Abstract
Ambient air, occupational settings, and the use and distribution of consumer products all serve as conduits for toxicant exposure through inhalation. While the pulmonary system remains a primary target following inhalation exposure, cardiovascular implications are exceptionally culpable for increased morbidity and mortality. The epidemiological evidence for cardiovascular dysfunction resulting from acute or chronic inhalation exposure to particulate matter has been well documented, but the mechanisms driving the resulting disturbances remain elusive. In the current review, we aim to summarize the cellular and molecular mechanisms that are directly linked to cardiovascular health following exposure to a variety of inhaled toxicants. The purpose of this review is to provide a comprehensive overview of the biochemical changes in the cardiovascular system following particle inhalation exposure and to highlight potential biomarkers that exist across multiple exposure paradigms. We attempt to integrate these molecular signatures in an effort to provide direction for future investigations. This review also characterizes how molecular responses are modified in at-risk populations, specifically the impact of environmental exposure during critical windows of development. Maternal exposure to particulate matter during gestation can lead to fetal epigenetic reprogramming, resulting in long-term deficits to the cardiovascular system. In both direct and indirect (gestational) exposures, connecting the biochemical mechanisms with functional deficits outlines pathways that can be targeted for future therapeutic intervention. Ultimately, future investigations integrating “omics”-based approaches will better elucidate the mechanisms that are altered by xenobiotic inhalation exposure, identify biomarkers, and guide in clinical decision making.
Keywords: engineered nanomaterial, genomics, gestation, heart, mitochondria, particulate matter, PM2.5, UFP
INTRODUCTION
Irrespective of diet, physical fitness, or other controllable risk factors, the quality and contents of the air we breathe are often unavoidable. In industrial cities and large metropolitan areas, the most pervasive mode of exposure to aerosolized toxicants is through the ambient air. Long-term exposure to ambient air pollution contributes to 7.6% of global mortality, with 91% of the world’s population residing in places where air quality exceeds the guideline limits put forth by the World Health Organization (WHO) (30, 166, 167). The number of annual premature deaths as a result of outdoor air pollution are staggering, with projections indicating six to nine million deaths in 2060 (17, 116). Subsequently, the predicted economic costs are increasing substantially as a repercussion of the illnesses resulting from exposure (116). The importance of lowering ambient fine particulate matter exposure is highlighted specifically by the notion that if all countries met the WHO Air Quality Guidelines, life expectancy could increase by more than 7 mo (2). This becomes a daunting task with increasing urbanization (166) and implementation of nanoparticles for their advantageous physical, chemical, and biological properties in industrial, commercial, and medical sectors (131).
There are numerous factors that contribute to the diminishing air quality, including industrial sources, energy power plants, traffic, agriculture, and fires (36, 76, 137). Particulate matter (PM) is the sum of all organic and inorganic compounds dispersed in the solid and liquid phases that can be carried by air (21, 77). PM components are typically categorized by particle size, including coarse (PM10; ≤10 µm in diameter), fine (PM2.5; ≤2.5 µm in diameter), and ultrafine particles (UFP; PM0.1; ≤0.1 µm in diameter). However, the surface area, chemical composition, number, solubility, and reactivity are some of the other characteristics that must be considered (15, 21, 102, 143). Decreasing the size of particulates from PM10 to PM2.5 increases the capacity for the material to travel further into the bronchoalveolar airways and allows for a broader interaction area (51, 170). Similarly to PM2.5, UFP also have the capacity to penetrate deeply into the alveoli of the tracheobronchial airways (112, 145), but UFP exhibit an increased capacity for interstitialization and can transport systemically throughout the body due to their high surface-to-volume ratios (21, 39, 102, 112, 143). Because the bulk of cardiovascular effects originates from fine PM and nanoparticles (51, 139), this review will focus on PM2.5, UFP, and engineered nanomaterials (ENM).
ENM (≥1 dimension ≤100 nm in diameter), which are similar in size to UFP, are anthropogenic materials that pose additional risk to public cardiovascular health (78, 120, 131, 141). Nanomaterials represent an ever-increasing vehicle for inhalation toxicology (81). Whether during the manufacturing of nanomaterials (81) or in consumer use (108), production and rate of application continue to rise (7, 64). Although inhalation studies examining ENMs and UFP typically differ in the number and standardization of compounds found within the mixtures, both share commonality in the nanometer scale that permits similar dispersion within the lungs during inhalation (143). Nanomaterials, similarly to UFP, exist exclusively at a biological scale that alters the dynamics of interactions with organic matter (107, 152), potentially altering toxicities compared with their micrometer-sized equivalents (21, 51, 139, 143). By integrating data from PM2.5, UFP, and ENM inhalation exposures, the capacity to delineate unique or common mechanisms altered during multicomponent exposures can be achieved.
According to the WHO, more than four million people die every year from outdoor air pollution, with cardiovascular disease accounting for ∼40% of those deaths (2, 87, 167). Although the pulmonary system is considered the primary site of interaction with these particles, secondary target organs, such as the heart and circulatory system, may be subject to the most detrimental long-term effects. The overt epidemiological risk of cardiovascular injury following chronic and acute inhalation exposure to PM2.5, UFP, and ENM has been well documented and includes increased propensity for ischemic heart disease, heart failure, out-of-hospital cardiac arrests, arrhythmias, atherosclerosis, and other cardiovascular complications (15, 27, 56, 123–125, 129, 147, 151, 182). The effects of PM inhalation exposure on the heart function of individuals who are susceptible to cardiovascular disease (CVD) have also gained recent interest. One of the main points of concern emanates from the increasing rate of patients with diet-induced obesity and type 2 diabetes (23). These populations with metabolic disorders can have further modifications to insulin-signaling pathways that arise from inhalation exposure, ultimately increasing susceptibility and aggravating the underlying pathophysiology (5, 14, 25, 70, 117, 119, 125).
Among those whom are most vulnerable to the repercussions of inhaled PM are those exposed at a critical point in development, such as during gestation. Although the main focus has been on the effects of toxicant inhalation exposure directly to an individual, recent studies have highlighted the importance of understanding how exposure during gestation impacts future progeny (12, 13, 50, 83, 96, 100, 103). As per the developmental origins of health and disease (DOHaD) hypothesis, a baleful gestational environment results in epigenetic alterations and has a notable influence on the health of the offspring (11, 47). Currently, there are limited studies defining the longitudinal cardiovascular effects of offspring exposed to PM during gestation and whether exposure increases susceptibility to metabolic diseases at a later point in life due to alterations in overlapping pathways (16, 68). Delineating the long-term effects of PM exposure on cardiovascular function, particularly in progeny, along with the molecular mechanisms governing predisposition to CVD, is critical for limiting premature mortality rates.
There are three prevailing theories on the mechanism by which inhaled pollutants result in deleterious effects on the cardiovascular system (lung inflammation, particle translocation, and autonomic regulation), which have been extensively reviewed elsewhere (102, 110, 112). Regardless of the initial response within the lungs, understanding the cardiovascular molecular pathways that exposure mediates is essential for identifying appropriate prophylactic and/or therapeutic strategies. In this review, we examine the molecular pathways that are implicated in cardiovascular adaptions to PM2.5, UFP, ENM, and coexposures and attempt to provide a cohesive framework of how each of these molecular pathways has been evaluated across multiple models. Summarized findings of these molecular mechanisms for various exposures and exposure models are presented in Table 1. Furthermore, there are limited studies examining the cardiovascular consequences of gestational PM exposure on progeny. Therefore, this review will also focus on the effects of PM exposure during gestation on the cardiovascular system in progeny and highlight the molecular pathways that PM exposure alters during this critical window of fetal development. The following sections are divided into categories, with each section interweaving similar molecular pathways/responses to PM inhalation exposure.
Table 1.
Summarized recent findings
| Study (Ref. No.) | Findings | Model | Exposure |
|---|---|---|---|
| PM2.5 | |||
| 6 | Fine PM2.5 and coarse PM decreased Alu and TLR4 methylation, respectively | 15 Local participants | PM2.5 collected in downtown Toronto, ON, Canada. Administered 250 and 200 μg/m3 per session |
| 16 | No changes seen in inflammatory markers related to PM2.5 exposure (monocytes, TNFα, IL-10, IL-6, IL-8, IL-1β) | 25 Healthy male and female participants 18–50 yr old | PM2.5 data obtained from Dearborn, Tecumesh, and Dexter, MI. Three subacute integrated 5-day-long exposure periods in Dearborn, MI (mean concentration of 11.5 ± 4.8 µg/m−3) |
| 18 | PM2.5 exposure was associated with mtDNA D-loop methylation in peripheral blood leukocytes | 48 Male participants | PM2.5 collected January 2007 to June 2012 in Boilermaker Union Local 29, Quincy, MA |
| 29 | Elemental carbon (48 genes), PM2.5 (49 genes), and organic carbon (260 genes) differentially affected the transcriptome | 63 Pickup and delivery drivers and dock workers | PM2.5 collected February 2009 and October 2010 from 10 trucking terminals in the northeastern US (CT, MA, MD, NJ, NY, and PA) |
| 32 | PM2.5 increased STAT3, which promoted microRNA-21 expression, leading to decreased TIMP3 and increased MMP9 | Male Sprague-Dawley rats, 6 wk old | PM2.5 collected in Beijing, China; trachea drip, 4 mg/kg body wt every 3 days for 36 days |
| 37 | PM2.5 reduced intracellular Ca2+ via decreased RYR2 and increased SERCA2a | Male Balb/c mice, 6–8 wk old | PM2.5 collected, Hebei, China; intratracheal instillation of 0.5 mg on days 0 and 2 |
| 38 | PM2.5 exposure with vitamin E and ω-3 polyunsaturated fatty acid administration decreased inflammation (TNFα, IL-1β, IL-6) | Sprague-Dawley rats, 6–8 wk old | PM2.5 collected May–September 2015 in Shanghai, China; intratracheal instillation every other day for 6 days at 10 mg/kg body wt or 1.5 mL/kg body wt |
| 42 | Increased LINE-1 methylation with increasing dose of PM2.5 | 66 Male participants | PM2.5 collected (pre- and postwelding shift) January 2010–June 2012 in Boilermaker Union Local 29, Quincy, MA |
| 49 | PM2.5 increasing concentration was associated with decreasing levels of ET-1 (negatively) and PF-4 (positively). | 15 Young, healthy, nonsmoking subjects | PM2.5 ambient air concentrations in Utah Valley from 2 monitoring sites (Lindon, North Provo) measured daily (January–March 2009); blood collections done during high (PM2.5 > 40 µg/m3), moderate (PM2.5 ∼20–40 µg/m3), and low (PM2.5 < 10 µg/m3) concentrations |
| 54 | Exercise during DE (PM2.5) exposure decreased ET-1 and increased NOx but was not modified by exercise intensity | 18 Male participants | PM2.5 collected a 5.5-kW diesel engine under a constant 2.5 kW load; exposure of 300 μg/m3 over 6/30-min low- and high-intensity cycling periods |
| 57 | PM2.5 increased expression of ICAM-1, VCAM, and CRP in sedentary mice and increased VCAM in exercised mice | Male ob/ob mice, 12 wk old | PM2.5 collected in Columbus, OH; average 32 μg/m3 for 6 h/day, 5 days/wk for 9 mo through a whole body inhalation system |
| 58 | PM2.5 decreased VEGF-induced Akt/eNOS phosphorylation and circulating levels of Flk-1+/Sca-1+ cells (EPCs) | C57BL/6J male mice, 8–12 wk old | PM2.5 collected June 2009 and December 2010 in Louisville, KY; whole body exposure, 6 h/day for 4–30 days at 30–100 μg/m3 |
| 59 | PM2.5 reduced insulin-stimulated Akt phosphorylation and circulating levels of Flk-1+/Sca-1+ cells (EPCs), elevated oxidative stress (SOD2, GST-P), and caused inflammasome activation (IL-1β, pro-IL-18 cleavage, activation of Casp-1) | C57BL/6J male mice, 8 wk old (control/high-fat diet), C57BL/6J male mice, 12 wk old (control diet, pre-treated with metformin/rosiglitazone | PM2.5 collected June 2009 and December 2010 in Louisville, KY; whole body exposure, 6 h/day for 9 or 30 days at 30–120 μg/m3 |
| 60 | PM2.5 decreased insulin-stimulated Akt/eNOS phosphorylation and IκBα, antioxidant treatment attenuated vascular insulin resistance and inflammation | C57BL/6J male mice, 12 wk old (control/high-fat diet), pretreated with Tempol | PM2.5 collected June 2009 and December 2010 in Louisville, KY; whole body exposure, 6 h/day for 9 or 30 days at 30–120 μg/m3 |
| 75 | PM2.5 increased phospho-EGFR (Tyr1068), phospho-Akt (Thr308), NLRP3, NF-κB-p52/p100, and NF-κB-p65 in heart, as well as CXCL1, IL-6, IL-18, and NLRP12 mRNA | BALB/c mice, 6–8 wk old | PM2.5 collected November 2014 in a major city in central China; intratracheal instillation of 4.0 mg/kg body wt for 5 consecutive days |
| 89 | PM2.5 decreased SOS1, CREB, GSK3b, and GRB2 expression; fucoidan treatment rescued all but CREB levels. | C57BL/6J mice, 8 wk old | PM2.5 collected November–December 2016 in Taipei, Taiwan; 100 μg/m3, 28 days, 6 h/day |
| 91 | PM2.5 caused mitochondrial size/cristae deformation (increased FIS1, MFN1, MFN2, DRP1, and OPA1), inflammation (increased TNF-α, IL-6, and IL-1β), decreased SOD, increased MDA, and iNOS | Sprague-Dawley rats | PM2.5 collected January 2013 in Taiyuan, China; intratracheal instillation of 0.375, 1.5, 6.0, and 24.0 mg/kg body wt performed 5 times |
| 94 | PM2.5 increased caspase-3, Bax, and Bcl-2 in heart and NF-κB in cardiac myocytes | Male C57/BL6 mice, 8 wk old | PM2.5 was collected in, Nanjing, China; 10 µg PM2.5 (10 µl) twice/wk per intranasal instillation. |
| 95 | Acute PM2.5 exposure decreased 5-mC methylation and DNMT1 mRNA expression in heart and blood; chronic PM2.5 exposure decreased these factors only in blood | C57BL/6J male mice, 7 wk old | PM2.5 (collection location not stated), whole body acute exposure (24 h; PM2.5 dose of 271.8 ± 86.8 µg/m3) and chronic exposure (140 days; PM2.5 dose of 271.8 ± 86.8 µg/m3) |
| 99 | PM2.5 increased inflammation; high-intensity exercise stimulated reduced inflammation (eHSP70) | Male B6.129SF2/J mice, 30 days old | PM2.5 collected in São Paulo, Brazil, 5 µg nasotropic instillation daily before exercise (12 wk) |
| 109 | DEP (PM2.5) increased PAI-1, fibrinogen, lipid peroxidation, and IL-6; Nootkatone pretreatment alleviated all and reduced thrombosis with increased Nrf2 and HO-1 activation. | BALB/C mice, 8 wk old | Diesel exhaust particles (DEP) with a geometric mean aerodynamic diameter of 215 nm from the National Institute of Standards and Technology; intratracheal instillation of 30 μg/mouse |
| 118 | PM2.5 exposure in atherosclerosis model increased MDA and NOX4 subunits (p22phox, p47phox) in cardiac tissue | Male ApoE−/− Tg mice, 8 wk old | PM2.5 collected June–October 2013 in Shanghai, China; intratracheal installation at 0–30 mg/kg body wt |
| 122 | PM2.5 exposure was associated with elevated levels of CD14+, CD16+, CD4+, CD8+, endothelial microparticles (annexin V+/CD41−/CD31+), antiangiogenic (TNFα, IP-10) and proinflammatory cytokines (MCP-1, MIP-1α/β, IL-6, IL-1β), and sICAM-1 and sVCAM-1, as well as decreased proangiogenic growth factors (EGF, sCD40L, PDGF, RANTES, GROα, and VEGF) | 72 Young, healthy, nonsmoking subjects (24 individuals per 3 winter/spring time periods (2013, 2014, and 2015)) | PM2.5 ambient air concentrations in Utah Valley from 3 monitoring sites (Lindon, North Provo, and Spanish Fork) measured daily; at Lindon and North Provo sites, hourly PM2.5 concentrations were used to estimate the average concentration for the ∼24-h period before each blood draw |
| 126 | PM2.5 increased fibrosis (Col1a1, Col3a1), oxidative stress (NOX4), and transcription factor binding (TGFβ1, SMAD3) | Female C57BL/6, 10 mo and 4 wk old | PM2.5 collected in 2012 and 2013 from Taiyuan, Northern China; 3 mg/kg body wt PM2.5 oropharyngeal aspiration every other day for 4 wk |
| 128 | CAPs exposure increased 7-KCh in plasma and aortic plaques and increased CD36 expression in plaque macrophages and CD36 uptake of oxidized lipids | ApoE−/− Tg and LDLR−/− Tg mice, 8 wk old | Concentrated ambient PM2.5 collected in Columbus, OH; mice exposed for 6 mo to 9.1 ± 7.3 μg/m3 |
| 130 | 12.4% Decrease in RHI for every 10 µg/m3 increase in PM2.5; IsoP, angiopoietin 1, VEGF, PlGF, MMP-9, and ICAM-1 positively associated with 10 µg/m3 increases in PM2.5, decreased VCAM-1 | 100 Male and female participants (44% had type 2 diabetes; 52% had diagnosis of hypertension) | PM2.5 levels obtained from 5 monitoring stations in Jefferson County, KY (June 2011–May 2013) with average concentration of 11.45µg/m3 |
| 135 | PM2.5 increased Hsp-70, HO-1, and MPO in heart as well as laminin, collagen, and calcium signaling proteins (181 upregulated, 178 downregulated genes) | Male BALB/c mice, 7–8 wk old | PM2.5 with high PAH, nitrite, and organic carbon levels collected during winter 2008 in an urban site (Milano, Italy); intratracheal instillation on days 0, 3, and 6, at 0.3 mg/mouse |
| 152 | TRPA1 activation increases negative cardiovascular responses to CAPs exposure through myocardial dyssynchrony | Trpa1tm1Kykw/J (Trpa1−/−) Tg female mice | PM2.5 collected November–December of 2014 in Triangle Park, NC; 3 h/day, 2 days/wk, 8 total whole body exposures |
| 156 | PM2.5 exposure increased oxidative stress (PRNP) and endoplasmic reticulum stress (GRP78). Nanoparticles found in myocardial ER and in abnormal mitochondria | 30 Children and young adults (∼20 yr old) | PM2.5 data obtained in Mexico City Metropolitan Area 1997–2012 |
| 160 | PM2.5 increased fibrosis and collagen genes, and oxidative stress (8-OHdg and 4-HNE) reduced antioxidant genes (PRDX5) | Male AMPK α2−/− Tg mice, 6–8 wk old | PM2.5 collected April–October 2016 in Beijing, China; intratracheal instillation for 6 mo at 10 mg/kg (64 µg/m3). |
| 162 | PM2.5 exposure in hyperlipidemic rats increased CRP, JNK, and P53 phosphorylation, increased Bax and caspase-3 in the heart, and decreased SOD | Male Wistar rats, 8 wk old, on high-fat diet | PM2.5 collected October–December 2012 in Beijing, China; endotracheal instillation of suspension at 0, 4, and 40 mg/kg |
| 165 | Higher PM2.5 resulted in myosin heavy chain isoform switch (increased β-MHC) and decreased SERCA2a | C57BL/6 male mice, 8 wk old | Concentrated PM2.5 in Columbus, OH; mean daily concentration of PM2.5 in the exposure chamber was 85.3 μg/m3; mice were exposed for 6 h/day, 5 days/wk, for 9 mo. |
| 168 | PM2.5 increased MDA, iNOS activity, TNFα, IL-1β, along with NOX4 and NOX subunits as p67phox, p47phox, and p22phox in the heart and reduced SOD activity | C57BL/6 mice, 6–8 wk old | PM2.5 collected September 2013 in Zhengzhou, Taiwan; instilled with 1.5, 3.0, 6.0 mg/kg body wt 5 days/wk for 2 wk |
| 176 | PM2.5 decreased GSH-Px, increased MDA and sICAM-1; SeY treatment reduced inflammation markers (TNFα, IL-1β, sICM-1) | Male Sprague-Dawley rats, 7 wk old | PM2.5 collected September–November 2015 in Shanghai, China; intratracheal instillation at 40 mg/kg |
| 180 | PM2.5 exposure in diabetic model induced NF-κB, COX-2, MAPK in heart, IκB inhibitor restored function through NF-κB reduction | KKay Tg mice, 7 wk old | PM2.5 collected Polaris in Columbus, OH; intratracheal instillation for 6 h/day, 5 day/wk, 8 wk at dose of 1.6 mg/kg |
| 181 | PM2.5 increased DNA damage (increased OGG1 and GADD153, decreased MTH1and XRCC1) in the heart and decreased SOD | Male Wistar rats | PM2.5 containing PAHs collected Taiyuan, China; intratracheal instillation of 1.5 mg/kg body wt PM2.5, 1.6 × 10− 4 mg/kg body wt 1-NP, 1.2 × 10− 4 mg/kg body wt 9-NA |
| 183 | Higher PM2.5 correlated with increased blood TLR2 methylation, flavonoid intake decreased TLR2 methylation | Normative Aging Study, 573 participants | PM2.5 data collected from Harvard School of Public Health |
| Gestational | |||
| 26 | PM2.5 exposure increased GATA4, NKX2–5, and inflammatory markers (TNFα and IL-1β); homocysteine exacerbates these effects | Female Sprague-Dawley rats, 10 wk old, neonatal progeny | PM2.5 collected Fujian, China; exposed during gestation and lactation (∼42 days) to 36.5 μg/m3 through a whole body inhalation system |
| 55 | DE PM2.5 caused promoter methylation of microRNA-133a-2 in progeny, and cardiovascular stress decreased microRNA 133a-2 expression | Female C57BL/6 12–14 wk old, 12-wk-old progeny | PM2.5 collected a single cylinder Yanmar diesel engine, operating at 82% load ∼300 μg/m3 for 6 h/day for a total of 5 days during pregnancy |
| 148 | PM2.5 preconception exposure increased inflammatory markers (IL‐6, IL‐15, NF-κB, CRP, CD26E, CD26P, VCAM-1, and MCP-1), fibrosis (Col3a1), Ca2+ regulation (SERCA2a, p‐PLN), and altered epigenetics (DNMT1↓, SIRT1↑, SIRT2↑) | Paternal and maternal FVB mice exposure; male 12 wk-old progeny | PM2.5 collected in Columbus, OH; average 38.58 μg/m3 for 6 h/day, 5 days/wk, for 3 mo through a whole body inhalation system preconception |
| 149 | PM2.5 induced changes in calcium dynamics by decreasing NCX and CaV1.2 | Female FVB mice, 12 wk old, 14-day-old progeny | PM2.5 collected in Columbus, OH; exposed 6 h/day, 5 days/wk, average 91.78 μg/m3, through a whole body inhalation system during pregnancy |
| 150 | PM2.5 decreased SIRT1 and SIRT2, increased DNMT1, DNMT3a, and DNMT3b, decreased Ca2+ markers (NCX, p-PLN, and SERCA2a), and increased IL-1 β, IL-6, Col-1, MMP9, and MMP13A | Female FVB mice, 12 wk old, 12-wk-old progeny | PM2.5 collected in Columbus, OH; 6 h/day, 7 days/wk, throughout pregnancy, average of 73.61 μg/m3 through a whole body inhalation system |
| 159 | PM2.5 caused a dose-dependent increase of OPA1, MFN1, DRP1, and FIS1 in progeny. | Female Wistar rats, 10–12 wk old, 1-day-old progeny | PM2.5 collected September 2014 to March 2016 in Harbin, China; administered dropwise, 3 doses (0.375 mg/kg, 1.5 mg/kg, and 6.0 mg/kg) during pregnancy |
| 169 | PM2.5 increased histone acetyltransferase (H3K9ac), GATA4, and MEF2c; exposure also increased histone acetylation of GATA4/MEF2c promoters | Female C57BL/6 mice, 12 wk old; 1-day- and 16-wk-old progeny | PM2.5 collected in Chongqing, China; gestational exposure by ultrasonic nebulization, 2 h/day during pregnancy, at ∼300 µg/m3 |
| UFP | |||
| 4 | UFP exposure increased AT1R protein along with Acta1 and Col3a1 in the heart, with decreased HO-1 expression | Male Sprague-Dawley rats | UFP collected north of Mexico City (May–July 2009); acute (3 days, 5 h/day) and subchronic (8 wk, 4 days/wk, 5 h/day) exposure to varying concentrations |
| 35 | UCAP exposure decreased blood plasminogen and thrombomodulin and increased CRP and SAA; GSTM1-null participants had worse outcomes | 34 Participants with metabolic syndrome, ∼48 yr old | UCAPs collected at EPA facility in Chapel Hill, NC; whole body exposure for 2 h at average particle concentration of 189,000 particles/cm3 |
| 66 | UFP exposure cause mitochondrial mPTP deficits, cyclosporin A resulted in attenuation | Male Sprague-Dawley rats | UFP collected at EPA facility in Chapel Hill, NC; 100 μg of UFP laryngopharynx instillation |
| 86 | TAA-PNC of UFP was positively associated with CRP and TNFR2 in white non-Hispanic participants | 408 individuals aged 40–91 yr old | UFP (near-highway, long-term exposure), Dorchester, South Boston, Chinatown (Boston, MA) |
| 142 | Elemental carbon UFP exposure increased platelet CD40L and vWF and decreased circulating CD40L | 19 type 2 diabetics, 30–60 yr old, never smoked | UFP particles were generated at 32 nm; elemental carbon UFP through mouthpiece for 2 h at 50 μg/m3 |
| 154 | UFCP exposure increased CRP and fibrinogen in systemic circulation | Male spontaneously hypertensive rats (SHRs), 12–13 mo old | UFCP exposure at 180 µg/m3 in a 24-h time frame |
| 178 | Increasing UFCP concentrations prevented the beneficial effects of captopril in reducing ANG II | Spontaneously hypertensive rats (SHRs), 10 wk old | UFP collected in Beijing, China; intratracheal instillation of UFCP (0.15 mg/kg, 0.45 mg/kg, and 1.35 mg/kg) and captopril administration |
| Gestational | |||
| 104 | UFP exposure induced placental HSD11B2 DNA methylation, elevated IL-1β, IL-6 and MCP-1, and caused increased activation of AT1R and ACE | Female C57BL/6J pun/pun mice, 6 wk old; gestational day 17.5 progeny | UFP collected in northern Mexico City, Mexico (April–June 2016); intratracheal instillation of 12 μg or 400 μg/kg during pregnancy |
| 134 | UFP exposure caused higher serum levels of IL-10 in C67BL/6 progeny | Female C57BL/6 and BALB/C mice; progeny 0–4 wk old | UFP generated with average mass concentration of 101.94 µg/m3; 24-h daily mean dose of 25 µg/m3 during pregnancy |
| Engineered nanomaterials and advanced materials | |||
| 52 | Carbon nanoparticle inhalation, compared with intra-arterial injection, was more detrimental to cardiac tissue with perturbation of inflammatory and endothelial/epithelial pathways | Female BALB/cJ mice, 10–12 wk old | Carbon nanoparticles with primary particle diameter of 10 ± 2 nm; administered through intra-arterial infusion (30 mm2/animal) and inhalation (lung deposited dose 10,000 mm2). Whole body exposure for 4 or 24 h |
| 61 | Nano-TiO2-exposed microRNA-378a Tg mice had higher MFN1 levels (fusion) and preserved metabolic profiles and ultrastructure in the heart | FVB and miRNA-378a knockout Tg mice aged 14–18 wk old | Nano-TiO2, particle size (21 nm)/surface area (48.08 m2/g);’ whole body inhalation exposure at a dose of 11.09 mg/m3 for 4 h |
| 67 | Nano-TiO2 exposure increased inflammatory markers (NF-κB, TNF-α, IL-4, IL-6, TGF-β, CK, CRP, and IFN-γ) and transcriptional regulators (STAT1, STAT3, STAT6, GATA3, and GATA4) | CD-1 (ICR) male mice, 4 wk old | Nano-TiO2 prepared through hydrolysis of Ti(OBu)4; administered via nasal instillation of mice every other day for 6 mo; treatment of 1.25–5 mg/kg TiO2 NPs |
| 73 | Nano-TiO2 exposure caused blood-specific activation of C3 and heart-specific activation of the complement cascade | Female C57BL/6 mice, 8 wk old | Nano-TiO2, UV-Titan L181; 6.0 mg of Ti/m3 – 10 mg TiO2/m3 administered through intratracheal instillation |
| 111 | Nano-TiO2-exposed mPHGPx Tg mice had reduced ROS and preserved electron transport chain complex constituents in the heart | Male FVB and male mPHGPx Tg mice, 10–12 wk | Nano-TiO2, particle size (21 nm)/surface area (48.08 m2/g); 11.58 ± 0.27 μg lung deposition, whole body inhalation exposure for 6 h |
| Gestational | |||
| 62 | Nano-TiO2 exposure increased cardiac mitochondrial proton leak (UCP2) and fatty acid metabolism (increased CPT1A and PDH phosphorylation) | Female Sprague-Dawley rats 10–12 wk,12-wk-old progeny | Nano-TiO2, particle size (21 nm)/surface area (48.08 m2/g); 7.79 ± 0.26 days, 5 h/day, 10.35 ± 0.13 mg/m3 through a whole body inhalation system during pregnancy |
| 83 | Nano-TiO2 exposure increased ROS (H2O2), global 5-mC methylation, DNMT1 protein expression, and HIF-1α activity and downregulated mPHGPx in the heart | Female FVB 12, wk old; gestational day 15 fetal progeny, 11-wk-old adult progeny | Nano-TiO2, particle size (21 nm)/surface area (48.08 m2/g); whole body inhalation exposure at a dose of 12.09 ± 0.26 mg/m3 for 6 days (over an 8-day period), 6 h/day, starting from gestational day 5 |
| 138 | Nano-TiO2 exposure resulted in cardiac mitochondrial epigenomic remodeling (increased H3K4Me3) and decreased transcriptomic immune response | Female Sprague-Dawley rats 10–12 wk old; gestational day 20 fetal progeny | Nano-TiO2, particle size (21 nm)/surface area (48.08 m2/g); whole body inhalation exposure at a dose of 10.35 ± 0.13 mg/m3 for 7.79 ± 0.26 days during gestation |
| 140 | Nano-TiO2 exposure decreased state 3 and State 4 mitochondrial respiration | Female Sprague-Dawley rats, female 11- to 16-wk-old progeny | Nano-TiO2, particle size (21 nm)/surface area (48.08 m2/g); whole body inhalation exposure at a dose of 10.6 ± 0.3 mg/m3 for 6.8 ± 0.5 days during gestation |
| Multicomponent exposure | |||
| 9 | Higher PM2.5 BC and mass PM2.5 exacerbated hypomethylation of IFNγ and ICAM-1 | Normative Aging Study, 777 elderly men | PM2.5 mass concentration and PM2.5 BC concentration measured hourly at the Harvard supersite, Boston, MA |
| 10 | Higher black carbon concentration associated with 12% reduction in F3 methylation | Normative Aging Study, 777 elderly men | PM2.5, BC, So4, and ozone concentrations of ambient air collected from Boston, MA, were measured hourly |
| 31 | Increases in PM2.5, BC, and UFP concentrations were positively associated with inflammation (CRP and fibrinogen) and coagulation, but not endothelial dysfunction; Delta-C increasing concentrations associated positively with fibrinogen and negatively with MPO | 135 Patients undergoing cardiac catheterization | Ambient air pollution, ΔC (wood smoke), PM2.5, BC, and UFP collected from New York State Department of Environmental Conservation (DEC) site in Rochester, NY |
| 41 | PM2.5 levels, but not UFP, were positively associated with hsa-miR-197-3p and hsa-miR-99a-5p | 59 volunteers (20 were healthy, 20 had COPD, and 19 had ischemic heart disease) | Ambient air pollution exposure (personal exposure level measurements of PM10 and PM2.5, UFP, nitrogen oxides, BC, and CO) during 2-h walk; Oxford Street or Hyde Park, London, UK |
| 46 | SiNP and Pb coexposure elevated markers of heart failure (ANP and BNP) and inflammation (CRP, IL-6, and TNFα) in heart and increased ANG II and ET-1 in serum; T-PA, TFPI, and ATIII decreased whereas fibrinogen and D2D increased with coexposure | Sprague-Dawley rats, 6 wk old | Stöber technique was used to synthesize SiNPs; intratracheal instillation of 2 mg/kg body SiNPs and/or 0.25 mg/kg of lead acetate (Pb) for 30 days |
| 80 | 54 Circulating miRNAs were correlated with dose of exposure and pollution type | 24 Nonsmoking adults | PM2.5, NO2, UFP, BC, and PM10 collected on Oxford Street in London, 2-h exposure |
| 82 | UFP, CO, and So2 exposure was associated with increased inflammatory markers (IL-6, IL-8, ET-1) in blood | 52 men and women 18–34 yr old | UFP, CO, and So2 concentrations collected in Sault Ste. Marie, ON, Canada (Summer 2010); occupational air pollution |
| 92 | PM2.5 and So4 increases associated with CRP levels, NOx positively associated with IL-6, BC, sulfate, ozone positively associated with TNFR2 | 3,996 non-smoking participants Framingham Offspring cohort cycle 7 and 8 and Third Generation cohort 1 | PM2.5, BC, , nitrogen oxides, and ozone collected at the Boston Harvard Supersite and assessed as 7-day moving average |
| 93 | Higher PM2.5 and BC concentrations were associated with lower P-selectin levels | 3,820 nonsmoking participants; Framingham Offspring cohort cycles 7 and 8 and third-generation cohort 1 | PM2.5, BC, , nitrogen oxides, and ozone collected at the Boston Harvard Supersite and assessed as 7-day moving average |
| 97 | Nickel, barium, and silver levels were positively correlated with VEGF, UCHL1, and cortisol | 53 Healthy participants, 18–60 yr old | Particulate matter collected in downtown Toronto, ON, Canada; 130-min exposure to PM10 (213 µg/m3), PM2.5 (238 µg/m3), and/or concentrated UFP (213 µg/m3) |
| 98 | Higher BC is associated with decreased methylation of Alu, and both increased So4 and BC resulted in decreased methylation of LINE-1 | Normative Aging Study, 706 individuals | PM2.5, BC, and So4 data collected from the Harvard School of Public Health from January 1995 to November 2007 |
| 105 | PM2.5 concentration was positively associated with 69 differentially methylated regions and 13 CpG sites, including altered methylation of KNDC1 and FAM50B; UFP was also associated with 15 differentially methylated regions | 157 Nonsmoking adults | PM2.5 and UFP levels collected in personal and ambient air pollution, measured in 24-h intervals, part of the EXPOsOMICS project conducted in 4 European countries (December 2013–February 2015) |
| 113 | BC was associated with sVCAM-1, sICAM-1, and vWF; PM2.5 was associated with sVCAM-1 and sICAM-1 in patients not taking statins; in smokers, PM2.5 and BC were positively associated with sVCAM-1 | 60 Male and 37 female participants with type 2 diabetes (smokers and nonsmokers) | PM2.5 (1998–2002) and BC (1999–2002) measured hourly and measured daily (1999–2002) at the Harvard School of Public Health (Boston, MA) |
| 127 | PM2.5 mass concentration shower positive trend with HDL-oxidant index (HOI); changes not seen with ozone coexposure; | 30 participants, 19–37 yr old | PM2.5 (149 µg/m3), ozone (221 µg/m3), and PM2.5 and ozone data collected from Toronto, Canada as part of Clean Air Research Center Project. |
| 153 | PM2.5 and quasi-ultrafine particles <0.50 µm dysregulated parasympathetic response and increased IFNγ methylation in blood | 12 Healthy participants | Particulate matter collected in Milan, Italy. Inhalation to mixture (PM10, PM2.5, PM1.0, and PM0.5µm) over 2 sessions |
| 155 | PM2.5 exposure was associated with higher inflammation (CRP) and coagulation (platelet count in blood) | 3,275 Participants | Ambient air pollution PM10 and PM2.5 levels from the German Heinz Nixdorf Recall Study, 3 adjacent cities (Essen, Mülheim, and Bochum, Germany) |
| 158 | Coexposure potentiated increases in CRP, IL-6, CK, LDH, and MDA and decreased SOD | Male Wistar rats (age not defined) | PM2.5 (varying doses), ozone (0.81 ppm), PM2.5, and ozone collected from June to October 2011 in Shanghai, China, administered as whole body inhalation exposure (ozone) and intratracheal instillation (PM2.5) |
| 164 | BC, CO, NOx, and PAHs positively associated with IL-6 and TNFα, and quasi-ultrafine particles <0.25 µm were also associated with IL-6; associations were stronger for haplotype H than haplotype U | 36 Participants, ∼84 yr old | Particulate matter (PM-varying sizes), nitrogen oxides (NOx), carbon monoxide (CO), and organic (OC) and elemental carbon (EC), collected from 4 retirement communities in the Los Angeles, CA, air basin |
| 171 | SiNP and MeHg coexposure caused cardiac injury through changes in serum biomarker activity, including increased SERCA2, cTnT, ANP, and BNP | Sprague-Dawley rats, 6 wk old | Silica nanoparticles (SiNPs) and methylmercury (MeHg) administered through intratracheal instillation 10 times over 30 days at doses of 0.25 mg/kg (MeHg) and 2 mg/kg (SiNPs) |
| 179 | C-exposure (PM2.5, So2, and NO2) causes endothelial dysfunction (increased ET-1 and decreased eNOS) and inflammation (increased COX-2, iNOS, TNFα, and IL-6) | Male C57BL/6 mice, 6–8 wk | PM2.5, So2, and NO2, collected in Taiyuan, China; intranasal instillation every other day for 6 h/28 days; 0.5/3.5 mg/m3 So2, 0.2/2 mg/m3 NO2, 1/10 mg/kg PM2.5 |
Summarized findings of the molecular mechanisms contributing to cardiovascular complications following particle inhalation exposure. Studies are organized by exposure (PM2.5, UFP, ENM, and multicomponent), including subsections for gestational and preconception exposures. All abbreviations are defined in glossary.
CARDIAC REMODELING
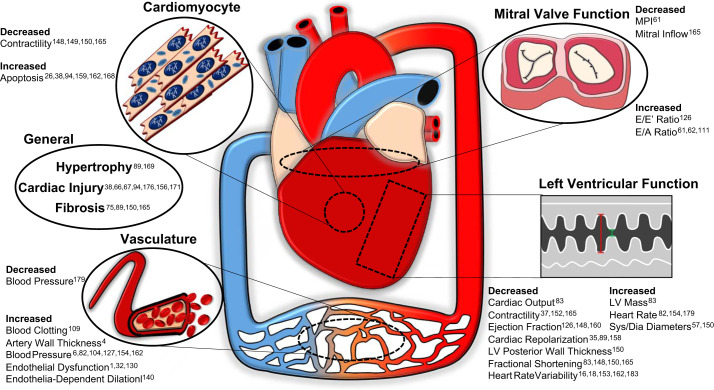
Whereas inhalation exposure directly impacts the lungs, the subsequent downstream adaptations of the heart can lead to sustained cardiac remodeling. This remodeling can be presented in the form of fibrosis, hypertrophy, and other structural changes to both the cardiomyocyte and the surrounding connective tissue of the heart. In understanding how this process occurs, examining the biochemical signature linked to cardiac remodeling changes associated with toxicant inhalation exposure can provide insight for future investigations into prevention and treatment. The reported cardiovascular adaptations that occur as a result of toxicant inhalation exposure are depicted in Fig. 1.
Fig. 1.
Summary of the cardiovascular changes following particulate inhalation exposures and the associated studies detailing these findings. Dia, diastolic; LV, left ventricular; MPI, myocardial performance index; Sys, systolic.
Cardiac fibrosis involves the imbalance of extracellular matrix production and degradation, which results in accumulation of scar tissue. Therefore, cardiac fibrosis, which occurs as a result of PM exposure, decreases compliance, impairing the ability of the heart to contract and relax properly. PM2.5 exposure results in elevation of several genes linked to collagen deposition [collagen type I α1 (Col1a1) and collagen type III α1 (Col3a1) and the fibrogenic growth factor transforming growth factor-β1 (TGFβ1)] (126). Simultaneously, NADPH oxidase (NOX4) is increased, matching the resultant cardiac dysfunction and prooxidative environment found in the heart as a result of PM exposure. Of note is that Qin et al. (126) also further demonstrates that the effects of PM2.5 are worse in older mice, making them more susceptible to prolonged cardiac systolic dysfunction marked by decreased ejection fraction and increased E/E′ ratio when compared with juveniles. PM2.5-induced fibrosis is also related to cardiac inflammation in the hearts of mice, which show elevated expression of inflammatory genes, including interleukin (IL)-6, IL-18, and C-X-C motif chemokine ligand 1 (CXCL1) (75). Inflammation and fibrosis in the heart may be attributed to increased phosphorylation and, therefore, activation of epidermal growth factor receptor (EGFR)/Akt signaling and increased expression of nuclear factor-κβ (NF-κB) following inhalation exposure. NF-κB expression in the heart is likewise increased as a result of PM2.5 exposure in diabetic mice, along with cyclooxygenase-2 (COX-2) and mitogen-activated protein kinase (MAPK), indicating high oxidative stress and inflammation (180). Intracerebroventricular injection of an inhibitor of IκB kinase-2 (IKK) analog (IMD-0354), which regulates the nuclear translocation of NF-κB, can remediate the inflammatory response that occurs following PM exposure. Whereas NF-κB plays an important role in the cardiovascular effects of PM inhalation exposure, other inflammatory markers contribute to cardiac remodeling. Cardiac injury and inflammation that occurred following PM2.5 exposure were attenuated with supplementation of vitamin E and ω-3 polyunsaturated fatty acids before the exposure in rats by decreasing expression of tumor necrosis factor-α (TNFα), IL-1β, and IL-6 (38). In addition to TNFα and IL-1β, PM2.5 exposure also induces soluble intercellular adhesion molecule 1 (ICAM-1) (176). Together, these inflammatory markers can form a positive feedback loop with NF-κB, amplifying PM-induced cardiac fibrosis and remodeling. Selenium yeast (SeY) supplementation can similarly serve as a pretreatment protective strategy, as reported in Sprague-Dawley rats that were subsequently exposed to PM2.5 (176). PM-exposed rats that were administered SeY pretreatment had lower levels of proinflammatory markers and significantly higher total antioxidant capacity, total superoxide dismutase (SOD), and total glutathione peroxidase (GSH-Px) compared with the PM2.5-exposed group without pretreatment (176). The ability of antioxidant therapy to thwart the perpetuation of inflammatory signaling associated with cardiac remodeling highlights the essential role of oxidative stress in the development of cardiac pathology following ambient air exposure. Understanding the proteins involved in the regulation of reactive oxygen species (ROS) may provide insight into long-term remodeling of the heart. Whereas most population-derived analyses assess cardiovascular impact in blood samples, a study of the effects of UFP urban air pollution used postmortem ventricular autopsies of 30 children and young adults to substantiate that early and prolonged cardiac stress can result in irreversible consequences (156). Compared with clean air controls, long-term UFP exposure results in significant upregulation of proteins involved in oxidative [prion protein (PRNP)] and endoplasmic reticulum (ER) [glucose regulated protein 78 (GRP78)] stress. These response markers of stress were disproportionately upregulated in the left ventricle compared with the right ventricle. Along with abnormal left-ventricular histopathology, increased oxidative and ER stress markers suggest a compensation by the heart to attenuate the inflammatory effects of air pollution in response to UFP exposure (156). GRP78 elevation in cardiomyocytes is sufficient to promote myocyte growth, potentially through stimulation of cardiac-specific transcriptional factor GATA sequence-binding protein 4 (GATA4) (177). Similarly to UFP, nano-TiO2 exposure in outbred mice can lead to an increase in cardiac lesions and a significant change in inflammation markers, including changes in transcription factors [signal transducer and activator of transcription (STAT) 1/3/6 and GATA 3/4] in the heart (67). Therefore, the ability for oxidative and inflammatory proteins to activate transcription factors such as GATA4 is notable, particularly following UFP/ENM exposure, as they appear to have strong correlations with cardiac hypertrophy. Fine PM exposures can also lead to sustained detrimental effects in the heart by altering the transcription factor profile. One example of this has been shown previously in a cohort of PM2.5-exposed mice (89). Cardiac remodeling through hypertrophy, QT interval prolongation, and fibrosis were correlated with elevated cAMP response element-binding protein (CREB) as well as SOS Ras/Rac guanine nucleotide exchange factor 1 (SOS1) and glycogen synthase kinase-3β (GSK3β) expression. Measuring biomarkers associated with myocardial infarction can also provide information on the extent of cardiac tissue dysfunction and death. An examination of the cardiovascular effects of a coexposure model using silica nanoparticles (SiNP) and methylmercury (MeHg) has shown increases in myocardial edema, myocardial gap expansion, and myofibril disorder as well as increases in activities of myocardial enzymes, including cardiac troponin T (cTnT), atrial natriuretic peptide (ANP), and brain natriuretic peptide (BNP) compared with the serum of single-exposure and sham-exposed rats (171). These authors also report increased sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2), a potential marker of endoplasmic reticulum stress (65), in the coexposure cohort along with oxidative stress. Similar to the preceding studies mentioned, coexposures can induce cardiac remodeling by increasing oxidative and ER stress and elevating prominent markers of heart failure. PM exposures, including UFP, ENM, and multicomponent exposure, all have the ability to alter the transcription factor profile in the heart and lead to sustained detrimental effects on cardiac structure. The molecular adaptations to particle exposures that result in cardiac remodeling center around a theme of inflammation and oxidative stress, which may ultimately be regulated by a greater transcriptional and epigenetic adaptation following toxicant inhalation exposure.
CARDIAC HEMODYNAMICS
The performance of the heart can highlight both the acute and chronic effects of toxicant inhalation exposure. Although cardiac remodeling provides an informative diagnostic standard for assessing the impacts of PM exposure, more immediate changes are captured in measures of heart function. Whether there is a sustained or transient perturbation to the heart is likely determined by the molecular cascades involved, such as transcriptional or epigenetic reprogramming.
Controlled studies on human exposure to PM allow for the most relevant simulation of pollutant exposure while also limiting interference of confounding factors. Measurements of blood methylation levels in 15 healthy participants revealed that both fine and coarse concentrated ambient particle (CAP) exposures induce hypomethylation of the Alu-repetitive transposable element and Toll-like receptor 4 (TLR4) (6). Decreased methylation of the short, interspersed nucleotide element Alu and TLR4 was also associated with higher systolic blood pressure postexposure, which is similar to previous findings that have shown a role for hypomethylation in atherosclerosis (22). Other studies have reported similar findings with specific components of PM2.5, including with black carbon (BC). Over a 90-day period, increased BC levels correlated with decreased methylation of Alu, whereas both carbon black and sulfate (So4) were associated with decreased methylation of long interspersed nuclear emlement-1 (LINE-1) (98). Interestingly, in a study examining occupational exposure to PM2.5, methylation of LINE-1 was significantly increased in peripheral blood leukocytes of participants but was not directly associated with declining heart rate variability (HRV) (42). When DNA methylation is altered, it has the potential to activate proinflammatory factors or repress anti-inflammatory factors, and therefore, it can activate pathways that are prevalent in the development of cardiovascular diseases. The discrepancy of whether toxicant exposure results in hypo- or hypermethylation can likely be attributed to different exposure environments (controlled or ambient), duration, and concentration. The concentration of PM2.5 during exposure was positively associated with Toll‐like receptor 2 (TLR2) methylation in older adults, further suggesting potentiation of the inflammatory response (183). Flavonoid supplementation can potentially limit TLR2 methylation, as correlations indicate and ameliorate PM2.5-induced low frequency HRV. Another study that aimed to address the epigenetic regulation of inflammatory markers by PM exposure used young, healthy subjects who inhaled an array of PM compositions (PM10, PM2.5, PM1.0, and PM0.5) over two sessions (153). These subjects presented with decreased HRV, indicating a stress response, which is consistent with previous studies of PM exposure. Furthermore, PM2.5 as well as quasi-ultrafine particles <0.50 µm were significantly associated with increased proinflammatory cytokine [interferon-γ (IFNγ)] methylation, correlating with alterations in the parasympathetic nervous system as an adaptive response (153). However, Bind et al. (9) reported that both PM2.5 BC and PM2.5 mass concentrations were associated with significantly lower methylation of immunoregulatory genes, IFNγ, and intercellular adhesion molecule 1 (ICAM-1) in individuals with low methylation levels before exposure. The main contributing factor that is likely responsible for these contradictory findings is the population that was studied. Whereas Tobaldini et al. (153) conducted a more controlled experiment with healthy, young males (n = 12), Bind et al. (9) used a large cohort (n = 777) of elderly men with varying health conditions. Other studies address epigenetic alterations of the cardiovascular system following PM inhalation exposure as well (95, 105), albeit without direct functional associations. Although the studies outlined provide a link between epigenetic mechanisms and cardiac function, specifically HRV, it remains unclear whether epigenetic reprogramming is a primary mechanism for sustaining adaptations to PM exposures or whether it is transiently changed with exposure. MicroRNA-378A and other microRNAs are potential molecular mediators of metabolic and cardiac dysfunction that ensue following ENM exposure (61). Nano-TiO2 inhalation exposure induced diastolic dysfunction and E-to-A ratio elevation and decreased myocardial performance indices, which were preserved in a microRNA-knockout transgenic mouse model (microRNA-378A). These authors point to regulation of mitofusin 1 (Mfn1) expression through microRNA-378A as a mechanism for controlling mitochondrial dynamics following exposure (61). Although the preceding studies examined epigenetic changes, the mechanisms of action were not elucidated. Multifaceted assessments of how pollutants impact the epigenome and inflammation-associated genes are critical, as both are implicated in processes mediating cardiovascular function impairment.
Inflammation plays a key role in pollutant exposure response in both individual particulate exposures and multicomponent exposures. Coexposure of sulfur dioxide (So2), nitrogen dioxide (NO2), and PM2.5, typical air pollutants produced as a result of coal combustion, results in an enhanced inflammatory response with upregulation of COX‐2, inducible nitric oxide synthase (iNOS), TNFα, and IL‐6 (179). The coexposure contributed to decreased blood pressure and increased heart rate when compared with PM2.5 alone. Similarly, IL-6, IL-8, and endothelin-1 (ET-1) were significantly associated with rises in carbon monoxide (CO), UFP, and So2 concentrations in healthy individuals near a steel mill (82). The precursor for ET-1 (BET-1) was positively correlated with systolic blood pressure, whereas C-reactive protein (CRP) was correlated with increased heart rate. Protein ontology revealed a significant elevation of inflammation specific pathways (82). Combined high-dose PM2.5 and ozone exposure also promote cardiovascular functional injuries that are presented as abnormal electrocardiogram (ECG) results (extended QRS complex and depression of ST segment) (158). The addition of ozone to the exposure paradigm propagated inflammatory and oxidative stress responses with elevated CRP, IL-6, creatine kinase (CK), lactate dehydrogenase (LDH), and malondialdehyde (MDA). These studies highlight the importance of controlled coexposure studies for delineating the risk of ambient pollutants and combustion materials on cardiovascular hemodynamics and the associated inflammatory and oxidative stress mechanisms.
Coexposure of CAPs and acrolein causes myocardial dyssynchrony, albeit through a less understood mechanism involving the activation of transient receptor potential cation channel A1 (TRPA1), which the authors report may act to increase cardiac risk, specifically in exposures with heterogeneous compositions (152). Along with channel proteins affecting cardiac conduction, modifications in the uptake and clearance of calcium can alter contractility within cardiomyocytes, further dysregulating the cardiac cycle. One of the more studied pathways is through SERCA2a. PM2.5 exposure increased intracellular free calcium (Ca2+) that was associated with increased ryanodine receptor 2 (RYR2) and decreased SERCA2a, which are responsible for shuttling Ca2+ from the sarcoplasmic reticulum to the cytoplasm and back, respectively (37). The changes in calcium handling may be a direct result of PM2.5-induced oxidative stress, thereby contributing to contractile dysfunction in cardiomyocytes. In response to long-term exposure to PM2.5, others have reported decreased fractional shortening, impaired mitral inflow patterns, and depressed contractile reserve, along with decreased peak shortening and relengthening of isolated cardiomyocytes in C57BL/6 mice (165). Downregulation of SERCA2a in PM2.5-exposed mice revealed abnormal Ca2+ cycling, as previously seen, but also elevation of β-myosin heavy chain (β-MHC) indicative of heart failure (165). Apart from contractility, tone of the vasculature within the heart can alter cardiac hemodynamics. A study of acute and subchronic inhalation exposure of Sprague-Dawley rats to PM2.5 and PM0.1 separately elevated angiotensin II (ANG II) type 1 receptor (AT1R) mRNA (4). The subchronic exposure to both sizes of PM further increased AT1R protein levels, expression of markers for myocardial adaptive response to damage [actin α1 (Acta1) and Col3a] and IL-6, while decreasing antioxidant heme-oxygenase 1 (HO-1). These authors suggest that angiotensin overexpression can promote coronary artery wall thickness, which is likely to influence blood pressure in exposure models (4). Another study aimed to elucidate the effects of toxicant exposure on the regulation of blood pressure using 12- to 13-mo-old spontaneously hypertensive rats (SHRs), which were exposed to ultrafine carbon particles (UFCP) (154). UFCP inhalation exposure resulted in increased blood pressure and heart rate, which were correlated with increased levels of serum CRP, plasma fibrinogen, and levels of ET-1 in the heart. UFCP exposure-induced hypertension in SHRs is also associated with elevated ANG II in the blood, with increasing doses of UFCP diminishing the effectiveness of captopril, an angiotensin-converting enzyme inhibitor (178). The diminished effectiveness of captopril suggests that increasing doses of UFP may activate a variety of pathways outside of angiotensin to induce changes in blood pressure. These data suggest that regulation of blood pressure, like other cardiovascular responses, is a multifactorial pathway that is influenced by other circumstances, including genetic makeup and various environmental influences.
One of these environmental influences, high-fat diet feeding, is often used to model hyperlipidemia. Hyperlipidemic rats exposed to PM2.5 are susceptible to higher blood pressure, lower HRV, and higher levels of cardiomyocyte apoptosis, accompanied by decreased expression of antioxidant proteins, including SOD (162). Markedly, high-fat diet-exposed mice, compared with high-fat diet alone, also revealed increased myocardial death through increased levels of cardiac troponin I (cTnI), LDH, and CK. Animal models with a high propensity for metabolic dysfunction, such as AMP-activated protein kinase-α2 knockout (AMPKα2−/−) mice that lack this crucial gene for fatty acid oxidation, are critical for understanding how PM2.5 inhalation exposure impairs cardiovascular function in vulnerable populations (160). PM2.5 exposure diminished left ventricular ejection fraction in AMPKα2−/− mice and promoted an oxidative and inflammatory environment through decreased expression of peroxiredoxin 5 (PRDX5) and increased expression of NF-κB and TNFα. These studies highlight the importance of understanding the molecular changes that occur and the downstream effects on oxidative stress regulation in susceptible populations exposed to PM. UFP inhalation exposure in human subjects with metabolic syndrome can be specifically detrimental to cardiac repolarization, as demonstrated by the QRS complexity in those without the glutathione s-transferase mu 1 (GTSM1) protein, a pivotal antioxidant gene (35). In the complete cohort (participants with and without the GTSM1 mutation), UFP exposure elevated CRP and serum amyloid A (SAA) with subsequent decreases in plasminogen and thrombomodulin within blood, indicating an upregulation of inflammatory pathways and the critical role of antioxidant genes. On the contrary, subacute exposure to PM2.5 in healthy adults was not correlated with increased inflammatory markers or altered vascular function (16). However, a 10 µg/m3 increase in exposure was associated with decreased HRV, correlating to increased homeostatic model assessment of insulin resistance (HOMA-IR). This suggests the possibility that chronic periods of exposure to PM2.5 could reduce insulin sensitivity and thus potentiate susceptibility to diabetes mellitus, although inflammation may not be the etiology implicated by this study (16). Particularly concerning is the notion that in obese mice, chronic exposure to PM2.5 can prevent the beneficial effects of exercise on cardiac function and anti-inflammatory pathways (57). Sedentary mice that were exposed to PM2.5 presented with increased left ventricular diameter (systolic and diastolic) that was not ameliorated in the exercised group. Moreover, PM2.5 exposure enhanced CRP, ICAM-1, and vascular cell adhesion protein 1 (VCAM-1), which were elevated in the PM2.5 exercised group as well (57). These results are similar to those seen in a previous study that reported the inability of moderate aerobic exercise to provide anti-inflammatory protection in mice exposed to PM2.5 (99), which may be linked to expression of heat shock protein 70 kilodalton (HSP70) (99, 135). The advantages of moderate exercise on cardiovascular function in obese mice (106, 121, 144), but not in obese mice exposed to PM2.5 (57), emphasize the consequential results of PM2.5 exposure in vulnerable populations. However, the use of a high-fat diet-induced obesity model may be more relevant in substantiating whether fine particulate exposure hinders the ability of exercise to mitigate the detrimental cardiovascular effects in diabetic models, as the ob/ob model may have impaired exercise capacity (57).
COAGULATION
Within the lungs, the circulatory system provides a necessary pathway for mediation of the inflammatory response. Due to this close association of the pulmonary and circulatory systems, changes arising in blood have the potential to alter homeostasis within blood vessels. Alterations in the coagulation pathway, specifically through fibrinogen, have been reported following PM exposure.
Levels of a pollutant from wood smoke, Delta-C, are positively correlated with fibrinogen and negatively correlated with myeloperoxidase (MPO), an enzyme involved in the inflammatory pathway and platelet activation (31). The study also demonstrated that PM2.5 and UFP retain a positive correlation with fibrinogen as well as CRP, sharing a similarity in the effects on blood coagulation with effects of Delta-C. In a coexposure model of SiNPs and lead acetate (Pb), Feng et al. (46) reported alterations to the blood-clotting cascade evidenced by decreased tissue-type plasminogen activator (t-PA), tissue factor pathway inhibitor (TFPI), and antithrombin III (ATIII) as well as elevated fibrinogen and D-dimer (D2D). Following coexposure, serum analyses revealed leukocytosis and thrombocytopenia, concomitant with increased expression of inflammatory markers (CRP, IL-6, and TNFα) and markers of heart failure (ANP and BNP). Overlap found between studies of varying particle sizes can help identify critical biomarkers of increased blood clotting that are a product of multicomponent exposure in both human and animal models. Furthermore, others have shown that levels of fibrinogen can be increased by other ambient air pollution constituents (8). Specific associations between PM2.5 BC and altered epigenetic modification status of tissue factor III (F3) were later reported by this group, indicating alterations to extrinsic blood coagulation (10). Decreased methylation of F3, which likely leads to increased transcription of the gene, can provide the initial stimulus for altering the response of thrombin and fibrinogen, thereby increasing coagulation (28). Similarly, in a meta-population study, including 3,275 participants, long-term rises in PM2.5, but not PM0.1, were matched with significantly increased CRP and platelet count (155), suggesting an increased propensity for blood clotting.
Interestingly, annual average UFP exposure levels and fibrinogen were negatively associated, albeit not significantly, in human blood samples (86), which may have been a result of microenvironment compared with ambient concentrations. To better elucidate the dynamic relationship between ambient exposure and the time/concentration of the exposure in terms of their effects on the cardiovascular system, Lane et al. (86) examined biomarkers in patients with varying levels of microenvironment exposures (time/activity adjusted) in addition to the annual average particle number concentration of UFPs. There were significant positive associations between time/concentration of exposure and inflammatory pathway genes in blood, including CRP and tumor necrosis factor receptor 2 (TNFR2) (86). These findings illustrate the significance of monitoring personal exposure levels, which may account for the differences seen in fibrinogen expression between studies that solely use annual outdoor particle concentrations and those that consider varying levels of individual microenvironment exposure. Modifying the coagulation pathway in individuals that are continuously exposed to high levels of pollutants through ambient air may provide a potential a potential prophylactic strategy to prevent the inevitable high risk of CVD. Nootkatone, a sesquiterpenoid in grapefruit, has potential as a pretreatment strategy for PM2.5 diesel exhaust exposure through its ability to reduce blood clotting by decreasing plasminogen activator inhibitor-1 (PAI-1) and fibrinogen and restoring thrombotic occlusion time in arterioles (109). These authors suggest that the pretreatment strategy activated nuclear factor erythroid-derived 2-like 2 (NRF2), which plays a key role in initiating HO-1, to provide protection from oxidative injury (109). Although the studies discussed thus far have primarily used healthy cohorts, it is important to understand how the coagulation pathway is altered in at-risk populations as well. Elemental carbon UFP inhalation exposure modifies platelet activation in type 2 diabetic patients by increasing CD40 ligand (CD40L) expression in platelets, with a resulting decrease in soluble CD40 (sCD40) in blood (142). Higher CD40L levels, as a result of UFP exposure, exerted a stimulatory role on von Willebrand factor (vWF) and elevated its expression. Although this study revealed the adaptations of those with diabetes mellitus, it did not provide a control cohort to elucidate whether these effects of PM on coagulation are exaggerated in the at-risk population. Nonetheless, genes involved in the activation of pathways involving fibrinogen and platelet activation are potential early biomarkers of increased CVD risk in healthy and vulnerable populations and require further clarification.
VASCULAR DYSFUNCTION
Along with mediating the blood clotting cascade, particulate exposure has the capacity to modify the integrity of vascular tissue, thus interfering with blood flow and cellular metabolism directly within the vasculature. Through direct damage to the vasculature or mediation of the inflammatory response, particulate matter can cause blood vessels to become predisposed to ultrastructural changes. Atherosclerosis, which involves the buildup of fatty acids within the intima layer of arteries, is linked to PM exposure.
In an atherosclerotic transgenic mouse model (ApoE−/−), PM2.5 exposure causes cardiac autonomic nervous system dysfunction concomitant with oxidative stress (118). Of note is that the high-fat diet fed atherosclerotic mice displayed a greater proclivity for oxidative damage, suggesting, once again the impairment of cellular protective mechanisms in those who already have an altered metabolic profile. Similarly, concentrated PM2.5 exposure significantly altered high-density lipoprotein (HDL) antioxidant and anti-inflammatory capacity [HDL oxidative index (HOI)], along with increased systolic blood pressure (127). However, ozone exposure in addition to the PM2.5 exposure in this study did not exacerbate these effects, suggesting that short-term CAP and ozone coexposure may not induce overt changes that are suggestive of atherosclerosis progression. On the other hand, long-term CAP exposure does contribute to the progression of atherosclerosis. Rao, et al. (128) discovered that PM2.5 exposure increased abnormal accumulation of an oxidized variant of cholesterol, 7-ketocholesterol (7-KCh), in macrophages and the aortic wall. PM2.5-exposed mice also presented with increased CD36 expression in plaque macrophages, implicating CD36 in the accumulation of oxidized lipids, promoting atherogenesis. By reducing the number of CD36-positive macrophages, internalization of 7-KCh can be limited, and therefore, the oxidized lipids can be efficiently cleared from the vasculature (128). Along with altered macrophage clearance, other immune responses have been implicated in vascular maladaptation following particle exposure, including changes to the innate immune response. ENM inhalation exposure resulted in translocation of nanomaterials to the heart, which initiated the complement cascade, setting off a local immune response, indicated by global complement factor 3 (C3) upregulation in the blood (73). This study demonstrates that alterations to the innate immune response may be a direct effect of translocated particles, but both studies (73, 128) highlight the importance of the inflammatory response and part of its role in altering vascular function. PM2.5 exposure can impact vascular endothelial cell permeability pathways by causing an inflammatory response, which is a hallmark of increased vascular permeability. A recent study has demonstrated that long-term changes in vascular permeability are promoted specifically by IL-6 and sustained in part by STAT3 phosphorylation (1). In rats, PM2.5 exposure induced phosphorylation and subsequently expression of STAT3, which upregulated microRNA-21 expression (32). Increased microRNA-21 expression reduced tissue inhibitor of metalloproteinase 3 (TIMP3) expression and enhanced matrix metalloproteinase 9 (MMP9) expression. Ultimately, vascular endothelial dysfunction was linked to extracellular matrix remodeling propagated by STAT3 (32), which was likely promoted by the PM-induced inflammatory response. Changes in the composition of the vascular matrix could result in alterations to immune cell binding and further changes to diapedesis, leading to vascular dysfunction.
In a 3,820 noncurrent smoking patient population that was part of the Framingham Heart Study, acute PM2.5 and BC exposure concentrations were inversely correlated with expression of P-selectin, suggesting decreased endothelial surface cell adhesion (93). Along with changes to vascular structure, the ability for the endothelium to regenerate and adapt to pollutant exposure can be compromised. Typically, this occurs through the production of endothelial progenitor cells (EPCs) in response to vascular injury (48). However, PM2.5 exposure diminishes circulating EPC levels, which may occur as a result of vascular endothelial growth factor (VEGF)-induced Akt and endothelial nitric oxide synthase (eNOS) phosphorylation (48, 58). Short-term CAP exposure in mice fed a control diet remarkably induced vascular insulin resistance, indicated by decreased Akt phosphorylation, and suppressed circulating levels of EPCs through activation of the NF-κB inflammatory pathway (59). Increasing insulin sensitivity prevented activation of these pathways, substantiating that PM2.5 exposure can lead to insulin resistance and, therefore, type 2 diabetes in healthy populations (48). Furthermore, CAP-induced vascular insulin resistance was accompanied by increased vascular oxidative stress marked by mitochondrial SOD2 and glutathione s-transferase-P (GST-P) mRNA levels and expression of protein-HNE adducts (59). Interestingly, 9-day CAP exposure did not exacerbate high-fat diet-induced changes in vascular insulin resistance (59), but a 30-day CAP exposure significantly aggravated systemic insulin resistance, elevating glucose intolerance and HOMA-IR in high-fat diet-fed mice (60). In aortic tissue, both 9-day and 30-day CAP exposures resulted in decreased insulin-stimulated phosphorylation of Akt and eNOS, subsequently suppressing inhibition of the transcription factor NF-κB (IκBα) and promoting an inflammatory response (60). By treating with an antioxidant, 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol), CAP-induced inflammation and the associated vascular insulin resistance were alleviated in high-fat diet-fed mice, leading to the conclusion that short-term exposure to PM2.5 triggers a pulmonary oxidative stress response that is able to elicit downstream vascular insulin resistance and inflammation (60).
Compounding environmental factors can further propagate the effects of particulate exposure. A study in type 2 diabetic residents of Boston, MA, provided evidence that both PM2.5 and BC are significantly associated with increased VCAM-1 and vWF in participants who have smoked in the past (113). These data indicate that prior exposure to smoking-related particulates may exacerbate the inflammatory response seen with ambient PM2.5 exposure, creating a greater risk for endothelial dysfunction and cardiovascular damage in type 2 diabetic patients. In an attempt to directly assess the physiological impact of PM2.5 exposure on vasculature in a CVD at-risk population, Riggs et al. (130) assessed individuals’ reactive hyperemia index as a measure of endothelial function. Increasing concentrations of PM2.5 significantly reduced the reactive hyperemia index and were associated with angiogenic signaling and inflammation markers, angiopoietin 1, placental growth factor (PlGF), VEGF, ICAM-1, and MMP9 and negatively associated with VCAM-1 and urinary F2-isoprostane (Isop), a measure of oxidative stress (130). Interestingly, incremental increases of PM2.5 by 10 μg/m3 were also associated with increased proinflammatory cytokines, including monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α/β (MIP-1α/β), and IFNγ-induced protein 10 (IP-10) in a cohort of young, healthy individuals with low CVD risk (122). Together, these studies indicate that PM2.5 initiates changes in cytokines and growth factors, which lead to endothelial injury and an immune response that may result in acute cardiovascular events in both high-risk and low-risk CVD populations (122, 130). Furthermore, PM2.5 exposure, in a subset of the same cohort, revealed an inverse association with ET-1 but a positive association with platelet factor 4 (PF-4) (49). Additionally, ET-1 was negatively associated with PF-4 following PM2.5 exposure, which the authors conclude may be the result of an antiangiogenic effect of the exposure that could also suppress endothelial repair (49, 122). Although this is contrary to previous studies reporting positive correlations between PM and ET-1 (46, 82, 179), another acute exposure reported similar findings (54). Conversely, chronic exposure paradigms appear to cause elevated ET-1 levels (46, 82, 179). Current literature provides convincing evidence that particulate matter exposure causes a remarkable maladaptive vascular response that can lead to increased risk of CVD. However, understanding the genome-wide effects of exposure may provide a better understanding of the molecular mechanisms that are responsible for these alterations.
CELLULAR STRESS AND DEATH
PM inhalation exposure-induced changes to the cardiovascular system can eventually trigger cell death pathways through varying mechanisms, including oxidative stress (90). These pathways include apoptosis and necrosis, leading to programmed or uncontrolled cell death and subsequently cardiovascular hypertrophy.
One of the mechanisms that initiates the cell death pathways is damage to nuclear DNA. Assessment of blood from trucking industry participants revealed that 48 [elemental carbon (EC)], 260 [organic carbon (OC)], and 49 (PM2.5) differentially regulated genes were associated with each of the exposures; these expression profiles implicate genes regulating apoptosis, DNA and metal binding, and chronic heart and lung disease pathways (29). Consistent with these findings, exposure to PM2.5-containing polycyclic aromatic hydrocarbons (PAHs) increased DNA damage and DNA damage response genes in a rodent model (181). These genes included 8-oxoguanine DNA glycosylase (OGG1) and growth arrest and DNA damage 153 (GADD153). PM exposure also altered genes involved in ROS regulation [glutathione S-transferase (GST) and SOD] (181). An increased expression of GST and decreased expression of SOD may promote a high-oxidative environment with reduced ability to scavenge ROS, which may help propagate the effects of DNA damage. NOX4 is a prominent contributor of oxidative stress in the failing heart and is associated with initiating increased DNA damage through this mechanism (85). PM2.5 exposure in mice enhances not only inflammatory markers, as previously reported (TNFα and IL-1β) but also myocardial apoptosis, NOX4, and NOX4-associated subunits, suggesting that the progression of cardiovascular disease may be dictated by ROS generation and the modification of antioxidant protein levels, which are initially regulated by PM2.5 exposure (168). Whereas unregulated levels of ROS may function through multiple pathways to cause cell death, programmed cell death through apoptosis has been linked to PM inhalation exposure through other channels. Hyperlipidemic rats exposed to PM2.5 are more prone to cardiac apoptosis (162), that is, mediated through c-Jun NH2-terminal kinase (JNK), P53 phosphorylation, and downstream activation of (Bcl-2-associated X) Bax and caspase-3 (162). The expression of both GST and JNK is modified following PM exposure, which implies the presence of an overlap among these pathways that mediate apoptosis, as previously suggested (53). Similar results are seen in other vulnerable models, such as mice that were challenged with myocardial infarction (94). PM inhalation exposure exacerbated left ventricular dysfunction and increased infarct size, with increased myocardial apoptosis through caspase-3, Bax, and B cell lymphoma 2 (Bcl-2). Moreover, PM exposure may not immediately stimulate cell death but rather result in reprogramming of the cell, making it susceptible to future insult. One-way reprogramming may be occurring is through changes in microRNAs, which are associated with PM2.5, but not UFP, in the blood of individuals residing in multiple urban regions (41). Specifically, PM2.5 is correlated with increased hsa-miR-197-3p and hsa-miR-99a-5p, which play roles in cell death and cancer (41, 175). PM2.5 inhalation exposure was also linked to microRNAs involved in cardiovascular function, including decreased expression of microRNA-133a, 145-5p, and 499a-5p in plasma (80). Overall, these studies outline some of the potential mechanisms that initiate myocardial apoptosis and eventually lead to cardiovascular abnormalities due to particulate exposure.
MITOCHONDRIAL BIOENERGETICS AND ULTRASTRUCTURE
In the cardiovascular system, slight perturbations to mitochondrial health could result in significant detriments and sustained pathology. The energy needed for the heart to contract is derived primarily through oxidative phosphorylation of mitochondrion (∼95%) (184). Therefore, alterations to mitochondrial function are implicated as a primary pathway for cardiovascular dysfunction and disease (112).
The integrity of mitochondrial DNA defines its respiratory potential, allowing for separation of individuals into a variety of haplogroups (133). Ambient air pollution has the potential to differentially affect individuals with diverse haplotype backgrounds (164). Haplogroup H (high ROS production) compared with haplogroup U (low ROS production) is more susceptible to quasi-ultrafine particle <0.25-µm exposure. Quasi-ultrafine particle exposure induced inflammation to a greater extent in haplogroup H than U, as demonstrated by increased IL-6 and TNFα (164). Furthermore, high doses of PM2.5 result in cardiac mitochondrial ultrastructure (size and cristae formation) changes in addition to increased inflammation (91). These ultrastructural changes are supported by increases in mitochondrial fission 1 (FIS1), MFN1, MFN2, dynamin-related protein 1 (DRP1), and mitochondrial dynamin like GTPase (OPA1), which suggest alterations to the fission/fusion pathway (91). UFP exposure has also been linked to mitochondrial dysfunction (66). Exposure to UFP exacerbated ischemia-reperfusion damage in the isolated hearts of rats. Isolated cardiac mitochondria of UFP-exposed rats displayed decreased Ca2+ buffering before the mitochondrial permeability transition pore (mPTP) opening, which indicates that exposure can increase mPTP Ca2+ sensitization and, therefore, mitochondrial permeability (66). The alterations to fission/fusion proteins (91) and mitochondrial permeability (66) showcase changes to the composition and functionality of mitochondrion following exposure, which can propagate oxidative damage and initiate mPTP-associated cell death pathways to instigate cardiac damage. A single dose of ENM through inhalation exposure in mice has the ability to trigger cardiac functional changes and mitochondrial dysregulation (111). However, a transgenic mouse model for mitochondrial phospholipid hydroperoxide glutathione peroxidase (mPHGPx) was used to restore cardiac function and mitochondrial respiratory function and abrogate ROS levels following nano-TiO2 exposure. By increasing antioxidant defense through mPHGPx overexpression, proteomics revealed that changes in mitochondrial composition, including individual electron transport chain complex constituents, were protected from the detrimental effects of exposure (111). This study further validates the importance of antioxidant protection in preservation of mitochondrial structure and function and, therefore, should be investigated as a potentially protective approach.
PM exposure can modify the transcriptional capacity of the mitochondrion and likewise incite dysfunction through this approach. Boilermakers exposed to PM2.5 were used in a study that aimed to elucidate the exposure-induced changes in methylation of mitochondrial DNA (18). Methylation of specific, mitochondrially transcribed genes involved in ATP synthesis, including mitochondrially encoded tRNA phenylalanine (MT‐TF) and mitochondrial 12s RNA (MT‐RNR1), were not associated with PM2.5 exposure. However, methylation of the mitochondrial promoter D-loop was associated with PM2.5 exposure. Participants with mitochondrial DNA hypermethylation were more vulnerable to the effects of PM2.5 exposure on HRV (18). Inhalation of toxicant particles may exert its effects on overall mitochondrial structure and function, and thus cardiovascular function, through ROS-regulated and inflammatory pathways, as the preceding studies indicate. Both increased inflammation and ROS have the ability to recruit methyltransferases, such as DNA methyltransferase 1 (DNMT1), and alter the methylome of various genes implicated in CVD, causing repression of the associated genes (79).
Although the molecular mechanisms behind cardiovascular impairment associated with particulate matter exposure vary based on the particle size, constituents, and physiochemical properties, there are common overlapping genes that appear to be modulated by multiple toxicants present in air pollution. Figure 2, created using ingenuity pathway analysis (IPA), provides an interaction network of genes that are dysregulated following particulate inhalation exposure. IPA included genes that were upregulated or downregulated or had diverse expression following more than one type of particulate exposure mentioned throughout this review. These genes can serve as potential early biomarkers of cardiovascular damage as a result of inhaled toxicant exposure.
Fig. 2.
Ingenuity pathway depicting the interaction network of genes that are dysregulated following particulate inhalation exposure. The genes included in the interaction network were associated with more than 1 type of particulate exposure. The interactions represent direct, validated experimental associations between genes that were increased (red), decreased (green), or found to have a diverse expression profile (yellow) following exposures. All abbreviations are defined in glossary.
GESTATIONAL EXPOSURES
There has been an increasing volume of literature investigating how particulate exposure can affect vulnerable populations, including women during pregnancy. Because of the coinciding metabolic changes prompted by both diabetes/obesity and air pollution, particularly related to oxidative stress and inflammation, understanding the dynamics of both pathologies is critical in determining how pollutant exposure can alter the metabolome (112). Particulate exposure during gestation is a major risk factor for gestational diabetes (101, 174). However, the effects of pollutant insult in utero on the offspring remain a critical gap in knowledge (11, 74, 96). This is particularly concerning for fine and ultrafine particles that have the ability to enter circulation and reach the fetal side of the human placenta (12). Recent studies indicate that maternal toxicant inhalation exposure during gestation is a predisposing factor for CVD in offspring.
OXIDATIVE STRESS AND INFLAMMATION
PM2 exposure results in activation of oxidative and inflammatory pathways, as has been described in the previous sections. Preconception toxicant exposure predisposes future offspring to the development of cardiac dysfunction (148). PM2.5 preconception exposure in both parents decreases ejection fraction, fractional shortening, and cardiomyocyte contractility in 3-mo-old progeny (148). Inflammatory and oxidative pathways are thereby activated, resulting in increased IL‐6, IL‐15, NF-κB, and CRP, along with elevated fibrosis (Col3a1), similar to the results found in many direct exposure studies. Notably, calcium-handling proteins SERCA2a and phosphorylated phospholamban (p‐PLN) are elevated in preconception PM2.5 exposed offspring, as increased p-PLN loses its ability to inhibit SERCA2a. Whereas PM-induced inflammation and oxidative stress are common in both direct and preconception exposures, SERCA2a is contrarily decreased in direct exposures (37, 165). One potential reason for this discrepancy may be that preconception exposure results in an adaptive response. Similar to direct and preconception exposure, PM2.5 exposure during gestation also results in cardiac remodeling with associated inflammation (increased IL‐6 and IL‐1β) (150). Furthermore, extracellular matrix remodeling, marked by increased collagen‐1, MMP9, and MMP13 expression, is also present in neonates following gestational exposure but was not assessed into adulthood. Nevertheless, long-term maternal exposure to PM2.5 throughout gestation and lactation enhanced activation of the inflammatory markers and myocardial apoptosis in both neonatal and weanling Sprague-Dawley rats (26). UFP exposure to pregnant C57BL/6J dams led to fetal reabsorption and increased blood pressure in adult progeny (104). Increases in maternal serum inflammation during gestation, along with AT1R and angiotensin I-converting enzyme (ACE) activation in the lungs, are suggested to contribute to progeny cardiovascular dysfunction both short and long term. Therefore, the notion that maternal and paternal exposure activates an inflammatory response in progeny from early life into adulthood is well established, but whether the changing maternal environment (104), particle translocation, or a combination of such factors is culpable in altering progeny development and metabolic function remains elusive.
In addition to inflammatory pathways, immune responses and oxidative stress pathways are changed in progeny who are exposed during gestation. Challenging progeny immune systems with house dust mite (HDM) allergen revealed a decreased immune response through lower IL-13 and IL-17, with higher serum levels of IL-10 following UFP exposure (134). This has also been demonstrated with UFP containing persistent free radicals (MCP230), which suppressed the T helper and T regulatory cells found within the lungs of offspring (161). Maternal ENM exposure during gestation may, however, modulate a regulatory axis involving enhanced hypoxia-inducible factor (HIF)-1α activity and decreased mPHGPx expression, which are critical in the regulation of ROS in fetal and young adult cardiac development (83). Understanding how gestational exposure, as opposed to direct exposure, affects individuals will contribute in the development of preventative measures for vulnerable populations, including pregnant women and children, and promote safer limits of exposure, thereby mitigating susceptibility to metabolic diseases and CVD in offspring.
CELLULAR FUNCTION
Calcium-regulated contraction is significantly affected throughout development in progeny exposed to particulate matter during gestation. Assessment of 14-day-old progeny whose dams were exposed to PM2.5 during gestation revealed decreased cardiomyocyte contractility through calcium dynamics, with changes to proteins involved in calcium flux in the heart, SERCA-2a, p-PLN, Na+/Ca2+ exchanger (NCX), calcium channel, voltage-dependent L type, and α1C subunit (CaV1.2) (149, 150). Offspring whose dams were exposed during gestation also present with major cardiac anomalies present into adulthood as a result of ENM-induced mitochondrial dysfunction. Nano-TiO2 gestational exposure decreased diastolic cardiac function, marked by a 15% increase in E-to-A ratio, and diminished mitochondrial respiration in young adult progeny (62). These results were simultaneous with mitochondrial proton leak and increased uncoupling protein 2 (UCP2) as well as upregulation of mitochondrial metabolism proteins, carnitine palmitoyltransferase 1A (CPT1A), and pyruvate dehydrogenase (PDH) phosphorylation. ENM exposure during gestation also affects mitochondrial bioenergetics by limiting state 3 respiration in the left ventricle of young adult progeny, coinciding with impaired endothelium-dependent dilation in coronary and uterine arterioles (140). PM2.5 gestational exposure can trigger myocardial apoptosis in a dose-dependent manner, as has been revealed in 1-day-old neonatal offspring from Wistar rats (159). Myocardial cell death likely occurred due to alterations in mitochondrial fission/fusion through OPA1, MFN1, DRP1, and FIS1 following PM2.5 exposure during gestation (159). Using functional genomics in addition to mitochondrial structural and bioenergetic assessment may provide insight into the mechanisms driving the deficits observed in progeny whose dams were exposed to particulate matter during gestation.
EPIGENETICS AND TRANSCRIPTIONAL REGULATION
Studies in progeny exposed to particulate exposure during a critical point in development, such as gestation, reveal that cellular regulatory networks are substantially altered, likely contributing to sustained detrimental effects into adulthood. These regulatory systems include epigenetic remodeling as well as noncoding RNA networks, which can act to reprogram the basal cellular function in progeny and cause predisposition to metabolic disorders that modulate analogous pathways. Maternal PM2.5 exposure during gestation can promote long-term cardiac remodeling and causes cardiac hypertrophy in young adult progeny, with an increase in both total histone acetylation and promoter acetylation of hypertrophy genes GATA4 and myocyte enhancer factor 2C (Mef2c) (169). This further suggests the ability of gestational PM exposure to induce global epigenetic remodeling through transcriptional activation of acetylated regions in offspring. Supporting these findings, multiple additional studies have reported a cardiac hypertrophic/fibrotic phenotype, cardiac functional changes, and epigenetic reprogramming in early development due to gestational PM exposure (148, 150, 169). Preconception exposure in both parents to PM2.5 resulted in progeny with decreased ejection fraction, fractional shortening, and cardiomyocyte contractility at 12 wk of age (148). Epigenetic assessments suggested changes that were indicative of reprogramming induced by the particulate exposure, with decreased expression of the maintenance methyltransferase DNMT1 but increased histone deacetylase sirtuin (SIRT) 1/2. This pattern of expression may be explained by the ability of SIRT to regulate DNMTs and alter their activity. Similarly to preconception exposure, PM2.5 exposure during gestation also impaired cardiac function of progeny at 12 wk of age, revealed by decreased fractional shortening and left ventricular posterior wall thickness and increased left ventricular systolic/diastolic diameters and collagen deposition in cardiac tissue (150). Epigenetic regulation likely contributed substantially to persistent cardiac detriments, as seen in the preceding study. In contrast with preconception PM exposure (148), gestational exposure decreased SIRT1/2 and increased DNMT1, DNMT3a, and DNMT3b expression (150). Although there are differences in expression profiles of these epigenetic regulators that likely stem from the time of exposure (preconception versus gestation), both are representative of profiles that can cause sustained biological detriments and/or susceptibility to pathological insult. ENM inhalation exposure during gestation causes similar changes to DNA methylation machinery (83). Maternal exposure to nano-TiO2 during gestation decreases cardiac output and increases left ventricular mass, with a subsequent decrease in fractional shortening in progeny. These functional alterations were linked to elevated levels of 5-methylcytosine (5-mC), a marker of global DNA methylation, DNMT1 protein expression, and HIF-1α activity (83). Along with DNA methylation, changes in the histone composition of DNA can contribute to modified gene expression profiles. Chromatin immunoprecipitation sequencing for histone 3 lysine 4/27 trimethyaltion (H3K4me3 and H3K27me3), coupled with the transcriptomic profiling of fetal cardiac tissue from dams exposed to ENM during gestation, has revealed significant changes to major pathways, including immune adaption and organismal growth (138). These authors suggest that cardiovascular outcomes at the young adult stage may actually be linked to liver and fetal development, as evidenced through IPA protein ontology. MicroRNAs have also been implicated in metabolic changes in offspring as a result of gestational particulate exposure (55). Diesel exhaust exposure in utero decreased methylation of microRNA133a-2 at the promoter region in isolated neonatal cardiomyocytes (55). As the progeny aged, introducing stress, such as transverse aortic constriction, enhanced microRNA-133a-2 and decreased protein tyrosine phosphatase receptor type F (PTPRF) and peptidase domain-containing associated with muscle regeneration 1 (PAMR1) expression, which mediates adult sensitivity to heart failure by inducing pathological hypertrophic signaling or preventing antihypertrophic signaling (55). Currently, longitudinal studies on the effects of maternal exposure to various pollutants on offspring cardiovascular function are limited. Figure 3 provides a summary of the factors involved in epigenetic regulation that have been discussed in this review, in the context of preconception/gestational particulate inhalation exposure. Functional genomics studies that combine transcriptomic, proteomic, and metabolomic data in cohorts presenting with particulate-induced cardiovascular alterations will provide a more thorough understanding of early epigenetic remodeling and its involvement in contributing to CVD predisposition later in life.
Fig. 3.
Epigenetic alterations observed following gestational and/or preconception particulate inhalation exposures. Gene expression of GATA4 and MEF2C is increased through promoter acetylation at H3K9. MicroRNA-133a-2 expression is repressed through elevated 5mC. DNA methyl transferases (DNMT1, DNMT3a, and DNMT3b) are all increased following exposure, along with increased expression of transcription factors (STAT3 and HIF-1α). H3K4me3 and H3K27me3 alterations are observed at promoter regions throughout the genome. The histone deacetylases SIRT1/2 reveal a dysregulated expression profile in progeny exposed during gestation. All abbreviations are defined in glossary.
FUTURE DIRECTIONS
From a morning commute, to an occupational setting, to our very homes, exposure to particulates remains a central issue for debate and therapeutic/lifestyle interventions. Industrialization and increased urbanization will continue to exacerbate issues concerning air quality and human health unless better-defined guidelines and limits are introduced. Although we provide a summary of the molecular mechanisms propagating cardiovascular decline following exposure in human and animal models, areas of research that are crucial in driving the field of molecular inhalation cardiovascular toxicology forward still remain uncharted. Future investigations should aim to address understudied populations.
There are limited studies investigating whether increased particulate matter exposure is associated with higher rates of cardiac arrest. One study that aimed to assess this association in New York City reported that a 10 µg/m3 increase in PM2.5 1 day before and on the day of the event significantly increased the relative risk for a cardiac arrest in the warm season (136). Similar results were seen in Melbourne (34), Copenhagen (163), Indianapolis, IN (132), and Houston, TX (40). Contrarily, a study done in Stockholm (129) and several studies by a group in Washington, DC (24, 88, 146), report no association between PM exposure and out-of-hospital cardiac arrests. Nonetheless, because of the high mortality rate associated with cardiac arrests, future studies should attempt to further clarify these associations through assessment of molecular markers.
There has been a growing body of literature exploring the adverse outcomes of PM exposure during gestation, including associations with metabolic dysregulation and cardiovascular deficits. In addition to a necessity for a better understanding of how particles induce specific changes during embryonic and fetal development, evaluation of the long-term effects is essential. This is particularly important for determining whether particulate exposure during gestation elicits alterations to the epigenome of offspring that can cause increased susceptibility to prevalent metabolic disorders, including diabetes mellitus (96). Notably, recent research has revealed the ability for prenatal and perinatal PM exposure to alter glucose metabolism (103) and elevate low-density lipoprotein cholesterol (LDL-C) (100), suggesting an increased risk of diabetes and atherosclerosis. However, the molecular mechanisms were not evaluated. To address these gaps in knowledge, focus must turn to the epigenetic effects following particle exposure during intrauterine and early childhood development concomitantly with metabolic assessments, which are currently meager (47, 74).
As we continue to shift the paradigm of cardiovascular research in inhalation toxicology, much of the cellular and molecular mechanisms are yet to be discerned. Cell culture models of exposure interactions can help provide a foundational understanding of cell and organelle function that cannot be fully captured through in vivo inhalation studies. Although it remains outside the scope of this review, in vitro models can provide a very granular and controlled approach to mechanistic evaluations. Of note, some authors have recently investigated the effects of PM2.5 exposure (19, 33, 44, 69, 172, 173, 179) as well as the toxicology of nanomaterials and advanced materials (45, 71, 72, 171) in cells. Additionally, in vitro studies can provide preliminary data for determining how complex and dynamic mixtures of particulate and gaseous substances affect cardiomyocytes, establishing potential molecular mechanisms and thus setting a path for future in vivo experiments (3, 63, 115).
Most of the current research is focused on ambient PM2.5 exposure, but with standards being set based on mass, UFP concentration is not accounted for thoroughly due to their low mass. However, the high surface area of UFP and ENM makes them considerably detrimental to health, potentially even more than fine and coarse PM (143). Because most ambient PM exposures are performed by collection and monitoring of PM in different regions, the problem of variable PM composition in these regions also arises. Integration of ENM exposure with UFP and PM multicomponent studies on both humans and mice will allow for a more thorough interpretation of the direct molecular mechanisms by which pollutants induce toxicity while also allowing the most relevant elucidation of overall health effects (143). Though coexposure studies are becoming more common in exploring the effects of present-day air pollution constituents on cardiac function in healthy and metabolically challenged systems (20, 43, 84, 114, 157), there is limited research assessing both cardiac function and the associated molecular mechanisms, leaving a critical gap in knowledge (3). This is one of the main limitations in many studies that could be addressed with the identification of altered candidate genes via functional genomic approaches.
A clear necessity exists for more encompassing “omics”-based approaches. Although investigators have begun to examine molecular modulators of the responses to inhalation exposure in the heart, including protein and gene markers for inflammation, few studies have examined the complete cellular protein, gene, or metabolic regulatory networks that coincide with the functional and structural changes. The integration of transcriptomics, epigenomics, proteomics, and other holistic approaches will provide a comprehensive understanding of cellular reprogramming, which can be impacted by the size, source, and duration of particle inhalation exposure (Fig. 4). The implementation of these approaches will outline early biomarkers induced by particulate exposure, ultimately encouraging exposure limits and standards that more precisely prevent the adverse effects of inhaled toxicants.
Fig. 4.
An overview of various contributing factors that can differentially alter the cardiovascular responses to toxicant inhalation exposure is provided. Current gaps in knowledge are outlined, including the lack of systemic integration of omics data across varying inhalation exposure paradigms.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-128485 (to J.M.H.), American Heart Association Grants AHA-20PRE35080170 (to A.K.) and AHA-17PRE33660333 (to Q.A.H.), and the Community Foundation for the Ohio Valley Whipkey Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K., Q.A.H., and J.M.H. conceived and designed research; A.K. and Q.A.H. prepared figures; A.K., Q.A.H., and M.V.P. drafted manuscript; A.K., Q.A.H., M.V.P., A.D.T., and J.M.H. edited and revised manuscript; A.K., Q.A.H., M.V.P., A.D.T., and J.M.H. approved final version of manuscript.
GLOSSARY
- 5-mC
5-Methylcytosine
- 7-KCh
7-Ketocholesterol
- ACE
Angiotensin I-converting enzyme
- Acta1
Actin α1 (ACTA1)
- AMPKα2−/−
AMP-activated protein kinase α2 knockout
- ANG II
Angiotensin II (ANG)
- ANP
Atrial natriuretic peptide (NPPA)
- ATIII
Antithrombin III (SERPINC1)
- AT1R
Angiotensin II type I receptor (ATGR1)
- Bax
Bcl-2-associated X protein
- BC
Black carbon
- Bcl-2
B cell lymphoma 2
- BNP
Brain natriuretic peptide (NPPB)
- β-MHC
β-myosin heavy chain
- C3
Complement factor 3
- Ca2+
Calcium
- CAP
Concentrated ambient particles
- CaV1.2
Calcium channel, voltage-dependent, L type, alpha 1C subunit
- CK
Creatine kinase
- CO
Carbon monoxide
- Col1a1
Collagen type I α1
- Col3a1
Collagen type III α1
- COX-2
Cyclooxygenase-2 (PTGS2)
- CPT1A
Carnitine palmitoyltransferase
- CREB
cAMP response element binding protein
- CRP
C-reactive protein
- CsA
Cyclosporin A
- cTnI
Cardiac troponin I
- cTnT
Cardiac troponin T
- CVD
Cardiovascular disease
- CXCL1
C-X-C motif chemokine ligand 1
- D2D
D-dimer
- DNMT
DNA methyltransferase
- DRP1
Dynamin-related protein 1
- EC
Elemental carbon
- EGFR
Epidermal growth factor receptor
- ENM
Engineered nanomaterials
- eNOS
Endothelial nitric oxide synthase
- EPC
Endothelial progenitor cells
- ER
Endoplasmic reticulum
- ET-1
Endothelin-1(EDN1)
- F3
Tissue factor III
- FBA/B/G
Fibrinogen genes
- FIS1
Fission 1
- GADD153
Growth arrest and DNA damage 153
- GATA
GATA sequence binding protein
- GRP78
Glucose-regulated protein 78
- GSH-Px
Glutathione peroxidase
- GSK3β
Glycogen synthase kinase-3β
- GST
Glutathione S-transferase
- GST-P
Glutathione s-transferase P
- GTSM1
Glutathione s-transferase mu 1
- H3K27me3
Histone 3 lysine 27 trimethyaltion
- H3K4me3
Histone 3 lysine 4 trimethyaltion
- HDL
High-density lipoprotein
- HDM
House dust mite
- HIF
Hypoxia inducible factor
- HO-1
Heme oxygenase-1(HMOX)
- HOI
HDL oxidative index
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- HRV
Heart rate variability
- HSP70
Heat-shock protein 70 kD
- ICAM-1
Intercellular adhesion molecule 1
- IFNγ
Interferon-γ (IFNG)
- IKK
Inhibitor of IκB kinase-2
- IL
Interleukin
- CXCL8
Interleukin 8
- iNOS
Inducible nitric oxide (NOS2)
- IP-10
IFNγ-induced protein 10
- IPA
Ingenuity Pathway Analysis
- Isop
F2-isoprostane
- JNK
C-Jun NH2-terminal kinase
- LDH
Lactate dehydrogenase (LDHB)
- LDL-C
Low-density lipoprotein cholesterol
- LINE-1
Long interspersed nuclear element-1
- MAPK
Mitogen activated protein kinase
- MCP-1
Monocyte chemoattractant protein 1
- MDA
Malondialdehyde
- Mef2c
Myocyte enhancer factor
- MeHg
Methylmercury
- Mfn1
Mitofusin 1
- MIP-1
Macrophage inflammatory protein
- MMP9
Matrix metalloproteinase
- mPHGPx
Mitochondrial phospholipid hydroperoxide glutathione peroxidase
- MPO
Myeloperoxidase
- mPTP
Mitochondrial permeability transition pore
- MT‐RNR1
Mitochondrial 12s RNA
- MT‐TF
Mitochondrially encoded tRNA phenylalanine
- NCX
Na+/Ca2+ exchanger
- NF-κB
Nuclear factor-κβ (NFKB1)
- NO2
Nitrogen dioxide
- NOX4
NADPH oxidase 4
- NRF2
Nuclear factor erythroid-derived 2-like 2
- OC
Organic carbon
- OGG1
8-oxoguanine DNA glycosylase
- OPA1
Mitochondrial dynamin like GTPase
- PAH
Polycyclic aromatic hydrocarbons
- PAI-1
Plasminogen activator inhibitor-1
- PAMR1
Peptidase domain containing associated with muscle regeneration 1
- Pb
Lead acetate
- PDH
Pyruvate dehydrogenase
- PF-4
Platelet factor 4
- PlGF
Placental growth factor
- PM
Particulate Matter
- p-PLN
Phosphorylated phospholamban
- PRDX5
Peroxiredoxin 5
- PRNP
Prion protein
- PTPRF
Protein tyrosine phosphatase receptor type F
- ROS
Reactive oxygen species
- RYR2
Ryanodine receptor 2
- SAA
Serum amyloid A
- SELP
P-selectin
- SERCA2
Sarco/endoplasmic reticulum Ca2+-ATPase (ATP2A2)
- SHR
Spontaneously hypertensive rats
- SiNP
Silica nanoparticles
- SIRT
Sirtuin
- So2
Sulfur dioxide
- So4
Sulfate
- SOD
Superoxide dismutase
- SOS1
SOS Ras/Rac Guanine Nucleotide Exchange Factor 1
- STAT
Signal transducer and activator of transcription
- TFPI
Tissue factor pathway inhibitor
- TGFβ1
Transforming growth factor beta 1
- TIMP3
Tissue inhibitor of metalloproteinase 3
- TiO2
Titanium dioxide
- TLR2
Toll-like receptor 2
- TLR4
Toll-like receptor 4
- TNFR2
Tumor necrosis factor receptor 2 (LTBR)
- TNFα
Tumor necrosis factor-α
- t-PA
Tissue-type plasminogen activator (PLAT)
- TRPA1
Transient receptor potential cation channel A1
- UCAP
Concentrated ambient ultrafine particles
- UCP2
Uncoupling protein 2
- UFCP
Ultrafine carbon particles
- UFP
Ultrafine particles
- VCAM-1
Vascular Cell Adhesion Protein 1
- VEGF
Vascular endothelial growth factor (VEGFA)
- vWF
Von Willebrand factor
REFERENCES
- 1.Alsaffar H, Martino N, Garrett JP, Adam AP. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am J Physiol Cell Physiol 314: C589–C602, 2018. doi: 10.1152/ajpcell.00235.2017. [DOI] [PubMed] [Google Scholar]
- 2.Apte JS, Brauer M, Cohen AJ, Ezzati M, Pope CA 3rd. Ambient PM2.5 reduces global and regional life expectancy. Environ Sci Technol Lett 5: 546–551, 2018. doi: 10.1021/acs.estlett.8b00360. [DOI] [Google Scholar]
- 3.Aragon MJ, Chrobak I, Brower J, Roldan L, Fredenburgh LE, McDonald JD, Campen MJ. Inflammatory and Vasoactive Effects of Serum Following Inhalation of Varied Complex Mixtures. Cardiovasc Toxicol 16: 163–171, 2016. doi: 10.1007/s12012-015-9325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aztatzi-Aguilar OG, Uribe-Ramírez M, Arias-Montaño JA, Barbier O, De Vizcaya-Ruiz A. Acute and subchronic exposure to air particulate matter induces expression of angiotensin and bradykinin-related genes in the lungs and heart: Angiotensin-II type-I receptor as a molecular target of particulate matter exposure. Part Fibre Toxicol 12: 17, 2015. doi: 10.1186/s12989-015-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basner M, Riggs DW, Conklin DJ. Environmental determinants of hypertension and diabetes mellitus: sounding off about the effects of noise. J Am Heart Assoc 9: e016048, 2020. doi: 10.1161/JAHA.120.016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellavia A, Urch B, Speck M, Brook RD, Scott JA, Albetti B, Behbod B, North M, Valeri L, Bertazzi PA, Silverman F, Gold D, Baccarelli AA. DNA hypomethylation, ambient particulate matter, and increased blood pressure: findings from controlled human exposure experiments. J Am Heart Assoc 2: e000212, 2013. doi: 10.1161/JAHA.113.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bierkandt FS, Leibrock L, Wagener S, Laux P, Luch A. The impact of nanomaterial characteristics on inhalation toxicity. Toxicol Res (Camb) 7: 321–346, 2018. doi: 10.1039/C7TX00242D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bind MA, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, Schwartz J. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology 23: 332–340, 2012. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bind MA, Coull BA, Peters A, Baccarelli AA, Tarantini L, Cantone L, Vokonas PS, Koutrakis P, Schwartz JD. Beyond the mean: quantile regression to explore the association of air pollution with gene-specific methylation in the Normative Aging Study. Environ Health Perspect 123: 759–765, 2015. doi: 10.1289/ehp.1307824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bind MA, Lepeule J, Zanobetti A, Gasparrini A, Baccarelli A, Coull BA, Tarantini L, Vokonas PS, Koutrakis P, Schwartz J. Air pollution and gene-specific methylation in the Normative Aging Study: association, effect modification, and mediation analysis. Epigenetics 9: 448–458, 2014. doi: 10.4161/epi.27584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bommarito PA, Martin E, Fry RC. Effects of prenatal exposure to endocrine disruptors and toxic metals on the fetal epigenome. Epigenomics 9: 333–350, 2017. doi: 10.2217/epi-2016-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, Van Eyken P, Plusquin M, Roeffaers MB, Ameloot M, Nawrot TS. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun 10: 3866, 2019. doi: 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowdridge EC, Abukabda AB, Engles KJ, McBride CR, Batchelor TP, Goldsmith WT, Garner KL, Friend S, Nurkiewicz TR. Maternal Engineered Nanomaterial Inhalation During Gestation Disrupts Vascular Kisspeptin Reactivity. Toxicol Sci 169: 524–533, 2019. doi: 10.1093/toxsci/kfz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brook RD, Cakmak S, Turner MC, Brook JR, Crouse DL, Peters PA, van Donkelaar A, Villeneuve PJ, Brion O, Jerrett M, Martin RV, Rajagopalan S, Goldberg MS, Pope CA III, Burnett RT. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 36: 3313–3320, 2013. doi: 10.2337/dc12-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism . Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 16.Brook RD, Xu X, Bard RL, Dvonch JT, Morishita M, Kaciroti N, Sun Q, Harkema J, Rajagopalan S. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ 448: 66–71, 2013. doi: 10.1016/j.scitotenv.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA 3rd, Apte JS, Brauer M, Cohen A, Weichenthal S, Coggins J, Di Q, Brunekreef B, Frostad J, Lim SS, Kan H, Walker KD, Thurston GD, Hayes RB, Lim CC, Turner MC, Jerrett M, Krewski D, Gapstur SM, Diver WR, Ostro B, Goldberg D, Crouse DL, Martin RV, Peters P, Pinault L, Tjepkema M, van Donkelaar A, Villeneuve PJ, Miller AB, Yin P, Zhou M, Wang L, Janssen NAH, Marra M, Atkinson RW, Tsang H, Quoc Thach T, Cannon JB, Allen RT, Hart JE, Laden F, Cesaroni G, Forastiere F, Weinmayr G, Jaensch A, Nagel G, Concin H, Spadaro JV. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA 115: 9592–9597, 2018. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byun HM, Colicino E, Trevisi L, Fan T, Christiani DC, Baccarelli AA. Effects of Air Pollution and Blood Mitochondrial DNA Methylation on Markers of Heart Rate Variability. J Am Heart Assoc 5: e003218, 2016. doi: 10.1161/JAHA.116.003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai C, Huang J, Lin Y, Miao W, Chen P, Chen X, Wang J, Chen M. Particulate matter 2.5 induced arrhythmogenesis mediated by TRPC3 in human induced pluripotent stem cell-derived cardiomyocytes. Arch Toxicol 93: 1009–1020, 2019. [Erratum in: Arch Toxicol 93: 2711, 2019.] doi: 10.1007/s00204-019-02403-y. [DOI] [PubMed] [Google Scholar]
- 20.Carll AP, Crespo SM, Filho MS, Zati DH, Coull BA, Diaz EA, Raimundo RD, Jaeger TNG, Ricci-Vitor AL, Papapostolou V, Lawrence JE, Garner DM, Perry BS, Harkema JR, Godleski JJ. Inhaled ambient-level traffic-derived particulates decrease cardiac vagal influence and baroreflexes and increase arrhythmia in a rat model of metabolic syndrome. Part Fibre Toxicol 14: 16, 2017. doi: 10.1186/s12989-017-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassee FR, Héroux ME, Gerlofs-Nijland ME, Kelly FJ. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal Toxicol 25: 802–812, 2013. doi: 10.3109/08958378.2013.850127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro R, Rivera I, Struys EA, Jansen EE, Ravasco P, Camilo ME, Blom HJ, Jakobs C, Tavares de Almeida I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem 49: 1292–1296, 2003. doi: 10.1373/49.8.1292. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services, 2020. [Google Scholar]
- 24.Checkoway H, Levy D, Sheppard L, Kaufman J, Koenig J, Siscovick D. A case-crossover analysis of fine particulate matter air pollution and out-of-hospital sudden cardiac arrest. Res Rep Health Eff Inst (99): 5–28, 2000. [PubMed] [Google Scholar]
- 25.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR, Copes R. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect 121: 804–810, 2013. doi: 10.1289/ehp.1205958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Chen X, Hong X, Liu C, Huang H, Wang Q, Chen S, Chen H, Yang K, Sun Q. Maternal exposure to ambient PM2.5 exaggerates fetal cardiovascular maldevelopment induced by homocysteine in rats. Environ Toxicol 32: 877–889, 2017. doi: 10.1002/tox.22287. [DOI] [PubMed] [Google Scholar]
- 27.Chin MT. Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart 101: 253–256, 2015. doi: 10.1136/heartjnl-2014-306379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflamm 2011: 1–30, 2011. doi: 10.4061/2011/367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu JH, Hart JE, Chhabra D, Garshick E, Raby BA, Laden F. Gene expression network analyses in response to air pollution exposures in the trucking industry. Environ Health 15: 101, 2016. doi: 10.1186/s12940-016-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389: 1907–1918, 2017. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croft DP, Cameron SJ, Morrell CN, Lowenstein CJ, Ling F, Zareba W, Hopke PK, Utell MJ, Thurston SW, Thevenet-Morrison K, Evans KA, Chalupa D, Rich DQ. Associations between ambient wood smoke and other particulate pollutants and biomarkers of systemic inflammation, coagulation and thrombosis in cardiac patients. Environ Res 154: 352–361, 2017. doi: 10.1016/j.envres.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai J, Chen W, Lin Y, Wang S, Guo X, Zhang QQ. Exposure to concentrated ambient fine particulate matter induces vascular endothelial dysfunction via miR-21. Int J Biol Sci 13: 868–877, 2017. doi: 10.7150/ijbs.19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai J, Sun C, Yao Z, Chen W, Yu L, Long M. Exposure to concentrated ambient fine particulate matter disrupts vascular endothelial cell barrier function via the IL-6/HIF-1α signaling pathway. FEBS Open Bio 6: 720–728, 2016. doi: 10.1002/2211-5463.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dennekamp M, Akram M, Abramson MJ, Tonkin A, Sim MR, Fridman M, Erbas B. Outdoor air pollution as a trigger for out-of-hospital cardiac arrests. Epidemiology 21: 494–500, 2010. doi: 10.1097/EDE.0b013e3181e093db. [DOI] [PubMed] [Google Scholar]
- 35.Devlin RB, Smith CB, Schmitt MT, Rappold AG, Hinderliter A, Graff D, Carraway MS. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol Sci 140: 61–72, 2014. doi: 10.1093/toxsci/kfu063. [DOI] [PubMed] [Google Scholar]
- 36.Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, MacNee W, Stone V. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol 2: 10, 2005. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong L, Sun W, Li F, Shi M, Meng X, Wang C, Meng M, Tang W, Liu H, Wang L, Song L. The harmful effects of acute PM2.5 exposure to the heart and a novel preventive and therapeutic function of CEOs. Sci Rep 9: 3495, 2019. doi: 10.1038/s41598-019-40204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du X, Jiang S, Bo L, Liu J, Zeng X, Xie Y, He Q, Ye X, Song W, Zhao J. Combined effects of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced cardiovascular injury in rats. Chemosphere 173: 14–21, 2017. doi: 10.1016/j.chemosphere.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 39.Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis 8: E8–E19, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ensor KB, Raun LH, Persse D. A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation 127: 1192–1199, 2013. doi: 10.1161/CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- 41.Espín-Pérez A, Krauskopf J, Chadeau-Hyam M, van Veldhoven K, Chung F, Cullinan P, Piepers J, van Herwijnen M, Kubesch N, Carrasco-Turigas G, Nieuwenhuijsen M, Vineis P, Kleinjans JC, de Kok TM. Short-term transcriptome and microRNAs responses to exposure to different air pollutants in two population studies. Environ Pollut 242, Pt A: 182–190, 2018. doi: 10.1016/j.envpol.2018.06.051. [DOI] [PubMed] [Google Scholar]
- 42.Fan T, Fang SC, Cavallari JM, Barnett IJ, Wang Z, Su L, Byun HM, Lin X, Baccarelli AA, Christiani DC. Heart rate variability and DNA methylation levels are altered after short-term metal fume exposure among occupational welders: a repeated-measures panel study. BMC Public Health 14: 1279, 2014. doi: 10.1186/1471-2458-14-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farraj AK, Walsh L, Haykal-Coates N, Malik F, McGee J, Winsett D, Duvall R, Kovalcik K, Cascio WE, Higuchi M, Hazari MS. Cardiac effects of seasonal ambient particulate matter and ozone co-exposure in rats. Part Fibre Toxicol 12: 12, 2015. doi: 10.1186/s12989-015-0087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng L, Yang X, Asweto CO, Wu J, Zhang Y, Hu H, Shi Y, Duan J, Sun Z. Genome-wide transcriptional analysis of cardiovascular-related genes and pathways induced by PM2.5 in human myocardial cells. Environ Sci Pollut Res Int 24: 11683–11693, 2017. doi: 10.1007/s11356-017-8773-3. [DOI] [PubMed] [Google Scholar]
- 45.Feng L, Yang X, Asweto CO, Wu J, Zhang Y, Hu H, Shi Y, Duan J, Sun Z. Low-dose combined exposure of nanoparticles and heavy metal compared with PM2.5 in human myocardial AC16 cells. Environ Sci Pollut Res Int 24: 27767–27777, 2017. doi: 10.1007/s11356-017-0228-3. [DOI] [PubMed] [Google Scholar]
- 46.Feng L, Yang X, Shi Y, Liang S, Zhao T, Duan J, Sun Z. Co-exposure subacute toxicity of silica nanoparticles and lead acetate on cardiovascular system. Int J Nanomedicine 13: 7819–7834, 2018. doi: 10.2147/IJN.S185259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrari L, Carugno M, Bollati V. Particulate matter exposure shapes DNA methylation through the lifespan. Clin Epigenetics 11: 129, 2019. doi: 10.1186/s13148-019-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finch J, Conklin DJ. Air pollution-induced vascular dysfunction: potential role of endothelin-1 (ET-1) system. Cardiovasc Toxicol 16: 260–275, 2016. doi: 10.1007/s12012-015-9334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finch J, Riggs DW, O’Toole TE, Pope CA 3rd, Bhatnagar A, Conklin DJ. Acute exposure to air pollution is associated with novel changes in blood levels of endothelin-1 and circulating angiogenic cells in young, healthy adults. AIMS Environ Sci 6: 265–276, 2019. doi: 10.3934/environsci.2019.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fong KC, Di Q, Kloog I, Laden F, Coull BA, Koutrakis P, Schwartz JD. Relative toxicities of major particulate matter constituents on birthweight in Massachusetts. Environ Epidemiol 3: e047, 2019. doi: 10.1097/EE9.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franck U, Odeh S, Wiedensohler A, Wehner B, Herbarth O. The effect of particle size on cardiovascular disorders–the smaller the worse. Sci Total Environ 409: 4217–4221, 2011. doi: 10.1016/j.scitotenv.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 52.Ganguly K, Ettehadieh D, Upadhyay S, Takenaka S, Adler T, Karg E, Krombach F, Kreyling WG, Schulz H, Schmid O, Stoeger T. Early pulmonary response is critical for extra-pulmonary carbon nanoparticle mediated effects: comparison of inhalation versus intra-arterial infusion exposures in mice. Part Fibre Toxicol 14: 19, 2017. doi: 10.1186/s12989-017-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh Dastidar S, Jagatheesan G, Haberzettl P, Shah J, Hill BG, Bhatnagar A, Conklin DJ. Glutathione S-transferase P deficiency induces glucose intolerance via JNK-dependent enhancement of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 315: E1005–E1018, 2018. doi: 10.1152/ajpendo.00345.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giles LV, Tebbutt SJ, Carlsten C, Koehle MS. The effect of low and high-intensity cycling in diesel exhaust on flow-mediated dilation, circulating NOx, endothelin-1 and blood pressure. PLoS One 13: e0192419, 2018. doi: 10.1371/journal.pone.0192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodson JM, Weldy CS, MacDonald JW, Liu Y, Bammler TK, Chien WM, Chin MT. In utero exposure to diesel exhaust particulates is associated with an altered cardiac transcriptional response to transverse aortic constriction and altered DNA methylation. FASEB J 31: 4935–4945, 2017. doi: 10.1096/fj.201700032R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon T, Reibman J. Cardiovascular toxicity of inhaled ambient particulate matter. Toxicol Sci 56: 2–4, 2000. doi: 10.1093/toxsci/56.1.2. [DOI] [PubMed] [Google Scholar]
- 57.Grimmer JA, Tanwar V, Youtz DJ, Adelstein JM, Baine SH, Carnes CA, Baer LA, Stanford KI, Wold LE. Exercise does not ameliorate cardiac dysfunction in obese mice exposed to fine particulate matter. Life Sci 239: 116885, 2019. doi: 10.1016/j.lfs.2019.116885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haberzettl P, Lee J, Duggineni D, McCracken J, Bolanowski D, O’Toole TE, Bhatnagar A, Conklin DJ. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ Health Perspect 120: 848–856, 2012. doi: 10.1289/ehp.1104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haberzettl P, McCracken JP, Bhatnagar A, Conklin DJ. Insulin sensitizers prevent fine particulate matter-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis. Am J Physiol Heart Circ Physiol 310: H1423–H1438, 2016. doi: 10.1152/ajpheart.00369.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haberzettl P, O’Toole TE, Bhatnagar A, Conklin DJ. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect 124: 1830–1839, 2016. doi: 10.1289/EHP212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hathaway QA, Durr AJ, Shepherd DL, Pinti MV, Brandebura AN, Nichols CE, Kunovac A, Goldsmith WT, Friend SA, Abukabda AB, Fink GK, Nurkiewicz TR, Hollander JM. miRNA-378a as a key regulator of cardiovascular health following engineered nanomaterial inhalation exposure. Nanotoxicology 13: 644–663, 2019. doi: 10.1080/17435390.2019.1570372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hathaway QA, Nichols CE, Shepherd DL, Stapleton PA, McLaughlin SL, Stricker JC, Rellick SL, Pinti MV, Abukabda AB, McBride CR, Yi J, Stine SM, Nurkiewicz TR, Hollander JM. Maternal-engineered nanomaterial exposure disrupts progeny cardiac function and bioenergetics. Am J Physiol Heart Circ Physiol 312: H446–H458, 2017. doi: 10.1152/ajpheart.00634.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helfenstein M, Miragoli M, Rohr S, Müller L, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Effects of combustion-derived ultrafine particles and manufactured nanoparticles on heart cells in vitro. Toxicology 253: 70–78, 2008. doi: 10.1016/j.tox.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Hendren CO, Mesnard X, Dröge J, Wiesner MR. Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ Sci Technol 45: 2562–2569, 2011. doi: 10.1021/es103300g. [DOI] [PubMed] [Google Scholar]
- 65.Højmann Larsen A, Frandsen A, Treiman M. Upregulation of the SERCA-type Ca2+ pump activity in response to endoplasmic reticulum stress in PC12 cells. BMC Biochem 2: 4, 2001. doi: 10.1186/1471-2091-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holland NA, Fraiser CR, Sloan RC 3rd, Devlin RB, Brown DA, Wingard CJ. Ultrafine particulate matter increases cardiac ischemia/reperfusion injury via mitochondrial permeability transition pore. Cardiovasc Toxicol 17: 441–450, 2017. doi: 10.1007/s12012-017-9402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong F, Wang L, Yu X, Zhou Y, Hong J, Sheng L. Toxicological effect of TiO2 nanoparticle-induced myocarditis in mice. Nanoscale Res Lett 10: 1029, 2015. doi: 10.1186/s11671-015-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hougaard KS, Campagnolo L, Chavatte-Palmer P, Tarrade A, Rousseau-Ralliard D, Valentino S, Park MV, de Jong WH, Wolterink G, Piersma AH, Ross BL, Hutchison GR, Hansen JS, Vogel U, Jackson P, Slama R, Pietroiusti A, Cassee FR. A perspective on the developmental toxicity of inhaled nanoparticles. Reprod Toxicol 56: 118–140, 2015. doi: 10.1016/j.reprotox.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 69.Hu H, Wu J, Li Q, Asweto C, Feng L, Yang X, Duan F, Duan J, Sun Z. Fine particulate matter induces vascular endothelial activation via IL-6 dependent JAK1/STAT3 signaling pathway. Toxicol Res (Camb) 5: 946–953, 2016. doi: 10.1039/C5TX00351B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang W, Zhu T, Pan X, Hu M, Lu SE, Lin Y, Wang T, Zhang Y, Tang X. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: interactions of systemic inflammation, overweight, and gender. Am J Epidemiol 176: 117–126, 2012. doi: 10.1093/aje/kwr511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huerta-García E, Ramos-Godinez MD, López-Saavedra A, Alfaro-Moreno E, Gómez-Crisóstomo NP, Colín-Val Z, Sánchez-Barrera H, López-Marure R. Internalization of titanium dioxide nanoparticles is mediated by actin-dependent reorganization and clathrin- and dynamin-mediated endocytosis in H9c2 rat cardiomyoblasts. Chem Res Toxicol 32: 578–588, 2019. doi: 10.1021/acs.chemrestox.8b00284. [DOI] [PubMed] [Google Scholar]
- 72.Huerta-García E, Zepeda-Quiroz I, Sánchez-Barrera H, Colín-Val Z, Alfaro-Moreno E, Ramos-Godinez MD, López-Marure R. Internalization of titanium dioxide nanoparticles is cytotoxic for H9c2 rat cardiomyoblasts. Molecules 23: 1955, 2018. doi: 10.3390/molecules23081955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Husain M, Wu D, Saber AT, Decan N, Jacobsen NR, Williams A, Yauk CL, Wallin H, Vogel U, Halappanavar S. Intratracheally instilled titanium dioxide nanoparticles translocate to heart and liver and activate complement cascade in the heart of C57BL/6 mice. Nanotoxicology 9: 1013–1022, 2015. doi: 10.3109/17435390.2014.996192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janssen BG, Madhloum N, Gyselaers W, Bijnens E, Clemente DB, Cox B, Hogervorst J, Luyten L, Martens DS, Peusens M, Plusquin M, Provost EB, Roels HA, Saenen ND, Tsamou M, Vriens A, Winckelmans E, Vrijens K, Nawrot TS. Cohort Profile: The ENVIRonmental influence ON early AGEing (ENVIRONAGE): a birth cohort study. Int J Epidemiol 46: 1387m, 2017. doi: 10.1093/ije/dyx033. [DOI] [PubMed] [Google Scholar]
- 75.Jin Y, Wu Z, Wang N, Duan S, Wu Y, Wang J, Wu W, Feng F. Association of EGF Receptor and NLRs signaling with Cardiac Inflammation and Fibrosis in Mice Exposed to Fine Particulate Matter. J Biochem Mol Toxicol 30: 429–437, 2016. doi: 10.1002/jbt.21806. [DOI] [PubMed] [Google Scholar]
- 76.Jørgensen RB, Buhagen M, Føreland S. Personal exposure to ultrafine particles from PVC welding and concrete work during tunnel rehabilitation. Occup Environ Med 73: 467–473, 2016. doi: 10.1136/oemed-2015-103411. [DOI] [PubMed] [Google Scholar]
- 77.Katsouyanni K. Ambient air pollution and health. Br Med Bull 68: 143–156, 2003. doi: 10.1093/bmb/ldg028. [DOI] [PubMed] [Google Scholar]
- 78.Kessler R. Engineered nanoparticles in consumer products: understanding a new ingredient. Environ Health Perspect 119: a120–a125, 2011. doi: 10.1289/ehp.119-a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kietzmann T, Petry A, Shvetsova A, Gerhold JM, Görlach A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br J Pharmacol 174: 1533–1554, 2017. doi: 10.1111/bph.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krauskopf J, Caiment F, van Veldhoven K, Chadeau-Hyam M, Sinharay R, Chung KF, Cullinan P, Collins P, Barratt B, Kelly FJ, Vermeulen R, Vineis P, de Kok TM, Kleinjans JC. The human circulating miRNome reflects multiple organ disease risks in association with short-term exposure to traffic-related air pollution. Environ Int 113: 26–34, 2018. doi: 10.1016/j.envint.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Kuhlbusch TA, Wijnhoven SW, Haase A. Nanomaterial exposures for worker, consumer and the general public. NanoImpact 10: 11–25, 2018. doi: 10.1016/j.impact.2017.11.003. [DOI] [Google Scholar]
- 82.Kumarathasan P, Vincent R, Blais E, Bielecki A, Guénette J, Filiatreault A, Brion O, Cakmak S, Thomson EM, Shutt R, Kauri LM, Mahmud M, Liu L, Dales R. Cardiovascular and inflammatory mechanisms in healthy humans exposed to air pollution in the vicinity of a steel mill. Part Fibre Toxicol 15: 34, 2018. doi: 10.1186/s12989-018-0270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kunovac A, Hathaway QA, Pinti MV, Goldsmith WT, Durr AJ, Fink GK, Nurkiewicz TR, Hollander JM. ROS promote epigenetic remodeling and cardiac dysfunction in offspring following maternal engineered nanomaterial (ENM) exposure. Part Fibre Toxicol 16: 24, 2019. doi: 10.1186/s12989-019-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurhanewicz N, McIntosh-Kastrinsky R, Tong H, Walsh L, Farraj AK, Hazari MS. Ozone co-exposure modifies cardiac responses to fine and ultrafine ambient particulate matter in mice: concordance of electrocardiogram and mechanical responses. Part Fibre Toxicol 11: 54, 2014. doi: 10.1186/s12989-014-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107: 15565–15570, 2010. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lane KJ, Levy JI, Scammell MK, Peters JL, Patton AP, Reisner E, Lowe L, Zamore W, Durant JL, Brugge D. Association of modeled long-term personal exposure to ultrafine particles with inflammatory and coagulation biomarkers. Environ Int 92-93: 173–182, 2016. doi: 10.1016/j.envint.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lelieveld J, Klingmüller K, Pozzer A, Pöschl U, Fnais M, Daiber A, Münzel T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 40: 1590–1596, 2019. doi: 10.1093/eurheartj/ehz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levy D, Sheppard L, Checkoway H, Kaufman J, Lumley T, Koenig J, Siscovick D. A case-crossover analysis of particulate matter air pollution and out-of-hospital primary cardiac arrest. Epidemiology 12: 193–199, 2001. doi: 10.1097/00001648-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 89.Li KL, Lin YC. PM2.5 induced cardiac hypertrophy via CREB/GSK3b/SOS1 pathway and metabolomics alterations. Oncotarget 9: 30748–30760, 2018. doi: 10.18632/oncotarget.25479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111: 455–460, 2003. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li R, Kou X, Geng H, Xie J, Tian J, Cai Z, Dong C. Mitochondrial damage: an important mechanism of ambient PM2.5 exposure-induced acute heart injury in rats. J Hazard Mater 287: 392–401, 2015. doi: 10.1016/j.jhazmat.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Li W, Dorans KS, Wilker EH, Rice MB, Ljungman PL, Schwartz JD, Coull BA, Koutrakis P, Gold DR, Keaney JF Jr, Vasan RS, Benjamin EJ, Mittleman MA. Short-Term exposure to ambient air pollution and biomarkers of systemic inflammation: The Framingham Heart Study. Arterioscler Thromb Vasc Biol 37: 1793–1800, 2017. doi: 10.1161/ATVBAHA.117.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li W, Dorans KS, Wilker EH, Rice MB, Ljungman PL, Schwartz JD, Coull BA, Koutrakis P, Gold DR, Keaney JF Jr, Vasan RS, Benjamin EJ, Mittleman MA. Short-term exposure to ambient air pollution and circulating biomarkers of endothelial cell activation: The Framingham Heart Study. Environ Res 171: 36–43, 2019. doi: 10.1016/j.envres.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X, Geng J, Chen Y, Chen F, Liu C, Xu Q, Zhao J, Hu J, Xie J, Xu B. Exposure to particulate matter induces cardiomyocytes apoptosis after myocardial infarction through NFκB activation. Biochem Biophys Res Commun 488: 224–231, 2017. doi: 10.1016/j.bbrc.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 95.Li Z, Li N, Guo C, Li X, Qian Y, Wu J, Yang Y, Wei Y. Genomic DNA methylation signatures in different tissues after ambient air particulate matter exposure. Ecotoxicol Environ Saf 179: 175–181, 2019. doi: 10.1016/j.ecoenv.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 96.Lim CC, Thurston GD. Air Pollution, Oxidative Stress, and Diabetes: a Life Course Epidemiologic Perspective. Curr Diab Rep 19: 58, 2019. doi: 10.1007/s11892-019-1181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu L, Urch B, Szyszkowicz M, Evans G, Speck M, Van Huang A, Leingartner K, Shutt RH, Pelletier G, Gold DR, Brook JR, Godri Pollitt K, Silverman FS. Metals and oxidative potential in urban particulate matter influence systemic inflammatory and neural biomarkers: A controlled exposure study. Environ Int 121: 1331–1340, 2018. doi: 10.1016/j.envint.2018.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Madrigano J, Baccarelli A, Mittleman MA, Wright RO, Sparrow D, Vokonas PS, Tarantini L, Schwartz J. Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ Health Perspect 119: 977–982, 2011. doi: 10.1289/ehp.1002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mai AS, Dos Santos AB, Beber LCC, Basso RDB, Sulzbacher LM, Goettems-Fiorin PB, Frizzo MN, Rhoden CR, Ludwig MS, Heck TG. Exercise training under exposure to low levels of fine particulate matter: effects on heart oxidative stress and extra-to-intracellular HSP70 Ratio. Oxid Med Cell Longev 2017: 1–13, 2017. doi: 10.1155/2017/9067875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McGuinn LA, Coull BA, Kloog I, Just AC, Tamayo-Ortiz M, Osorio-Yáñez C, Baccarelli AA, Wright RJ, Téllez-Rojo MM, Wright RO. Fine particulate matter exposure and lipid levels among children in Mexico city. Environ Epidemiol 4: e088, 2020. doi: 10.1097/EE9.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Melody SM, Ford JB, Wills K, Venn A, Johnston FH. Maternal exposure to fine particulate matter from a large coal mine fire is associated with gestational diabetes mellitus: A prospective cohort study. Environ Res 183: 108956, 2020. doi: 10.1016/j.envres.2019.108956. [DOI] [PubMed] [Google Scholar]
- 102.Miller MR, Newby DE. Air pollution and cardiovascular disease: car sick. Cardiovasc Res 116: 279–294, 2020. doi: 10.1093/cvr/cvz228. [DOI] [PubMed] [Google Scholar]
- 103.Moody EC, Cantoral A, Tamayo-Ortiz M, Pizano-Zárate ML, Schnaas L, Kloog I, Oken E, Coull B, Baccarelli A, Téllez-Rojo MM, Wright RO, Just AC. Association of prenatal and perinatal exposures to particulate matter with changes in hemoglobin A1c levels in children aged 4 to 6 years. JAMA Netw Open 2: e1917643, 2019. doi: 10.1001/jamanetworkopen.2019.17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morales-Rubio RA, Alvarado-Cruz I, Manzano-León N, Andrade-Oliva MD, Uribe-Ramirez M, Quintanilla-Vega B, Osornio-Vargas Á, De Vizcaya-Ruiz A. In utero exposure to ultrafine particles promotes placental stress-induced programming of renin-angiotensin system-related elements in the offspring results in altered blood pressure in adult mice. Part Fibre Toxicol 16: 7, 2019. doi: 10.1186/s12989-019-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mostafavi N, Vermeulen R, Ghantous A, Hoek G, Probst-Hensch N, Herceg Z, Tarallo S, Naccarati A, Kleinjans JCS, Imboden M, Jeong A, Morley D, Amaral AFS, van Nunen E, Gulliver J, Chadeau-Hyam M, Vineis P, Vlaanderen J. Acute changes in DNA methylation in relation to 24 h personal air pollution exposure measurements: A panel study in four European countries. Environ Int 120: 11–21, 2018. doi: 10.1016/j.envint.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 106.Musman J, Pons S, Barau C, Caccia C, Leoni V, Berdeaux A, Ghaleh B, Morin D. Regular treadmill exercise inhibits mitochondrial accumulation of cholesterol and oxysterols during myocardial ischemia-reperfusion in wild-type and ob/ob mice. Free Radic Biol Med 101: 317–324, 2016. doi: 10.1016/j.freeradbiomed.2016.10.496. [DOI] [PubMed] [Google Scholar]
- 107.Nature Nanotechnology Nanoplastic should be better understood. Nat Nanotechnol 14: 299, 2019. doi: 10.1038/s41565-019-0437-7. [DOI] [PubMed] [Google Scholar]
- 108.Nazarenko Y, Zhen H, Han T, Lioy PJ, Mainelis G. Nanomaterial inhalation exposure from nanotechnology-based cosmetic powders: a quantitative assessment. J Nanopart Res 14: 1229, 2012. doi: 10.1007/s11051-012-1229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. Thrombosis and systemic and cardiac oxidative stress and DNA damage induced by pulmonary exposure to diesel exhaust particles and the effect of nootkatone thereon. Am J Physiol Heart Circ Physiol 314: H917–H927, 2018. doi: 10.1152/ajpheart.00313.2017. [DOI] [PubMed] [Google Scholar]
- 110.Nemmar A, Holme JA, Rosas I, Schwarze PE, Alfaro-Moreno E. Recent advances in particulate matter and nanoparticle toxicology: a review of the in vivo and in vitro studies. BioMed Res Int 2013: 1–22, 2013. doi: 10.1155/2013/279371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nichols CE, Shepherd DL, Hathaway QA, Durr AJ, Thapa D, Abukabda A, Yi J, Nurkiewicz TR, Hollander JM. Reactive oxygen species damage drives cardiac and mitochondrial dysfunction following acute nano-titanium dioxide inhalation exposure. Nanotoxicology 12: 32–48, 2018. doi: 10.1080/17435390.2017.1416202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, Kovacic JC. Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: part 3 of a 3-part series. J Am Coll Cardiol 70: 230–251, 2017. doi: 10.1016/j.jacc.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med 64: 373–379, 2007. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Occelli F, Lanier C, Cuny D, Deram A, Dumont J, Amouyel P, Montaye M, Dauchet L, Dallongeville J, Genin M. Exposure to multiple air pollutants and the incidence of coronary heart disease: A fine-scale geographic analysis. Sci Total Environ 714: 136608, 2020. doi: 10.1016/j.scitotenv.2020.136608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Okayama Y, Kuwahara M, Suzuki AK, Tsubone H. Role of reactive oxygen species on diesel exhaust particle-induced cytotoxicity in rat cardiac myocytes. J Toxicol Environ Health A 69: 1699–1710, 2006. doi: 10.1080/15287390600631078. [DOI] [PubMed] [Google Scholar]
- 116.Organisation for Economic Co-operation and Development The Economic Consequences of Outdoor Air Pollution Paris: OECD Publishing, 2016. [Google Scholar]
- 117.Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care 33: 2196–2201, 2010. doi: 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pei Y, Jiang R, Zou Y, Wang Y, Zhang S, Wang G, Zhao J, Song W. Effects of fine particulate matter (PM2.5) on systemic oxidative stress and cardiac function in ApoE(−/−) Mice. Int J Environ Res Public Health 13: 484, 2016. doi: 10.3390/ijerph13050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peters A, Hampel R, Cyrys J, Breitner S, Geruschkat U, Kraus U, Zareba W, Schneider A. Elevated particle number concentrations induce immediate changes in heart rate variability: a panel study in individuals with impaired glucose metabolism or diabetes. Part Fibre Toxicol 12: 7, 2015. doi: 10.1186/s12989-015-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pietroiusti A, Stockmann-Juvala H, Lucaroni F, Savolainen K. Nanomaterial exposure, toxicity, and impact on human health. Wiley Interdiscip Rev Nanomed Nanobiotechnol 10: e1513, 2018. doi: 10.1002/wnan.1513. [DOI] [PubMed] [Google Scholar]
- 121.Pons S, Martin V, Portal L, Zini R, Morin D, Berdeaux A, Ghaleh B. Regular treadmill exercise restores cardioprotective signaling pathways in obese mice independently from improvement in associated co-morbidities. J Mol Cell Cardiol 54: 82–89, 2013. doi: 10.1016/j.yjmcc.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 122.Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 119: 1204–1214, 2016. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109: 71–77, 2004. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 124.Pope CA 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation 114: 2443–2448, 2006. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 125.Pope CA 3rd, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res 116: 108–115, 2015. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- 126.Qin G, Xia J, Zhang Y, Guo L, Chen R, Sang N. Ambient fine particulate matter exposure induces reversible cardiac dysfunction and fibrosis in juvenile and older female mice. Part Fibre Toxicol 15: 27, 2018. doi: 10.1186/s12989-018-0264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramanathan G, Yin F, Speck M, Tseng CH, Brook JR, Silverman F, Urch B, Brook RD, Araujo JA. Effects of urban fine particulate matter and ozone on HDL functionality. Part Fibre Toxicol 13: 26, 2016. doi: 10.1186/s12989-016-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena FA, Chen LC, Harkema JR, Sun Q, Rajagopalan S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ Res 115: 770–780, 2014. doi: 10.1161/CIRCRESAHA.115.304666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raza A, Bellander T, Bero-Bedada G, Dahlquist M, Hollenberg J, Jonsson M, Lind T, Rosenqvist M, Svensson L, Ljungman PL. Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm. Eur Heart J 35: 861–868, 2014. doi: 10.1093/eurheartj/eht489. [DOI] [PubMed] [Google Scholar]
- 130.Riggs DW, Zafar N, Krishnasamy S, Yeager R, Rai SN, Bhatnagar A, O’Toole TE. Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ Res 180: 108890, 2020. doi: 10.1016/j.envres.2019.108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol 43: 4227–4233, 2009. doi: 10.1021/es8032549. [DOI] [PubMed] [Google Scholar]
- 132.Rosenthal FS, Carney JP, Olinger ML. Out-of-hospital cardiac arrest and airborne fine particulate matter: a case-crossover analysis of emergency medical services data in Indianapolis, Indiana. Environ Health Perspect 116: 631–636, 2008. doi: 10.1289/ehp.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science 303: 223–226, 2004. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- 134.Rychlik KA, Secrest JR, Lau C, Pulczinski J, Zamora ML, Leal J, Langley R, Myatt LG, Raju M, Chang RC, Li Y, Golding MC, Rodrigues-Hoffmann A, Molina MJ, Zhang R, Johnson NM. In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proc Natl Acad Sci USA 116: 3443–3448, 2019. doi: 10.1073/pnas.1816103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sancini G, Farina F, Battaglia C, Cifola I, Mangano E, Mantecca P, Camatini M, Palestini P. Health risk assessment for air pollutants: alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). PLoS One 9: e109685, 2014. doi: 10.1371/journal.pone.0109685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Silverman RA, Ito K, Freese J, Kaufman BJ, De Claro D, Braun J, Prezant DJ. Association of ambient fine particles with out-of-hospital cardiac arrests in New York City. Am J Epidemiol 172: 917–923, 2010. doi: 10.1093/aje/kwq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol 52: 719–726, 2008. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 138.Stapleton PA, Hathaway QA, Nichols CE, Abukabda AB, Pinti MV, Shepherd DL, McBride CR, Yi J, Castranova VC, Hollander JM, Nurkiewicz TR. Maternal engineered nanomaterial inhalation during gestation alters the fetal transcriptome. Part Fibre Toxicol 15: 3, 2018. doi: 10.1186/s12989-017-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stapleton PA, Minarchick VC, McCawley M, Knuckles TL, Nurkiewicz TR. Xenobiotic particle exposure and microvascular endpoints: a call to arms. Microcirculation 19: 126–142, 2012. doi: 10.1111/j.1549-8719.2011.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stapleton PA, Nichols CE, Yi J, McBride CR, Minarchick VC, Shepherd DL, Hollander JM, Nurkiewicz TR. Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure. Nanotoxicology 9: 941–951, 2015. doi: 10.3109/17435390.2014.984251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stebounova LV, Morgan H, Grassian VH, Brenner S. Health and safety implications of occupational exposure to engineered nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol 4: 310–321, 2012. doi: 10.1002/wnan.174. [DOI] [PubMed] [Google Scholar]
- 142.Stewart JC, Chalupa DC, Devlin RB, Frasier LM, Huang LS, Little EL, Lee SM, Phipps RP, Pietropaoli AP, Taubman MB, Utell MJ, Frampton MW. Vascular effects of ultrafine particles in persons with type 2 diabetes. Environ Health Perspect 118: 1692–1698, 2010. doi: 10.1289/ehp.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stone V, Miller MR, Clift MJ, Elder A, Mills NL, Møller P, Schins RPF, Vogel U, Kreyling WG, Alstrup Jensen K, Kuhlbusch TA, Schwarze PE, Hoet P, Pietroiusti A, De Vizcaya-Ruiz A, Baeza-Squiban A, Teixeira JP, Tran CL, Cassee FR. Nanomaterials versus ambient ultrafine particles: an opportunity to exchange toxicology knowledge. Environ Health Perspect 125: 106002, 2017. doi: 10.1289/EHP424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stoyell-Conti FF, Irigoyen MC, Sartori M, Ribeiro AA, Dos Santos F, Machi JF, Figueroa DMT, Rodrigues B, De Angelis K. Aerobic training is better than resistance training on cardiac function and autonomic modulation in female ob/ob mice. Front Physiol 10: 1464, 2019. doi: 10.3389/fphys.2019.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sturm R. Local lung deposition of ultrafine particles in healthy adults: experimental results and theoretical predictions. Ann Transl Med 4: 420, 2016. doi: 10.21037/atm.2016.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sullivan J, Ishikawa N, Sheppard L, Siscovick D, Checkoway H, Kaufman J. Exposure to ambient fine particulate matter and primary cardiac arrest among persons with and without clinically recognized heart disease. Am J Epidemiol 157: 501–509, 2003. doi: 10.1093/aje/kwg015. [DOI] [PubMed] [Google Scholar]
- 147.Sun Q, Hong X, Wold LE. Cardiovascular effects of ambient particulate air pollution exposure. Circulation 121: 2755–2765, 2010. doi: 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tanwar V, Adelstein JM, Grimmer JA, Youtz DJ, Katapadi A, Sugar BP, Falvo MJ, Baer LA, Stanford KI, Wold LE. Preconception exposure to fine particulate matter leads to cardiac dysfunction in adult male offspring. J Am Heart Assoc 7: e010797, 2018. doi: 10.1161/JAHA.118.010797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tanwar V, Adelstein JM, Grimmer JA, Youtz DJ, Sugar BP, Wold LE. PM2.5 exposure in utero contributes to neonatal cardiac dysfunction in mice. Environ Pollut 230: 116–124, 2017. doi: 10.1016/j.envpol.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tanwar V, Gorr MW, Velten M, Eichenseer CM, Long VP 3rd, Bonilla IM, Shettigar V, Ziolo MT, Davis JP, Baine SH, Carnes CA, Wold LE. In utero particulate matter exposure produces heart failure, electrical remodeling, and epigenetic changes at adulthood. J Am Heart Assoc 6: e005796, 2017. doi: 10.1161/JAHA.117.005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tanwar V, Katapadi A, Adelstein JM, Grimmer JA, Wold LE. Cardiac pathophysiology in response to environmental stress: a current review. Curr Opin Physiol 1: 198–205, 2018. doi: 10.1016/j.cophys.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thompson LC, Walsh L, Martin BL, McGee J, Wood C, Kovalcik K, Pancras JP, Haykal-Coates N, Ledbetter AD, Davies D, Cascio WE, Higuchi M, Hazari MS, Farraj AK. Ambient particulate matter and acrolein co-exposure increases myocardial dyssynchrony in mice via TRPA1. Toxicol Sci 167: 559–572, 2019. doi: 10.1093/toxsci/kfy262. [DOI] [PubMed] [Google Scholar]
- 153.Tobaldini E, Bollati V, Prado M, Fiorelli EM, Pecis M, Bissolotti G, Albetti B, Cantone L, Favero C, Cogliati C, Carrer P, Baccarelli A, Bertazzi PA, Montano N. Acute particulate matter affects cardiovascular autonomic modulation and IFN-γ methylation in healthy volunteers. Environ Res 161: 97–103, 2018. doi: 10.1016/j.envres.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 154.Upadhyay S, Stoeger T, George L, Schladweiler MC, Kodavanti U, Ganguly K, Schulz H. Ultrafine carbon particle mediated cardiovascular impairment of aged spontaneously hypertensive rats. Part Fibre Toxicol 11: 36, 2014. doi: 10.1186/s12989-014-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Möhlenkamp S, Nonnemacher M, Jakobs H, Erbel R, Jöckel KH, Hoffmann B; Heinz Nixdorf Recall Investigator Group . Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med 72: 656–663, 2015. doi: 10.1136/oemed-2014-102800. [DOI] [PubMed] [Google Scholar]
- 156.Villarreal-Calderon R, Franco-Lira M, González-Maciel A, Reynoso-Robles R, Harritt L, Pérez-Guillé B, Ferreira-Azevedo L, Drecktrah D, Zhu H, Sun Q, Torres-Jardón R, Aragón-Flores M, Calderón-Garcidueñas A, Diaz P, Calderón-Garcidueñas L. Up-regulation of mRNA ventricular PRNP prion protein gene expression in air pollution highly exposed young urbanites: endoplasmic reticulum stress, glucose regulated protein 78, and nanosized particles. Int J Mol Sci 14: 23471–23491, 2013. doi: 10.3390/ijms141223471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wagner JG, Allen K, Yang HY, Nan B, Morishita M, Mukherjee B, Dvonch JT, Spino C, Fink GD, Rajagopalan S, Sun Q, Brook RD, Harkema JR. Cardiovascular depression in rats exposed to inhaled particulate matter and ozone: effects of diet-induced metabolic syndrome. Environ Health Perspect 122: 27–33, 2014. doi: 10.1289/ehp.1307085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang G, Zhen L, Lü P, Jiang R, Song W. [Effects of ozone and fine particulate matter (PM2.5) on rat cardiac autonomic nervous system and systemic inflammation]. Wei Sheng Yan Jiu 42: 554–560, 2013. [PubMed] [Google Scholar]
- 159.Wang H, Peng X, Cao F, Wang Y, Shi H, Lin S, Zhong W, Sun J. Cardiotoxicity and Mechanism of Particulate Matter 2.5 (PM2.5) Exposure in Offspring Rats During Pregnancy. Med Sci Monit 23: 3890–3896, 2017. doi: 10.12659/MSM.903006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang H, Shen X, Tian G, Shi X, Huang W, Wu Y, Sun L, Peng C, Liu S, Huang Y, Chen X, Zhang F, Chen Y, Ding W, Lu Z. AMPKα2 deficiency exacerbates long-term PM2.5 exposure-induced lung injury and cardiac dysfunction. Free Radic Biol Med 121: 202–214, 2018. doi: 10.1016/j.freeradbiomed.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 161.Wang P, You D, Saravia J, Shen H, Cormier SA. Maternal exposure to combustion generated PM inhibits pulmonary Th1 maturation and concomitantly enhances postnatal asthma development in offspring. Part Fibre Toxicol 10: 29, 2013. doi: 10.1186/1743-8977-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Q, Gan X, Li F, Chen Y, Fu W, Zhu X, Xu D, Long M, Xu D. PM2.5 Exposure Induces More Serious Apoptosis of Cardiomyocytes Mediated by Caspase3 through JNK/ P53 Pathway in Hyperlipidemic Rats. Int J Biol Sci 15: 24–33, 2019. doi: 10.7150/ijbs.28633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wichmann J, Folke F, Torp-Pedersen C, Lippert F, Ketzel M, Ellermann T, Loft S. Out-of-hospital cardiac arrests and outdoor air pollution exposure in Copenhagen, Denmark. PLoS One 8: e53684, 2013. doi: 10.1371/journal.pone.0053684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wittkopp S, Staimer N, Tjoa T, Gillen D, Daher N, Shafer M, Schauer JJ, Sioutas C, Delfino RJ. Mitochondrial genetic background modifies the relationship between traffic-related air pollution exposure and systemic biomarkers of inflammation. PLoS One 8: e64444, 2013. doi: 10.1371/journal.pone.0064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wold LE, Ying Z, Hutchinson KR, Velten M, Gorr MW, Velten C, Youtz DJ, Wang A, Lucchesi PA, Sun Q, Rajagopalan S. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circ Heart Fail 5: 452–461, 2012. doi: 10.1161/CIRCHEARTFAILURE.112.966580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.World Health Organization Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease. Geneva: World Health Organization, 2016. [Google Scholar]
- 167.World Health Organization Exposure to Ambient Air Pollution from Particulate Matter for 2016. Geneva: World Health Organization, 2018. [Google Scholar]
- 168.Wu F, Zhang J. The involvement of Nox4 in fine particulate matter exposure-induced cardiac injury in mice. J Toxicol Sci 43: 171–181, 2018. doi: 10.2131/jts.43.171. [DOI] [PubMed] [Google Scholar]
- 169.Wu X, Pan B, Liu L, Zhao W, Zhu J, Huang X, Tian J. In utero exposure to PM2.5 during gestation caused adult cardiac hypertrophy through histone acetylation modification. J Cell Biochem 120: 4375–4384, 2019. doi: 10.1002/jcb.27723. [DOI] [PubMed] [Google Scholar]
- 170.Xing YF, Xu YH, Shi MH, Lian YX. The impact of PM2.5 on the human respiratory system. J Thorac Dis 8: E69–E74, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang X, Feng L, Zhang Y, Hu H, Shi Y, Liang S, Zhao T, Cao L, Duan J, Sun Z. Co-exposure of silica nanoparticles and methylmercury induced cardiac toxicity in vitro and in vivo. Sci Total Environ 631-632: 811–821, 2018. doi: 10.1016/j.scitotenv.2018.03.107. [DOI] [PubMed] [Google Scholar]
- 172.Yang X, Feng L, Zhang Y, Hu H, Shi Y, Liang S, Zhao T, Fu Y, Duan J, Sun Z. Cytotoxicity induced by fine particulate matter (PM2.5) via mitochondria-mediated apoptosis pathway in human cardiomyocytes. Ecotoxicol Environ Saf 161: 198–207, 2018. doi: 10.1016/j.ecoenv.2018.05.092. [DOI] [PubMed] [Google Scholar]
- 173.Yang X, Feng L, Zhang Y, Shi Y, Liang S, Zhao T, Sun B, Duan J, Sun Z. Integrative analysis of methylome and transcriptome variation of identified cardiac disease-specific genes in human cardiomyocytes after PM2.5 exposure. Chemosphere 212: 915–926, 2018. doi: 10.1016/j.chemosphere.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 174.Ye B, Zhong C, Li Q, Xu S, Zhang Y, Zhang X, Chen X, Huang L, Wang H, Zhang Z, Huang J, Sun G, Xiong G, Yang X, Hao L, Yang N, Wei S. The association of ambient fine particulate matter exposure during pregnancy with blood glucose levels and gestational diabetes mellitus risk: a prospective cohort study in Wuhan, China. Am J Epidemiol kwaa056, 2020. doi: 10.1093/aje/kwaa056. [DOI] [PubMed] [Google Scholar]
- 175.Yoshimura A, Sawada K, Nakamura K, Kinose Y, Nakatsuka E, Kobayashi M, Miyamoto M, Ishida K, Matsumoto Y, Kodama M, Hashimoto K, Mabuchi S, Kimura T. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC Cancer 18: 1065, 2018. doi: 10.1186/s12885-018-4974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zeng X, Liu J, Du X, Zhang J, Pan K, Shan W, Xie Y, Song W, Zhao J. The protective effects of selenium supplementation on ambient PM2.5-induced cardiovascular injury in rats. Environ Sci Pollut Res Int 25: 22153–22162, 2018. doi: 10.1007/s11356-018-2292-8. [DOI] [PubMed] [Google Scholar]
- 177.Zhang G, Wang X, Bi X, Li C, Deng Y, Al-Hashimi AA, Luo X, Gillette TG, Austin RC, Wang Y, Wang ZV. GRP78 (glucose-regulated protein of 78 kDa) promotes cardiomyocyte growth through activation of GATA4 (GATA-binding protein 4). Hypertension 73: 390–398, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang X, Chen Y, Wei H, Qin Y, Hao Y, Zhu Y, Deng F, Guo X. Ultrafine carbon black attenuates the antihypertensive effect of captopril in spontaneously hypertensive rats. Inhal Toxicol 26: 853–860, 2014. doi: 10.3109/08958378.2014.965558. [DOI] [PubMed] [Google Scholar]
- 179.Zhang Y, Ji X, Ku T, Sang N. Inflammatory response and endothelial dysfunction in the hearts of mice co-exposed to SO2, NO2, and PM2.5. Environ Toxicol 31: 1996–2005, 2016. doi: 10.1002/tox.22200. [DOI] [PubMed] [Google Scholar]
- 180.Zhao J, Liu C, Bai Y, Wang TY, Kan H, Sun Q. IKK inhibition prevents PM2.5-exacerbated cardiac injury in mice with type 2 diabetes. J Environ Sci (China) 31: 98–103, 2015. doi: 10.1016/j.jes.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 181.Zhao L, Zhang L, Chen M, Dong C, Li R, Cai Z. Effects of ambient atmospheric PM2.5, 1-nitropyrene and 9-nitroanthracene on DNA damage and oxidative stress in hearts of rats. Cardiovasc Toxicol 19: 178–190, 2019. doi: 10.1007/s12012-018-9488-5. [DOI] [PubMed] [Google Scholar]
- 182.Zheng Q, Liu H, Zhang J, Chen D. The effect of ambient particle matters on hospital admissions for cardiac arrhythmia: a multi-city case-crossover study in China. Environ Health 17: 60, 2018. doi: 10.1186/s12940-018-0404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhong J, Colicino E, Lin X, Mehta A, Kloog I, Zanobetti A, Byun HM, Bind MA, Cantone L, Prada D, Tarantini L, Trevisi L, Sparrow D, Vokonas P, Schwartz J, Baccarelli AA. Cardiac autonomic dysfunction: particulate air pollution effects are modulated by epigenetic immunoregulation of Toll-like receptor 2 and dietary flavonoid intake. J Am Heart Assoc 4: e001423, 2015. doi: 10.1161/JAHA.114.001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest 128: 3716–3726, 2018. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]