Prevention and therapy of COVID-19 via exogenous estrogen treatment for both male and female patients. J Pharm Pharm Sci 23: 75–85, 2020. doi: 10.18433/jpps31069. [DOI] [PubMed] [Google Scholar]
- 39.Sumi D, Ignarro LJ. Estrogen-related receptor α1 up-regulates endothelial nitric oxide synthase expression. Proc Natl Acad Sci USA 100: 14451–14456, 2003. doi: 10.1073/pnas.2235590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tasker C, Ding J, Schmolke M, Rivera-Medina A, García-Sastre A, Chang TL. 17β-estradiol protects primary macrophages against HIV infection through induction of interferon-alpha. Viral Immunol 27: 140–150, 2014. doi: 10.1089/vim.2013.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J 56: 729–737, 2009. doi: 10.1507/endocrj.K09E-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B. Identification of a new human coronavirus. Nat Med 10: 368–373, 2004. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wambier CG, Goren A, Vaño-Galván S, Ramos PM, Ossimetha A, Nau G, Herrera S, McCoy J. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev Res. In press. doi: 10.1002/ddr.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Syrett CM, Kramer MC, Basu A, Atchison ML, Anguera MC. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci USA 113: E2029–E2038, 2016. doi: 10.1073/pnas.1520113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Shoemaker R, Thatcher SE, Batifoulier-Yiannikouris F, English VL, Cassis LA. Administration of 17β-estradiol to ovariectomized obese female mice reverses obesity-hypertension through an ACE2-dependent mechanism. Am J Physiol Endocrinol Metab 308: E1066–E1075, 2015. doi: 10.1152/ajpendo.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells 9: 920, 2020. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei X, Xiao YT, Wang J, Chen R, Zhang W, Yang Y, Lv D, Qin C, Gu D, Zhang B, Chen W, Hou J, Song N, Zeng G, Ren S. Sex differences in severity and mortality among patients with covid-19: evidence from pooled literature analysis and insights from integrated bioinformatic analysis (Preprint) arXiv 2020. doi:arXiv:2003.13547v1.
- 48.World Health Organization Public health emergency SOLIDARITY trial of treatments for SARS-COV-2 infection in hospitalized patients (Online). doi: 10.1186/ISRCTN83971151. [DOI]
- 49.World Health Organization Draft landscape of COVID-19 candidate vaccines (Online). https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 50.Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W, Li Y, Ni Q, Zou R, Li X, Xu M, Zhang Y, Zhao H, Zhang X, Yu L, Su J, Lang G, Liu J, Wu X, Guo Y, Tao J, Shi D, Yu L, Cao Q, Ruan B, Liu L, Wang Z, Xu Y, Liu Y, Sheng J, Li L. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19). Clin Infect Dis 71: 799–806, 2020. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X, Xiao JC, Luo LF, Wang S, Zhang JP, Huang JJ, Liu ML, Liu CG, Xu KQ, Li YJ, Song HP. Effects of ovariectomy and 17β-estradiol treatment on the renin-angiotensin system, blood pressure, and endothelial ultrastructure. Int J Cardiol 130: 196–204, 2008. doi: 10.1016/j.ijcard.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 52.Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci 78: 2166–2171, 2006. [Erratum in: Life Sci 79: 2499, 2006.] doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-α mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 54.Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J Neurosci 29: 13823–13836, 2009. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ziegler S, Altfeld M. Sex differences in HIV-1-mediated immunopathology. Curr Opin HIV AIDS 11: 209–215, 2016. doi: 10.1097/COH.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]



