Abstract
Obesity is associated with dyslipidemia and subclinical inflammation that promotes metabolic disturbances including insulin resistance and pancreatic β-cell dysfunction. The nuclear protein, transcriptional regulator 1 (NUPR1) responds to cellular stresses and features tissue protective properties. To characterize the role of NUPR1 in endocrine pancreatic islets during inflammatory stress, we generated transgenic mice with β-cell-specific Nupr1 overexpression (βNUPR1). Under normal conditions, βNUPR1 mice did not differ from wild type (WT) littermates and display normal glucose homeostasis and β-cell mass. For induction of inflammatory conditions, mice were treated with multiple low-dose streptozotocin (mld-STZ) and/or fed a high-fat diet (HFD). All treatments significantly worsened glycaemia in WT mice, while βNUPR1 mice substantially preserved insulin secretion and glucose tolerance. HFD increased β-cell mass in all animals, with βNUPR1 mice tending to show higher values. The improved outcome of βNUPR1 mice was accompanied by decreased NF-κB activation and lymphocyte infiltration in response to mld-STZ. In vitro, isolated βNUPR1 islets preserved insulin secretion and content with insignificantly low apoptosis during culture stress and IL-1β exposure. These findings suggest that NUPR1 plays a vital role in the protection of β-cells from apoptosis, related degradation of insulin storages and subsequent secretion during inflammatory and obesity-related tissue stress.
Keywords: high-fat diet, IL-1β, insulin secretion, NF-κB, NUPR1
INTRODUCTION
Chronic high-caloric malnutrition in combination with physical inactivity promotes obesity with varying degrees of dyslipidemia and a state of systemic low-grade inflammation. Upon severity, susceptive individuals develop diverse metabolic disorders including type 2 diabetes (13). In this regard, chronically high levels of circulating free fatty acids (FFA) can activate immune responses via toll-like receptors (TLR) 2 and 4 (11, 25) and impair insulin resistance as well as insulin secretion by pancreatic β-cells. Consequently, Tlr2−/− mice and mice with non-functional TLR4 are insensitive to HFD-mediated activation of NF-κB and display improved insulin sensitivity and insulin responses.
FFA-mediated immune responses can further promote M1-like polarization of macrophages and potential recruitment of immune cells leading to local production of inflammatory cytokines (10). This may also influence β-cells since they express cytokine receptors such as the IL-1 receptor type I (3), which like TLR2 and 4 activates NF-κB (30) and thereby impaires insulin secretion (16). However, histologic signs of islets infiltration in rodent and human type 2 diabetes were generally small and transcriptome studies did not consistently identify pro-inflammatory gene expression signatures (20). This indicates dyslipidemia as the principle trigger in this scenario and accordingly the efficacy of anti-inflammatory therapies remained modest (20).
Plausibly, the individual type 2 diabetes risk is modulated by increasing numbers of disease-related gene loci (14) and the efficacy of protective molecular mechanisms that counteract lipotoxic and inflammatory stress by controlling NF-κB and other intracellular mediators. In this respect, we tested the β-cell-protective potential of the nuclear protein, transcriptional regulator, 1 (NUPR1; former aliases p8 and com1). NUPR1 is expressed in many tissues including endocrine β-cells and its gene expression is acutely upregulated by glucose and different cellular stresses (17, 22, 23, 26). A functional NF-κB site within the Nupr1 promoter region links inflammation to Nupr1 gene expression (18) and NUPR1 has been shown in turn to inhibit nuclear NF-κB translocation (27) and apoptosis (21). Consequently, Nupr1−/− mice displayed enhanced mortality during sepsis and increased tissue damage after pancreatitis or carbon tetrachloride-mediated liver injury (26–28).
Based on these findings, NUPR1 has been considered a part of the cellular responses against tissue stress and inflammation. To characterize its protective role in the endocrine pancreas, we generated βNUPR1 mice with β-cell-specific Nupr1 overexpression and challenged these mice and their WT littermates with mld-STZ and/or feeding a HFD.
MATERIALS AND METHODS
Animals.
Animal experiments were performed with governmental permission by the Regional Commission of Freiburg. βNUPR1 mice were generated on C57BL/6NCrl background by pronucleus injection of a DNA construct with a 500 bp rat insulin gene 1 promoter (RIP1) fragment, the mouse Nupr1 coding sequence and a SV40 poly A site (IBF/Biotechnology Laboratory, University of Heidelberg, Germany). Genotyping by PCR used a RIP1 forward (5′-GAGCCCTTAATGGGCCAAAC-3′) and a mouse Nupr1 reverse primer (5′-CTCTCTTGGTCCGACCTTTC-3′). This was combined with a commercial Mm_RPS13_1_SG housekeeping primer (Qiagen, Hilden, Germany) to avoid false-negative results for the WT genotpye. A plasmid containing the transgenic construct served as positive control. βNUPR1 mice displayed a heterozygous genotype since their mating results in both transgenic and WT littermates. The latter were used as experimental controls. If not otherwise noted, experimental groups consist of about equally distributed females and males of about same age.
HFD and mld-STZ.
Mice were fed standard chow (4.5% energy from fat) or a HFD (60% energy from fat; Altromin Spezialfutter, Lage, Germany). STZ (Sigma-Aldrich, Taufkirchen, Germany) was always freshly dissolved in ice-cold citrate buffer before immediate intraperitoneal (i.p.) injection of 40 µg STZ per g body weight per day on consecutive days 1–5.
Blood glucose, ipGTT, ipITT, insulin secretion and cleaved poly (ADP-ribose) polymerase (PARP).
Blood glucose was measured from tail blood using a wide-range glucometer (Gluco Smart Swing; MSP Brodmann, Bodingen, Germany). Non-fasting blood glucose levels were analyzed between 9:00–10:30 AM For ipGTT and ipITT, mice were fasted for 6 h before i.p. injection of 1 mg glucose or 0.75 international units insulin per g body weight, respectively. Same glucose challenge was also used in non-fasted animals (see Fig. 3, induced) with blood collected after 30 min for measurement of serum insulin. Isolated islets were sequentially exposed to 2.8 mM and 20 mM glucose with supernatants collected after 1 h. Islets were then lysed with RIPA to determine protein concentration for normalization. Insulin was measured using the Ultra Sensitive Mouse ELISA Kit (Chrystal Chem Inc.). PARP cleavage was analyzed in RIPA samples using PathScan Cleaved PARP (Asp 214) Sandwich ELISA Kit (Cell Signaling Technologies/New England Biolabs).
Fig. 3.
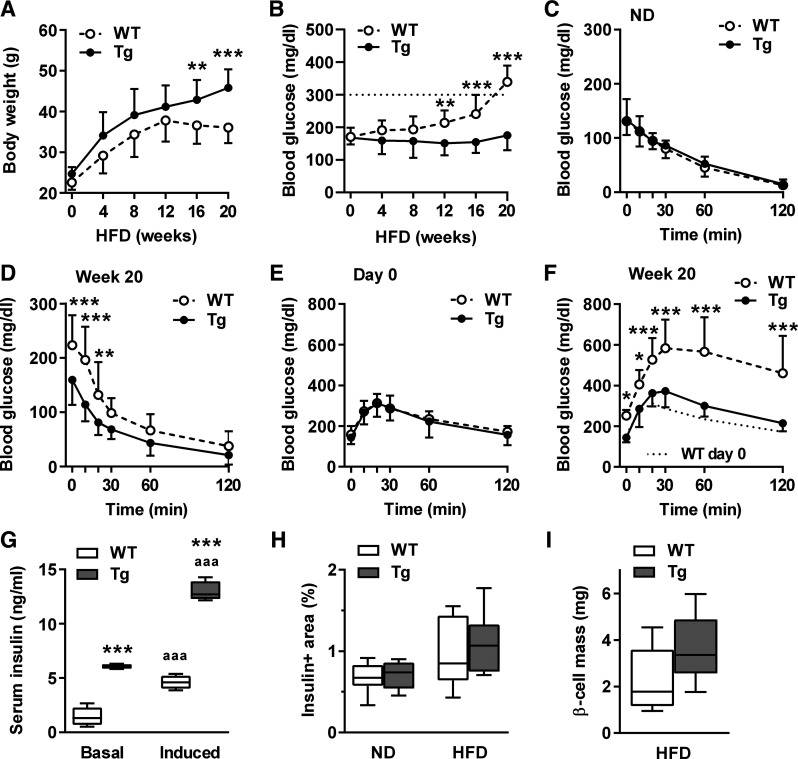
β-Cell-protective potential of βNUPR1 (transgenic mice with β-cell-specific Nupr1 overexpression) (Tg) preserved glucose homeostasis during 20 wk high-fat diet (HFD) with 60% energy from fat. Mice were ~2–3 mo at start of diet (n = 13 per group if not otherwise noted). A: body weight during HFD. Means in week 20: 45.8 g βNUPR1 and 36.1 g wild-type (WT). B: nonfasting blood glucose levels during HFD. Dotted line indicates diabetic threshold of 300 mg/dl. C and D: ip insulin tolerance tests (IpITT) in normal diet (ND)-fed mice and in 20-wk HFD-fed mice. E and F: ip glucose tolerance tests (IpGTT) on day 0 and in week 20 of HFD. G: nonfasting basal and glucose-induced serum insulin levels after 20 wk HFD (basal/induced, n = 7/4). H: β-cell area after 20 wk HFD (n = 8). ND, data of 5-mo-old normal-diet-fed mice from Fig. 1F for comparison. I: β-mass after normalization of insulin+ area shown in H to pancreas wet weight. Data are means ± SD (A–F) or box plots with min/max whiskers (G–I). *P < 0.05, **P < 0.01 and ***P < 0.001 (WT vs. Tg); aaaP < 0.001 (basal vs. induced).
Immunostaining, β-cell area and lymphocyte infiltration.
Pancreases were fixed with 2% paraformaldehyde, embedded in paraffin and cut into 5 µm longitudinally sections. β-cell area was evaluated on 10 slides with equal distribution over the organ. Deparaffined sections (3/slide) were digested with pepsin and stained with a polyclonal guinea pig anti-swine insulin antiserum (Dako), Vectastain ABC Kit (Guinea Pig IgG), DAB Peroxidase Substrate Kit (Vector Laboratories/Biozol, Eching, Germany) and hematoxylin/eosin counterstain before evalution of insulin+ β-cell area in percent of total pancreas area using photographed sections and free ImageJ software (http://rsb.info.nih.gov/ij/). CD45+ lymphocytes were stained as above using a purified anti-mouse CD45 antibody (rat 30-F11; Biolegend, London, UK). Numbers were analyzed on 3 equally distributed slides per organ.
Islet isolation and culture.
Islets were isolated according to Zmuda et al. (31) with modifications and cultured at 37°C in glucose-free RPMI 1640 supplemented with 5.6 mM glucose, 10 mM HEPES, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 1 mM sodium pyruvate and 0.5% bovine serum albumin. Islets were seeded in inserts with 12-µm pores (Merck Millipore, Darmstadt, Germany) and placed in 24-well plates. Some islets were exposed to recombinant murine IL-1β (PromoKine, Heidelberg, Germany).
qPCR.
RNA was isolated using RNeasy Mini Kit (Qiagen) and reversely transcribed with Superscript III kit and oligo-(dT) primers (Invitrogen). Samples were analyzed using Lightcycler 2.0, Faststart DNA Master+ SYBR Green I kit (Roche, Mannheim, Germany) and Quantitect Mm_B2m and Mm_Nupr1 primers (Qiagen). Transcript levels were calculated from primer-specific standard curves (dilution series 1:1 to 1:10,000) using Light Cycler Software 3.5 (Roche). qPCR conditions: 10 min at 95°C, 40 cycles with 15 s at 95°C, 10 s at 55°C and 20 s at 72°C followed by built-in melting curve analysis of product specificity.
Western blot.
Islets were lysed using RIPA buffer (Upstate/Biomol, Hamburg, Germany) containing Complete Mini EDTA-free protease inhibitor cocktail (Roche, Mannheim, Germany). Protein concentration was analyzed by Bradford assay (Sigma-Aldrich). Samples were separated by SDS-PAGE and transferred to PVDF membranes by tank blotting. Blots were incubated overnight at 4–8°C with goat polyclonal antibody p8 (T-14) (Santa Cruz Biotechnology, Heidelberg, Germany) or phospho-NF-κB p65 (Ser536) (93H1) rabbit monoclonal antibody (Cell Signaling Technologies/New England Biolabs, Frankfurt, Germany). Actin I-19 (Santa Cruz Biotechnology) staining served as loading control. Signals were visualized on films after 1 h exposure to donkey anti-rabbit or anti-goat IgG-HRP and ECL+ treatment (GE Healthcare, Freiburg, Germany).
Data analysis.
Statistical differences between two or more groups were analyzed by t test or ANOVA with post hoc test using GraphPad Prism 6 (GraphPad Software, San Diego, California, USA).
RESULTS
Basic characterization.
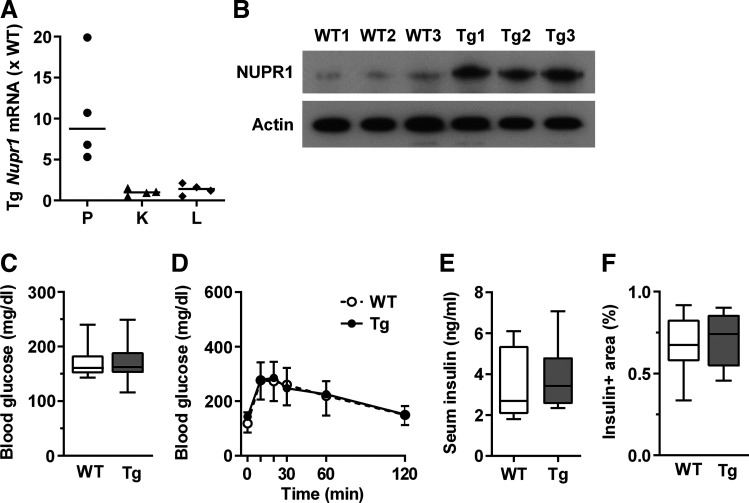
βNUPR1 mice exhibit increased pancreatic Nupr1 gene expression and NUPR1 protein levels in isolated islets while overexpression was absent in kidney and liver (Fig. 1, A and B). Their non-fasted blood glucose, glucose tolerance, insulin levels, and β-cell mass did not differ from WT littermates (Fig. 1, C–F). We never observed insulinomas, not even in aged βNUPR1 mice monitored for 22 mo (n = 14 WT/14 βNUPR1).
Fig. 1.
Basic characteristics of βNUPR1 (transgenic mice with β-cell-specific Nupr1 overexpression) (Tg) mice. Age was ~3 mo except in E. A: Nupr1 mRNA levels in pancreas (P), kidney (K), and liver (L) of transgenic (Tg) mice relative to mean of wild-type (WT) mice (n = 4). B: NUPR1 protein levels in isolated islets (n = 3). C: nonfasting blood glucose levels (n = 12). D: ip glucose tolerance tests (IpGTT; n = 12). E: nonfasting serum insulin (n = 8 WT and 6 Tg). F: β-cell area of ~5-mo-old mice (n = 8 WT and 5 Tg). Data are individual mice (A, B) with median in (A), box plots with min/max whiskers (C, E, F) or means ± SD (D).
βNUPR1 mice were less susceptive to mld-STZ.
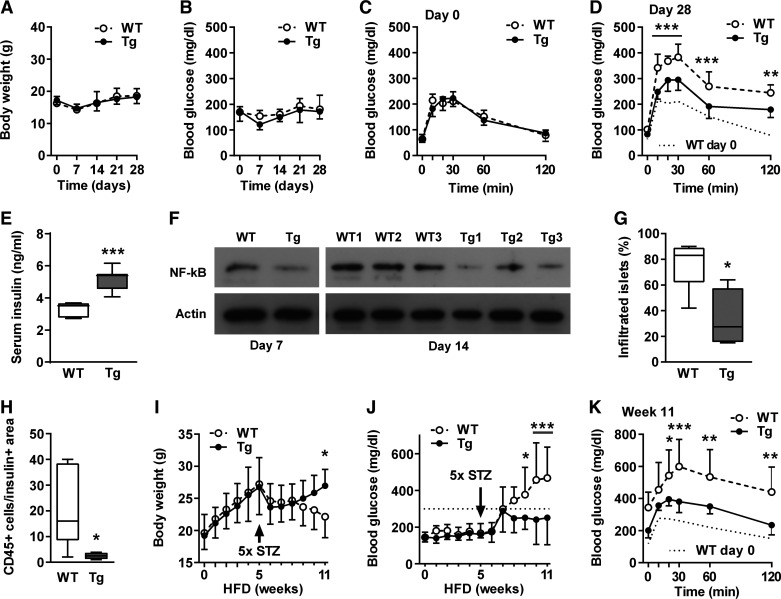
The mld-STZ treatment did not affect body weight and non-fasting blood glucose levels in normal-fed mice (Fig. 2, A and B). The latter may indicate lower sensitivity to STZ than in other strains. Nevertheless, WT mice displayed significantly impaired glucose tolerance and reduced non-fasting serum insulin levels on day 28 after start of treatment (Fig. 2, C–E). In comparison, βNUPR1 mice were much less affected and their substantially preserved insulin secretion was associated with reduced NF-κB activation and significantly less lymphocyte infiltration in islets (Fig. 2, F–H).
Fig. 2.
β-Cell-protective potential of βNUPR1 (transgenic mice with β-cell-specific Nupr1 overexpression) (Tg) mice were less susceptive to multiple low-dose streptozotocin (mld-STZ). Mice were ~2 mo at start of treatments. One group received STZ on normal diet (days 1–5) and was monitored for 28 days [A–H; n = 7 wild-type (WT) and 8–12 transgenic (Tg) if not otherwise noted]. A second group received STZ in week 5 of 11 wk high-fat diet (HFD) with 60% energy from fat (I–K; n = 10 WT and 9 Tg). A: body weight. B: nonfasting blood glucose. C and D: ip glucose tolerance tests (IpGTT) on days 0 and 28. E: nonfasting serum insulin levels on day 28. F: active NF-κB (phosphorylated p65) levels in islets isolated on day 7 (representative) and 14 (individual mice; n = 3). G and H: percentages of infiltrated islets with CD45+ lymphocytes and numbers of CD45+ lymphocytes per insulin+ area in the same sections on day 7 (n = 4–5). I: body weight during HFD. J: nonfasting blood glucose levels during HFD. Dotted line indicates the diabetic threshold of 300 mg/dl. K: IpGTT in week 11 of HFD. Data are means ± SD (A-C and H-J) or box plots with min/max whiskers (D, F, and G). *P < 0.05, **P < 0.01, and ***P < 0.001 (WT vs. Tg).
We further tested the impact of mld-STZ in animals fed an HFD for 11 wk. All mice similarly gained weight during the first 5 wk without becoming obese in this short period (Fig. 2I). Administration of mld-STZ in week 5 caused an immediate weight loss in all animals. Until week 11 WT mice continuously lost further weight and developed severe diabetes. In comparison, βNUPR1 mice gained weight again and their recovery was associated with subdiabetically non-fasting blood glucose levels below 300 mg/dl and accordingly improved glucose tolerance (Fig. 2, J and K). These findings demonstrate that HFD boosted the impact of mld-STZ and the combination of both stresses strongly accelerated the development of diabetes if compared with HFD alone (see Fig. 3).
βNUPR1 mice preserved glucose homeostasis during 20 wk HFD.
We further analyzed the consequences of prolonged HFD without additional stress by STZ. Within the first 12 wk, the body weight of all animals increased comparably (Fig. 3A). In weeks 12–20, WT mice stopped weight gain and non-fasting blood glucose levels rised to diabetic levels above 300 mg/dl in week 20 (Fig. 3B). Obese WT mice demonstrated increased values for insulin resistance during ipITT in week 20 since they start with with elevated fasting blood glucose levels. However, the course of glucose clearance was similar to that of βNUPR1 mice (Fig. 3, C and D).
The development of obesity-related diabetes in WT mice goes along with impaired glucose tolerance as well as substantially reduced basal and glucose-induced insulin secretion in week 20 (Fig. 3, E–G). In contrast, βNUPR1 mice continuously increased in weight and preserved almost normal glycaemia. Their remarkably beneficial outcome was associated with preserved insulin secretion (Fig. 3G). We further observed a roughly doubled median of β-cell mass in βNUPR1 mice, but the spread of individual data avoids significance (P = 0.074; Fig. 3, H and I).
Sex differences upon 20 wk HFD.
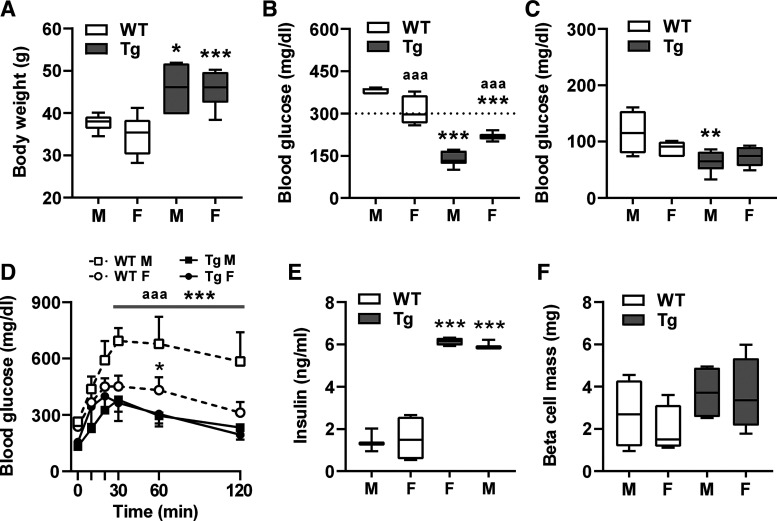
Female mice may gain less weight with less insulin resistance than males and their ovarian hormones mediate some protection from HFD-induced degradation of glucose homeostasis (8, 15). Therefore, we revisited the findings from HFD week 20 in Fig. 3 and compared males and females (Fig. 4). WT females showed significantly improved non-fasting blood sugar levels and glucose tolerance than their males (Fig. 4B and D), but remained significantly worse compared with βNUPR1 mice. However, differences in glucose tolerance were still significant at 60 min but became a trend at 30 and 120 min (P = 0.3819 and 0.0525).
Fig. 4.
Sex differences upon 20-wk high-fat diet (HFD). Data of week 20 from Fig. 3 after separation of males (M) and females (F). A: body weight. B: nonfasting blood glucose. C: ip insulin tolerance tests (IpITT) values at 30-min postinsulin injection. D: ip glucose tolerance tests (IpGTT). E: nonfasting basal serum insulin levels. F: β-cell mass. Data are means ± SD (D) or box plots with min/max whiskers. *P < 0.05, **P < 0.01, and ***P < 0.001 [wild-type (WT) vs. corresponding transgenic (Tg) gender]; aaaP < 0.001 (M vs. F).
The improved outcome of WT females compared with WT males correlates with tendentially lower body weight and reduced insulin resistance (P = 0.2068 and 0.1092) (Fig. 4, A and C), but not with non-fasting serum insulin levels, which were similarly low in both WT sexes (Fig. 4E). In contrast, βNUPR1 mice showed no sex differences except for slightly but significantly elevated non-fasting blood glucose levels in females (Fig. 4B). This somewhat unexpected difference appears to be negligible since glucose tolerance, insulin secretion and β-cell mass were very similar between sexes Fig. 4, D–F).
Islets from βNUPR1 mice were less susceptive to culture stress and IL-1β.
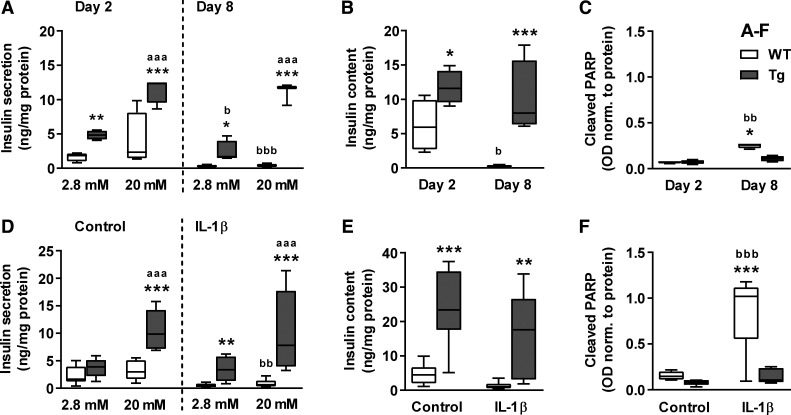
Upon isolation and loss of blood supply, islets quickly degrade in culture (6, 7). Note that investigated islets were cultured in the presence of 5,6 mM glucose and 0.5% bovine serum albumin, although higher glucose concentrations and serum supplementation are well known to support β-cell viability and maintenance of secretory function in vitro (1, 5, 12). Under these reduced conditions, WT islets showed a functional insulin release on day 2, loss of stimulated insulin response on day 4 and complete loss of secretory function along with completely emptied insulin storages on day 8 (Fig. 5, A and B, and controls in Fig. 5D). This degradation was associated with increased caspase-mediated cleavage of the DNA repair enzym PARP (Fig. 5, A–C).
Fig. 5.
Islets from βNUPR1 mice were less susceptive to culture stress and IL-1β. Isolated islets from ~3-mo-old normal-fed mice were exposed for 2–8 days to culture stress (A–C) or for 24 h to 10 ng/ml IL-1β on culture day 4 (D–F) before measurement of glucose-induced insulin secretion/content and PARP cleavage. A: insulin secretion after 1-h incubation with 2.8 and 20 mM glucose (n = 4–5). B: insulin content in islets from A. C: PARP cleavage (n = 4). D: insulin secretion after 1-h incubation with 2.8 and 20 mM glucose (n = 6–8). E: insulin content in islets from D. F: PARP cleavage (n = 5). Data are box plots with min/max whiskers. *P < 0.05, **P < 0.01, and ***P < 0.001 [wild-type (WT) vs. transgenic (Tg)]; aaaP < 0.001 (2.8 vs. 20 mM); bP < 0.05, bbP < 0.01, and bbbP < 0.001 (day 2 vs. day 8 or Con vs. IL-1β).
βNUPR1 islets were much less affected by culture stress since they displayed more than twofold enhanced insulin secretion and content on day 2 and further maintained these values until day 8 without significant rise in cleaved PARP. IL-1β as an additional strong stress on day 4 induced massive apoptosis and quickly exhausted insulin storages and secretion in WT islets (Fig. 5, D and E). Again, βNUPR1 islets effectively maintained insulin function along with still insignificantly low apoptosis (Fig. 5F).
DISCUSSION
Elevated NUPR1 levels are associated with proliferation during pancreatic organogenesis, organ recovery after subtotal pancreatectomy (22) and high glucose conditions (23). Accordingly, Nupr1 overexpression promotes proliferation in isolated mouse islets, AR42J acinar cells and INS-1E β-cells (22–24, 29), but has also been associated with tumor progression (4). Despite this, βNUPR1 mice exhibit a normal β-cell mass and did not develop insulinomas, not even in aged mice.
In contrast, a study reported that Nupr1 overexpression reduced proliferation in MIN6 β-cells and enhanced β-cell proliferation and mass in Nupr1−/− mice (2). These opposite findings may be related to inherent characteristics of individual tumor cell lines and interfering systemic effects of the global knock out. For example, Nupr1−/− mice were highly resistant to HFD-induced weight gain. We assume that NUPR1-mediated proliferation is well controlled and underlies a tissue and context-dependent regulation.
Nupr1−/− mice further displayed enhanced mortality during sepsis and increased tissue damage after carbon tetrachloride-mediated liver injury or pancreatitis (26–28). Therefore we tested the anti-inflammatory properties of Nupr1 overexpression by mld-STZ and found substantially reduced NF-κB activation and lymphocyte infiltration in transgenic islets. Consequently, βNUPR1 mice preserved insulin secretion and showed improved glucose tolerance compared with WT.
The investigated mice were somewhat resistant to mld-STZ since there was no change in non-fasting blood glucose levels in normal-fed mice. Such reduced sensitivity may result from low numbers of the low-affinity glucose transporter (GLUT)2 on the cell surface since this transporter mediates the selective uptake of STZ into β-cells (19). However, the toxic impact of the same STZ regimen dramatically worsened glycaemia in HFD-fed WT animals, thereby demonstrating the synergistical impact of two risk factors.
Though more delayed as in combination with STZ, also HFD alone worsened glycaemia in WT to type 2 diabetic levels in week 20. The HFD-mediated degradation of metabolic health was reflected by restricted weight gain from week 12 indicating an unhealthy metabolic state. In contrast, βNUPR1 mice remained almost unaffected. They continuously gained weight, substantially maintained insulin secretion and glucose homeostasis, and demonstrated a tendentially increased β-cell mass. Likely, NUPR1 overexpression reduced β-cell stress by limiting obesity-related NF-κB activation and inflammatory islet infiltration as observed for mld-STZ, but this needs to be finally confirmed in obese animals.
We further analyzed potential sex differences upon HFD since less weight gain of females may result in less insulin resistance than in males. In addition, female mice have been shown to profit from their ovarian hormones such as estrogen that protect glucose homeostasis in obesesity (8, 15). In this regard, obese WT females from HFD week 20 showed improved non-fasting blood glucose levels and glucose tolerance compared with WT males, and this improvement was associated with tendentially higher insulin sensitivity. However, βNUPR mice did not show relevant sex differences and their serum insulin levels and glucose homeostasis were still further improved than in WT females.
Mld-STZ-related inflammation and HFD-induced lipotoxic conditions have in common that both activate NF-κB, either via cytokine receptors or TLR2/4, respectively (30). This is of importance since NUPR1 overexpression has been demonstrated to inhibit NF-κB signaling in primary alveolar macrophages and AR42J pancreatic acinar cells (27). Accordingly, mld-STZ-treated βNUPR1 islets displayed substantially reduced NF-κB activation as well as reduced immune cell infiltration.
With respect to NUPR1-mediated inhibition of NF-κB, we finally tested whether Nupr1 overexpression protects insulin secretion of isolated islets during culture stress and IL-1β exposure since both of which are known to quickly emerge apoptosis and to degrade islets (6, 7, 9). Upon culture stress, WT islets expectedly demonstrated such degradation and lost their insulin secretion and content until culture day 8. This loss was much accelerated in the presence of IL-1β and related to massive apoptosis, while NUPR1 overexpression effectively inhibits such apoptosis in βNUPR1 islets. Plausibly, this enabled their remarkable maintainance of insulin storages and secretory function in response to IL-1β. In this regard, it has been shown that NUPR1 in complex with prothymosin α reduced the activity of staurosporine-induced apoptotic caspases-3, −7 and −9 in HeLa cells (21).
In summary, β-cell-specific Nupr1 overexpression effectively maintained insulin secretion during tissue stress by mld-STZ and prolonged HFD in vivo as well as during culture stress and IL-1β exposure in vitro. This was associated with reduced NF-κB and islet infiltration in response to mld-STZ, a tendentially more enlarged β-cell mass after HFD and low apoptosis in vitro. Though not all of these parameters have been analyzed in all settings, we consider inhibition of NF-κB and related apoptosis a plausible underlying molecular mechanism, which, however, remains to be finally elucidated in HFD. We conclude that, at least in rodents, NUPR1 plays a vital role in the protection of β-cells and their secretory function from inflammatory and obesity-related tissue stress. Future research has to investigate whether the presented findings translate into the human context.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.P. conceived and designed research; G.P., A.E.M., I.H.P., M.A., J.B., I.S., and A.H. performed experiments; G.P., A.E.M., and I.H.P. analyzed data; G.P., A.E.M., and I.H.P. interpreted results of experiments; G.P. prepared figures; G.P. drafted manuscript; G.P. edited and revised manuscript; G.P., A.E.M., I.H.P., M.A., J.B., I.S., A.H., and J.S. approved final version of manuscript.
REFERENCES
- 1.Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia 14: 397–404, 1978. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa-Sampaio HC, Liu B, Drynda R, Rodriguez de Ledesma AM, King AJ, Bowe JE, Malicet C, Iovanna JL, Jones PM, Persaud SJ, Muller DS. Nupr1 deletion protects against glucose intolerance by increasing beta cell mass. Diabetologia 56: 2477–2486, 2013. doi: 10.1007/s00125-013-3006-x. [DOI] [PubMed] [Google Scholar]
- 3.Böni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150: 5218–5229, 2009. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 4.Cano CE, Hamidi T, Sandi MJ, Iovanna JL. Nupr1: the Swiss-knife of cancer. J Cell Physiol 226: 1439–1443, 2011. doi: 10.1002/jcp.22324. [DOI] [PubMed] [Google Scholar]
- 5.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online 11: 3–31, 2009. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronel MM, Geusz R, Stabler CL. Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials 129: 139–151, 2017. doi: 10.1016/j.biomaterials.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui YF, Ma M, Wang GY, Han DE, Vollmar B, Menger MD. Prevention of core cell damage in isolated islets of Langerhans by low temperature preconditioning. World J Gastroenterol 11: 545–550, 2005. doi: 10.3748/wjg.v11.i4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dakin RS, Walker BR, Seckl JR, Hadoke PW, Drake AJ. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int J Obes 39: 1539–1547, 2015. doi: 10.1038/ijo.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 17: 314–321, 2010. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 10.Eguchi K, Nagai R. Islet inflammation in type 2 diabetes and physiology. J Clin Invest 127: 14–23, 2017. doi: 10.1172/JCI88877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rütti S, Schuit FC, Lutz TA, Böni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia 53: 1795–1806, 2010. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 12.Eizirik DL, Strandell E, Sandler S. Culture of mouse pancreatic islets in different glucose concentrations modifies B cell sensitivity to streptozotocin. Diabetologia 31: 168–174, 1988. doi: 10.1007/BF00276851. [DOI] [PubMed] [Google Scholar]
- 13.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445, 2011. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 14.Hara K, Shojima N, Hosoe J, Kadowaki T. Genetic architecture of type 2 diabetes. Biochem Biophys Res Commun 452: 213–220, 2014. doi: 10.1016/j.bbrc.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Hong J, Stubbins RE, Smith RR, Harvey AE, Núñez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J 8: 11, 2009. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes JH, Colca JR, Easom RA, Turk J, McDaniel ML. Interleukin 1 inhibits insulin secretion from isolated rat pancreatic islets by a process that requires gene transcription and mRNA translation. J Clin Invest 86: 856–863, 1990. doi: 10.1172/JCI114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang YF, Vaccaro MI, Fiedler F, Calvo EL, Iovanna JL. Lipopolysaccharides induce p8 mRNA expression in vivo and in vitro. Biochem Biophys Res Commun 260: 686–690, 1999. doi: 10.1006/bbrc.1999.0953. [DOI] [PubMed] [Google Scholar]
- 18.Kallwellis K, Grempler R, Günther S, Päth G, Walther R. Tumor necrosis factor alpha induces the expression of the nuclear protein p8 via a novel NF kappaB binding site within the promoter. Horm Metab Res 38: 570–574, 2006. doi: 10.1055/s-2006-950503. [DOI] [PubMed] [Google Scholar]
- 19.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51: 216–226, 2008. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 20.Lytrivi M, Igoillo-Esteve M, Cnop M. Inflammatory stress in islet β-cells: therapeutic implications for type 2 diabetes? Curr Opin Pharmacol 43: 40–45, 2018. doi: 10.1016/j.coph.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Malicet C, Giroux V, Vasseur S, Dagorn JC, Neira JL, Iovanna JL. Regulation of apoptosis by the p8/prothymosin α complex. Proc Natl Acad Sci USA 103: 2671–2676, 2006. doi: 10.1073/pnas.0508955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V, Morisset J, Iovanna JL. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem 272: 32360–32369, 1997. doi: 10.1074/jbc.272.51.32360. [DOI] [PubMed] [Google Scholar]
- 23.Päth G, Opel A, Gehlen M, Rothhammer V, Niu X, Limbert C, Romfeld L, Hügl S, Knoll A, Brendel MD, Bretzel RG, Seufert J. Glucose-dependent expansion of pancreatic beta-cells by the protein p8 in vitro and in vivo. Am J Physiol Endocrinol Metab 291: E1168–E1176, 2006. doi: 10.1152/ajpendo.00436.2005. [DOI] [PubMed] [Google Scholar]
- 24.Päth G, Opel A, Knoll A, Seufert J. Nuclear protein p8 is associated with glucose-induced pancreatic beta-cell growth. Diabetes 53, Suppl 1: S82–S85, 2004. doi: 10.2337/diabetes.53.2007.S82. [DOI] [PubMed] [Google Scholar]
- 25.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taïeb D, Malicet C, Garcia S, Rocchi P, Arnaud C, Dagorn JC, Iovanna JL, Vasseur S. Inactivation of stress protein p8 increases murine carbon tetrachloride hepatotoxicity via preserved CYP2E1 activity. Hepatology 42: 176–182, 2005. doi: 10.1002/hep.20759. [DOI] [PubMed] [Google Scholar]
- 27.Vasseur S, Folch-Puy E, Hlouschek V, Garcia S, Fiedler F, Lerch MM, Dagorn JC, Closa D, Iovanna JL. p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the anti-inflammatory protein pancreatitis-associated protein I. J Biol Chem 279: 7199–7207, 2004. doi: 10.1074/jbc.M309152200. [DOI] [PubMed] [Google Scholar]
- 28.Vasseur S, Hoffmeister A, Garcia-Montero A, Barthet M, Saint-Michel L, Berthézène P, Fiedler F, Closa D, Dagorn JC, Iovanna JL. Mice with targeted disruption of p8 gene show increased sensitivity to lipopolysaccharide and DNA microarray analysis of livers reveals an aberrant gene expression response. BMC Gastroenterol 3: 25, 2003. doi: 10.1186/1471-230X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasseur S, Vidal Mallo G, Fiedler F, Bodeker H, Canepa E, Moreno S, Iovanna JL. Cloning and expression of the human p8, a nuclear protein with mitogenic activity. Eur J Biochem 259: 670–675, 1999. doi: 10.1046/j.1432-1327.1999.00092.x. [DOI] [PubMed] [Google Scholar]
- 30.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-κB: variations on a common theme. Cell Mol Life Sci 65: 2964–2978, 2008. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zmuda EJ, Powell CA, Hai T. A method for murine islet isolation and subcapsular kidney transplantation. J Vis Exp 50: 2096, 2011. doi: 10.3791/2096. [DOI] [PMC free article] [PubMed] [Google Scholar]