Abstract
Naked mole-rats (NMRs) are mammalian champions of hypoxia tolerance that enter metabolic suppression to survive in low oxygen environments. Common physiological mechanisms used by animals to suppress metabolic rate include downregulating energy metabolism (ATP supply) as well as ion pumps (primary cellular ATP consumers). A recent goldfish study demonstrated that remodeling of membrane lipids may mediate these responses, but it is unknown if NMR employs the same strategies; therefore, we aimed to test the hypotheses that these fossorial mammals 1) downregulate the activity of key enzymes of glycolysis, tricarboxylic acid (TCA) cycle, and β-oxidation, 2) inhibit sodium-potassium-ATPase, and 3) alter membrane lipids in response to chronic hypoxia. We found that NMRs exposed to 11% oxygen for 4 wk had a lower metabolic rate by 34%. This suppression occurs concurrently with tissue-specific 25–99% decreases in metabolic enzymes activities, a 77% decrease in brain sodium/potassium-ATPase activity, and widespread changes in membrane cholesterol abundance. By reducing glycolytic and β-oxidation fluxes, NMRs decrease the supply of acetyl-CoA to the TCA cycle. By contrast, there is a 94% upregulation of citrate synthase in the heart, possibly to support circulation and thus oxygen supply to other organs. Taken together, these responses may reflect a coordinated physiological response to hypoxia, but a clear functional link between changes in membrane composition and enzyme activities could not be established. Nevertheless, this is the first demonstration that hypometabolic NMRs alter the lipid composition of their membranes in response to chronic in vivo exposure to hypoxia.
Keywords: enzymes, hypoxia tolerance, membrane lipids, metabolic suppression, sodium/potassium ATPase
INTRODUCTION
Hypoxia-tolerant organisms use metabolic suppression as a key strategy to cope with reduced oxygen levels, which are otherwise deleterious to most animals (4, 47, 49). The primary physiological mechanisms that promote suppression include downregulating energy metabolism [tricarboxylic acid (TCA) cycle, glycolysis, and β-oxidation (38, 51, 52)] as well as major cellular consumers of ATP such as ion pumps (26). It has recently been suggested that membrane remodeling may play a role in the overall inhibition of energy metabolism in hypoxic goldfish (17). The lipid composition of membranes affects the activity of integral proteins and could therefore play a role in mediating metabolic suppression. For example, changes in cholesterol (a modulator of Na+-K+-ATPase) and percent docosahexaenoic acid (22:6 or DHA; an activator of Na+-K+-ATPase) could be involved in reducing metabolic rate (2, 17, 24). We have recently shown that chronic hypoxia causes extensive changes in the membrane lipid composition of goldfish (17): a hypoxia-tolerant vertebrate that utilizes significant metabolic rate depression to tolerate severe hypoxia and anoxia (46). However, the plasticity of membrane lipids in response to hypoxia has never been investigated in mammals.
Naked mole-rats (NMRs; Heterocephalus glaber, Linnaeus 1758), are fossorial and hypoxia-tolerant mammals that live in poorly ventilated underground burrows where temperatures are high (25–49°C) and oxygen levels are putatively hypoxic (27, 44). In laboratory conditions, acute exposure to a few hours of 3% O2 causes an 85% decrease in metabolic rate (43): the strongest suppression of metabolism among hypoxia-tolerant mole-rat species (31). However, it is unclear how NMRs cope with more chronic hypoxia.
Whereas the impact of hypoxia on membrane lipid composition is largely unexplored in mammals, the effects of hypoxia acclimation on the activities of key enzymes of energy metabolism have received more attention. For example, in rats, mice, and high-altitude deer mice, hypoxia generally stimulates glycolysis (13, 15, 37, 45) and, apart from deer mice, downregulates β-oxidation (10, 15, 19). Most investigations of TCA cycle enzymes suggest that their activity is not modified by low oxygen (6, 9, 13, 19), although inhibition was observed in one study (51). Several reports also show that Na+-K+-ATPase activity is decreased in the lungs of hypoxic rats (8, 36, 54). However, none of these enzymes have been explored in the hypoxia-tolerant NMR. Therefore, the goals of this study were to test the hypotheses that during chronic hypoxia, NMRs: 1) downregulate key enzymes of TCA cycle, glycolysis, and β-oxidation; 2) inhibit Na+-K+-ATPase; and 3) alter the composition of membrane lipids concomitantly with metabolic suppression.
METHODS
Animals
Adult NMRs (n = 37; body mass: 45 ± 3.1 g; 2–5 yr old) were group-housed in interconnected multicage systems (30°C; 70% humidity; 12:12-h light-dark cycle) and were fed fresh tubers, vegetables, fruit, and Pronutro cereal supplement ad libitum. All experimental procedures were approved by the University of Ottawa’s Animal Care Committee (Protocol No. 2535) in accordance with the Animals for Research Act and the Canadian Council on Animal Care.
Experimental Design
Subordinate animals were randomly allocated to respirometry experiments or enzyme/membrane experiments. For each set of experiments, the animals were randomly divided into normoxic controls and a hypoxic treatment group. Both groups were placed in separate chambers with controlled, continuous air flow either normoxic (21% O2; 0.04% CO2; balance N2) or hypoxic (11% O2; 0.04% CO2; balance N2) and were kept under these conditions for 4–6 wk. This duration was selected to provide enough time for potential membrane restructuring, given that homeoviscous adjustments to changes in environmental temperature can take 3 wk in ectotherms (50).
Whole Body Respirometry
After normoxic or hypoxic acclimation, animals were individually placed unrestrained into a 450-mL plexiglass respirometer, which was held inside a larger environmental chamber held at 30°C. Animals were provided a thin layer of corn cob bedding. The respirometer was continuously ventilated with gas mixtures set to the desired fractional gas composition by calibrated rotameters (Krohne, Duisburg, Germany). Inflowing gas was set at a flow rate of 100 mL/min, determined using a calibrated mass flow meter (Alicat Scientific, Tuscon, AZ). The excurrent gas was passed through a desiccant (Drierite, W.A. Hammond Drierite, Xenia, OH) before entering the cells of the CO2 and O2 analyzers (FC-10 O2 and CA-10 CO2 Analyzers, Sable Systems), which were used to determine the gas concentrations of inspired and expired air. Before each trial the CO2 and O2 analyzers were calibrated using 100% N2, compressed air (20.95% O2), and a span gas (1.5% CO2; balance N2). The animals were placed in the respirometer for 1 h before measurements to familiarize them with their new surroundings. Oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were then recorded for the next hour. V̇o2 and V̇co2 measured during the last 30 min (three 10-min intervals) of the recording period were averaged to determine baseline values for each animal. For 5 min at the end of the recording period, incurrent gas concentrations were measured by bypassing the experimental chamber and diverting air flow directly to the CO2 and O2 analyzers. Body temperature was recorded noninvasively every 10 min using an RFID microchip reader (Allflex Inc., Dallas, TX) to scan previously implanted RFID microchips (Destron Fearing, Langeskov, Denmark). Normoxic controls (33.1 ± 0.13°C) and hypoxic animals (33 ± 0.12°C) had the same body temperature. Chamber temperature was recorded every 2 s using a custom-designed thermocouple (range 29.8°C-30.2°C).
Enzyme Assays
At the end of the experiments, NMRs were quickly euthanized by cervical dislocation. Because different tissues do not always respond similarly to physiological stresses, the brain, heart, liver, and skeletal (temporalis) muscles and kidney were sampled in <2 min, immediately frozen in liquid N2, and stored at −80°C until analysis. All enzyme activities were measured using a Spectra Max Plus384 Absorbance Microplate Reader (Molecular Devices, Sunnyvale, CA). To measure the activities of key enzymes involved in 1) glycolysis [pyruvate kinase (PK) and lactate dehydrogenase (LDH)], 2) the tricarboxylic acid cycle [citrate synthase (CS)], and 3) β-oxidation [carnitine palmitoyl transferase (CPT) and 3-hydroxyacyl CoA dehydrogenase (HOAD)], 50 mg of each frozen tissue were weighed and homogenized on ice in 19 vol of extraction medium [25 mM Tris·HCl + 1 mM EDTA as well as 5 mM dithiothreitol (DTT) and 0.05% (vol/vol) Triton X-100, which were added on the day of the experiment to complete the enzyme extraction]. Homogenates were then centrifuged at 4°C at 2,400 g for 5 min, and the resulting supernatant was stored at −80°C until analysis. All assay conditions were first optimized to give maximal rates with the skeletal muscle and thus may not yield the maximal rate in all tissues. All homogenates were subjected to a freeze/thaw cycle. Preliminary experiments were carried out to ensure that all substrate and cofactor concentrations were saturating but not inhibitory. Control reactions (containing no substrate) were run simultaneously for each enzyme to measure background activity if present. All assays were run in triplicate at 32°C.
Assay conditions were as follows: PK: [A340; pH 7.35 (56)]: 0.17 mM NADH, 5 mM ADP, 80 mM KCl, 10 mM MgCl2, and 5 mM phospho(enol)pyruvate (PEP) (omitted from the control), excess coupling enzyme (LDH) in 160 mM triethanolamine/HCl; LDH: [A340; pH 7.3 (57)]: 0.17 mM NADH, 1 mM KCN, and 2 mM pyruvate (omitted from the control) in 50 mM Tris·HCl. CS: [A412; pH 8.1 (1)]: 0.2 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 0.1 mM acetyl CoA, and 0.5 mM oxaloacetate (omitted from the control) in 50 mM Tris·HCl; CPT: [A412; pH 8 (23)]: 0.15 mM DTNB, 0.035 mM palmitoyl CoA, and carnitine (omitted from the control) in 50 mM Tris; and HOAD: [A340; pH 7.4 (23)]: 0.2 mM NADH and 0.1 mM acetoacetyl CoA (omitted from the control) in 50 mM imidazole + 1 mM EDTA. Our measurements of CPT activity most likely reflect the behavior of CPT2 because CPT1 is inactivated by freezing.
We measured Na+-K+-ATPase activity (A340) using a modified protocol from Ref. 40. Frozen tissue was weighed (~100 mg) and homogenized on ice with a sonicator (Fisher Scientific Sonic Dismembrator model 100, San Diego, CA) in a 4:1 SEI:SEID buffer (SEI: 250 mM sucrose, 10 mM EDTA and 42 mM imidazole, pH 7.3; SEID: 100 mL SEI + 0.5 g sodium deoxycholate). Homogenates were then centrifuged at 10,000 g for 5 min at 4°C and the resulting supernatant was directly used in the assay. The assay was performed as previously described (40) in quadruplicate [2 replicates contained 10 µL of homogenate + 200 µL of assay solution A (50 mM imidazole, 2.8 mM PEP, 0.7 mM ATP, 0.22 mM NADH, 5 mM PK, and 4 mM LDH, pH 7.5) and 2 replicates contained 10 µL of homogenate + 200 µL of assay solution B (solution A + 0.5 mM ouabain]. Ouabain was added to block Na+-K+-ATPase and measure any detectable ATP use not associated with this enzyme. All enzyme measurements performed in this study provide information on capacity for flux in different key pathways. Because enzyme Vmax was measured under optimal in vitro conditions, the observed changes may not necessarily reflect the effects of chronic hypoxia on in vivo fluxes.
Membrane Lipid Analyses
Total lipids were extracted from ~30 mg of each frozen tissue sample as described previously (35). Briefly, tissues were homogenized with a Polytron (Kinematica, Luzern, Switzerland) and total lipids were extracted twice in chloroform-methanol (2:1 vol/vol). After filtration, 0.25% KCl was added and the mixture centrifuged to separate aqueous and organic phases. The aqueous phase was discarded and the organic phase containing the lipids was dried on a rotating evaporator (Büchi Rotavapor, Flawil, Switzerland). After extraction and drying, total lipids were resuspended in chloroform before being loaded on solid-phase extraction columns (Supelclean 3 mL 500 mg LC-NH2; Sigma-Aldrich; St. Louis, MO). Neutral lipids, nonesterified fatty acids (FAs) and phospholipids (PLs) were separated by sequential elution using solvents of increasing polarity: chloroform:isopropanol (3:2 vol/vol), isopropyl ether:acetic acid (98:2 vol/vol), and methanol (35). Total PL concentration was determined by gas chromatography as a measure of tissue membrane abundance by adding a PL internal standard before solid-phase column separation (40 mg/100 mL phosphatidyl choline 17:0/17:0; Avanti Polar Lipids; Alabaster, AL). The PL fraction was then used for analysis of its FA composition, which was measured after acid transesterification in acetyl chloride and methanol (90°C for 2 h). FA methyl esters were analyzed on an Agilent Technologies 6890N gas chromatograph (Mississauga, Ontario, Canada) equipped with a flame-ionization detector and a fused silica capillary column (Supelco DB-23, 60 m, 0.25-mm inner diameter, 0.25-μm film thickness; Sigma-Aldrich), using hydrogen as carrier gas. The following conditions were used during analysis: 1) oven temperature was programmed for 1 min at 130°C and raised up to 170°C at a rate of 6.5°C/min, then up to 215°C at 2.75°C/min, and maintained at 215°C for 12 min, then up to 230°C at 40°C/min, and maintained at 230°C for 3 min; 2) injector temperature was 270°C using a 50:1 split ratio; and 3) detector temperature was 280°C. Individual FAs were identified by determining exact retention times with pure standards (Supelco, Bellefonte, PA). Only the FAs accounting for >1% of total FAs in total PLs are reported.
Membrane cholesterol was measured as nonesterified (free) cholesterol in ~30 mg of tissue. Each tissue was homogenized in chloroform:methanol (2:1 vol/vol). Separation of aqueous and organic phases was achieved by adding 2 M KCl/5 mM EDTA before centrifugation (10 min at 3,000 g). The organic phase was dried under N2, resuspended in 2-methoxyethanol, and cholesterol was measured by fluorometry (SpectraMax Gemini XS, Molecular Devices, Sunnyvale, CA) using a commercial assay kit (Cayman Chemical, Ann Arbor, MI). This kit was selected because it allows the measurement of membrane (free, nonesterified) cholesterol separately from cholesterol esters that are only found outside membranes.
Calculations and Statistics
Respirometry data were collected using LabChart software and analyzed in PowerLab (AD Instruments, Colorado Springs, CO). With the use of these measurements, V̇o2 was then calculated using Eq. 10.6 in Ref. 34: V̇o2 = FRi[( – ) – ( – )]/(1 – ). Also, V̇o2 was calculated using Eq. 10.7 in Ref. 34: V̇co2 = FRi[( – ) – ( – )]/(1 – ). In both equations FRi is the incurrent flow rate (mL/min), and are the fractional concentrations of incurrent O2 and CO2 of dry gas, and and are the fractional concentrations of excurrent O2 and CO2 from the experimental chamber. Total PL concentration was calculated as total number of moles of fatty acids in the PL fraction divided by 2 as follows: [PL] = {Σ[(area under the curve of individual FA) × (PL 17:0/17:0 internal standard concentration)]/(individual FA molar weight)}/2. The double bond index (DBI) of membranes was calculated as the average number of double bonds in PLs divided by percent saturated fatty acids. Absolute concentration of membrane cholesterol in a tissue (expressed in μmol/g) is not indicative of the relative amount of cholesterol in membranes if treatment causes changes in membrane abundance. To address this problem, relative cholesterol concentration was calculated as moles of cholesterol per mole of PL and expressed as a unitless ratio (55). Statistical analyses were performed using SigmaPlot 12.5 (Systat, San Jose, CA). Normoxic and hypoxic animals were compared using a two-tailed t test. Normality was assessed using the Shapiro-Wilk test and homoscedasticity by the Levene test. When the assumptions of normality or equality of variances were not met, the data were normalized by log10 or square root transformation. If transformation was unsuccessful, nonparametric Mann-Whitney U test was performed. Values presented are means ± SE, and a level of significance of P < 0.05 was used in all tests.
RESULTS
Metabolic Rate
The effects of chronic hypoxia acclimation on NMR metabolic rate are presented in Fig. 1. Chronic hypoxia decreased V̇o2 by 34% and V̇co2 by 33% (P < 0.01).
Fig. 1.
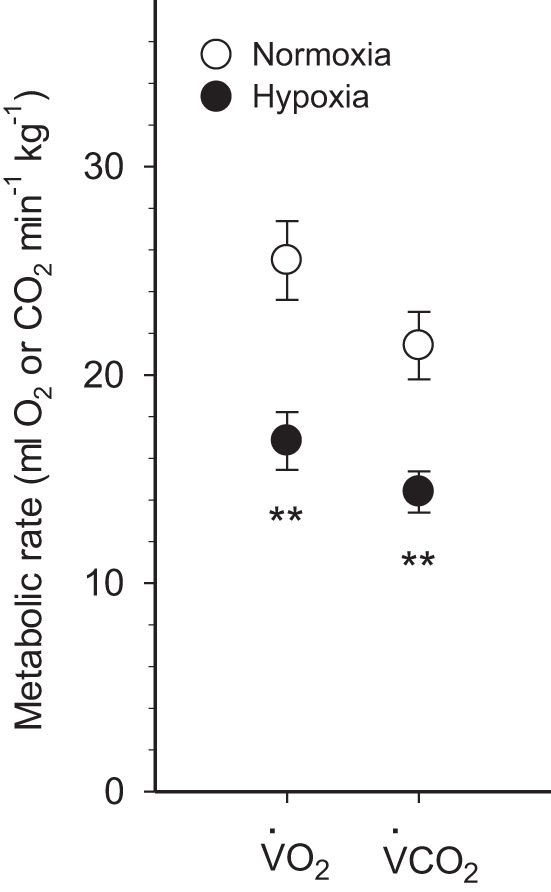
Metabolic rates of normoxic controls and hypoxia-acclimated naked mole-rats. Oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) are presented. Values are means ± SE (n = 9 mole-rats in normoxia and n = 7 mole-rats in hypoxia). **P < 0.01, significant effects of hypoxia.
Enzyme activities
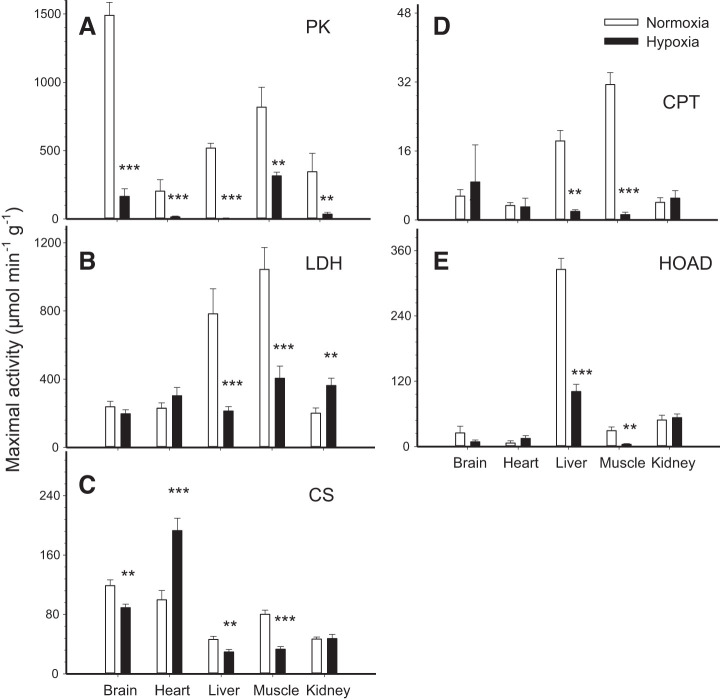
Glycolysis.
Observed changes in PK and LDH activities suggest that glycolytic capacity was reduced by hypoxia (Fig. 2, A and B). PK activity was strongly downregulated by chronic hypoxia and this response was observed in all tissues (Fig. 2A; P < 0.01). LDH activity decreased in liver and muscle (P < 0.001), increased in kidney (P < 0.01), and was unchanged in brain and heart (P > 0.05) (Fig. 2B). Overall, enzyme activities calculated per gram tissue or per gram protein were affected very similarly and led to the same conclusions apart from LDH in brain and kidney (see Table 1).
Fig. 2.
Maximal enzymatic activity per gram tissue of pyruvate kinase (PK; A), lactate dehydrogenase (LDH; B), citrate synthase (CS; C), carnitine palmitoyl transferase (CPT; D), and 3-hydroxyacyl CoA dehydrogenase (HOAD; E) in the tissues of normoxic controls and hypoxia-acclimated naked mole-rats. Values are means ± SE (n = 12 mole-rats in normoxia and n = 9 mole-rats in hypoxia). **P < 0.01 and ***P < 0.001, significant effects of hypoxia.
Table 1.
Effects of chronic hypoxia on the activities of key enzymes of energy metabolism in various tissues of naked mole-rats
| PK |
LDH |
CS |
CPT |
HOAD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| /gtissue | /µgprotein | /gtissue | /µgprotein | /gtissue | /µgprotein | /gtissue | /µgprotein | /gtissue | /µgprotein | |
| Brain | −89%*** | −97%*** | NS | −73%*** | −25%** | −76%*** | −94%* | −98%* | NS | NS |
| Heart | −93%*** | −90%*** | NS | NS | +94%*** | +115%*** | NS | NS | NS | NS |
| Liver | −99%*** | −99%*** | −73%*** | −82%*** | −36%** | −57%** | −89%** | −98%** | −69%*** | −80%*** |
| Muscle | −61%** | −79%** | −62%*** | −80%*** | −59%*** | −78%*** | −96%*** | −98%** | −86%** | −93%*** |
| Kidney | −90%** | −96%** | +81%** | NS | NS | −56%*** | NS | NS | NS | NS |
Effects of chronic hypoxia on the activities of key enzymes of energy metabolism (standardized either per gram tissue or per gram protein) in various tissues of naked mole-rats (n = 12 mole-rats in normoxia and n = 9 mole-rats in hypoxia) are shown. No effect of hypoxia is indicated by NS (P > 0.05). Significant effects of hypoxia are indicated as
P < 0.05,
P < 0.01, and
P < 0.001, presented as percent differences between treatments.
TCA cycle.
CS activity was decreased in brain (P < 0.01), liver (P < 0.01), and muscle (P < 0.001). It was almost doubled in heart (P < 0.001) but remained unchanged in kidney (P > 0.05) (Fig. 2C). Results were relatively similar when correcting per gram protein except for a decrease in kidney CS activity per gram protein (Table 1).
β-Oxidation.
β-Oxidation capacity was reduced in liver and muscle where the activities of CPT (Fig. 2D) and HOAD (Fig. 2E) were downregulated by hypoxia (P < 0.01 or P < 0.001). These same enzymes were not affected in heart and kidney (P > 0.05). In the brain, CPT was downregulated (P < 0.05) but HOAD remained at normoxic levels (P > 0.05). Standardizations per gram tissue and per gram protein showed the same effects of hypoxia on CPT and HOAD (Table 1).
Na+-K+-ATPase.
Hypoxia downregulated Na+-K+-ATPase in the brain but upregulated it in liver (P < 0.05) without affecting muscle and heart (P > 0.05) (Fig. 3).
Fig. 3.
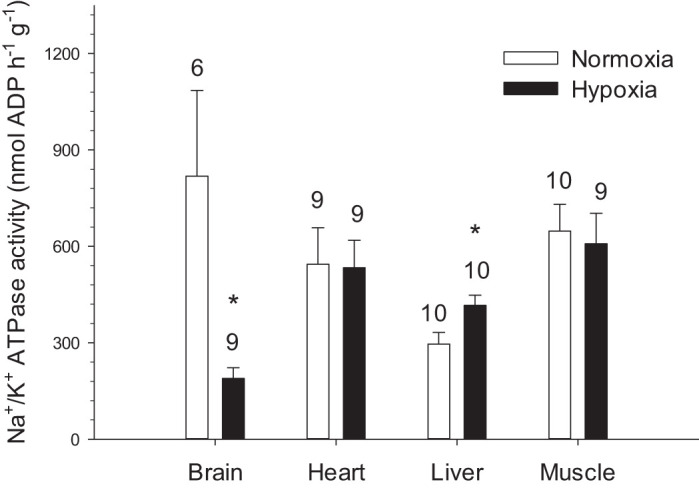
Na+-K+-ATPase activity per gram tissue in the tissues of normoxic controls and hypoxia-acclimated naked mole-rats. Values are means ± SE. Sample sizes are indicated on the figure. *P < 0.05, significant effects of hypoxia.
Membrane Lipids
Hypoxia caused large changes in cholesterol abundance in all tissues. Relative cholesterol increased in heart, muscle (P < 0.05), and kidney (P < 0.01), and it decreased in brain (P < 0.05) and liver (P < 0.001) (Fig. 4). The effects of hypoxia acclimation on the total PL content per gram tissue (an index of membrane abundance in [PL]/g), the DBI, and the absolute concentration of saturated (SFA), monounsaturated (MUFA), and polyunsaturated fatty acids (PUFA) of NMR membranes are shown in Table 2. Hypoxia elicited a decrease in DBI in liver but an increase in muscle without affecting the other tissues. There was an increase in [PL] in brain and liver, but a decrease in muscle. Chronic hypoxia had no effect on the PL concentration of heart and kidney. Percent SFA increased in liver, decreased in muscle, but did not change in the other tissues. Percent MUFA did not change in all tissues except liver where it decreased. %PUFA decreased in liver and kidney, increased in muscle but did not change in the other tissues (Table 2). Surprisingly, NMR membranes of all tissues only contained trace amounts of 22:6 in both normoxic and hypoxic animals (Supplemental Figs. S1–S5; see https://doi.org/10.6084/m9.figshare.11988768).
Fig. 4.
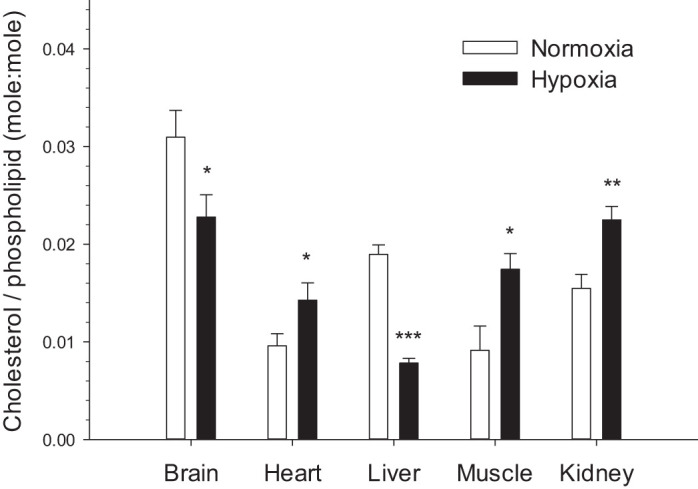
Relative membrane cholesterol levels in the tissues of normoxic controls and hypoxia-acclimated naked mole-rats. Values are means ± SE (n = 12 mole-rats in normoxia and n = 9 mole-rats in hypoxia). *P < 0.05, **P < 0.01, and ***P < 0.001, significant effects of hypoxia.
Table 2.
Relative effects of chronic hypoxia on the membrane phospholipids of naked mole-rat tissues
| DBI | PL/g | SFA | MUFA | PUFA | |
|---|---|---|---|---|---|
| Brain | NS | +18%** | NS | NS | NS |
| Heart | NS | NS | NS | NS | NS |
| Liver | −23%* | +50%** | +24%* | −43%*** | −12%* |
| Muscle | +22%* | −28%*** | −27%* | NS | +11%* |
| Kidney | NS | NS | NS | NS | −5%* |
Relative effects of chronic hypoxia on the membrane phospholipids of naked mole-rat tissues (n = 12 mole-rats in normoxia and n = 9 mole-rats in hypoxia). Double bond index (DBI), phospholipid/gtissue (PL/g), saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) are indicated separately. Absolute concentrations of PL and FAs in moles per gram tissue (Supplemental Table 1; see https://doi.org/10.6084/m9.figshare.11988768.v2) were used to calculate the percent changes presented. No effect of hypoxia is indicated by NS (P > 0.05). Significant effects of hypoxia are indicated as
P < 0.05,
P < 0.01, and
P < 0.001.
DISCUSSION
Several weeks of hypoxia cause a 34% decrease in the metabolic rate of NMRs (Fig. 1). We show that this suppression occurs simultaneously with a major decrease in the capacity for energy metabolism, the downregulation of brain Na+-K+-ATPase, and widespread changes in membrane lipids. Chronic hypoxia decreases the activities of key enzymes in glycolysis, the TCA cycle, and the β-oxidation pathway but also induces important changes in the relative abundance of membrane cholesterol in all tissues. Together, these changes in protein activities and membrane composition may reflect a coordinated physiological response to hypoxia, although a clear functional link between membrane changes and enzyme downregulation could not be established in this study. Nevertheless, this is the first demonstration that hypometabolic NMRs alter the lipid composition of their membranes in response to chronic in vivo exposure to hypoxia.
Metabolic Suppression in Hypoxia
The degree of metabolic rate suppression observed after 4 wk at 11% O2 is consistent with the only previous report of NMR metabolic rate measured during chronic hypoxia (25–33% reduction after 10 days at 8% O2) (11). More information is available for acute exposure of a few hours only. NMRs rapidly exposed to progressive hypoxia from 9 to 3% O2 experience a stepwise decrease in metabolic rate, with the strongest suppression occurring at the lowest oxygen level (55% decrease in Ṁo2 at 9% O2 but a more than 80% decline at 3% O2) (42). In addition to tolerating extremely low oxygen, NMRs have the capacity to survive in complete anoxia for up to 18 min (44). However, the goal of our study was not to examine the effects of anoxia (when reliance on anaerobic glycolysis becomes essential for survival) but to characterize those of chronic hypoxia on aerobic metabolism.
Downregulation of Glycolysis
Chronic hypoxia causes large tissue-specific changes in the activities of the glycolytic enzymes PK and LDH (Table 1 and Fig. 2, A and B). The general downregulation of PK suggests that glycolytic capacity is reduced, but this enzyme shares flux control with phosphofructokinase and hexokinase, which were not measured here. If PK behavior is indicative of overall changes in pathway capacity, NMRs respond very differently than hypoxic rats (13, 15, 37, 45), mice (6), and deer mice (9, 33), who either activate or do not modulate glycolysis in hypoxia. Conversely, NMRs respond by downregulating the glycolytic supply of pyruvate to the TCA cycle. In NMRs, the strong downregulation of PK observed in all tissues (61–99% decrease in activity; Fig. 2A) would concomitantly slow the aerobic production of ATP. The 62–73% downregulation of LDH observed in liver and muscle also shows that NMRs do not rely on anaerobic metabolism at this level of hypoxia (Fig. 2B). This is perhaps not surprising because the anaerobic use of total carbohydrate reserves could only last minutes to hours, not several weeks. A recent study reported that NMRs store more cardiac glycogen than mice (18), and this observation was interpreted as an indication that cardiac glycolysis is activated in hypoxia. Our results do not support this idea because heart PK activity is strongly decreased (Fig. 2A), suggesting that glycolysis is downregulated rather than upregulated, at least in chronic hypoxia. Hypoxic NMRs can afford to slow down glycolysis because they rely on the suppression of aerobic metabolism that likely spares small carbohydrate stores and minimizes the accumulation of anaerobic end products.
Effects of Chronic Hypoxia on Citrate Synthase
In concert with the downregulation of glycolysis, the activity of CS is decreased in NMR brain, liver, and muscle (25–59%) (Fig. 2C). These responses were not observed in chronically hypoxic rats (13, 19), mice (6), or deer mice (9, 33), which instead maintain normoxic CS activity. Sustaining aerobic ATP supply from the TCA cycle becomes problematic when oxygen is scarce, and NMRs can afford to downregulate this pathway because ATP demand is lowered by metabolic suppression. Tissue CS activity can be modulated by changing mitochondrial density to adjust enzyme abundance (14). This is supported here because acute hypoxia has no effect on CS activity in NMR brains (43), whereas chronic hypoxia downregulates the enzyme (this study; Fig. 2C), and a few hours of acute hypoxia does not provide sufficient time to alter mitochondrial density.
In contrast to other tissues, activity is upregulated by chronic hypoxia in NMR heart (94%; Fig. 2C), indicating that the TCA cycle is stimulated in this organ. The utility of this response is not intuitively obvious; however, similar responses have been observed in the hearts of other animals such as high-altitude Andean mice (48) and sablefish (21). It is possible that activating the TCA cycle in this key organ is necessary because cardiac output must be increased to compensate for the decreased arterial O2 saturation caused by hypoxia. However, such a scenario is not consistent with lower heart rate during acute hypoxia (42), although it is unclear whether more chronic hypoxia could have the opposite effect on heart rate. Taken together, CS upregulation and PK downregulation suggest that the chronically hypoxic NMR heart switches to using more lipids and less carbohydrates, thus increasing its reliance on acetyl CoA from β-oxidation rather than glycolysis. Such a change in fuel selection precludes NMR hearts from taking advantage of the 10–25% higher ATP yield per mole O2 provided by carbohydrates over lipids (25, 29, 39), and it has also been reported for various cardiac pathologies (32). Here, this response is consistent with the idea that the higher cardiac glycogen reserves of NMR (18) are used for acute and severe hypoxia, rather than for coping with chronic low oxygen stress. These observations suggest that metabolically suppressed NMRs can survive at 11% O2 without harnessing all the oxygen-saving mechanisms available to them.
Tissue-Specific Downregulation of β-Oxidation
Chronic hypoxia causes the downregulation of the β-oxidation enzymes CPT (Fig. 2D) and HOAD (Fig. 2E) in most tissues except the heart and kidney. These findings agree with previous studies in rats (13, 15, 19) and mice (41) but not high-altitude deer mice in which either increased (9, 10) or sustained HOAD activity has been reported (33). Reducing flux through β-oxidation logically follows the downregulation of 1) the other main pathways of energy metabolism (glycolysis and TCA cycle), and 2) multiple ATP-utilizing processes (overall metabolic suppression) observed in most NMR tissues. The absence of a change in the β-oxidation pathway in kidney and heart may be related to the potentially higher energy demands of these critical organs. However, the same could be said about the brain, which exhibits a 94% decrease in CPT activity. More research will be needed to examine the underlying physiological reasons why some tissues maintain β-oxidation and others do not.
Na+-K+-ATPase Activity Is Downregulated in NMR Brains
Chronic hypoxia reduces Na+-K+-ATPase activity in NMR brain (77%) but not in muscle, liver, or heart (Fig. 3). This localized response may be a conserved adaptation shared by hypoxia-tolerant organisms because similar changes also occur in anoxic turtle brain (30) but not in chronically hypoxic mice (6). The brain is highly metabolically active [accounting for ~20% of whole body metabolic rate in NMRs (22)] and uses ~60% of its total ATP supply for pumping ions to ensure normal electric activity. The brain relies on Na+-K+-ATPase to maintain Na+ and K+ gradients and, indirectly, to regulate the transport of Ca2+ and neurotransmitters (16). Any failure of this pump in hypoxia-sensitive neurons leads to a spike in intracellular calcium concentration that can eventually cause cell death (26). The robust reduction of Na+-K+-ATPase activity observed here in NMR brains must occur together with a decrease in ion channel leak, so that ATP supply and demand can remain in balance (3, 5). However, the potential inhibition of ion channels in the hypoxic NMR brain has not been explored.
Changes in Membrane Composition Caused by Chronic Hypoxia
This study is the first to demonstrate that in vivo exposure to chronic hypoxia can alter the composition of membrane lipids in mammals. It is unclear whether such changes only occur in hypoxia-tolerant species or if it is a general mammalian response. In NMRs, chronic hypoxia caused widespread changes in the membrane cholesterol abundance of all tissues. This result is intriguing because studies on artificial membranes (20, 55) and on manipulated fish membranes (12) show that changes in intrinsic, baseline cholesterol generally downregulate Na+-K+-ATPase: possibly contributing to metabolic suppression. NMR brains may use this mechanism because a large decrease in membrane cholesterol (Fig. 4) occurs together with the strong downregulation of Na+-K+-ATPase (Fig. 3). However, results from other NMR tissues do not support this idea because membrane cholesterol is modified without downregulating Na+-K+-ATPase activity.
In contrast to previous findings on goldfish (17), chronic hypoxia does not cause major changes in the fatty acid composition of NMR membranes (Table 2). Because 22:6 is a known activator of ion pumps (7, 53), decreasing its relative abundance could be used to suppress metabolism (17). However, our study shows that NMRs cannot rely on this mechanism because they have no room to reduce membrane 22:6 from an intrinsically low level of < 2% in nonhypometabolic animals (Supplemental Figs. S1–S5; see https://doi.org/10.6084/m9.figshare.11988768.v2). Interestingly, 22:6 is much more abundant in mouse PLs (11–26%), and the very low levels of this peroxidation-prone polyunsaturated fatty acid found in NMRs may explain their longer lifespan and lower metabolic rate (28).
Perspectives and Significance
This study shows that the downregulation of energy metabolism and brain Na+-K+-ATPase, as well as the widespread restructuring of membranes are coordinated physiological responses that accompany metabolic suppression in NMRs. Instead of activating anaerobic metabolism, chronic hypoxia downregulates the aerobic supply of acetyl-CoA from glycolysis and β-oxidation to the TCA cycle in brain, muscle, and liver. By contrast, the NMR heart maintains aerobic metabolism, possibly to keep adequate oxygen supply to the other organs. These tissue-specific responses suggest that local metabolic requirements vary greatly. Therefore, characterizing the effects of chronic hypoxia on the metabolic capacity and fuel preference of isolated mitochondria from different tissues may be a productive avenue for future research. Hypoxia-induced changes in membrane lipids occur in NMRs (Fig. 4) and goldfish (17), but the physiological significance of this response is still unclear. Do the observed changes in NMR membrane cholesterol play a role in promoting metabolic suppression? A common membrane signal regulating the joint inhibition of ion pumps and ion channels could be an exquisite way to preserve the balance between ATP supply and demand in the hypometabolic state, and it could serve as a neuroprotective mechanism in NMR brain. To determine whether membrane restructuring and metabolic suppression are physiologically linked, it may be useful to mimic the membrane changes observed in vivo on artificial membranes to characterize how ion pumps and channels are affected.
GRANTS
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants 05955-2017 (to J. M. Weber) and 2015-04229 (to M. Pamenter), a Canada Research Chairs Program grant (to M. E. Pamenter), and an Ontario Early Research Award (to M. E. Pamenter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.F., M.E.P., and J.-M.W. conceived and designed research; E.F. and M.E.D. performed experiments; E.F. and M.E.D. analyzed data; E.F., M.E.P., and J.-M.W. interpreted results of experiments; E.F. and M.E.D. prepared figures; E.F. drafted manuscript; E.F., M.E.P., and J.-M.W. edited and revised manuscript; E.F., M.E.P., and J.-M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Tama Davis for expert care of the animals. We thank Ariane Rondot for help with the enzyme assays as well as Daniel Munro, Hang Cheng, and Liam Eaton for help with handling the animals.
REFERENCES
- 1.Alp PR, Newsholme EA, Zammit VA. Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem J 154: 689–700, 1976. doi: 10.1042/bj1540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastiaanse EM, Höld KM, Van der Laarse A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc Res 33: 272–283, 1997. doi: 10.1016/S0008-6363(96)00193-9. [DOI] [PubMed] [Google Scholar]
- 3.Bickler PE, Buck LT. Adaptations of vertebrate neurons to hypoxia and anoxia: maintaining critical Ca2+ concentrations. J Exp Biol 201: 1141–1152, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu Rev Physiol 69: 145–170, 2007. doi: 10.1146/annurev.physiol.69.031905.162529. [DOI] [PubMed] [Google Scholar]
- 5.Boutilier RG, St-Pierre J. Surviving hypoxia without really dying. Comp Biochem Physiol A Mol Integr Physiol 126: 481–490, 2000. doi: 10.1016/S1095-6433(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 6.Cáceda R, Gamboa JL, Boero JA, Monge-C C, Arregui A. Energetic metabolism in mouse cerebral cortex during chronic hypoxia. Neurosci Lett 301: 171–174, 2001. doi: 10.1016/S0304-3940(01)01630-5. [DOI] [PubMed] [Google Scholar]
- 7.Calhoon EA, Ro J, Williams JB. Perspectives on the membrane fatty acid unsaturation/pacemaker hypotheses of metabolism and aging. Chem Phys Lipids 191: 48–60, 2015. doi: 10.1016/j.chemphyslip.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter TC, Schomberg S, Nichols C, Stenmark KR, Weil JV. Hypoxia reversibly inhibits epithelial sodium transport but does not inhibit lung ENaC or Na-K-ATPase expression. Am J Physiol Lung Cell Mol Physiol 284: L77–L83, 2003. doi: 10.1152/ajplung.00181.2002. [DOI] [PubMed] [Google Scholar]
- 9.Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc Natl Acad Sci USA 109: 8635–8640, 2012. doi: 10.1073/pnas.1120523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheviron ZA, Connaty AD, McClelland GB, Storz JF. Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68: 48–62, 2014. doi: 10.1111/evo.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung D, Dzal YA, Seow A, Milsom WK, Pamenter ME. Naked mole rats exhibit metabolic but not ventilatory plasticity following chronic sustained hypoxia. Proc Biol Sci 283: 20160216, 2016. doi: 10.1098/rspb.2016.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crockett EL, Hazel JR. Cholesterol affects physical properties and (Na+,K+)-ATPase in basolateral membranes of renal and intestinal epithelia from thermally acclimated rainbow trout. J Comp Physiol B 167: 344–351, 1997. doi: 10.1007/s003600050083. [DOI] [Google Scholar]
- 13.Daneshrad Z, Garcia-Riera MP, Verdys M, Rossi A. Differential responses to chronic hypoxia and dietary restriction of aerobic capacity and enzyme levels in the rat myocardium. Mol Cell Biochem 210: 159–166, 2000. doi: 10.1023/A:1007137909171. [DOI] [PubMed] [Google Scholar]
- 14.DiMauro S, Moraes CT. Mitochondrial encephalomyopathies. Arch Neurol 50: 1197–1208, 1993. doi: 10.1001/archneur.1993.00540110075008. [DOI] [PubMed] [Google Scholar]
- 15.Dutta A, Vats P, Singh VK, Sharma YK, Singh SN, Singh SB. Impairment of mitochondrial β-oxidation in rats under cold-hypoxic environment. Int J Biometeorol 53: 397–407, 2009. doi: 10.1007/s00484-009-0224-5. [DOI] [PubMed] [Google Scholar]
- 16.Erecińska M, Silver IA. Ions and energy in mammalian brain. Prog Neurobiol 43: 37–71, 1994. doi: 10.1016/0301-0082(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 17.Farhat E, Turenne ED, Choi K, Weber JM. Hypoxia-induced remodelling of goldfish membranes. Comp Biochem Physiol B Biochem Mol Biol 237: 110326, 2019. doi: 10.1016/j.cbpb.2019.110326. [DOI] [PubMed] [Google Scholar]
- 18.Faulkes CG, Eykyn TR, Aksentijevic D. Cardiac metabolomic profile of the naked mole-rat-glycogen to the rescue. Biol Lett 15: 20190710, 2019. doi: 10.1098/rsbl.2019.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galbès O, Goret L, Caillaud C, Mercier J, Obert P, Candau R, Py G. Combined effects of hypoxia and endurance training on lipid metabolism in rat skeletal muscle. Acta Physiol (Oxf) 193: 163–173, 2008. doi: 10.1111/j.1748-1716.2007.01794.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia A, Lev B, Hossain KR, Gorman A, Diaz D, Pham TH, Cornelius F, Allen TW, Clarke RJ. Cholesterol depletion inhibits Na+,K+-ATPase activity in a near-native membrane environment. J Biol Chem 294: 5956–5969, 2019. doi: 10.1074/jbc.RA118.006223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerber L, Clow KA, Katan T, Emam M, Leeuwis RH, Parrish CC, Gamperl AK. Cardiac mitochondrial function, nitric oxide sensitivity and lipid composition following hypoxia acclimation in sablefish. J Exp Biol 222: jeb208074, 2019. doi: 10.1242/jeb.208074. [DOI] [PubMed] [Google Scholar]
- 22.Gesser H, Johansen K, Maloiy GM. Tissue metabolism and enzyme activities in the rodent Heterocephalus glaber, a poor temperature regulator. Comp Biochem Physiol B 57: 293–296, 1977. doi: 10.1016/0305-0491(77)90056-6. [DOI] [PubMed] [Google Scholar]
- 23.Guglielmo CG, Haunerland NH, Hochachka PW, Williams TD. Seasonal dynamics of flight muscle fatty acid binding protein and catabolic enzymes in a migratory shorebird. Am J Physiol Regul Integr Comp Physiol 282: R1405–R1413, 2002. doi: 10.1152/ajpregu.00267.2001. [DOI] [PubMed] [Google Scholar]
- 24.Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol 19: 281–296, 2018. doi: 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- 25.Hochachka PW, Stanley C, Matheson GO, McKenzie DC, Allen PS, Parkhouse WS. Metabolic and work efficiencies during exercise in Andean natives. J Appl Physiol (1985) 70: 1720–1730, 1991. doi: 10.1152/jappl.1991.70.4.1720. [DOI] [PubMed] [Google Scholar]
- 26.Hochachka PW. Defense strategies against hypoxia and hypothermia. Science 231: 234–241, 1986. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- 27.Holtze S, Braude S, Lemma A, Koch R, Morhart M, Szafranski K, Platzer M, Alemayehu F, Goeritz F, Hildebrandt TB. The microenvironment of naked mole-rat burrows in East Africa. Afr J Ecol 56: 279–289, 2018. doi: 10.1111/aje.12448. [DOI] [Google Scholar]
- 28.Hulbert AJ, Faulks SC, Buffenstein R. Oxidation-resistant membrane phospholipids can explain longevity differences among the longest-living rodents and similarly-sized mice. J Gerontol A Biol Sci Med Sci 61: 1009–1018, 2006. doi: 10.1093/gerona/61.10.1009. [DOI] [PubMed] [Google Scholar]
- 29.Hütter JF, Piper HM, Spieckerman PG. Effect of fatty acid oxidation on efficiency of energy production in rat heart. Am J Physiol Heart Circ Physiol 249: H723–H728, 1985. doi: 10.1152/ajpheart.1985.249.4.H723. [DOI] [PubMed] [Google Scholar]
- 30.Hylland P, Milton S, Pek M, Nilsson GE, Lutz PL. Brain Na+/K+-ATPase activity in two anoxia tolerant vertebrates: crucian carp and freshwater turtle. Neurosci Lett 235: 89–92, 1997. doi: 10.1016/S0304-3940(97)00727-1. [DOI] [PubMed] [Google Scholar]
- 31.Ivy CM, Sprenger RJ, Bennett NC, van Jaarsveld B, Hart DW, Kirby AM, Yaghoubi D, Storey KB, Milsom WK, Pamenter ME. The hypoxia tolerance of eight related African mole‐rat species rivals that of naked mole‐rats, despite divergent ventilatory and metabolic strategies in severe hypoxia. Acta Physiol (Oxf) 228: 13436, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Kolwicz SC Jr, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovasc Res 90: 194–201, 2011. doi: 10.1093/cvr/cvr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau DS, Connaty AD, Mahalingam S, Wall N, Cheviron ZA, Storz JF, Scott GR, McClelland GB. Acclimation to hypoxia increases carbohydrate use during exercise in high-altitude deer mice. Am J Physiol Regul Integr Comp Physiol 312: R400–R411, 2017. doi: 10.1152/ajpregu.00365.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lighton JR. Measuring Metabolic Rates: A Manual for Scientists. Oxford, UK: Oxford University Press, 2018. [Google Scholar]
- 35.Maillet D, Weber JM. Performance-enhancing role of dietary fatty acids in a long-distance migrant shorebird: the semipalmated sandpiper. J Exp Biol 209: 2686–2695, 2006. doi: 10.1242/jeb.02299. [DOI] [PubMed] [Google Scholar]
- 36.Mairbäurl H, Wodopia R, Eckes S, Schulz S, Bärtsch P. Impairment of cation transport in A549 cells and rat alveolar epithelial cells by hypoxia. Am J Physiol Lung Cell Mol Physiol 273: L797–L806, 1997. doi: 10.1152/ajplung.1997.273.4.L797. [DOI] [PubMed] [Google Scholar]
- 37.Malthankar-Phatak GH, Patel AB, Xia Y, Hong S, Chowdhury GM, Behar KL, Orina IA, Lai JC. Effects of continuous hypoxia on energy metabolism in cultured cerebro-cortical neurons. Brain Res 1229: 147–154, 2008. doi: 10.1016/j.brainres.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martínez ML, Landry C, Boehm R, Manning S, Cheek AO, Rees BB. Effects of long-term hypoxia on enzymes of carbohydrate metabolism in the Gulf killifish, Fundulus grandis. J Exp Biol 209: 3851–3861, 2006. doi: 10.1242/jeb.02437. [DOI] [PubMed] [Google Scholar]
- 39.McClelland GB, Hochachka PW, Weber JM. Carbohydrate utilization during exercise after high-altitude acclimation: a new perspective. Proc Natl Acad Sci USA 95: 10288–10293, 1998. doi: 10.1073/pnas.95.17.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCormick SD. Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50: 656–658, 1993. doi: 10.1139/f93-075. [DOI] [Google Scholar]
- 41.Morash AJ, Kotwica AO, Murray AJ. Tissue-specific changes in fatty acid oxidation in hypoxic heart and skeletal muscle. Am J Physiol Regul Integr Comp Physiol 305: R534–R541, 2013. doi: 10.1152/ajpregu.00510.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pamenter ME, Dzal YA, Thompson WA, Milsom WK. Do naked mole rats accumulate a metabolic acidosis or an oxygen debt in severe hypoxia? J Exp Biol 222: jeb191197, 2019. doi: 10.1242/jeb.191197. [DOI] [PubMed] [Google Scholar]
- 43.Pamenter ME, Lau GY, Richards JG, Milsom WK. Naked mole rat brain mitochondria electron transport system flux and H+ leak are reduced during acute hypoxia. J Exp Biol 221: jeb171397, 2018. doi: 10.1242/jeb.171397. [DOI] [PubMed] [Google Scholar]
- 44.Park TJ, Reznick J, Peterson BL, Blass G, Omerbašić D, Bennett NC, Kuich PH, Zasada C, Browe BM, Hamann W, Applegate DT, Radke MH, Kosten T, Lutermann H, Gavaghan V, Eigenbrod O, Bégay V, Amoroso VG, Govind V, Minshall RD, Smith ES, Larson J, Gotthardt M, Kempa S, Lewin GR. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 356: 307–311, 2017. doi: 10.1126/science.aab3896. [DOI] [PubMed] [Google Scholar]
- 45.Pastoris O, Dossena M, Foppa P, Arnaboldi R, Gorini A, Villa RF, Benzi G. Modifications by chronic intermittent hypoxia and drug treatment on skeletal muscle metabolism. Neurochem Res 20: 143–150, 1995. doi: 10.1007/BF00970538. [DOI] [PubMed] [Google Scholar]
- 46.Regan MD, Gill IS, Richards JG. Calorespirometry reveals that goldfish prioritize aerobic metabolism over metabolic rate depression in all but near-anoxic environments. J Exp Biol 220: 564–572, 2017. doi: 10.1242/jeb.145169. [DOI] [PubMed] [Google Scholar]
- 47.Richards JG. HYPOXIA| metabolic rate suppression as a mechanism for surviving hypoxia. In: Encyclopedia of Fish Physiology, edited by Farrell AP. San Diego, CA: Academic, 2011, p. 1764–1770. [Google Scholar]
- 48.Schippers MP, Ramirez O, Arana M, Pinedo-Bernal P, McClelland GB. Increase in carbohydrate utilization in high-altitude Andean mice. Curr Biol 22: 2350–2354, 2012. doi: 10.1016/j.cub.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 49.Seibel BA. Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J Exp Biol 214: 326–336, 2011. doi: 10.1242/jeb.049171. [DOI] [PubMed] [Google Scholar]
- 50.Sellner PA, Hazel JR. Time course of changes in fatty acid composition of gills and liver from rainbow trout (Salmo gairdneri) during thermal acclimation. J Exp Zool Part A Ecol Genet Physiol 221: 159–168, 1982. doi: 10.1002/jez.1402210206. [DOI] [Google Scholar]
- 51.Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta 1797: 1171–1177, 2010. doi: 10.1016/j.bbabio.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Storey KB. Metabolic regulation in mammalian hibernation: enzyme and protein adaptations. Comp Biochem Physiol A Physiol 118: 1115–1124, 1997. doi: 10.1016/S0300-9629(97)00238-7. [DOI] [PubMed] [Google Scholar]
- 53.Turner N, Haga KL, Hulbert AJ, Else PL. Relationship between body size, Na+-K+-ATPase activity, and membrane lipid composition in mammal and bird kidney. Am J Physiol Regul Integr Comp Physiol 288: R301–R310, 2005. doi: 10.1152/ajpregu.00297.2004. [DOI] [PubMed] [Google Scholar]
- 54.Wodopia R, Ko HS, Billian J, Wiesner R, Bärtsch P, Mairbäurl H. Hypoxia decreases proteins involved in epithelial electrolyte transport in A549 cells and rat lung. Am J Physiol Lung Cell Mol Physiol 279: L1110–L1119, 2000. doi: 10.1152/ajplung.2000.279.6.L1110. [DOI] [PubMed] [Google Scholar]
- 55.Yeagle PL, Young J, Rice D. Effects of cholesterol on (Na+,K+)-ATPase ATP hydrolyzing activity in bovine kidney. Biochemistry 27: 6449–6452, 1988. doi: 10.1021/bi00417a037. [DOI] [PubMed] [Google Scholar]
- 56.Zammit VA, Beis I, Newsholme EA. Maximum activities and effects of fructose bisphosphate on pyruvate kinase from muscles of vertebrates and invertebrates in relation to the control of glycolysis. Biochem J 174: 989–998, 1978. doi: 10.1042/bj1740989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zammit VA, Newsholme EA. The maximum activities of hexokinase, phosphorylase, phosphofructokinase, glycerol phosphate dehydrogenases, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxykinase, nucleoside diphosphatekinase, glutamate-oxaloacetate transaminase and arginine kinase in relation to carbohydrate utilization in muscles from marine invertebrates. Biochem J 160: 447–462, 1976. doi: 10.1042/bj1600447. [DOI] [PMC free article] [PubMed] [Google Scholar]