Abstract
Angiotensin II (ANG II) is the key contributor to renal fibrosis and injury. The present study investigated the role of endothelium prolyl hydroxylase 2 (PHD2) in ANG II-mediated renal fibrosis and injury. In vitro, endothelial cells (ECs) were isolated from PHD2f/f control [wild-type (WT)] mice or PHD2 EC knockout (PHD2ECKO) mice. In vivo, WT and PHD2ECKO mice were infused with ANG II (1,000 ng·kg−1·min−1) for 28 days. Renal fibrosis, reactive oxygen species (ROS), and iron contents were measured. Knockout of PHD2 resulted in a significant increase in the expression of hypoxia-inducible factor (HIF)-1α and HIF-2α in ECs. Intriguingly, knockout of PHD2 significantly reduced expression of the ANG II type 1 receptor (AT1R) in ECs. WT mice infused with ANG II caused increases in renal fibrosis, ROS formation, and iron contents. ANG II treatment led to a downregulation of PHD1 expression and upregulation of HIF-1α and HIF-2α in the renal cortex and medulla. Knockout of PHD2 in EC blunted ANG II-induced downregulation of PHD1 expression. Furthermore, knockout of PHD2 in ECs attenuated ANG II-induced expression of HIF-1α, HIF-2α, transforming growth factor-β1, p47phox, gp91phox, heme oxygenase-1, and ferroportin. This was accompanied by a significant suppression of renal fibrosis, ROS formation, and iron accumulation. In summary, knockout of endothelial PHD2 suppressed the expression of AT1R in ECs and blunted ANG II-induced downregulation of PHD1 and upregulation of HIF-α in the kidney. Our study, for the first time, demonstrates a necessary role of endothelial PHD2 in ANG II-mediated renal fibrosis and injury.
Keywords: angiotensin II, hypoxia-inducible factor, prolyl hydroxylase 2, reactive oxygen species, renal fibrosis
INTRODUCTION
Chronic kidney disease (CKD) is recognized as a worldwide epidemic and is an important global health challenge (7, 20). Angiotensin II (ANG II), an endogenous vasoconstrictor, is one of the major contributors to the progression of CKD (4). ANG II has been shown to increase renal fibrosis (19), reactive oxygen species (ROS) formation (26), iron content (34), and apoptosis (45). Hypoxia-inducible factor (HIF) is a well-known master regulator of hypoxia-adaptive responses in a variety of pathophysiological processes, including renal diseases (16). Under hypoxic conditions, HIF, and its oxygen-regulated isoforms, HIF-1α and HIF-2α, are stabilized, promote cellular adaptation to the reduced oxygen supply, and influence cell proliferation, survival, and metabolism (31). Accumulating evidence has implicated the crucial role of HIFs in ANG II-induced renal fibrosis and injury (17, 43). The ANG II type 1 receptor (AT1R) is the specific receptor of angiotensin. The angiotensin receptor is activated by the vasoconstricting peptide ANG II. AT1R has been shown to mediate the major cardiovascular effects of ANG II (9). AT1R has an important role in renal oxidative stress and ROS formation (24). Several studies have demonstrated that ANG II-induced upregulation of HIF-α is mainly through activation of AT1R (14, 31, 35). A previous study (18) has also shown that pharmacological blockade of prolyl hydroxylases (PHDs) suppressed ANG II-induced perivascular fibrosis of the aorta and coronary artery via downregulation of AT1R.
PHDs are oxygen sensor proteins, and HIF-α stabilization is regulated by the PHD (PHD1–PHD3) family. Among the PHD isoforms, PHD2 is considered to be the most important oxygen sensor in the PHD family. Increased levels of HIF-1α and HIF-2α were strongly correlated with glomerular injury, renal fibrosis, and hypertensive CKD (10, 27, 31). Pharmacological inhibition of PHDs increased the stability of HIF-1α, promoted angiogenesis, and protected human renal epithelial cells and mouse kidneys against hypoxic injury (3, 49). PHD inhibitors also regulate HIF-2α stability and enhance erythropoietin production to ameliorate anemia in CKD (32). So far, all PHD inhibitors used in research experiments and clinical trials are nonselective PHD isoform inhibitors, and some exhibit detrimental effects (29, 32, 36). Moreover, nothing is known about the functional roles of individual PHD isoforms in ANG II-induced renal injury. Therefore, it is imperative to investigate the specific role of PHD2 in the kidney. Gene knockout (KO) technology provides a unique tool to address this problem. A recent study (2) has shown that endothelium-specific depletion of PHD2/3 promoted cardiomyocyte proliferation and prevented ventricular failure induced by myocardial ischemia. Using an endothelial PHD2-specific KO mouse model, we and other investigators have demonstrated that PHD2 in endothelial cells (ECs) has a critical role in the regulation of pulmonary vascular remodeling, hepatic steatosis, and fibrosis (1, 8, 41, 50). Therefore, delineating the precise function of each PHD in vivo will facilitate the design of therapeutic strategies against hypertension-induced injury. Furthermore, unraveling the roles of ANG II in the regulation of PHD/HIF-α expression and renal injury will provide the foundation for future therapies to prevent and reverse hypertensive CKD and end-organ failure.
The aim of the present study was to investigate the specific role of endothelium PHD2 in ANG II-mediated renal fibrosis, ROS formation, and iron metabolism. Our data clearly demonstrated that KO of PHD2 significantly reduced AT1R expression in ECs. Most important, ANG II-induced renal fibrosis and ROS formation were abolished in PHD2 EC KO (PHD2ECKO) mice, suggesting a necessary role of endothelial PHD2 in ANG II-mediated renal fibrosis and injury in mice.
MATERIALS AND METHODS
All procedures conformed with the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals. The study was also approved by the Animal Care and Use Committee of the University of Mississippi Medical Center (protocol identifier: 1280B). The investigation conformed with the National Institutes of Health (NIH; Bethesda, MD) Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996).
Experimental animal model and treatment.
Both PHD2f/f control mice [wild-type (WT)] and PHD2ECKO mice were generated using the Cre-LoxP system as previously described (40, 50). Absence of PHD2 in the vascular endothelium was confirmed by Western blot analysis using cultured ECs isolated from the aorta of PHD2ECKO mice. Experiments were performed on male mice at 16 wk of age. Both WT and PHD2ECKO mice were infused with ANG II at 1,000 ng·kg−1·min−1 for 28 days (WT + ANG II group and PHD2ECKO + ANG II group) by subcutaneously implanted Alzet miniosmotic pumps (Durect, Cupertino, CA).
Measurement of blood pressure by the tail-cuff method.
The tail-cuff method was used to measure mean arterial pressure (MAP) and systolic and diastolic blood pressures in conscious mice using an MC1000 BP Analysis System (Hatteras Instruments). Mice were trained and acclimated to restraint for 20–30 min for 4 consecutive days at the same time of day before the actual measurements.
Isolation and culture of mouse aortic ECs.
Mouse aortic ECs (MAECs) were isolated from the thoracic aortas of WT or PHD2ECKO mice as previously described (39). Briefly, mice were anesthetized with ketamine (100 mg/kg) and xylazine (15 mg/kg). The thoracic aorta was removed using microdissection forceps and cut into ~1-mm rings. The rings were then embedded in 25 µL of Geltrex basement membrane matrix (GIBCO) in a 96-well plate and covered with 150 µL of endothelial growth medium (EGM-2; Lonza) supplemented with growth factors and 10% FBS at 37°C and 5% CO2 in a humidified incubator. After 4 days of incubation, EC sprouting was observed, and the aortic rings were removed without interrupting the growing ECs. ECs were then replaced with fresh medium and allowed to proliferate until 90% confluent on matrix. ECs were trypsinized, transferred to a six-well plate coated with 0.1% gelatin, and maintained in EGM-2 at 37°C and 5% CO2. Cells between passages 4 and 10 were used for the study.
Western blot analysis.
Mouse kidneys were placed over dry ice and carefully dissected under a microscope to detach the medulla from the cortex. Tissues or cultured ECs were collected and homogenized in lysis buffer. Homogenates were centrifuged at 16,000 g at 4°C for 15 min. A BCA protein assay kit (Pierce, Rockford, IL) was used to analyze protein concentrations. An aliquot (25 µg) of the protein was separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline and incubated with the following primary antibodies overnight: HIF-1α (1:1,000, GeneTex, Irvine, CA), HIF-2α, ferroportin, and PHD3 (1:1,000, Novus Bio, Littleton, CO), PHD1, acetyl-p53, and AT1R (1:1,000; Abcam, Cambridge, MA), transforming growth factor (TGF)-β1, caspase-3, and p47phox (1:1,000; Santa Cruz Biotechnology), heme oxygenase (HO)-1 and gp91phox (1:1,000; BD Transduction, San Jose, CA), and PHD2 and p53 (1:1,000, Cell Signaling, Danvers, MA). After being washed, membranes were incubated for 2 h with anti-rabbit or anti-mouse secondary antibody coupled to horseradish peroxidase (1:5,000; Santa Cruz Biotechnology). Densitometric analyses were carried out with image acquisition and analysis software (Bio-Rad, Hercules, CA).
Histological analysis.
Renal tissues were fixed with neutral-buffered 10% formalin solution (SF93-20, Fisher Scientific, Pittsburgh, PA) and embedded in frozen OCT compound (no. 4585, Fisher Health Care, Houston, TX). Frozen sections and paraffin sections were prepared (10 µm thickness). Masson’s trichrome staining and sirius red staining (paraffin section) were performed to measure the degree of fibrosis (blue and red) in the kidney. Masson’s trichrome-stained sections also were examined with a fluorescent light source. The areas that exhibited red fluorescence at the corticomedullary junction were measured to assess the formation of protein casts. ROS (frozen sections) was measured by dihydroethidium staining. Iron content (frozen sections) was measured by Prussian blue staining (Abcam). The area percentage of fibrosis, iron content, and ROS fluorescence intensity were quantified by measuring six random microscopic fields using image-analysis software (Image J, NIH).
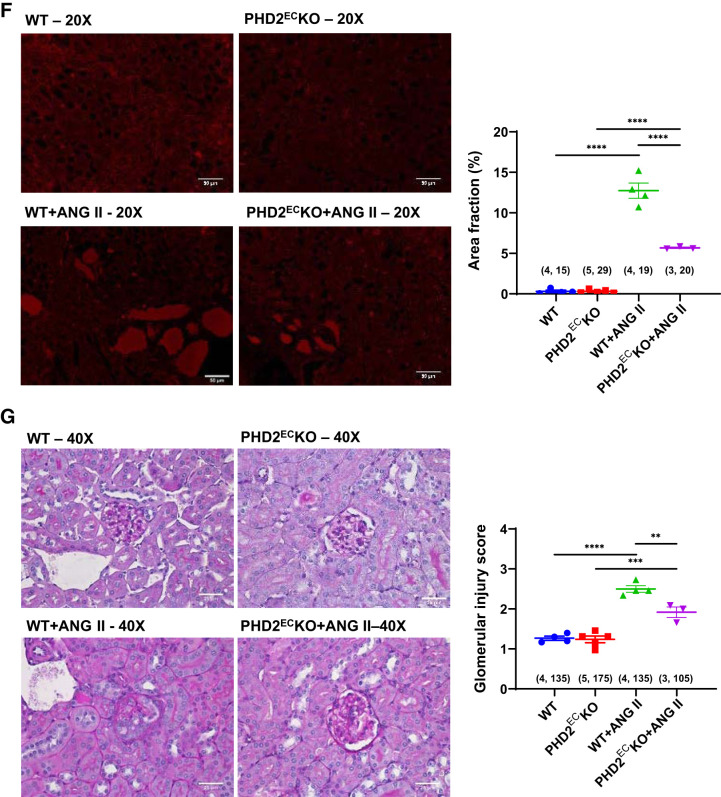
To examine glomerular injury, slides were stained with periodic acid-Schiff reagents and scored semiquantitatively in a blinded fashion (33, 38); 30–35 glomeruli/mice were scored for the presence of mesangial matrix expansion and loss of capillaries on a scale of 0–4, with 0 representing a normal glomerulus, 1 representing 1–25%, 2 representing 26–50%, 3 representing 51–75%, and 4 representing >75% of mesangial expansion and loss of glomerular capillaries in the glomerular tuft.
Statistical analysis.
All data are expressed as means ± SE. Two-group comparisons were analyzed by an unpaired Student’s t test. Differences between the means of multiple groups were compared by one-way ANOVA followed by Tukey’s multiple-comparisons test. P < 0.05 was considered statistically significant. GraphPad Prism 8.0 software (GraphPad Software, La Jolla, CA) was used for statistical analysis.
RESULTS
PHD2ECKO reduced expression of AT1R in MAECs.
KO of PHD2 in EC was confirmed by Western blot analysis using cultured MAECs isolated from the aorta of PHD2ECKO mice (Fig. 1A). KO of endothelial PHD2 resulted in a significant increase in expression of HIF-1α and HIF-2α in cultured ECs (Fig. 1, B and C). KO of PHD2 further downregulated AT1R expression in cultured ECs (Fig. 1D).
Fig. 1.
Knockout (KO) of endothelial prolyl hydroxylase 2 (PHD2) suppressed expression of angiotensin II type 1 receptor (AT1R) in cultured endothelial cells (ECs). A: Western blot analysis confirmed expression of PHD2 in ECs isolated from endothelial PHD2-specific KO (PHD2ECKO) mice was significantly reduced, indicating a knockdown of PHD2 in ECs. B and C: KO of endothelial PHD2 increased expression of hypoxia-inducible factor (HIF)-1α and HIF-2α in ECs. D: KO of endothelial PHD2 downregulated AT1R expression in ECs. WT, wild type. Male mice at 16 wk of age were used; n = 4 mice/group. *P < 0.05; **P < 0.01.
PHD2ECKO blunted ANG II-induced renal fibrosis.
Infusion of mice with ANG II for 28 days resulted in a significant elevation of MAP both in WT and PHD2ECKO mice. However, there were no significant differences in MAP between WT and PHD2ECKO mice with or without ANG II infusion (see Supplemental Fig. S1 in the Supplemental Material, available online at https://doi.org/10.6084/m9.figshare.11688864.v6). Because the levels of AT1R expression were decreased in ECs from PHD2ECKO mice, we then further investigated whether this downregulation impacted ANG II-induced renal fibrosis. Both Masson’s trichrome staining and sirius red staining showed that there was significantly increased renal and perivascular fibrosis in WT mice infused with ANG II compared with WT mice. Interestingly, the areas of renal and perivascular fibrosis were significantly reduced in PHD2ECKO mice infused with ANG II (Fig. 2, A–D). Western blot analysis showed that ANG II infusion increased expression of TGF-β1, whereas knockout of endothelial PHD2 blunted this increase in both the renal cortex and medulla (Fig. 2E). In addition, there was no statistically significant difference of renal fibrosis between WT mice and PHD2ECKO mice without ANG II infusion. Infusion with ANG II resulted in a significant increase in protein cast area in mouse kidneys. KO of PHD2 in ECs significantly reduced protein cast areas in ANG II-treated mice (Fig. 2F). Glomerular injury score analysis further showed that infusion with ANG II caused a significant increase in glomerular injury score. KO of PHD2 in ECs significantly attenuated ANG II-induced glomerular injury score (Fig. 2G).
Fig. 2.
Knockout (KO) of prolyl hydroxylase 2 (PHD2) in endothelial cells (ECs) reduced angiotensin II (ANG II)-induced renal fibrosis. A: Masson’s trichrome staining of a renal section. B: Masson’s trichrome staining of a perivascular section. C: sirius red staining of a renal section. D: sirius red staining of a perivascular section. The percent area of renal and perivascular fibrosis was significantly increased in wild-type (WT) mice infused with ANG II, but this was dramatically suppressed in endothelial PHD2-specific KO (PHD2ECKO) mice with ANG II infusion (n = 3–5 mice/group). E: ANG II increased expression of transforming growth factor-β1 (TGF-β1), whereas KO of endothelial PHD2 reduced this increase in both the renal cortex and medulla (n = 3–4 mice/group). Male mice at 16 wk of age were used. *P < 0.05; **P < 0.01. F: assessment of the formation of protein casts by red fluorescence at the corticomedullary junction area. The area of protein casts was significantly increased in WT mice infused with ANG II, but this was dramatically suppressed in PHD2ECKO mice with ANG II infusion (n = 3–5 mice/group, numbers in parentheses indicate the number of glomeruli scored/number of mice in each group). G: assessment of the glomerular injury score by periodic acid-Schiff staining. The glomerular injury score was significantly higher in WT mice infused with ANG II, but this was dramatically suppressed in PHD2ECKO mice with ANG II infusion (n = 3–5 mice/group, numbers in parentheses indicate the number of fields analyzed/number of mice in each group). ***P < 0.001; ****P < 0.0001.
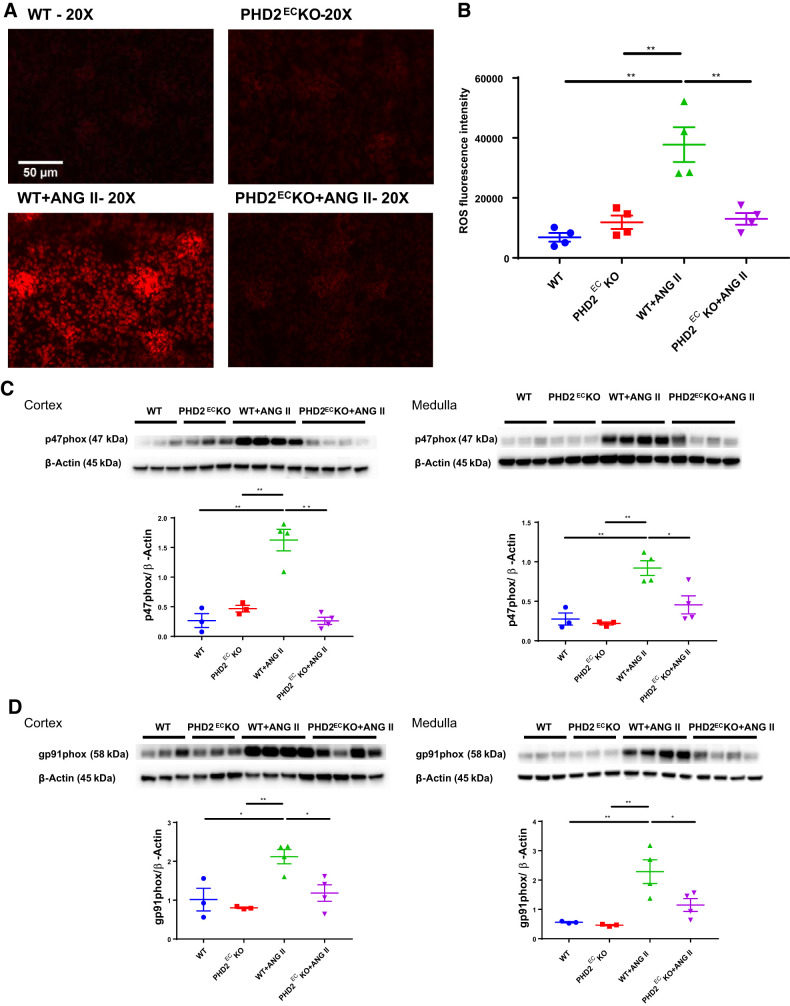
PHD2ECKO reduced ANG II-induced NADPH oxidase expression and ROS formation.
ROS formation measured by dihydroethidium staining showed that WT mice infused with ANG II had significantly higher ROS levels than WT or PHD2ECKO mice without ANG II infusion. ANG II-induced ROS formation was significantly reduced in PHD2ECKO mice (Fig. 3, A and B). To further investigate the molecular events by which KO of endothelial PHD2 modulated ANG II-induced ROS formation, expression of NADPH oxidase subunits p47phox and gp91phox was examined in both the renal cortex and medulla. ANG II infusion led to a significant upregulation of expression of p47phox and gp91phox, but these upregulations were abolished in PHD2ECKO mice infused with ANG II (Fig. 3, C and D). There was no statistical difference among WT, PHD2ECKO, and PHD2ECKO + ANG II mice.
Fig. 3.
Knockout (KO) of prolyl hydroxylase 2 (PHD2) in endothelial cells (ECs) reduced angiotensin II (ANG II)-induced renal reactive oxygen species (ROS) formation. A and B: dihydroethidium staining showed that ANG II infusion enhanced ROS formation, whereas KO of endothelial PHD2 significantly attenuated ANG II-induced ROS formation (n = 4 mice/group). C and D: ANG II infusion increased expression of p47phox and gp91phox in the renal cortex and medulla, but these changes were abolished in endothelial PHD2-specific KO (PHD2ECKO) mice (n = 3–4 mice/group). WT, wild type. Male mice at 16 wk of age were used. *P < 0.05; **P < 0.01.
PHD2ECKO reduced ANG II-induced renal iron accumulation.
Prussian blue staining showed that ANG II infusion increased the percentage of the iron staining area, whereas KO of PHD2 in ECs reduced ANG II-induced iron accumulation (Fig. 4, A and B). Western blot analysis showed that ANG II infusion significantly increased expression of HO-1 in the renal cortex and medulla. KO of PHD2 in ECs significantly suppressed ANG II-induced HO-1 expression (Fig. 4C). ANG II infusion also increased expression of ferroportin, an iron transporter, whereas KO of endothelial PHD2 reduced this increase in the renal cortex. Intriguingly, ANG II infusion failed to upregulate ferroportin in the medulla when endothelial PHD2 was absent (Fig. 4D). We also found that ANG II infusion resulted in a significant upregulation of caspase-3, p53, and acetyl-p53 in the renal cortex. KO of PHD2 in ECs reduced ANG II-mediated upregulation of caspase-3, p53, and acetyl-p53 in the cortex (see Supplemental Fig. S2 in the Supplemental Material, available online at https://doi.org/10.6084/m9.figshare.11688864.v6).
Fig. 4.
Knockout (KO) of prolyl hydroxylase 2 (PHD2) in endothelial cells (ECs) reduced angiotensin II (ANG II)-induced renal iron accumulation. A and B: Prussian blue staining showed that ANG II infusion increased the percentage of iron staining area, whereas KO of PHD2 in ECs reduced ANG II-induced iron accumulation. C: Western blot showed that ANG II infusion increased the expression of heme oxygenase (HO)-1, but KO of PHD2 in ECs suppressed this increase. D: ANG II infusion increased the expression of ferroportin in the cortex and medulla. KO of endothelial PHD2 reduced ANG II-induced ferroportin expression in the cortex but not in the medulla. WT, wild type; PHD2ECKO, endothelial PHD2-specific KO. Male mice at 16 wk of age were used; n = 3–4 mice/group. *P < 0.05; **P < 0.01.
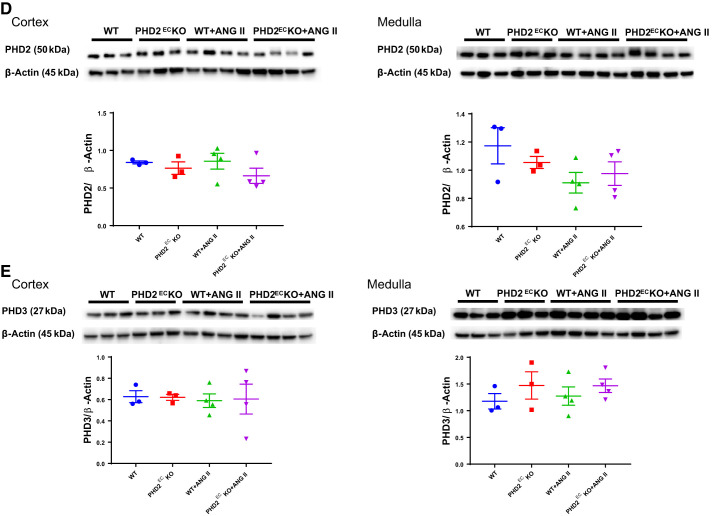
PHD2ECKO reversed ANG II-induced downregulation of PHD1 and upregulation of HIFs in the kidney.
Expression of HIF-1α and HIF-2α was significantly increased in WT mice infused with ANG II. KO of endothelial PHD2 suppressed these increases in both the renal cortex and medulla. In the cortex or medulla, expression of HIF-1α and HIF-2α was not statistically significant among WT, PHD2ECKO, and PHD2ECKO + ANG II mice (Fig. 5, A and B). To explore which ANG II infusion upregulated HIF-1α and HIF-2α expression, levels of PH1, PHD2, and PHD3 in the renal cortex and medulla were examined. Interestingly, ANG II infusion resulted in a significant downregulation of PHD1 expression in both the cortex and medulla. Furthermore, KO of endothelial PHD2 reversed this downregulation in both the cortex and medulla. Surprisingly, KO of PHD2 in ECs alone reduced expression of PHD1 in the cortex (Fig. 5C). Expression of PHD2 and PHD3 was not significantly altered after ANG II infusion in both the cortex and medulla (Fig. 5, D and E).
Fig. 5.
Expression of hypoxia-inducible factor (HIF)-α and prolyl hydroxylases (PHDs) in the kidney. A and B: expression of HIF-1α and HIF-2α was increased in wild-type (WT) mice infused with angiotensin II (ANG II), whereas knockout (KO) of endothelial PHD2 significantly suppressed these increases in both the renal cortex and medulla. C: ANG II infusion downregulated the expression of PHD1 in both the cortex and medulla, whereas KO of endothelial PHD2 prevented this decline. D and E: there were no statistical differences in expression of PHD2 or PHD3. PHD2ECKO, endothelial PHD2-specific KO. Male mice at 16 wk of age were used; n = 3–4 mice/group. *P < 0.05; **P < 0.01.
Effect of PHD2ECKO on the expression of AT1R in the kidney.
We further examined whether KO of PHD2 in ECs affected levels of AT1R in the cortex and medulla in vivo. In the renal cortex, ANG II infusion led to a significant elevation of AT1R levels in WT and PHD2ECKO mice. There was no statistical difference between WT and PHD2ECKO mice infused with ANG II. Levels of AT1R did not reach a significant difference in the medulla in WT or PHD2ECKO mice either infused with or without ANG II (Fig. 6).
Fig. 6.
Expression of angiotensin II (ANG II) type 1 receptor (AT1R) in the kidney. In the cortex, ANG II infusion resulted in a significant increase in AT1R expression in wild-type (WT) mice and endothelial prolyl hydroxylase (PHD2)-specific KO (PHD2ECKO) mice. There was no statistical difference between WT and PHD2ECKO mice. In the medulla, expression of AT1R showed no significant difference between WT and PHD2ECKO mice. Male mice at 16 wk of age were used; n = 3–4 mice/group. *P < 0.05; **P < 0.01.
DISCUSSION
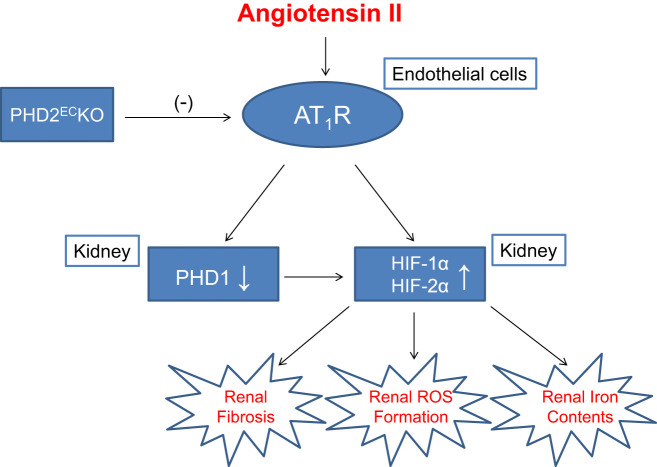
For the first time to our knowledge, we have demonstrated that ANG II infusion failed to induce renal fibrosis, ROS formation, and iron accumulation in the absence of endothelial PHD2. Mechanistically, KO of endothelial PHD2 suppressed expression of AT1R in ECs. Inhibition of AT1R expression attenuated ANG II-induced downregulation of PHD1 and upregulation of HIF-1α and HIF-2α in both the cortex and medulla (Fig. 7). Our results suggest that endothelial PHD2 is necessary for ANG II-mediated renal fibrosis and injury.
Fig. 7.
Potential mechanisms and cross talk of endothelium prolyl hydroxylase 2 (PHD2) in regulation of angiotensin II-mediated renal fibrosis, reactive oxygen species (ROS) formation, and iron metabolism. AT1R, angiotensin II type 1 receptor; HIF, hypoxia-inducible factor; PHD2ECKO, endothelial PHD2-specific knockout.
HIF-α stabilization is regulated by PHDs. Under normoxic conditions, HIF-α is hydroxylated by specific oxygen-dependent PHDs and ultimately degraded rapidly by inducing the α-subunit to undergo E3 ubiquitination. In hypoxic conditions, the enzymatic activities of PHDs are inhibited. As a result, unmodified HIF-α escapes from entering the destructive process, resulting in the stabilization and accumulation of HIF-α (30). So far, three isoforms of PHD (PHD1–PHD3) that modulate the stabilization and accumulation of HIF-α have been identified. Among them, PHD2 is a major negative regulator for HIF-1α stabilization under hypoxia, whereas PHD1 and PHD3 are more active on HIF-2α. Several studies have demonstrated that pharmacological inhibition of PHDs and induction of HIF-1α recapitulate various cellular and physiological responses to hypoxia or preconditioning stimuli in cardiovascular diseases (13, 37, 46). A previous study by Luo et al. (17) revealed that ANG II-induced upregulation of endothelial HIF-1α contributed to glomerular injury and promoted hypertensive CKD. In their study, endothelial HIF-1α was deleted, which showed a lowering of blood pressure and protective effects against hypertension-induced renal injury (17). In our present study, endothelial PHD2 was the specific KO, but not endothelial HIF-1α. KO of endothelial PHD2 led to increases in both HIF-1α and HIF-2α expression in ECs. Most importantly, KO of PHD2 significantly reduced AT1R expression in ECs, which blunted ANG II-mediated effects on the renal cortex and medulla. This was validated by the increased expression of HIF-1α and HIF-2α in WT mice infused with ANG II, whereas this increase was suppressed in PHD2ECKO mice infused with ANG II in the renal cortex and medulla. Furthermore, PHD2ECKO attenuated ANG II-mediated renal fibrosis and injury. Previous studies (31, 35) have indicated that ANG II-induced vascular remodeling was mainly through activation of the AT1R and HIF signaling pathways. One study showed that inhibition of PHD by treatment with cobalt chloride downregulated AT1R expression and attenuated the ANG II-mediated signaling pathway in vascular smooth muscle cells. Furthermore, treatment with PHD inhibitor attenuated perivascular fibrosis of coronary arteries (18). Using gene-specific KO approaches (endothelium-specific KO), we examined the specific role of endothelial PHD2 on AT1R expression. We found a direct inhibitory effect of PHD2 KO on AT1R expression in ECs. Keep in mind that pharmacological PHD inhibitors are nonspecific agents and generally target all cell types in the kidney, whereas tissue-specific KO of PHD isoforms by a genetic approach may only target these specific renal cells, which may exhibit distinctly phenotype during the pathogenesis of renal fibrosis in response to ANG II. Although the expression of AT1R was no statistically different between WT + ANG II and PHD2ECKO + ANG II mice in the kidney, ANG II-mediated renal fibrosis, ROS formation, and iron accumulation were suppressed by KO of PHD2 in ECs. KO of PHD2 has been shown to upregulate apelin expression in ECs (40). Apelin is a negative regulator of ANG II-induced cardiovascular remodeling (48). We postulate that the protection of endothelial PHD2 KO might be ascribed, at least in part, to downregulation of AT1R expression in ECs, which may have cross talk with renal cells via a paracrine mechanism, such as release apelin in ECs, and further blunt ANG II-induced HIF-α upregulation and fibrosis in the kidney.
Although PHDs have been reported to be involved in AT1R expression in vitro (18), our knowledge of a specific isoform of PHDs in renal injury under pathological conditions such as hypertension and ANG II infusion in vivo is still very limited. Therefore, it is important to examine the specific role of PHD isoforms through functional genomic and tissue specific processes in animal models. Our study, for the first time, demonstrated that expression of PHD1 in the renal cortex and medulla was downregulated, whereas PHD2 and PHD3 remained unchanged, in mice infused with ANG II. Moreover, their targeting genes HIF-1α and HIF-2α were upregulated, suggesting a regulatory role of ANG II on the PHD1/HIF-α signaling pathway, but not PHD2/PHD3, in the renal cortex and medulla. We found that KO of PHD2 in ECs alone suppressed expression of PHD1 in the renal cortex but in not medulla. Most intriguingly, ANG II-mediated alterations of the PHD1/HIF-α signaling pathway in the renal cortex and medulla were significantly reversed in PHD2ECKO mice. These results further indicated that there may be a cross talk and inhibitory mechanism among PHD2-AT1R in the renal vascular endothelium and PHD1-HIF-α in the renal cortex and medulla in response to ANG II. So far, how the endothelial PHD/HIF cross talk with signaling in renal cells remains unknown. Our present data indicate that KO of endothelial PHD2 inhibits endothelial AT1R, which subsequently leads to suppression of ANG II-induced PHD1 downregulation and HIF-α upregulation in renal cells, eventually attenuating ANG II-mediated renal fibrosis and injury (Fig. 7).
ANG II is a well-recognized contributor that promotes renal fibrosis and injury in hypertensive CKD (16, 43). HIF-α, especially HIF-1α, is a key regulator of renal fibrosis under various pathological conditions (15). Studies have shown that treatment with ANG II leads to an increase of HIF-1α expression in renal glomerular and medullary cells (17, 43). Also, HIF-1α has been attributed to the profibrotic action of ANG II in renal medullary interstitial cells (43). Moreover, ANG II caused hypoxic conditions in the tubule interstitium of the kidney and led to renal injury (21). TGF-β is a master regulator of the profibrotic signaling pathway in hypertensive CKD. Emerging evidence has revealed a strongly causative relationship between HIF-α and TGF-β signaling pathways in renal fibrosis (5, 6, 25). In the present study, we found that KO of PHD2 in ECs reduced ANG II-induced upregulation of HIF-1α and TGF-β1. We speculated that KO of PHD2 in ECs may block the profibrotic signaling pathway of ANG II via downregulation of HIF-1α/TGF-β.
In addition, we did not find significant differences in renal fibrosis between WT and PHD2ECKO mice under physiological conditions. In our previous study, EC-specific PHD2 KO promoted renal fibrosis by the accumulation of HIF-α and significantly upregulated TGF-β1 expression in renal cortex tissues (40). The discrete results may be because of the different age of the mice that we had used. Our previous study was performed on aged male mice that were ~15 mo old. In the present study, young male mice at 16 wk were used. A recent study also showed that endothelium-specific depletion of PHD2/3 stimulates cardiomyocyte proliferation and enhances cardiac function in both neonatal (postnatal days 2–12) and adult (8–12 wk old) mice (2). We reasoned that short-term activation of HIF-α is beneficial and protective while long-term activation of HIF-α is detrimental. It is still debatable whether HIF-α is profibrotic or antifibrotic (15). A previous study has shown that long-term overactivation of HIF-1α is detrimental in chronic renal injury associated with ischemia/hypoxia (44). We hypothesized that deactivation of PHD2 in ECs possesses protective effects on ANG II-induced renal injury at the early stage of PHD2ECKO mice, whereas, during aging, excessive accumulation of HIF-1α promotes a profibrotic effect in CKD and hypertension. Similarly, the balance of beneficial and detrimental effects was also observed in pharmacological PHD inhibitor treatment. Treatment of rats with PHD inhibitors also has dual roles in the development of CKD depending on the timing of administration (47).
It has been suggested that ANG II promotes ROS formation via the activation of NADPH oxidase (22). NADPH oxidase subunits p47phox and gp91phox represent the levels of NADPH oxidation generating ROS formation (37, 46). Our data demonstrated that KO of PHD2 in ECs reduced ANG II-induced ROS formation. This was accompanied by a significant reduction of ANG II-mediated upregulation of p47phox, gp91phox, caspase-3, p53, and acetyl-p53 expression in the kidney. These results revealed that the ANG II-induced response to hypoxia, such as ROS and apoptosis, was diminished in the absence of endothelial PHD2, further suggesting a necessary role of endothelial PHD2 in ANG II-induced renal injury. Previous studies have demonstrated intracellular interactions among ROS formation, apoptosis, and iron metabolism in renal injury (13, 23). The HIF-α signaling pathway has an important role in the regulation of iron metabolism (28, 32). HO-1 was found to be involved both in iron homeostasis and oxidative stress responses (12). Upregulation of HO-1 and ferroportin expression is associated with excessive iron accumulation and overloading during ischemic renal injury (11, 42). Our data showed that KO of PHD2 in ECs significantly suppressed ANG II-induced HO-1 and ferroportin expression in the renal cortex. It was indicated that KO of PHD2 in ECs reduced ANG II-induced iron accumulation in the renal cortex.
Our study has some limitations. First, we focused on the role of endothelial PHD2 in ANG II-mediated renal fibrosis and injury in vivo. It remains to be determined whether endothelial PHD2 has the same effects on ANG II treatment in vitro. Second, the present study revealed that KO of endothelial PHD2 suppressed expression of AT1R in ECs but had little effect on AT1R in the kidney. Surprisingly, KO of PHD2 in ECs had no significant impact on ANG II-induced hypertension. These results indicate that endothelial PHD2 is necessary for ANG II-induced renal fibrosis and injury, which may be independent of blood pressure. The underlying mechanism of difference of PHD2 between ECs and the renal cortex or medulla remains to be further studied. Third, our results showed that KO of endothelial PHD2 had little effect on ferroportin in the medulla in response to ANG II. This may be the result of less abundance of ECs in the renal medulla than in the cortex. The exact mechanisms by which endothelial PHD2 affect iron metabolism in the medulla also warrant further study.
Our present study demonstrated a necessary role of endothelial PHD2 in ANG II-mediated renal fibrosis and injury. Specific KO of endothelial PHD2 protects the kidney against ANG II-induced fibrosis, ROS formation, and iron accumulation by mechanisms involving EC-renal cell cross talk and the AT1R/PHD1/HIF-α signaling pathway. Our data suggest that endothelial PHD2 is an important mediator of hypertension-associated CKD and is a promising therapeutic target.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant 2R01HL102042 and the University of Mississippi Medical Center Intramural Research Support Program (to J. X. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.-X. C. and H. Z conceived and designed research; Y.Z., B.L and H.Z. performed experiments; Y.Z. analyzed data; J.-X. C., Y.Z., and H.Z. interpreted results of experiments and prepared figures; Y.Z. drafted manuscript; J.-X. C. and Y.Z. edited and revised manuscript; Y.Z., H.Z., and J.-X. C. approved final version of manuscript.
REFERENCES
- 1.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2α. Circulation 133: 2447–2458, 2016. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan Q, Mao H, Angelini A, Coarfa C, Robertson MJ, Lagor WR, Wehrens XHT, Martin JF, Pi X, Xie L. Depletion of endothelial prolyl hydroxylase domain protein 2 and 3 promotes cardiomyocyte proliferation and prevents ventricular failure induced by myocardial infarction. Circulation 140: 440–442, 2019. doi: 10.1161/CIRCULATIONAHA.118.039276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang Y, Zhang H, Zhong Y, Ding X. Prolyl hydroxylase 2 (PHD2) inhibition protects human renal epithelial cells and mice kidney from hypoxia injury. Oncotarget 7: 54317–54328, 2016. doi: 10.18632/oncotarget.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase VH. Angiotensin II: breathtaking in the renal medulla. Kidney Int 79: 269–271, 2011. doi: 10.1038/ki.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han WQ, Zhu Q, Hu J, Li PL, Zhang F, Li N. Hypoxia-inducible factor prolyl-hydroxylase-2 mediates transforming growth factor beta 1-induced epithelial-mesenchymal transition in renal tubular cells. Biochim Biophys Acta 1833: 1454–1462, 2013. doi: 10.1016/j.bbamcr.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna C, Hubchak SC, Liang X, Rozen-Zvi B, Schumacker PT, Hayashida T, Schnaper HW. Hypoxia-inducible factor-2α and TGF-β signaling interact to promote normoxic glomerular fibrogenesis. Am J Physiol Renal Physiol 305: F1323–F1331, 2013. doi: 10.1152/ajprenal.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet 382: 260–272, 2013. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 8.Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, Shay S, French JL, West J, Haase VH. The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol 36: 1584–1594, 2016. doi: 10.1128/MCB.01055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai T, Forrester SJ, O’Brien S, Baggett A, Rizzo V, Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res 125: 4–13, 2017. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong KH, Oh HJ, Lim BJ, Kim M, Han KH, Choi YH, Kwon K, Nam BY, Park KS, Park JT, Han SH, Yoo TH, Lee S, Kim SJ, Kang DH, Choi KB, Eremina V, Quaggin SE, Ryu DR, Kang SW. Selective tubular activation of hypoxia-inducible factor-2α has dual effects on renal fibrosis. Sci Rep 7: 11351, 2017. doi: 10.1038/s41598-017-11829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawen A, Lane DJ. Mammalian iron homeostasis in health and disease: uptake, storage, transport, and molecular mechanisms of action. Antioxid Redox Signal 18: 2473–2507, 2013. doi: 10.1089/ars.2011.4271. [DOI] [PubMed] [Google Scholar]
- 12.Lever JM, Boddu R, George JF, Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal 25: 165–183, 2016. doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang M, Li A, Lou A, Zhang X, Chen Y, Yang L, Li Y, Yang S, Hou FF. Advanced oxidation protein products promote NADPH oxidase-dependent β-cell destruction and dysfunction through the Bcl-2/Bax apoptotic pathway. Lab Invest 97: 792–805, 2017. doi: 10.1038/labinvest.2017.24. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Zhang JW, Hu L, Song YC, Zhou L, Fan Y, Zhu HY, Wang Y, Li QP. Activation of the AT1R/HIF-1α/ACE axis mediates angiotensin II-induced VEGF synthesis in mesenchymal stem cells. BioMed Res Int 2014: 627380, 2014. doi: 10.1155/2014/627380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Wei Q, Guo C, Dong G, Liu Y, Tang C, Dong Z. Hypoxia, HIF, and associated signaling networks in chronic kidney disease. Int J Mol Sci 18: 0950, 2017. doi: 10.3390/ijms18050950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Ning X, Li R, Yang Z, Yang X, Sun S, Qian Q. Signalling pathways involved in hypoxia-induced renal fibrosis. J Cell Mol Med 21: 1248–1259, 2017. doi: 10.1111/jcmm.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo R, Zhang W, Zhao C, Zhang Y, Wu H, Jin J, Zhang W, Grenz A, Eltzschig HK, Tao L, Kellems RE, Xia Y. Elevated endothelial hypoxia-inducible factor-1α contributes to glomerular injury and promotes hypertensive chronic kidney disease. Hypertension 66: 75–84, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuura H, Ichiki T, Ikeda J, Takeda K, Miyazaki R, Hashimoto T, Narabayashi E, Kitamoto S, Tokunou T, Sunagawa K. Inhibition of prolyl hydroxylase domain-containing protein downregulates vascular angiotensin II type 1 receptor. Hypertension 58: 386–393, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167106. [DOI] [PubMed] [Google Scholar]
- 19.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension 38: 635–638, 2001. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- 20.Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, Chen J, He J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int 88: 950–957, 2015. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res 31: 175–184, 2008. doi: 10.1291/hypres.31.175. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal 19: 1110–1120, 2013. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renassia C, Peyssonnaux C. New insights into the links between hypoxia and iron homeostasis. Curr Opin Hematol 26: 125–130, 2019. doi: 10.1097/MOH.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rincón J, Correia D, Arcaya JL, Finol E, Fernández A, Pérez M, Yaguas K, Talavera E, Chávez M, Summer R, Romero F. Role of angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci 124: 81–90, 2015. doi: 10.1016/j.lfs.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozen-Zvi B, Hayashida T, Hubchak SC, Hanna C, Platanias LC, Schnaper HW. TGF-β/Smad3 activates mammalian target of rapamycin complex-1 to promote collagen production by increasing HIF-1α expression. Am J Physiol Renal Physiol 305: F485–F494, 2013. doi: 10.1152/ajprenal.00215.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439–2446, 2007. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 27.Schietke RE, Hackenbeck T, Tran M, Günther R, Klanke B, Warnecke CL, Knaup KX, Shukla D, Rosenberger C, Koesters R, Bachmann S, Betz P, Schley G, Schödel J, Willam C, Winkler T, Amann K, Eckardt KU, Maxwell P, Wiesener MS. Renal tubular HIF-2α expression requires VHL inactivation and causes fibrosis and cysts. PLoS One 7: e31034, 2012. doi: 10.1371/journal.pone.0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schödel J, Ratcliffe PJ. Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol 15: 641–659, 2019. doi: 10.1038/s41581-019-0182-z. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber T, Salhöfer L, Quinting T, Fandrey J. Things get broken: the hypoxia-inducible factor prolyl hydroxylases in ischemic heart disease. Basic Res Cardiol 114: 16, 2019. doi: 10.1007/s00395-019-0725-2. [DOI] [PubMed] [Google Scholar]
- 30.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med 365: 537–547, 2011. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 31.Sievers LK, Eckardt KU. Molecular mechanisms of kidney injury and repair in arterial hypertension. Int J Mol Sci 20: 2138, 2019. doi: 10.3390/ijms20092138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugahara M, Tanaka T, Nangaku M. Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int 92: 306–312, 2017. doi: 10.1016/j.kint.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Jia Z, Liu G, Zhou L, Liu M, Yang B, Yang T. PPARγ agonist rosiglitazone suppresses renal mPGES-1/PGE2 pathway in db/db mice. PPAR Res 2013: 612971, 2013. doi: 10.1155/2013/612971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tajima S, Tsuchiya K, Horinouchi Y, Ishizawa K, Ikeda Y, Kihira Y, Shono M, Kawazoe K, Tomita S, Tamaki T. Effect of angiotensin II on iron-transporting protein expression and subsequent intracellular labile iron concentration in human glomerular endothelial cells. Hypertens Res 33: 713–721, 2010. doi: 10.1038/hr.2010.63. [DOI] [PubMed] [Google Scholar]
- 35.Takenaka T, Inoue T, Miyazaki T, Kobori H, Nishiyama A, Ishii N, Hayashi M, Suzuki H. Klotho ameliorates medullary fibrosis and pressure natriuresis in hypertensive rat kidneys. Hypertension 72: 1151–1159, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka T, Eckardt KU. HIF activation against CVD in CKD: novel treatment opportunities. Semin Nephrol 38: 267–276, 2018. doi: 10.1016/j.semnephrol.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Trujillo J, Molina-Jijón E, Medina-Campos ON, Rodríguez-Muñoz R, Reyes JL, Barrera D, Pedraza-Chaverri J. Superoxide anion production and expression of gp91(phox) and p47(phox) are increased in glomeruli and proximal tubules of cisplatin-treated rats. J Biochem Mol Toxicol 29: 149–156, 2015. doi: 10.1002/jbt.21679. [DOI] [PubMed] [Google Scholar]
- 38.Uil M, Scantlebery AML, Butter LM, Larsen PWB, de Boer OJ, Leemans JC, Florquin S, Roelofs JJTH. Combining streptozotocin and unilateral nephrectomy is an effective method for inducing experimental diabetic nephropathy in the ‘resistant’ C57Bl/6J mouse strain. Sci Rep 8: 5542, 2018. doi: 10.1038/s41598-018-23839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang JM, Chen AF, Zhang K. Isolation and primary culture of mouse aortic endothelial cells. J Vis Exp 118: e52965, 2016. doi: 10.3791/52965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Zeng H, Chen ST, Zhou L, Xie XJ, He X, Tao YK, Tuo QH, Deng C, Liao DF, Chen JX. Ablation of endothelial prolyl hydroxylase domain protein-2 promotes renal vascular remodelling and fibrosis in mice. J Cell Mol Med 21: 1967–1978, 2017. doi: 10.1111/jcmm.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Zeng H, Xie XJ, Tao YK, He X, Roman RJ, Aschner JL, Chen JX. Loss of prolyl hydroxylase domain protein 2 in vascular endothelium increases pericyte coverage and promotes pulmonary arterial remodeling. Oncotarget 7: 58848–58861, 2016. doi: 10.18632/oncotarget.11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Zheng X, Zhang J, Zhao S, Wang Z, Wang F, Shang W, Barasch J, Qiu A. Physiological functions of ferroportin in the regulation of renal iron recycling and ischemic acute kidney injury. Am J Physiol Renal Physiol 315: F1042–F1057, 2018. doi: 10.1152/ajprenal.00072.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li PL, Li N. Hypoxia-inducible factor-1α contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int 79: 300–310, 2011. doi: 10.1038/ki.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Zhu Q, Li PL, Dhaduk R, Zhang F, Gehr TW, Li N. Silencing of hypoxia-inducible factor-1α gene attenuates chronic ischemic renal injury in two-kidney, one-clip rats. Am J Physiol Renal Physiol 306: F1236–F1242, 2014. doi: 10.1152/ajprenal.00673.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid Redox Signal 7: 1337–1345, 2005. doi: 10.1089/ars.2005.7.1337. [DOI] [PubMed] [Google Scholar]
- 46.Yousefian M, Shakour N, Hosseinzadeh H, Hayes AW, Hadizadeh F, Karimi G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 55: 200–213, 2019. doi: 10.1016/j.phymed.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Yu X, Fang Y, Liu H, Zhu J, Zou J, Xu X, Jiang S, Ding X. The balance of beneficial and deleterious effects of hypoxia-inducible factor activation by prolyl hydroxylase inhibitor in rat remnant kidney depends on the timing of administration. Nephrol Dial Transplant 27: 3110–3119, 2012. doi: 10.1093/ndt/gfr754. [DOI] [PubMed] [Google Scholar]
- 48.Zhang ZZ, Wang W, Jin HY, Chen X, Cheng YW, Xu YL, Song B, Penninger JM, Oudit GY, Zhong JC. Apelin is a negative regulator of angiotensin II-mediated adverse myocardial remodeling and dysfunction. Hypertension 70: 1165–1175, 2017. doi: 10.1161/HYPERTENSIONAHA.117.10156. [DOI] [PubMed] [Google Scholar]
- 49.Zhao L, Liu Z, Yang F, Zhang Y, Xue Y, Miao H, Liao X, Huang H, Li G. Intrabody against prolyl hydroxylase 2 promotes angiogenesis by stabilizing hypoxia-inducible factor-1α. Sci Rep 9: 11861, 2019. doi: 10.1038/s41598-019-47891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou LY, Zeng H, Wang S, Chen JX. Regulatory role of endothelial PHD2 in the hepatic steatosis. Cell Physiol Biochem 48: 1003–1011, 2018. doi: 10.1159/000491968. [DOI] [PMC free article] [PubMed] [Google Scholar]