Abstract
White adipose tissue (WAT) dysfunction in obesity is implicated in the onset of whole body insulin resistance. Alterations in mitochondrial bioenergetics, namely impaired mitochondrial respiration and increased mitochondrial reactive oxygen species (mtROS) production, have been suggested to contribute to this metabolic dysregulation. However, techniques investigating mitochondrial function are classically normalized to tissue weight, which may be confounding when considering obesity-related adipocyte hypertrophy. Furthermore, the effect of long-term high-fat diet (HFD) on mtROS in WAT has yet to be elucidated. Therefore, we sought to determine the HFD-mediated temporal changes in mitochondrial respiration and mtROS emission in WAT. C57BL/6N mice received low-fat diet or HFD for 1 or 8 wk and changes in inguinal WAT (iWAT) and epididymal WAT (eWAT) were assessed. While tissue weight-normalized mitochondrial respiration was reduced in iWAT following 8-wk HFD-feeding, this effect was mitigated when adipocyte cell size and/or number were considered. These data suggest HFD does not impair mitochondrial respiratory capacity per adipocyte within WAT. In support of this assertion, within eWAT compensatory increases in lipid-supported and maximal succinate-supported respiration occurred at 8 wk despite cell hypertrophy and increases in WAT inflammation. Although these data suggest impairments in mitochondrial respiration do not contribute to HFD-mediated WAT phenotype, lipid-supported mtROS emission increased following 1-wk HFD in eWAT, while both lipid and carbohydrate-supported mtROS were increased at 8 wk in both depots. Combined, these data establish that while HFD does not impair adipocyte mitochondrial respiratory capacity, increased mtROS is an enduring physiological occurrence within WAT in HFD-induced obesity.
Keywords: high-fat diet, insulin resistance, mitochondrial function, obesity, white adipose tissue
INTRODUCTION
White adipose tissue (WAT) plays a fundamental role in the maintenance of whole body glucose homeostasis and energy metabolism. Under healthy conditions, WAT is a dynamic metabolic organ that functions as a site of lipid storage in conditions of energy surplus and reciprocally, as a reservoir of free fatty acids (FFA) during fasting or periods of high-energy demand (11). In obesity, however, a positive energy balance exerts substantial metabolic stress on WAT, necessitating rapid expansion, which primarily occurs by adipocyte hypertrophy (44). This expansion is sometimes accompanied by inflammation, macrophage infiltration, the accumulation of oxidative stress markers, and insulin resistance (16, 63, 68). As a consequence of insulin resistance, lipolysis becomes largely unregulated, and a surplus of FFAs in circulation results in ectopic fat storage within the liver and skeletal muscle (37, 46), implicating metabolic dysregulation within WAT as a key contributor in the progression of whole body insulin resistance.
While mechanisms involved in the development of insulin resistance within WAT are, indeed, multifactorial, mitochondria have become a central focus. Genetically reducing mitochondrial content through the ablation of the transcription factor peroxisome proliferator-activated receptor (PPAR)-γ coactivator (PGC)-1α promotes the induction of insulin resistance (33), while upregulation of PGC-1α preserves insulin sensitivity in the presence of a high-fat environment (40). Moreover, there is increasing evidence that the production of mitochondrial reactive oxygen species (mtROS) is an early event in the development of WAT insulin resistance. In humans, WAT mitochondria isolated from obese individuals with Type 2 diabetes display increased reactive oxygen species (ROS) emission and oxidative stress compared with lean healthy controls (10). Likewise, the induction of mtROS is sufficient to produce insulin resistance in cultured adipocytes and myotubes (18, 26), while pharmacological or genetic interventions that attenuate mtROS production in rodent models confer protection against acute high-fat feeding (3, 51, 52). Collectively, these studies emphasize the importance of mtROS and altered redox imbalance in the development of insulin resistance within WAT.
In addition to mitochondria-mediated events, endoplasmic reticulum (ER) stress and inflammation have been implicated in the development of WAT insulin resistance. Inflammation is mediated in part by leukocytes that infiltrate WAT and form crown-like structures (CLS), which are recognized as a hallmark of WAT inflammation (47, 56). At a molecular level, a common signaling pathway between these models is the activation of JNKs, as ER stress and TNF-α/NF-κB signaling converge at the phosphorylation of JNK (29, 61). Importantly, ablating JNK1 prevents high-fat diet (HFD)-induced metabolic derangements within WAT, highlighting the importance of this signaling event (5). However, mitochondria appear to influence these signaling events, as temporally, the consumption of an HFD increases markers of oxidative stress and insulin resistance in WAT in the absence of ER stress and inflammation (6), while attenuating mtROS prevents the development of inflammation within WAT (51). Collectively, a sufficient amount of data has been generated to implicate mtROS as a key signal in the initiation of HFD-diet-induced insulin resistance and inflammation within WAT.
While recent literature has established a role for mtROS in the induction of WAT insulin resistance, the effect of long-term HFD feeding on mtROS in WAT has yet to be elucidated. Rapid expansion of this tissue due to chronic overnutrition results in inadequate vascularization and reduced perfusion capacity (28), which in combination with adipocyte hypertrophy, is believed to induce hypoxia (66). Although hypoxia has been associated with attenuated oxidative phosphorylation and increased oxidative stress in cell culture preparations (9, 15, 54, 69), in vitro mitochondrial preparations have suggested ROS production is attenuated when oxygen is limiting (27, 53). Given these divergent possibilities, the aim of the present study was to investigate the temporal adaptations of mitochondrial biogenetics and mtROS production in HFD-fed animals.
MATERIALS AND METHODS
Experimental design.
Fifteen-week-old male C57BL/6N mice were randomly assigned to receive either 1 or 8 wk of low-fat diet (LFD; 10% kcal from fat; D12450J) or sucrose-matched high-fat diet (HFD; 60% kcal from fat; D12492) (Research Diets, New Brunswick, NJ), which has been widely applied in diet-induced obesity research (13, 25, 31, 38). Animals were group housed in a temperature-controlled (22°C) barrier facility under a 12:12-h light-dark cycle and used for a variety of measurements (animal numbers listed in each corresponding figure legend, as all measurements were not performed on each animal). Animals had ad libitum access to food and water. All experimental protocols were approved by Committee on Animal Care at the University of Guelph.
Glucose tolerance test.
After a 4-h fast, mice underwent an intraperitoneal glucose tolerance test (ipGTT), as previously reported (64). Blood glucose was measured using a hand-held glucometer (FreeStyle Lite, Abbott Diabetes Care, Alameda, CA) through the tail vein before and up to 120 min after glucose injection (2 g/kg body wt). The area under the curve (AUC) was calculated above the baseline glucose levels of each animal (45).
Adipose tissue response to insulin.
In a distinct subset of animals, WAT-specific insulin signaling was assessed, as previously described (51). Inguinal WAT (iWAT) and epididymal WAT (eWAT) were excised from anesthetized animals before (basal) and 15 min after an intraperitoneal injection of 1 U/kg body wt of insulin (NovoRapid, Novo Nordisk Canada). Tissue was rapidly frozen in liquid nitrogen for Western blot analysis, as described below. Blood glucose was sampled before and after administration of insulin, and one LFD animal, which did not respond to insulin, was removed from the successive analysis.
Adipose tissue histology.
Adipose tissue samples were fixed, embedded, and stained with hematoxylin and eosin, as previously described (4, 51). Images were captured using an Olympus FSX light microscope (Tokyo, Japan) at ×40 magnification for adipocyte cross-sectional area (CSA) measurements and at ×20 magnification to assess leukocyte infiltration via CLS count. The average of three fields per animal were analyzed using ImageJ 1.48 (National Institutes of Health) software for CSA and CLS indices. For the latter, the average number of CLS were counted from four equal quadrants per image, and the average of these fields was expressed as the number of CLS/field (2).
High-resolution respirometry.
Mitochondrial respiration analysis was performed as previously described, with minor changes (8, 34, 58). Briefly, adipose tissue was collected, immediately transferred to biopsy preservation solution (BIOPS) buffer, minced with sharp scissors, and washed in mitochondrial respiration buffer MiR05 for 5 min. Tissue was then blotted, weighed, and ~15 mg (wet weight) of adipose tissue was added to a high-resolution respirometer chamber at 37°C (Oroboros Oxygraph-2K, Innsbruck, Austria). Substrate additions (listed as final concentrations) were performed in the following sequence in permeabilized (50 μg/mL saponin) WAT: 5 mM glycerol 3-phosphate (G3P), 5 mM ADP, 5 mM pyruvate + 2 mM malate, 5 mM glutamate, 10 mM succinate, and 166.7 μM 2,4-dinitrophenol (DNP). G3P is an essential component of TAG synthesis and is consequently paramount to cellular metabolic homeostasis, as greater G3P utilization within the mitochondria could limit TAG synthesis, while also participating in redox imbalance, two mechanisms directly linked to insulin resistance. To explore this and while distinct from classical protocols, G3P was used as an initial substrate to maximize the signal-to-noise ratio due to the low respiratory drive of this substrate. In a second set of experiments, lipid-supported respiration was tested in the presence of 2 mM malate + 5 mM L-carnitine + 50 μM palmitoyl-CoA (P-CoA), 5 mM ADP, and 166.7 μM DNP. Cytochrome c (10 μM) was added to the chamber at the end of each experimental protocol to evaluate the integrity of the outer mitochondrial membrane, which was not elevated >10% in any of the experiments. Mitochondrial respiration and ROS emission data were normalized by 1) wet tissue weight and 2) WAT cell number. Cell number was calculated using an estimate of cell volume derived from two-dimensional (2D) histology images in combination with the relative density of fat (1 g/1.0869 cm3). These calculations were carried out under the assumption of spherical adipocyte architecture.
ROS measurements.
Mitochondrial H2O2 emission was determined fluorometrically (Lumina, Thermo Scientific, Waltham, MA), as previously described in skeletal muscle (24) and adapted to WAT (51). Immediately after collection, WAT was transferred to BIOPS buffer, minced with sharp scissors, and washed for 5 min in Buffer Z (105 mM K-MES, 30 mM KCl, 1 mM EGTA, 10 mM K2HPO4, 5 mM MgCl2, 5 μM glutamate, 5 μM malate, and 0.5% BSA, pH 7.4). Tissue was then blotted and weighed, and ~5 mg (wet weight) of adipose tissue was loaded into a cuvette containing Amplex Red, 1 U/mL horseradish peroxidase, 40 U/mL superoxide dismutase, 10 μg/mL digitonin, and 6.7 μg/mL oligomycin in Buffer Z (51). Four distinct protocols were run at 37°C to measure 1) pyruvate + malate-supported (5 mM pyruvate + 2 mM malate), 2) glycerol 3-phosphate-supported (G3P; 5 mM), 3) succinate-supported (10 mM succinate), and 4) lipid-supported (5 mM l-carnitine + 50 μM palmitoyl-CoA) mitochondrial H2O2 emission rates. These experiments have previously been shown to have a high specificity for mitochondria-specific ROS production in WAT, whereby the addition of DNP (166.7 μM) dissipated H2O2 emission rates by ~70% (51). Mitochondrial H2O2 was calculated from the slope (absorbance/min) using a standard curve for each substrate with known concentrations of H2O2. To determine whether increases in mtROS can be explained by increases in mitochondrial respiration, the G3P-supported H2O2/JO2 ratio was calculated, as this was the only experiment that yielded identical substrate additions, allowing us to reasonably compare the two metrics.
Western blot analysis.
Adipose tissue was homogenized in lysis buffer, diluted to 1 µg/µL, and loaded equally for total-Akt (1:1,000; Cell Signaling, no. 4691), phosphorylated Akt-Ser-473 (1:1,000; Cell Signaling, no. 9271), phosphorylated Akt-Thr-308 (1:1,000, Cell Signaling, no. 9275), and mitochondrial oxidative phosphorylation complexes cocktail (OXPHOS; 1:500; Mitosciences, Ab110413). Proteins were separated by SDS-PAGE, blocked, and incubated with appropriate primary, and corresponding secondary antibodies. All membrane signals were detected using FluorChem HD imaging chemiluminescence (Alpha Innotech, Santa Clara), with α-tubulin or Ponceau S staining as a loading control. Protein carbonylation (Oxyblot, Millipore) was carried out according to instructions provided by the manufacturer.
Statistical analysis.
Results are presented as means ± SE and were analyzed using unpaired two-tailed Student’s t tests comparing LFD vs. HFD at each timepoint. Significance was considered when P < 0.05. All graphs and statistical analyses were performed using Prism 6.0 (GraphPad Software, La Jolla, CA).
RESULTS
Animal characterization.
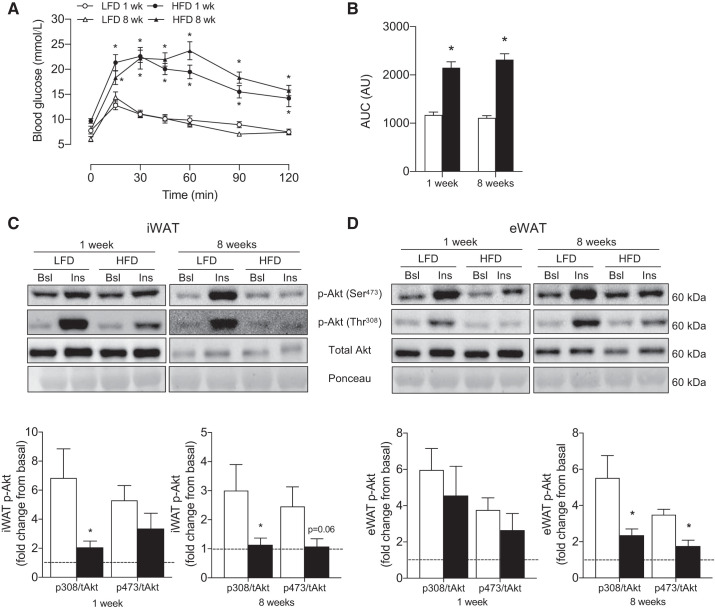
We first aimed to establish the effects of consuming an HFD on whole body metabolism before initiating an examination of depot-specific changes within iWAT and eWAT. Although body weight was not different before the dietary intervention (LFD: 33 ± 1 g, HFD: 32 ± 1 g), body weight was higher (P < 0.05) following 1 wk (LFD: 34 ± 1 g, HFD: 37 ± 1 g) and 8 wk (LFD: 42 ± 1 g, HFD: 52 ± 1 g) of HFD consumption, such that the weight gain during the 8 wk intervention was ~2.5-fold greater in HFD animals (ΔLFD: 8 ± 1 g, ΔHFD: 20 ± 1 g). In addition, while fasting blood glucose was similar between groups after 1 wk of HFD (data not shown), blood glucose was higher (P < 0.05) in animals following 8 wk of HFD (LFD: 9 ± 1 mM, HFD: 11 ± 1 mM). Compared with control, whole body glucose tolerance was impaired (P < 0.0001) after 1 wk (AUC; LFD: 703 ± 75, HFD: 1,567 ± 129) and 8 wk (AUC; LFD: 744 ± 38, HFD: 1,844 ± 101) of HFD feeding (Fig. 1, A and B).
Fig. 1.
Effects of 1 and 8 wk of HFD on whole body glucose homeostasis and white adipose tissue depot-specific insulin signaling. GTT (A), AUC (B), insulin-stimulated phosphorylated Akt fold change from basal in iWAT (C) and eWAT (D) (n = 5–7/experiment). Open bars denote LFD-fed mice, whereas solid bars denote HFD-fed mice. Data are expressed as means ± SE *P < 0.05 compared with LFD group. AUC, area under the curve; Bsl, basal; eWAT, epididymal white adipose tissue; GTT, glucose tolerance test; Ins, insulin-stimulated; iWAT, inguinal white adipose; t, total.
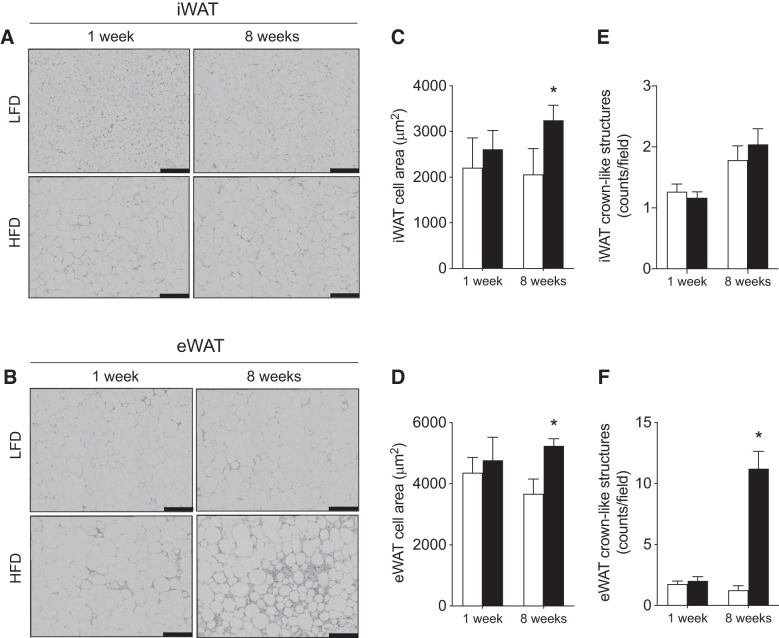
We next investigated the effects of HFD on WAT-specific insulin signaling and adipocyte hypertrophy within iWAT and eWAT. After 1 wk of HFD, insulin-stimulated Akt phosphorylation was attenuated in iWAT (P < 0.05 for Thr-308), while after 8 wk of consuming an HFD, Akt phosphorylation was attenuated in both iWAT and eWAT (Fig. 1, C and D). In support of the greater cellular stress following 8 wk, whereas 1 wk of HFD did not induce adipocyte hypertrophy, 8 wk of HFD feeding increased the average CSA in both depots (Fig. 2, A–D) and increased leukocyte infiltration (CLS) approximately three-fold within eWAT (Fig. 2, B and F).
Fig. 2.
Effects of 1 and 8 wk of HFD on adipocyte size and white adipose tissue leukocyte infiltration. Representative images of hematoxylin-and-eosin (H&E) stained iWAT (top) and eWAT (bottom) from LFD- and HFD-fed mice imaged at ×40 magnification (A and B), cross-sectional adipocyte area (C and D), crown-like structure quantification (E and F) (n = 4–6/experiment). Open bars denote LFD-fed mice, while solid bars denote HFD-fed mice. Scale bars are 100 μm. Data are expressed as means ± SE *P < 0.05 compared with LFD group. eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose.
Mitochondrial respiration.
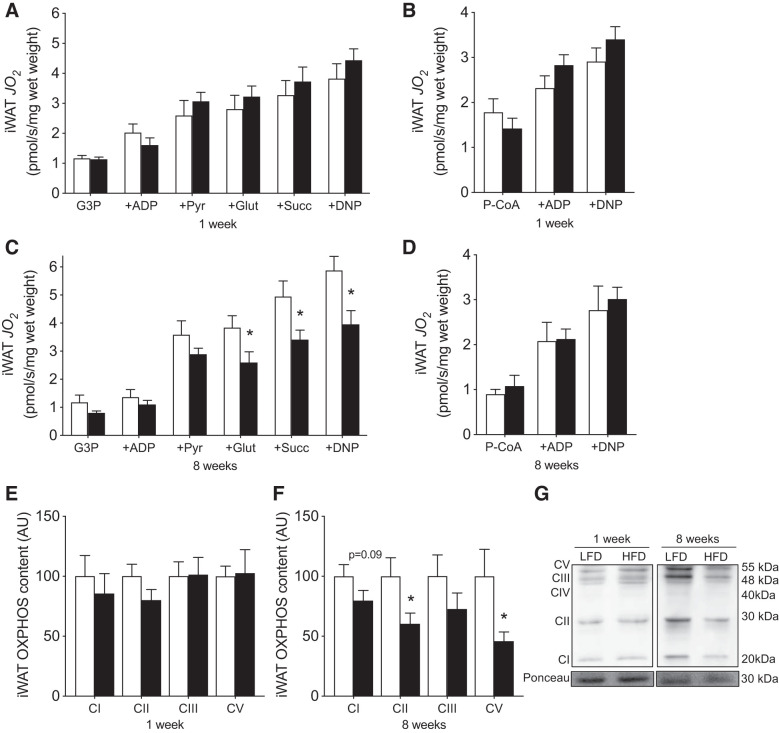
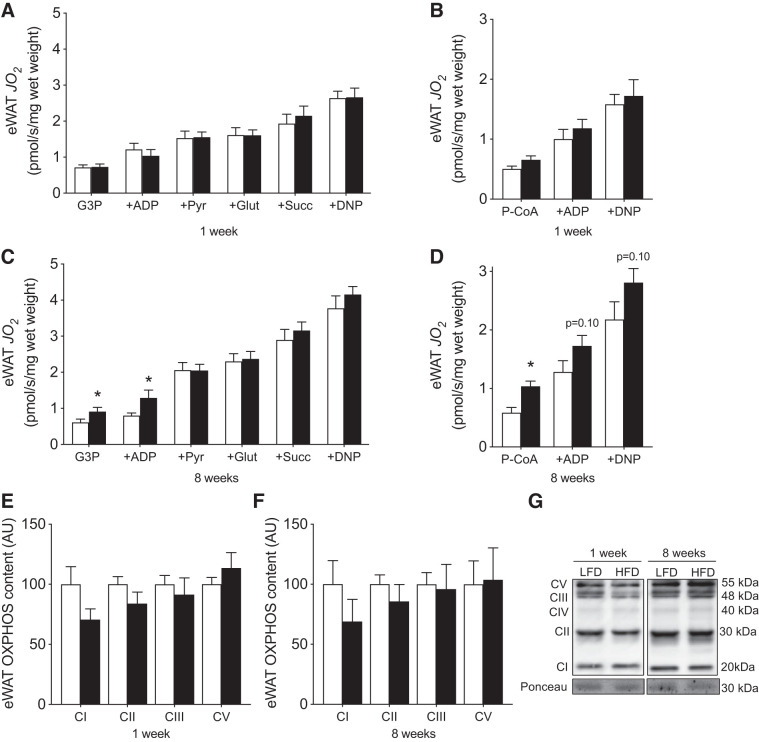
We subsequently examined the potential depot-specific effects of HFD on mitochondrial respiration. Our absolute values of maximal respiration were comparable or higher than previous reports (4, 34, 51). In permeabilized iWAT tissue, 1 wk of HFD consumption did not alter maximal ADP-stimulated respiration (supported by G3P, pyruvate, malate, and glutamate), maximal succinate-supported or lipid-supported (P-CoA, l-carnitine) respiration (Fig. 3, A and B). In contrast, tissue weight-normalized maximal ADP-stimulated and succinate-supported, but not lipid-supported, respiration was reduced following 8 wk of HFD consumption (Fig. 3, C and D). In support of these findings, OXPHOS protein content was not affected by 1 wk of HFD but was reduced following 8 wk (Fig. 3, E–G). Altogether, these data suggest mitochondrial respiratory capacity is reduced in iWAT following 8 wk of HFD consumption. However, since 8 wk of HFD-feeding also induced iWAT hypertrophy, it is not clear whether the apparent “mitochondrial dysfunction” manifests from fewer WAT cells being present per gram of tissue.
Fig. 3.
Mitochondrial respiration and mitochondrial content after 1 and 8 wk of HFD-feeding in iWAT. Maximal ADP and succinate-supported (A) and lipid-supported (B) mitochondrial respiration at 1 wk, maximal ADP and succinate-supported (C) and lipid-supported mitochondrial respiration at 8 wk (D), OXPHOS protein content at 1 (E) and 8 wk (F), and representative Western blots (G) in permeabilized iWAT (n = 5 or 6/experiment for respiration, n = 11 or 12/experiment for Western blots). CIV band was faint and unable to be reliably quantified. Open bars denote LFD-fed mice, while solid bars denote HFD-fed mice. Data are expressed as means ± SE. *P < 0.05 compared with LFD group. AU, arbitrary units/µg protein; CI–CV, complexes I–V of the electron transport chain; DNP, 2,4-dinitrophenol; Glut, glutamate; G3P, glycerol-3-phosphate; iWAT, inguinal white adipose tissue; JO2, oxygen flux; P-CoA, palmitoyl-CoA; Pyr, pyruvate; Succ, succinate; OXPHOS, oxidative phosphorylation.
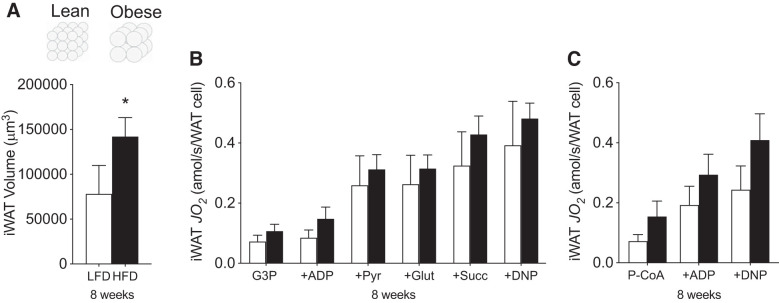
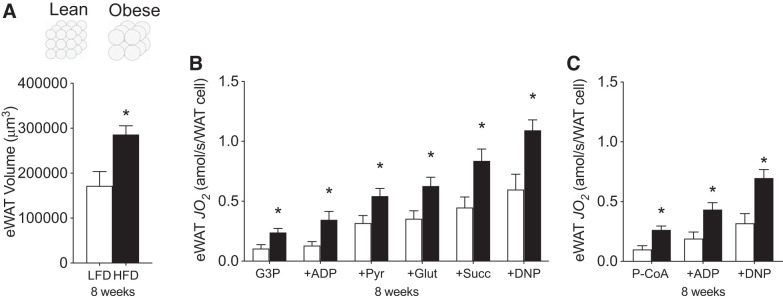
Therefore, we next attempted to normalize respiration per cell. To achieve this, we calculated the average volume of iWAT based on the 2D-CSA and the relative density of fat. As expected, iWAT volume increased nearly twofold following 8 wk of HFD (Fig. 4A), which would proportionally result in a twofold reduction in the number of WAT cells/g tissue. As a result, when respiration is normalized to the number of WAT cells, as opposed to gram weight, the apparent mitochondrial respiratory dysfunction is lost (Fig. 4, B and C). Altogether, these data indicate the absence of HFD-induced adipocyte-specific mitochondrial respiratory dysfunction within iWAT.
Fig. 4.
Mitochondrial respiration relative to changes in cell volume induced by HFD feeding at 8 wk in iWAT. Adipocyte cell volume (A), carbohydrate-supported (B), and lipid-supported (C) mitochondrial respiration in iWAT (n = 4–6/experiment). Open bars denote LFD-fed mice, while solid bars denote HFD-fed mice. Data are expressed as means ± SE *P < 0.05 compared with LFD group. DNP, 2,4-dinitrophenol; Glut, glutamate; G3P, glycerol-3-phosphate; iWAT, inguinal white adipose tissue; JO2, oxygen flux; P-CoA, palmitoyl-CoA; Pyr, pyruvate; Succ, succinate.
Thereafter, we examined the temporal responses of mitochondrial respiration within eWAT to determine whether a dysfunction in this tissue coincided with the observed leukocyte infiltration within this depot. However, unlike iWAT, mitochondrial respiration and OXPHOS content in eWAT were not reduced following either 1 or 8 wk of HFD consumption (Fig. 5, A–G). Moreover, since eWAT cellular volume increased following 8 wk, when respiration was normalized to cell number, a compensatory increase was observed in the presence of all substrates tested (Fig. 6, A–C). Combined, these data further support the interpretation that adipose tissue mitochondrial respiratory dysfunction does not coincide with obesity or intracellular signaling events associated with insulin resistance (e.g., leukocyte infiltration).
Fig. 5.
Mitochondrial respiration and mitochondrial content after 1 and 8 wk of HFD-feeding in eWAT. Maximal ADP and succinate-supported (A) and lipid-supported mitochondrial respiration at 1 wk (B), ADP and succinate-supported (C), and lipid-supported mitochondrial respiration at 8 wk (D), OXPHOS protein content at 1 (E) and 8 wk (F), and representative Western blots (G) in permeabilized eWAT (n = 5–6/experiment for respiration, n = 11–12/experiment for Western blots). CIV band was faint and unable to be reliably quantified. Open bars denote LFD-fed mice, whereas solid bars denote HFD-fed mice. Data are expressed as means ± SE. *P < 0.05, compared with LFD group. AU, arbitrary units/µg protein; CI–CV, complexes I–V of the electron transport chain; DNP, 2,4-dinitrophenol; eWAT, epididymal white adipose tissue; Glut, glutamate; G3P, glycerol-3-phosphate; JO2, oxygen flux; OXPHOS, oxidative phosphorylation; P-CoA, palmitoyl-CoA; Pyr, pyruvate; Succ, succinate.
Fig. 6.
Mitochondrial respiration relative to changes in cell volume induced by HFD-feeding at 8 wk in eWAT. Adipocyte cell volume (A), carbohydrate-supported (B), and lipid-supported (C) mitochondrial respiration in eWAT (n = 4–6/experiment). Open bars denote LFD-fed mice, whereas solid bars denote HFD-fed mice. Data are expressed as means ± SE. *P < 0.05 compared with LFD group. DNP, 2,4-dinitrophenol; eWAT, epididymal white adipose tissue; Glut, glutamate; G3P, glycerol-3-phosphate; JO2, oxygen flux; P-CoA, palmitoyl-CoA; Pyr, pyruvate; Succ, succinate.
Mitochondrial H2O2 emission.
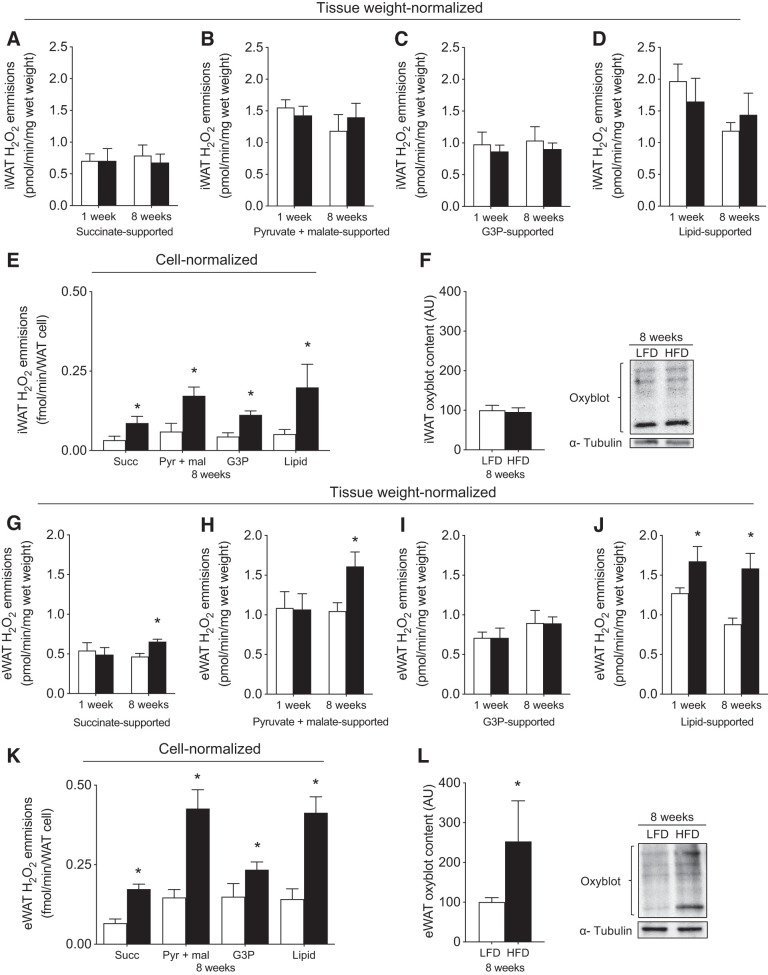
WAT hypoxia and hypertrophy have been associated with redox imbalance and several detrimental cellular events that contribute to the induction of insulin resistance (48). Therefore, we also examined the potential influence of an HFD on mitochondrial H2O2 emission. Within iWAT, tissue weight-normalized H2O2 emission was not increased following 1 or 8 wk of HFD (Fig. 7, A–D). While HFD increased cell-normalized H2O2 emission within iWAT (Fig. 7E), this did not result in global changes in protein carbonylation (Fig. 7F). In contrast, within eWAT, tissue weight-normalized H2O2 emission was increased after consuming both 1-wk (lipid-supported) and 8-wk (succinate, pyruvate, and lipid-supported) of an HFD (Fig. 7, G–J). Moreover, these responses were more pronounced within individual cells, as cell-normalized H2O2 emission following 8 wk of HFD was increased approximately twofold (Fig. 7K) in association with an approximately twofold increase in protein carbonylation (Fig. 7L). Altogether, while HFD does not attenuate electron transport chain respiratory capacity within WAT, HFD rapidly increases mitochondrial H2O2 emission rates within eWAT, which is exacerbated with a chronic HFD in association with leukocyte infiltration.
Fig. 7.
Mitochondrial reactive oxygen species (mtROS) emission and oxidative stress in iWAT and eWAT. In iWAT (top) and eWAT (bottom): tissue weight-normalized carbohydrate-supported (A–C, G–I) and lipid-supported (D, J) and cell-normalized (E, K) mtROS emission and protein carbonylation (F, L) (n = 5–8/experiment for mtROS, n = 6–10/experiment for oxyblot). Open bars denote LFD-fed mice, whereas solid bars denote HFD-fed mice. Data are expressed as means ± SE. *P < 0.05 compared with LFD group. AU, arbitrary units/µg protein; DNP, 2,4-dinitrophenol; eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose tissue; Glut, glutamate; G3P, glycerol-3-phosphate; H2O2, hydrogen peroxide; P-CoA, palmitoyl-CoA; Pyr, pyruvate; Succ, succinate.
DISCUSSION
In the current study, we aimed to determine mitochondrial responses to short-term and long-term HFD-feeding interventions. Herein, we demonstrate that 1) HFD consumption does not impair mitochondrial respiratory capacity within WAT, as cell-normalized eWAT respiration was, in fact, increased following HFD, 2) HFD rapidly increased mtROS in eWAT, while 3) chronically consuming HFD increased cell-normalized mtROS in both iWAT and eWAT. Our data also highlight 4) the necessity of cognizant normalization with respect to hypertrophied WAT when investigating mitochondrial function, as while tissue-level WAT respiration was impaired following HFD (iWAT), this appeared to manifest secondary to WAT hypertrophy and the associated dilution of mitochondria per gram of tissue.
Molecular approaches that alter mitochondrial content have suggested a cause-and-effect relationship with respect to WAT insulin sensitivity (33, 40), a notion strengthened by the finding that mitochondrial respiration and content are reduced in obesity (12, 59, 65, 67). In the present study, iWAT respiration and OXPHOS content were reduced at the tissue level, supporting the concept that mitochondrial respiratory capacity contributes to metabolic abnormalities within WAT. Interestingly, despite the reduction of OXPHOS in iWAT, P-CoA-supported respiration was unaffected. It can be reasoned that while experiments use saturating levels of lipids to assess maximal lipid-supported oxidation, this process is not saturating for the maximal capacity of the electron transport chain (ETC). Therefore, while a reduction in OXPHOS decreases maximal respiration of the ETC observed by succinate-supported respiration, lipid-supported respiration is not concomitantly reduced since the upper limit of the system is not met by lipids. Consequently, palmitate oxidation is likely not limited by the capacity of the ETC system, but rather reflects carnitine palmitoyltransferase (CPT)-I transport or β-oxidation. However, cellular WAT hypertrophy occurs during HFD consumption, which we reasoned would “dilute” the number of mitochondria per gram wet weight tissue, confounding the interpretation that HFD impaired mitochondrial respiration. To examine this possibility, we normalized iWAT respiration to cellular number to estimate cell-normalized respiration. By doing so, we aimed to address an aspect of WAT biology distinct from other metrics, such as correction to mitochondrial DNA or citrate synthase, which may diminish any compensatory changes related to mitochondrial content that may be biologically relevant. Rather than normalizing to each mitochondrion, our method highlights the contribution of respiratory function from each adipocyte. This approach mitigated the detriment of iWAT respiration from HFD-fed animals, suggesting mitochondrial respiratory capacity was not impaired following HFD.
In further support of this interpretation, both weight-normalized and cell-normalized eWAT respiration were not reduced following HFD, and the latter was, in fact, elevated. This compensatory increase in mitochondrial respiration within eWAT may support the higher metabolic demand of the adipocytes (i.e., protein transport, lipolysis, FFA reesterification, adipokine synthesis, and endocrine signaling) (36, 42), and while the mechanism for this response remains unknown, the induction of mitochondrial biogenesis within eWAT appears to recapitulate the phenotypic changes observed in muscle, where short-term HFD has also been shown to increase mitochondrial ROS as a mechanism to induce mitochondrial biogenesis (21). It should be acknowledged that eWAT OXPHOS content was not altered by HFD, which could be interpreted as the absence of mitochondrial biogenesis. However, it is our contention that similar to respiration, Western blot analysis can be influenced by hypertrophy, as OXPHOS content is normalized to protein loading, which will largely reflect plasma membrane content within WAT. In this respect, a Western blot is essentially determining a ratio of OXPHOS protein to cellular protein content, and since hypertrophy would predicate an increase in total plasma membrane protein per cell, a logical inference would be that cellular OXPHOS protein content has similarly increased within eWAT following HFD. While the notion that HFD-induced mtROS regulates mitochondrial biogenesis with eWAT remains speculative, the present data do indicate that reductions in mitochondrial content/respiratory capacity are not required for the induction of insulin resistance in WAT.
While respiratory capacity was not reduced following HFD, there was a preferential increase in lipid-supported mitochondrial respiration following HFD feeding, which may represent a compensatory response to sustain ATP production while sparing glucose from ATP synthesis. This shift in fuel selection could drive glucose to alternative pathways, such as lactate and glycerol synthesis, which serve to dispose of surplus glucose in WAT (30, 35, 55). Notably, glycerol synthesis is mandatory for fatty acid esterification to triacylglycerol; therefore, glucose-derived glycerol and mitochondria contribute substantially to this process as the necessary substrates/precursors to 3-glycerol-P synthesis are produced within this organelle (49).
In addition to respiratory capacity and glycerol synthesis, mitochondria contribute substantially to cellular redox balance (22, 32). Within WAT, mtROS emission has been linked to inflammation, cellular damage, and the development of insulin resistance (18, 48, 51). Intriguingly, hypoxia has been shown to increase redox stress within adipose tissue, and while tempting to suggest that hypertrophy contributes to this response, in the present study, mtROS increased before CSA changed. Alternatively, the treatment of primary eWAT adipocytes from HFD-fed mice with palmitate has been shown to result in adenine nucleotide translocase 2 (ANT 2)-dependent uncoupling and greater oxygen consumption, which may contribute to a hypoxic environment (39). However, mitochondrial uncoupling would be expected to decrease mtROS (7, 43, 50), likely accounting for well-established protective effect of dinitrophenol (DNP) in the context of insulin resistance and diabetes (14, 60). Therefore, whereas lipid-mediated uncoupling may contribute to hypoxia-induced signaling events, it is unlikely to directly contribute to the observed increase in mtROS in the present study. This is supported by our findings that the increase in mtROS could not be explained by an increase in OXPHOS capacity, as HFD did not change the H2O2/JO2 ratio in either iWAT (P = 0.47; LFD: 1.02 ± 0.14, HFD 1.16 ± 0.12) or eWAT (P = 0.12; LFD: 1.61 ± 0.19, HFD: 1.13 ± 0.20). Alternatively, the simple increase in lipid availability with prolonged HFD may contribute to the increase in mtROS and redox stress, as visceral WAT (e.g., eWAT) is more metabolically active, has a higher capacity for lipolysis, and appears specific for the accumulation of the aldehyde product of lipid peroxidation, trans-4-hydroxy-2-nonenal (4-HNE), at 1, 9, and 12 wk of HFD feeding (23, 41, 51). The biological availability of substrates/lipids is not recapitulated in the present in vitro approach that relies on the exogenous provision of saturating substrates, and therefore, the actual differences between iWAT and eWAT ROS may be underestimated with these approaches. It remains possible that a longer duration of HFD is required for redox stress to manifest within iWAT (19).
While the mechanisms for the increase in mtROS remain unknown, subtle changes in lipid concentrations have been shown to rapidly increase ROS through the electron transfer flavoprotein (ETF) and ETF-oxidoreductase in skeletal muscle (57), suggesting this is a likely site for ROS production. While reductions in coenzymeQ (CoQ) content have also been shown to drive mtROS production and insulin resistance in WAT of both rodents and humans, this is unlikely to account for the present findings, which appear specific for P-CoA-supported mtROS, as reductions in CoQ would be expected to affect several substrates (17). However, a reduction in CoQ, or alterations in Complex I or III, may contribute to the apparent amplification of mtROS following an 8-wk HFD. While further investigation is clearly required to solidify the site of mitochondrial oxidant production in HFD-fed models, the present data nonetheless support a role for mtROS during the development of WAT insulin resistance.
Importantly, mtROS is an integrated physiological signal in the adipocyte, regulating nutrient, hormonal, and metabolic fluxes (36). In this scenario, adipocyte differentiation is dependent on ROS (1, 62); therefore, it would be reasonable to consider mtROS emission as a signal for preadipocytes to differentiate into adipocytes, and ultimately permit storage of triacylglycerol due to higher caloric intake. Furthermore, the effects of ROS on adipose tissue mitochondrial content under HFD conditions have been tested in a specific deletion model of manganese superoxide dismutase (MnSOD), in which mtROS clearance is impaired through alteration of the antioxidant system. In this model, mitochondrial content and oxygen consumption were increased in mice exposed to HFD, which reinforces the notion of mtROS as a required signaling molecule (20). Therefore, while mtROS may promote compensatory adaptations, including hypertrophy and mitochondrial biogenesis, augmented production of mtROS has also been linked to inflammation and the induction of WAT insulin resistance.
While our model of permeabilized WAT explants provides a biologically relevant and undisturbed cellular environment for assessment of mitochondrial bioenergetics, we acknowledge the presence of leukocytes in eWAT as a limitation of the model, which may contribute, in part, to the observed elevated respiration and mtROS emission at the 8-wk time point. However, our data are consistent with a previous report that determined that maximal respiration is elevated at 6 wk in eWAT explants before the presence of leukocyte infiltration, suggesting that this potential contribution is likely negligible (13). Therefore, the present data suggest that when classically analyzed by normalizing to wet weight, HFD induces an apparent dysfunction in mitochondrial respiratory capacity within WAT. However, when we consider HFD-induced adipocyte hypertrophy in our normalization approach, the data present a dramatically different result, and instead suggest that mitochondrial respiratory function within WAT is not reduced with HFD feeding, beginning to divorce the notion that a dysfunction in mitochondrial respiratory capacity is a contributor to insulin resistance within WAT. Indeed, in the latter circumstance we observe apparent compensatory changes in mitochondrial respiration within eWAT, which could be assumed to sustain more demanding metabolic requirements imposed by hypertrophy and the expanding tissue, although it should be acknowledged that this increase in oxygen utilization could, in theory, contribute to WAT hypoxia, as previously suggested (39). Additionally, we show that while lipid-supported mtROS emission is increased after 1 wk of HFD feeding, long-term HFD consumption globally increased mtROS in the presence of various substrates, therefore implicating elevated mtROS emission in the induction of insulin-resistant WAT. Overall, the normalization approach is a key consideration in studies of HFD feeding focused on mitochondrial bioenergetics.
GRANTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada (to G.P.H.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.P.-B., H.S.B., S.P., and G.P.H. conceived and designed research; V.P.-B., H.S.B., S.P., and H.L.P. performed experiments; V.P.-B., H.S.B., S.P., H.L.P., and G.P.H. analyzed data; V.P.-B., H.S.B., S.P., H.L.P., and G.P.H. interpreted results of experiments; V.P.-B., H.S.B., and G.P.H. prepared figures; V.P.-B. and G.P.H. drafted manuscript; V.P.-B., H.S.B., H.L.P., and G.P.H. edited and revised manuscript; V.P.-B., H.S.B., S.P., H.L.P., and G.P.H. approved final version of manuscript.
REFERENCES
- 1.Alcala M, Calderon-Dominguez M, Serra D, Herrero L, Ramos MP, Viana M. Short-term vitamin E treatment impairs reactive oxygen species signaling required for adipose tissue expansion, resulting in fatty liver and insulin resistance in obese mice. PLoS One 12: e0186579, 2017. doi: 10.1371/journal.pone.0186579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, Reiser J, Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res 52: 480–488, 2011. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin C-T, Price JW III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudoin MS, Snook LA, Arkell AM, Simpson JA, Holloway GP, Wright DC. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol 305: R542–R551, 2013. doi: 10.1152/ajpregu.00200.2013. [DOI] [PubMed] [Google Scholar]
- 5.Becattini B, Zani F, Breasson L, Sardi C, D’Agostino VG, Choo MK, Provenzani A, Park JM, Solinas G. JNK1 ablation in mice confers long-term metabolic protection from diet-induced obesity at the cost of moderate skin oxidative damage. FASEB J 30: 3124–3132, 2016. doi: 10.1096/fj.201600393R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boden G, Homko C, Barrero CA, Stein TP, Chen X, Cheung P, Fecchio C, Koller S, Merali S. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci Transl Med 7: 304re7, 2015. doi: 10.1126/scitranslmed.aac4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg 1859: 940–950, 2018. doi: 10.1016/j.bbabio.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Cantó C, Garcia-Roves PM. High-resolution respirometry for mitochondrial characterization of ex vivo mouse tissues. Curr Protoc Mouse Biol 5: 135–153, 2015. doi: 10.1002/9780470942390.mo140061. [DOI] [PubMed] [Google Scholar]
- 9.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay M, Khemka VK, Chatterjee G, Ganguly A, Mukhopadhyay S, Chakrabarti S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol Cell Biochem 399: 95–103, 2015. doi: 10.1007/s11010-014-2236-7. [DOI] [PubMed] [Google Scholar]
- 11.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 7: 30, 2016. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 49: 784–791, 2006. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 13.Cummins TD, Holden CR, Sansbury BE, Gibb AA, Shah J, Zafar N, Tang Y, Hellmann J, Rai SN, Spite M, Bhatnagar A, Hill BG. Metabolic remodeling of white adipose tissue in obesity. Am J Physiol Endocrinol Metab 307: E262–E277, 2014. doi: 10.1152/ajpendo.00271.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutting WC, Mehrtens HG, Tainter ML. Actions and uses of dinitrophenol: Promising metabolic applications. J Am Med Assoc 101: 193–195, 1933. doi: 10.1001/jama.1933.02740280013006. [DOI] [Google Scholar]
- 15.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273: 11619–11624, 1998. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 16.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal 7: 1040–1052, 2005. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 17.Fazakerley DJ, Chaudhuri R, Yang P, Maghzal GJ, Thomas KC, Krycer JR, Humphrey SJ, Parker BL, Fisher-Wellman KH, Meoli CC, Hoffman NJ, Diskin C, Burchfield JG, Cowley MJ, Kaplan W, Modrusan Z, Kolumam G, Yang JYH, Chen DL, Samocha-Bonet D, Greenfield JR, Hoehn KL, Stocker R, James DE. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. eLife 7: e32111, 2018. doi: 10.7554/eLife.32111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazakerley DJ, Minard AY, Krycer JR, Thomas KC, Stöckli J, Harney DJ, Burchfield JG, Maghzal GJ, Caldwell ST, Hartley RC, Stocker R, Murphy MP, James DE. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J Biol Chem 293: 7315–7328, 2018. doi: 10.1074/jbc.RA117.001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48, 2007. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 20.Han YH, Buffolo M, Pires KM, Pei S, Scherer PE, Boudina S. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Diabetes 65: 2639–2651, 2016. doi: 10.2337/db16-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105: 7815–7820, 2008. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal 16: 1323–1367, 2012. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauck AK, Zhou T, Hahn W, Petegrosso R, Kuang R, Chen Y, Bernlohr DA. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. J Biol Chem 293: 13464–13476, 2018. doi: 10.1074/jbc.RA118.003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst EAF, Paglialunga S, Gerling C, Whitfield J, Mukai K, Chabowski A, Heigenhauser GJF, Spriet LL, Holloway GP. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol 592: 1341–1352, 2014. doi: 10.1113/jphysiol.2013.267336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, Vallabhajosyula P, Kambayashi T, Won KJ, Lazar MA. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci USA 115: E5096–E5105, 2018. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 106: 17787–17792, 2009. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol 292: H101–H108, 2007. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 28.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 29.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol Cell Biol 26: 3071–3084, 2006. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansson PA, Larsson A, Smith U, Lönnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest 89: 1610–1617, 1992. doi: 10.1172/JCI115756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-κB and c-Jun NH2-terminal kinase pathways. Diabetes 58: 104–115, 2009. doi: 10.2337/db07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo SH, Son MK, Koh HJ, Lee SM, Song IH, Kim YO, Lee YS, Jeong KS, Kim WB, Park JW, Song BJ, Huh TL. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem 276: 16168–16176, 2001. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- 33.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc Natl Acad Sci USA 109: 9635–9640, 2012. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraunsøe R, Boushel R, Hansen CN, Schjerling P, Qvortrup K, Støckel M, Mikines KJ, Dela F. Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. J Physiol 588: 2023–2032, 2010. doi: 10.1113/jphysiol.2009.184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krycer JR, Quek LE, Francis D, Fazakerley DJ, Elkington SD, Diaz-Vegas A, Cooke KC, Weiss FC, Duan X, Kurdyukov S, Zhou PX, Tambar UK, Hirayama A, Ikeda S, Kamei Y, Soga T, Cooney GJ, James DE. Lactate production is a prioritized feature of adipocyte metabolism. J Biol Chem 295: 83–98, 2020. doi: 10.1074/jbc.RA119.011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab 23: 435–443, 2012. doi: 10.1016/j.tem.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langin D. In and out: adipose tissue lipid turnover in obesity and dyslipidemia. Cell Metab 14: 569–570, 2011. doi: 10.1016/j.cmet.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Leduc-Gaudet JP, Reynaud O, Chabot F, Mercier J, Andrich DE, St-Pierre DH, Gouspillou G. The impact of a short-term high-fat diet on mitochondrial respiration, reactive oxygen species production, and dynamics in oxidative and glycolytic skeletal muscles of young rats. Physiol Rep 6: e13548, 2018. doi: 10.14814/phy2.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, Quehenberger O, Johnson RS, Olefsky JM. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157: 1339–1352, 2014. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R, Parker MG. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci USA 101: 8437–8442, 2004. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long EK, Olson DM, Bernlohr DA. High-fat diet induces changes in adipose tissue trans-4-oxo-2-nonenal and trans-4-hydroxy-2-nonenal levels in a depot-specific manner. Free Radic Biol Med 63: 390–398, 2013. doi: 10.1016/j.freeradbiomed.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu RH, Ji H, Chang ZG, Su SS, Yang GS. Mitochondrial development and the influence of its dysfunction during rat adipocyte differentiation. Mol Biol Rep 37: 2173–2182, 2010. doi: 10.1007/s11033-009-9695-z. [DOI] [PubMed] [Google Scholar]
- 43.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med 51: 1106–1115, 2011. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin T, Craig C, Liu LF, Perelman D, Allister C, Spielman D, Cushman SW. Adipose cell size and regional fat deposition as predictors of metabolic response to overfeeding in insulin-resistant and insulin-sensitive humans. Diabetes 65: 1245–1254, 2016. doi: 10.2337/db15-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miotto PM, LeBlanc PJ, Holloway GP. High-fat diet causes mitochondrial dysfunction as a result of impaired ADP sensitivity. Diabetes 67: 2199–2205, 2018. doi: 10.2337/db18-0417. [DOI] [PubMed] [Google Scholar]
- 46.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 125: 259–266, 2016. doi: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res 49: 1562–1568, 2008. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Netzer N, Gatterer H, Faulhaber M, Burtscher M, Pramsohler S, Pesta D. Hypoxia, oxidative stress and fat. Biomolecules 5: 1143–1150, 2015. doi: 10.3390/biom5021143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nye C, Kim J, Kalhan SC, Hanson RW. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol Metab 19: 356–361, 2008. doi: 10.1016/j.tem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Okuda M, Lee HC, Kumar C, Chance B. Comparison of the effect of a mitochondrial uncoupler, 2,4-dinitrophenol and adrenaline on oxygen radical production in the isolated perfused rat liver. Acta Physiol Scand 145: 159–168, 1992. doi: 10.1111/j.1748-1716.1992.tb09351.x. [DOI] [PubMed] [Google Scholar]
- 51.Paglialunga S, Ludzki A, Root-McCaig J, Holloway GP. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia 58: 1071–1080, 2015. doi: 10.1007/s00125-015-3531-x. [DOI] [PubMed] [Google Scholar]
- 52.Perriotte-Olson C, Adi N, Manickam DS, Westwood RA, Desouza CV, Natarajan G, Crook A, Kabanov AV, Saraswathi V. Nanoformulated copper/zinc superoxide dismutase reduces adipose inflammation in obesity. Obesity (Silver Spring) 24: 148–156, 2016. doi: 10.1002/oby.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrick HL, Pignanelli C, Barbeau PA, Churchward-Venne TA, Dennis KMJH, van Loon LJC, Burr JF, Goossens GH, Holloway GP. Blood flow restricted resistance exercise and reductions in oxygen tension attenuate mitochondrial H2O2 emission rates in human skeletal muscle. J Physiol 597: 3985–3997, 2019. doi: 10.1113/JP277765. [DOI] [PubMed] [Google Scholar]
- 54.Priyanka A, Nisha VM, Anusree SS, Raghu KG. Bilobalide attenuates hypoxia induced oxidative stress, inflammation, and mitochondrial dysfunctions in 3T3-L1 adipocytes via its antioxidant potential. Free Radic Res 48: 1206–1217, 2014. doi: 10.3109/10715762.2014.945442. [DOI] [PubMed] [Google Scholar]
- 55.Rotondo F, Ho-Palma AC, Remesar X, Fernández-López JA, Romero MDM, Alemany M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci Rep 7: 8983, 2017. doi: 10.1038/s41598-017-09450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 19: 1109–1117, 2011. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seifert EL, Estey C, Xuan JY, Harper ME. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem 285: 5748–5758, 2010. doi: 10.1074/jbc.M109.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snook LA, MacPherson REK, Monaco CMF, Frendo-Cumbo S, Castellani L, Peppler WT, Anderson ZG, Buzelle SL, LeBlanc PJ, Holloway GP, Wright DC. Prior exercise training blunts short-term high-fat diet-induced weight gain. Am J Physiol Regul Integr Comp Physiol 311: R315–R324, 2016. doi: 10.1152/ajpregu.00072.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutherland LN, Capozzi LC, Turchinsky NJ, Bell RC, Wright DC. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab 295: E1076–E1083, 2008. doi: 10.1152/ajpendo.90408.2008. [DOI] [PubMed] [Google Scholar]
- 60.Tainter ML, Stockton AB, Cutting WC. Use of dinitrophenol in obesity and related conditions: A progress report. J Am Med Assoc 101: 1472–1475, 1933. doi: 10.1001/jama.1933.02740440032009. [DOI] [Google Scholar]
- 61.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Zhang Y, Lu W, Liu K. Mitochondrial reactive oxygen species regulate adipocyte differentiation of mesenchymal stem cells in hematopoietic stress induced by arabinosylcytosine. PLoS One 10: e0120629, 2015. doi: 10.1371/journal.pone.0120629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitfield J, Paglialunga S, Smith BK, Miotto PM, Simnett G, Robson HL, Jain SS, Herbst EAF, Desjardins EM, Dyck DJ, Spriet LL, Steinberg GR, Holloway GP. Ablating the protein TBC1D1 impairs contraction-induced sarcolemmal glucose transporter 4 redistribution but not insulin-mediated responses in rats. J Biol Chem 292: 16653–16664, 2017. doi: 10.1074/jbc.M117.806786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114: 1281–1289, 2004. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc 68: 370–377, 2009. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 67.Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab 99: E209–E216, 2014. doi: 10.1210/jc.2013-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Ebenezer PJ, Dasuri K, Fernandez-Kim SO, Francis J, Mariappan N, Gao Z, Ye J, Bruce-Keller AJ, Keller JN. Aging is associated with hypoxia and oxidative stress in adipose tissue: implications for adipose function. Am J Physiol Endocrinol Metab 301: E599–E607, 2011. doi: 10.1152/ajpendo.00059.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]