Abstract
Prolonged sitting, which is known to impair peripheral vascular function, often occurs in spaces (e.g., offices) with mild hypercapnic atmospheres. However, the effects of prolonged sitting in hypercapnic conditions on vascular function are unknown. Therefore, the purpose of this study was to investigate the effects of prolonged sitting in mild hypercapnic conditions on vascular and autonomic function in humans. Twelve healthy young adults participated in two experimental visits that consisted of sitting for 2.5 h in a control condition [normal atmospheric conditions sitting (PSIT)] or a mild hypercapnic condition (HCAP; CO2 = 1,500 ppm). During each visit, heart rate variability (HRV), blood pressure (BP), pulse wave velocity (PWV), augmentation index (AIx), brachial and popliteal artery flow-mediated dilation (FMD), and near-infrared spectroscopy (NIRS) were assessed before and after prolonged sitting. Sitting significantly decreased AIx in both groups (P < 0.05). Brachial and popliteal FMD were reduced with sitting (P < 0.05), and the reduction in popliteal FMD was amplified by HCAP (P < 0.05). Baseline microvascular oxygenation was decreased following sitting in both groups (P < 0.05). However, microvascular reoxygenation upon cuff release was slower only in HCAP (P < 0.05). HRV, HR, BP, and PWV did not significantly change with sitting in either group (P > 0.05). We conclude that prolonged sitting attenuated both brachial and popliteal endothelial function and was associated with perturbed microcirculation. Additionally, mild hypercapnic conditions further impaired peripheral endothelial and microvascular function. Together, these findings suggest that prolonged sitting is accompanied by a host of deleterious effects on the vasculature, which are exacerbated by mild hypercapnia.
NEW & NOTEWORTHY The results of this study reveal that prolonged sitting attenuates endothelial function and microvascular function. Additionally, prolonged sitting with mild hypercapnia, which is similar to everyday environments, further exacerbates peripheral endothelial function and microvascular function.
Keywords: arterial stiffness, endothelial function, hypercapnia, microvascular oxygenation, prolonged sitting
INTRODUCTION
Approximately half of all adults in the United States currently do not meet the daily recommended level of physical activity (53). Physical inactivity and sedentary lifestyles have been shown to impair vascular function (60), and these lifestyles are believed to be responsible for 6% of worldwide cardiovascular disease (CVD) (49). Sedentary behaviors, specifically spending time in a seated position, have significantly increased in the United States (62) and are common among all age groups (19, 21, 35). Approximately 25% of Americans sit for more than 8 h a day (87). Sitting time has been shown to be an independent risk factor for CVD (18, 34, 40) and is associated with an increase in risk factors for metabolic disease (30, 66) and all-cause mortality (6). In contrast to other models of sedentary behavior, such as laying down, sitting is associated with distinctly different physiological mechanisms such as low shear rate, blood pooling, and bent artery system in the lower limbs (86). Prolonged sitting, previously identified as uninterrupted sitting for at least 1 h or longer, has been shown to impair endothelial function in the legs (20, 85, 94) and increase peripheral blood pressure (BP) (78, 79), and it may also be a significant contributor to the increased propensity of atherosclerotic development in the lower extremities (1, 45, 73).
Prolonged periods of sitting oftentimes occur during work and leisure activities in classrooms, offices, auditoriums, etc., which have previously been identified as hypercapnic environments or having elevated levels of atmospheric carbon dioxide (CO2). These densely populated and enclosed spaces tend to have a CO2 concentration of ∼1,500 ppm, i.e., four to five times the atmospheric CO2 concentration (32, 51, 95). Although hypercapnia is involved in local blood flow (BF) regulation (72), previous studies have also reported that acute exposure to elevated CO2 concentrations increased mean arterial pressure (MAP), heart rate (HR), cardiac output (CO), and sympathetic nerve activity (43, 80). This evidence suggests that exposure to mild hypercapnic environments can disturb the homeostatic regulation of the cardiovascular and sympathetic nervous system activity and, in turn, exacerbate the negative effects of prolonged sitting. However, despite previous research on the effects of sedentary behavior and sitting on cardiovascular health, the effects of prolonged sitting in a mild hypercapnic environment on cardiovascular and autonomic function remain unknown.
Therefore, the purpose of this study was to examine the potential additive effects of mild hypercapnia during prolonged sitting on vascular function, microvascular oxygenation, and autonomic nervous system activity. We hypothesized that mild hypercapnia during prolonged sitting will 1) increase the activity of the sympathetic nervous system, resulting in greater arterial stiffness and peripheral resistance (elevated MAP), and 2) further impair endothelial and microvascular function in comparison with sitting.
MATERIALS AND METHODS
Participants.
To complete this study, 12 healthy, young participants (n = 12, 6 men and 6 women) were recruited. All participants were between the ages of 19 and 35 yr old, nonsmokers, and free of any known medical condition, such as, but not limited to, CVD, dyslipidemia, kidney/renal disease, neuromuscular disease, cancer, obesity, or diabetes mellitus. Additional exclusion criteria included pregnant or nursing women and the use of hormonal contraceptives. Written, informed consent was obtained from all participants before the beginning of the study, and all experiments were examined in accordance with the protocol approved by the University of Nebraska Medical Center Institutional Review Board and carried out in accordance with the Declaration of Helsinki.
Study design.
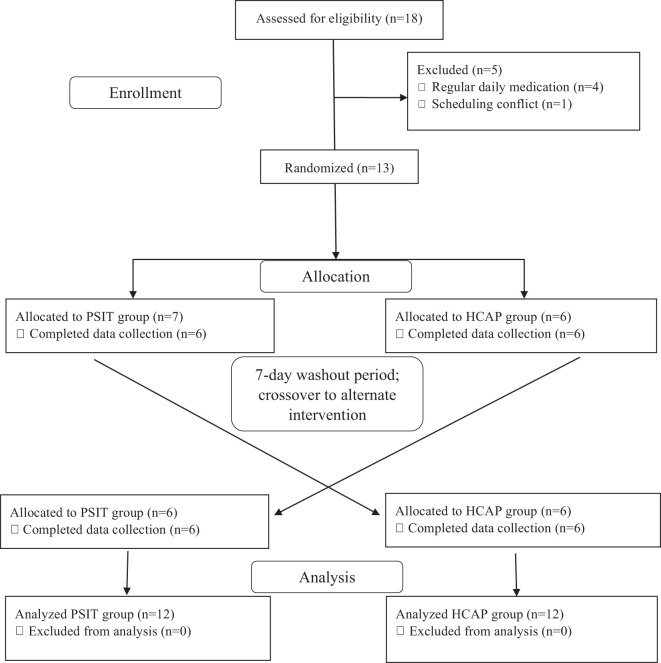
Each experimental visit was completed at ∼8 am (±1 h) after an overnight fast, and subjects were asked to abstain from excessive physical activity, caffeine, and alcohol for ≥24 h before each visit. Experimental visits were completed in a randomized crossover design, with ≥1 wk between each visit (Fig. 1). Participant characteristics such as HR, BP, hand grip strength, anthropometrics, and body composition were assessed at the beginning of each visit. Additionally, each participant completed a physical activity questionnaire that provided self-reported levels of weekly physical activity, daily step count from a fitness tracker, and estimated volume of maximal oxygen consumption (V̇o2max) from previously completed submaximal exercise testing (YMCA cycle ergometer test). Measurements of cardiovascular function, microvascular oxygenation, and autonomic nervous system activity were obtained before and after 2.5 h of sitting in both conditions. Following the assessment of baseline experimental measurements, each participant sat for 2.5 h in either a control ambient environment [normal atmospheric conditions sitting (PSIT)] or in a mild hypercapnic environment (HCAP) with a CO2 concentration of ∼1,500 ppm, i.e., four to five times the atmospheric CO2 concentration (32, 51, 95). Participants sat uninterrupted for 2.5 h to ensure that the induction of the effects of prolonged sitting, as previous studies have shown that the negative effects of prolonged sitting are induced after 1 h of sitting (85), but no further attenuation is observed when sitting for periods of time longer than 2 h (69). During sitting, the participant was instructed to move their lower body as little as possible while also restricting upper limb movement to reading, writing, or video entertainment, which induce minimal muscular contraction. Measurements of HR and BP and recordings of CO2 concentrations were obtained every 15 min during the prolonged sitting. All female participants (n = 6) were tested during the early- to mid-follicular (days 1–10) and late-luteal phases (>day 19) of the menstrual cycle to avoid confounding effects of endogenous estrogen on autonomic function (76).
Fig. 1.
Randomized crossover study design, study participant allocation, and analysis. HCAP, mild hypercapnic conditions sitting; PSIT, normal atmospheric conditions sitting.
Experimental conditions.
Following baseline measurements, the subject sat for 2.5 h in either control conditions or mild hypercapnic conditions. PSIT visits using a normal, ambient environment took place in the laboratory. Atmospheric conditions (temperature, humidity, and CO2 concentration) and participant movement, HR, and BP were monitored during sitting time. Temperature and humidity were monitored using a handheld weather meter (Kestrel Meters, Brooklyn, PA). HCAP visits took place in an environmentally controlled chamber (Darwin Chambers Company, St. Louis, MO), using natural respiration to generate a mild hypercapnic environment. The participant and multiple investigators entered and remained in the environmental chamber, allowing natural respiration to increase the CO2 concentration to the experimental value of 1,500 ppm. Temperature and humidity in the chamber were controlled at 22°C and 50% relative humidity (RH) respectively. During sitting, CO2 concentrations and participant movement were monitored. CO2 concentrations were monitored during both visits with a CO2 meter (Extech CO260 CO/CO2 Meter+; Psychrometer, Nashua, NH).
Anthropometric measures.
The height, to the nearest 1.0 cm, and weight, to the nearest 0.1 kg, of each participant were obtained using a standard stadiometer and scale (Detecto Physician’s Scale; DETECTO Webb City, MO). Body mass index (BMI) was calculated using the common formula body mass divided by the square of height (kg/m2). Body composition was measured using bioelectrical impedance analysis (Omron HBF-306C Handheld Scanner; OMRON Healthcare, Kyoto, Japan), which simultaneously records fat-free mass and body fat percentage (%BF).
Heart rate variability.
Autonomic nervous system function was assessed using a head-up tilt test and measurements of heart rate variability (HRV). Each participant was transferred into a supine position on the 9520 Manual Economy Tilt Table and fastened to the table for 20 min at 180° (supine). A 2-min recording of HR was obtained using a wearable HR monitor and Suunto Spartan Sport fitness tracker (Suunto, Vantaa, Finland). The participant was then tilted to a vertical position of 70° while remaining securely fastened to the tilt table. The participant remained in this vertical position for 20 min. After 20 min, a 2-min recording of HR was obtained. Raw RR interval data was extracted from HR recordings and analyzed via Kubios HRV Standard (Kubios Oy, Boston, MA) software (42).
Blood pressure.
Resting systolic blood pressure (SBP; mmHg) and diastolic blood pressure (DBP; mmHg) were measured using an automatic sphygmomanometer (Omron BP786N; Omron Corp., Kyoto, Japan). The measurements were performed in duplicate, and the average of the two measurements were recorded as the resting BP. The measurement procedures were repeated every 15 min during sitting time. Additionally, mean arterial pressure (MAP) was calculated from BP recordings using the common method: MAP = DBP + 1/3(SBP – DBP).
Pulse wave velocity and augmentation index.
Commercially available applanation tonometers (SphygmoCor XCEL; AtCor Medical Ltd., Sydney, NSW, Australia; and Complior Analyse; Alam Medical, Saint-Quentin-Fallavier, France) were used to measure carotid-to-radial pulse wave velocity (crPWV; m/s) and carotid-to-ankle PWV (cdPWV; m/s), indicators of peripheral arterial stiffness, and carotid-to-femoral PWV (cfPWV, m/s); an indicator of central arterial stiffness.
Augmentation index (AIx) and AIx adjusted to 75 beats/min (AIx@75) were also assessed (SphygmoCor XCEL; AtCor Medical Ltd.) using applanation tonometry, which is calculated by the pulse arterial wave reflection method using the ratio of the magnitude of the wave reflection and the central pulse pressure (41, 59).
Endothelial function.
Endothelial function of the brachial and popliteal artery was assessed before and after prolonged sitting using flow-mediated dilation (FMD). Baseline measurements of resting artery diameter were obtained for 5 min using an ultrasound imager. An occlusive was placed distal to the ultrasound probe over the brachial or popliteal arteries and inflated to 250 mmHg for 5 min to induce ischemic conditions. The cuff was then deflated, and an ultrasound image was measured to assess the endothelium-dependent vasodilatory response of the blood vessel for 3 min. The most stable 30–60 s of the baseline measurement were averaged and used as the resting brachial diameter, which included a minimum of 10 cardiac cycles (36). Images of the brachial and popliteal arteries were analyzed using an automated edge detection software, Vascular Research Tools 6 (Medical Imaging Applications, LLC, Coralville, IA).
Microvascular oxygenation.
Microvascular oxygenation was assessed using near-infrared spectroscopy (NIRS), which provides continuous, noninvasive measurements of oxygenated ([HbO2]), deoxygenated ([HHb]), and total ([Hbtot]) hemoglobin levels as well as a tissue oxygenation index (TOI; i.e., [HbO2]/[Hbtot]). Due to identical spectral characteristics, hemoglobin and myoglobin are not separated using NIRS. However, the signal is usually considered as being derived mainly from Hb (44). A portable NIRS device (Artinis, Eisteinweg, The Netherlands) was adhered to the participant’s skin over the soleus muscle of the dominant leg while the participant sat in an upright position. The NIRS probe consisted of a light source and a light detector separated by 30 mm. Light absorption was measured at different wavelengths (775, 810, 850, and 910 nm), and the whole set of signals was analyzed according to a modified Beer-Lambert’s law. Given that assumption about constant optical scattering of the photons has been shown to significantly affect changes in NIRS variables (27), we did not use a constant differential pathlength factor, and the data were displayed as relative changes with respect to baseline ([HHb]) or as percentage (TOI). Baseline measurements of tissue oxygen saturation were obtained. An occlusive cuff placed above the knee was inflated to 275 mmHg for 5 min. After 5 min the cuff was deflated, and measurements of tissue oxygen recovery were measured until the recorded values stabilized (74, 75), and the initial slope of the TOI recovery was calculated to estimate tissue reoxygenation, an index of microvascular function (8, 31). Measurements of tissue oxygen saturation were obtained before and after 2.5 h of sitting. The estimated baseline tissue oxygen (O2) consumption was calculated from a modified version of the method proposed by Ryan et al. (74) and van Beekvelt et al. (88). Specifically, upon cuff occlusion, the rate of decline in tissue oxygenation reflects tissue O2 use and was therefore used to estimate basal tissue O2 consumption before and after 2.5 h of sitting.
Statistical analysis.
The Shapiro-Wilk’s test was used to determine the normality of the data. Student’s t tests were used for group comparisons at baseline (PSIT and HCAP). A 2 × 2 analysis of variance with repeated measures [group (PSIT and HCAP) × time (before and after prolonged sitting)] was used to compare the difference in changes at pre- and post-CO2 exposure within and between groups (2 × 2 crossover design). When a significant interaction or main effect was noted, a paired t test was used for post hoc analysis. SPSS25.0 (SPSS, Inc., Chicago, IL) was used for all data analyses. Associations between variables were assessed with Pearson’s product-moment correlation coefficient. Statistical significance was set at a P value of <0.05. Additional analyses were completed to examine the differential responses of cardiovascular and autonomic function between sexes before and after prolonged sitting.
RESULTS
Participant characteristics.
The participant characteristics between the PSIT (age = 22.3 ± 2.0 yr, BMI = 23.9 ± 3.0 kg/m2, %BF = 16.5 ± 4.8%) and HCAP (age = 22.3 ± 2.0 yr, BMI = 23.9 ± 2.8 kg/m2, %BF = 17.1 ± 5.1%) visits were not significantly different (P > 0.05). According to the US Surgeon General’s guidelines, all participants were considered physically active. Each participant reported completing ≥30 min of moderate physical activity on most days of the week. The participants also reported an average daily step count of 11,995 ± 3,155 steps and an average estimated V̇o2max of 45.9 ± 9.3 mL·kg−1·min−1 (Table 1). Additionally, each participant completed each visit in full, reporting no adverse side effects to the prolonged sitting in either condition (Fig. 1). V̇o2max was positively correlated with resting brachial FMD (r = 0.494, P > 0.05) and resting popliteal FMD (r = 0.447, P > 0.05) and was weakly associated with changes in brachial FMD (r = −0.100, P > 0.05) and changes in popliteal FMD (r = −0.073, P > 0.05) following sitting. Participant lean body mass exhibited a positive correlation with resting brachial FMD (r = 0.654, P < 0.05) as well as a positive correlation with resting popliteal FMD (r = 0.305, P > 0.05). Participant lean body mass also exhibited a negative correlation with changes in brachial FMD (r = −0.393, P > 0.05) and was weakly associated with changes in popliteal FMD following sitting (r = −0.132, P > 0.05; Table 3).
Table 1.
Participant descriptive characteristics, physical activity, and cardiorespiratory fitness
| PSIT |
HCAP |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| n | 12 | 12 | ||
| Age, yr | 22.3 ± 2.0 | 22.3 ± 2.0 | ||
| Height, kg | 172.0 ± 4.7 | 171.4 ± 4.8 | ||
| Body mass, kg | 70.8 ± 9.8 | 70.6 ± 9.4 | ||
| Body fat, % | 16.5 ± 4.8 | 17.1 ± 5.1 | ||
| BMI, kg/m2 | 23.9 ± 3.0 | 23.9 ± 2.8 | ||
| HGS, right, kg | 37.2 ± 8.1 | 37.2 ± 8.1 | ||
| HGS, left, kg | 35.7 ± 6.8 | 35.7 ± 6.8 | ||
| Average daily steps | 11995.7 ± 3154.6 | 11995.7 ± 3154.6 | ||
| V̇o2max, mL·kg−1·min−1 | 45.9 ± 9.3 | 45.9 ± 9.3 | ||
| Heart rate, beats/min | 61.3 ± 10.3 | 61.4 ± 10.3 | 59.2 ± 9.6 | 60.4 ± 9.2 |
| SBP, mmHg | 113.2 ± 9.1 | 110.7 ± 11.0 | 112.5 ± 7.8 | 110.3 ± 12.7 |
| DBP, mmHg | 67.3 ± 4.2 | 70.1 ± 5.1 | 70.5 ± 6.8 | 68.8 ± 5.3 |
| MAP, mmHg | 82.6 ± 4.9 | 81.9 ± 5.6 | 84.5 ± 5.8 | 82.6 ± 7.3 |
Values are means ± SD; n = 12 (6 men and 6 women). PSIT, normal atmospheric conditions sitting; HCAP, mild hypercapnic environment; BMI, body mass index; DBP, diastolic blood pressure; HGS, hand grip strength; MAP, mean arterial pressure; SBP, systolic blood pressure; V̇o2max, volume of maximal oxygen consumption.
Table 3.
Pearson correlation coefficients for participant characteristics and brachial FMD and popliteal FMD (resting and changes following sitting)
| V̇o2max (mL·min−1·kg−1) | Lean body mass (kg) | |
|---|---|---|
| Resting brachial FMD (%) | r = 0.494 | r = 0.654* |
| Resting popliteal FMD (%) | r = 0.447 | r = 0.305 |
| ΔBrachial FMD (%) | r = −0.100 | r = −0.393 |
| ΔPopliteal FMD (%) | r = −0.073 | r = −0.132 |
n = 12. FMD, flow-mediated dilation. V̇o2max, volume of maximal oxygen consumption.
P < 0.05.
Heart rate variability.
Indices of sympathetic and parasympathetic activity, low-frequency (LF) and high-frequency (HF) power, respectively, were recorded and analyzed as values normalized to participant total power (84). No significant changes (P > 0.05) were observed in LF, HF, or LF/HF ratio (Table 2) between conditions or within groups pre- and postsitting.
Table 2.
Heart rate, total power, LF and HF power, and LF/HF ratio before and after prolonged sitting
| PSIT |
HCAP |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | Δ | Pre | Post | Δ | |
| Heart rate, beats/min | 61.30 ± 10.30 | 61.4 ± 10.30 | −1.62 ± 1.20 | 59.20 ± 9.60 | 60.40 ± 9.20 | 0.92 ± 1.42 |
| Total power, ms2 | 25382.27 ± 27,276.60 | 22362.11 ± 28,112.90 | −3020.16 ± 3473.25 | 47804.33 ± 74151.94 | 25333.55 ± 28746.67 | −22470.79 ± 24084.52 |
| LF power, NU | 73.92 ± 16.92 | 79.13 ± 13.09 | 5.21 ± 4.72 | 79.05 ± 18.91 | 70.94 ± 15.89 | −8.10 ± 8.03 |
| HF power, AU | 25.98 ± 16.82 | 20.82 ± 13.08 | −5.16 ± 4.70 | 20.39 ± 17.80 | 28.98 ± 15.87 | 8.59 ± 7.71 |
| LF/HF ratio, ms2 | 5.94 ± 5.24 | 6.44 ± 4.77 | 0.50 ± 1.73 | 8.12 ± 4.29 | 4.75 ± 4.72 | −3.36 ± 1.61 |
Values are means ± SE; n = 12. Pre and Post, before and after prolonged sitting, respectively; AU, arbitrary units; HCAP, mild hypercapnic conditions sitting; HF, high frequency; LF, low frequency; NU, normalized units; PSIT, normal atmospheric conditions sitting. LF and HF power are normalized to total power and are presented as NU.
Blood pressure and heart rate.
There was no significant difference in participant resting HR, SBP, DBP, or MAP between each experimental visit (P > 0.05). There was no significant change observed for HR, SBP, DBP, or MAP (P > 0.05) before and after sitting in either condition. Additionally, there was no significant change observed for HR (Fig. 2A), SBP (Fig. 2B), or DBP (Fig. 2C) (P > 0.05) at any time during prolonged sitting in either condition.
Fig. 2.
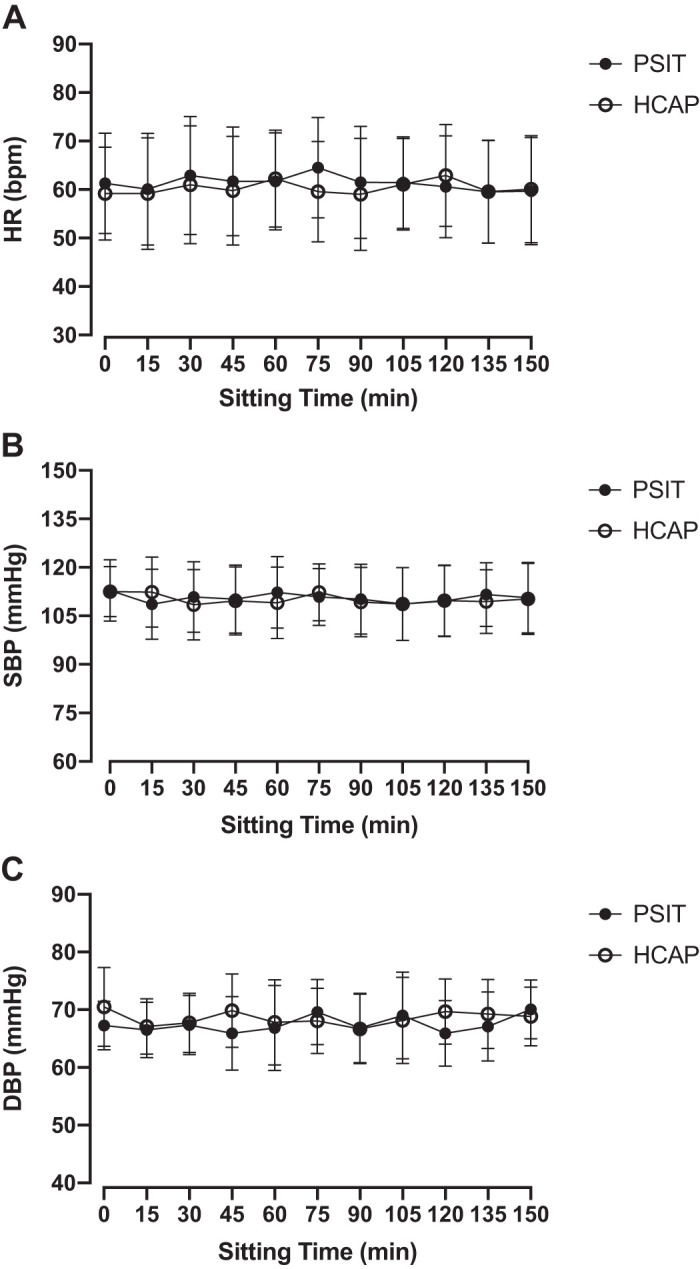
Heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were assessed every 15 min during prolonged sitting in normal ambient conditions (PSIT) and mild hypercapnic conditions (HCAP). A: HR during prolonged sitting did not significantly change in either condition (n = 12). B: SBP during prolonged sitting did not significantly change in either condition (n = 12). C: DBP during prolonged sitting did not significantly change in either condition (n = 12). Values are means ± SD.
Vascular function.
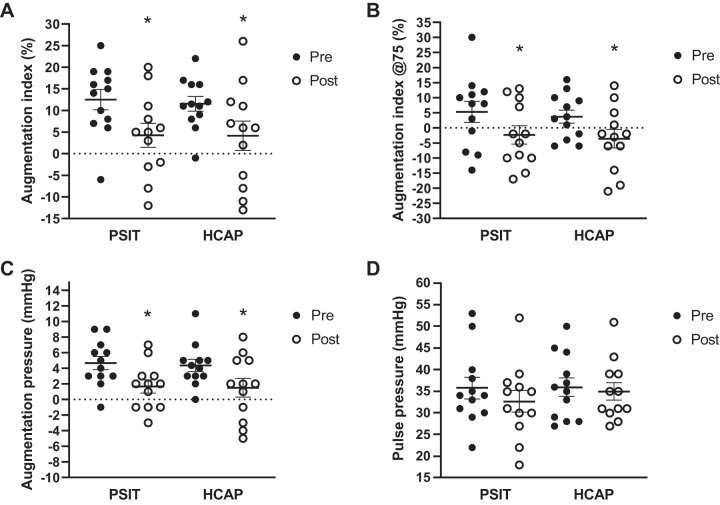
There were no significant changes observed in cfPWV, crPWV, cdPWV, or pulse pressure (PP) (P > 0.05; Fig. 3D) between conditions or within groups pre- and postsitting. There were significant reductions in AIx (Fig. 3A), AIx@75 (Fig. 3B), and augmentation pressure (AP) (Fig. 3C) pre- and postsitting (P < 0.05) in both groups. In the PSIT condition AIx decreased from 12.5 ± 2.2% to 4.25 ± 2.6%, and in the HCAP condition AIx decreased from 11.5 ± 1.6% to 4.2 ± 3.3%. In the PSIT condition AIx@75 decreased from 5.3 ± 3.3% to −2.3 ± 2.9%, and in the HCAP condition AIx@75 decreased from 11.5 ± 1.6% to −3.6 ± 3.0%. In the PSIT condition AP decreased from 4.7 ± 0.8 mmHg to 1.7 ± 0.8 mmHg, and in the HCAP condition AP decreased from 4.4 ± 0.8mmHg to 1.5 ± 1.1 mmHg. There was no significant effect of the hypercapnic condition (P > 0.05). The hypercapnic condition had no significant effect on AIx, AIx@75, or AP (P > 0.05).
Fig. 3.
Augmentation index (AIx), augmentation index adjusted to 75 beats/min (AIx@75), augmentation pressure (AP), and pulse pressure (PP) pre- (Pre) and postsitting (post) in normal ambient conditions (PSIT) and mild hypercapnic conditions (HCAP). A: AIx was significantly decreased following sitting; however, there was no significant difference between conditions (n = 12). B: AIx@75 was significantly decreased following sitting; however, there was no significant difference between conditions (n = 12). C: AP was significantly decreased following sitting; however, there was no significant difference between conditions (n = 12). D: PP was unchanged following sitting in either condition (n = 12). Values are means ± SE. *P < 0.05 vs. Pre.
Endothelial function.
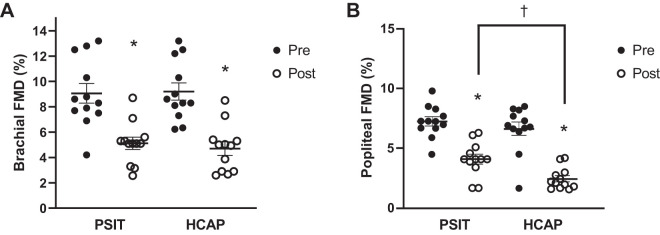
There was no significant difference in resting diameter of the brachial or popliteal arteries pre- or postsitting or between visits (P > 0.05). Following prolonged sitting there was a significant reduction in brachial artery FMD (P < 0.05) in both groups. In the PSIT condition brachial artery FMD decreased from 9.1 ± 0.7% to 5.1 ± 0.5%, and in the HCAP condition brachial artery FMD decreased from 9.2 ± 0.7% to 4.7 ± 0.5%. The reductions in brachial artery FMD were not significantly different between conditions (Fig. 4A). Following prolonged sitting there was a significant reduction in popliteal artery FMD (P < 0.05) in both groups. In the PSIT condition, popliteal artery FMD decreased from 7.2 ± 0.4% to 4.9 ± 0.4%, and in the HCAP condition popliteal artery FMD decreased from 6.6 ± 0.5% to 2.5 ± 0.2%. The reduction of popliteal artery FMD was significantly greater following prolonged sitting in a mild hypercapnic environment when compared with prolonged sitting in the normal ambient conditions (P < 0.05) (Fig. 4B).
Fig. 4.
Flow-mediated dilation (FMD; %) of the brachial and popliteal arteries pre- (Pre) and postsitting (Post) in normal ambient conditions (PSIT) and mild hypercapnic conditions (HCAP). A: %brachial artery FMD dilation significantly decreased in both conditions following sitting; however, there was no significant difference between conditions (n = 12). B: %popliteal artery FMD dilation significantly decreased in both conditions following sitting, and the decrease was significantly greater in the HCAP condition (n = 12). Values are means ± SE. *P < 0.05 vs. Pre; †P < 0.05 PSIT vs. HCAP.
Microvascular oxygenation.
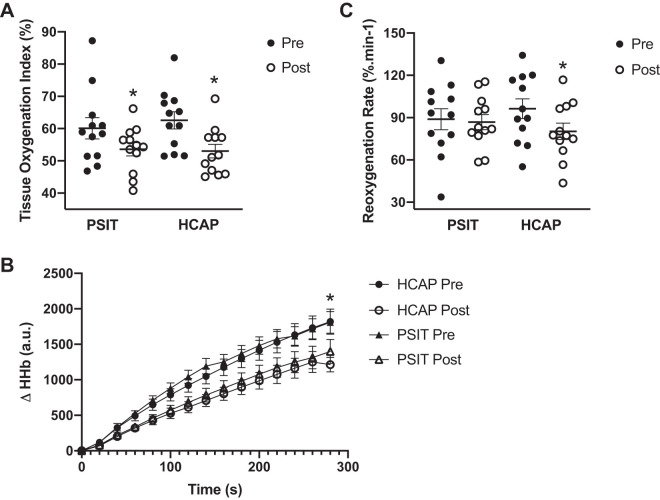
Baseline TOI was significantly lower following sitting in the PSIT and HCAP conditions, falling from 62.6 ± 9.2% to 53.0 ± 7.3% (P < 0.05) and 60.1 ± 11.5% to 53.6 ± 7.2% (P < 0.05), respectively, with no significant difference between conditions (Fig. 5A). Relative changes in [HHb] were significantly attenuated following sitting in the PSIT condition (pre-PSIT 1,018 ± 355 Δ[HHb] and post-PSIT 684 ± 271 Δ[HHb], P < 0.05) and the HCAP condition (pre-HCAP 1,023 ± 272 Δ[HHb] and post-HCAP 755 ± 307 Δ[HHb], P < 0.05), with no significant difference between conditions (Fig. 5B). Upon cuff release, the slope of TOI was significantly attenuated following sitting in the HCAP condition (pre-HCAP 95.3 ± 7.0%/min and post-HCAP 80.2 ± 5.8%/min, P < 0.05) but was unaffected in the PSIT condition (pre-PSIT 90.1 ± 7.5%/min and post-PSIT 86.8 ± 5.3%/min, P > 0.05) (Fig. 5C).
Fig. 5.
Measurements of microvascular and tissue oxygenation were completed before (Pre) and after (Post) prolonged sitting in normal ambient conditions (PSIT) and mild hypercapnic conditions (HCAP). A: following sitting, resting tissue oxygen index (TOI; %) was significantly decreased; however, there was no significant difference between conditions (n = 12). B: following sitting, changes in deoxygenated hemoglobin (Δ[HHb]) were significantly attenuated; however, there was no difference between conditions (n = 12). C: following sitting in mild hypercapnic conditions, there was a significant attenuation in the slope of TOI recovery (%/min); however, sitting in normal ambient conditions had no effect on the slope of TOI recovery (n = 12). Values are means ± SE. *P < 0.05 vs. Pre. AU, arbitrary units.
Sex differences.
Additional analyses were completed to compare the differences in responses to prolonged sitting in a normal ambient condition and a mild hypercapnic condition between sexes. There were no significant differences observed between male and female responses to prolonged sitting in either condition in HRV (P > 0.05), HR, SBP, DBP, MAP (P > 0.05), AIx, AIx@75, AP, or PP (P > 0.05), cfPWV, crPWV, or cdPWV (P > 0.05), brachial or popliteal FMD (P > 0.05), or microvascular oxygenation (P > 0.05). When the associations between variables within each sex were examined, no significant associations between variables were identified (see Supplemental Materials; available online at https://doi.org/10.6084/m9.figshare.12555080.v1).
DISCUSSION
Previous evidence suggests that prolonged sitting and exposure to hypercapnic environments may independently disturb the homeostatic regulation of the cardiovascular system. Accordingly, the purpose of this study was to examine the potential deleterious interactions between mild hypercapnia and prolonged sitting on autonomic nervous system activity, vascular function, and microvascular oxygenation. The main findings of the present study were that 1) the activity of the autonomic nervous system appeared unchanged by both interventions, as indicated by the unaltered LF and HF power and LF/HF ratio, which were associated with unaltered baseline HR, SBP, or DBP, 2) central and peripheral arterial stiffness was unchanged by prolonged sitting regardless of the CO2 levels, and 3) endothelial function (measured via FMD) and the microvasculature were significantly impaired following prolonged sitting, with hypercapnia worsening these effects. Together, these findings partially confirm our hypothesis that mild hypercapnia exerts an aggravating effect on endothelial and microvascular function during prolonged sitting in healthy individuals. Our findings may provide clinical insights into the negative effects of prolonged sitting with increased CO2 levels relevant to workspaces and classroom environments in our daily lives on cardiovascular and autonomic function.
Autonomic nervous system activity.
We found that the balance between the sympathetic and parasympathetic nervous system was unaltered by sitting or hypercapnia used in the present study, as indicated by the lack of significant changes in HR, BP, or HRV, measured as LF and HF power and LF/HF ratio (Table 2). Additionally, HR (Fig. 2A), SBP (Fig. 2B), and DBP (Fig. 2C) remained unchanged in either condition over time. These results may be due to the moderate level of hypercapnia used in the experimental condition, which reflects a CO2 concentration similar to everyday offices and classrooms. This concentration may be below the threshold to elicit an autonomic response in our healthy young participants.
When compared with the general US population (aged 20–39 yr old), our participants (average age ≈22 yr old) have a more favorable %BF of 16.8% compared with the US population average %BF of 27.5% (50). Activity questionnaires from our participants revealed that they exceed the recommended level of physical activity and live significantly more active lifestyles than the average American taking ∼12,000 steps/day compared with 4,000 steps/day (5). Additionally, average V̇o2max in these participants was 45.9 ± 9.3 mL·kg−1·min−1 (men: 50.5 ± 9.7 mL·kg−1·min−1; women: 40.32 ± 4.3 mL·kg−1·min−1). These values are considered “good” when compared with normal values for their age group and may play a significant role in cardiovascular regulation, as greater cardiorespiratory fitness is known to be strongly related to indices of autonomic regulation (77) and protects against CVD and all-cause mortality (4). Therefore, since our participants were all young, heathy, and fit, we believe that their active lifestyle and superior cardiorespiratory fitness may also positively affect their autonomic regulatory function, which may explain the insignificant effects of prolonged sitting and hypercapnic condition in either condition on BP, HR, and autonomic activity, as they are capable of efficiently regulating cardiovascular and nervous system homeostasis. Therefore, additional studies are warranted to determine whether populations with greater basal sympathetic activity (e.g., older adults, patients with hypertension or heart failure) are more susceptible to the effects of CO2.
Arterial stiffness and central vascular response to prolonged sitting.
In the present study, we observed no significant effects of prolonged sitting on central or peripheral PWV in either the PSIT or the HCAP condition. Arterial stiffness is characterized by vascular compliance and distensibility and is an important factor in vascular reactivity, BP, and vascular health (14). PWV has been suggested as a gold standard measurement of arterial stiffness for both small and larger arteries since the early 2000s (47, 97). To our knowledge, there is only a single previous study investigating the effects of prolonged sitting on arterial stiffness (PWV) and the central vascular (AIx) response. This previous study suggested that prolonged sitting increases arterial stiffness (23), reporting a significant increase in central arterial stiffness (cfPWV); however, the observed increase was an increase of ∼0.4 ± 0.03 m/s, which does not reach the clinically significant threshold of 1.0 m/s (91). We believe that an acute exposure to prolonged sitting may not produce a powerful enough effect to induce a clinically significant change in PWV or arterial stiffness, which represents a measurement of an arterial vessel’s structural elasticity that tends to change progressively over time (13, 48). However, longer exposure to sitting or repeated exposure to prolonged sitting over a longer period of time may change those structural factors and contribute to increases in arterial stiffness (3, 38), which has been shown to increase the likelihood of the development of CVD (61, 92).
Unlike PWV, a significant reduction in AIx (Fig. 3A) and AIx@75 (Fig. 3B) was observed following prolonged sitting independently from the level of CO2. AIx is a measurement of central vascular health, which is calculated with pulse arterial wave reflections (41). Pulse wave reflections are affected by both structural and hemodynamic factors, including vascular impedance, aortic length, and ejection dynamics (98). In the present study, a significant reduction in AIx from 12.5 ± 2.2% to 4.25 ± 2.6% and 11.5 ± 1.6% to 4.2 ± 3.3% was observed following sitting in the PSIT and HCAP conditions, respectively. Consistent with our findings, Credeur et al. (23) reported that, following 3 h of uninterrupted sitting, AIx was significantly reduced from 13 ± 3% to 3 ± 3% in healthy young adults. These results are consistent with other studies that assess the AIx response to orthostatic stressors, such as a head-up tilt test (37, 82). Similarly, to the seated position, an acute prolonged upright position leads to increased venous pooling in the lower extremities (81) and reduced stroke volume (83), which subsequently blunts pulse wave reflection (82) and reduces AIx. We also found that the AP was significantly reduced (Fig. 3C) and PP unchanged (Fig. 3D) following acute exposure to prolonged sitting, which together supports the observed reduction in AIx. AP is the difference between the initial inflection point of a pulse waveform and the maximum pressure of that waveform. Changes in the magnitude of the wave reflection of a pulse waveform are represented proportionally by changes in AP, and AP and PP are the primary components for calculating AIx [AIx = (AP/PP)] (41, 59). These results indicate that the reduced AIx is due mainly to the increased venous pooling in the lower extremities, which alters central hemodynamics and may contribute to the reduced shear stress in the vasculature and endothelial-mediated dilation observed following prolonged sitting.
Endothelial function.
Unique to our study, we found that brachial endothelial function was significantly reduced (Fig. 4A) following prolonged sitting, whereas the mild hypercapnic environment had no additional effects on brachial endothelial function. It has been suggested that the carotid and brachial arteries are less vulnerable to endothelial dysfunction (10) and the development of CVD (45) when compared with peripheral vasculature such as arteries in the lower extremities. Previous research also indicates that brachial artery FMD is largely unaffected by prolonged physical inactivity (10) and by acute exposure to prolonged sitting (20, 69, 93). Such discrepancy may be in part due to our research protocols, which limited upper limb movement to reading, writing, or video entertainment, which induce minimal muscular contraction. Additionally, these sedentary activities set the arm position below the level of the heart. This may have resulted in a similar hydrostatic effect observed by Padilla et al. (64), who found that an arm-hanging protocol meant to mimic the lower-limb effects of prolonged sitting produced similar hydrostatic forces and reduced endothelial function in the brachial artery (64). Additionally, these results may be explained in part by increased venous pooling in the lower limbs (69, 93), which would result in reduced cardiac output due to reduced venous return and subsequently decreased BF in the brachial artery, which has been previously observed following prolonged sitting (69). Although brachial artery BF and shear rate were not directly measured in this study, the observed alteration in central hemodynamics, reduced AIx and AP, supports the notion of reduced BF and subsequently shear rate in the brachial artery due to decreased venous return and reduced stroke volume and cardiac output. This may explain the reduced brachial endothelial function observed, as reduced BF and shear rate have been identified as contributing factors to the reduced popliteal endothelial function associated with prolonged sitting (70).
In the present study, popliteal artery endothelial function measured by FMD was significantly attenuated following an acute exposure to prolonged sitting, resulting in decreased basal tissue oxygenation (Fig. 5A) assessed by NIRS, whereas the decrease in popliteal endothelial function induced by sitting was exaggerated in the HCAP condition (Fig. 4B) but had no additional effect on basal tissue oxygenation. Previous studies suggest that attenuated endothelial function in the popliteal artery following prolonged sitting may be mediated by a reduction in shear stress (70). BF and shear stress are recognized as important stimuli for normal endothelial function (15, 17), and reduced BF and shear stress have been identified as major mediators for reduced endothelial function in lower-limb arteries following prolonged sitting (20, 54, 58, 70). Additionally, shear stress regulates vascular tone through the production of endothelial derived vasoactive molecules such as nitric oxide (NO) and endothelin-1 (ET-1) (17). Unidirectional laminar shear regulates vascular tone by stimulating mechanoreceptors on the endothelial layer (33), which then signal for alterations in the production and release of endothelial-derived NO (24, 52, 55, 56) by activating endothelial NO synthase (eNOS) in the vasculature that induces vasorelaxation.
A reduction in BF and shear stress results in reduced NO synthesis activity and a subsequent increase in ET-1 activity (16, 86). The reduced NO bioavailability and subsequent NO and ET-1 imbalance is one of the feasible explanations for the vascular impairment observed in response to prolonged sitting (63), since previous studies demonstrated that shear stress-mediated reduction of ET-1 production is NO dependent (46). Additionally, it is also noteworthy that reduced endothelial function is associated with reduced O2 transport capacity (99). O2 is required for endothelial-derived NO production and a reduction in resting TOI (Fig. 5A), which suggests a reduction in microvascular oxygenation that may be associated with imbalance between endothelial mediated NO bioavailability and ET-1. Clinical studies also suggested that impaired endothelial function (26, 65) often presents with an increase in ET-1 (9, 12, 71) that is associated with the development of atherosclerotic CVD. Together, this evidence suggests that prolonged sitting promotes a proatherogenic endothelial cell phenotype through the reduced shear stress and ET-1-dependent mechanism. However, unfortunately, we have not directly assessed shear rate and blood ET-1 levels following prolonged sitting, and therefore, this proposed mechanism warrants further investigation.
The effects of substantial increases in blood CO2 concentration on oxygen saturation and toxicity have been well documented; however, research investigating the physiological responses to mild hypercapnic environments is very limited. To our knowledge, this is the first study to investigate the effects of prolonged sitting in a mild hypercapnic environment. We found that the mild hypercapnic environment exacerbates attenuated popliteal artery endothelial function when compared with prolonged sitting in normal ambient conditions. Although a bit speculative, this finding may be explained by further reduced shear stress, as indirectly suggested by the documentation of a slower reoxygenation rate following occlusion (Fig. 5C). Additionally, mild hypercapnic conditions may potentially increase sympathetic output over parasympathetic output that may increase vascular resistance during prolonged sitting (89). Previous research has shown that hypercapnic conditions induced through CO2 rebreathing led to increases in BP and vascular resistance (22) through chemoreceptor-mediated increases in sympathetic outflow (57), as increased sympathetic tone is known to increase BP variability by increasing peripheral vascular resistance (39, 90). Additionally, obstructive sleep apnea is associated with an increase in arterial CO2 content that contributes to increased sympathetic output associated with sleep apnea (22) and is suggested to be a major contributing factor to the increased likelihood of developing CVD observed in this population as well (28). However, we did not directly measure hypercapnia-mediated alterations in sympathetic output in the present study, and further investigation is warranted using muscle sympathetic nerve activity (MSNA) measurements to identify the effect of prolonged sitting with the hypercapnic condition on the sympathetic activity and endothelial function.
It is also important to note that following sitting there was a significant attenuation in the calculated slope of TOI recovery in the HCAP condition but not in the PSIT condition (Fig. 5C). The initial slope of the TOI recovery is an estimate of tissue reoxygenation, an index of microvascular function (8, 31). Therefore, these results indicate that sitting in mild hypercapnic conditions may further attenuate microvascular function (Fig. 5C). Reduced blood flow and the subsequent reduction in O2 delivery to skeletal muscle has been identified as a key player in functional decline associated with aging and heart failure (67, 96). These results confirm the role of hypercapnic condition in further exacerbating microvascular dysfunction induced by sitting.
O2 supply and demand.
To our knowledge, we are the first group to investigate the effects of prolonged sitting and prolonged sitting in a mild hypercapnic environment on microvascular oxygenation measurements in skeletal muscle in the lower extremities. Microvascular oxygenation and O2 use were assessed using NIRS, which is a noninvasive method of determining tissue [HbO2], [HHb], and [Hbtot] as well as TOI (7). TOI represents the balance between O2 delivery and utilization, which is affected by the balance between blood flow to the tissue and tissue metabolic activity (25). Following sitting, basal TOI was significantly reduced (Fig. 5A), suggesting an imbalance between O2 supply and use, which may be the consequence of the attenuated popliteal artery endothelial function (Fig. 4B). This finding is particularly intriguing, as it suggests that O2 supply did not meet the lower metabolic need of the skeletal muscle in a condition where there is yet ample vasodilatory reserve.
It is also particularly interesting that the relative changes in [HHb] during occlusion were reduced (Fig. 5B). The changes in [HHb] using NIRS have been identified as an index of tissue O2 extraction (2); therefore, under an arterial occlusion, as in the present study, it directly reflects muscle O2 consumption. Together, these findings strongly support a decrease in lower-limb metabolism following sitting, thus confirming the role of posture on muscle metabolism and the implication of prolonged period of inactivity in facilitating weight gain by decreasing basal energy expenditure.
Fitness level and cardiovascular response.
We also found that V̇o2max was positively correlated with resting brachial and popliteal FMD (r = 0.494, and r = 0.447, P > 0.05, respectively) but was weakly associated with changes in brachial and popliteal FMD (r = −0.100 and r = −0.073, P > 0.05, respectively) following sitting. Participant lean body mass exhibited a moderate to strong correlation with resting brachial FMD (r = 0.654, P < 0.05) and a moderate correlation with resting popliteal FMD (r = 0.305, P > 0.05). Additionally, participant lean body mass was negatively correlated with changes in brachial FMD (r = −0.393, P > 0.05) and was weakly associated with changes in popliteal FMD following sitting (r = −0.132, P > 0.05) (Table 3). V̇o2max has been known as the gold standard measure of cardiorespiratory fitness, and a greater V̇o2max generally indicates that an individual is more fit, which likely results in a more ideal body composition. V̇o2max has been shown to be positively associated with endothelial function (11), which supports the idea that more fit individuals may have better endothelial function and vice versa. Furthermore, regular exercise and increased cardiorespiratory fitness may protect endothelial function from degrading. The present study confirmed that there is undoubtedly a significant positive relationship present between cardiorespiratory fitness and endothelial function, and to our knowledge, this is the first study that has identified the relationship between V̇o2max and the attenuated endothelial function associated with prolonged sitting. Interestingly, we also found that the hypercapnic condition has no additive effects on the relationship between V̇o2max versus changes in FMDs and lean body mass versus changes in FMDs.
Sex differences.
We observed no significant difference in the effects of prolonged sitting on brachial or popliteal FMD, central or peripheral PWV, AIx, HR, SBP, DBP, or nervous system activity between sexes in either condition. However, previous studies have noted that young adult females experience less profound vascular effects when compared with young males following acute prolonged sitting (93). It was hypothesized that sex hormones play a protective role in the vasculature of adult women, as it was previously reported that prepubescent females were not protected against the negative effects of prolonged sitting (54). Additionally, lower prevalence of atherosclerosis in the lower extremities of women (29, 68) has been reported compared with men (76). In our study, all female participants performed experimental measures during the early to mid-follicular (days 1–10) and late-luteal phases (>day 19) of the menstrual cycle, which is the most stable estrogen level, and this may be the major factor that leads to the different results from the previous study. Because there is only limited literature available comparing the cardiovascular and autonomic effects of acute prolonged sitting between sexes, further investigation is warranted.
Experimental considerations.
There were several experimental considerations that could have affected the outcomes of the study. First, we measured the concentration of CO2 in the atmosphere (room CO2 concentration), which is not a direct measurement of the concentration of CO2 that was inspired and dissolved in the blood of each participant. We could not perform blood gas analysis for blood pH or CO2 levels, which should be included in future research. Although this is the major experimental consideration, the purpose of the study was to examine the cardiovascular and autonomic responses to the prolonged sitting in hypercapnic environments that are relevant to common environments experienced in our daily lives. However, future investigation is warranted to examine the cardiovascular and autonomic response to specific blood CO2 levels. Second, unfortunately, due to technical limitations, we were unable to administer an NO donor drug, such as nitroglycerin, to measure endothelium-independent vasodilation. Additionally, we were unable to measure blood markers of endothelial dysfunction; therefore, our results cannot be definitively attributed to the endothelium or vascular smooth muscle. Additionally, we were unable to assess shear rate and shear stress in the brachial and popliteal arteries during FMD, and thus we are unable to directly compare the shear stress-mediated attenuated FMD between prolonged sitting in the normal atmospheric condition and the hypercapnic condition. However, previous studies have already established that following prolonged sitting there is a reduction in shear stress in the arms and legs. Third, MSNA is the gold standard for measuring skeletal muscle nervous system activity but was not able to be used in the present study due to technical issues. Despite not using the gold standard of nervous system activity measurements, we did use a previously validated HRV measurement method (84), and to our knowledge we are the first group to examine HRV changes in response to prolonged sitting with mild hypercapnic conditions. Finally, since the participants are all young, healthy, and fit, the conclusions drawn may not be extrapolated to other populations.
Conclusion.
This study confirmed that sitting attenuates peripheral endothelial function while also identifying that sitting results in decreased resting microvascular oxygenation in lower-limb skeletal muscle. Additionally, this study revealed that mild hypercapnic environments may exacerbate the deleterious effects of prolonged sitting on the endothelial function in the peripheral vasculature in healthy young adults. The aggravating effects of mild hypercapnic conditions may be explained by an increase in sympathetic output to peripheral skeletal muscle. However, mild hypercapnic conditions did not significantly affect any other measure of central cardiovascular function or autonomic nervous system activity in healthy young adults. This is the first study to report that mild hypercapnic conditions may further attenuate the reduced macrovascular endothelial function associated with prolonged as well as reduced microvascular function. Additionally, these findings are relevant, as the experimental conditions are more representative of everyday environments, such as offices and classrooms, and provide clinical insight into the cardiovascular and autonomic responses to acute exposure to prolonged sitting with mild hypercapnia in humans.
GRANTS
This study was supported by a University of Nebraska at Omaha Graduate Research and Creative Activity grant; National Institute of General Medical Sciences Grant U54-GM-115458 which funds the Great Plains IDeA-CTR Network; and NASA Nebraska Space Grant NNX15AI09H.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.H. and S.-Y.P. conceived and designed research; R.J.H., E.J.P., W.-M.S., J.S., and S.-Y.P. performed experiments; R.J.H., E.J.P., T.K.W., W.-M.S., G.L., J.S., and S.-Y.P. analyzed data; R.J.H., E.J.P., T.K.W., W.-M.S., G.L., J.S., and S.-Y.P. interpreted results of experiments; R.J.H. and T.K.W. prepared figures; R.J.H. and S.-Y.P. drafted manuscript; R.J.H., G.L., and S.-Y.P. edited and revised manuscript; R.J.H., E.J.P., T.K.W., W.-M.S., G.L., J.S., and S.-Y.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our study participants.
REFERENCES
- 1.Aboyans V, McClelland RL, Allison MA, McDermott MM, Blumenthal RS, Macura K, Criqui MH; The Multi-Ethnic Study of Atherosclerosis . Lower extremity peripheral artery disease in the absence of traditional risk factors. Atherosclerosis 214: 169–173, 2011. doi: 10.1016/j.atherosclerosis.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adami A, Koga S, Kondo N, Cannon DT, Kowalchuk JM, Amano T, Rossiter HB. Changes in whole tissue heme concentration dissociates muscle deoxygenation from muscle oxygen extraction during passive head-up tilt. J Appl Physiol (1985) 118: 1091–1099, 2015. doi: 10.1152/japplphysiol.00918.2014. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadi-Abhari S, Sabia S, Shipley MJ, Kivimäki M, Singh-Manoux A, Tabak A, McEniery C, Wilkinson IB, Brunner EJ. Physical activity, sedentary behavior, and long-term changes in aortic stiffness: The Whitehall II Study. J Am Heart Assoc 6: e005974, 2017. doi: 10.1161/JAHA.117.005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Mallah MH, Sakr S, Al-Qunaibet A. Cardiorespiratory Fitness and Cardiovascular Disease Prevention: an Update. Curr Atheroscler Rep 20: 1, 2018. doi: 10.1007/s11883-018-0711-4. [DOI] [PubMed] [Google Scholar]
- 5.Althoff T, Sosič R, Hicks JL, King AC, Delp SL, Leskovec J. Large-scale physical activity data reveal worldwide activity inequality. Nature 547: 336–339, 2017. doi: 10.1038/nature23018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biddle SJH, Bennie JA, Bauman AE, Chau JY, Dunstan D, Owen N, Stamatakis E, van Uffelen JGZ. Too much sitting and all-cause mortality: is there a causal link? BMC Public Health 16: 635, 2016. doi: 10.1186/s12889-016-3307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boezeman RP, Boersma D, Wille J, Kelder JC, Visscher MI, Waanders FG, Moll FL, de Vries JP. The significance of regional hemoglobin oxygen saturation values and limb-to-arm ratios of near-infrared spectroscopy to detect critical limb ischemia. Vascular 24: 492–500, 2016. doi: 10.1177/1708538115613936. [DOI] [PubMed] [Google Scholar]
- 8.Bopp CM, Townsend DK, Warren S, Barstow TJ. Relationship between brachial artery blood flow and total [hemoglobin+myoglobin] during post-occlusive reactive hyperemia. Microvasc Res 91: 37–43, 2014. doi: 10.1016/j.mvr.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Bossard M, Pumpol K, van der Lely S, Aeschbacher S, Schoen T, Krisai P, Lam T, Todd J, Estis J, Risch M, Risch L, Conen D. Plasma endothelin-1 and cardiovascular risk among young and healthy adults. Atherosclerosis 239: 186–191, 2015. doi: 10.1016/j.atherosclerosis.2014.12.061. [DOI] [PubMed] [Google Scholar]
- 10.Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol (1985) 115: 1519–1525, 2013. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buscemi S, Canino B, Batsis JA, Buscemi C, Calandrino V, Mattina A, Arnone M, Caimi G, Cerasola G, Verga S. Relationships between maximal oxygen uptake and endothelial function in healthy male adults: a preliminary study. Acta Diabetol 50: 135–141, 2013. doi: 10.1007/s00592-010-0229-x. [DOI] [PubMed] [Google Scholar]
- 12.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 106: 1783–1787, 2002. doi: 10.1161/01.CIR.0000032260.01569.64. [DOI] [PubMed] [Google Scholar]
- 13.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 57: 1511–1522, 2011. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis 1: 1–10, 2012. doi: 10.1258/cvd.2012.012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49: 2379–2393, 2007. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA 107: 10268–10273, 2010. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol (Oxf) 219: 382–408, 2017. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 18.Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, Garcia L, Allison MA, Sims ST, LaMonte MJ, Johnson KC, Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. J Am Coll Cardiol 61: 2346–2354, 2013. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One 6: e19657, 2011. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Climie RE, Wheeler MJ, Grace M, Lambert E, Cohen N, Owen N, Kingwell B, Dunstan DW, Green DJ. Simple intermittent resistance activity mitigates the detrimental effect of prolonged unbroken sitting on arterial function in overweight and obese adults. J Appl Physiol (1985) 125: 1787–1794, 2018. doi: 10.1152/japplphysiol.00544.2018. [DOI] [PubMed] [Google Scholar]
- 21.Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian children and youth: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep 22: 15–23, 2011. [PubMed] [Google Scholar]
- 22.Cooper VL, Pearson SB, Bowker CM, Elliott MW, Hainsworth R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia - a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol 568: 677–687, 2005. doi: 10.1113/jphysiol.2005.094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Credeur DP, Miller SM, Jones R, Stoner L, Dolbow DR, Fryer SM, Stone K, McCoy SM. Impact of Prolonged Sitting on Peripheral and Central Vascular Health. Am J Cardiol 123: 260–266, 2019. doi: 10.1016/j.amjcard.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res 89: 1073–1080, 2001. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- 25.De Santis V, Singer M. Tissue oxygen tension monitoring of organ perfusion: rationale, methodologies, and literature review. Br J Anaesth 115: 357–365, 2015. doi: 10.1093/bja/aev162. [DOI] [PubMed] [Google Scholar]
- 26.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115: 1285–1295, 2007. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira LF, Hueber DM, Barstow TJ. Effects of assuming constant optical scattering on measurements of muscle oxygenation by near-infrared spectroscopy during exercise. J Appl Physiol (1985) 102: 358–367, 2007. doi: 10.1152/japplphysiol.00920.2005. [DOI] [PubMed] [Google Scholar]
- 28.Floras JS. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ Res 122: 1741–1764, 2018. doi: 10.1161/CIRCRESAHA.118.310783. [DOI] [PubMed] [Google Scholar]
- 29.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 20: 384–392, 1991. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 30.Frydenlund G, Jørgensen T, Toft U, Pisinger C, Aadahl M. Sedentary leisure time behavior, snacking habits and cardiovascular biomarkers: the Inter99 Study. Eur J Prev Cardiol 19: 1111–1119, 2012. doi: 10.1177/1741826711419999. [DOI] [PubMed] [Google Scholar]
- 31.Gayda M, Juneau M, Tardif JC, Harel F, Levesque S, Nigam A. Cardiometabolic and traditional cardiovascular risk factors and their potential impact on macrovascular and microvascular function: preliminary data. Clin Hemorheol Microcirc 59: 53–65, 2015. doi: 10.3233/CH-141816. [DOI] [PubMed] [Google Scholar]
- 32.Guan J, Li Z, Yang XD. Net in-cabin emission rates of VOCs and contributions from outside and inside the aircraft cabin. Atmos Environ 111: 1–9, 2015. doi: 10.1016/j.atmosenv.2015.04.002. [DOI] [Google Scholar]
- 33.Gulino-Debrac D. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers 1: e24180, 2013. doi: 10.4161/tisb.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 35.Harrington DM, Barreira TV, Staiano AE, Katzmarzyk PT. The descriptive epidemiology of sitting among US adults, NHANES 2009/2010. J Sci Med Sport 17: 371–375, 2014. doi: 10.1016/j.jsams.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes WE, Casey DP. Aortic Wave Reflection During Orthostatic Challenges: Influence of Body Position and Venous Pooling. Am J Hypertens 30: 166–172, 2017. doi: 10.1093/ajh/hpw138. [DOI] [PubMed] [Google Scholar]
- 38.Huynh QL, Blizzard CL, Sharman JE, Magnussen CG, Dwyer T, Venn AJ. The cross-sectional association of sitting time with carotid artery stiffness in young adults. BMJ Open 4: e004384, 2014. doi: 10.1136/bmjopen-2013-004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Julius S, Sanchez R, Malayan S, Hamlin M, Elkins M, Brant D, Bohr DF. Sustained blood pressure elevation to lower body compression in pigs and dogs. Hypertension 4: 782–788, 1982. doi: 10.1161/01.HYP.4.6.782. [DOI] [PubMed] [Google Scholar]
- 40.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 41: 998–1005, 2009. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 41.Kaya M, Balasubramanian V, K-J Li J. Augmentation index in the assessment of wave reflections and systolic loading. Comput Biol Med 113: 103418, 2019. doi: 10.1016/j.compbiomed.2019.103418. [DOI] [PubMed] [Google Scholar]
- 42.Kenny RA, O’Shea D, Parry SW. The Newcastle protocols for head-up tilt table testing in the diagnosis of vasovagal syncope, carotid sinus hypersensitivity, and related disorders. Heart 83: 564–569, 2000. doi: 10.1136/heart.83.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiely DG, Cargill RI, Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest 109: 1215–1221, 1996. doi: 10.1378/chest.109.5.1215. [DOI] [PubMed] [Google Scholar]
- 44.Koga S, Kano Y, Barstow TJ, Ferreira LF, Ohmae E, Sudo M, Poole DC. Kinetics of muscle deoxygenation and microvascular PO(2) during contractions in rat: comparison of optical spectroscopy and phosphorescence-quenching techniques. J Appl Physiol (1985) 112: 26–32, 2012. doi: 10.1152/japplphysiol.00925.2011. [DOI] [PubMed] [Google Scholar]
- 45.Kröger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology 50: 649–654, 1999. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 46.Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol 264: H150–H156, 1993. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- 47.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 48.Lee HY, Oh BH. Aging and arterial stiffness. Circ J 74: 2257–2262, 2010. doi: 10.1253/circj.CJ-10-0910. [DOI] [PubMed] [Google Scholar]
- 49.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT; Lancet Physical Activity Series Working Group . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380: 219–229, 2012. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr 90: 1457–1465, 2009. doi: 10.3945/ajcn.2009.28141. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Guan J, Yang XD, Lin CH. Source apportionment of airborne particles in commercial aircraft cabin environment: Contributions from outside and inside of cabin. Atmos Environ 89: 119–128, 2014. doi: 10.1016/j.atmosenv.2014.01.042. [DOI] [Google Scholar]
- 52.Lüscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 102: 2434–2440, 2000. doi: 10.1161/01.CIR.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 53.McGuire S. Centers for Disease Control and Prevention. State indicator report on Physical Activity, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Adv Nutr 5: 762–763, 2014. doi: 10.3945/an.114.007211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McManus AM, Ainslie PN, Green DJ, Simair RG, Smith K, Lewis N. Impact of prolonged sitting on vascular function in young girls. Exp Physiol 100: 1379–1387, 2015. doi: 10.1113/EP085355. [DOI] [PubMed] [Google Scholar]
- 55.Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol 61: 391–415, 1999. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- 56.Morawietz H, Talanow R, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J Physiol 525: 761–770, 2000. doi: 10.1111/j.1469-7793.2000.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 577: 369–386, 2006. doi: 10.1113/jphysiol.2006.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol 311: H177–H182, 2016. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol 17: 543–551, 2002. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M, Grenon SM. Short-term physical inactivity impairs vascular function. J Surg Res 190: 672–682, 2014. doi: 10.1016/j.jss.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, Nakamura M, Ohkubo T, Watada H, Munakata M, Ohishi M, Ito N, Nakamura M, Shoji T, Vlachopoulos C, Yamashina A, Nagano M, Yukiyo O, Kabutoya T, Asayama K, Takashima N, Chowdhury TT, Mitsuki-Shinohara K, Yamashita T; Collaborative Group for J-BAVEL (Japan Brachial-Ankle Pulse Wave Velocity Individual Participant Data Meta-Analysis of Prospective Studies)* . Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension 69: 1045–1052, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09097. [DOI] [PubMed] [Google Scholar]
- 62.Owen N, Bauman A, Brown W. Too much sitting: a novel and important predictor of chronic disease risk? Br J Sports Med 43: 81–83, 2009. doi: 10.1136/bjsm.2008.055269. [DOI] [PubMed] [Google Scholar]
- 63.Padilla J, Fadel PJ. Prolonged sitting leg vasculopathy: contributing factors and clinical implications. Am J Physiol Heart Circ Physiol 313: H722–H728, 2017. doi: 10.1152/ajpheart.00326.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 65.Park SY, Ives SJ, Gifford JR, Andtbacka RH, Hyngstrom JR, Reese V, Layec G, Bharath LP, Symons JD, Richardson RS. Impact of age on the vasodilatory function of human skeletal muscle feed arteries. Am J Physiol Heart Circ Physiol 310: H217–H225, 2016. doi: 10.1152/ajpheart.00716.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinto Pereira SM, Ki M, Power C. Sedentary behaviour and biomarkers for cardiovascular disease and diabetes in mid-life: the role of television-viewing and sitting at work. PLoS One 7: e31132, 2012. doi: 10.1371/journal.pone.0031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol (1985) 85: 68–75, 1998. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- 68.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 97: 1–37, 2017. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 69.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reynolds LJ, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP. Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J Appl Physiol (1985) 122: 38–47, 2017. doi: 10.1152/japplphysiol.00286.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richardson DW, Wasserman AJ, Patterson JL Jr. General and regional circulatory responses to change in blood pH and carbon dioxide tension. J Clin Invest 40: 31–43, 1961. doi: 10.1172/JCI104234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93, 1984. [PMC free article] [PubMed] [Google Scholar]
- 74.Ryan TE, Brizendine JT, McCully KK. A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. J Appl Physiol (1985) 114: 230–237, 2013. doi: 10.1152/japplphysiol.01043.2012. [DOI] [PubMed] [Google Scholar]
- 75.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985) 113: 175–183, 2012. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saeki Y, Atogami F, Takahashi K, Yoshizawa T. Reflex control of autonomic function induced by posture change during the menstrual cycle. J Auton Nerv Syst 66: 69–74, 1997. doi: 10.1016/S0165-1838(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 77.Sala R, Malacarne M, Pagani M, Lucini D. Association between aerobic fitness and indices of autonomic regulation: cardiovascular risk implications. J Sports Med Phys Fitness 56: 794–801, 2016. [PubMed] [Google Scholar]
- 78.Shvartz E, Gaume JG, White RT, Reibold RC. Hemodynamic responses during prolonged sitting. J Appl Physiol Respir Environ Exerc Physiol 54: 1673–1680, 1983. [DOI] [PubMed] [Google Scholar]
- 79.Shvartz E, Reibold RC, White RT, Gaume JG. Hemodynamic responses in orthostasis following 5 hours of sitting. Aviat Space Environ Med 53: 226–231, 1982. [PubMed] [Google Scholar]
- 80.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985) 67: 2101–2106, 1989. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 81.Stone KJ, Fryer SM, Ryan T, Stoner L. The validity and reliability of continuous-wave near-infrared spectroscopy for the assessment of leg blood volume during an orthostatic challenge. Atherosclerosis 251: 234–239, 2016. doi: 10.1016/j.atherosclerosis.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 82.Stoner L, Stone K, Hanson ED, Faulkner J, Fryer S, Credeur D. Reliability of pulse waveform separation analysis responses to an orthostatic challenge. Hypertens Res 41: 176–182, 2018. doi: 10.1038/s41440-017-0005-1. [DOI] [PubMed] [Google Scholar]
- 83.Tahvanainen A, Koskela J, Tikkakoski A, Lahtela J, Leskinen M, Kähönen M, Nieminen T, Kööbi T, Mustonen J, Pörsti I. Analysis of cardiovascular responses to passive head-up tilt using continuous pulse wave analysis and impedance cardiography. Scand J Clin Lab Invest 69: 128–137, 2009. doi: 10.1080/00365510802439098. [DOI] [PubMed] [Google Scholar]
- 84.Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17: 354–381, 1996. doi: 10.1093/oxfordjournals.eurheartj.a014868. [DOI] [PubMed] [Google Scholar]
- 85.Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc 47: 843–849, 2015. doi: 10.1249/MSS.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 86.Thosar SS, Johnson BD, Johnston JD, Wallace JP. Sitting and endothelial dysfunction: the role of shear stress. Med Sci Monit 18: RA173–RA180, 2012. doi: 10.12659/MSM.883589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ussery EN, Fulton JE, Galuska DA, Katzmarzyk PT, Carlson SA. Joint prevalence of sitting time and leisure-time physical activity among US adults, 2015-2016. JAMA 320: 2036–2038, 2018. doi: 10.1001/jama.2018.17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Beekvelt MC, Borghuis MS, van Engelen BG, Wevers RA, Colier WN. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci (Lond) 101: 21–28, 2001. doi: 10.1042/cs1010021. [DOI] [PubMed] [Google Scholar]
- 89.Victor RG, Leimbach WN Jr. Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol (1985) 63: 2558–2562, 1987. doi: 10.1152/jappl.1987.63.6.2558. [DOI] [PubMed] [Google Scholar]
- 90.Vissing SF, Scherrer U, Victor RG. Relation between sympathetic outflow and vascular resistance in the calf during perturbations in central venous pressure. Evidence for cardiopulmonary afferent regulation of calf vascular resistance in humans. Circ Res 65: 1710–1717, 1989. doi: 10.1161/01.RES.65.6.1710. [DOI] [PubMed] [Google Scholar]
- 91.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 92.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension 60: 556–562, 2012. doi: 10.1161/HYPERTENSIONAHA.112.194779. [DOI] [PubMed] [Google Scholar]
- 93.Vranish JR, Young BE, Kaur J, Patik JC, Padilla J, Fadel PJ. Influence of sex on microvascular and macrovascular responses to prolonged sitting. Am J Physiol Heart Circ Physiol 312: H800–H805, 2017. doi: 10.1152/ajpheart.00823.2016. [DOI] [PubMed] [Google Scholar]
- 94.Walsh LK, Restaino RM, Martinez-Lemus LA, Padilla J. Prolonged leg bending impairs endothelial function in the popliteal artery. Physiol Rep 5: e13478, 2017. doi: 10.14814/phy2.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weisel CP, Fiedler N, Weschler CJ, Ohman-Strickland PA, Mohan KR, McNeil K, Space DR. Human symptom responses to bioeffluents, short-chain carbonyls/acids, and long-chain carbonyls in a simulated aircraft cabin environment. Indoor Air 27: 1154–1167, 2017. doi: 10.1111/ina.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wray DW, Amann M, Richardson RS. Peripheral vascular function, oxygen delivery and utilization: the impact of oxidative stress in aging and heart failure with reduced ejection fraction. Heart Fail Rev 22: 149–166, 2017. doi: 10.1007/s10741-016-9573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 25: 359–364, 2002. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 98.Zachariah JP. Pulse wave reflection in children: amplification through the lifecourse. J Hypertens 35: 1363–1365, 2017. doi: 10.1097/HJH.0000000000001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zinchuk VV, Pronko TP, Lis MA. Blood oxygen transport and endothelial dysfunction in patients with arterial hypertension. Clin Physiol Funct Imaging 24: 205–211, 2004. doi: 10.1111/j.1475-097X.2004.00549.x. [DOI] [PubMed] [Google Scholar]