Abstract
Understanding mouse thermal physiology informs the usefulness of mice as models of human disease. It is widely assumed that the mouse tail contributes greatly to heat loss (as it does in rat), but this has not been quantitated. We studied C57BL/6J mice after tail amputation. Tailless mice housed at 22°C did not differ from littermate controls in body weight, lean or fat content, or energy expenditure. With acute changes in ambient temperature from 19 to 39°C, tailless and control mice demonstrated similar body temperatures (Tb), metabolic rates, and heat conductances and no difference in thermoneutral point. Treatment with prazosin, an α1-adrenergic antagonist and vasodilator, increased tail temperature in control mice by up to 4.8 ± 0.8°C. Comparing prazosin treatment in tailless and control mice suggested that the tail’s contribution to total heat loss was a nonsignificant 3.4%. Major heat stress produced by treatment at 30°C with CL316243, a β3-adrenergic agonist, increased metabolic rate and Tb and, at a matched increase in metabolic rate, the tailless mice showed a 0.72 ± 0.14°C greater Tb increase and 7.6% lower whole body heat conductance. Thus, the mouse tail is a useful biomarker of vasodilation and thermoregulation, but in our experiments contributes only 5–8% of whole body heat dissipation, less than the 17% reported for rat. Heat dissipation through the tail is important under extreme scenarios such as pharmacological activation of brown adipose tissue; however, non-tail contributions to heat loss may have been underestimated in the mouse.
Keywords: ambient temperature, body temperature, energy expenditure, heat conductance, tail
INTRODUCTION
Mice are widely used for obesity and diabetes research. However, mouse and human thermal physiology differ, and a better understanding of mouse thermal biology is needed to improve the predictive validity of mice as a model for human disease (22). Mice and humans defend similar core body temperatures (Tb) but differ in how this is accomplished. Humans usually generate the required heat as a by-product of metabolism, with Tb regulated by adjusting the thermal microenvironment and changes in heat loss (39). Humans effectively dissipate heat by evaporation using diaphoresis, hence, they can even live in environments where ambient temperature (Ta) is greater than Tb (10, 11). In contrast, mice generally regulate Tb by increasing energy expenditure to generate warmth (20, 39). Mice do not sweat or pant efficiently. Their Tb regulation in hot Tas depends mostly on dry heat loss, which is limited, so mice can die after 2–3 h at a Ta of 40°C (2, 45).
Nonevaporative heat dissipation is regulated by cutaneous vasodilation, with species-specific specialized organs (“radiators”) for this purpose, such as elephant and rabbit ears, head vasculature in large dinosaurs (34), chicken combs, stegosaurus plates (12), and rat and mouse tails. These organs are typically covered by glabrous (non-hairy) skin, have large surface area-to-volume ratios, and exhibit dense vascularization with frequent arteriovenous anastomoses. At warm Ta, blood flow redistribution and vasodilation increase surface warming and heat transfer to the environment. When Ta drops, these are reversed, reducing heat loss (16, 40, 48).
It is assumed that the mouse tail has a major role in thermoregulation, based largely on extrapolation from the rat. The rat tail comprises 9% of the rat’s surface area (23) and can dissipate 17% of body heat (35). Mouse and rat tails share structural features (15, 16, 38, 49), and increased mouse tail temperatures indicate vasodilation and heat loss (14, 47). One mouse study showed that tail amputation caused death earlier in a heat challenge (26). From these data, it is assumed that thermoregulatory properties of mouse and rat tails are similar or equivalent. However, a quantitative analysis of mouse tail heat dissipation has not been reported.
Here we study the ability of the mouse tail to dissipate heat. Under most conditions there was no detected thermal phenotype in tailless mice. The relative contribution of the mouse tail to heat dissipation is modest.
MATERIALS AND METHODS
Mice and reagents.
C57BL/6J (#000664, Jackson Laboratories, Bar Harbor, ME) mice were singly housed with bedding (7099-TEK-fresh, Envigo, Indianapolis, IN) at 21–22°C with a 12:12-h dark:light cycle (lights on at 0600) in a clean, conventional facility with water and chow (NIH-07 Envigo Inc) provided ad libitum. Experiments were approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Animal Care and Use Committee (protocol K016-DEOB-17). Tail amputation was performed with a scalpel at 24 to 48 h after birth. This age was chosen to be less traumatic, because nociceptive sensory neurons are not yet matured (43). Half of each litter underwent tail amputation, and the controls were handled similarly but without amputation. Amputation was well tolerated, with minimal bleeding, full healing in 1 week, no maternal rejection of pups, and no pup mortality. Intraperitoneal implantation of G2 E-Mitter (Starr Life Sciences, Oakmont, PA) or IPTT-300 (Bio Medic Data Systems, Seaford, DE) temperature sensors was performed under isoflurane anesthesia (5% induction, 1.2% maintenance; Baxter Healthcare Corporation, Deerfield, IL) with Prevail (flunixin meglumine) analgesia (2.2 mg/kg sc at operation and daily for 2 days). Mice were studied at least 1 week after telemeter implantation.
Prazosin (vehicle, ultra-pure water) was obtained from Sigma (P7791). CL316243 (vehicle, saline) was obtained from Tocris (1499).
Determination of thermal physiology parameters.
Tb and activity were measured in home cages by telemetry using intraperitoneal G2 E-Mitter transponders and ER4000 Energizer/Receivers (Starr Life Sciences, Oakmont, PA) as 1-min means collected with VitalView software (v 5.0, Starr Life Sciences).
TEE, Tb, and activity (beam breaks) were measured by indirect calorimetry (CLAMS using Oxymax software v5.52, Columbus Instruments, Columbus, OH) in chambers without bedding or nesting material (2.5 L volume, flow rate 0.5 L/min, sampling flow 0.4 L/min, settle time 55 s, measure time 5 s, each chamber sampled every 13 min).
Heat capacity (Cp) and heat conductance after death were measured as described (1). Heat loss was defined as the difference between heat produced and heat retained: = TEE – Cp(ΔTb), where ΔTb is the difference between current and previous measurements (Tbn – Tbn-1). Heat conductance, which is the reciprocal of insulation, was calculated as = heat loss/(meanTb – Ta), where mean Tb is the mean Tb of the interval [(Tbn + Tbn-1)/2].
Body composition was measured by EchoMRI (EchoMRI LLC, Houston, TX). Long-term energy expenditure in home cages was estimated by energy balance (calculated from the metabolizable caloric intake, corrected for the change in caloric content of the mouse) (37). Surface areas were estimated as described (23). Tail volume and density was determined using Archimedes law. Scholander analysis (studying mice over a range of Tas) was performed as previously described (44). The control mice from the current study were included in Ref. 44. Heat conductance in the Scholander analysis was calculated as TEE/(Tb – Ta) (31).
Infrared thermography.
One day before study, 2 × 2 cm midline areas 2 cm above the tail and in the interscapular region over the brown adipose tissue (BAT) were shaved under isoflurane anesthesia. The next day, mice were placed in a 20 × 20 cm open-top cage with bedding, 170 cm below a T650sc infrared (IR) camera (FLIR Systems, Wilsonville, OR, resolution 640 × 480) and allowed to acclimate for 1 h. At intervals, IR video (~30 s at 7.5 frames per second) was collected using ResearchIR software (FLIR Systems). As needed, mice were gently nudged until they moved so the tail was visible to the overhead camera. Tb was measured by telemetry (IPTT-300) immediately after the IR recording. The maximal temperatures of shaved areas (TBAT, TLumbar) and average temperature of a 2-mm circle located 1 cm from tail base (TTail) and a 4 mm circle between the shaved areas (TFur) were determined by FLIR Tools software (FLIR, Wilsonville, OR). Emissivity of fur and shaved skin was set to 0.88 and 0.97, respectively (33). The tail base and tip have been used (13, 38, 47); however, we selected 1 cm from the tail base as reproducible and reliable. The 0-cm point was variable and partially reflected Tb, and the tail tip was close to Ta even after vasodilation. Measurements at 1 or 2 cm from the tail base gave comparable results. Positioning the camera to view the BAT and lumbar areas meant the feet were visible inconsistently, precluding quantitative analysis of extremity/foot temperature. We did not quantitate ear temperature since we could not reliably measure pinna temperature (it is small, variably positioned, and sometimes the deep ear is visible, which likely measures Tb, as it does in humans).
Statistics.
Data are represented as means ± SE, unless otherwise indicated. Unpaired t tests were used for comparisons between control and tailless mice or the effect of drug versus vehicle treatment. The effect of Ta on TEE, Tb, and heat conductance were analyzed by segmented line regression as described (44). Statistical power analysis was performed using https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html.
RESULTS
Tail surface area is disproportionately lower in mice compared with rats.
We compared mouse and rat body characteristics to understand heat loss in each. Mice have a ~1.9-fold higher mass-specific surface area than rats, and mouse tail surface area is 6.0% of total body surface area (Table 1) compared with 8.9% in rat (23), so relatively less tail surface is available for heat dissipation. In addition, the rat pelt is 2.4-fold more insulating (0.13°C·kcal−1·h−1·m−2 in mice vs. 0.31°C·kcal−1·h−1·m−2 in rats) (7, 8, 27). These factors all suggest that mice, compared with rats, lose relatively more heat from the trunk and less from the tail.
Table 1.
Structural properties of mice and rats
| Parameter | Mice, This Study | Rats, from Ref. 22 |
|---|---|---|
| Body weight, g | 31.5 ± 1.8 | 219 ± 12 |
| Body volume, cm3 | 31.3 ± 2.1 | |
| Body total surface area, cm2 | 97.9 ± 3.0 | 357 ± 13 |
| Body surface area/weight, cm2/g | 3.12 ± 0.24 | 1.62 |
| Body length (without tail), mm | 96.0 ± 3.6 | 199 ± 8 |
| Tail weight, g | 0.57 ± 0.03 | |
| Tail volume, cm3 | 0.50 ± 0.02 | |
| Tail surface area, cm2 | 5.84 ± 0.20 | 31.7 ± 3.7 |
| Tail surface area, % of total | 6.0% | 8.9% |
| Tail length, mm | 80 ± 5 |
Data are means ± SD, n = 3 mice. Body and tail parameters were measured in 7-mo-old male C57BL/6J mice.
Tail loss has no effect on growth or body weight.
Tails were amputated at 1–2 days of age and tailless and control mice of both sexes housed at 22°C showed no difference in body weight, body composition, food intake, or energy expenditure calculated by mass balance (Fig. 1). In addition, at 8 mo there was no difference in limb or foot length (Table 2). Thus, under typical baseline vivarium conditions (22°C), no in vivo difference was detected in tailless mice.
Fig. 1.
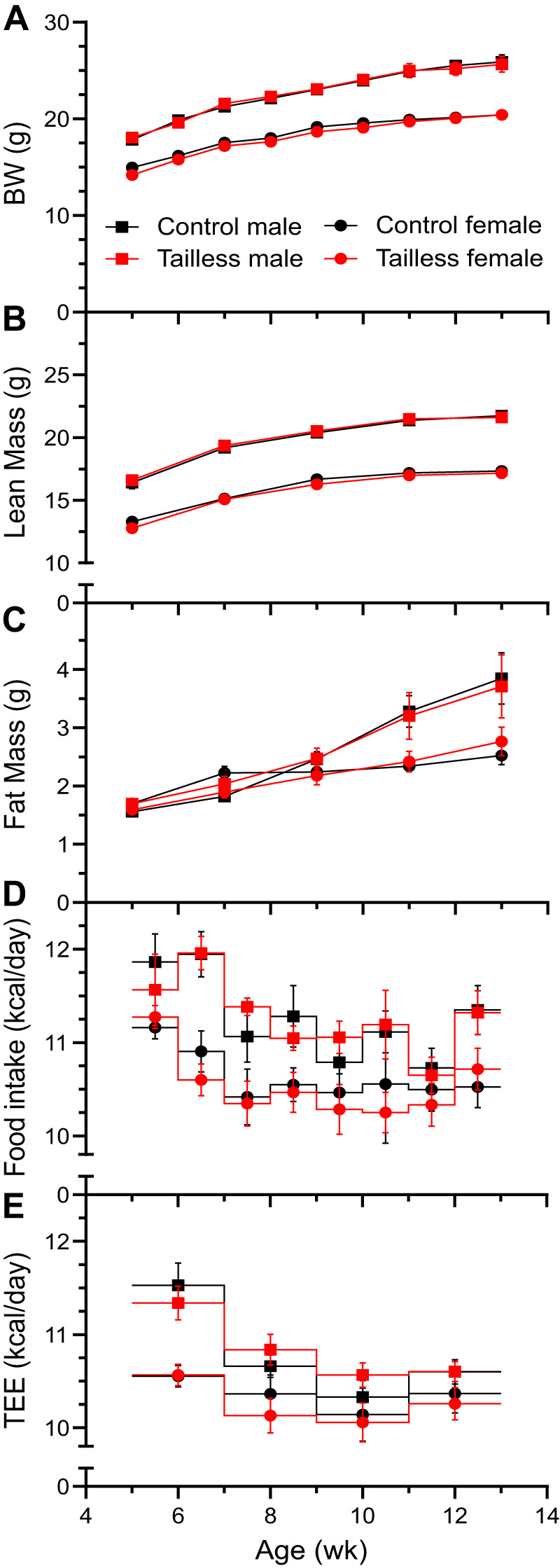
No effect of tail loss on growth. Body weight (A; BW), lean mass (B), fat mass (C), food intake (D), and total energy expenditure (E; TEE) by mass balance were measured in C57BL/6J mice. Littermate control and tailless male and female mice were singly housed and fed a chow diet. Data are means ± SE, n = 7–9 mice/group.
Table 2.
Body characteristics of control and tailless mice
| Parameter | Control | Tailless | P |
|---|---|---|---|
| Body weight, g | 32.0 ± 0.8 | 31.6 ± 0.6 | 0.66 |
| Body length, mm | 101.0 ± 0.6 | 100.4 ± 0.6 | 0.51 |
| Front paw length, mm | 7.9 ± 0.2 | 7.6 ± 0.1 | 0.37 |
| Hind paw length, mm | 18.2 ± 0.3 | 18.3 ± 0.1 | 0.81 |
| Tibia length, mm | 19.1 ± 0.4 | 18.7 ± 0.1 | 0.29 |
| Femur length, mm | 15.6 ± 0.3 | 15.7 ± 0.2 | 0.95 |
| Ulna length, mm | 14.8 ± 0.2 | 14.9 ± 0.1 | 0.66 |
| Humerus length, mm | 12.2 ± 0.1 | 12.2 ± 0.2 | 0.85 |
Data are means ± SE, n = 7 mice/group. Body and limbs parameters were measured in 8-mo-old male C57BL/6J mice. All measurements were performed by a single investigator.
The mouse tail contribution to heat loss in the absence of biologically regulated processes such as heat production and blood flow was quantitated as heat conductance after death. No difference was detected between control [0.0493 ± 0.0065 kcal·h−1·°C(Tb-Ta)−1, n = 6] and tailless [0.0471 ± 0.0034 kcal·h−1·°C(Tb-Ta)−1, n = 8, P = 0.42] mice. Thus, the postmortem conditions did not reveal a detectable contribution of the mouse tail to heat dissipation.
No detected effect of high ambient temperatures on tailless mice.
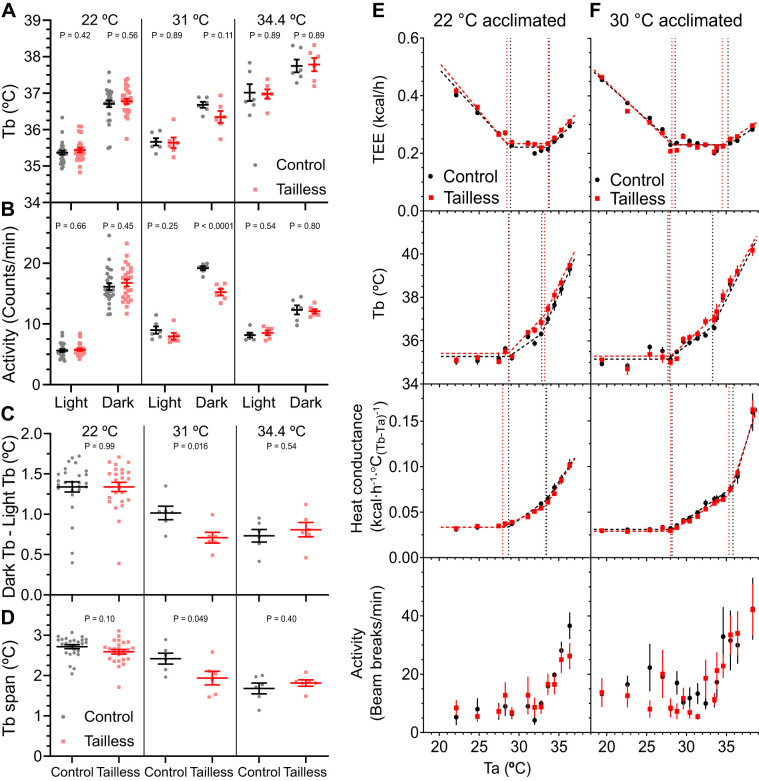
Mouse thermal physiology depends on the ambient temperature (Ta). The thermoneutral point (TNP) is a discrete ambient temperature below which energy expenditure increases and above which body temperature increases. Qualitatively different rules apply below the light-phase TNP (TNPL, ~29°C), between the TNPL and dark-phase TNP (TNPD, ~33°C), and above the TNPD (44). We studied mice for 72 h at a single Ta in each of the three zones (22°C, <TNPL; 31°C, between TNPL and TNPD; 34.4°C, >TNPD). At 22°C, the tailless and control mice had similar light and dark phase Tb and activity, dark-light Tb difference (~1.4°C), and Tb span (the difference between the 5th and 95th Tb percentiles, ~2.6°C) (Fig. 2, A–D). At 31°C, the tailless mice had a reduction in dark phase activity, dark-light Tb difference, and Tb span. The activity reduction may be driving the non-significantly lower Tb. Housing at 34.4°C, compared with 22°C, produced the expected increase in light and dark phase Tb and reduction of the dark-light difference and Tb span. However, there was no difference between the control and tailless mice. Thus, at 34.4°C, where mice are under heat stress and have an elevated Tb, there was no detected effect of the absence of the tail.
Fig. 2.
Effect of ambient temperature (Ta) on tailless mice. Light and dark phase core body temperature (Tb) and physical activity (A and B), difference between dark and light Tb (C), and Tb span (the 95th minus the 5th percentile) (D) in male control and tailless mice. Tb and activity were monitored continuously by telemetry for 72 h in singly housed mice at Ta of 22, 31, or 34.4°C, as indicated. Data are means ± SE, n = 6–26 mice/group (male). E and F: control and tailless mice previously housed at 22°C or 30°C for 10 days were acclimated to indirect calorimetry chambers for 2 days and then exposed to a range of Ta. Total energy expenditure (TEE), Tb, and heat conductance were analyzed by mixed model segmented regression (see materials and methods and Ref. 44). For visual clarity, only Ta plateau mean ± SE data points are depicted; however, all data were included in the regression models. Complete regression results are in Table 3.
The above studies used multi-day exposure to each Ta. Since acute exposure might be a more sensitive test of thermal physiology, mice were next exposed for short times to a wide range of Ta, with the TEE, Tb, and whole body heat conductance analyzed by segmented line regression. This technique yields multiple thermal biology parameters, including the TNPL, Tb change per Ta change, and TEE change per Ta change. For mice acclimated to 22°C, no difference between control and tailless mice was observed for any of the parameters (Fig. 2E, Table 3). For example, the thermoneutral point, below which TEE increases (TlcEE) and above which Tb increases (Tbinc), was 28.4 to 28.9°C in light phase in both groups. The second breakpoints in the Tb graph, the Ta above which Tb increases rapidly (Tb_R; 32.9 vs. 33.2°C, control vs. tailless) and the Tb slope above this point (Tb Slope, >Tb_R; 0.84 vs. 0.78°C Tb per °C Ta, control vs. tailless) were also similar in both groups. Mice acclimated to 30°C for 10 days were similarly studied, and again the loss of the tail had no effect on any of the parameters (Fig. 2F, Table 3). These results suggest that the tail’s thermoregulatory contribution is below the detection limits of these assays.
Table 3.
Light phase thermal biology parameters of control and tailless mice acclimated to 22°C or 30°C
| Control | Tailless | Control | Tailless | |
|---|---|---|---|---|
| 22°C | 30°C | |||
| n of mice | 5 | 6 | 5 | 6 |
| n of data points in data set | 585 | 702 | 725 | 870 |
| TlcEE, °C | 28.92 ± 0.19 | 28.43 ± 0.15 | 28.60 ± 0.01 | 28.19 ± 0.20 |
| Tbinc, °C | 28.74 ± 0.33 | 28.69 ± 0.18 | 27.69 ± 0.19 | 27.92 ± 0.01 |
| Tlccond, °C | 28.70 ± 0.21 | 27.95 ± 0.22 | 28.19 ± 0.22 | 28.00 ± 0.02 |
| TEE, at TlcEE, kcal/h | 0.221 ± 0.004 | 0.233 ± 0.008 | 0.230 ± 0.008 | 0.230 ± 0.008 |
| Cond, <Tlccond, kcal·h−1·Δ°C−1 | 0.0335 ± 0.0012 | 0.0334 ± 0.0010 | 0.0310 ± 0.0022 | 0.0290 ± 0.0014 |
| Tb, <Tbinc, °C | 35.27 ± 0.08 | 35.31 ± 0.11 | 35.15 ± 0.08 | 35.30 ± 0.16 |
| defended Tb, °C | 36.20 ± 0.47 | 35.43 ± 0.44 | 37.86 ± 0.41 | 36.85 ± 0.52 |
| TEE Slope, <TlcEE, kcal·h−1·°C−1 | −0.0304 ± 0.0013 | −0.0334 ± 0.0013 | −0.0248 ± 0.0006 | −0.0266 ± 0.0009 |
| Tb Slope, >Tbinc <Tb_R, °C Tb/°C | 0.256 ± 0.030 | 0.391 ± 0.023 | 0.272 ± 0.021 | 0.345 ± 0.024 |
| Cond Slope, >Tlccond <Cond_R, kcal·h−1·Δ°C−1·°C−1 | 0.0063 ± 0.0004 | 0.0044 ± 0.0003 | 0.0061 ± 0.0003 | 0.0055 ± 0.0001 |
| TEE_R, °C | 33.78 ± 0.37 | 33.68 ± 0.02 | 35.24 ± 0.39 | 34.50 ± 0.34 |
| Tb_R, °C | 32.85 ± 0.16 | 33.21 ± 0.23 | 33.31 ± 0.15 | 33.29 ± 0.19 |
| Cond_R, °C | 33.47 ± 0.24 | 33.36 ± 0.13 | 35.83 ± 0.11 | 35.33 ± 0.06 |
| TEE Slope, >TEE_R, kcal·h−1·°C−1 | 0.0275 ± 0.0058 | 0.0299 ± 0.0027 | 0.0181 ± 0.0037 | 0.0178 ± 0.0027 |
| Tb Slope, >Tb_R, °C Tb/°C | 0.841 ± 0.033 | 0.776 ± 0.035 | 0.728 ± 0.049 | 0.684 ± 0.029 |
| Cond Slope, >Cond_R, kcal·h−1·Δ°C−1·°C−1 | 0.0131 ± 0.0007 | 0.0141 ± 0.0006 | 0.0296 ± 0.0014 | 0.0267 ± 0.0006 |
| Body wt, g | 29.78 ± 1.37 | 27.97 ± 1.00 | 31.32 ± 1.44 | 30.47 ± 0.84 |
| Age, wk | 33 | 33 | 28 | 28 |
Data are means ± SE (see materials and methods and Fig. 2). Parameters were calculated from analysis of total energy expenditure (TEE), body temperature (Tb), and conductance as a function of ambient temperature (Ta) by mixed model segmented linear regression. Cond Slope, >Cond_R, conductance vs. Ta slope for Ta >Cond_R; Cond Slope, >Tlccond <Cond_R, conductance slope in the region >Tlccond and <Cond_R; Cond, <Tlc, mean conductance at Ta <Tlccond; Cond_R, second breakpoint of the conductance vs. Ta graph; defended Tb, X intercept of TEE vs. Ta line (using only Ta <TlcEE); Tb Slope, >Tb_R, Tb vs. Ta slope for Ta >Tb_R; Tb Slope, >Tbinc <Tb_R, Tb slope in the region >Tbinc and <Tb_R; Tb, <Tbinc, mean Tb at Ta < Tbinc; Tb_R, second breakpoint of the Tb vs. Ta graph, where Tb starts to steeply rise with Ta; Tbinc, Ta above which the Tb first increases; TEE at TlcEE, mean TEE at Ta = TlcEE; TEE Slope > TEE_R, TEE vs. Ta slope for Ta >TEE_R; TEE Slope, <TlcEE, TEE vs. Ta slope for Ta <TlcEE; TEE_R, breakpoint of the TEE vs. Ta graph, where TEE starts to rise with Ta; TlcEE, lower critical temperature, the (first) breakpoint of the TEE vs. Ta graph; Tlccond, lower critical temperature, the (first) breakpoint of the conductance vs. Ta graph.
Prazosin-induced vasodilation increases heat loss.
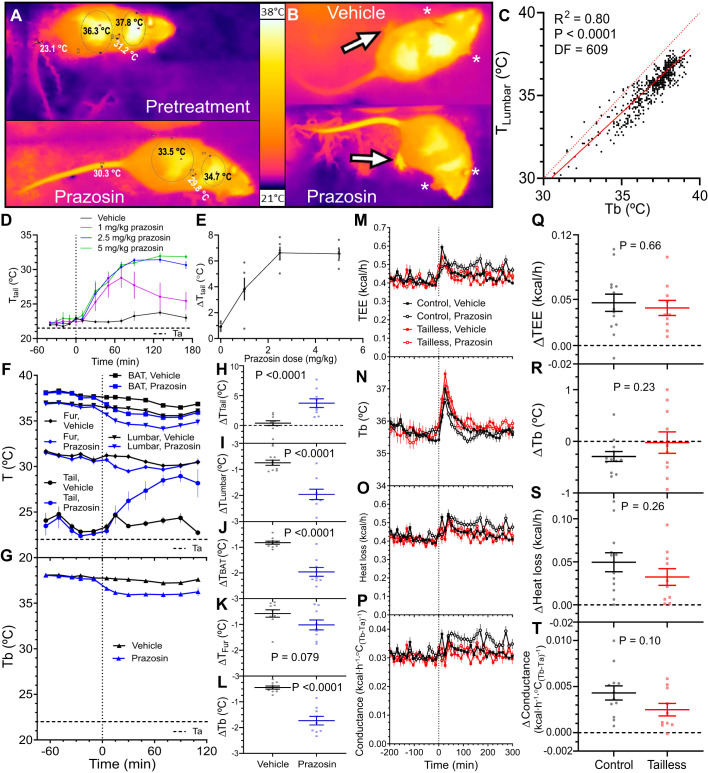
Infrared thermography (IR) allows simultaneous measurement of body surface temperatures at multiple sites (Fig. 3, A and B). Lumbar skin temperature (TLumbar) correlated with intraperitoneal Tb measured simultaneously by telemetry (R2 = 0.80, P < 0.0001), with TLumbar being ~1°C lower than Tb (Fig. 3C). This correlation supports using TLumbar as a biomarker of core body temperature.
Fig. 3.
Prazosin-induced vasodilation increases heat loss. A: infrared thermography (IR) of a mouse pretreatment (top) or 70 min after prazosin (bottom). Skin surface temperature measurements are on the tail (1 cm from base, TTail), shaved lumbar (TLumbar), and interscapular (TBAT) areas and unshaved fur (TFur) between the TLumbar and TBAT areas. B: IR images 70 min after treatment with prazosin or vehicle (water). Prazosin increased temperature of the feet (arrows) but not the ears (asterisks). C: correlation between TLumbar and intraperitoneal temperature (Tb) measured concurrently. Solid line is regression TLumbar = (0.88 ± 0.02)Tb + (3.31 ± 0.65), R2 = 0.80, df = 608, n = 14 mice (mixed sex). Dotted reference line is TLumbar = Tb. D: TTail after treatment with the indicated doses of prazosin, n = 5 mice (mixed sex). E: prazosin dose response. ΔTTail is the change from baseline, calculated as TTail mean of 10 to 170 min minus TTail mean of −40 to −10 min, (treatment10to170 – baseline−40to−10). F and G: effect of prazosin on TTail, TLumbar, TBAT, TFur, and Tb in control mice. The 22°C ambient temperature (Ta) is indicated, n = 9 or 10 mice/group (mixed sex). H–L: drug effect was calculated as the change from baseline (treatment5to110 – baseline−65to−10). Prazosin and vehicle effect were compared by unpaired t test. M–T: effect of prazosin on total energy expenditure (TEE), Tb, heat loss, and heat conductance of control and tailless mice. Drug effect was calculated as the vehicle-corrected change from baseline [(prazosin60to180 – prazosin−150to−30) – (vehicle60to180 – vehicle−150to−30)]. n = 10–13 mice/group (male). Data are means ± SE, compared by unpaired t test. Prazosin dose was 2.5 mg/kg, ip, except in dose response.
At 22°C, the tail is vasoconstricted and prazosin, an α1-adrenoceptor antagonist, blocks the vasoconstricting signal (47) (Fig. 3, A and B). A pilot dose-response experiment demonstrated a prazosin ED50 of ~1.2 mg/kg, ip. The maximum tail skin temperature was reached at 2.5 mg/kg (Fig. 3, D and E), so this prazosin dose was used for further experiments.
In mice at 22°C, tail temperatures at baseline (23.5 ± 0.9°C) and after vehicle injection (23.9 ± 0.3°C) were only slightly above Ta (22°C). Prazosin injection increased tail temperature by up to 4.6 ± 0.8°C (at 90 min postinjection) compared with vehicle (Fig. 3, F and H). Prazosin also increased the temperature of the feet, but not the ears (Fig. 3B). Opposite of its effect on the extremities, prazosin treatment reduced lumbar (TLumbar) and interscapular (TBAT, reflecting BAT) skin temperatures, fur temperature (TFur tailless vs. controls; P = 0.011 at 15 min and P = 0.0008 at 30 min), and Tb (Fig. 3, F–L). In a pooled data set, TLumbar correlated better with Tb than did TFur (Tb = 0.8508 TLumbar + 4.1603; R2 = 0.57 vs. Tb = 0.5566 TFur + 10.219; R2 = 0.20; df = 542). The worse correlation with Tb and the blunted prazosin effect demonstrate that shaved skin, rather than fur, is a better IR measure of Tb (for a contrasting view, see Ref. 30). Thus, prazosin-induced vasodilation increased tail and extremity temperatures and decreased core and BAT temperatures, while maintaining the BAT-Tb gradient. These results suggest that prazosin caused generalized vasodilation, increasing heat loss and reducing Tb, with continued BAT heat production.
We next tested the effect of prazosin in tailless mice. Prazosin increased TEE similarly in control and tailless mice by ~11% (Fig. 3, M and Q). Tb was less reduced by prazosin (−0.29 ± 0.10°C in controls, P = 0.011; vs. −0.02 ± 0.21°C in tailless; P = 0.92) (Fig. 3, N and R) in the indirect calorimetry chambers than in the IR measurements (above). Prazosin increased heat loss by 0.050 ± 0.011 kcal/h in controls (P = 0.0003) and 0.032 ± 0.010 kcal/h in tailless (P = 0.005) (Fig. 3, O and S). The increase in heat loss was lower in tailless mice by 0.017 ± 0.015 kcal/h (P = 0.26), which is 3.4% of total heat produced (0.017/0.498). Whole body heat conductance was increased by prazosin in controls by 0.0043 ± 0.0008 kcal·h−1·°C(Tb-Ta)−1 (P = 0.0002) and nonsignificantly less in tailless [0.0025 ± 0.0007 kcal·h−1·°C(Tb-Ta)−1, P = 0.0004; P control vs. tailless = 0.097] (Fig. 3, P and T). While not statistically significant, the difference in heat conductance between control and tailless mice was 4.9% [(0.0043–0.0025)/0.037] of whole body heat conductance. These data indicate that a sample size of 31 is needed for an 80% power to detect a difference of this magnitude.
Tail contributes to heat dissipation under severe heat stress.
Activation of adipose tissue thermogenesis and lipolysis with a β3-adrenergic agonist such as CL316243 massively increases heat production. At 22.5°C, CL316243 (0.1 mg/kg, ip) increased TTail by 2.3 ± 1.0°C compared with vehicle (Fig. 4, A and C). No significant effect of CL316243 treatment was observed on TBAT, TLumbar, TFur, or core Tb (Fig. 4, A, B, D–G). This is presumably due to elevated baseline temperatures, as IR thermography sometimes requires prodding the mouse so that its tail is visible. These results demonstrate that the tail contributes to dissipating the heat generated by treatment with a β3-adrenergic agonist at 22°C.
Fig. 4.
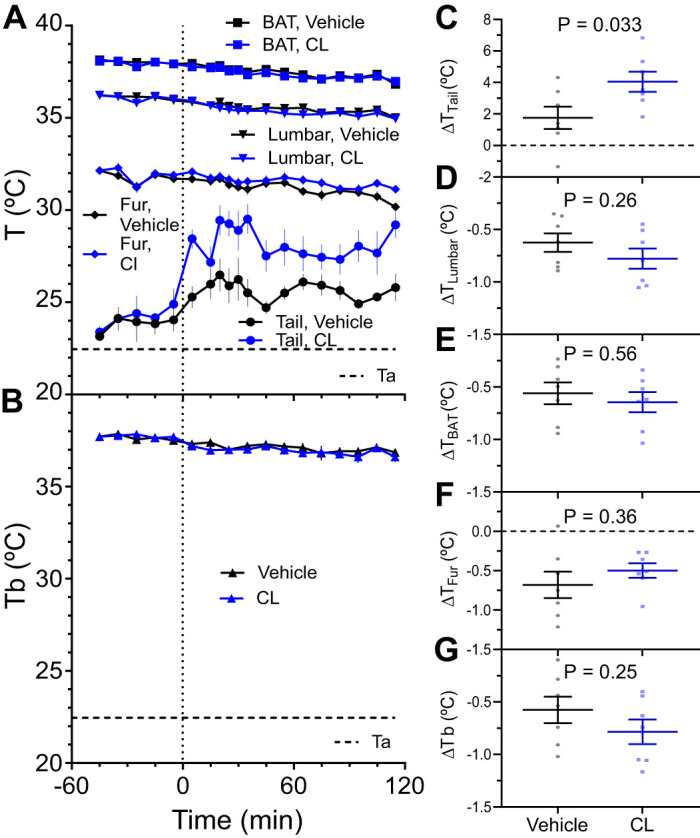
Effect of a β3-adrenergic agonist, CL316243, on skin and body temperatures. A and B: effect of CL316243 (0.1 mg/kg, ip) or vehicle (saline) on interscapular (TBAT), lumbar (TLumbar), tail (TTail), and unshaved fur (TFur) surface temperatures and intraperitoneal temperature by telemetry (Tb) in control mice. The 22.5°C ambient temperature (Ta) is indicated. C–G: drug effect was calculated as the change from baseline (treatment5to115 – baseline−45to−5). Data are means ± SE, n = 7 mice/group (mixed sex), compared by unpaired t test.
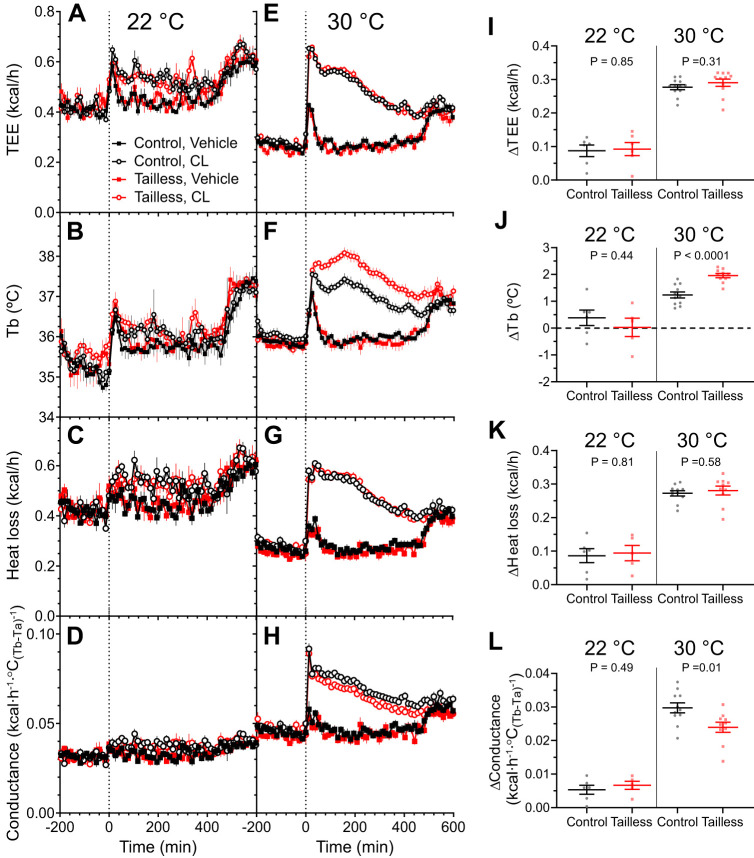
The effect of CL316243 in tailless mice was measured by telemetry and indirect calorimetry. Treatment with CL316243 at 22°C had similar effects in control and tailless mice, increasing TEE by ~27%, heat loss by ~26%, thermal conductance by ~20%, and not significantly affecting Tb (Fig. 5, A–D, and I–L).
Fig. 5.
Effect of CL316243 on the thermal metabolism. Mice were treated with CL316243 (0.1 mg/kg, ip) or vehicle (saline) at 22°C (A–D) or 30°C (E–H) and the effect on total energy expenditure (TEE), body temperature (Tb), heat loss, and heat conductance were measured. I–L: drug effect was calculated as the vehicle-corrected change from baseline [(CL31624360to180 – CL316243−150to−30) – (vehicle60to180 – vehicle−150to−30)]. Data are means ± SE, n = 5 or 6 mice/group (22°C) or n = 10 or 11 mice/group (30°C) (male); control and tailless were compared by unpaired t test.
However, using a Ta of 22°C underestimates the effects of CL316243, since the baseline TEE is already increased by cold-induced thermogenesis. To minimize cold-induced thermogenesis, mice were treated with CL316243 at 30°C. At 30°C, TEE was massively and similarly increased (by ~110%) in both groups (Fig. 5, E and I). Despite the similar increase in TEE, the Tb increased substantially more (by 0.72 ± 0.14°C, P < 0.0001) in tailless mice (Fig. 5, F and J). Heat loss was similar in control and tailless mice (Fig. 5, G and K). The difference in whole body heat conductance between the control and tailless mice [0.0058 ± 0.0021 kcal·h−1·°C(Tb-Ta)−1, P = 0.014; Fig. 5, H and L] measures the contribution of the tail. Assuming that CL316243 treatment at 30°C is a maximal heat stress, the tail’s contribution to maximal heat conductance [0.076 ± 0.02 kcal·h−1·°C(Tb-Ta)−1] is ~7.6% (0.0058/0.076).
DISCUSSION
To inform the translatability of mouse obesity studies, we studied the role of the mouse tail in thermoregulation. Tailless mice studied in a range of Tas did not differ from controls in Tb, TEE, or whole body heat conductance. Prazosin caused vasodilation and increased heat dissipation, with the tail’s contribution being a nonsignificant 4.9% of whole body heat conductance. Only under severe heat stress, produced by pharmacologic BAT activation at a Ta of 30°C, did tailless mice demonstrate a 7.6% reduction in whole body heat conductance.
Comparing mouse and rat thermal biology.
Rats regulate heat loss by adjusting tail blood flow, with a fully vasodilated tail able to dissipate 17% of energy produced (35). Similar thermoregulatory features are generally assumed for the mouse tail. However, mice have disproportionately greater trunk surface area, thinner fur, and a smaller body size and thus greater mass-specific surface area. Thus, one could predict that there would be relatively less heat loss from the tail and more from the trunk in mice compared with rats.
How mice cope with hot environments.
In thermal preference studies, mice choose a Ta at their TNPL in the light phase and well below the TNPD in the dark phase (19), suggesting that mice also live at and below thermoneutrality in their natural environment. Below thermoneutrality, mice support their Tb with regulated increases in energy expenditure, chiefly from BAT (1, 9, 39). Indeed, mice can even adapt to environments below 0°C, demonstrating the extent of their heat-generating abilities (6). Mice respond to a warm environment in two stages. In the light phase, starting with Ta just above the TNPL, the Tb target (or “set”) point is regulated upwards, storing some of the excess heat, reducing the demand for heat loss, and facilitating heat loss due to the larger Tb − Ta difference. The second stage occurs when the Ta exceeds the TNPD (in either the light or dark phase), where heat loss mechanisms are insufficient and overwhelmed (44). Mice do not efficiently sweat or pant (2). Some mouse studies show greater water loss at hot temperatures (18, 21, 32), but the regulation and mechanism(s) are unclear, relying on mechanistic studies from other species (17, 46). Mice can accomplish evaporative heat loss by grooming with saliva or water (25).
Nonevaporative heat loss (convection, conduction, radiation) mechanisms require heat loss from the body surface, facilitated by posture and behavioral responses, and also determined by surface area and surface temperature. The temperature of the vasodilated tail and trunk fur are comparable, so the temperature gradients of tail and trunk are similar. As the tail is ~6% of the body surface, it likely dissipates a similar percentage of total heat, consistent with the observed tail contribution of 5–8% to whole body heat conductance. Our qualitative results suggest that the feet, but not the ears, contribute to regulated heat loss, which agrees with reports that mouse ears do not vasodilate at high Tb, but do dilate with inflammation (4, 36). This is also true for rat ears, which do not have the arteriovenous anastomoses typically found in thermoregulatory sites (24). In the mouse it is likely that the largest fraction of regulated heat loss comes from the body trunk, with a contribution from the tail and feet, but not the ears. These principles of thermal biology likely apply generally to similarly small mammals.
The tail as a biomarker of thermal physiology and thermoregulation.
The mouse tail is clearly a thermoregulatory organ. However, the phenotype of the tailless mice is subtle, requiring dual stressors (Ta = 30°C and doubling energy expenditure with a β3-adrenergic agonist) to elicit a clear phenotype. The subtle phenotype suggests that, while mouse tail temperature and blood flow are valuable biomarkers of thermoregulatory vasodilation, it is unlikely that tail heat dissipation per se is causing whole body effects. This suggests that non-tail contributions may have been underestimated, with possible examples being leptin deficiency (14), thyroid hormone receptor α1 deficiency (47), transient receptor potential M8 (TRPM8) deletion (38), NK3 receptor agonism (29), and evodiamine treatment (28). Tail amputation would permit explicitly testing this hypothesis in each example. Besides vasodilation, factors outside the tail that contribute to thermal physiology include changes in activity, fur insulation, body size, energy expenditure, and behavioral thermoregulation. An elegant example of using mouse tail temperature/vasoconstriction status as a biomarker was to estimate thermoneutrality (16).
An interesting aspect of mouse thermal physiology is that the tail and limbs are longer when mice are raised at high ambient temperatures (3, 5, 6, 42). The mechanism is unknown, but increased tail and limb blood flow (with its nutrients, growth factors, and hormones) could contribute. We measured limb length to reveal possible adaptation to tail amputation and did not observe any increased length. However, the tailless mice were housed at 22°C, which is ≥7°C below thermoneutrality. Since 22°C causes constant tail vasoconstriction (16, 41), there was likely maximal recruitment of heat preservation processes, with no pressure to increase heat dissipation. It is possible that limb-length adaptations would be detected if the tailless mice were raised in a hot environment during their growth phase, which we have not investigated, a limitation of our investigation. More generally, we cannot rule out some sort of thermoregulatory adaptation to compensate for neonatal tail loss. As noted above, this seems unlikely for mice housed at 22°C. Nevertheless, if adaptation occurs, our results would become a lower bound, rather than a quantitation, of the tail’s contribution to heat dissipation in unadapted mice.
In conclusion, the mouse tail contributes less to whole body heat dissipation than is assumed, one-third to one-half of the percentage reported in rat. Despite the tail’s modest quantitative role as a thermoregulatory organ, it is important as a biomarker indicative of whole body thermal physiology. The tail’s role in thermal biology enriches the use of the mouse as a model organism for studying energy homeostasis, diabetes, and obesity.
GRANTS
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (ZIA DK075062; ZIA DK075063, ZIA DK075064, ZIA DK070002).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.Š., O.G., and M.L.R. conceived and designed research; V.Š., N.L., and O.G. performed experiments; V.Š., N.L., J.G., O.G., and M.L.R. analyzed data; V.Š., O.G., and M.L.R. interpreted results of experiments; V.Š. prepared figures; V.Š. and M.L.R. drafted manuscript; V.Š., N.L., O.G., and M.L.R. edited and revised manuscript; V.Š., N.L., J.G., O.G., and M.L.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alice Franks for superb technical assistance; Yuning Huang for surgical implantation of E-Mitters; and Drs. Ramón Piñol, Cuiying Xiao, Aaron Cypess, and Kong Chen for helpful discussions.
References
- 1.Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab 4: 461–470, 2015. doi: 10.1016/j.molmet.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adolph EF. Tolerance to heat and dehydration in several species of mammals. Am J Physiol 151: 564–575, 1947. doi: 10.1152/ajplegacy.1947.151.2.564. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hilli F, Wright EA. The effects of changes in the environmental temperature on the growth of bone in the mouse. Radiological and morphological study. Br J Exp Pathol 64: 43–52, 1983. [PMC free article] [PubMed] [Google Scholar]
- 4.Aubdool AA, Graepel R, Kodji X, Alawi KM, Bodkin JV, Srivastava S, Gentry C, Heads R, Grant AD, Fernandes ES, Bevan S, Brain SD. TRPA1 is essential for the vascular response to environmental cold exposure. Nat Commun 5: 5732, 2014. doi: 10.1038/ncomms6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker RL, Cockrem FR. Selection for body weight in the mouse at three temperatures and the correlated response in tail length. Genetics 65: 505–523, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett SA. Genotype and environment in tail length in mice. Q J Exp Physiol Cogn Med Sci 50: 417–429, 1965. doi: 10.1113/expphysiol.1965.sp001807. [DOI] [PubMed] [Google Scholar]
- 7.Barnett SA. The skin and hair of mice living at a low environmental temperature. Q J Exp Physiol Cogn Med Sci 44: 35–42, 1959. doi: 10.1113/expphysiol.1959.sp001374. [DOI] [PubMed] [Google Scholar]
- 8.Birkebak RC. Heat transfer in biological systems. Int Rev Gen Exp Zool 2: 269–344, 1966. doi: 10.1016/B978-1-4831-9978-8.50011-6. [DOI] [Google Scholar]
- 9.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 10.Costello JT, Rendell RA, Furber M, Massey HC, Tipton MJ, Young JS, Corbett J. Effects of acute or chronic heat exposure, exercise and dehydration on plasma cortisol, IL-6 and CRP levels in trained males. Cytokine 110: 277–283, 2018. doi: 10.1016/j.cyto.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Cramer MN, Jay O. Biophysical aspects of human thermoregulation during heat stress. Auton Neurosci 196: 3–13, 2016. doi: 10.1016/j.autneu.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Farlow JO, Thompson CV, Rosner DE. Plates of the dinosaur stegosaurus: forced convection heat loss fins? Science 192: 1123–1125, 1976. doi: 10.1126/science.192.4244.1123. [DOI] [PubMed] [Google Scholar]
- 13.Fischer AW, Cannon B, Nedergaard J. Leptin-deficient mice are not hypothermic, they are anapyrexic. Mol Metab 6: 173, 2016. doi: 10.1016/j.molmet.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer AW, Hoefig CS, Abreu-Vieira G, de Jong JMA, Petrovic N, Mittag J, Cannon B, Nedergaard J. Leptin raises defended body temperature without activating thermogenesis. Cell Rep 14: 1621–1631, 2016. doi: 10.1016/j.celrep.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 15.Gabra P, Shen G, Xuan J, Lee TY. Arterio-venous anastomoses in mice affect perfusion measurements with dynamic contrast enhanced CT. Physiol Meas 31: 249–260, 2010. doi: 10.1088/0967-3334/31/2/010. [DOI] [PubMed] [Google Scholar]
- 16.Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci 31: 1721–1733, 2011. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Getz LL. Relationship between ambient temperature and respiratory water loss of small mammals. Comp Biochem Physiol 24: 335–342, 1968. doi: 10.1016/0010-406X(68)90986-9. [DOI] [PubMed] [Google Scholar]
- 18.Gordon CJ. Effect of heating rate on evaporative heat loss in the microwave-exposed mouse. J Appl Physiol 53: 316–323, 1982. doi: 10.1152/jappl.1982.53.2.316. [DOI] [PubMed] [Google Scholar]
- 19.Gordon CJ. The mouse thermoregulatory system: Its impact on translating biomedical data to humans. Physiol Behav 179: 55–66, 2017. doi: 10.1016/j.physbeh.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon CJ. The mouse: An “average” homeotherm. J Therm Biol 37: 286–290, 2012. doi: 10.1016/j.jtherbio.2011.06.008. [DOI] [Google Scholar]
- 21.Gordon CJ. Open-loop gain of evaporative heat loss during radiant heat exposure in the mouse. Am J Physiol Regul Integr Comp Physiol 242: R275–R279, 1982. doi: 10.1152/ajpregu.1982.242.3.R275. [DOI] [PubMed] [Google Scholar]
- 22.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol 37: 654–685, 2012. doi: 10.1016/j.jtherbio.2012.08.004. [DOI] [Google Scholar]
- 23.Gouma E, Simos Y, Verginadis I, Lykoudis E, Evangelou A, Karkabounas S. A simple procedure for estimation of total body surface area and determination of a new value of Meeh’s constant in rats. Lab Anim 46: 40–45, 2012. doi: 10.1258/la.2011.011021. [DOI] [PubMed] [Google Scholar]
- 24.Grant RT. Vasodilation and body warming in the rat. J Physiol 167: 311–317, 1963. doi: 10.1113/jphysiol.1963.sp007151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hainsworth FR. Saliva spreading, activity, and body temperature regulation in the rat. Am J Physiol 212: 1288–1292, 1967. doi: 10.1152/ajplegacy.1967.212.6.1288. [DOI] [PubMed] [Google Scholar]
- 26.Harrison GA. The adaptability of mice to high environmental temperatures. J Exp Biol 35: 892–901, 1958. [Google Scholar]
- 27.Heroux O, Depocas F, Hart JS. Comparison between seasonal and thermal acclimation in white rats. Can J Biochem Physiol 37: 473–478, 1959. doi: 10.1139/o59-048. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi Y, Nakano Y, Kizaki M, Hoshikuma K, Yokoo Y, Kamiya T. Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Med 67: 628–633, 2001. doi: 10.1055/s-2001-17353. [DOI] [PubMed] [Google Scholar]
- 29.Krajewski-Hall SJ, Blackmore EM, McMinn JR, Rance NE. Estradiol alters body temperature regulation in the female mouse. Temperature (Austin) 5: 56–69, 2017. doi: 10.1080/23328940.2017.1384090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer CW, Ootsuka Y, Romanovsky AA. Body temperature measurements for metabolic phenotyping in mice. Front Physiol 8: 520, 2017. doi: 10.3389/fphys.2017.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mount LE. Metabolic rate and thermal insulation in albino and hairless mice. J Physiol 217: 315–326, 1971. doi: 10.1113/jphysiol.1971.sp009573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oufara S, Barré H, Rouanet JL, Chatonnet J. Adaptation to extreme ambient temperatures in cold-acclimated gerbils and mice. Am J Physiol Regul Integr Comp Physiol 253: R39–R45, 1987. doi: 10.1152/ajpregu.1987.253.1.R39. [DOI] [PubMed] [Google Scholar]
- 33.Piñol RA, Zahler SH, Li C, Saha A, Tan BK, Škop V, Gavrilova O, Xiao C, Krashes MJ, Reitman ML. Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat Neurosci 21: 1530–1540, 2018. doi: 10.1038/s41593-018-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter WR, Witmer LM. Vascular patterns in the heads of dinosaurs: evidence for blood vessels, sites of thermal exchange, and their role in physiological thermoregulatory strategies. Anat Rec 303: 1075–1103, 2020. doi: 10.1002/ar.24234. [DOI] [PubMed] [Google Scholar]
- 35.Rand RP, Burton AC, Ing T. The tail of the rat, in temperature regulation and acclimatization. Can J Physiol Pharmacol 43: 257–267, 1965. doi: 10.1139/y65-025. [DOI] [PubMed] [Google Scholar]
- 36.Ravnic DJ, Konerding M, Pratt JP, Wolloscheck T, Huss HT, Mentzer SJ. Inflammation-responsive focal constrictors in the mouse ear microcirculation. J Anat 209: 807–816, 2006. doi: 10.1111/j.1469-7580.2006.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravussin Y, Gutman R, LeDuc CA, Leibel RL. Estimating energy expenditure in mice using an energy balance technique. Int J Obes 37: 399–403, 2013. [Erratum in Int J Obesity 37: 473, 2013]. doi: 10.1038/ijo.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimúndez A, Fernández-Peña C, García G, Fernández R, Ordás P, Gallego R, Pardo-Vazquez JL, Arce V, Viana F, Señarís R. Deletion of the cold thermoreceptor TRPM8 increases heat loss and food intake leading to reduced body temperature and obesity in mice. J Neurosci 38: 3643–3656, 2018. doi: 10.1523/JNEUROSCI.3002-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reitman ML. Of mice and men - environmental temperature, body temperature, and treatment of obesity. FEBS Lett 592: 2098–2107, 2018. doi: 10.1002/1873-3468.13070. [DOI] [PubMed] [Google Scholar]
- 40.Romanovsky AA. The thermoregulation system and how it works. Handb Clin Neurol 156: 3–43, 2018. doi: 10.1016/B978-0-444-63912-7.00001-1. [DOI] [PubMed] [Google Scholar]
- 41.Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol (1985) 92: 2667–2679, 2002. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- 42.Serrat MA, Williams RM, Farnum CE. Exercise mitigates the stunting effect of cold temperature on limb elongation in mice by increasing solute delivery to the growth plate. J Appl Physiol (1985) 109: 1869–1879, 2010. doi: 10.1152/japplphysiol.01022.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman J, Hendricks G. Sensory neuron development in mouse coccygeal vertebrae and its relationship to tail biopsies for genotyping. PLoS One 9: e88158, 2014. doi: 10.1371/journal.pone.0088158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Škop V, Guo J, Liu N, Xiao C, Hall KD, Gavrilova O, Reitman ML. Mouse thermoregulation: introducing the concept of the thermoneutral point. Cell Rep 31: 107501, 2020. doi: 10.1016/j.celrep.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szymusiak R, Satinoff E. Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate. Physiol Behav 26: 687–690, 1981. doi: 10.1016/0031-9384(81)90145-1. [DOI] [PubMed] [Google Scholar]
- 46.Tennent DM. A study of the water losses through the skin in the rat. Am J Physiol 145: 436–440, 1946. doi: 10.1152/ajplegacy.1946.145.3.436. [DOI] [PubMed] [Google Scholar]
- 47.Warner A, Rahman A, Solsjö P, Gottschling K, Davis B, Vennström B, Arner A, Mittag J. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor α1. Proc Natl Acad Sci USA 110: 16241–16246, 2013. doi: 10.1073/pnas.1310300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams TM. Heat transfer in elephants: thermal partitioning based on skin termperature profiles. J Zool (Lond) 222: 235–245, 1990. doi: 10.1111/j.1469-7998.1990.tb05674.x. [DOI] [Google Scholar]
- 49.Young AA, Dawson NJ. Evidence for on-off control of heat dissipation from the tail of the rat. Can J Physiol Pharmacol 60: 392–398, 1982. doi: 10.1139/y82-057. [DOI] [PubMed] [Google Scholar]