Abstract
Bone morphogenetic protein (BMP) receptor signaling is critical for the regulation of the endocrine system and cardiovascular structure and function. The objective of this study was to investigate whether Bmp3b, a glycoprotein synthetized and secreted by adipose tissue, is necessary to regulate glucose and lipid metabolism, adipogenesis, and cardiovascular remodeling. Over the course of 4 mo, Bmp3b-knockout (Bmp3b−/−) mice gained more weight than wild-type (WT) mice. The plasma levels of cholesterol and triglycerides were higher in Bmp3b−/− mice than in WT mice. Bmp3b−/− mice developed insulin resistance and glucose intolerance. The basal heart rate was higher in Bmp3b−/− mice than in WT mice, and echocardiography revealed eccentric remodeling in Bmp3b−/− mice. The expression of adipogenesis-related genes in white adipose tissue was higher in Bmp3b−/− mice than in WT control mice. In vitro studies showed that Bmp3b modulates the activity of the C/ebpα promoter, an effect mediated by Smad2/3. The results of this study suggest that Bmp3b is necessary for the maintenance of homeostasis in terms of age-related weight gain, glucose metabolism, and left ventricular (LV) remodeling and function. Interventions that increase the level or function of BMP3b may decrease cardiovascular risk and pathological cardiac remodeling.
Keywords: adipokines, adipose tissue, cardiac remodeling, diabetes, metabolic syndrome
INTRODUCTION
Worldwide, more than 1.9 billion adults are overweight and 650 million adults are obese; obesity-related diseases are responsible for 2.8 million deaths each year (48a). Obesity is closely associated with metabolic and cardiovascular abnormalities, including dyslipidemia, insulin resistance, diabetes, and hypertension. Several risk factors associated with the development of cardiovascular disease can cluster into what is defined as the metabolic syndrome, which includes dyslipidemia, increased blood pressure, and dysregulated glucose homeostasis (17). Presence of the metabolic syndrome is associated with a 2-fold increase in the risk of cardiovascular diseases and a 1.5-fold increase in the risk of all causes of mortality in adults (30).
Adipose tissue plays a key role in the regulation of body weight and metabolism, not only as a lipid storing and mobilizing tissue but also through the secretion of multiple adipokines, which mediate feeding behavior, absorption of nutrients from the digestive system, and insulin sensitivity (5). Brown adipose tissue, mostly present in the interscapular region in rodents and the subclavicular region in humans, contributes to nonshivering thermogenesis by converting the energy of free fatty acids and glucose oxidation into heat (31). In mice, activation of brown adipose tissue increases insulin sensitivity and resistance to weight gain associated with a high-fat diet (7). The regulation of adipogenesis involves a complex and highly organized program of transcription factors and epigenomic regulators of gene transcription (21). Crucial metabolic regulators expressed in adipose tissue include peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα), both key activators of adipogenesis. Together, these two transcription factors synergistically promote adipogenesis by increasing the expression of genes involved in adipocyte differentiation (49). C/EBPα is considered a key transcription factor regulating the development of fat cells because expression of C/EBPα is sufficient to trigger the differentiation of preadipocytes into mature adipocytes in cell culture (9). C/EBPα is active in mature adipocytes and activates the gene transcription program that drives insulin-stimulated glucose uptake (49).
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor (TGF)-β superfamily. Several members of the family are involved in the development of cardiovascular diseases, where BMP signaling plays an important role in pulmonary arterial hypertension, structural integrity of the vessels, and postinfarction remodeling of the heart (29). Treatment of mice with BMP2 or overexpression of BMP4 in transgenic mice increases adipogenesis in white adipose tissue and induces weight gain and diabetes (16, 28). In contrast, overexpression of BMP7 induces adipogenesis of brown adipose tissue, leading to an increase in energy expenditure and a reduction in weight gain (45). In addition, several BMP family members have shown direct cardioprotective effects, such as BMP4 in heart failure (13) and BMP10 and BMP2 in myocardial infarction (11, 35).
BMP3b, also known as Growth and Differentiation Factor 10 (GDF10), is expressed in both brown and white adipose tissue, as well as the aorta, brain, and bone, and modestly expressed in the stomach, lung, and heart (14). Hino et al. (15) observed that mice overexpressing Bmp3b in adipocytes and fed a high-fat diet had less weight gain, lower glucose intolerance, and higher energy expenditure than wild-type mice fed the same diet. Additional effects of treatment with BMP3b include induction of axonal sprouting and improvement in functional recovery after stroke (23). Depletion of Bmp3b increases mortality in a murine model of neonatal hypoxic-ischemic encephalopathy (32).
The objective of the present study was to investigate the role of Bmp3b in the regulation of weight gain, glucose metabolism, and cardiovascular function. We compared the metabolic and cardiovascular phenotype of Bmp3b-deficient (Bmp3b−/−) and wild-type (WT) mice. To elucidate how adipogenesis may be modified in Bmp3b−/− mice, we investigated the effect of Bmp3b treatment and depletion on the C/ebpα signaling pathway.
METHODS
Protocol.
Bmp3b−/− whole body knockout mice were obtained courtesy of Dr. Se-Jin Lee (Johns Hopkins University) and have been described previously (54). Both Bmp3b−/− and WT mice are on a C57BL/6J genetic background and were bred in the Animal Facility at the University of Pennsylvania. All animals were fed a standard diet with food and water given ad libitum and maintained on a standard 12:12-h light-dark cycle. Mouse body weight was recorded weekly. At 2 or 6 mo of age male and female mice were euthanized with pentobarbital (200 mg/kg), and interscapular brown adipose tissue (BAT), epididymal and subcutaneous adipose tissue, and left ventricle (LV) were excised, weighed immediately, and stored at −80°C. Liver and skeletal muscle (quadriceps) were excised and stored immediately at −80°C. The protocol was approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee.
Metabolic analysis.
Mice were fasted for 6 h, and blood glucose was measured in awake mice by tail bleeding at 0, 15, 30, 60, 90, and 120 min after injection. For glucose tolerance tests, mice received an intraperitoneal injection of glucose (2 mg/kg body wt). For insulin tolerance tests, mice received an intraperitoneal injection of insulin (0.3 U/kg body wt or 0.5 U/kg body wt at 2 mo or 6 mo of age, respectively). The glucose disappearance rate (kITT) was calculated with the formula (0.693/t1/2) × 100, where t1/2 is the half-life of the process.
Metabolic cage studies were conducted with a Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments) at the University of Pennsylvania Rodent Metabolic Phenotyping Core. Male mice were acclimated in the metabolic chambers before the start of the experiments. Food intake, movement, and CO2 and O2 levels were measured for 72 h.
Blood pressure measurements.
Male mice at 24 wk of age were implanted with radiotelemeters (model No. TA11PA-C20; DSI) as previously described (52). After 1-wk recovery, continuous mean pressure, systolic pressure, diastolic pressure, pulse pressure, activity, and heart rate were monitored for 3 days with the DataQuest LabPro Acquisition System.
Plasma cholesterol, triglyceride, nonesterified fatty acid, and insulin determination.
Mice were fasted for 4 h, and blood samples were collected. Plasma was separated by centrifugation and stored at −80°C until being analyzed. Commercial kits were used to measure total cholesterol and triglycerides (TG) (Alfa Wassermann Diagnostic Technologies), high-density lipoprotein cholesterol (HDL-C), and nonesterified fatty acids (NEFA) (Wako Diagnostics) according to the manufacturer’s instructions. Insulin was measured with an ELISA kit (Mercodia) according to the manufacturer’s instructions.
Echocardiography.
Transthoracic echocardiography was performed as described previously with isoflurane sedation (1%) (44). Left ventricular ejection fraction (LVEF), posterior wall thickness (PWT), intraventricular septum (IVS), LV end-diastolic (LVEDD) and end-systolic (LVESD) diameters, and heart rate were obtained from M-mode tracings at the level of the papillary muscles. LV end-diastolic (LVEDV) and end-systolic (LVESV) internal volumes were obtained with a parasternal long-axis two-dimensional view. Relative wall thickness (RWT) was calculated with the following formula: RWT = (PWT + IVS)/LVEDD. Three measurements were averaged for each animal.
Histology.
Mouse tissues were fixed in 4% paraformaldehyde (PFA) overnight, dehydrated in 100% ethanol, and embedded in paraffin for sectioning (5 µm). Adipose tissue and liver were stained with hematoxylin and eosin, and adipocyte area was measured as an average of 20 cells per section in eight mice per group with ImageJ software. Heart tissue was stained with Sirius Red and Masson’s trichrome to evaluate fibrosis.
Cardiomyocyte isolation.
Primary adult ventricular cardiomyocytes were isolated from 6-mo-old male WT and Bmp3b−/− mice. After euthanasia, the heart was rapidly isolated and cardiomyocytes were obtained by enzymatic digestion. Briefly, the aorta was cannulated and the heart was rinsed for 5 min with a Tyrode solution [in mM: 120.4 NaCl, 14.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4·7H2O, 10 Na-HEPES, 4.6 NaHCO3, 30 taurine, 10 butanedione monoxime (BDM), 5.5 glucose; pH adjusted to 7]. The heart was then perfused with an enzymatic solution containing 2.4 mg/mL collagenase II (Worthington Biochemical). The LV was isolated, minced, and filtered through a 100-µm filter. Finally, calcium was gradually reintroduced to physiological concentrations.
Cell contractility.
Cardiomyocytes were suspended in Tyrode buffer (in mM: 132 NaCl, 4.8 KCl, 1.2 MgCl2, 10 HEPES, 1 CaCl2, 5 glucose). In contractility assays, cells were stimulated in a 37°C incubation chamber with an electrical field at 1 Hz with a myopacer (IonOptix MYP100) through carbon electrodes lowered into a glass-bottom dish containing the cells and mounted on a Nikon Eclipse TE2000-U inverted confocal microscope with a ×40 objective and a transmitted light camera (IonOptix MyoCam-S). Sarcomere length was measured optically by Fourier transform analysis (IonWizard; IonOptix). After 20 s of 1-Hz pacing to achieve steady state, five traces were recorded and analyzed. IonWizard software was used to analyze and quantify the data (IonOptix LLC, Milton, MA).
Gene expression analysis.
Total RNA was isolated from tissues with TRIzol reagent (Life Technologies), and complementary DNA was synthesized with a High Capacity cDNA Archive Kit (Thermo Fisher Scientific). Quantification of mRNA levels was performed by real-time PCR. Forward and reverse primers used were as follows (5′→3′): Bmp2 forward: GCTAGATCTGTACCGCAGGC; Bmp2 reverse: CTCCACGGCTTCTTCGTGAT; Bmp3 forward: AACAATGAGCTACCTGGGGC; Bmp3 reverse: CCTACTCTTCTCAGGGGGCT; Bmp4 forward: GCTACCAGGCCTTCTACTGC; Bmp4 reverse: ACTAGGGTCTGCACAATGGC; C/ebpα forward: GTGGACAAGAACAGCAACGAG; C/ebpα reverse: ACGTTGCGTTGTTTGGCTTTA; Cd36 forward: GCCAGTCGGAGACATGCTTA; Cd36 reverse: CCACGTCATCTGGGTTTTGC; Cidea forward: TGCTCTTCTGTATCGCCCAGT; Cidea reverse: GCCGTGTTAAGGAATCTGCTG; Col1α1 forward: CGCCATCAAGGTCTACTGC; Col1α1 reverse: GAATCCATCGGTCATGCTCT; Cox5b forward: GCTGCATCTGTGAAGAGGACAAC; Cox5b reverse: CAGCTTGTAATGGGTTCCACAGT; Cox7a1 forward: CAGCGTCATGGTCAGTCTGT; Cox7a1 reverse: AGAAAACCGTGTGGCAGAGA; Cpt1b forward: GCTCATTTCCGGGACAAAGG; Cpt1b reverse: TTGGTACAGGAACGCACAGT; Cycs forward: GCAAGCATAAGACTGGACCAAA; Cycs reverse: TTGTTGGCATCTGTGTAAGAGAATC; Dgat1 forward: AAGAGGACGAGGTGCGAGAC; Dgat1 reverse: TGGCACCTCAGATCCCAGTAG; Glut4 forward: ACGGATAGGGAGCAGAAA; Glut4 reverse: AAGGGTGAGTGAGGCATT; Pgc1α forward: CCCTGCCATTGTTAAGACC; Pgc1α reverse: TGCTGCTGTTCCTGTTTTC; Pparα forward: GCGTACGGCAATGGCTTTAT; Pparα reverse: GAACGGCTTCCTCAGGTTCTT; Pparγ2 forward: GCATGGTGCCTTCGCTGA; Pparγ2 reverse: TGGCATCTCTGTGTCAACCATG; Tgfβ1 forward: CTGCTGACCCCCACTGATAC; Tgfβ1 reverse: AGCCCTGTATTCCGTCTCCT. PrimeTime qPCR probe assays (Integrated DNA Technologies) were used for Bmp3b (Mm.PT.58.31156962) and Ucp1 (Mm.58.7088262). TATA-binding protein (TBP) (Mm.PT.39a.22214839) was used as endogenous control.
Immunoblotting analysis.
Total proteins were extracted in RIPA buffer containing protease inhibitors, and their concentrations were determined with the BCA Protein Assay Kit. Protein extracts were resolved by SDS-PAGE and transferred to Immobilon PVDF membranes. Blots were incubated with antibodies against phospho-Akt (no. 9271), Akt (no. 9272), phospho-ERK1/2 (no. 9101), ERK1/2 (no. 9102), phospho-IRS1 (no. 3203), IRS1 (no. 3407), phospho-Smad2/3 (no. 8828), total-Smad2/3 (no. 5678), β-actin (no. 3700), and Gapdh (no. 2118), all purchased from CST. Detection was performed with the SuperSignal detection system.
Small interfering RNA transfection.
3T3-L1 cells were transfected with BMP3b Silencer Select Pre-designed small interfering RNA (siRNA) (Ambion, siRNA ID no. s66568) or Silencer Select Negative Control no. 1 with Lipofectamine RNAiMAX. After 24 h, cells were scraped to corroborate Bmp3b inhibition or lipofected with the corresponding plasmid.
Generation of C/ebpα promoter construct.
Mouse C/ebpα promoter (1,437-bp fragment, from −1440 to −3 relative to the ATG) was amplified by PCR from genomic DNA. The primers used were 5′-TTAACTCGAGGGTCTTCGGGGTGCAAAAAC-3′ (forward; XhoI site is underlined) and 5′-TTCAAAGCTTGGAGTTAGAGTTCTCCCGGC-3′ (reverse; HindIII site is underlined). The PCR product was cloned into the pGL3-Enhancer vector (Promega) (pGL3/pCebpα).
Transient transfection and luciferase assays.
The pGL3/pCebpα was transfected in 3T3-L1 cells with Lipofectamine 2000. The pRL-SV40 was included as an internal control. Luciferase activity was measured after 6-h BMP3b treatment in cell lysates with the Dual-Luciferase Reporter Assay System.
Statistical analysis.
Statistical data analysis was performed with GraphPad Prism. For the comparison between genotypes, significant differences were established by unpaired Student’s t test. For the analysis of changes over time in the different genotypes, as weight and glucose and insulin tolerance tests, we used a two-way ANOVA followed by Bonferroni correction for multiple comparisons. To compare telemetry parameters, we used multiple unpaired t test. Data are expressed as means ± SE unless otherwise stated. In all experiments, differences were considered significant at P < 0.05.
RESULTS
Bmp3b deficiency is associated with weight gain, increased adipocyte size, and increased adipose tissue.
Obesity is characterized by an increase in white adipose tissue (WAT) accumulation, which results from an increase in adipocyte size and/or an increase in adipocyte number. When WT and Bmp3b-overexpressing mice were fed a high-fat diet, the Bmp3b-overexpressing mice gained less weight (15). To investigate the effect of Bmp3b deficiency on body weight, we obtained Bmp3b−/− mice (54) and assessed weight gain while the mice were fed a standard (low fat) chow diet.
In 6-mo-old WT mice, Bmp3b was highly expressed in the aorta and expressed in the white adipose tissue (inguinal and subcutaneous, iWAT and sWAT, respectively) and brain (Supplemental Fig. S1A; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.11563254).
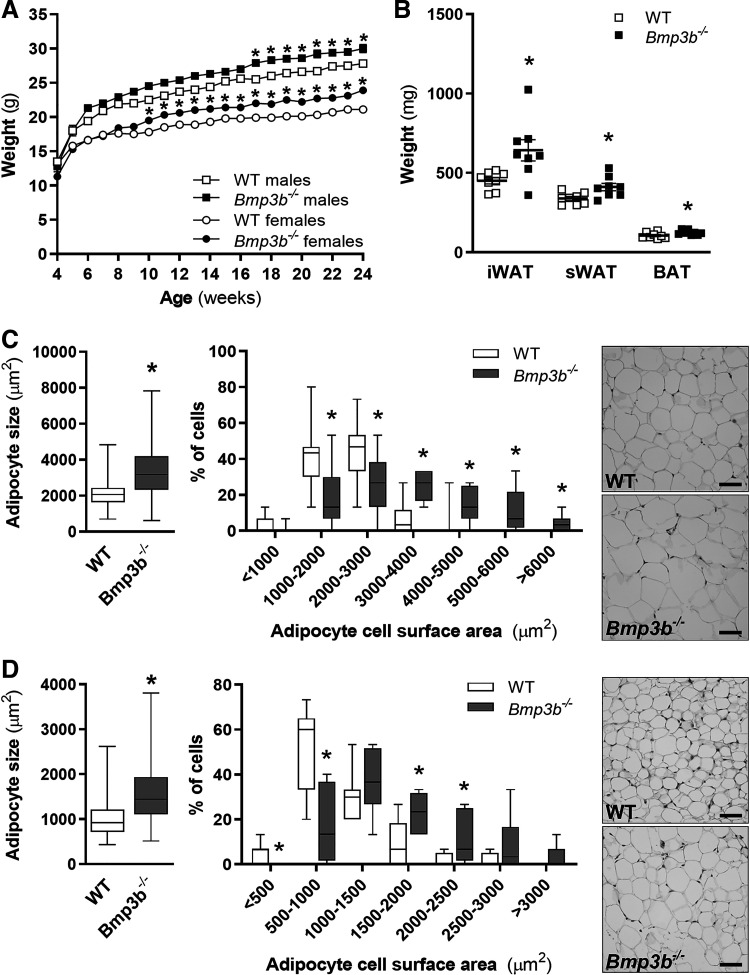
To consider the possibility that Bmp3b−/− mice might overexpress other BMPs to compensate for the absence of Bmp3b, the levels of Bmp2, Bmp3, and Bmp4 mRNA were measured in adipose tissue. Compared with WT mice, Bmp3b−/− mice had no increase in the levels of Bmp3 and Bmp4 mRNA in iWAT, sWAT, or brown adipose tissue (BAT) (Supplemental Fig. S1). There was a modest increase in the expression of Bmp2 in the sWAT of Bmp3b−/− mice. Mice were weighed weekly while eating a standard diet. At 4 and 6 mo of age, male Bmp3b−/− mice weighed more than male WT mice [WT vs. Bmp3b−/−: 25.6 ± 0.4 vs. 27.0 ± 0.4 g (P < 0.05) and 27.8 ± 0.3 vs. 30.0 ± 0.6 g (P < 0.05); 4 and 6 mo, respectively] (Fig. 1A). Female Bmp3b−/− mice also weighed more than female WT mice, starting at an earlier age (10-wk-old WT vs. Bmp3b−/−: 17.8 ± 0.3 vs. 19.5 ± 0.3 g; P < 0.05) (Fig. 1A). At 6 mo, the excised weight of iWAT, sWAT, and BAT was greater in male Bmp3b−/− mice than in age- and sex-matched control mice (Fig. 1B). There was no difference in the weight of iWAT, sWAT, and BAT tissue between male Bmp3b−/− and male WT mice at 2 mo of age (Supplemental Fig. S2A).
Fig. 1.
Bmp3b deficiency is associated with weight gain and increased adipocyte size. Mice were fed regular chow. A: body weight of male and female wild-type (WT) and Bmp3b-knockout (Bmp3b−/−) mice was measured weekly (n = 10–15 mice in each group). B: inguinal white adipose tissue (iWAT), subcutaneous white adipose tissue (sWAT), and brown adipose tissue (BAT) were weighed in 6-mo-old WT and Bmp3b−/− male mice (n = 8 mice in each group). C and D: adipocyte size was measured in iWAT (C) and sWAT (D) in 6-mo-old WT and Bmp3b−/− male mice (n = 160 cells, 8 mice in each group). Representative hematoxylin and eosin staining is shown on right. Scale bars, 50 µm. Values are means ± SE. *P < 0.05 vs. WT.
To investigate the possibility that the cross-sectional area of adipocytes differed between WT and Bmp3b−/− mice, iWAT, sWAT, and BAT were excised, fixed, sectioned, and stained with hematoxylin and eosin. At 2 mo of age, there was no difference in adipocyte area between WT and Bmp3b−/− mice (Supplemental Fig. S2B). At 6 mo of age, the cross-sectional area of adipocytes was greater in iWAT and sWAT obtained from Bmp3b−/− mice compared with iWAT (Fig. 1C) and sWAT (Fig. 1D) obtained from WT mice. Taken together, the results show a progressive development of obesity with an increase in body weight, adipose tissue weight, and adipocyte cross-sectional area in Bmp3b−/− mice.
Bmp3b deficiency is associated with dyslipidemia, impaired glucose tolerance, and increased insulin resistance.
Because Bmp3b−/− mice had a greater increase in weight gain over time than WT mice, we considered the possibility that the deficiency of Bmp3b was associated with alterations in lipid, insulin, and glucose levels. There was no difference in the levels of plasma cholesterol, high-density lipoprotein (HDL), and triglycerides between Bmp3b−/− mice and WT mice at 2 mo of age (Supplemental Table S1). Compared with age- and sex-matched WT mice, 6-mo-old Bmp3b−/− male mice had increased plasma levels of cholesterol, HDL-cholesterol, and triglycerides (Table 1). In contrast, the levels of nonesterified fatty acids (NEFA) and non-HDL-cholesterol were the same in 6-mo-old Bmp3b−/− and WT male mice. Compared with WT mice, 6-mo-old female Bmp3b−/− mice had increased plasma levels of cholesterol, HDL-cholesterol, non-HDL-cholesterol, triglycerides, and NEFA (Table 1). These results show that both male and female Bmp3b−/− mice develop hyperlipidemia, which can be detected at 6 mo of age.
Table 1.
Plasma biochemical analysis in 4-h fasted male and female WT and Bmp3b−/− mice at 6 mo of age
| Males (n = 8) |
Females (n = 7) |
|||||
|---|---|---|---|---|---|---|
| WT | Bmp3b−/− | P | WT | Bmp3b−/− | P | |
| Cholesterol, mg/dL | 97 ± 2 | 106 ± 3 | 0.03 | 85 ± 3 | 99 ± 3 | 0.01 |
| HDL-C, mg/dL | 66 ± 2 | 73 ± 2 | <0.01 | 54 ± 3 | 63 ± 1 | 0.01 |
| Non-HDL-C, mg/dL | 31 ± 1 | 33 ± 1 | n.s. | 31 ± 1 | 37 ± 2 | 0.03 |
| Triglycerides, mg/dL | 77 ± 2 | 94 ± 8 | 0.05 | 58 ± 2 | 69 ± 3 | 0.02 |
| NEFA, meq/L | 1.05 ± 0.10 | 1.00 ± 0.10 | n.s. | 0.75 ± 0.08 | 1.03 ± 0.05 | 0.01 |
| Insulin, µg/L | 0.40 ± 0.01 | 0.57 ± 0.04 | <0.01 | 0.35 ± 0.01 | 0.34 ± 0.01 | n.s. |
Data represent means ± SE for n mice. Bmp3b−/−, Bmp3b− knockout; HDL-C, high-density lipoprotein cholesterol; NEFA, nonesterified fatty acids; n.s., not significant; WT, wild type.
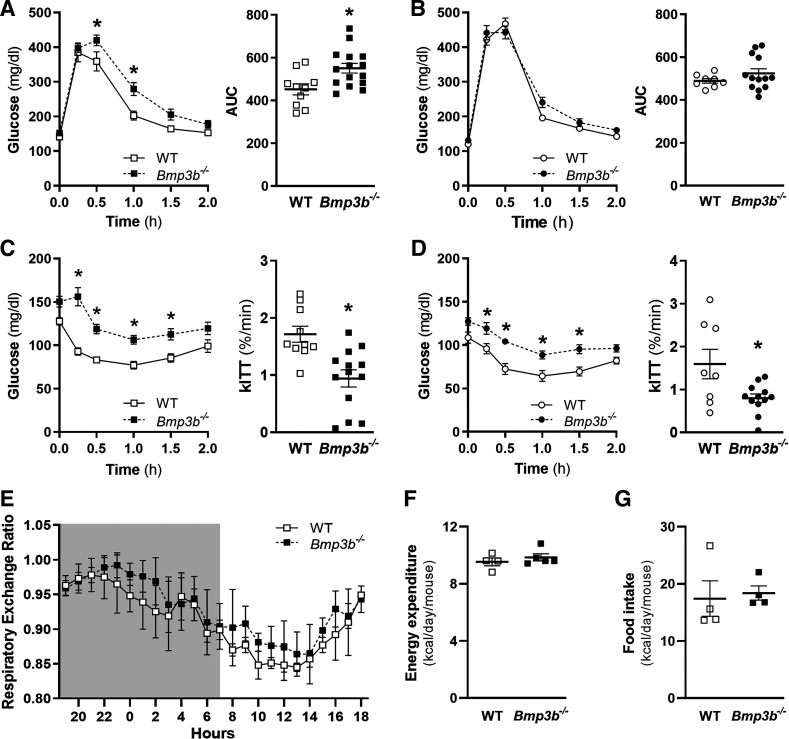
At 2 mo of age, there was no difference in fasting glucose and insulin levels in male or female Bmp3b−/− mice compared with age- and sex-matched WT mice. In 6-mo-old male mice, there was an increase in fasting insulin in Bmp3b−/− compared with WT mice (Table 1), but no differences were observed in females, and fasting glucose also remained without alteration in male and female WT and Bmp3b−/− mice. After glucose or insulin injections, neither male nor female 2-mo-old Bmp3b−/− mice had differences in plasma glucose concentrations compared with their WT counterparts (Supplemental Fig. S2, C and D). However, compared with age- and sex-matched WT mice, 6-mo-old male Bmp3b−/− mice had decreased glucose tolerance and increased insulin resistance after glucose or insulin injection (Fig. 2, A and C). In 6-mo-old female mice, there was no difference in glucose tolerance, but insulin resistance was noted in Bmp3b−/− mice compared with WT mice (Fig. 2, B and D). The constant for the rate of glucose disappearance during the ITT (kITT) was also decreased in male and female Bmp3b−/− mice compared with WT mice. These results demonstrate age-dependent metabolic abnormalities of glucose tolerance in male Bmp3b−/− mice and insulin resistance in male and female Bmp3b−/− mice.
Fig. 2.
Glucose tolerance (GTT) and insulin tolerance (ITT) tests and energy expenditure in 6-mo-old Bmp3b-knockout (Bmp3b−/−) mice. GTT (A and B) and ITT (C and D) were performed on 6-mo-old male (A and C) and female (B and D) wild-type (WT) and Bmp3b−/− mice. Mice were fasted for 6 h and injected with 2 g/kg body wt glucose (GTT) or 0.5 U/kg body wt insulin (ITT). (n = 8–12 mice). Values are means ± SE. *P < 0.05 vs. WT. AUC, area under curve; kITT, glucose disappearance rate. E–G: metabolic cage studies were performed in WT and Bmp3b−/− male mice fed a chow diet for 72 h. Respiratory exchange ratio (E), energy expenditure (F), and food consumption (G) were measured (n = 4 or 5 mice). Data are represented as means ± SE.
To further investigate metabolic differences between WT and Bmp3b−/− mice, 6-mo-old WT and Bmp3b−/− male mice were placed in metabolic cages. There were no significant differences in respiratory exchange ratio [RER = carbon dioxide production (V̇co2)/oxygen consumption (V̇o2)] (Fig. 2E), energy expenditure (Fig. 2F), and food intake (Fig. 2G) between the WT and Bmp3b−/− mice. Therefore, the energy expenditure and the respiratory exchange ratio do not appear to be major contributors to the metabolic differences observed between WT and Bmp3b−/− mice.
Bmp3b−/− mice present alterations in the insulin and glucose pathways.
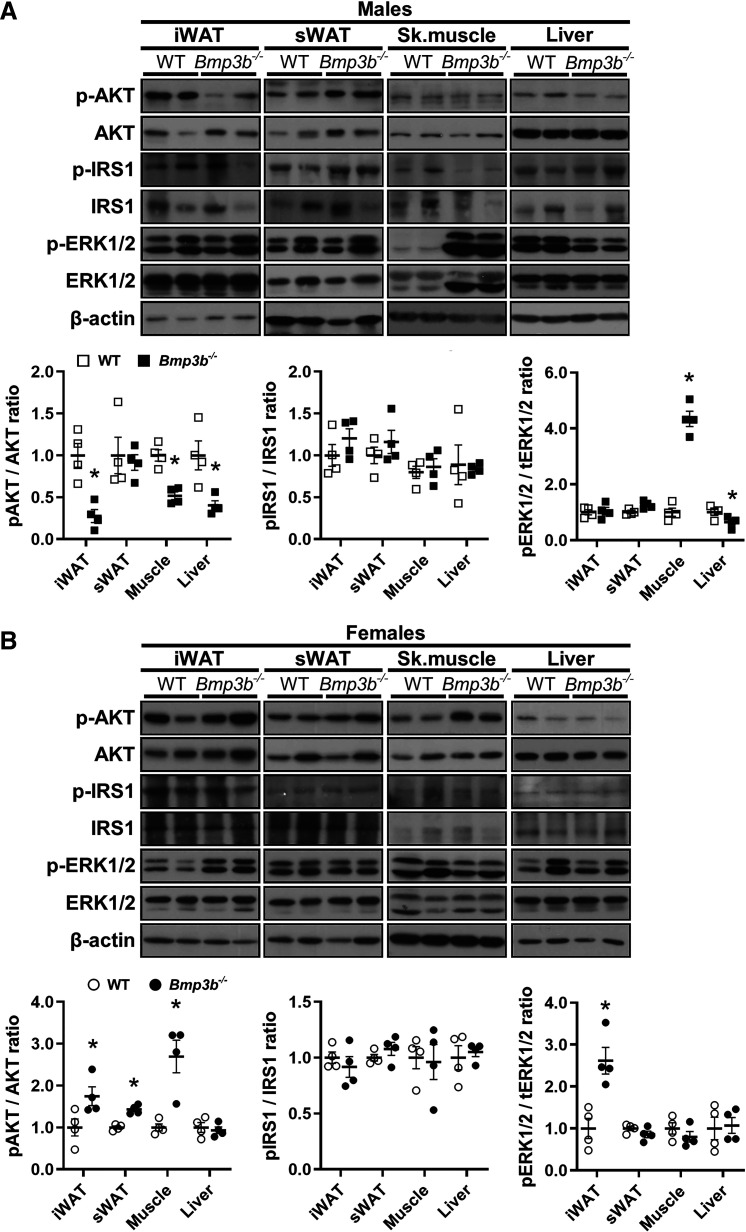
To further investigate which tissues might contribute to the glucose metabolism alterations observed in Bmp3b−/− mice, we analyzed the protein expression of key regulators of glucose and insulin metabolism in the inguinal and subcutaneous adipose tissue, liver, and skeletal muscle (quadriceps). Several differences in the insulin pathways were noted in the tissues of Bmp3b−/− mice compared with the WT mice at 6 mo of age. The phosphorylation of Akt was decreased in the iWAT, liver, and skeletal muscle of the Bmp3b−/− male mice compared with WT male mice (Fig. 3A). ERK1/2 phosphorylation was higher in the skeletal muscle of Bmp3b−/− male mice compared with WT male mice but was lower in the liver of Bmp3b−/− male mice and unchanged in the iWAT and sWAT. The expression of insulin receptor substrate 1 (IRS1) and its tyrosine phosphorylation were not modified in the different tissues analyzed (WAT, skeletal muscle, and liver) (Fig. 3A).
Fig. 3.
Glucose and insulin signaling pathway in inguinal white adipose tissue (iWAT), subcutaneous white adipose tissue (sWAT), liver, and skeletal muscle in 6-mo-old Bmp3b-knockout (Bmp3b−/−) mice. Protein expression of Akt, insulin receptor substrate 1 (IRS1), and ERK1/2 phosphorylation (p) was analyzed by Western blot in iWAT, sWAT, skeletal muscle, and liver tissues from wild-type (WT) and Bmp3b−/− male mice at 6 mo of age (A) or WT and Bmp3b−/− female mice at 6 mo of age (B). Total Akt, IRS1, and ERK1/2 are also shown. Levels of β-actin are shown as loading controls. Densitometric analyses of Western blots are shown at bottom (n = 4 mice). Data are expressed as means ± SE. *P < 0.05 vs. WT.
The analysis of the same pathways and tissues in 6-mo-old Bmp3b−/− females revealed an increased phosphorylation of Akt in the iWAT, sWAT, and skeletal muscle of the Bmp3b−/− female mice compared with WT female mice; Akt phosphorylation was not modified in the liver (Fig. 3B). The phosphorylation of ERK1/2 was increased in the iWAT of Bmp3b−/− female mice compared with WT female mice, but it was not altered in the other tissues (Fig. 3B). Phosphorylated and total IRS1 were not altered in the different tissues analyzed between the Bmp3b−/− and WT female mice (Fig. 3B). Thus, Bmp3b deficiency is associated with different responses of the Akt and ERK pathways in male and female mice.
Bmp3b−/− mice have an increased resting heart rate.
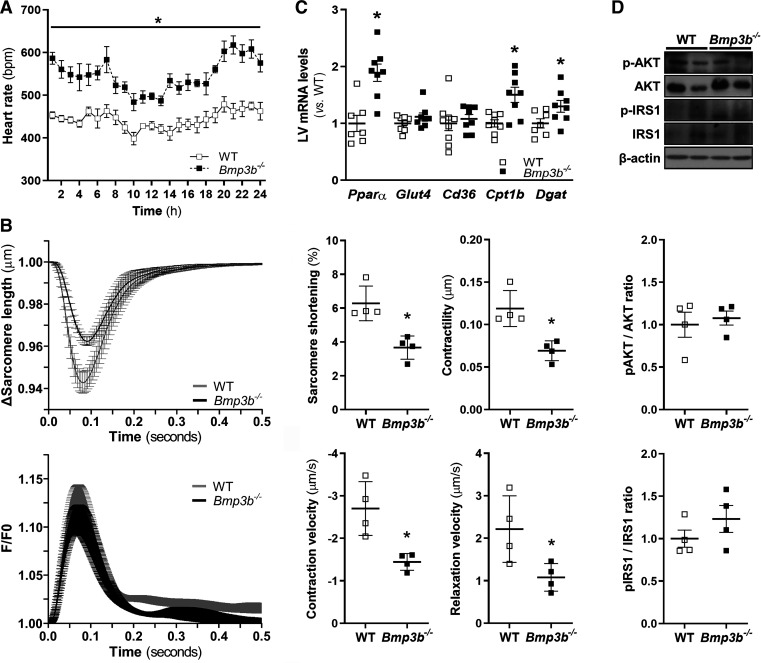
To investigate the effect of Bmp3b deficiency on blood pressure and heart rate, 6-mo-old mice were implanted with a radiotelemetry device to measure blood pressure and heart rate under normal physiological conditions during three consecutive days. Heart rate was higher in Bmp3b−/− male mice than in WT male mice (Fig. 4A). No differences were observed in mean arterial, systolic, diastolic, and pulse pressure, as well as overall motor activity, between Bmp3b−/− and WT male mice (Supplemental Fig. S3).
Fig. 4.
Heart rate, cardiomyocyte contractility, and fatty acid metabolism are altered in Bmp3b−/− mice. A: heart rate [beats per minute (bpm)] was measured by telemetry over a 72-h period in 6-mo-old wild-type (WT) and Bmp3b-knockout (Bmp3b−/−) male mice. Values are plotted as the average of data collected every 5 min during the course of an hour. B: average traces of sarcomere shortening (top left) and Ca2+ transients [fluorescence intensity ratio (F/F0); bottom left] in 6-mo-old male WT and Bmp3b−/− cardiomyocytes (1 Hz; field stimulation). Sarcomere length and contraction and relaxation velocities were measured in Bmp3b−/− (■) and WT (□) (n = 4 mice). All values are means ± SD. *P < 0.05 vs. WT. C: mRNA levels were analyzed by real-time PCR in left ventricular (LV) tissue from WT and Bmp3b−/− mice (n = 8 mice). Values are means ± SE. *P < 0.05 vs. WT. D: Akt and insulin receptor substrate 1 (IRS1) phosphorylation were analyzed by Western blot in LV tissue from WT (□) and Bmp3b−/− (■) male mice. Total Akt and IRS1 are also shown. β-Actin is shown as a loading control. The graphs show the densitometric analysis of Western blots. Results are expressed as means ± SE (n = 4 mice).
Bmp3b−/− mice develop LV hypertrophy.
Recent studies showed that BMP signaling modulates myocyte function and has a protective role during postinfarction remodeling of the heart (29). We tested the hypothesis that Bmp3b−/− mice develop altered cardiac function over time. At 2 mo of age, there was no difference in systolic function or LV weight between Bmp3b−/− and WT male mice (Supplemental Table S2). In contrast, compared with WT control mice, 6-mo old Bmp3b−/− male mice had increased left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) and decreased left ventricular ejection fraction (LVEF). In addition, the relative wall thickness (RWT) was decreased in Bmp3b−/− mice (Table 2). At necropsy, the left ventricular weight relative to total body weight (LVW/BW) or to tibia length (LVW/TL) was greater in the 6-mo-old Bmp3b−/− mice compared with control mice. These findings suggest that eccentric cardiac hypertrophy develops in Bmp3b−/− mice over time.
Table 2.
Echocardiographic measurements and postmortem analysis of LV mass in male WT and Bmp3b−/− mice at 6 mo of age
| WT (n = 8) | Bmp3b−/− (n = 8) | P | |
|---|---|---|---|
| HR, beats/min | 441 ± 11 | 458 ± 7 | 0.241 |
| LVEF, % | 58 ± 1 | 53 ± 2 | 0.037 |
| PWT, mm | 0.68 ± 0.02 | 0.67 ± 0.03 | 0.895 |
| IVS, mm | 0.74 ± 0.02 | 0.68 ± 0.03 | 0.104 |
| LVEDD, mm | 3.7 ± 0.1 | 4.1 ± 0.1 | 0.002 |
| LVESD, mm | 2.6 ± 0.1 | 2.9 ± 0.1 | 0.014 |
| LVEDV, µL | 55 ± 1 | 72 ± 3 | <0.0001 |
| LVESV, µL | 23 ± 1 | 33 ± 2 | <0.001 |
| SV, µL | 32 ± 1 | 38 ± 2 | 0.015 |
| CO, mL/min | 14 ± 1 | 17 ± 1 | 0.007 |
| RWT, % | 0.38 ± 0.01 | 0.33 ± 0.02 | 0.013 |
| LVW/BW, mg/g | 3.7 ± 0.1 | 4.4 ± 0.2 | 0.031 |
| LVW/TL, mg/mm | 5.3 ± 0.1 | 6.7 ± 0.3 | 0.001 |
Data represent means ± SE for n mice. Bmp3b−/−, Bmp3b knockout; BW, body weight; CO, cardiac output; HR, heart rate; IVS, end-diastolic interventricular septal thickness; LVEDD, left ventricular (LV) end-diastolic internal diameter; LVEDV, LV end-diastolic internal volume; LVEF, LV ejection fraction; LVESD, LV end-systolic internal diameter; LVESV, LV end-systolic internal volume; LVW, LV weight; PWT, end-diastolic posterior wall thickness; RWT, relative wall thickness; SV, stroke volume; TL, tibia length; WT, wild type.
Cardiomyocyte contractility is reduced in Bmp3b−/− mice.
To investigate the mechanism by which Bmp3b deficiency causes eccentric remodeling, we studied the contractility of isolated ventricular cardiomyocytes from WT and Bmp3b−/− mice. There was no difference in contractility between cardiomyocytes obtained from 2-mo-old Bmp3b−/− and WT mice (Supplemental Fig. S4). In cardiomyocytes isolated from 6-mo-old Bmp3b−/− mice, there was decreased contractility (WT vs. Bmp3b−/−: 0.12 ± 0.01 vs. 0.07 ± 0.01 μm; P < 0.05), contraction velocity (WT vs. Bmp3b−/−: −2.70 ± 0.32 vs. −1.45 ± 0.10 μm/s; P < 0.05), and relaxation velocity (WT vs. Bmp3b−/−: 2.22 ± 0.39 vs. 1.08 ± 0.16 μm/s; P < 0.05) compared with cardiomyocytes from WT mice (Fig. 4B). The results suggest that cardiomyocytes isolated from Bmp3b−/− mice have decreased contractility compared with cardiomyocytes from WT control mice.
Lipid metabolism is altered in the heart and adipose tissue of Bmp3b−/− mice.
In patients with diabetes, there is a shift in the source of energy used by the heart, from glycolysis to fatty acid oxidation. Heart failure is associated with changes in myocardial metabolism, dependent on the stage of heart failure. Increased expression of the gene encoding Pparα results in the increased uptake and oxidation of fatty acids (18). To investigate whether cardiac energy production is shifted to an increased oxidation of fatty acids in Bmp3b−/− mice, we measured the left ventricular mRNA levels of Pparα, Glut4, Cd36, Cpt1b, and Dgat1. The expressions of Glut4 and Cd36 were not modified between WT and Bmp3b−/− mice (Fig. 4C). However, the expression of Pparα, Cpt1b, and Dgat1 was higher in the left ventricle of Bmp3b−/− mice than that of WT mice (Fig. 4C), suggesting an increase in the genes encoding proteins involved in cardiac fatty acid uptake and oxidation and esterification pathways.
To determine whether there were changes in myocardial glucose metabolism, we measured the levels of several proteins involved in the insulin pathways. The levels of phosphorylated insulin receptor substrate 1 (IRS1) and phosphorylated Akt were unchanged in the LV of Bmp3b−/− mice compared with the LV of WT mice (Fig. 4D). These findings suggest that myocardial glucose uptake was not markedly changed.
Although we could not observe any histological difference in fibrosis between WT and Bmp3b−/− mice (Supplemental Fig. S5A), a trend toward an increased expression of genes involved in profibrotic pathways (Tgfβ1 and Col1α1) was observed in the LV of Bmp3b−/− compared with WT mice (Supplemental Fig. S5B).
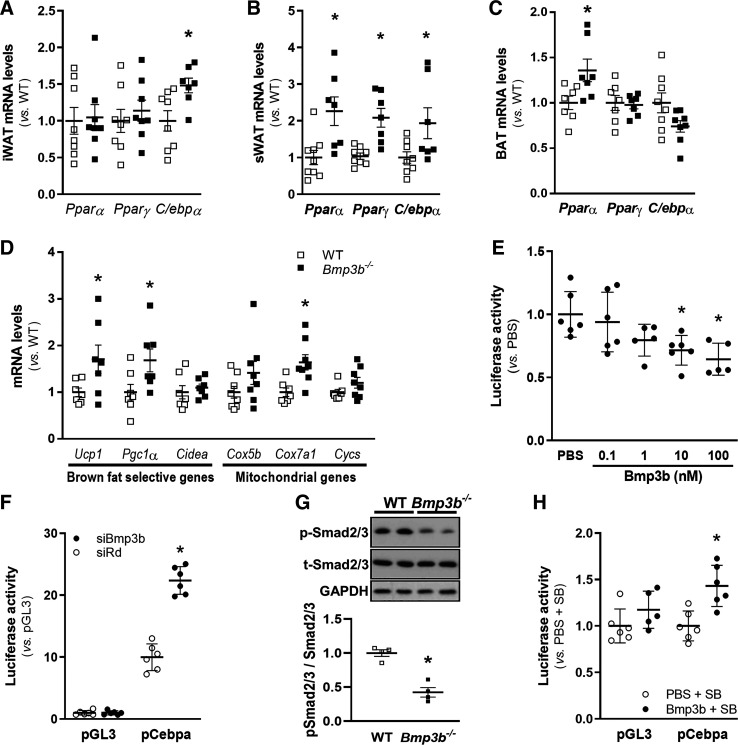
To determine whether the increase in adipose tissue weight was associated with an increase in adipogenesis, we measured the expression of adipogenesis-related genes in iWAT, sWAT, and BAT. The expression of C/ebpα was increased in the iWAT of Bmp3b−/− mice (Fig. 5A), and the expression of Pparγ and C/ebpα was increased in the sWAT of Bmp3b−/− mice (Fig. 5B) compared with WT mice. Therefore, the expression of key genes in adipogenesis is upregulated in Bmp3b−/− mice. The expression of markers of BAT activation (Ucp1, Pparα, Pgc1a, and Cox7a1) was increased in Bmp3b−/− mice compared with WT mice (Fig. 5, C and D).
Fig. 5.
Bmp3b regulates adipogenesis gene expression in adipose tissue and C/ebpα promoter activity through Smad2/3. A–D: mRNA levels were assessed by real-time PCR in wild-type (WT) and Bmp3b-knockout (Bmp3b−/−) male mouse inguinal white adipose tissue (iWAT; A), subcutaneous white adipose tissue (sWAT; B), and brown adipose tissue (BAT; C and D) (n = 7 or 8 mice). Results are expressed as means ± SE. *P < 0.05 vs. WT. E: luciferase activity was evaluated in 3T3-L1 preadipocyte cells transfected with the pGL3/pCebpα plasmid and treated with Bmp3b at different dosage levels over 6 h (n = 6 mice). Data are means ± SD. *P < 0.05 vs. PBS. F: 3T3-L1 preadipocyte cells were transfected with a small interfering (si)RNA against Bmp3b (siBmp3b) or a control siRNA (siRd) and after 24 h were transfected with the pGL3/pCebpα plasmid and the luciferase activity was analyzed (n = 6 mice). Results are expressed as means ± SD. *P < 0.05 vs. pGL3. G: phospho (p)-Smad2/3 and total (t)-Smad2/3 protein levels were determined by Western blot in sWAT in Bmp3b−/− (■) or WT (□) male mice. Levels of Gapdh are shown as the loading control. Results are expressed as means ± SD (n = 4 mice). *P < 0.05 vs. WT. H: 3T3-L1 cells were transfected with the pGL3/pCebpα (●) or the corresponding empty plasmid (pGL3, ○). After 24 h, cells were treated with SB431542 (SB, 5 µM) and Bmp3b (10 nM) or PBS. After 6 h, luciferase activity was analyzed (n = 6 mice). Results are expressed as means ± SD. *P < 0.05 vs. pGL3 + SB.
Bmp3b regulates C/ebpα promoter activity through Smad2/3.
The effect of Bmp3b treatment or depletion on the C/ebpα signaling pathway was further investigated through the study of Cebpα promoter in 3T3-L1 preadipocytes by luciferase assays. BMP3b treatment dose-dependently decreased the transcriptional activity of Cebpα promoter in 3T3-L1 preadipocytes transfected with the Cebpα promoter reporter plasmid. Treatment of transfected cells with 10 and 100 nM Bmp3b produced a 30% and 35% reduction in Cebpα promoter activity, respectively, compared with control cells treated with PBS (Fig. 5E). Conversely, Cebpα promoter activity was increased twofold when expression of Bmp3b was inhibited with siRNA (Fig. 5F).
Previous investigators showed that the effects of Bmp3b are mediated by Smad transcription factors (15, 23). sWAT tissue was obtained from WT and Bmp3b−/− mice, and the level of phospho-Smads was measured by immunoblot. Compared with WT mice, phospho-Smad2/3 levels were reduced in the sWAT of Bmp3b−/− mice (Fig. 5G). To further investigate the potential involvement of Smad2/3 in activation of the Cebpα promoter, 3T3-L1 cells containing the Cebpα reporter were treated with BMP3b and SB431542, a specific inhibitor of phosphorylation of Smad2/3. Treatment with SB431542 inhibited the BMP3b-induced decrease in Cebpα promoter activity (Fig. 5H). The results show that BMP3b decreases C/ebpα promoter activity through Smad2/3.
DISCUSSION
In the present study, we report that Bmp3b has an important role in the regulation of age-related weight gain and metabolic homeostasis in mice. Although a prior study reported that overexpression of Bmp3b in adipocytes limited weight gain and glucose intolerance in mice fed a high-fat diet (15), our study is the first to reveal that Bmp3b has a physiological role and is necessary in the regulation of metabolism and myocardial function.
Compared with WT mice, Bmp3b−/− mice gained more weight, had increased plasma levels of cholesterol and triglycerides, developed insulin resistance and glucose intolerance, and demonstrated LV eccentric hypertrophy with decreased cardiomyocyte contractility. The deficiency in Bmp3b was associated with an increase in C/ebpα and Pparγ expression in white adipose tissue. Moreover, inhibition of Bmp3b in 3T3-L1 cells induced C/ebpα transcriptional activation through the Smad2/3 pathway.
Bmp3b deficiency was accompanied by a progressive increase in body weight in mice fed a regular chow diet. Although Bmp3−/− mice have been reported to have increased bone density (8), no difference in bone weight was noted between Bmp3b−/− and WT mice (data not shown). In addition to an increased weight, Bmp3b−/− mice presented hypertrophy of their adipose tissue, with an augmentation in adipose tissue weight and adipocyte size in the epididymal and subcutaneous fat pads.
Bmp3b−/− mice also developed metabolic abnormalities, including increased plasma levels of lipids and a state of prediabetes with altered glucose metabolism. The mechanism underlying the glucose intolerance appears to be insulin resistance, rather than a failure to produce insulin, as demonstrated by the preserved (in females) or increased (in males) insulin plasma levels in Bmp3b−/− mice. The insulin resistance and glucose intolerance were accompanied by higher plasma levels of lipids, including cholesterol, triglycerides, and NEFA.
The presence of the metabolic abnormalities in Bmp3b−/− mice may be caused by the increase in adipose tissue and obesity. A direct role of Bmp3b deficiency on glucose metabolism is also possible, as Bmp3b deficiency increases the expression of C/ebpα, which regulates glucose metabolism; both prenatal and postnatal ablation of C/ebpα induces severe hypoglycemia through a pleiotropic response, where C/ebpα-knockout mice fail to accumulate lipid in adipose tissue and liver (47, 51). The metabolic phenotype of Bmp3b deficiency appears more pronounced in males, in which glucose intolerance and insulin resistance are present at 6 mo of age. In females, we did not detect differences in glucose tolerance between Bmp3b−/− and WT mice. In genetically and high-fat diet-induced rodent models of obesity, females developed a milder metabolic phenotype than males (34), possibly because estrogens have a protective role in females, improving glucose tolerance and increasing insulin sensitivity (25).
Phosphorylated Akt was lower in the liver, skeletal muscle, and adipose tissue of Bmp3b−/− male mice compared with the tissues of WT mice. Akt activation promotes glucose uptake in the skeletal muscle (42), activates glycogen synthesis (19), limits gluconeogenesis in the liver (24), and contributes to the insulin-induced inhibition of lipolysis in adipocytes (10). Therefore, the decrease in Akt activation observed in the liver, skeletal muscle, and adipose tissue may contribute to the alterations in glucose metabolism and the insulin resistance observed in Bmp3b−/− male mice. Bmp3b−/− male mice also had increased phosphorylation of ERK in the skeletal muscle, which may contribute to insulin resistance (41).
A different response of Akt and ERK was noted in Bmp3b−/− female mice. The increased level of phosphorylated Akt noted in the iWAT, sWAT, and skeletal muscle of Bmp3b−/− female mice may contribute to the finding that the Bmp3b−/− female mice have preserved glucose tolerance whereas Bmp3b−/− male mice do not (53). An increase in the phosphorylation of ERK was also noted in the inguinal adipose tissue of Bmp3b−/− female mice compared with WT female mice. Increased phosphorylation of ERK in adipose tissue has been reported in obese mice, and its inhibition decreases insulin resistance and glucose intolerance in these mice (33). It has been noted that sex differences in adipocytes include increased Akt and ERK phosphorylation at a lower insulin dose in adipocytes from female mice than in adipocytes from male mice (25); however, the difference in the effect of Bmp3b deficiency on the levels of pAKT and pERK between males and females is not clearly explained. The findings demonstrated a markedly altered metabolic phenotype in Bmp3b−/− male mice but a milder altered metabolic phenotype in Bmp3b−/− female mice. As female hormones have a proven protective role not only for the metabolic phenotype but also at cardiac level (25), we focused the exploration of the cardiac phenotype and molecular biology on Bmp3b−/− male mice.
The cardiac phenotype developed by Bmp3b−/− mice included decreased cardiomyocyte contractility, eccentric left ventricular hypertrophy, and increased heart rate. At 6 mo of age, the cardiomyocytes of Bmp3b−/− mice had impaired contractility and relaxation. A direct effect of Bmp3b on cardiomyocyte function is conceivable. Several BMPs have shown effects on the cardiovascular system: in particular, BMP10 has recently been reported to preserve cardiac function and promote cardiomyocyte survival in models of cardiomyocyte injury (35). The metabolic phenotype observed in the Bmp3b−/− mice could contribute to the decrease in cardiomyocyte contractility. A decreased contractility and relaxation in cardiomyocytes has been noted in some mouse models of high-fat diet-induced obesity (37). It is noteworthy, however, that the impairment of contractility in obesity is modest unless severe diabetes is present (39), which was not the case in Bmp3b−/− mice. Therefore, the metabolic abnormalities may not fully explain the marked impairment of cardiomyocyte function.
Bmp3b−/− mice developed LV eccentric hypertrophy, defined as an increase in LV mass, a decrease in relative wall thickness, and LV dilation (12). Eccentric hypertrophy can be a cardiac remodeling response to decreased cardiomyocyte contractility as an attempt to preserve cardiac output and is seen in dilated cardiomyopathies (2). Eccentric hypertrophy is present in ~10% of the general human population and is a powerful marker of poor prognosis, with increased development of cardiovascular diseases and mortality, even after adjustment for cardiovascular risk factors (55). Eccentric hypertrophy is also frequent in obesity and has been attributed to obesity-related volume overload (27) and increases in catecholamines, insulin, leptin, as well as other growth factors. Hyperinsulinemia, in turn, can induce LV hypertrophy by several mechanisms, including increased angiotensin II levels (4).
Increased heart rate was noted in Bmp3b−/− mice compared with WT mice. Tachycardia is frequently present in heart failure; however, it is not an early symptom and would be unlikely to be present in the mild LV dysfunction with preserved stroke volume observed in Bmp3b−/− mice. Elevated heart rate has been reported in obese patients compared with nonobese patients in several studies (36, 50). Investigators have suggested that there is an imbalance between the cardiac sympathetic and parasympathetic systems (38); this imbalance could explain the increased heart rate. Finally, as Bmp3b is expressed in the brain (54), a direct effect of Bmp3b deficiency on the sympathetic/parasympathetic balance would be conceivable. No increase in blood pressure was noted in the Bmp3b−/− mice; however, in humans, an increase in heart rate may precede an increase in blood pressure in obesity-prone individuals (4).
The modifications in the expression of genes involved in cardiac metabolism observed in the Bmp3b−/− mice were also similar to those reported in murine models of diabetes. The expression of Cpt1 and Dgat1, genes involved in fatty acid oxidation and triglyceride synthesis and turnover (39), respectively, was higher in the LV of Bmp3b−/− mice than in the LV of WT mice. Furthermore, an increase in the expression of Pparα was noted in the LV of Bmp3b−/− mice. Activation of Pparα is reported in the heart of diabetic mice and increases the uptake and oxidation of fatty acids (18). These findings suggest that Bmp3b deficiency is associated with an increase in the myocardial utilization of free fatty acids, as noted in patients with prediabetes and diabetes (1). Heart failure is associated with a switch from fatty acid utilization to carbohydrate utilization (3). There were no changes in the levels of phosphorylated IRS or AKT in the LV of Bmp3b−/− mice compared with that of WT mice. The cardiac phenotype of Bmp3b−/− mice may have been too mild to detect any changes in myocardial glucose metabolism (6). Moreover, no direct measurements of glucose oxidation were obtained in the present study.
Bmp-7, -8b, and -9 have been reported to limit obesity and insulin resistance in high-fat diet-induced obesity through an increase in mass or activation of brown adipose tissue (20, 45, 48). It would be conceivable that Bmp3b similarly augments brown adipose tissue mass or activity and Bmp3b deficiency decreases brown adipose tissue mass or activity. Such a mechanism, however, is unlikely to play a role, as the brown adipose tissue mass was increased in Bmp3b−/− mice, with an upregulation of Ucp1 and Pgc1α in the BAT, suggesting activation of the BAT rather than inhibition.
The expression of C/ebpα was increased in the WAT of Bmp3b−/− compared with WT mice. The induction of this proadipogenic transcription factor could provide a mechanism by which the lack of Bmp3b can induce the adipogenic differentiation and the increased weight gain in Bmp3b−/− mice. The divergence in Pparγ expression between iWAT and sWAT could originate in the difference in the activation of gene expression programs depending on the anatomical origin of the WAT (22).
To further investigate the mechanism by which the expression of Pparγ and C/ebpα was increased in WAT, we inhibited the expression of Bmp3b in 3T3-L1 cells that produced an increase on the transcriptional activity of C/ebpα promoter. Because C/EBPs have an important role in adipogenesis and adipocyte differentiation, this increase may potentially augment adipogenesis and weight gain. The mechanism by which Bmp3b promotes the activation of C/ebpα promoter was unknown. We observed that Bmp3b−/− mice had decreased phospho-Smad2/3 in their adipose tissue. Our findings also demonstrated that the inhibition of Smad2/3 by SB431542 prevented the effect of Bmp3b on C/ebpα transcriptional activity, suggesting that Bmp3b regulates C/ebpα through Smad2/3. Previously, Smad3 was reported to regulate the adipogenic function of C/ebpα in mesenchymal stem cells and 3T3-L1 preadipocytes (26). The region of the C/ebpα promoter interacting with Smad2/3 is unknown, but Smad3 binds directly to the DNA sequences AGAC or CAGAC in the Pparγ promoter (43); these SMAD-binding elements are also present in the C/ebpα promoter and may be regions of interest for future studies examining the regulation of C/ebpα expression.
In summary, Bmp3b has an important role in the regulation of adipogenesis and metabolism in mice. Bmp3b deficiency induces weight gain, prediabetes, and cardiac hypertrophy and dysfunction, and the presence or absence of Bmp3b can modulate the activity of C/ebpα promoter, an important adipogenesis-related gene. These findings suggest that Bmp3b signaling may be targeted to decrease obesity and its associated metabolic abnormalities.
GRANTS
This study was supported by a grant from the National Institutes of Health (NIH) (R01-HL-131613 to M. S.-C.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.-P., R.T., P.S., E.S.B., D.B.B., and M.S.-C. conceived and designed research; I.M.-P., A.V., A.C., J.T., L.G., and W.H. performed experiments; I.M.-P., A.V., A.C., and W.H. analyzed data; I.M.-P. and M.S.-C. interpreted results of experiments; I.M.-P. and A.V. prepared figures; I.M.-P., D.B.B., and M.S.-C. drafted manuscript; I.M.-P., E.S.B., D.B.B., and M.S.-C. edited and revised manuscript; I.M.-P., R.T., P.S., A.V., A.C., J.T., L.G., W.H., E.S.B., D.B.B., and M.S.-C. approved final version of manuscript.
REFERENCES
- 1.Bayeva M, Sawicki KT, Ardehali H. Taking diabetes to heart--deregulation of myocardial lipid metabolism in diabetic cardiomyopathy. J Am Heart Assoc 2: e000433, 2013. doi: 10.1161/JAHA.113.000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin IJ, Schuster EH, Bulkley BH. Cardiac hypertrophy in idiopathic dilated congestive cardiomyopathy: a clinicopathologic study. Circulation 64: 442–447, 1981. doi: 10.1161/01.CIR.64.3.442. [DOI] [PubMed] [Google Scholar]
- 3.Birkenfeld AL, Jordan J, Dworak M, Merkel T, Burnstock G. Myocardial metabolism in heart failure: purinergic signalling and other metabolic concepts. Pharmacol Ther 194: 132–144, 2019. doi: 10.1016/j.pharmthera.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Brady TM. The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr Hypertens Rep 18: 3, 2016. doi: 10.1007/s11906-015-0608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmean CM, Cohen RN, Brady MJ. Systemic regulation of adipose metabolism. Biochim Biophys Acta 1842: 424–430, 2014. doi: 10.1016/j.bbadis.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Chandler MP, Kerner J, Huang H, Vazquez E, Reszko A, Martini WZ, Hoppel CL, Imai M, Rastogi S, Sabbah HN, Stanley WC. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol 287: H1538–H1543, 2004. doi: 10.1152/ajpheart.00281.2004. [DOI] [PubMed] [Google Scholar]
- 7.Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099, 2014. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet 27: 84–88, 2001. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 9.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem 273: 30057–30060, 1998. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- 10.Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, Stenson L, Manganiello V. From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol 11: 676–682, 2011. doi: 10.1016/j.coph.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebelt H, Hillebrand I, Arlt S, Zhang Y, Kostin S, Neuhaus H, Müller-Werdan U, Schwarz E, Werdan K, Braun T. Treatment with bone morphogenetic protein 2 limits infarct size after myocardial infarction in mice. Shock 39: 353–360, 2013. doi: 10.1097/SHK.0b013e318289728a. [DOI] [PubMed] [Google Scholar]
- 12.Grant C, Greene DG, Bunnell IL. Left ventricular enlargement and hypertrophy. A clinical and angiocardiographic study. Am J Med 39: 895–904, 1965. doi: 10.1016/0002-9343(65)90111-7. [DOI] [PubMed] [Google Scholar]
- 13.Guo WT, Dong DL. Bone morphogenetic protein-4: a novel therapeutic target for pathological cardiac hypertrophy/heart failure. Heart Fail Rev 19: 781–788, 2014. doi: 10.1007/s10741-014-9429-8. [DOI] [PubMed] [Google Scholar]
- 14.Hino J, Miyazawa T, Miyazato M, Kangawa K. Bone morphogenetic protein-3b (BMP-3b) is expressed in adipocytes and inhibits adipogenesis as a unique complex. Int J Obes 36: 725–734, 2012. doi: 10.1038/ijo.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hino J, Nakatani M, Arai Y, Tsuchida K, Shirai M, Miyazato M, Kangawa K. Overexpression of bone morphogenetic protein-3b (BMP-3b) in adipose tissues protects against high-fat diet-induced obesity. Int J Obes 41: 483–488, 2017. doi: 10.1038/ijo.2017.15. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA 106: 12670–12675, 2009. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2: 231–237, 2009. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly DP. PPARs of the heart: three is a crowd. Circ Res 92: 482–484, 2003. doi: 10.1161/01.RES.0000064382.46274.95. [DOI] [PubMed] [Google Scholar]
- 19.Kim KH, Song MJ, Yoo EJ, Choe SS, Park SD, Kim JB. Regulatory role of glycogen synthase kinase 3 for transcriptional activity of ADD1/SREBP1c. J Biol Chem 279: 51999–52006, 2004. doi: 10.1074/jbc.M405522200. [DOI] [PubMed] [Google Scholar]
- 20.Kuo MM, Kim S, Tseng CY, Jeon YH, Choe S, Lee DK. BMP-9 as a potent brown adipogenic inducer with anti-obesity capacity. Biomaterials 35: 3172–3179, 2014. doi: 10.1016/j.biomaterials.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 21.Lee JE, Schmidt H, Lai B, Ge K. Transcriptional and epigenomic regulation of adipogenesis. Mol Cell Biol 39: e00601-18, 2019. doi: 10.1128/MCB.00601-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab 25: 293–302, 2014. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, Bahjat FR, Stenzel-Poore MP, Kawaguchi R, Coppola G, Carmichael ST. GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci 18: 1737–1745, 2015. doi: 10.1038/nn.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature 447: 1012–1016, 2007. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 25.Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58: 803–812, 2009. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchildon F, St-Louis C, Akter R, Roodman V, Wiper-Bergeron NL. Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. J Biol Chem 285: 13274–13284, 2010. doi: 10.1074/jbc.M109.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messerli FH, Sundgaard-Riise K, Reisin ED, Dreslinski GR, Ventura HO, Oigman W, Frohlich ED, Dunn FG. Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann Intern Med 99: 757–761, 1983. doi: 10.7326/0003-4819-99-6-757. [DOI] [PubMed] [Google Scholar]
- 28.Modica S, Straub LG, Balaz M, Sun W, Varga L, Stefanicka P, Profant M, Simon E, Neubauer H, Ukropcova B, Ukropec J, Wolfrum C. Bmp4 promotes a brown to white-like adipocyte shift. Cell Rep 16: 2243–2258, 2016. doi: 10.1016/j.celrep.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 29.Morrell NW, Bloch DB, ten Dijke P, Goumans MJ, Hata A, Smith J, Yu PB, Bloch KD. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol 13: 106–120, 2016. doi: 10.1038/nrcardio.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 56: 1113–1132, 2010. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 11: 268–272, 2010. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa Y, Tsuji M, Tanaka E, Miyazato M, Hino J. Bone morphogenetic protein (BMP)-3b gene depletion causes high mortality in a mouse model of neonatal hypoxic-ischemic encephalopathy. Front Neurol 9: 397, 2018. doi: 10.3389/fneur.2018.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozaki KI, Awazu M, Tamiya M, Iwasaki Y, Harada A, Kugisaki S, Tanimura S, Kohno M. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab 310: E643–E651, 2016. doi: 10.1152/ajpendo.00445.2015. [DOI] [PubMed] [Google Scholar]
- 34.Pettersson US, Waldén TB, Carlsson P-O, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 7: e46057, 2012. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu X, Liu Y, Cao D, Chen J, Liu Z, Ji H, Chen Y, Zhang W, Zhu P, Xiao D, Li X, Shou W, Chen H. BMP10 preserves cardiac function through its dual activation of SMAD-mediated and STAT3-mediated pathways. J Biol Chem 294: 19877–19888, 2019. doi: 10.1074/jbc.RA119.010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabbia F, Silke B, Conterno A, Grosso T, De Vito B, Rabbone I, Chiandussi L, Veglio F. Assessment of cardiac autonomic modulation during adolescent obesity. Obes Res 11: 541–548, 2003. doi: 10.1038/oby.2003.76. [DOI] [PubMed] [Google Scholar]
- 37.Relling DP, Esberg LB, Fang CX, Johnson WT, Murphy EJ, Carlson EC, Saari JT, Ren J. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J Hypertens 24: 549–561, 2006. doi: 10.1097/01.hjh.0000203846.34314.94. [DOI] [PubMed] [Google Scholar]
- 38.Riva P, Martini G, Rabbia F, Milan A, Paglieri C, Chiandussi L, Veglio F. Obesity and autonomic function in adolescence. Clin Exp Hypertens 23: 57–67, 2001. doi: 10.1081/CEH-100001197. [DOI] [PubMed] [Google Scholar]
- 39.Roe ND, Handzlik MK, Li T, Tian R. The role of diacylglycerol acyltransferase (DGAT) 1 and 2 in cardiac metabolism and function. Sci Rep 8: 4983, 2018. doi: 10.1038/s41598-018-23223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Alcaraz AJ, Lipina C, Petrie JR, Murphy MJ, Morris AD, Sutherland C, Cuthbertson DJ. Obesity-induced insulin resistance in human skeletal muscle is characterised by defective activation of p42/p44 MAP kinase. PLoS One 8: e56928, 2013. doi: 10.1371/journal.pone.0056928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295: E29–E37, 2008. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Y, Wang YF, Jayaraman L, Yang H, Massagué J, Pavletich NP. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-β signaling. Cell 94: 585–594, 1998. doi: 10.1016/S0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- 44.Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A, Chen C, Chao W, Panagia M, Sosnovik DE, Puppala D, Armoundas AA, Hindle A, Bloch KD, Buys ES, Scherrer-Crosbie M. Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol 84: 202–211, 2015. doi: 10.1016/j.yjmcc.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454: 1000–1004, 2008. [Erratum in Nature 459: 122, 2009.] doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 269: 1108–1112, 1995. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 48.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, López M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149: 871–885, 2012. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.World Health Organization 10 Facts on Obesity (Online). https://www.who.int/features/factfiles/obesity/en/ [16 January 2019].
- 49.Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP α and PPAR γ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell 3: 151–158, 1999. doi: 10.1016/S1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 50.Yadav RL, Yadav PK, Yadav LK, Agrawal K, Sah SK, Islam MN. Association between obesity and heart rate variability indices: an intuition toward cardiac autonomic alteration—a risk of CVD. Diabetes Metab Syndr Obes 10: 57–64, 2017. doi: 10.2147/DMSO.S123935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J, Croniger CM, Lekstrom-Himes J, Zhang P, Fenyus M, Tenen DG, Darlington GJ, Hanson RW. Metabolic response of mice to a postnatal ablation of CCAAT/enhancer-binding protein α. J Biol Chem 280: 38689–38699, 2005. doi: 10.1074/jbc.M503486200. [DOI] [PubMed] [Google Scholar]
- 52.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, Landesberg G, Crichton I, Wu W, Puré E, Funk CD, FitzGerald GA. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci Transl Med 4: 132ra54, 2012. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z, Liu H, Liu J. Akt activation: a potential strategy to ameliorate insulin resistance. Diabetes Res Clin Pract 156: 107092, 2019. doi: 10.1016/j.diabres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Zhao R, Lawler AM, Lee SJ. Characterization of GDF-10 expression patterns and null mice. Dev Biol 212: 68–79, 1999. doi: 10.1006/dbio.1999.9326. [DOI] [PubMed] [Google Scholar]
- 55.Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community-dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail 2: 512–522, 2014. [Erratum in JACC Heart Fail 2: 678, 2014.] doi: 10.1016/j.jchf.2014.03.016. [DOI] [PubMed] [Google Scholar]