Abstract
Cold viruses have generally been considered fairly innocuous until the appearance of the severe acute respiratory coronavirus 2 (SARS-CoV-2) in 2019, which caused the coronavirus disease 2019 (COVID-19) global pandemic. Two previous viruses foreshadowed that a coronavirus could potentially have devastating consequences in 2002 [severe acute respiratory coronavirus (SARS-CoV)] and in 2012 [Middle East respiratory syndrome coronavirus (MERS-CoV)]. The question that arises is why these viruses are so different from the relatively harmless cold viruses. On the basis of an analysis of the current literature and using bioinformatic approaches, we examined the potential human miRNA interactions with the SARS-CoV-2’s genome and compared the miRNA target sites in seven coronavirus genomes that include SARS-CoV-2, MERS-CoV, SARS-CoV, and four nonpathogenic coronaviruses. Here, we discuss the possibility that pathogenic human coronaviruses, including SARS-CoV-2, could modulate host miRNA levels by acting as miRNA sponges to facilitate viral replication and/or to avoid immune responses.
Keywords: coronaviruses, coronavirus disease 2019 (COVID-19), miRNA sponges, SARS-CoV, UPR
INTRODUCTION
Coronaviruses (CoVs) are single‐stranded RNA viruses that infect a wide variety of animals including humans (HCoV). They have generally been considered to be relatively innocuous until the outbreaks of severe acute respiratory coronavirus (SARS-CoV) in 2002 (18, 31, 108), the Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 (120), and the recent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), which resulted in a global pandemic (130).
Each of these dangerous human coronaviruses (HCoVs) has been reported to differ significantly in their pathomechanisms of infection, despite the similarities in their RNA sequences (17). SARS-CoV-2’s active viral replication occurs in the upper respiratory tract (111), and its severe presentation requires a virus receptor expressed in lung epithelial cells (39, 46). Furthermore, 75% of people with severe COVID-19 disease develop evidence of pneumonia (102) and evidence of a strong inflammatory response in the lungs. Unfortunately, a fraction of COVID-19 patients can further develop into severe acute respiratory distress syndrome (20, 36, 77, 110). Previous studies indicate that both SARS-CoV and MERS-CoV infections are accompanied by a delayed/deregulated interferon response in primary human airway epithelial cells. This indicates that these HCoVs have developed the ability to attenuate innate immunity (58, 67), whereas harmless HCoVs seem to lack this capability (117). Similar to SARS-CoV and MERS-CoV infections, elevated levels of proinflammatory cytokines, the so-called “cytokine storm,” have been reported in COVID-19 patients (47). These findings stress the critical roles of the expression of lung cell-specific receptors for viral entry and the importance of innate immunity in determining the mechanisms controlling the inflammatory responses during SARS-CoV-2 infections.
Given that there is no vaccine currently available for COVID-19, although there are promising candidates, research into the pathogenesis of this viral infection has focused on understanding the mechanisms involved in viral entry and replication, and importantly, in restoring and enhancing innate and adaptive immunity. Following virus attachment and entry into host cells, the viral particle is uncoated and its positive-sense single-stranded RNA genome is released into cytosol where it serves as a matrix for the host translation machinery to produce viral proteins (33). A virus replication cycle such as this potentially exposes its RNA to an antiviral cellular defense that relies on the host’s endogenous microRNAs (miRNAs). These miRNAs could prevent viral protein translation and/or directly degrade its RNA and prevent viral protein translation, such as has been reported for the influenza virus (82). Furthermore, miRNAs, through their role in posttranscriptional gene regulation, modulate cellular signaling that regulates immune responses (100). Notably, a recent study proposed that bats, considered as the SARS-CoV-2 host of origin, have tolerance to potentially deadly viruses because of specific miRNAs (25). Furthermore, viruses can also take advantage of either their host’s or their own miRNAs to facilitate viral replication or to inhibit the host’s antiviral responses (3). miRNA regulation or manipulation could therefore provide a novel basis for antiviral drug therapies.
miRNA expression profiles are often cell-type specific and differ between individuals, and importantly, can be affected by cellular stress responses (11, 12). In our recent study, we demonstrated that exposure of human lung epithelial cells to endoplasmic reticulum stress (ER stress) results in a global reduction of miRNA expression levels (37) and may also lead to activation of a miRNA-based reduction of antigen presentation (7). Notably, ER stress and the downstream activation of stress responses has been reported during coronavirus infections (16, 35), and has been proposed to facilitate SARS-CoV replication (16). Thus, the individual and epigenetic differences in the miRNA profiles during an infection in human lung epithelial cells could affect the effectiveness of the antiviral responses and the disease severity.
Although underappreciated, miRNAs expressed in human lung cells may be an important factor determining the COVID-19’s severity. Using a bioinformatic analysis of human miRNA potential interactions with the SARS-CoV-2’s genome, we examined the potential miRNA target sites in seven coronavirus genomes that include SARS-CoV-2, MERS-CoV, SARS-CoV, and four nonpathogenic coronaviruses.
COMPARISON OF THE miRNA TARGET SITES IN HUMAN CORONAVIRUSES
To examine whether SARS-CoV-2 could be differentially targeted by human endogenous miRNAs compared with other HCoVs, we analyzed and compared the potential microRNA target sites (MTSs) within a set of seven HCoVs RNA genomes. Our approach was to examine and compare three pathogenic and four nonpathogenic strains of HCoVs. The HCoV RNA genomes of pathogenic strains were SARS-CoV-2 (NC_045512.2), SARS-CoV (NC_004718.3), and MERS-CoV (NC_019843.3). The nonpathogenic strains were HCoV-OC43 (KU131570.1), HCoV-229E (NC_002645.1), HCoV-HKU1 (KF686346.1), and HCoV-NL63 (NC_005831.2). These coronaviruses were tested against the set of 896 confident mature human miRNA sequences that were obtained from the miRBbase v2.21 (54, 55) using the RNA22 v2 microRNA target discovery tool web server (70). To reduce the false discovery rate of the MTS predictions, the strictest parameters were applied to the default computation workflow using a specificity of 92% versus a sensitivity of 22% (70). The results of the MTS mapping to viral RNAs are provided in Supplemental Data Set S1 (see https://doi.org/10.5281/zenodo.3966446).
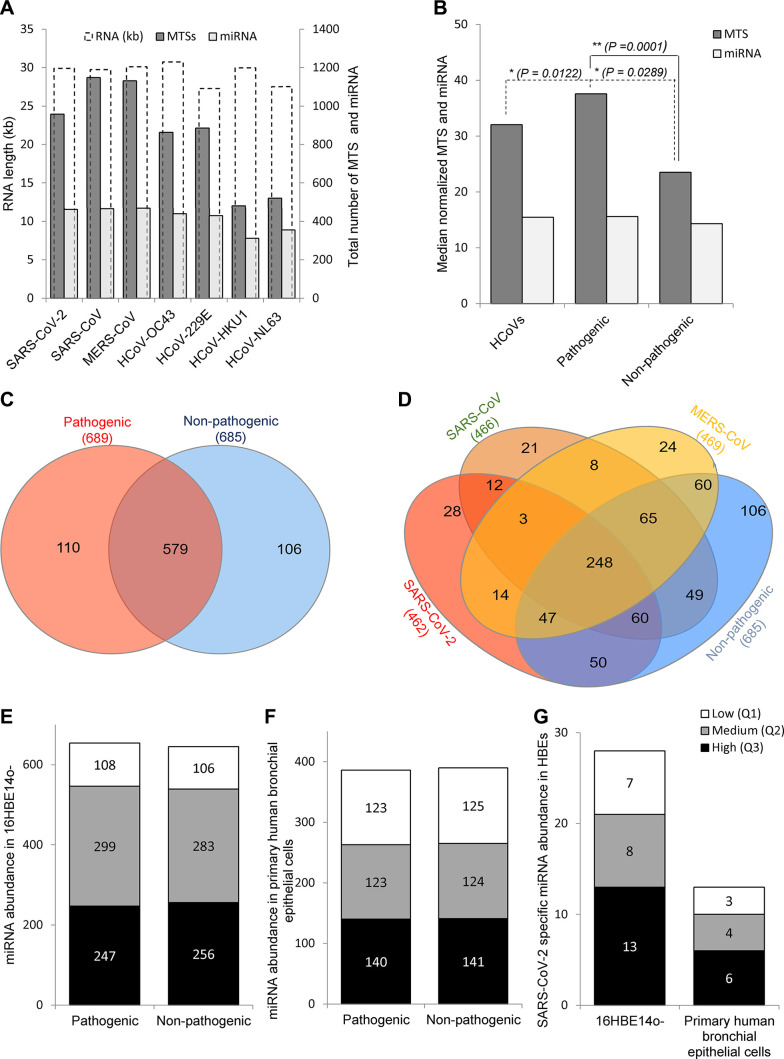
This analysis illustrates the relative genome size for all seven HCoVs (Fig. 1A, white bars), and indicates that the potential number of MTSs was elevated in the pathogenic strains compared with the nonpathogenic strains (Fig. 1A, black bars). Interestingly, there were no major differences in miRNA numbers when normalized to the genome size (Fig. 1B). The observed differences in MTS number between pathogenic and nonpathogenic HCoVs could be an artifact of small experimental numbers since only seven unique viral genomes were analyzed. Given that concern, we next examined the number of miRNAs that were common or different that could potentially bind to each of the coronavirus RNAs. As shown in Fig. 1C, the pathogenic HCoVs attract a set of miRNAs that differ from the nonpathogenic HCoVs. A detailed analysis defines a set of 28 miRNAs that are unique for SARS-CoV-2, as well as the set of another 21 and 24 miRNAs that are unique for SARS-CoV and MERS-CoV, respectively (Fig. 1D).
Fig. 1.
Distribution of human miRNAs and microRNA (miRNA) target sites (MTSs) in human coronavirus (HCoV) RNAs. A: the number of miRNAs is marked in light gray bars, the MTS number is marked in dark gray bars, and the virus RNA length (in kb) is marked in white bars. B: the pathogenic HCoV miRNA and MTS numbers [severe acute respiratory coronavirus 2 (SARS-CoV-2), SARS-CoV, and Middle East respiratory syndrome coronavirus (MERS-CoV)] were normalized to the length of the viral RNAs and were compared with nonpathogenic numbers (HCoV-OC43, HCoV-229E, HCoV- HKU1, and HCoV-NL63). The results of the MTS mapping to viral RNAs are provided in Supplemental Data Set S1 (https://doi.org/10.5281/zenodo.3966446). C: the Venn diagram (43) represents distribution of miRNAs between the pathogenic and nonpathogenic HCoVs. D: the distribution of miRNAs between the COVID-19, SARS-CoV, MERS-CoV, and the group of nonpathogenic HCoVs is shown. *P value compared with all HCoVs (P = 0.0122 and P = 0.0289). **P value for the pathogenic compared with the nonpathogenic groups (P = 0.0001). A P value less than 0.05 was considered significant. E and F: comparison of the miRNA expression profiles is shown for 16HBE14o- cells (median of raw reads; 31M/sample; n = 10) and for primary human bronchial epithelial cells (median of raw reads; 12M/sample; n = 24). G: miRNAs that have MTSs specific for SARS-CoV-2 are well represented in human lung epithelial cells. Low abundance miRNAs (first quartile Q1, low expression) are marked white, medium abundance miRNAs (Q2) are marked in gray, and high abundance miRNAs are marked in black. The numbers of miRNAs in each group are provided inside the bars.
On the basis of the above analysis, we envision three different possibilities.
Host miRNAs Serve as an Antiviral Shield
In this possibility, the miRNAs that are specific for nonpathogenic HCoVs are abundant in bronchial epithelial cells and thus provide one type of defense mechanism for viral infection. Whereas, the levels of miRNA specific for the pathogenic coronaviruses are either low or lowered during these viral infections. To test this hypothesis, we compared miRNA abundances between the miRNA specific for pathogenic and nonpathogenic HCoVs. Our approach was to use miRNA expression profiles of normal human bronchial epithelial (HBE) cells. For these studies, we analyzed data from a previous next-generation sequencing analysis of 16HBE14o-cells and primary HBEs. The deep sequencing data are deposited in the Gene Expression Omnibus (GEO) at accession number GSE117629) (37).
As shown in Fig. 1, E and F, the abundance of miRNAs that could recognize pathogenic versus nonpathogenic HCoVs were similar, and the vast majority of miRNAs were well expressed in 16HBE14o- and primary HBE cells (bronchial epithelial cells differentiated at an air-liquid interface). This is despite the fact that the next-generation sequencing depths for these two sample sets were different [12 million (M) reads per sample (primary HBE cells) versus 31 M reads per sample (16HBE14o- cells)]. The miRNAs that potentially could target SARS-CoV-2 were well expressed and similar in both primary HBE and 16HBE14o- cells (Fig. 1G). Hence, the hypothesis that the lung cells lack specific miRNA directed against pathogenic HCoVs seems rather unlikely, although it still cannot be excluded for specific miRNAs. This observation questions the use of miRNA overexpression (miRNA mimics) as a therapeutic approach against the COVID-19 infection since siRNAs directed against viral RNA would be more specific and therefore less prone to have off-target effects (12).
HCoVs RNAs Serve as microRNA Sponges
Given the observation that the pathogenic HCoVs have more potential MTSs in their RNA than the nonpathogenic ones, we speculate that these viruses work like specific microRNA sponges that reduce cellular miRNA levels and therefore modulate the host’s cellular processes to facilitate viral replication. Viral infections result in high abundance of viral RNA and the higher potential miRNA binding sites these pathogenic viruses harbor could offer a very effective defense by lowering the cellular miRNA levels during the early stages of an infection.
How likely is it that a viral RNA can act as a miRNA sponge? Host miRNAs constitute ~0.01% of total cellular and tissue RNA (81), while the viral RNA in infected lung cells can reach more than 50% of total cellular RNA (13). Furthermore, evidence that viral RNA sponges are capable of being efficient in removing host miRNAs is demonstrated in studies on Epstein-Barr virus which illustrates that EBV miRNA sponges regulate virus infection as well as oncogenesis by downregulating a number of target miRNAs (85). Viral RNA sponge activity has also been reported for Herpes virus (15) and hepatitis C virus (64).
Additionally, the pathogenic HCoVs could remove host-specific miRNAs to modulate specific gene expression to suppress immunity or prevent activation of unfolded protein response (UPR)-related apoptosis. To follow this hypothesis, we focused on the potential role of the 28 miRNAs that were uniquely specific for SARS-CoV-2 (Fig. 1D). As shown in Table 1, the majority of these miRNAs are well expressed in bronchial epithelial cells, and their dysregulation has been reported for various human lung pathologies that include lung cancers, chronic obstructive pulmonary disease, cystic fibrosis, and tuberculosis. Furthermore, many of these miRNAs have been proposed to act as tumor suppressors that target apoptosis-related pathways, and thus their reduction has been associated with poor cancer prognosis. Hence, SARS-CoV-2, by its potential reduction of the host’s miRNA pool, may promote infected cell survival and thus continuity of its replication cycle.
Table 1.
Human miRNAs that could potentially interact with severe acute respiratory coronavirus 2 and their biological roles
| miRNA | Abundance in HBE | MTS | Potential Impact on Cellular Processes |
|---|---|---|---|
| hsa-let-7a-3p | Q3/Q2 | 1 | Reduced expression in human lung cancers is associated with shortened postoperative survival (96). |
| hsa-miR-10a-5p | Q3/Q3 | 1 | Constrains the plasticity of helper T cells (95). Is deregulated in breast cancer (19). |
| hsa-miR-126–3p | Q3/Q3 | 1 | Downregulated in cystic fibrosis airway epithelial cells and regulates TOM1 expression (78). Downregulates VEGF and inhibits the growth of lung cancer cell lines in vitro and in vivo (62). |
| hsa-miR-154–3p | Q2/nd | 1 | Is profibrotic in pulmonary fibrosis (69). |
| hsa-miR-195–3p | Q1/nd | 2 | Deregulated in cancer (119) |
| hsa-miR-200a-5p | Q3/Q3 | 1 | Suppresses MMP9 expression in lung cancer (23). |
| hsa-miR-345–5p | Q3/Q3 | 1 | Deregulated in colorectal cancer (97). Suppresses cell invasion and migration in NSCLC (125). Low tissue expression was associated with progression and poor prognosis of lung cancer (22). |
| hsa-miR-34a-3p | Q3/Q2 | 1 | Deregulated in cancers (44). Inhibits lung fibrosis by inducing lung fibroblast senescence (26) Control of vertebrate multiciliogenesis (66, 93) UPR related—proposed to target XBP1 (6). |
| hsa-miR-3664–5p | Q2/Q1 | 2 | Altered in sputum of patients with active pulmonary tuberculosis (116). |
| hsa-miR-376a-3p | Q3/Q1 | 1 | Targets insulin growth factor 1 receptor (121). Facilitates immune elimination of HCMV infected cells (76). Inhibits lung cancer progression (107). |
| hsa-miR-376b-3p | Q3/nd | 1 | Controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1 (53). Associated with COPD (79). |
| hsa-miR-3939 | Q1/Q1 | 1 | Unknown |
| hsa-miR-4746–5p | Q1/Q1 | 1 | Deregulated in lung cancer (114, 129). |
| hsa-miR-495–5p | Q2/nd | 1 | Deregulated in cancers (24, 48). Targets UPR in lung cancer cells -reduction of these miRNA might be the reason for upregulation of GRP78 in NSCLC patients (1). |
| hsa-miR-513a-3p | Q2/nd | 1 | Contributes to the controlling of cellular migration processes in the A549 lung tumor cells by modulating integrin β-8 expression (27). |
| hsa-miR-513b-3p | Q3/nd | 1 | Deregulated in lung cancer (105). |
| hsa-miR-513c-3p | Q2/nd | 1 | Lung cancer biomarker (65). |
| hsa-miR-514b-5p | Q1/nd | 1 | Deregulated in colorectal cancer (88). |
| hsa-miR-548av-5p | Q1/nd | 1 | Potentially associated with transition from immune tolerance to immune activation of chronic hepatitis B (113). |
| hsa-miR-582–5p | Q3/Q2 | 3 | Suppresses non-small cell lung cancer cells growth and invasion via downregulating NOTCH1 (63). |
| hsa-miR-605–5p | Q3/Q1 | 1 | Mediates p53 related cellular responses to stress (112). miR-605 genetic polymorphisms are a risk factors of lung cancer susceptibility and gastrointestinal cancer risk (123, 124). |
| hsa-miR-664a-3p | Q3/Q3 | 1 | Associated with cigarette smoke-induced COPD (128). |
| hsa-miR-6733–5p | Q2/nd | 1 | Unknown |
| hsa-miR-6758–3p | Q2/nd | 1 | Predicted miRNAs from Toxoplasma gondii potentially regulates the hosts’ gene expression (89). |
| hsa-miR-6793–3p | Q1/nd | 1 | Unknown |
| hsa-miR-6802–3p | Q1/nd | 1 | Unknown |
| hsa-miR-766–3p | Q3/Q2 | 1 | A potential biomarker for predicting survival in lung adenocarcinoma (61). |
| hsa-miR-99b-5p | Q3/Q1 | 1 | DNA damage response (74). Mycobacterium tuberculosis controls miR-99b expression in infected murine dendritic cells to modulate host immunity (92). Suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation (94). |
The miRNA abundance in human bronchial epithelial cells is provided as a quartile (Q) of raw next generation sequencing reads distribution for 16HBE14o-/primary HBE. nd, not detected. COPD, chronic obstructive pulmonary disease; HCMV, human cytomegalovirus; MTS, miRNA target site; mTOR, mammalian target of rapamycin; NSCLC, non-small cell lung cancer; UPR, unfolded protein response; VEGF, vascular endothelial growth factor; XBP1, X-box binding protein transcription factor.
Another possibility is the enrichment of MTSs in the virus RNA that are sites for specific miRNAs. The 10 miRNAs that have the highest MTS number in SARS-CoV-2’s RNA genome are listed in Table 2. Although they are not unique for this virus, the MTSs for them are more frequent in the pathogenic HCoVs. Again, some of these miRNAs are either UPR regulators (miR-34c-5p; miR-34a-5) or modulators of immune responses (miR-149-3p).
Table 2.
miRNAs having the most miRNA target sites in severe acute respiratory coronavirus 2 RNA
| miRNA | Abundance in HBE | MTS |
Potential Impact on Cellular Processes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | SARS | MERS | NL63 | OC43 | 229E | HKU1 | |||
| hsa-miR-449c-5p | Q2/Q3 | 9 | 5 | 3 | 2 | 1 | 3 | 2 | Targets c-Myc and inhibits NSCLC cell progression (68) |
| hsa-miR-3940–5p | Q1/nd. | 9 | 11 | 5 | 7 | 2 | 3 | 3 | Tumor Suppressor in NSCLCs (87) |
| hsa-miR-34c -5p | Q3/Q3 | 9 | 5 | 11 | 1 | 6 | 7 | 3 | UPR-dependent and targets XBP1 (4, 6). |
| hsa-miR-34a -5p | Q3/Q3 | 9 | 8 | 11 | 0 | 4 | 6 | 5 | UPR-IRE1 dependent and targets Caspase 2 (60). |
| hsa-miR-149-3p | Q2/Q2 | 8 | 4 | 6 | 2 | 4 | 1 | 2 | Deregulated in COPD (32). Suppresses hepatic inflammatory response through antagonizing the STAT3 signaling pathway (126). |
| hsa-miR-92a-1-5p | Q3/Q2 | 8 | 4 | 4 | 5 | 5 | 2 | 0 | Unknown |
| hsa-miR-138-5p | Q3/Q3 | 8 | 6 | 4 | 0 | 4 | 2 | 1 | Deregulated in colorectal and lung cancers (42, 127). |
| hsa-miR-4433b-3p | Q1/nd | 8 | 6 | 13 | 7 | 5 | 3 | 5 | Unknown |
| hsa-miR-766-5p | Q3/Q1 | 7 | 4 | 3 | 1 | 5 | 4 | 3 | Unknown |
| hsa-miR-6741-5p | Q1/Q1 | 7 | 6 | 9 | 3 | 9 | 7 | 3 | Unknown |
Abundance in human bronchial epithelial cells is provided as a quartile (Q) of raw next generation sequencing reads distribution for 16HBE14o-/primary HBE. HBE, human bronchial epithelial; IRE, inositol-requiring enzyme; nd, not detected; NSCLC, non-small cell lung cancer; UPR, unfolded protein response.
Virus proteins require and extensively use the endoplasmic reticulum (ER) and Golgi system for proper protein folding and assembly. Except for the N protein, all coronavirus structural proteins are transmembrane proteins synthesized in the ER (35). Furthermore, ER membranes are crucial for the formation of virus double‐membrane vesicles (DMVs) (28) that require both virus transmembrane proteins and massive morphological rearrangement of the ER (52, 86). Both the increased ER membrane and the protein folding demand can result in coronavirus-related ER stress and activation of a dedicated signaling pathway, the unfolded protein response (UPR). Importantly, the UPR has the ability to promote cellular survival by increasing ER membranes and folding capacity, but also has the ability to induce cell death if the stress is persistent (5, 8, 10, 37, 45, 51, 104).
During the UPR, the increased demand for chaperone proteins in ER results in the dissociation of the glucose-regulated protein 78 [GRP78 also known as BiP (binding immunoglobin protein)] from luminal domains of three ER proteins, protein kinase RNA-like endoplasmic reticulum kinase (PERK), the inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) (45). When not associated with BiP, PERK and IRE1α undergo multimerization and transautophosphorylation to become active, whereas ATF6 is proteolytically processed to its transcriptionally active form (90). ATF6 promotes protein chaperone and lipid synthesis and enhances N-glycosylation (59, 122). PERK gains control on the cellular translation processes to reduce ER load (38, 41), and eventually facilitates autophagy. If the stress is persistent, PERK and ATF6 induce cell death via accumulation of CCAAT/enhancer binding homologous protein (CHOP) (2, 8, 49, 80). IRE1α uses its endoribonuclease properties to selectively reduce ER mRNA (40, 72) and to produce the active isoform of the X-box binding protein transcription factor (XBP1s) (118). XBP1s increases the ER’s folding capacity and ER membrane biosynthesis, as well as vesicular trafficking (4, 7, 118). XBP1s can also lead to decreased antigen presentation (7). Importantly, IRE1α activates JNK and this leads to an inflammatory response and apoptosis (40, 101).
Indeed, the pathogenic HCoVs infections lead to ER stress and UPR activation (35). The induction of adaptive ER-stress chaperones such as glucose-regulated protein 94 (GRP94) and BiP was observed in cells infected with SARS-CoV (29, 50, 115), along with increased levels of homocysteine-inducible, ER stress-inducible, ubiquitin-like domain member 1 (HERPUD1), a protein involved in the ER degradation pathway (ERAD) (103). Nevertheless, the HCoV effects on the specific UPR branches remain limited and rather unclear, and differ depending on virus and cell culture model used (reviewed in Ref. 35). Notably, however, NF-κB interplay with both IRE1 and PERK pathways has also been proposed to contribute to the cytokine storm that accompanies SARS-CoV (84, 103). The detailed mechanisms underlying this observation, however, remain poorly understood (30, 106).
Taken together, the viral strategies to increase the ER membranes and ER folding capacity and block UPR-associated translational attenuation, inflammatory responses, and apoptosis are critical components for virus production. This strategy could be achieved through modulation of host miRNAs that are involved in the UPR and/or in inflammatory responses. As shown in Table 1, COVID-19-mediated reduction of host miR-34a-3p and miR-495-5p levels could increase XBP1s and BiP expression, respectively, by increasing the ER folding capacity and promoting survival. Through miR-376b-3p, the virus could also potentially modulate the mTOR and autophagy pathways. Furthermore, some of these miRNAs have been proposed to modulate immune responses that include miR-376a-3p, miR-99b-5p, miR-10a-5p, miR-376a-3p, miR-548av-5p, and miR-99b-5p (Table 1). Although these predictions will require further experimental validation, they support the hypothesis that SARS-CoV-2 and other HCoVs modulate host miRNAs to favorably fine-tune the ER of infected cells and protect themselves from the immune system.
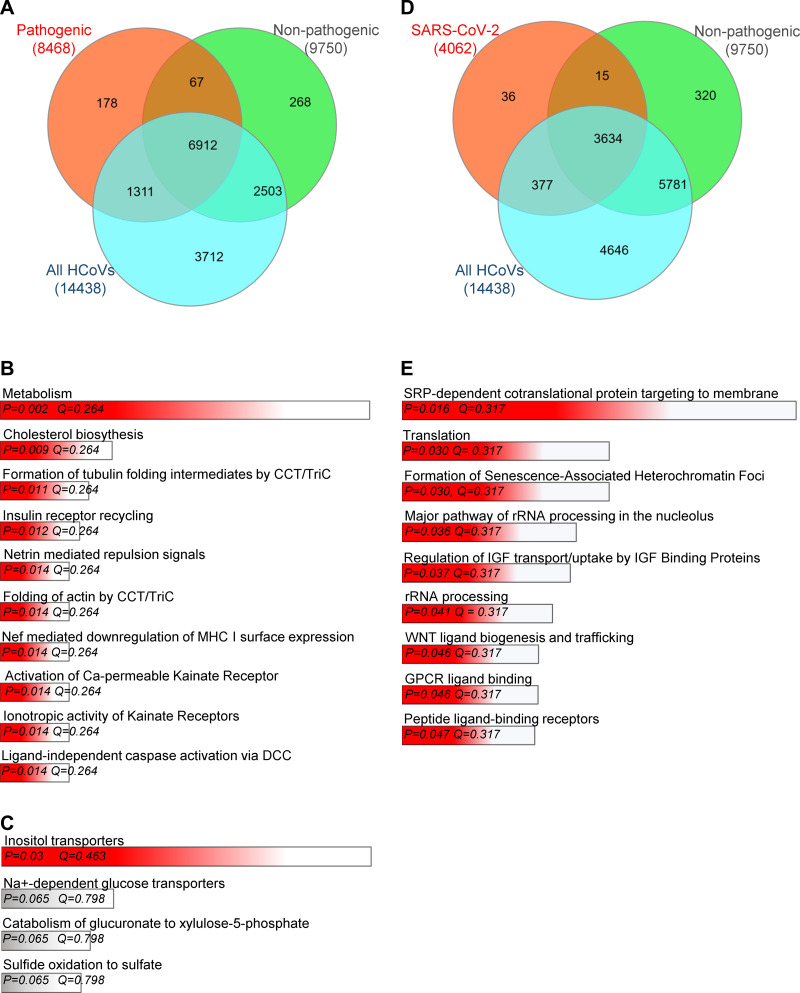
Our initial assessment of virus-controlled miRNA effects was focused on literature reporting miRNA effects; however, miRNAs have multiple mRNA targets and thus a wide range of cellular responsibilities. To address this, using the miRDIP database (99) with only the top 1% of the most probable targets considered, we analyzed the potential targets of miRNA that could be bound to either the pathogenic, the nonpathogenic, or both groups of HCoVs. As shown in Fig. 2A, the host miRNAs potentially controlled by the pathogenic HCoVs may be the key to gaining control over a very limited and specific set of mRNAs targets. The specific details for target predictions are provided in the Supplemental Data Set S2 (see https://doi.org/10.5281/zenodo.3966446). From this analysis, we asked how could the pathogenic HCoVs affect the cellular pathways that would provide an advantage to the virus? To put the predicted mRNA targets into a Gene Ontology (GO) context, we used the Enrichr web server (21, 57). As shown in Fig. 2B, the genes encoded by the mRNAs that were potential targets of the miRNAs specific for the pathogenic HCoVs included those that regulate cellular membrane synthesis, the cytoskeleton, antigen presentation, and apoptosis, as well as insulin receptor recycling. Interestingly, a similar analysis set for the nonpathogenic HCoVs had only one significant assignment and that was in the inositol transporters GO category (Fig. 2C). More importantly, we also performed a more specific analysis to identify mRNA targets that could be specific for SARS-CoV-2-controlled miRNAs. As shown in Fig. 2, D and E, only 36 specific mRNAs were considered, but their GO assignments included SRP-dependent processes, translation and rRNA processing, and cellular senescence processes. This analysis further supports the hypothesis that pathogenic HCoVs, including SARS-CoV-2, use the host miRNAs to adjust cellular processes to facilitate their viral protein production.
Fig. 2.
The impact of the pathogenic human coronaviruses (HCoV)-dependent modulation of hosts miRNA profiles and their potential consequences on cellular mRNA levels. A: Venn diagram (43) represents the potential general distribution of human mRNAs that could be affected by the miRNAs that target the pathogenic (orange), the nonpathogenic (green) HCoVs, and all seven coronaviruses (blue). The hypothesis is that the miRNA sponge effect used by the individual viruses could lead to an increase in these human mRNAs since these are also targets of these miRNAs. B: the Gene Ontology assignment of the cellular functions of mRNAs defines very specific targets for the miRNAs potentially regulated by the pathogenic HCoVs as assigned by the Enrichr web server (21, 57). C: the Gene Ontology assignment of the cellular function of mRNAs defines specific targets for the miRNAs potentially regulated by the harmless HCoVs. D: the Venn diagram represents the general distribution of mRNAs that are targets of miRNAs modulated by severe acute respiratory coronavirus 2 (SARS-CoV-2) (orange) and the nonpathogenic (green) HCoVs, and all 7 HCoVs (blue). E: the Gene Ontology assignment of the cellular functions of mRNAs that are high probability targets for the miRNAs potentially modulated by the SARS-CoV-2, as assigned by the Enrichr web server (21, 57). All of the mRNA targets were predicted with the miRDIP database (99) with only the top 1% of the most probable targets considered. The predicted targets lists are provided in Supplemental Data Set S2 (https://doi.org/10.5281/zenodo.3966446). The red bar color depicts the P value less than 0.05. The longer bars have the lower P values. The false discovery rate is provided as a Q value.
HCoVs Encoded Small Noncoding RNAs Use the Hosts RNAi Pathway to Modulate Cellular Responses
A third possibility is that HCoVs encode in their own pre-miRNA sequences that could mature in human cells by entering the human RISC complex. miRNAs have been shown to be produced by both DNA viruses and positive- and negative-strand RNA viruses (reviewed in Ref. 14). Viral miRNAs have been identified for many human viruses, including influenza (83), EV71 (109), hepatitis A (91), and SARS-CoV (71). Although the abundance of these viral noncoding RNAs was shown to be relatively low in infected tissues (below 0.1%), their role in changing the phenotype of SARS-CoV infected cells was confirmed through the use of specific miRNA inhibitors that reduced inflammation and lung damage in vivo (71). Using the miRNAFold web server (98), we identified 10 pre-miRNA sequences in the SARS-CoV-2 RNA sequence that could potentially enter the human RNAi pathway (Supplemental Data Set S3; see https://doi.org/10.5281/zenodo.3966446). Clearly, however, further experimental research will be required to verify their presence in infected tissues as well as their functional roles.
Concluding Notes and Future Prospects
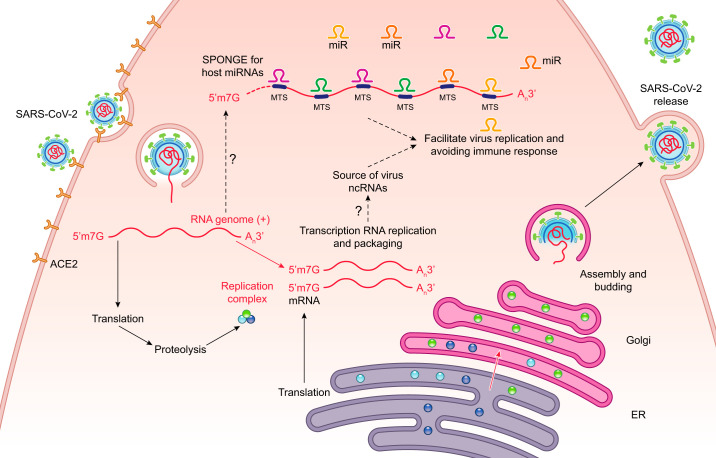
Despite the difficult and hopefully successful fight with the COVID-19 pandemic, there is no guarantee that other novel HCoV-related dangers will not appear in the future, and thus understanding the mechanisms that determine their pathogenicity will remain medically important. This is especially true with the subject of the host-virus interactions during infections. Here, based on an analysis of the current literature and using bioinformatic approaches, we discussed the potential mechanisms by which pathogenic HCoVs including SARS-CoV-2 could modulate host miRNA levels by acting as miRNA sponges to facilitate their replication and to avoid immune responses (Fig. 3). Because our studies focused on human bronchial epithelial cells (16HBE14o- cells and primary HBEs), it is important to note that there may be differences seen in other cell types such as nasal epithelial cells and alveolar type II epithelial cells. Our hypothesis will require validations starting with the assessment of these miRNA levels in infected tissues and ending with restoring the host miRNA balance with miRNA analogs. Furthermore, completely understanding how viruses take advantage of the ER and UPR pathway may also lead to novel therapeutic strategies. Nevertheless, other questions remain including determining how these viruses control the UPR, analyzing the role of the MTS frequencies in relation to the miRNA profile and infection stage, and finally, deciphering the complicated potential cooperation between numerous miRNAs that are affected by the virus. Hopefully, noncoding RNAs of virus origin that are easier to target and test with synthetic analogs may turn out to be effective drug candidates as well. Finally, numerous reports have illustrated the large diversity in terms of responses and clinical outcomes to COVID-19 infections. One possible variable that has not been considered is the individual differences in patient’s miRNA profiles or polymorphisms in either MTSs or miRNA sequences (9, 11, 56, 73). In that regard, a recent study has suggested that COVID-19 virulence in aged patients may be due to a lower abundance of miRNAs and this may be a contributing factor in disease severity (34). This certainly supports the idea that the normal function of miRNAs is to effectively regulate mRNA levels and thus promote cellular homeostasis. Understanding these types of differences in patients is important for developing personalized antiviral therapies.
Fig. 3.
The hypothesis is that severe acute respiratory coronavirus 2 (SARS-CoV-2) regulates cellular responses through depletion of specific host microRNAs (miRNAs). The SARS-CoV-2 uptake and replication cycle is illustrated and our model is that noncoding viral RNAs serve as sponges for the host miRNAs and this accomplishes two goals. First, it disrupts normal cellular homeostasis by upregulating specific host mRNA levels that are normally controlled by host miRNAs. And second, by downregulating certain host miRNAs, the virus enhances its own viral replication cycle and also attenuates immune responses. ER, endoplasmic reticulum; MTS, microRNA target sites.
GRANTS
This work has been supported by National Science Center Sonata Bis and OPUS Programs under contracts 2015/18/E/NZ3/00687 and 2015/17/B/NZ3/01485 (to R.B.), the National Science Foundation, National Science Center Program Opportunities for Promoting Understanding through Synthesis (OPUS) under contract 2014/13/B/NZ3/02393 to (B.J.) and by the NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK072482 and the Research Development Program (ROWE15R0) from the Cystic Fibrosis Foundation (to J.F.C.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.B. prepared figures; R.B. and J.F.C. drafted manuscript; R.B., S.M., K.S.H., M.S., and J.F.C. edited and revised manuscript; R.B., M.D., B.J., S.M., K.S.H., M.S., and J.F.C. approved final version of manuscript.
REFERENCES
- 1.Ahmadi A, Khansarinejad B, Hosseinkhani S, Ghanei M, Mowla SJ. miR-199a-5p and miR-495 target GRP78 within UPR pathway of lung cancer. Gene 620: 15–22, 2017. doi: 10.1016/j.gene.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 2.B’chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 41: 7683–7699, 2013. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbu MG, Condrat CE, Thompson DC, Bugnar OL, Cretoiu D, Toader OD, Suciu N, Voinea SC. MicroRNA involvement in signaling pathways during viral infection. Front Cell Dev Biol 8: 143, 2020. doi: 10.3389/fcell.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartoszewska S, Cabaj A, Dąbrowski M, Collawn JF, Bartoszewski R. miR-34c-5p modulates X-box-binding protein 1 (XBP1) expression during the adaptive phase of the unfolded protein response. FASEB J 33: 11541–11554, 2019. doi: 10.1096/fj.201900600RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoszewska S, Collawn JF. Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia. Cell Mol Biol Lett 25: 18, 2020. doi: 10.1186/s11658-020-00212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartoszewska S, Kochan K, Madanecki P, Piotrowski A, Ochocka R, Collawn JF, Bartoszewski R. Regulation of the unfolded protein response by microRNAs. Cell Mol Biol Lett 18: 555–578, 2013. doi: 10.2478/s11658-013-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartoszewski R, Brewer JW, Rab A, Crossman DK, Bartoszewska S, Kapoor N, Fuller C, Collawn JF, Bebok Z. The unfolded protein response (UPR)-activated transcription factor X-box-binding protein 1 (XBP1) induces microRNA-346 expression that targets the human antigen peptide transporter 1 (TAP1) mRNA and governs immune regulatory genes. J Biol Chem 286: 41862–41870, 2011. doi: 10.1074/jbc.M111.304956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartoszewski R, Gebert M, Janaszak-Jasiecka A, Cabaj A, Króliczewski J, Bartoszewska S, Sobolewska A, Crossman DK, Ochocka R, Kamysz W, Kalinowski L, Dąbrowski M, Collawn JF. Genome-wide mRNA profiling identifies RCAN1 and GADD45A as regulators of the transitional switch from survival to apoptosis during ER stress. FEBS J 287: 2923–2947, 2020. doi: 10.1111/febs.15195. [DOI] [PubMed] [Google Scholar]
- 9.Bartoszewski R, Króliczewski J, Piotrowski A, Jasiecka AJ, Bartoszewska S, Vecchio-Pagan B, Fu L, Sobolewska A, Matalon S, Cutting GR, Rowe SM, Collawn JF. Codon bias and the folding dynamics of the cystic fibrosis transmembrane conductance regulator. Cell Mol Biol Lett 21: 23, 2016. doi: 10.1186/s11658-016-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartoszewski R, Matalon S, Collawn JF. Ion channels of the lung and their role in disease pathogenesis. Am J Physiol Lung Cell Mol Physiol 313: L859–L872, 2017. doi: 10.1152/ajplung.00285.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartoszewski R, Sikorski AF. Editorial focus: entering into the non-coding RNA era. Cell Mol Biol Lett 23: 45, 2018. doi: 10.1186/s11658-018-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartoszewski R, Sikorski AF. Editorial focus: understanding off-target effects as the key to successful RNAi therapy. Cell Mol Biol Lett 24: 69, 2019. doi: 10.1186/s11658-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181: 1036–1045.e9, 2020. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruscella P, Bottini S, Baudesson C, Pawlotsky JM, Feray C, Trabucchi M. Viruses and miRNAs: more friends than foes. Front Microbiol 8: 824, 2017. doi: 10.3389/fmicb.2017.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cazalla D, Steitz JA. Down-regulation of a host microRNA by a viral noncoding RNA. Cold Spring Harb Symp Quant Biol 75: 321–324, 2010. doi: 10.1101/sqb.2010.75.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CP, Siu KL, Chin KT, Yuen KY, Zheng B, Jin DY. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J Virol 80: 9279–9287, 2006. doi: 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9: 221–236, 2020. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan MC, Chan RW, Tsao GS, and Peiris JS. Pathogenesis of SARS coronavirus infection using human lung epithelial cells: an in vitro model. Hong Kong Med J 17 Suppl 6: 31–35, 2011. [PubMed] [Google Scholar]
- 19.Chang CH, Fan TC, Yu JC, Liao GS, Lin YC, Shih AC, Li WH, Yu AL. The prognostic significance of RUNX2 and miR-10a/10b and their inter-relationship in breast cancer. J Transl Med 12: 257, 2014. doi: 10.1186/s12967-014-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chau VQ, Oliveros E, Mahmood K, Singhvi A, Lala A, Moss N, Gidwani U, Mancini DM, Pinney SP, Parikh A. The imperfect cytokine storm: severe COVID-19 with ARDS in patient on durable LVAD support. JACC Case Rep 2: 1315–1320. 2020. doi: 10.1016/j.jaccas.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14: 128, 2013. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Li X, Chen X. Prognostic significance of tissue miR-345 downregulation in non-small cell lung cancer. Int J Clin Exp Med 8: 20971–20976, 2015. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Zhu H, Yin L, Wang T, Wu J, Xu J, Tao H, Liu J, He X. lncRNA-PVT1 facilitates invasion through upregulation of MMP9 in nonsmall cell lung cancer cell. DNA Cell Biol 36: 787–793, 2017. doi: 10.1089/dna.2017.3725. [DOI] [PubMed] [Google Scholar]
- 24.Chu H, Chen X, Wang H, Du Y, Wang Y, Zang W, Li P, Li J, Chang J, Zhao G, Zhang G. MiR-495 regulates proliferation and migration in NSCLC by targeting MTA3. Tumour Biol 35: 3487–3494, 2014. doi: 10.1007/s13277-013-1460-1. [DOI] [PubMed] [Google Scholar]
- 25.Cowled C, Stewart CR, Likic VA, Friedländer MR, Tachedjian M, Jenkins KA, Tizard ML, Cottee P, Marsh GA, Zhou P, Baker ML, Bean AG, Wang LF. Characterisation of novel microRNAs in the Black flying fox (Pteropus alecto) by deep sequencing. BMC Genomics 15: 682, 2014. doi: 10.1186/1471-2164-15-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, Thannickal VJ, Liu G. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol 56: 168–178, 2017. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Silveira MB, Lima KF, da Silva AR, Dos Santos RAS, Moraes KCM. Mir-513a-3p contributes to the controlling of cellular migration processes in the A549 lung tumor cells by modulating integrin β-8 expression. Mol Cell Biochem 444: 43–52, 2018. doi: 10.1007/s11010-017-3229-0. [DOI] [PubMed] [Google Scholar]
- 28.David-Ferreira JF, Manaker RA. An electron microscope study of the development of a mouse hepatitis virus in tissue culture cells. J Cell Biol 24: 57–78, 1965. doi: 10.1083/jcb.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeDiego ML, Nieto-Torres JL, Jiménez-Guardeño JM, Regla-Nava JA, Alvarez E, Oliveros JC, Zhao J, Fett C, Perlman S, Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PLoS Pathog 7: e1002315, 2011. doi: 10.1371/journal.ppat.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dosch SF, Mahajan SD, Collins AR. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res 142: 19–27, 2009. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguière AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348: 1967–1976, 2003. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 32.Dutta RK, Chinnapaiyan S, Unwalla H. Aberrant microRNAomics in pulmonary complications: implications in lung health and diseases. Mol Ther Nucleic Acids 18: 413–431, 2019. doi: 10.1016/j.omtn.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 1282: 1–23, 2015. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulzele S, Sahay B, Yusufu I, Lee TJ, Sharma A, Kolhe R, Isales CM. COVID-19 virulence in aged patients might be impacted by the host cellular microRNAs abundance/profile. Aging Dis 11: 509–522, 2020. doi: 10.14336/AD.2020.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fung TS, Liu DX. Coronavirus infection, ER stress, apoptosis and innate immunity. Front Microbiol 5: 296, 2014. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care 24: 154, 2020. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebert M, Bartoszewska S, Janaszak-Jasiecka A, Moszyńska A, Cabaj A, Króliczewski J, Madanecki P, Ochocka RJ, Crossman DK, Collawn JF, Bartoszewski R. PIWI proteins contribute to apoptosis during the UPR in human airway epithelial cells. Sci Rep 8: 16431, 2018. doi: 10.1038/s41598-018-34861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonen N, Sabath N, Burge CB, Shalgi R. Widespread PERK-dependent repression of ER targets in response to ER stress. Sci Rep 9: 4330, 2019. doi: 10.1038/s41598-019-38705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy D, Sidorov IA, Sola I, Ziebuhr J. Severe acute respiratory syndrome-related coronavirus: The species and its viruses—a statement of the Coronavirus Study Group (Preprint). bioRxiv 2020. doi: 10.1101/2020.02.07.937862. [DOI]
- 40.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1α kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138: 562–575, 2009. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15: 481–490, 2013. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y, Yang Y, Kuang P, Ren S, Rozeboom L, Rivard CJ, Li X, Zhou C, Hirsch FR. Seven-microRNA panel for lung adenocarcinoma early diagnosis in patients presenting with ground-glass nodules. OncoTargets Ther 10: 5915–5926, 2017. doi: 10.2147/OTT.S151432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 16: 169, 2015. doi: 10.1186/s12859-015-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ 17: 193–199, 2010. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 45.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89–102, 2012. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells (Preprint). bioRxiv 2020. doi: 10.1101/2020.01.31.929042. [DOI]
- 47.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang-Verslues WW, Chang PH, Wei PC, Yang CY, Huang CK, Kuo WH, Shew JY, Chang KJ, Lee EYHP, Lee WH. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene 30: 2463–2474, 2011. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 49.Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J 283: 2640–2652, 2016. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 50.Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the α-subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stresses. Mol Cell Biol 23: 5651–5663, 2003. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis 11: 5–13, 2006. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 52.Knoops K, Kikkert M, Worm SH, Zevenhoven-Dobbe JC, van der Meer Y, Koster AJ, Mommaas AM, Snijder EJ. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol 6: e226, 2008. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korkmaz G, le Sage C, Tekirdag KA, Agami R, Gozuacik D. miR-376b controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1. Autophagy 8: 165–176, 2012. doi: 10.4161/auto.8.2.18351. [DOI] [PubMed] [Google Scholar]
- 54.Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res 47: D155–D162, 2019. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42: D68–D73, 2014. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Króliczewski J, Sobolewska A, Lejnowski D, Collawn JF, Bartoszewski R. microRNA single polynucleotide polymorphism influences on microRNA biogenesis and mRNA target specificity. Gene 640: 66–72, 2018. doi: 10.1016/j.gene.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 57.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44: W90–W97, 2016. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lau SKP, Lau CCY, Chan KH, Li CPY, Chen H, Jin DY, Chan JFW, Woo PCY, Yuen KY. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol 94: 2679–2690, 2013. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 59.Li M, Baumeister P, Roy B, Phan T, Foti D, Luo S, Lee AS. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol Cell Biol 20: 5096–5106, 2000. doi: 10.1128/MCB.20.14.5096-5106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Q, Liu T, Yang S, Zhang Z. Upregulation of miR-34a by Inhibition of IRE1α has protective effect against Aβ-induced injury in SH-SY5Y cells by targeting caspase-2. Oxid Med Cell Longev 2019: 2140427, 2019. doi: 10.1155/2019/2140427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Shi Y, Yin Z, Xue X, Zhou B. An eight-miRNA signature as a potential biomarker for predicting survival in lung adenocarcinoma. J Transl Med 12: 159, 2014. doi: 10.1186/1479-5876-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B, Peng XC, Zheng XL, Wang J, Qin YW. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer 66: 169–175, 2009. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Liu J, Liu S, Deng X, Rao J, Huang K, Xu G, Wang X. MicroRNA-582-5p suppresses non-small cell lung cancer cells growth and invasion via downregulating NOTCH1. PLoS One 14: e0217652, 2019. doi: 10.1371/journal.pone.0217652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luna JM, Scheel TK, Danino T, Shaw KS, Mele A, Fak JJ, Nishiuchi E, Takacs CN, Catanese MT, de Jong YP, Jacobson IM, Rice CM, Darnell RB. Hepatitis C virus RNA functionally sequesters miR-122. Cell 160: 1099–1110, 2015. doi: 10.1016/j.cell.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mairinger FD, Ting S, Werner R, Walter RF, Hager T, Vollbrecht C, Christoph D, Worm K, Mairinger T, Sheu-Grabellus SY, Theegarten D, Schmid KW, Wohlschlaeger J. Different micro-RNA expression profiles distinguish subtypes of neuroendocrine tumors of the lung: results of a profiling study. Modern Pathol 27: 1632–1640, 2014. doi: 10.1038/modpathol.2014.74. [DOI] [PubMed] [Google Scholar]
- 66.Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, Giovannini-Chami L, Nawrocki-Raby B, Birembaut P, Waldmann R, Kodjabachian L, Barbry P. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Cell Biol 13: 1280, 2011. doi: 10.1038/ncb2358. [DOI] [PubMed] [Google Scholar]
- 67.Menachery VD, Eisfeld AJ, Schäfer A, Josset L, Sims AC, Proll S, Fan S, Li C, Neumann G, Tilton SC, Chang J, Gralinski LE, Long C, Green R, Williams CM, Weiss J, Matzke MM, Webb-Robertson BJ, Schepmoes AA, Shukla AK, Metz TO, Smith RD, Waters KM, Katze MG, Kawaoka Y, Baric RS. Pathogenic influenza viruses and coronaviruses utilize similar and contrasting approaches to control interferon-stimulated gene responses. MBio 5: e01174-314, 2014. doi: 10.1128/mBio.01174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miao LJ, Huang SF, Sun ZT, Gao ZY, Zhang RX, Liu Y, Wang J. MiR-449c targets c-Myc and inhibits NSCLC cell progression. FEBS Lett 587: 1359–1365, 2013. doi: 10.1016/j.febslet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, Yu G, Vuga LJ, Weksler B, Benos PV, Gibson KF, McMillan M, Kahn M, Kaminski N. Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol 47: 879–887, 2012. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell 126: 1203–1217, 2006. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 71.Morales L, Oliveros JC, Fernandez-Delgado R, tenOever BR, Enjuanes L, Sola I. SARS-CoV-encoded small RNAs contribute to infection-associated lung pathology. Cell Host Microbe 21: 344–355, 2017. doi: 10.1016/j.chom.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moszyńska A, Collawn JF, Bartoszewski R. IRE1 endoribonuclease activity modulates hypoxic HIF-1α signaling in human endothelial cells. Biomolecules 10: 895, 2020. doi: 10.3390/biom10060895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moszyńska A, Gebert M, Collawn JF, Bartoszewski R. SNPs in microRNA target sites and their potential role in human disease. Open Biol 7: 170019, 2017. doi: 10.1098/rsob.170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mueller AC, Sun D, Dutta A. The miR-99 family regulates the DNA damage response through its target SNF2H. Oncogene 32: 1164–1172, 2013. doi: 10.1038/onc.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nachmani D, Zimmermann A, Oiknine Djian E, Weisblum Y, Livneh Y, Khanh Le VT, Galun E, Horejsi V, Isakov O, Shomron N, Wolf DG, Hengel H, Mandelboim O. MicroRNA editing facilitates immune elimination of HCMV infected cells. PLoS Pathog 10: e1003963, 2014. doi: 10.1371/journal.ppat.1003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ñamendys-Silva SA. ECMO for ARDS due to COVID-19. Heart Lung 49: 348–349, 2020. doi: 10.1016/j.hrtlng.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oglesby IK, Bray IM, Chotirmall SH, Stallings RL, O’Neill SJ, McElvaney NG, Greene CM. miR-126 is downregulated in cystic fibrosis airway epithelial cells and regulates TOM1 expression. J Immunol 184: 1702–1709, 2010. doi: 10.4049/jimmunol.0902669. [DOI] [PubMed] [Google Scholar]
- 79.Ong J, Faiz A, Timens W, van den Berge M, Terpstra MM, Kok K, van den Berg A, Kluiver J, Brandsma CA. Marked TGF-β-regulated miRNA expression changes in both COPD and control lung fibroblasts. Sci Rep 9: 18214, 2019. doi: 10.1038/s41598-019-54728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 81.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA 14: 844–852, 2008. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng S, Wang J, Wei S, Li C, Zhou K, Hu J, Ye X, Yan J, Liu W, Gao GF, Fang M, Meng S. Endogenous cellular microRNAs mediate antiviral defense against influenza A virus. Mol Ther Nucleic Acids 10: 361–375, 2018. doi: 10.1016/j.omtn.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perez JT, Varble A, Sachidanandam R, Zlatev I, Manoharan M, García-Sastre A, tenOever BR. Influenza A virus-generated small RNAs regulate the switch from transcription to replication. Proc Natl Acad Sci USA 107: 11525–11530, 2010. doi: 10.1073/pnas.1001984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 5: 917–927, 2005. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qiao Y, Zhao X, Liu J, Yang W. Epstein-Barr virus circRNAome as host miRNA sponge regulates virus infection, cell cycle, and oncogenesis. Bioengineered 10: 593–603, 2019. doi: 10.1080/21655979.2019.1679698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reggiori F, Monastyrska I, Verheije MH, Calì T, Ulasli M, Bianchi S, Bernasconi R, de Haan CAM, Molinari M. Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe 7: 500–508, 2010. doi: 10.1016/j.chom.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren K, Li Y, Lu H, Li Z, Han X. miR-3940-5p functions as a tumor suppressor in non-small cell lung cancer cells by targeting cyclin D1 and ubiquitin-specific peptidase-28. Transl Oncol 10: 80–89, 2017. doi: 10.1016/j.tranon.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ren LL, Yan TT, Shen CQ, Tang JY, Kong X, Wang YC, Chen J, Liu Q, He J, Zhong M, Chen HY, Hong J, Fang JY. The distinct role of strand-specific miR-514b-3p and miR-514b-5p in colorectal cancer metastasis. Cell Death Dis 9: 687, 2018. doi: 10.1038/s41419-018-0732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saçar MD, Bağcı C, Allmer J. Computational prediction of microRNAs from Toxoplasma gondii potentially regulating the hosts’ gene expression. Genomics Proteomics Bioinformatics 12: 228–238, 2014. doi: 10.1016/j.gpb.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789, 2005. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 91.Shi J, Sun J, Wang B, Wu M, Zhang J, Duan Z, Wang H, Hu N, Hu Y. Novel microRNA-like viral small regulatory RNAs arising during human hepatitis A virus infection. FASEB J 28: 4381–4393, 2014. doi: 10.1096/fj.14-253534. [DOI] [PubMed] [Google Scholar]
- 92.Singh Y, Kaul V, Mehra A, Chatterjee S, Tousif S, Dwivedi VP, Suar M, Van Kaer L, Bishai WR, Das G. Mycobacterium tuberculosis controls microRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J Biol Chem 288: 5056–5061, 2013. doi: 10.1074/jbc.C112.439778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song R, Walentek P, Sponer N, Klimke A, Lee JS, Dixon G, Harland R, Wan Y, Lishko P, Lize M, Kessel M, He L. miR-34/449 miRNAs are required for motile ciliogenesis by repressing cp110. Nature 510: 115–120, 2014. doi: 10.1038/nature13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun D, Lee YS, Malhotra A, Kim HK, Matecic M, Evans C, Jensen RV, Moskaluk CA, Dutta A. miR-99 family of microRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res 71: 1313–1324, 2011. doi: 10.1158/0008-5472.CAN-10-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciumè G, Muljo SA, Kuchen S, Casellas R, Wei L, Kanno Y, O’Shea JJ. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol 13: 587–595, 2012. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64: 3753–3756, 2004. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 97.Tang JT, Wang JL, Du W, Hong J, Zhao SL, Wang YC, Xiong H, Chen HM, Fang JY. MicroRNA 345, a methylation-sensitive microRNA is involved in cell proliferation and invasion in human colorectal cancer. Carcinogenesis 32: 1207–1215, 2011. doi: 10.1093/carcin/bgr114. [DOI] [PubMed] [Google Scholar]
- 98.Tav C, Tempel S, Poligny L, Tahi F. miRNAFold: a web server for fast miRNA precursor prediction in genomes. Nucleic Acids Res 44: W181–W184, 2016. doi: 10.1093/nar/gkw459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tokar T, Pastrello C, Rossos AEM, Abovsky M, Hauschild AC, Tsay M, Lu R, Jurisica I. mirDIP 4.1-integrative database of human microRNA target predictions. Nucleic Acids Res 46: D360–D370, 2018. doi: 10.1093/nar/gkx1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsitsiou E, Lindsay MA. microRNAs and the immune response. Curr Opin Pharmacol 9: 514–520, 2009. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 102.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT, Fu H, Dighe A, Griffin JT, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon D, Nedjati-Gilani G, Riley S, van Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly CA, Ghani AC, Ferguson NM. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 20: 669–677, 2020. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Versteeg GA, van de Nes PS, Bredenbeek PJ, Spaan WJM. The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. J Virol 81: 10981–10990, 2007. doi: 10.1128/JVI.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086, 2011. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 105.Wang J, Sheng Z, Cai Y. Effects of microRNA-513b on cell proliferation, apoptosis, invasion, and migration by targeting HMGB3 through regulation of mTOR signaling pathway in non-small-cell lung cancer. J Cell Physiol 234: 10934–10941, 2019. doi: 10.1002/jcp.27921. [DOI] [PubMed] [Google Scholar]
- 106.Wang W, Ye L, Ye L, Li B, Gao B, Zeng Y, Kong L, Fang X, Zheng H, Wu Z, She Y. Up-regulation of IL-6 and TNF-α induced by SARS-coronavirus spike protein in murine macrophages via NF-κB pathway. Virus Res 128: 1–8, 2007. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Cong W, Wu G, Ju X, Li Z, Duan X, Wang X, Gao H. MiR-376a suppresses the proliferation and invasion of non-small-cell lung cancer by targeting c-Myc. Cell Biol Int 42: 25–33, 2018. doi: 10.1002/cbin.10828. [DOI] [PubMed] [Google Scholar]
- 108.Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res 81: 85–164, 2011. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weng KF, Hung CT, Hsieh PT, Li ML, Chen GW, Kung YA, Huang PN, Kuo RL, Chen LL, Lin JY, Wang RYL, Chen SJ, Tang P, Horng JT, Huang HI, Wang JR, Ojcius DM, Brewer G, Shih SR. A cytoplasmic RNA virus generates functional viral small RNAs and regulates viral IRES activity in mammalian cells. Nucleic Acids Res 42: 12789–12805, 2014. doi: 10.1093/nar/gku952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost 18: 1548–1555, 2020. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465–469, 2020. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 112.Xiao J, Lin H, Luo X, Luo X, Wang Z. miR-605 joins p53 network to form a p53:miR-605:Mdm2 positive feedback loop in response to stress. EMBO J 30: 5021, 2011. doi: 10.1038/emboj.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xing TJ, Xu HT, Yu WQ, Wang B, Zhang J. MiRNA-548ah, a potential molecule associated with transition from immune tolerance to immune activation of chronic hepatitis B. Int J Mol Sci 15: 14411–14426, 2014. doi: 10.3390/ijms150814411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Z, Yin H, Shi L, Qian X. A novel microRNA signature for pathological grading in lung adenocarcinoma based on TCGA and GEO data. Int J Mol Med 45: 1397–1408, 2020. doi: 10.3892/ijmm.2020.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yeung YS, Yip CW, Hon CC, Chow KYC, Ma ICM, Zeng F, Leung FCC. Transcriptional profiling of Vero E6 cells over-expressing SARS-CoV S2 subunit: insights on viral regulation of apoptosis and proliferation. Virology 371: 32–43, 2008. doi: 10.1016/j.virol.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yi Z, Fu Y, Ji R, Li R, Guan Z. Altered microRNA signatures in sputum of patients with active pulmonary tuberculosis. PLoS One 7: e43184, 2012. doi: 10.1371/journal.pone.0043184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 23: 130–137, 2018. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891, 2001. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 119.Yu W, Liang X, Li X, Zhang Y, Sun Z, Liu Y, Wang J. MicroRNA-195: a review of its role in cancers. OncoTargets Ther 11: 7109–7123, 2018. doi: 10.2147/OTT.S183600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367: 1814–1820, 2012. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 121.Zehavi L, Avraham R, Barzilai A, Bar-Ilan D, Navon R, Sidi Y, Avni D, Leibowitz-Amit R. Silencing of a large microRNA cluster on human chromosome 14q32 in melanoma: biological effects of mir-376a and mir-376c on insulin growth factor 1 receptor. Mol Cancer 11: 44, 2012. doi: 10.1186/1476-4598-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 279: 25935–25938, 2004. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 123.Zhang MW, Jin MJ, Yu YX, Zhang SC, Liu B, Jiang X, Pan YF, Li QI, Ma SY, Chen K. Associations of lifestyle-related factors, hsa-miR-149 and hsa-miR-605 gene polymorphisms with gastrointestinal cancer risk. Mol Carcinog 51, Suppl 1: E21–E31, 2012. doi: 10.1002/mc.20863. [DOI] [PubMed] [Google Scholar]
- 124.Zhang MW, Yu YX, Jin MJ, Pan YF, Jiang X, Li QL, Ma XY, Zhang SC, Chen K. [Association of miR-605 and miR-149 genetic polymorphisms with related risk factors of lung cancer susceptibility]. Zhejiang Da Xue Xue Bao Yi Xue Ban 40: 265–271, 2011. [DOI] [PubMed] [Google Scholar]
- 125.Zhang MY, Lin J, Kui YC. MicroRNA-345 suppresses cell invasion and migration in non-small cell lung cancer by directly targeting YAP1. Eur Rev Med Pharmacol Sci 23: 2436–2443, 2019. doi: 10.26355/eurrev_201903_17390. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Q, Su J, Wang Z, Qi H, Ge Z, Li Z, Chen WD, Wang YD. MicroRNA-149* suppresses hepatic inflammatory response through antagonizing STAT3 signaling pathway. Oncotarget 8: 65397–65406, 2017. doi: 10.18632/oncotarget.18541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z, Liu R, Tang A, Li X, Liu F, Shen S. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget 7: 45370–45384, 2016. doi: 10.18632/oncotarget.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhong S, Chen C, Liu N, Yang L, Hu Z, Duan P, Shuai D, Zhang Q, Wang Y. Overexpression of hsa-miR-664a-3p is associated with cigarette smoke-induced chronic obstructive pulmonary disease via targeting FHL1. Int J Chron Obstruct Pulmon Dis 14: 2319–2329, 2019. doi: 10.2147/COPD.S224763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou R, Zhou X, Yin Z, Guo J, Hu T, Jiang S, Liu L, Dong X, Zhang S, Wu G. Tumor invasion and metastasis regulated by microRNA-184 and microRNA-574-5p in small-cell lung cancer. Oncotarget 6: 44609–44622, 2015. doi: 10.18632/oncotarget.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]