Abstract
Exercise is often used as a strategy for weight loss maintenance. In preclinical models, we have shown that exercise may be beneficial because it counters the biological drive to regain weight. However, our studies have demonstrated sex differences in the response to exercise in this context. In the present study, we sought to better understand why females and males exhibit different compensatory food eating behaviors in response to regular exercise. Using a forced treadmill exercise paradigm, we measured weight gain, energy expenditure, food intake in real time, and the anorectic effects of leptin. The 4-wk exercise training resulted in reduced weight gain in males and sustained weight gain in females. In male rats, exercise decreased intake, whereas it increased food intake in females. Our results suggest that the anorectic effects of leptin were not responsible for these sex differences in appetite in response to exercise. If these results translate to the human condition, they may reveal important information for the use and application of regular exercise programs.
Keywords: body weight, exercise, food intake, sex differences
INTRODUCTION
Obesity continues to be a major health concern in the United States, costing more than $342.2 billion annually in medical costs (3). Prediction models anticipate nearly half of US adults will have obesity by 2030 (60). Aside from the increased financial burden, one of the greatest issues facing society and individuals with obesity is the unsustainability of long-term weight loss (18, 36), and the majority of those that lose weight regain the lost weight within a year (62). Preclinical data from our laboratory indicate the presence of profound physiological adaptations in response to weight loss that elevate appetite, suppress energy expenditure, and promote weight regain (35, 48, 51). This mismatch between appetite and energy requirements likely involves both peripheral and central systems (27).
The benefits of exercise on general health are well known (21), but it may be particularly beneficial as a strategy for facilitating weight loss maintenance (52). Observational clinical studies have identified regular exercise as a key component of successful weight loss management (18). In preclinical research, we (18, 26, 35) and others (33) have reported that chronic exercise counters the biological drive to regain lost weight in male rats that are allowed to refeed ad libitum. This occurs, in part, through affecting food intake (18, 26, 35), which results in decreased weight regain over time compared with sedentary controls.
This effect of exercise is not exclusive to the weight-reduced state. In preclinical models, others (7, 37, 38) have found that exercising rats defend a lower body weight even without prior weight loss. The mechanisms driving the attenuation in food intake in either the weight-reduced or nonweight-reduced state have not been elucidated. However, research findings indicate a potential role for insulin (5, 24), leptin (33, 42, 47), motivational aspects of palatable diet (32), or immunoregulation (5) in mediating the effects of exercise on energy balance. Given the pleotropic effects of exercise, the relationship between exercise and energy balance is complex and considerable individual variability arises in the response to exercise interventions (31). We (18) and others (40, 41, 46) have demonstrated that sex differences may be one factor that imparts variability in response to exercise. The objective of the present study was to elucidate the mechanisms underlying the differential response to exercise in male and female rats without necessarily producing weight loss.
To accomplish this, we performed a short-term exercise study in male and female rats and examined the impact of training on appetite, energy expenditure, and several nodes of the homeostatic system involved in energy balance regulation. Our original hypothesis was that regular exercise would enhance the anorectic effects of leptin in males but not females. When changes in responsivity to leptin did not appear to mediate the sex-specific differences, we investigated aspects of meal patterns, response to highly palatable foods, and hypothalamic inflammation as alternative explanations. Furthermore, because the treadmill exercise was forced (which may be stressful for rodents), we also investigated aspects of stress and anxiety-like behaviors in mediating the feeding response to exercise.
Our observations indicate that the underlying mechanisms affecting the feeding response to exercise are likely to be transient in nature and uniquely different for males and females. The culmination of these differential effects is that male rats exhibit an attenuation of food intake and the rate of weight gain, whereas female rats compensate for the exercise bout and consume more resulting in a sustained rate of weight gain. Our results may have implications for how we develop and implement exercise regimens.
METHODS
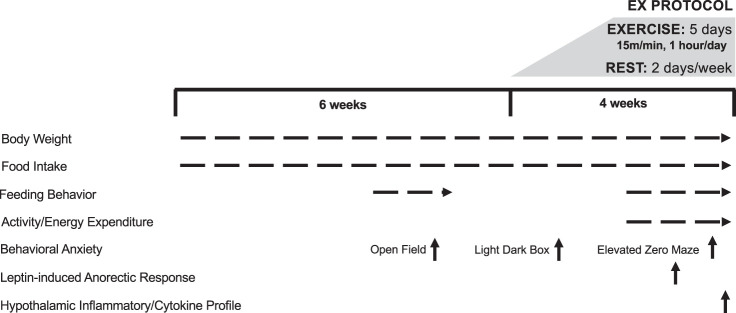
An outline of the study design and timing of individual experiments can be found in Fig. 1. All procedures were approved by the Institutional Animal Care and Use Committee at University of Colorado, Anschutz Medical Campus.
Fig. 1.
Study design and timeline. Ex, exercise.
Animals.
We purchased 8-wk-old male and female Wistar rats from Charles River Laboratories (Wilmington, MA). Rats were individually housed in metabolic caging on a 14:10 light-dark cycle (Lights off at 4 PM; on at 2 AM). Rats were placed on either a high- or low-fat diet (HFD 46% kcal fat, D12344; LFD 11.5% kcal fat, D11724; Research Diets, New Brunswick, NJ) with free access to water. Diet was available ad libitum throughout the entirety of the study with the exception of the leptin responsiveness experiment (see Leptin responsiveness). After 6 wk on the respective diets, rats were split into sedentary or exercise groups. In an effort to control for animal handling and the novel environment, sedentary rats were placed in clean polycarbonate cages for a duration equivalent to the exercise bout. The exercise regimen consisted of 4 wk of forced treadmill exercise, similar to what we have previously used (23, 26, 35, 49, 51). Over the first 2 wk, the speed and duration of exercise were gradually increased to 15 m/min, 1 h/day, 5 day/wk, with the bout occurring in the last 2 h of the light cycle. Exercising rats were rested (REST) 2 consecutive days per week. To objectively monitor compliance to the exercise regimen, rats were scored on a scale of 1–10 each time they were exercised. A score of 10 indicated the rat ran at the front of the treadmill for the entire exercise bout and did not require motivation during the hour. A score of 1 indicated the rat refused to run the majority of the time despite strong encouragement. We utilize a variety of techniques to encourage exercise compliance, including tapping on the plexiglass, placing the rat back on the treadmill, placing ping pong balls on the electrical grid, providing water at the front of the treadmill, and very sparingly administering electric shock. At the end of study, rats were euthanized immediately after a final exercise bout for the expressed purpose of studying the molecular changes that reflect the combination of both acute and training adaptations. Exercise start times were staggered to ensure no more than 3 min passed between when the animals were removed from the treadmill and when they were euthanized (see Tissue collection). Groups were: male/female, LFD/HFD, and sedentary (SED)/exercised (EX). The total animals per group were n = 16 (except LFD females, which had only n = 8 sedentary and n = 8 exercised); totals for individual experiments are noted below.
Body composition.
Lean and fat mass were measured using quantitative magnetic resonance (EchoMRI, Houston, TX) before the start of exercise and again at the end of the study (35).
Estrus cycle monitoring.
The female estrus cycle was monitored by daily vaginal lavages as described in Bouchet et al. (4). The estrus cycle affects food intake and energy expenditure (22), so monitoring the cycle allowed us to stage the female rats during the behavioral and leptin responsiveness testing. Testing took place daily between 7 AM and 9 AM daily (middle of the light cycle). Male rats were handled daily in a similar manner to mimic and control for the handling the females experienced for estrus cycle monitoring.
Metabolic monitoring.
Total energy expenditure (TEE), resting energy expenditure (REE), and spontaneous physical activity (SPA) were measured in a metabolic monitoring system (Oxymax CLAMS-8M; Columbus Instruments, Columbus, OH). Individual metabolic cages included an animal activity meter (Opto-Max, Columbus Instruments, Columbus, OH) to allow for the calculation of total, ambulatory, and nonambulatory activity by monitoring beam breaks within a one-dimensional series of infrared beams. Metabolic monitoring was performed in exercising rats after the full exercise protocol was achieved, for 6–10 days. Metabolic rate (MR) was calculated from gas exchange measurements acquired every 18 min using the Weir (61) equation: MR = 3.941 × V̇o2 + 1.106 × V̇co2 – 2.17 × N, where N is urinary nitrogen. MR was averaged and extrapolated over 24 h to estimate TEE. REE was determined by taking the average of the three lowest EE measurements, which generally occurred during the light cycle. Energy intake was measured daily while the rat was in the metabolic monitoring system. Energy balance was calculated as the difference between energy intake and TEE (n = 8 rats per group).
Exercise energy expenditure.
Energy expended during the exercise bout was assessed in an indirect calorimetry treadmill chamber (Opto-Max, Columbus Instruments, Columbus, OH) in a subset of rats (both males and females) after the full exercise protocol was achieved (26, 35). Gas exchange data were collected every 30 s for the first 30 min of the exercise bout or until a steady state was achieved. Measurements were then extrapolated to estimate the energetic cost of the remainder of the bout, and the total energy expended during the exercise bout was added to the calculations of TEE (n = 4 rats per group).
Meal pattern monitoring.
Food intakes were monitored using 16 feeding modules consisting of a food hopper, a load cell, and associated hardware/software (BioDAQ: Research Diets). Each of the 16 feeding units was interfaced to a serial bus controller, which in turn reported data to a microcomputer. A proprietary software program (Research Diets) was used to analyze the meal pattern data. In this system, a meal was initiated upon the load cell sensing movement of the food hopper. A meal was deemed to have terminated when there were no additional movements of the food hopper for 240 s. The minimum meal amount was set at 0.1 g. The load cells were calibrated daily. The accuracy of the BioDAQ continuous feeding method was verified by weighing the hopper (including food pellets) at various time points. Food spillage was collected on paper positioned on metal pans beneath each hanging cage. Spillage was collected daily and was minimal over 24 h (typically less than 0.14 ± 0.04 g) (n = 8 rats per group).
Highly palatable food test.
Before data collection, rats were trained in the two-bottle choice for 3 consecutive days. Rats were provided access to 5 mL of the isocaloric solutions of sucrose (25% sucrose solution) and intralipid (10% intralipid solution) in their home cage for 1 h. Data collection began on the fourth day in which both solutions were provided in excess for 1 h at the start of the dark cycle for 7 consecutive days [4 days with exercise (exercise was performed just before the start of the test), 3 days without exercise]. Bottles were weighed before and at the end of the hour to determine the amount of liquid consumed. Bottle placement was alternated each day to avoid the development of a side preference. During this time frame, rats also had ad libitum access to diet and water. We measured 24-h diet intake daily during the two-choice bottle test. This experiment was performed in HFD-fed rats only (n = 8 per group).
Behavioral testing.
All behavioral testing was carried out under the guidance of the Animal Behavior Core and took place during the morning hours of 7 AM–11 AM (middle of the light cycle). Females were tested during proestrus. See Fig. 1 for the timing of each experiment. Briefly, rats were tested at three timepoints: first, before the start of exercise; second, during the initial introduction to the exercise protocol; and finally, after the full exercise protocol was achieved. The last exercise bout occurred between 16 and 19 h before the tests (n = 8 rats per group for every test).
Open field.
The open-field test was conducted before the start of exercise. The testing apparatus was an 80 × 80 cm arena with 40-cm high walls. Rats were tested under bright lighting conditions (900–1,000 lux) for one 20-min session. All sessions were videotaped for later analysis. Time spent in the center was assessed using video-tracking digitizing system (EthoVision XT, Noldus, The Netherlands).
Light/dark box.
The light/dark box test was run during the initial introduction to exercise. The light/dark box consisted a rectangle (75 × 35 × 45 cm) divided into two compartments. A fully enclosed dark box (26.6 × 35 cm) and light box (49.3 × 35 cm) were connected by a small (8 × 8 cm) opening. The light compartment was illuminated with bright light (900–1,000 lux). To start the experiment, rats were placed in the dark box compartment. The 1-h session was videotaped and scored for time spent in the light compartment, latency to enter the light compartment, and the total number of entries into the light compartment.
Elevated zero maze.
The elevated zero maze was implemented once the full exercise protocol was achieved. The maze was a circular runway (100 cm in diameter and 10 cm wide) elevated 60 cm from the ground. The runway was divided into four 90-degree quadrants. Two opposite quadrants had high walls (40 cm high), whereas the other two quadrants had no walls. The rat was introduced to the runway with its head facing the entrance of one of the “closed” quadrants. Each 20-min session was videotaped and scored for time spent in the open quadrants, latency to enter the open quadrant, and the number of times crossing into the open quadrants.
Leptin responsiveness.
To determine the anorexic response to a peripheral leptin injection, rats were fasted for 6 h. One hour before the dark cycle, rats were randomized to receive a leptin (2.5 mg/kg; cat. no. 400-21, Peprotech, Rocky Hill, NJ) or equal volume saline intraperitoneal injection. Exercisers then exercised (15 m/min for 1 h) and were returned to their home cage. Nonexercising rats were placed in a clean polycarbonate cage for the duration of the exercise bout. All rats were given access to food at the start of the dark cycle. Food intake and body weight were measured at 4, 16, and 24 h. After a minimum 3-day washout period, rats received the second injection (leptin or saline). Female rats were tested during proestrus. Food intake from both injections (leptin and saline) was calculated as a percentage of average energy intake overall (males) or on equivalent estrous cycle days (females) to control for differences in the estrous cycle. The 24-h intake following the leptin injection was normalized to 24-h intake after the saline injection (n = 8 rats per group).
Serum hormone and substrates.
Trunk blood was collected at the time of euthanasia (immediately following an exercise bout for the EX groups), and plasma was stored at −80°C until analysis. All analyses were performed in duplicate. Plasma insulin and leptin were measured by ELISA (80‐INSRT‐E01 and 22‐LEPMS‐E01, respectively, ALPCO, Salem, NH). Limits of detection were 0.124 ng/mL for insulin and 10 pg/mL for leptin. Colorimetric assays were used to measure plasma nonesterified fatty acids (NEFA; Wako Chemicals USA, Richmond, VA), glucose, triglycerides (TG), and total cholesterol [cat. nos. TR15421, TR22421, and TR13421, respectively, Thermo Fisher Scientific, Waltham, MA; n = 16 rats per group, except LFD females (sedentary and exercised, n = 8 rats)]. Plasma interleukin-6 (IL-6) was measured by ELISA in HFD rats only (n = 8 per group) [cat. no. R6000B, R&D Systems, Minneapolis, MN; n = 16 per group except LFD females (sedentary and exercised, n = 8 per group)].
Tissue collection.
At time of euthanization, rats received an injection of Fatal Plus (0.7 mL) (Vortech Pharmaceuticals, Dearborn, MI) and were quickly decapitated (see Animals section for exercise timing at euthanization). Trunk blood, brain, and adipose tissue were subsequently collected. Specifically, subcutaneous, retroperitoneal, and mesenteric fat depots were excised and weighed. Brains were removed and placed in a prechilled brain matrix on ice. Two razor cuts were made, one at the caudal end of the hypothalamus at approximately −4.8 mm caudal from Bregma (39) and the other at the caudal end of the ventral tegmental area (VTA) at approximately −6 mm caudal from Bregma (39). The brain section containing the VTA was placed on a glass plate in ice-cold saline with the caudal side facing up, and ~1 mm of tissue was dissected from each side of midline (medial lemniscus served as the lateral border) with a 1-mm brain punch. The hypothalamus was removed from the rostral portion of the brain at a depth of ~6 mm (from 1.3 mm to −4.8 mm from Bregma) on a prechilled glass plate using the optic track as a reference point. Approximately 2 mm of tissue were dissected on each side of midline with the top of the 3rd ventricle serving as the dorsal border. All brain dissections were completed by the same experimenter, and care was taken to ensure the same anatomical boundaries were used for each rat. Tissues were frozen in liquid nitrogen and stored at −80°C until analyses [n = 16 rats per group except LFD females (sedentary and exercised, n = 8 rats per group)].
Hypothalamic and VTA measurements.
The hypothalamus and VTA tissues were each homogenized on ice in 1.5 mL sonication buffer (Tris buffer with 10% 10× Enzyme Cocktail and 1% 20 mM PMSF). Homogenates were centrifuged at 14,000 rpm at 4°C for 10 min. The supernatant was removed and stored at −20°C until ELISA or WES analysis was performed. VTA IL-6 was measured by ELISA in HFD rats only (n = 8 per group) (cat. no. R6000B; R&D Systems, Minneapolis, MN). In the hypothalamus, total protein concentrations of homogenates were determined by bicinchoninic acid analysis (BCA) (Pierce BCA Protein Assay; Thermo Fisher Scientific, Rockford, IL). IL-6, interleukin-10 (IL-10), interleukin-1β (IL-1β), tumor necrosis factor-α (TNFα), glial fibrillary acidic protein (GFAP), and ionized calcium-binding adaptor molecule 1 (IBA1) protein concentrations were measured in the hypothalamus using the WES capillary electrophoresis system (ProteinSimple, San Jose, CA) according to the manufacturer’s instructions (8, 43). Data were analyzed with Compass software (ProteinSimple). Primary antibodies used were: IL-6 (1:10; cat. no. 500-P73, Peprotech, Rocky Hill, NJ), IL-10 (1:10; cat. no. 500-P139, Peprotech, Rocky Hill, NJ), IL-1B (1:10; cat. no. 500-P80, Peprotech, Rocky Hill, NJ), TNFa (1:10; cat. no. 500-P72, Peprotech), GFAP (1:200; NB300-141, Novus, Centenial, CO), and IBA1 (1:50; ab15690, Abcam, Cambridge, UK). All proteins were normalized to the total loaded protein (ProteinSimple, San Jose, CA) as measured according to the manufacturer’s instructions [n = 16 per group except LFD females (sedentary and exercised, n = 8 per group)].
Statistical analysis.
Data were analyzed using SPSS version 25. Three-factor (sex, activity, diet) ANOVAs were used to examine the effects of sex, diet, and activity status for most end points. In the case of a significant two-factor interaction, significant simple effects of the effect of activity or diet within sex were confirmed using the general linear model syntax option with SPSS. Significant main effects were considered in the absence of significant two-factor interaction. A three-factor repeated measure ANOVA was used to assess weight gain over time. When a significant interaction involving time was found, a least-significant difference post hoc within activity/diet group was performed to confirm simple effects. In some analyses, measures of the exercise group were performed on the days they were rested, and this is specified as the REST group. Pearson correlation analyses were used to examine relationships among outcome variables. Data are presented as means ± SE. The threshold for a statistically significant difference was set at P < 0.05. For ease of presentation, in the broad absence of significant three-way interaction and activity × diet interaction effects, most of the results are presented with a focus on the main effect of sex (SEX) and activity status (ACT) (diet is collapsed); however, some specific analyses were presented to show the main effect of diet (DIET) (activity status is collapsed).
RESULTS
Exercise reduced body weight gain in male rats but did not affect body weight gain in female rats.
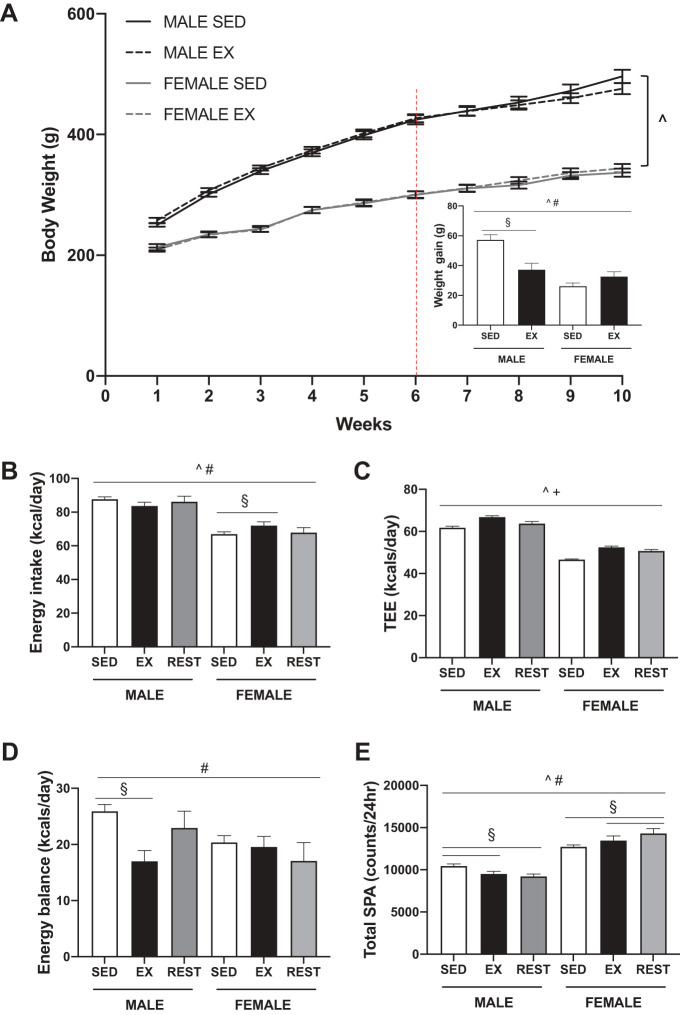
Over the 4-wk exercise training period, exercising male rats gained less weight compared with sedentary males (Fig. 2A, SEX × ACT, P < 0.001, post hoc, males P < 0.001). In contrast, weight gain in exercising females was comparable to that of sedentary females. Lean mass and body fat percentage were not different between exercising or sedentary rats, although there was a trend (P = 0.060) for a lower body fat percentage in exercising male rats (Table 1). The individual fat depots were differentially affected by exercise. The subcutaneous fat depot was lower in weight in exercising male but not female rats compared with respective sedentary controls (SEX × ACT, P = 0.026, post hoc, males P = 0.016). The retroperitoneal and mesenteric fat pads were not altered with exercise in either sex.
Fig. 2.
Metabolic phenotyping of sedentary (SED), exercised (EX), and exercise rested (REST) male and female Wistar rats. The exercise protocol consisted of forced treadmill exercise at 15 m/min, 1 h/day, 5 day/wk (2 day/wk were rest days). A: body weight curves during the 10-wk study. The dotted line indicates the beginning of exercise training. Inset: change in body weight over the 4 wk of exercise training. Exercising males gained less weight over the 4 wk compared with sedentary controls. Energy intake (B), total energy expenditure (TEE; C), energy balance (D), and total spontaneous physical activity (SPA; E) were measured using indirect calorimetry in SED and EX rats. Exercise took place 5 day/wk allowing for the presentation of the data based on whether the rat was exercised or rested (REST). Energy intake, energy balance, and total SPA were differed by sex and exercise status. TEE was elevated by exercise (EX and REST) as compared with SED rats. P < 0.05, ^main effect of sex, +main effect of activity, #sex by activity interaction, §post hoc simple effect.
Table 1.
End of study body weight and body composition in male and female Wistar rats with and without exercise
| Male |
Female |
|||
|---|---|---|---|---|
| SED | EX | SED | EX | |
| Body weight, g | 509 ± 7 | 484 ± 9§ | 320 ± 6 | 327 ± 7^# |
| Lean mass, g | 387 ± 9 | 389 ± 8 | 233 ± 5 | 238 ± 5^ |
| Body fat, % | 20.0 ± 1.0 | 16.9 ± 1.3 | 21.0 ± 1.0 | 22.3 ± 1.9^ |
| Mesenteric fat pad, g | 4.9 ± 0.4 | 4.3 ± 0.4 | 3.3 ± 0.3 | 3.4 ± 0.3^ |
| Retroperitoneal fat pad, g | 7.7 ± 0.4 | 6.6 ± 0.4 | 4.5 ± 0.3 | 4.4 ± 0.3^ |
| Subcutaneous fat pad, g | 6.2 ± 0.3 | 5.2 ± 0.3§ | 2.7 ± 0.2 | 3.2 ± 0.3^# |
EX; exercised; SED; sedentary. P < 0.05,
main effect of sex,
sex by activity interaction,
post hoc simple effect.
Overall, energy intake differed significantly by sex and activity status (SEX × ACT, P = 0.032) (Fig. 2B). Total energy expenditure (TEE) followed a similar pattern in both males and females depending on activity status (ACT, P < 0.001) (Fig. 2C). Exercise resulted in an increase in TEE compared with sedentary controls (P < 0.001). On days the exercisers were rested, TEE was lower (P < 0.001) but remained elevated above that of the sedentary rats (P = 0.002). Although the speed and duration of the exercise bout was held constant, the average workload for males and female rats differed (4.0 and 2.3 kcal/bout, respectively). The differences in energy intake and expenditure resulted in sex- and activity-dependent differences in energy balance (SEX × ACT, P = 0.023) (Fig. 2D). Exercising males were in less of a positive energy balance on the days they exercised compared with sedentary rats (P < 0.001). Energy balance did not differ in females regardless of activity status.
Total spontaneous physical activity (SPA) differed by sex and activity (SEX × ACT, P < 0.001) (Fig. 2E) and was driven by differences in both ambulatory (Supplemental Fig. S1; all supplemental material is available at https://doi.org/10.6084/m9.figshare.9716966.v2) and nonambulatory (data not shown) activity (SEX × ACT, P = 0.001 and P = 0.004, respectively). Exercising males moved less than sedentary males both on the days they were exercised (P = 0.026) and the days they were rested (P = 0.031). Exercising females moved more than sedentary females on days they were exercised (P = 0.048) and rested (P = 0.001). These patterns were evident during both the dark and light periods (Supplemental Fig. S1).
Feeding behaviors are differentially affected by exercise in male and female rats.
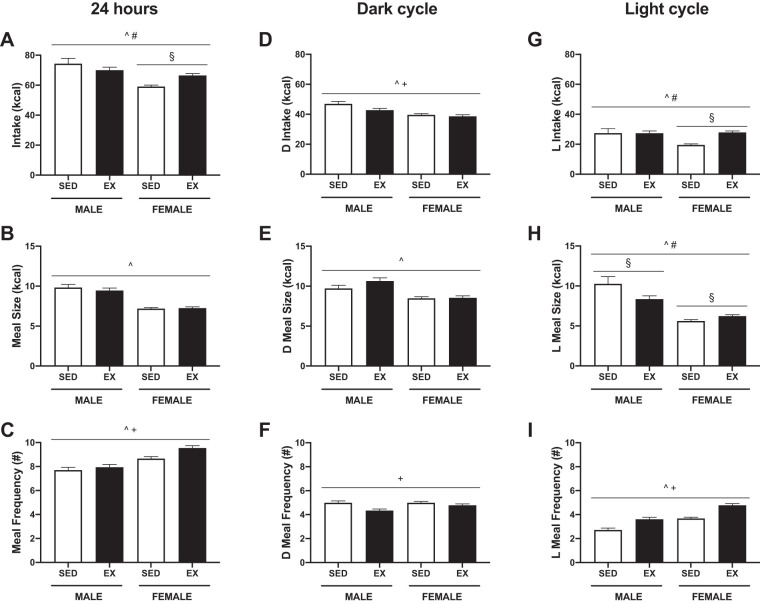
The 24-h real-time measurements of feeding behavior also showed that exercise resulted in a sex difference in food intake (SEX × ACT, P = 0.001) (Fig. 3, A–C and Supplemental Fig. S2). In exercising females, the compensatory increase in food intake (P < 0.001) was mainly driven by an increase in meal number (P = 0.002). Intake in males was not significantly different (P = 0.080). When food intake data obtained during the metabolic monitoring and meal patterning experiments were combined, statistically significant differences were found (SEX × ACT, P < 0.001). Exercising males had a lower calorie intake compared with sedentary males (P < 0.001) and the days they were rested (P = 0.005). On the days they exercised, females had a higher calorie intake compared with sedentary females (P < 0.001).
Fig. 3.
Feeding behavior in male and female Wistar rats with and without exercise. Intake (A), meal size (B), and meal frequency (C) over 24 h. Exercise differentially affected 24-h food intake in males and females. Intake (D), meal size (E), and meal frequency (F) during the dark cycle (D; 10 h). Exercising male rats decreased food intake during the dark cycle through a decrease in meal frequency. Intake (G), meal size (H), and meal frequency (I) during the light cycle (L; 14 h). Exercise in female rats increased food intake during the light cycle through an increase in meal size and frequency. P < 0.05, ^main effect of sex, +main effect of activity, #sex by activity interaction, §post hoc simple effect. SED; sedentary, EX; exercised.
When the 24-h meal pattern data were separated between the dark (Fig. 3, D–F) and light (Fig. 3, G–I) periods, additional sex differences in feeding behaviors emerged. During the dark cycle, intake differed by activity (ACT, P = 0.005) whereas there was a SEX × ACT interaction (P < 0.001) during the light cycle. Exercising male rats consumed less during the dark period as compared with sedentary males (P = 0.012), driven mainly by a decreased meal frequency (P = 0.001). In contrast, there were no significant differences in feeding behaviors between sedentary and exercised females during the dark period. There was no significant difference in caloric intake between exercised and sedentary males during the light cycle. However, the meal patterns between the two groups differed: exercising males had an increased meal frequency (P = 0.001) that was accompanied by a decrease in meal size (P = 0.004) compared with sedentary males. Exercising females increased their intake in the light cycle (P < 0.001) through an increase in meal size (P = 0.046) and frequency (P < 0.001).
Exercise does not prevent overconsumption of highly palatable foods.
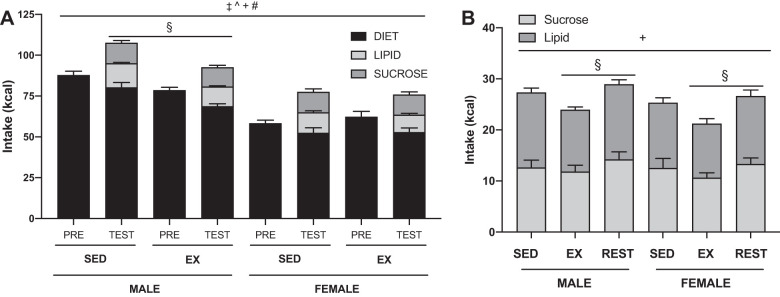
Regardless of sex or activity status, all rats overconsumed on average 16.8 ± 1.0 kcals (P < 0.001) when presented with the individual highly palatable macronutrients (Fig. 4A). Total calorie intake depended on sex and activity (SEX × ACT P = 0.008): exercising males consumed fewer total calories than sedentary males (post hoc, males P < 0.001). Total intake was similar between sedentary and exercised females. There was no difference in preference between the sucrose and lipid solutions regardless of sex or activity status. Caloric consumption of the palatable solutions differed depending on activity status (ACT, P = 0.027) (Fig. 4B). Exercisers consumed more of the palatable solutions on the days they rested compared with the days they were exercised (post hoc, P = 0.008).
Fig. 4.
Feeding response to highly palatable foods. A: 24-h energy intake of sedentary (SED) and exercised (EX) male and female Wistar rats prior (PRE) to the start of the highly palatable food exposure and averaged across the 4-day test (TEST). All groups, regardless of sex or exercise, overconsumed when presented with the highly palatable foods. Total intake on the day of the palatable food test differed depending on sex and activity: exercising males consumed less than sedentary males. B: energy intake of isocaloric sucrose and lipid solutions in sedentary, exercised, or rested male and female Wistar rats. Exercised male and female rats consumed more on the days they were rested than they did on the days they exercised. P < 0.05, ‡main effect of the test, ^main effect of sex, +main effect of activity, #sex by activity interaction, §post hoc simple effect.
Exercise increases some aspects of anxiety-like behaviors in both male and female rats.
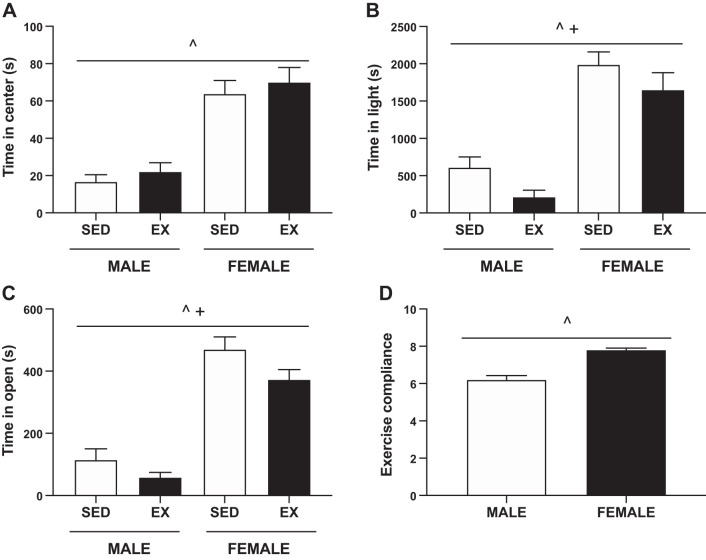
Three different behavioral tests were utilized at three different time points to assess anxiety-like behaviors. Before the start of exercise, the time spent in the center during the open-field test did not differ between sedentary and exercised rats (Fig. 5A). Females spent significantly more time in the center compared with male rats (SEX, P < 0.001). This sex difference was evident in all tests of behavioral anxiety.
Fig. 5.
Behavioral measures of anxiety and exercise compliance. A: time spent in the open during the open-field behavioral test. There were no significant differences between sedentary (SED) and exercised (EX) groups before the exercise intervention. During all of the behavioral tests, females spent more time in the center/light/open compared with males. B: time spent in the light during the light/dark box behavior test at the start of exercise. Exercising male and female rats spent less time in the light. C: time spent in the open during the elevated zero maze behavioral test at the full exercise protocol. Both exercising male and female rats spent less time in the open arms. D: forced treadmill exercise compliance scored on a scale of 1–10, 1 being the least compliant. Exercising females outperform males. P < 0.05, ^main effect of sex, +main effect of activity.
Using the light/dark box during the first week of exercise training, we observed that exercising rats spent less time in the light (ACT, P = 0.02) (Fig. 5B). Exercising rats had fewer total entries into the light area (Male: SED 18 ± 3 vs. EX 6 ± 2, Female: SED 33 ± 2 vs. EX 20 ± 2; ACT, P < 0.001), and exercising males demonstrated an increased latency (Male: SED 769 ± 264 vs. EX 2,195 ± 378 s, Female: SED 222 ± 56 vs. EX 253 ± 52 s; SEX × ACT P = 0.004, post hoc males P < 0.001).
At the end of exercise training, exercising rats spent less time in the open arms of the elevated zero maze (ACT, P = 0.022) (Fig. 5C). However, exercise did not affect the number of crossing into the open arms or the latency to the first entry into the open arm (data not shown). Male rats scored lower in exercise compliance compared with female rats (SEX, P < 0.001) (Fig. 5D).
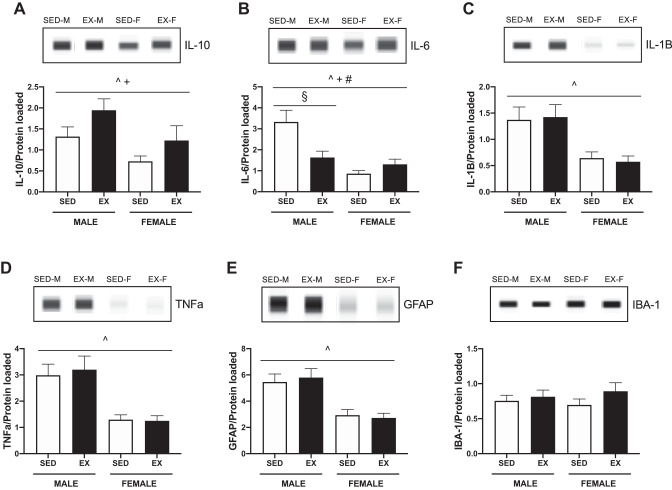
Only hypothalamic IL-10 and IL-6 profiles are altered with exercise.
Acute exercise increased hypothalamic IL-10 in both male and female rats compared with sedentary rats (ACT, P = 0.030) (Fig. 6A). Exercise decreased hypothalamic IL-6 in male rats (SEX × ACT, P = 0.002, post hoc males P < 0.001) (Fig. 6B). Ventral tegmental area (VTA) (Male: SED 18.9 ± 1.9 vs. EX 18.4 ± 2.2, Female: SED 12.1 ± 1.4 vs. EX 13.0 ± 1.4, pg/mL) and plasma (Male: SED 7.7 ± 1.3 vs. EX 7.7 ± 1.2, Female: SED 17.8 ± 1.1 vs. EX 19.8 ± 2.2, pg/mL) IL-6 concentrations were unaffected by exercise. Exercise had no effect on hypothalamic IL-1B, TNFa, GFAP, or IBA-1 protein concentrations (Fig. 6, C–F). Females had lower concentrations of IL-1B (SEX, P < 0.001), IL-10 (P < 0.001), IL-6 (P < 0.001), TNFa (P < 0.001), and GFAP (P < 0.001) as compared with males.
Fig. 6.
Hypothalamic inflammatory profile in sedentary (SED) and exercised (EX) male and female Wistar rats. Protein was measured using the WES capillary system from Protein Simple and normalized to total protein. A: hypothalamic interleukin-10 (IL-10) protein content. Exercising male and female rats displayed increased hypothalamic IL-10. B: hypothalamic interleukin-6 (IL-6) protein content. Exercising males displayed decreased hypothalamic IL-6. C: hypothalamic interleukin-1β (IL-1β) protein content. D: hypothalamic tumor necrosis factor-α (TNFα) protein content. E: hypothalamic glial fibrillary acidic protein (GFAP) content. There was no effect of exercise on IL-1B, TNFa, or GFAP; however, females had significantly lower concentrations of each protein compared with males. F: hypothalamic ionized calcium-binding adaptor molecule 1 (IBA-1) protein content. P < 0.05, ^main effect of sex, +main effect of activity, #sex by activity interaction, §post hoc simple effect.
Exercise lowers plasma leptin, triglycerides, and cholesterol in male rats.
Concentrations of glucose and insulin were not significantly affected by exercise (Table 2). Leptin and triglycerides were decreased in exercising males compared with sedentary male rats but unchanged in females (SEX × ACT, P = 0.008 post hoc males P = 0.046 and SEX × ACT, P = 0.003 post hoc males P < 0.001, respectively). Leptin concentrations significantly correlated with fat mass in both sexes (P < 0.001, r value = 0.432). Cholesterol decreased (ACT, P < 0.001) and NEFA increased (ACT, P < 0.001) with exercise in both male and female rats.
Table 2.
Plasma concentrations of hormones and metabolites at end of study
| Male |
Female |
|||
|---|---|---|---|---|
| SED | EX | SED | EX | |
| Glucose, mmol/L | 10.9 ± 0.2 | 10.4 ± 0.2 | 10.5 ± 0.3 | 10.5 ± 0.2 |
| Insulin, mg/mL | 3.7 ± 0.4 | 2.9 ± 0.2 | 2.6 ± 0.3 | 2.4 ± 0.3 |
| Leptin, pg/mL | 4,738 ± 278 | 3,600 ± 252§ | 4,097 ± 600 | 4,794 ± 632^# |
| TG, mg/dL | 79.1 ± 5.3 | 46.5 ± 3.8§ | 50.5 ± 4.9 | 46.4 ± 4.9+^# |
| Cholesterol, mg/dL | 84.3 ± 3.9 | 71.4 ± 3.0 | 58.3 ± 2.7 | 48.9 ± 1.6+^ |
| NEFA, meq/L | 0.29 ± 0.01 | 0.51 ± 0.02 | 0.35 ± 0.04 | 0.56 ± 0.03+^ |
EX, exercised; NEFA, nonesterified fatty acids; SED; sedentary; TG, triglycerides. P < 0.05,
main effect of activity;
main effect of sex;
sex by activity interaction;
post hoc simple effect.
Leptin responsiveness is unaffected by exercise.
All rats, regardless of sex, diet, or activity status, consumed less energy over 24 h following the intraperitoneal leptin injection as compared with the control injection (main effect of injection, P < 0.001) (Fig. 7). Exercise not did affect the anorectic response to the leptin injection, as measured by food intake over 24 h (Fig. 7) or when intakes were examined at either 4 or 16 h (data not shown).
Fig. 7.
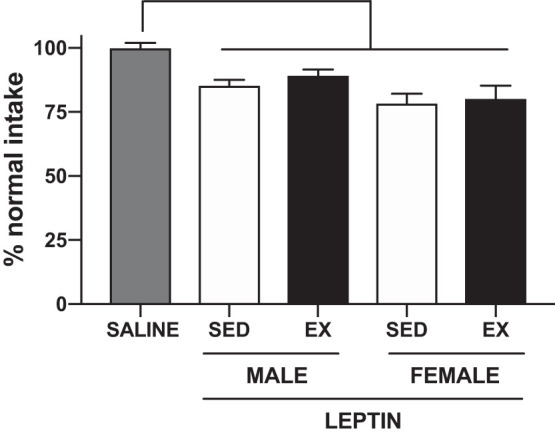
Anorectic response to a peripheral leptin injection. 24-h food intake in response to an intraperitoneal saline or leptin (2.5 mg/kg) injection in sedentary (SED) and exercised (EX) male and female Wistar rats. Data are presented as a percentage of average energy intake overall (males) or on equivalent estrous cycle days (females) to control for differences in the estrous cycle across the leptin and control injection days. All groups saw a similar and significant decrease in food intake following the leptin injection as compared with saline. P < 0.001, main effect of injection.
Male rats are susceptible to negative effects of high-fat diet.
During the 10-wk treatment period, HFD increased adiposity, the magnitude of positive energy balance, and 24-h and light cycle food intake in male but not female rats (Supplemental Tables S1 and S2). Both HFD-fed male and female rats consumed smaller, more frequent meals compared with LFD controls (Supplemental Table S2). Neither anxiety-like behaviors nor the anorectic response to leptin were affected by diet (data not shown). Diet did not affect endocrine or metabolite panels, with the exception of plasma cholesterol (Supplemental Table S1). Even so, HFD feeding in this paradigm increased hypothalamic concentrations of IL-10, IL-6, TNFa, and GFAP in both sexes (Supplemental Table S3).
DISCUSSION
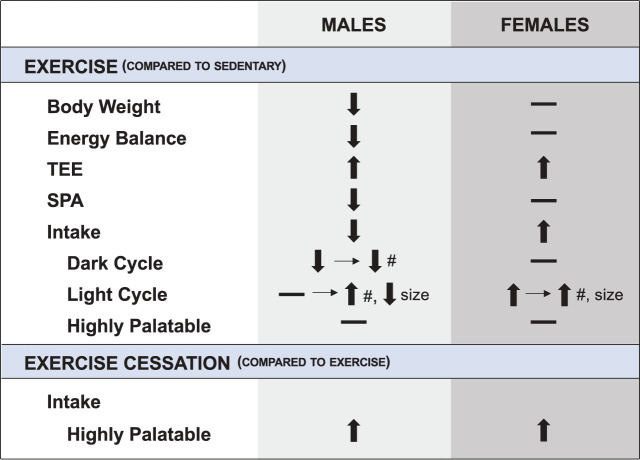
The novel observations of this study are that exercise affects energy balance in both a sex-dependent and independent manner and that its sex-specific effects on eating behaviors may work through distinctly different mechanisms. In this preclinical paradigm, we performed a battery of tests examining the effects of exercise on metabolism, feeding behaviors, palatable food intake, anxiety-like behaviors, and the anorectic response to a leptin injection. Some of those results are summarized in Fig. 8. Our observations clearly show that regular exercise affects the biological control of body weight and food intake in a sexually dimorphic manner. We found that exercised males exhibited an early (dark cycle) decrease in meal frequency followed by a delayed (light cycle) increase in meal frequency and decrease in meal size. In contrast, exercising females had a delayed (light cycle) increase in meal size and frequency. These sex differences in total intake and feeding behaviors in response to exercise do not appear to be mediated by anxiety-like behaviors, hypothalamic inflammation, or leptin sensitivity. Finally, this work suggests there are limits to the effectiveness of exercise in limiting caloric intake, even in males, as all rats, regardless of sex or activity, overconsumed in the face of varied, highly palatable foods.
Fig. 8.
Summary of the metabolic and feeding effects of exercise on male and female rats. SPA, spontaneous physical activity; TEE, total energy expenditure; #, meal number; –, no change. Horizontal arrow indicates how overall intake was affected by changes in meal size or meal number.
Effects of exercise on energy balance appear to be acute.
An acute suppression of food intake in response to exercise has been seen in male rats given alternate day access to a running wheel (37), but the acute effects of exercise on food intake have not been studied in females before this work. We observed that only on the days the rats exercised is food intake acutely suppressed in males and potentiated in females. Intakes on the days the exercisers are rested reflected those of sedentary rats, regardless of sex. These results suggest that the effect of exercise on energy intake, and subsequently energy balance, in response to exercise are not a chronic adaptation to training, but instead an acute and transient response.
The summation of these transient effects on appetite lead to sex differences in weight gain over time. Several studies have demonstrated that regular voluntary exercise decreased weight gain in males with little effect on female body weight (40, 41, 46). The present study is consistent with these results. In exercising males, positive energy balance was reduced, resulting in an attenuated weight gain over time compared with sedentary males. Over this relatively short (4-wk) intervention, exercise did not significantly change body composition in either sex. It is possible that a 7 day/wk exercise paradigm, compared with the 5 day/wk protocol used in the present study or an exercise intervention lasting longer than 4 wk, would have resulted in a greater effect on body composition, particularly in the males, as there was a trend (P = 0.060) for a decreased body fat percentage in the exercise group.
Sex differences in the temporal effects of exercise on meal patterns.
Previous reports have shown an exercise-induced (voluntary and forced) decrease in food intake in male rats (7, 18, 26, 35, 37, 38) and increase in food intake in female rats (7, 18, 38). However, to our knowledge, our study provides the first in-depth analysis of the sex differences in meal patterns in response to forced treadmill exercise. Interpretation of the changes in feeding behavior suggest an initial (dark cycle) role of satiety (interval between meals or meal frequency) followed by a delayed (light cycle) decrease in satiety and increase in satiation (termination of eating during a single meal or meal size) in the male feeding response to exercise. Alternatively, exercise in females resulted in a delayed decrease in satiation (increased meal size) and satiety (increased meal frequency). The orexigenic hormone ghrelin, secreted by the stomach in increasing concentrations during fasting, plays a role in meal initiation and satiety (10). Although we did not measure ghrelin, others (15) have reported a decrease in ghrelin following moderate exercise in male rats and could be mediating the increased satiety we observed. Future studies should investigate a potential role of gastrointestinal hormones and peptides in mediating the sex differences in energy intake and balance in response to moderate exercise.
Rats are nocturnal and will generally consume the majority of their food during the dark cycle. We consistently exercise our rats just before the start of the dark cycle to take advantage of anticipatory foraging behaviors while not decreasing the amount of time they have to consume food during the dark cycle. It is unclear, however, whether these differences in feeding behaviors in response to exercise would be altered if the timing of the exercise bout had been adjusted. Despite these limitations, these data suggest that males and females could differentially benefit from adjusting the timing of either the exercise bout or meals to maximize (or minimize) the biological sex differences in satiation and satiety in response to exercise. Further studies are needed to understand how changing the timing of exercise could affect subsequent food intake.
Overconsumption of highly palatable lipid and sucrose solutions were not prevented by exercise.
It is well established that rodents and humans will overconsume in the presence of highly palatable food (30), although in the present study, only the male rats overconsumed on the HFD. Sexually dimorphic responses to HFD are known in preclinical rodent models (19, 29, 53) and should be considered when interpreting the results of this study. The reduction in food intake in males in response to exercise did not appear to be dependent on diet, as there was a lack of a significant activity × diet interaction. Given the ability of exercise to decrease high-fat diet intake in our male rats, we expected exercise to limit consumption of these highly palatable foods in the male rats. We hypothesized that exercise would shift macronutrient preference, as has been seen by others (9, 32, 34, 44, 45, 47, 63, 64) when providing diets of differing macronutrient compositions; however, we did not find an effect of exercise on macronutrient preference in this context. Instead, we observed that exercise was unable to prevent overconsumption regardless of sex or activity status when provided highly palatable sucrose and lipid solutions. Furthermore, we observed that exercise cessation (rest days) resulted in increased intake of the palatable solutions.
Anxiety is unlikely to mediate exercise-induced effects on food intake.
Depending on the exercise paradigm, exercise can be either anxiolytic (13, 20, 56) or anxiogenic (6, 25). We repeatedly find that male rats score lower in exercise compliance compared with females and hypothesized that the exercising male rats would display enhanced anxiety-like behaviors in response to exercise training, which we hypothesized could be responsible for the exercise-induced decrease in food intake. However, we found that exercise training similarly increased anxiety-like behaviors in both male and female rats. Additionally, data indicating similar effects on appetite (7, 18, 26, 35, 37, 38) and body weight (40, 41, 46) in male rodents given access to voluntary running wheels suggests that these findings are not strictly the result of a stress response to forced treadmill exercise but a biological response to exercise. We therefore conclude that it is unlikely that potential anxiogenic effects or stress of the exercise bout (as measured by approach vs. avoidant anxiety-like behaviors) are contributing to the sex differences we observe in food intake.
Hypothalamic inflammation does not appear to explain sexually dimorphic response to exercise.
The work of the Schwartz laboratory, among others, showed that hypothalamic inflammation occurs rapidly in response to high-fat diet (54, 55, 57). This inflammation can be reversed by removal of high-fat diet (2). We hypothesized that the exercising male rats, which showed a reduction in food intake and body weight, would display a reduction in markers of hypothalamic inflammation relative to sedentary males. We showed, however, that exercise had no effect on hypothalamic inflammation in either our male or female rats. Based on these data, it seems unlikely that sex differences in hypothalamic inflammation are mediating the differential eating behaviors in response to exercise. It is possible we would have been able to detect more nuanced changes in hypothalamic inflammation had we performed immunohistochemical analysis to measure reactive gliosis. An additional limitation to our work is that we extracted and homogenized the whole hypothalamus, which leaves the possibility that we may have missed nuclei-specific differences in inflammation in response to exercise.
Leptin unlikely to play a role in the sex differences in appetite in response to exercise.
Acute exercise has been seen to increase leptin sensitivity in males (42, 50) through an increase in hypothalamic IL-6 and IL-10 (42). Based on this literature, we hypothesized that the decrease in food intake we see in exercising male rats was brought about by an increase in the anorectic effect of leptin due to an increase in hypothalamic IL-6 and IL-10. Instead, we found no effect of exercise on the anorectic response to leptin in either sex, which suggests that leptin signaling is not likely mediating the sex differences in food intake in response to exercise. Potentially, a central leptin injection or different measurement output of leptin responsiveness (i.e., STAT3 phosphorylation) may have produced different results, as has been seen by some (50) but not all studies (11), particularly given that the exercising male rats had significantly lower plasma leptin concentrations.
We believe the differences between our results and the published data (42) stem from differences in the exercise protocol. As described in methods, our study utilized a chronic (4 wk), moderate, forced treadmill exercise paradigm, which is in contrast to the acute (single bout), extreme (6 h), forced swimming exercise paradigm used in the Ropelle et al. study (42). The extreme nature of the acute swimming exercise protocol suggests that the measurements taken may not have captured only an acute exercise response but also a stress response. The decrease in hypothalamic IL-6 we see in males may be a chronic adaptation to the moderate exercise training. IL-6 is a major signaling molecule within the central nervous system and has functions involved in both pathological and normal situations, including a complex role in regulating appetite, energy expenditure, and body weight (16). Clinical research demonstrates an association with high-intensity exercise-induced IL-6 release and reduced appetite (1, 28). Full-body IL-6 knockout animals have been shown to develop obesity late in life through increased food intake in some (59) but not all studies (12), and muscle-specific IL-6 knockout mice display body weights similar to wild-type controls (17). Intracerebral ventricular injections of IL-6 decrease food intake and body weight (58). In the present study, we cannot explain how or if the decrease in hypothalamic IL-6 concentrations in exercising male rats contributes to differences in energy intake or balance, but future work should seek to understand the role for exercise-induced changes in hypothalamic IL-6 in appetite regulation.
Perspectives and Significance
The use of this preclinical model allows for the analysis of the biological effects of exercise on physiology and behavior while minimizing the role of environmental and psychosocial modifiers that can greatly influence human behavior. There is some evidence that these biologically driven sex differences in appetite in response to exercise translate to humans. In one such study, 16 wk of exercise produced on average a 5.2-kg weight loss in male participants and no net change in body weight of female participants (14). These overlapping findings demonstrate that sex is an important variable that may be responsible for some of the variability in response to exercise and should be considered when designing future studies. Although the present study was unable to pinpoint a specific mechanism responsible for the sex differences in appetite in response to moderate exercise, if these observations translate to the human condition, we may find that women, generally, may need to utilize greater cognitive control to consciously minimize maladaptive behaviors (i.e., increased intake or decreased physical activity) to realize benefits of exercise on body weight.
GRANTS
This work was supported by National Institutes of Health Grants F31 DK115238 and TL1 TR001081 (to R. M. Foright); KL2 TR002534 (to V. D. Sherk); and P50 HD073063, R01 CA164166, U54 AG062319, and P30 NORC DK48520 (to P. S. MacLean). This work was supported by the Animal Behavior Core at the University of Colorado, Anschutz Medical Campus.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.F., M.R.J., B.N.G., and P.S.M. conceived and designed research; R.M.F., G.C.J., D.K., C.A.C., and C.A.B. performed experiments; R.M.F. analyzed data; R.M.F., M.R.J., B.N.G., and P.S.M. interpreted results of experiments; R.M.F. prepared figures; R.M.F. drafted manuscript; R.M.F., D.M.P., E.A.W., V.D.S., B.N.G., and P.S.M. edited and revised manuscript; R.M.F., G.C.J., D.K., C.A.C., D.M.P., C.A.B., E.A.W., V.D.S., M.R.J., B.N.G., and P.S.M. approved final version of manuscript.
REFERENCES
- 1.Almada C, Cataldo LR, Smalley SV, Diaz E, Serrano A, Hodgson MI, Santos JL. Plasma levels of interleukin-6 and interleukin-18 after an acute physical exercise: relation with post-exercise energy intake in twins. J Physiol Biochem 69: 85–95, 2013. doi: 10.1007/s13105-012-0191-x. [DOI] [PubMed] [Google Scholar]
- 2.Berkseth KE, Guyenet SJ, Melhorn SJ, Lee D, Thaler JP, Schur EA, Schwartz MW. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology 155: 2858–2867, 2014. doi: 10.1210/en.2014-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biener A, Cawley J, Meyerhoefer C. The impact of obesity on medical care costs and labor market outcomes in the US. Clin Chem 64: 108–117, 2018. doi: 10.1373/clinchem.2017.272450. [DOI] [PubMed] [Google Scholar]
- 4.Bouchet CA, Lloyd BA, Loetz EC, Farmer CE, Ostrovskyy M, Haddad N, Foright RM, Greenwood BN. Acute exercise enhances the consolidation of fear extinction memory and reduces conditioned fear relapse in a sex-dependent manner. Learn Mem 24: 358–368, 2017. doi: 10.1101/lm.045195.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab 295: E586–E594, 2008. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res 1019: 84–96, 2004. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 7.Carrera O, Cerrato M, Vazquez R, Sineiro C, Gutierrez E. Gender dimorphic effects of voluntary running in laboratory rats depends on maturational status. Q J Exp Psychol (Hove) 64: 823–832, 2011. doi: 10.1080/17470218.2010.523473. [DOI] [PubMed] [Google Scholar]
- 8.Checkley LA, Rudolph MC, Wellberg EA, Giles ED, Wahdan-Alaswad RS, Houck JA, Edgerton SM, Thor AD, Schedin P, Anderson SM, MacLean PS. metformin accumulation correlates with organic cation transporter 2 protein expression and predicts mammary tumor regression in vivo. Cancer Prev Res (Phila) 10: 198–207, 2017. doi: 10.1158/1940-6207.CAPR-16-0211-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordeira J, Monahan D. Voluntary wheel running reduces weight gain in mice by decreasing high-fat food consumption. Physiol Behav 207: 1–6, 2019. doi: 10.1016/j.physbeh.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 11.de Carvalho FP, Moretto TL, Benfato ID, Barthichoto M, Ferreira SM, Costa-Júnior JM, de Oliveira CA. Central and peripheral effects of physical exercise without weight reduction in obese and lean mice. Biosci Rep 38: BSR20171033, 2018. doi: 10.1042/BSR20171033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab 287: E182–E187, 2004. doi: 10.1152/ajpendo.00189.2003. [DOI] [PubMed] [Google Scholar]
- 13.Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, Wilson MA. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiol Behav 60: 699–705, 1996. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, Heelan K, Hise M, Fennessey PV, Sonko B, Sharp T, Jakicic JM, Blair SN, Tran ZV, Mayo M, Gibson C, Washburn RA. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med 163: 1343–1350, 2003. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 15.Ebal E, Cavalie H, Michaux O, Lac G. Effect of a moderate exercise on the regulatory hormones of food intake in rats. Appetite 49: 521–524, 2007. doi: 10.1016/j.appet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8: 1254–1266, 2012. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrer B, Navia B, Giralt M, Comes G, Carrasco J, Molinero A, Quintana A, Señarís RM, Hidalgo J. Muscle-specific interleukin-6 deletion influences body weight and body fat in a sex-dependent manner. Brain Behav Immun 40: 121–130, 2014. doi: 10.1016/j.bbi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Foright RM, Presby DM, Sherk VD, Kahn D, Checkley LA, Giles ED, Bergouignan A, Higgins JA, Jackman MR, Hill JO, MacLean PS. Is regular exercise an effective strategy for weight loss maintenance? Physiol Behav 188: 86–93, 2018. doi: 10.1016/j.physbeh.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuente-Martín E, Argente-Arizón P, Ros P, Argente J, Chowen JA. Sex differences in adipose tissue: it is not only a question of quantity and distribution. Adipocyte 2: 128–134, 2013. doi: 10.4161/adip.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med 25: 78–82, 2004. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- 21.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP; American College of Sports Medicine . American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43: 1334–1359, 2011. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 22.Giles ED, Jackman MR, Johnson GC, Schedin PJ, Houser JL, MacLean PS. Effect of the estrous cycle and surgical ovariectomy on energy balance, fuel utilization, and physical activity in lean and obese female rats. Am J Physiol Regul Integr Comp Physiol 299: R1634–R1642, 2010. doi: 10.1152/ajpregu.00219.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giles ED, Steig AJ, Jackman MR, Higgins JA, Johnson GC, Lindstrom RC, MacLean PS. Exercise decreases lipogenic gene expression in adipose tissue and alters adipocyte cellularity during weight regain after weight loss. Front Physiol 7: 32, 2016. doi: 10.3389/fphys.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, Goodyear LJ. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab 297: E495–E504, 2009. doi: 10.1152/ajpendo.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hicks JA, Hatzidis A, Arruda NL, Gelineau RR, De Pina IM, Adams KW, Seggio JA. Voluntary wheel-running attenuates insulin and weight gain and affects anxiety-like behaviors in C57BL6/J mice exposed to a high-fat diet. Behav Brain Res 310: 1–10, 2016. doi: 10.1016/j.bbr.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JA, Jackman MR, Brown IL, Johnson GC, Steig A, Wyatt HR, Hill JO, Maclean PS. Resistant starch and exercise independently attenuate weight regain on a high fat diet in a rat model of obesity. Nutr Metab (Lond) 8: 49, 2011. doi: 10.1186/1743-7075-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 299: 853–855, 2003. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 28.Hunschede S, Kubant R, Akilen R, Thomas S, Anderson GH. Decreased appetite after high-intensity exercise correlates with increased plasma interleukin-6 in normal-weight and overweight/obese boys. Curr Dev Nutr 1: e000398, 2017. doi: 10.3945/cdn.116.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, Chiou LC. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring) 18: 463–469, 2010. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- 30.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron 69: 664–679, 2011. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King NA, Horner K, Hills AP, Byrne NM, Wood RE, Bryant E, Caudwell P, Finlayson G, Gibbons C, Hopkins M, Martins C, Blundell JE. Exercise, appetite and weight management: understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br J Sports Med 46: 315–322, 2012. doi: 10.1136/bjsm.2010.082495. [DOI] [PubMed] [Google Scholar]
- 32.Lee JR, Tapia MA, Nelson JR, Moore JM, Gereau GB, Childs TE, Vieira-Potter VJ, Booth FW, Will MJ. Sex dependent effects of physical activity on diet preference in rats selectively bred for high or low levels of voluntary wheel running. Behav Brain Res 359: 95–103, 2019. doi: 10.1016/j.bbr.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 286: R771–R778, 2004. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- 34.Liang NC, Bello NT, Moran TH. Wheel running reduces high-fat diet intake, preference and mu-opioid agonist stimulated intake. Behav Brain Res 284: 1–10, 2015. doi: 10.1016/j.bbr.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackman MR, Giles ED, Brown IE, Hill JO. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 297: R793–R802, 2009. doi: 10.1152/ajpregu.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melby CL, Paris HL, Foright RM, Peth J. Attenuating the biologic drive for weight regain following weight loss: must what goes down always go back up? Nutrients 9: 468, 2017. doi: 10.3390/nu9050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller DT, Loft A, Eikelboom R. Alternate-day wheel access: effects on feeding, body weight, and running. Physiol Behav 62: 905–908, 1997. doi: 10.1016/S0031-9384(97)00266-7. [DOI] [PubMed] [Google Scholar]
- 38.Nance DM, Bromley B, Barnard RJ, Gorski RA. Sexually dimorphic effects of forced exercise on food intake and body weight in the rat. Physiol Behav 19: 155–158, 1977. doi: 10.1016/0031-9384(77)90173-1. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). Amsterdam, The Netherlands: Elsevier, 2007. [Google Scholar]
- 40.Pitts GC. Body composition in the rat: interactions of exercise, age, sex, and diet. Am J Physiol Regul Integr Comp Physiol 246: R495–R501, 1984. doi: 10.1152/ajpregu.1984.246.4.R495. [DOI] [PubMed] [Google Scholar]
- 41.Rolls BJ, Rowe EA. Exercise and the development and persistence of dietary obesity in male and female rats. Physiol Behav 23: 241–247, 1979. doi: 10.1016/0031-9384(79)90361-5. [DOI] [PubMed] [Google Scholar]
- 42.Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, Morari J, de Souza CT, Moraes JC, Prada PO, Guadagnini D, Marin RM, Oliveira AG, Augusto TM, Carvalho HF, Velloso LA, Saad MJ, Carvalheira JB. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLoS Biol 8: e1000465, 2010. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Rudolph MC, Jackman MR, Presby DM, Houck JA, Webb PG, Johnson GC, Soderborg TK, de la Houssaye BA, Yang IV, Friedman JE, MacLean PS. Low neonatal plasma n-6/n-3 PUFA ratios regulate offspring adipogenic potential and condition adult obesity resistance. Diabetes 67: 651–661, 2018. doi: 10.2337/db17-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarpace ET, Matheny M, Strehler KY, Shapiro A, Cheng KY, Tümer N, Scarpace PJ. Simultaneous introduction of a novel high fat diet and wheel running induces anorexia. Physiol Behav 105: 909–914, 2012. doi: 10.1016/j.physbeh.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scarpace PJ, Matheny M, Zhang Y. Wheel running eliminates high-fat preference and enhances leptin signaling in the ventral tegmental area. Physiol Behav 100: 173–179, 2010. doi: 10.1016/j.physbeh.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroeder M, Shbiro L, Gelber V, Weller A. Post-weaning voluntary exercise exerts long-term moderation of adiposity in males but not in females in an animal model of early-onset obesity. Horm Behav 57: 496–505, 2010. doi: 10.1016/j.yhbeh.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Shapiro A, Cheng KY, Gao Y, Seo DO, Anton S, Carter CS, Zhang Y, Tumer N, Scarpace PJ. The act of voluntary wheel running reverses dietary hyperphagia and increases leptin signaling in ventral tegmental area of aged obese rats. Gerontology 57: 335–342, 2011. doi: 10.1159/000321343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherk VD, Jackman MR, Giles ED, Higgins JA, Foright RM, Presby DM, Johnson GC, Houck JA, Houser JL, Oljira R, MacLean PS. Prior weight loss exacerbates the biological drive to gain weight after the loss of ovarian function. Physiol Rep 5: e13272, 2017. doi: 10.14814/phy2.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherk VD, Jackman MR, Higgins JA, Giles ED, Foright RM, Presby DM, Carpenter RD, Johnson GC, Oljira R, Houck JA, MacLean PS. Impact of exercise and activity on weight regain and musculoskeletal health post-ovariectomy. Med Sci Sports Exerc 51: 2465–2473, 2019. doi: 10.1249/MSS.0000000000002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiuchi T, Miyatake Y, Otsuka A, Chikahisa S, Sakaue H, Séi H. Role of orexin in exercise-induced leptin sensitivity in the mediobasal hypothalamus of mice. Biochem Biophys Res Commun 514: 166–172, 2019. doi: 10.1016/j.bbrc.2019.04.145. [DOI] [PubMed] [Google Scholar]
- 51.Steig AJ, Jackman MR, Giles ED, Higgins JA, Johnson GC, Mahan C, Melanson EL, Wyatt HR, Eckel RH, Hill JO, MacLean PS. Exercise reduces appetite and traffics excess nutrients away from energetically efficient pathways of lipid deposition during the early stages of weight regain. Am J Physiol Regul Integr Comp Physiol 301: R656–R667, 2011. doi: 10.1152/ajpregu.00212.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis 61: 206–213, 2018. doi: 10.1016/j.pcad.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Taraschenko OD, Maisonneuve IM, Glick SD. Sex differences in high fat-induced obesity in rats: Effects of 18-methoxycoronaridine. Physiol Behav 103: 308–314, 2011. doi: 10.1016/j.physbeh.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes 62: 2629–2634, 2013. doi: 10.2337/db12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thaler JP, Schwartz MW. Minireview: inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151: 4109–4115, 2010. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tharp GD, Carson WH. Emotionality changes in rats following chronic exercise. Med Sci Sports 7: 123–126, 1975. doi: 10.1249/00005768-197500720-00021. [DOI] [PubMed] [Google Scholar]
- 57.Velloso LA, Schwartz MW. Altered hypothalamic function in diet-induced obesity. Int J Obes 35: 1455–1465, 2011. doi: 10.1038/ijo.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun 293: 560–565, 2002. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 59.Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 60.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 381: 2440–2450, 2019. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 61.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 82, Suppl 1: 222S–225S, 2005. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 63.Yang TY, Gardner JC, Gentile JD, Liang NC. Sex and individual differences in meal patterns mediate the persistency of running-associated high-fat diet avoidance in rats. Am J Physiol Regul Integr Comp Physiol 316: R130–R143, 2019. doi: 10.1152/ajpregu.00231.2018. [DOI] [PubMed] [Google Scholar]
- 64.Yang TY, Liang NC. Ovarian hormones mediate running-induced changes in high fat diet choice patterns in female rats. Horm Behav 100: 81–93, 2018. doi: 10.1016/j.yhbeh.2018.02.010. [DOI] [PubMed] [Google Scholar]