Abstract
The purpose of this study was to investigate the effect of race and subclinical elevations in blood pressure (i.e., prehypertension) on cutaneous sensory nerve-mediated and nitric oxide (NO)-dependent vasodilation. We recruited participants who self-identified as either non-Hispanic black (n = 16) or non-Hispanic white (n = 16). Within each group, participants were subdivided as either normotensive (n = 8 per group) or prehypertensive (n = 8 per group). Each participant was instrumented with four intradermal microdialysis fibers: 1) control (lactated Ringer’s), 2) 5% lidocaine (sensory nerve inhibition), 3) 20 mM Nω-nitro-l-arginine methyl ester (l-NAME) (NO synthase inhibition), and 4) lidocaine + l-NAME. Skin blood flow was assessed via laser-Doppler flowmetry, and each site underwent local heating from 33°C to 39°C. At the plateau, 20 mM l-NAME were infused at control and lidocaine sites to quantify NO-dependent vasodilation. Maximal vasodilation was induced via 54 mM sodium nitroprusside and local heating to 43°C. Data are means ± SD. Sensory nerve-mediated cutaneous vasodilation was reduced in prehypertensive non-Hispanic white (34 ± 7%) and both non-Hispanic black groups (normotensive, 20 ± 9%, prehypertensive, 24 ± 15%) relative to normotensive non-Hispanic whites (54 ± 12%). NO-dependent vasodilation was also reduced in prehypertensive non-Hispanic white (41 ± 7%) and both non-Hispanic black groups (normotensive, 44 ± 7%, prehypertensive, 19 ± 7%) relative to normotensive non-Hispanic whites (60 ± 11%). The decrease in NO-dependent vasodilation in prehypertensive non-Hispanic blacks was further reduced relative to all other groups. These data suggest subclinical increases in blood pressure adversely affect sensory-mediated and NO-dependent vasodilation in both non-Hispanic blacks and whites.
NEW & NOTEWORTHY Overt hypertension is known to reduce cutaneous sensory nerve-mediated and nitric oxide (NO)-dependent vasodilation, but the effect of subclinical increases in blood pressure (i.e., prehypertension) is unknown. The combined effect of race and prehypertension is also unknown. In this study, we found that prehypertension reduces cutaneous sensory nerve-mediated and NO-dependent vasodilation in both non-Hispanic white and black populations, with the greatest reductions observed in prehypertensive non-Hispanic blacks.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/race-blood-pressure-and-microvascular-function/.
Keywords: axon reflex, endothelium, human
INTRODUCTION
Although cardiovascular disease (CVD) is the leading cause of death in the composite population in the United States, rates of CVD are higher in the non-Hispanic black population relative to non-Hispanic whites (3). Rates of hypertension (a risk factor for CVD) are also higher in non-Hispanic blacks (3), and prehypertensive non-Hispanic blacks are more likely to develop hypertension faster and earlier in life relative to non-Hispanic whites (46).
Impaired vascular function and endothelial nitric oxide (NO)-dependent vasodilation are known risk factors for CVD across all populations. Reduced NO-dependent vasodilation in non-Hispanic blacks relative to non-Hispanic whites in both health and disease and across different vascular beds, including the cutaneous microvasculature, is strongly supported by several lines of data (2, 4, 7, 8, 29, 30, 35, 36, 40, 43–45, 47). Understanding mechanisms of microvascular function is important, as regulation of total peripheral resistance and glucose uptake occur largely in the microvasculature. Microvascular dysfunction may precede conduit vessel dysfunction and can thus result in hypertension and type 2 diabetes.
The cutaneous circulation is an easily accessible microvascular bed and appears to be representative of less accessible microvascular beds such as the kidney (1, 12, 24, 37, 41). Local heating of the skin results in substantial cutaneous vasodilation and is highly reproducible. This cutaneous vasodilation is a biphasic response with each phase mediated largely by two distinct mechanisms. The initial peak occurs within a few seconds of increasing skin temperature and is largely mediated by cutaneous sensory nerve mechanisms with modest endothelial NO-dependent and -independent components (6, 9, 18, 23, 39, 42, 55–57). The plateau phase is more prolonged and is ~60–80% dependent on endothelial NO (6, 9, 10, 18, 31–34, 42, 55–57).
Cutaneous sensory nerves are important for detecting temperature and other potentially noxious stimuli, and sensory nerve function is widely known to be reduced in type 2 diabetes and following chemotherapy (i.e., diabetic sensory neuropathy and chemotherapy-induced peripheral neuropathy, respectively). Whether race and/or blood pressure status adversely affect sensory nerve function is not completely known; however, some evidence suggests overt hypertension and race may be risk factors for the development of sensory neuropathy (11, 17, 21). Our laboratory recently demonstrated that the temperature threshold eliciting cutaneous sensory nerve activation is shifted to a higher temperature in non-Hispanic blacks relative to non-Hispanic whites, and this rightward shift is largely due to a reduction in bioavailable NO (52). In this previous study we used a slow, ramp local heating protocol and were unable to determine the magnitude of sensory nerve-mediated vasodilation (27, 52). Although microvascular NO-dependent vasodilation is reduced in hypertension (5, 12, 15, 16, 20, 37, 49) and healthy young non-Hispanic blacks (30, 35, 36, 45), it remains unknown whether subclinical elevations in blood pressure (i.e., prehypertension) affect NO-dependent vasodilation in either non-Hispanic whites or non-Hispanic blacks. It is also unknown whether cutaneous sensory nerve-mediated vasodilation is affected by subclinical prehypertension or race. Importantly, rapid, multipainful local heating of the skin allows for simultaneous assessment of both sensory nerve-mediated and NO-dependent vasodilation.
The purpose of this study was to investigate the effect of race and subclinical prehypertension on cutaneous sensory nerve-mediated and NO-dependent vasodilation. We hypothesized both cutaneous sensory nerve-mediated and NO-dependent vasodilation would be reduced in normotensive and prehypertensive non-Hispanic blacks relative to normotensive and prehypertensive non-Hispanic whites.
METHODS
Ethical approval.
This study was approved by Advarra Institutional Review Board (Columbia, MD; No. Pro00024265), the Georgia State University Institutional Review Board, and the United States Food and Drug Administration (IND 138231). All experimental procedures conformed with the Declaration of Helsinki. Each participant provided written and verbal consent before participating in any experimental procedure.
Participants.
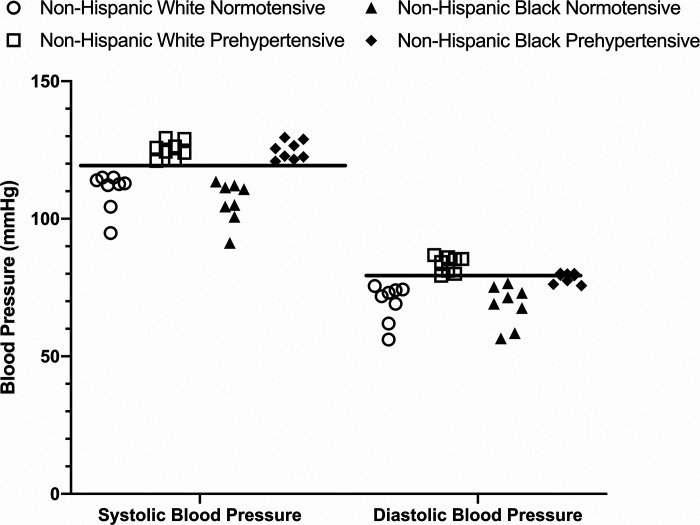
Participant demographics are shown in Table 1. Participants who self-identified as either non-Hispanic black (n = 16) or non-Hispanic white (n = 16) were recruited and tested. Within each group, participants were further subdivided as either normotensive (n = 8 per group) or prehypertensive (n = 8 per group). Participants’ blood pressure was assessed at two time points before the experimental protocol: 1) before enrollment in the study (usually 7–10 days before the experimental session) and 2) the day before, or day of, the experimental session. Each blood pressure assessment was performed following at least 15 min of quiet, seated rest and three measurements were made, with each measurement separated by at least 2 to 3 min. These blood pressure assessment periods were performed at approximately the same time (within 1 to 2 h) of day as the participants’ experimental session. Blood pressure groupings were based on the 2017 American Heart Association guidelines (normotensive, systolic blood pressure <120 mmHg and diastolic blood pressure <80 mmHg; prehypertensive, systolic blood pressure between 120 and 129 mmHg or diastolic blood pressure between 80 and 89 mmHg) (53). Individual participant systolic and diastolic blood pressures from the experimental protocol are shown in Fig. 1 (the horizontal bar indicates the cutoff point for prehypertension for systolic and diastolic blood pressure). Thus, there were four groups with eight participants in each group. Participants were required to refrain from alcohol, strenuous/high-intensity exercise, and high-fat meals for at least 8 h before the experimental protocol. For the duration of the experiment, participants were seated in the semirecumbent position and the experimental arm was positioned and secured at heart level to minimize the effect of hydrostatic pressure on blood flow.
Table 1.
Participant demographics
| Non-Hispanic Black |
Non-Hispanic White |
|||
|---|---|---|---|---|
| Normotensive | Prehypertensive | Normotensive | Prehypertensive | |
| n | 8 | 8 | 8 | 8 |
| Women, men, n | 5, 3 | 4, 4 | 5, 3 | 3, 5 |
| Menstrual cycle/OC phase | ||||
| Menstrual/low hormone (placebo) | 3 (1 OC) | 2 (1 OC) | 2 (2 OC) | 2 |
| Luteal/high hormone | 2 (1 OC) | 2 | 3 (1 OC) | 1 (1 OC) |
| Age, yr | 23 ± 4 | 20 ± 2 | 26 ± 6 | 24 ± 3 |
| Height, cm | 1.72 ± 0.07 | 1.70 ± 0.10 | 1.70 ± 0.12 | 1.77 ± 0.07 |
| Mass, kg | 78.40 ± 14.72 | 72.73 ± 16.97 | 69.04 ± 14.11 | 77.78 ± 9.62 |
| Body mass index, kg/m2 | 26.44 ± 3.70 | 25.11 ± 4.39 | 23.60 ± 2.17 | 24.90 ± 3.09 |
| Systolic blood pressure, mmHg | 106 ± 8 (91–114) | 125 ± 4 (122–129) | 110 ± 7 (95–115) | 127 ± 6 (120–129) |
| Diastolic blood pressure, mmHg | 69 ± 7 (57–77) | 79 ± 2 (76–82) | 70 ± 7 (56–76) | 84 ± 3 (80–87) |
| Mean arterial pressure, mmHg | 81 ± 7 (68–89) | 93 ± 4 (84–98) | 83 ± 7 (69–88) | 98 ± 4 (93–103) |
| Heart rate, beats/min | 70 ± 11 | 69 ± 9 | 67 ± 5 | 62 ± 7 |
Values are means ± SD (range for blood pressure variables); 7 of the 17 female participants were taking oral contraceptives (OCs).
Fig. 1.
Individual systolic and diastolic blood pressure responses during the experimental protocol (n = 8 participants per group; n = 32 total). The solid horizontal line indicates the lower limit for prehypertension for both systolic (120–129 mmHg) and diastolic (80–89 mmHg) blood pressure. Both systolic and diastolic blood pressure were below the lower limit for normotensive participants. Systolic blood pressure for all prehypertensive participants was at, or above, the lower limit for prehypertension. Diastolic blood pressure for all non-Hispanic white prehypertensive participants was at, or above, the lower limit for prehypertension, whereas 5 of 8 prehypertensive non-Hispanic black participants were at, or above, the lower diastolic blood pressure limit. Circles, normotensive non-Hispanic white [5 women (W)/3 men (M)]; squares, prehypertensive non-Hispanic white (3W/5M); triangles, normotensive non-Hispanic black (5W/3M); diamonds, prehypertensive non-Hispanic black (4W/4M).
All participants were free of cardiovascular, pulmonary, and metabolic diseases and had no history of nerve pain/damage, cancer (chemotherapy or radiation therapy), or skin disorders (e.g., psoriasis). Self-report health history was obtained; however, blood analyses (lipids, glucose, etc.) were not performed. All participants reported engaging in moderate physical activity at least 3 days/wk. Menstrual cycle and oral contraceptive phase were not controlled in this study; however, 11 of the 17 female participants were tested during the early follicular phase of the normal menstrual cycle or low hormone (no pill) phase of oral contraceptive use. Of the 17 female participants, 7 were taking oral contraceptives. All female participants not using oral contraceptives were required to submit a urine pregnancy test (McKesson hCG Combo Test Cassette, Consult Diagnostics; Richmond, VA) to confirm negative pregnancy status.
Instrumentation.
Participants were instrumented with four microdialysis fibers (CMA 30; Harvard Apparatus, Hollister, MA) on the ventral forearm. Ice was used to numb the skin (22), after which a 23-gauge needle was placed into the dermal layer of the skin. The microdialysis fiber was threaded through the lumen of the needle, the microdialysis membrane was left in the skin, and the needle was removed. Microdialysis sites were randomly assigned to receive 1) lactated Ringer’s solution (Baxter Healthcare, Deerfield, IL) to serve as a control (48), 2) 5% topical lidocaine (LMX5; Ferndale Healthcare, Ferndale, MI) to inhibit cutaneous sensory nerve function, 3) 20 mM Nω-nitro-l-arginine methyl ester (l-NAME) to inhibit NO synthase [EMD Millipore (10, 31, 38)], or 4) combined 5% lidocaine and 20 mM l-NAME. The experimental design (heating rate and drug administration) was based on previous studies (10, 42). All drugs were diluted in sterile Ringer’s solution and drawn through filter needles (BD Filter Needle; Becton Dickinson, Franklin Lakes, NJ). Drugs were infused for at least 60 min before the experimental protocol at a rate of 2 μL/min (Beehive Controller and Baby Bee syringe pumps; Bioanalytical Systems, West Lafayette, IN). Topical lidocaine was placed directly above each microdialysis fiber and covered with a sterile dressing (Tegaderm; 3M Healthcare, St. Paul, MN). Lidocaine was placed on the skin for 60 min (13, 14), wiped clean, and sensation to the pointed edge of surgical scissors was assessed and compared with a site not treated with lidocaine. Sensation was also assessed at the end of the experimental protocol. During lidocaine application, each microdialysis fiber was perfused with its respective drug treatment (lactated Ringer’s or 20 mM l-NAME). Previous studies from our laboratory and others used EMLA (2.5% lidocaine + 2.5% prilocaine) to inhibit cutaneous sensory nerve function (42, 54); however, EMLA requires a prescription whereas lidocaine is available over the counter, and recent studies demonstrated it is equally effective at inhibiting cutaneous sensory nerve function (13, 14). Pilot studies from our laboratory also indicated that 60 min of 5% lidocaine was effective at inhibiting the initial peak of the local heating response without affecting the plateau phase.
To control local skin temperature, local heater units (VHP1 heater units and VMS-HEAT controller; Moor Instruments, Axminster, UK) were placed directly over each microdialysis membrane. Integrated laser-Doppler probes (VP7b probes and VMS-LDF2 monitor; Moor Instruments) were placed in the center of the local heating unit to obtain red blood cell flux, an index of skin blood flow, at each microdialysis site.
Blood pressure was measured from the contralateral (right) arm using an automated brachial oscillometric device, and heart rate was derived from the pulse detection (Welch Allyn Vital Signs Series 6000; Skaneatelles Falls, NY). Blood pressure and heart rate measurements were made every 10 min, and mean arterial pressure (MAP) was calculated as one-third pulse pressure plus diastolic pressure.
Experimental protocol.
Baseline skin blood flow was assessed for 10–15 min, during which time the local heater units were set to 33°C. Following baseline measurements, local heater temperature was increased from 33°C to 39°C at a rate of 0.1°C/s (10). None of the participants reported any pain sensation during the local heating protocol. Once a plateau in skin blood flow was achieved (~40 min into local heating), 20 mM l-NAME was perfused through the control and lidocaine sites to quantify NO-dependent vasodilation (31, 38, 55). Once a new post-l-NAME plateau was achieved at the control and lidocaine sites (~20–30 min into l-NAME infusion), maximal vasodilation was induced by heating the skin from 39°C to 43°C (0.1°C/s) and infusing 54 mM nitroprusside (31, 38).
Data analysis.
Skin blood flow data were continuously recorded at 40 Hz using commercially available equipment and software (Power Laboratory 16/35 data acquisition and Laboratory Chart 8 software; ADInstruments, Colorado Springs, CO). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by MAP and standardized to maximal vasodilation (%CVCmax).
Baseline data were averaged over a 3–5-min period immediately preceding the onset of the local heating protocol. Three main components of the skin blood flow response to local heating were analyzed: initial peak, plateau, and post-l-NAME plateau. The initial peak is a robust yet rapid and transient response and, as such, 30–75 s of data were analyzed. For all sites, the plateau was analyzed by averaging a 5-min period immediately preceding the infusion of l-NAME at the control and lidocaine sites. The post-l-NAME plateau was analyzed over a 3-min period immediately preceding initiation of maximal vasodilation. Maximal skin blood flow was analyzed over a 3–5-min period. In addition to the skin blood flow values, we also calculated the percent contribution of sensory nerves to the initial peak, percent contribution of NO to the initial peak, and percent contribution of NO to the plateau (55).
Statistical analysis.
Sample size was determined with an a priori power analysis. Effect sizes were specified based on preliminary data from pilot studies completed in our laboratory. Assuming an α level of 0.05, 80% power, and mean %NO-dependent vasodilation of 52% (SD = 8.96%) and 67% (SD = 13.44%) for non-Hispanic blacks and whites, respectively, the needed sample size to detect this difference in means is 8 per group. Skin blood flow (%CVCmax) data were analyzed using a three-way ANOVA with factors of race (non-Hispanic black and non-Hispanic white), blood pressure (normotensive and prehypertensive), and microdialysis treatment (control, lidocaine, l-NAME, and lidocaine + l-NAME). For the three-way ANOVA, we began with a model of main effects and explored additive interaction terms for each two-way interaction. We planned a priori to only consider a three-way interaction if all two-way interaction terms were statistically significant. The percent contribution of sensory nerves and NO to the initial peak and percent contribution of NO to the plateau were analyzed using a two-way ANOVA with factors of race and blood pressure. Tukey correction factors were used to account for multiple pairwise comparisons. All data were analyzed and graphed using commercially available software (SAS, Cary, NC and GraphPad Prism 8, San Diego, CA). All data are presented as means ± SD. Differences in means (Δmeans) are presented for data where there were nonsignificant P values but had a difference in means of at least 15% (19, 50). Exact P values for all between group comparisons are provided in Supplemental Table S1 (see https://doi.org/10.6084/m9.figshare.11968887).
RESULTS
Baseline and maximal data.
Baseline and maximal data are shown in Table 2.
Table 2.
Baseline and maximal data
| Non-Hispanic Black |
Non-Hispanic White |
|||
|---|---|---|---|---|
| Normotensive | Prehypertensive | Normotensive | Prehypertensive | |
| n | 8 | 8 | 8 | 8 |
| Baseline | ||||
| Control | 15 ± 7 | 13 ± 9 | 17 ± 7 | 18 ± 11 |
| Lidocaine | 13 ± 6 | 11 ± 4 | 12 ± 7 | 18 ± 9 |
| l-NAME | 8 ± 4 | 10 ± 5 | 10 ± 2 | 14 ± 5 |
| Lidocaine + l-NAME | 9 ± 4 | 13 ± 10 | 12 ± 5 | 15 ± 8 |
| Maximal CVC | ||||
| Control | 2.27 ± 0.81 | 2.30 ± 0.61 | 2.16 ± 0.69 | 2.03 ± 0.55 |
| Lidocaine | 2.51 ± 0.52 | 2.62 ± 0.70 | 2.67 ± 0.62 | 2.60 ± 0.34 |
| l-NAME | 2.30 ± 0.58 | 1.96 ± 0.55 | 2.25 ± 0.50 | 2.26 ± 0.51 |
| Lidocaine + l-NAME | 2.52 ± 0.45 | 1.94 ± 0.56 | 2.02 ± 0.89 | 1.89 ± 0.58 |
Values are means ± SD. Baseline are maximal vasodilation (%CVCmax), and maximal values are cutaneous vascular conductance (CVC) (flux/mean arterial pressure). l-NAME, Nω-nitro-l-arginine methyl ester.
Initial peak data.
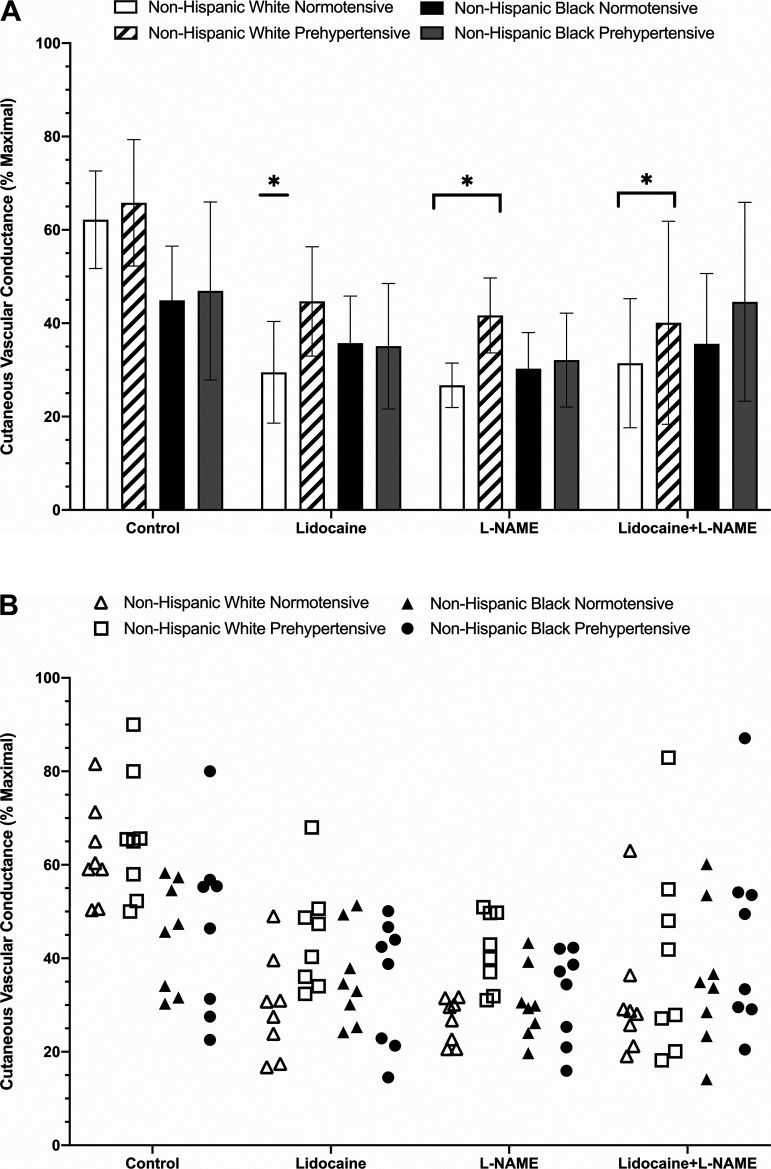
The initial peak response to local heating is shown in Fig. 2. Initial peak at control sites averaged 63 ± 10% CVCmax for normotensive non-Hispanic whites, 66 ± 14% CVCmax for prehypertensive non-Hispanic whites, 45 ± 12% CVCmax for normotensive non-Hispanic blacks, and 47 ± 19% CVCmax for prehypertensive non-Hispanic blacks. There was no statistical difference observed at control sites between prehypertensive non-Hispanic whites and normotensive non-Hispanic blacks (P = 0.0996; Δmeans = 28%) and no other observed between-group differences (Supplemental Table S1).
Fig. 2.
A: group initial maximal vasodilation (peak %CVCmax) responses shown as means ± SD (n = 8 participants per group; n = 32 total). Normotensive non-Hispanic white [5 women (W)/3 men (M)], open bars; prehypertensive non-Hispanic white (3W/5M), dashed bars; normotensive non-Hispanic black (5W/3M), black bars; prehypertensive non-Hispanic black (4W/4M), gray bars. B: individual initial peak responses for each group and treatment site. Normotensive non-Hispanic white (5W/3M), open triangles; prehypertensive non-Hispanic white (3W/5M), open squares; normotensive non-Hispanic black, filled triangles (5W/3M); prehypertensive non-Hispanic black (4W/4M), filled circles. Lidocaine significantly reduced the initial peak compared with control in normotensive non-Hispanic whites. Nω-nitro-l-arginine methyl ester (l-NAME) and lidocaine + l-NAME reduced the initial peak compared with control in both non-Hispanic white groups. *Significant at P < 0.05 vs. respective control sites (significance markers are only shown on A for clarity). Comparisons assessed via three-way ANOVA.
At lidocaine sites, initial peak averaged 30 ± 11% CVCmax (P = 0.0003 vs. control) for normotensive non-Hispanic whites, 45 ± 12% CVCmax (P = 0.0640 vs. control; Δmeans = 32%) for prehypertensive non-Hispanic whites, 36 ± 10% CVCmax (P = 0.9927 vs. control) for normotensive non-Hispanic blacks, and 35 ± 14% CVCmax (P = 0.9310 vs. control) for prehypertensive non-Hispanic blacks. There were no observed between-group differences at lidocaine sites (Supplemental Table S1).
At l-NAME sites, the initial peak averaged 27 ± 5% CVCmax (P = 0.0032 vs. control, P > 0.9999 vs. lidocaine) for normotensive non-Hispanic whites, 42 ± 8% CVCmax (P = 0.0438 vs. control, P > 0.9999 vs. lidocaine) for prehypertensive non-Hispanic whites, 30 ± 8% CVCmax (P = 0.8800 vs. control and >0.9999 vs. lidocaine) for normotensive non-Hispanic blacks, and 32 ± 10% CVCmax (P = 0.6319 vs. control and >0.9999 vs. lidocaine) for prehypertensive non-Hispanic blacks. There were no observed between group differences at l-NAME sites (Supplemental Table S1).
The initial peak at combined lidocaine plus l-NAME sites averaged 31 ± 14% CVCmax (P = 0.0011 vs. control, P > 0.9999 vs. lidocaine and l-NAME) for normotensive non-Hispanic whites, 40 ± 22% CVCmax; P = 0.0006 vs. control, 0.9927 vs. lidocaine, and 0.9975 vs. l-NAME) for prehypertensive non-Hispanic whites, 36 ± 15% CVCmax (P = 0.3364 vs. control, 0.9924 vs. lidocaine, and >0.9999 vs. l-NAME) for normotensive non-Hispanic blacks, and 46 ± 21% CVCmax (P > 0.9999 vs. control, 0.9898 vs. lidocaine, and 0.8471 vs. l-NAME) for prehypertensive non-Hispanic blacks. There were no observed between group differences at combined lidocaine plus l-NAME sites (Supplemental Table S1).
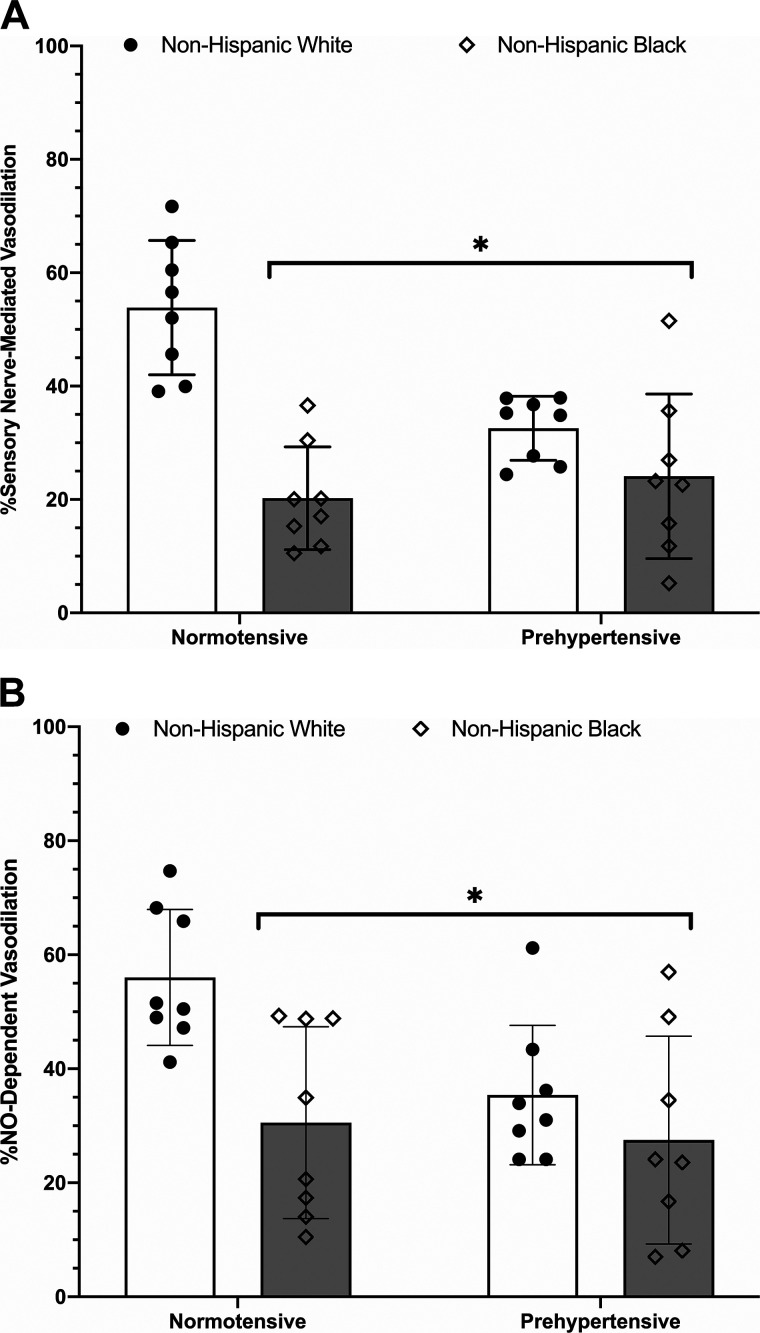
The calculated percent contribution of sensory nerves to the initial peak (Fig. 3A) averaged 54 ± 12% for normotensive non-Hispanic whites, 33 ± 6% for prehypertensive non-Hispanic whites, 20 ± 9% for normotensive non-Hispanic blacks, and 24 ± 15% for prehypertensive non-Hispanic blacks. Relative to normotensive non-Hispanic whites, the percent contribution of sensory nerves to the initial peak was reduced in prehypertensive non-Hispanic whites (P = 0.0045), normotensive non-Hispanic blacks (P < 0.0001), and prehypertensive non-Hispanic blacks (P < 0.0001). There was no observed statistical difference (P = 0.0972; Δmeans = 24%) between prehypertensive non-Hispanic white and non-Hispanic black groups. There were no observed differences between normotensive and prehypertensive non-Hispanic black groups (P = 0.8931) or between prehypertensive non-Hispanic black and white groups (P = 0.3386).
Fig. 3.
A: percent contribution of sensory nerves to the initial peak. Bars depict means ± SD (n = 8 participants per group; n = 32 total) with individual responses overlaid on the group data. The percent contribution of sensory nerves to the initial peak was reduced in normotensive [5 women (W)/3 men (M)] and prehypertensive non-Hispanic black (4W/4M) and prehypertensive non-Hispanic whites (3W/5M) relative to normotensive non-Hispanic whites (5W/3M). Non-Hispanic white, open bars, filled circles; non-Hispanic black, gray bars, diamonds. *Significant at P < 0.05 vs. normotensive non-Hispanic white via two-way ANOVA. B: percent contribution of nitric oxide (NO) to the initial peak. Bars depict means ± SD (n = 8 participants per group; n = 32 total) with individual responses overlaid on the group data. The percent NO contribution to the initial peak was reduced in both normotensive (5W/3M) and prehypertensive (4W/4M) non-Hispanic blacks and prehypertensive non-Hispanic whites (3W/5M) relative to normotensive non-Hispanic whites (5W/3M). Non-Hispanic white, open bars, filled circles; non-Hispanic black, gray bars, diamonds. *Significant at P < 0.05 vs. normotensive non-Hispanic white via two-way ANOVA.
The percent contribution of NO to the initial peak (Fig. 3B) was 56 ± 12% in normotensive non-Hispanic whites, which was statistically greater than that in prehypertensive non-Hispanic whites (35 ± 12%; P = 0.0488), normotensive non-Hispanic blacks (31 ± 17%; P = 0.0108), and prehypertensive non-Hispanic blacks (28 ± 18%; P = 0.0039). There were no observed differences between normotensive non-Hispanic black, prehypertensive non-Hispanic white, or prehypertensive non-Hispanic black groups (Supplemental Table S1).
Plateau data.
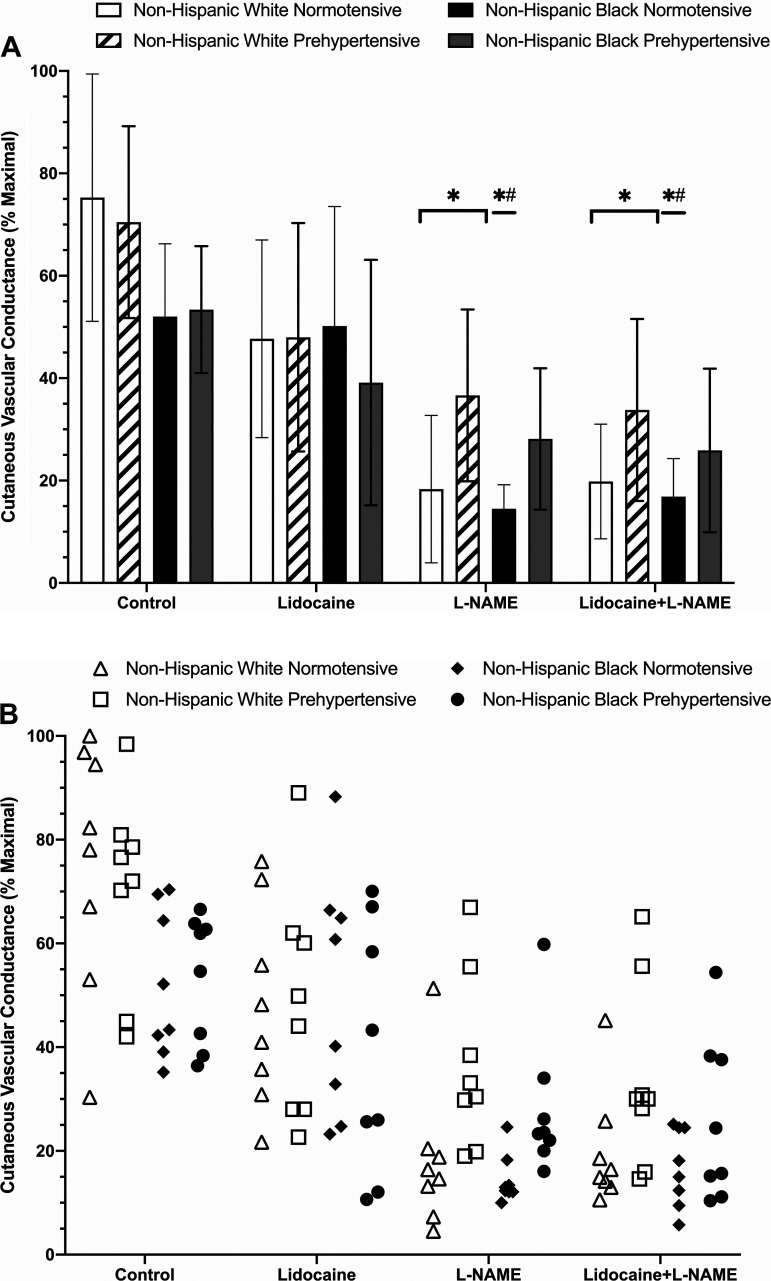
The plateau phase of the local heating response is depicted in Fig. 4. The plateau response at control sites averaged 75 ± 24% CVCmax for normotensive non-Hispanic whites, 71 ± 19% CVCmax for prehypertensive non-Hispanic whites, 52 ± 14% CVCmax for normotensive non-Hispanic blacks, and 53 ± 12% CVCmax for prehypertensive non-Hispanic blacks; there were no observed differences between groups (Supplemental Table S1).
Fig. 4.
A: group plateau maximal vasodilation (%CVCmax) responses shown as means ± SD (n = 8 participants per group; n = 32 total). Normotensive non-Hispanic white [5 women (W)/3 men (M)], open bars; prehypertensive non-Hispanic white (3W/5M), dashed bars; normotensive non-Hispanic black (5W/3M), black bars; prehypertensive non-Hispanic black (4W/4M), gray bars. B: individual plateau responses for each group and treatment site. Normotensive non-Hispanic white (5W/3M), open triangles; prehypertensive non-Hispanic white (3W/5M), open squares; normotensive non-Hispanic black (5W/3M), filled diamonds; prehypertensive non-Hispanic black (4W/4M), filled circles. Nω-nitro-l-arginine methyl ester (l-NAME) and lidocaine + l-NAME were reduced compared with control in both non-Hispanic white groups. In normotensive non-Hispanic blacks, l-NAME and lidocaine + l-NAME sites were reduced compared with control and lidocaine only sites. *Significant at P < 0.05 vs. respective control sites; #significant at P < 0.05 vs. respective lidocaine sites (significance markers are only shown on A for clarity). Comparisons assessed via three-way ANOVA.
At lidocaine-treated sites, plateau values averaged 48 ± 19% CVCmax (P = 0.3128 vs. control) for normotensive non-Hispanic whites, 48 ± 22% CVCmax (P = 0.3994 vs. control) for prehypertensive non-Hispanic whites, 50 ± 23% CVCmax (P > 0.999 vs. control) for normotensive non-Hispanic blacks, and 39 ± 24% CVCmax (P = 0.9532 vs. control) for prehypertensive non-Hispanic blacks; there were no observed between group differences at lidocaine-treated sites between groups (Supplemental Table S1).
Plateau values at l-NAME-treated sites averaged 18 ± 14% CVCmax (P < 0.001 vs. control and lidocaine) for normotensive non-Hispanic whites, 37 ± 17% CVCmax (P = 0.0131 vs. control, P = 0.9944 vs. lidocaine) for prehypertensive non-Hispanic whites, 15 ± 5% CVCmax (P = 0.0028 vs. control, P = 0.0062 vs. lidocaine) for normotensive non-Hispanic blacks, and 28 ± 14% CVCmax (P = 0.2138 vs. control, P > 0.9959 vs. lidocaine) for prehypertensive non-Hispanic blacks; there were no observed differences between groups at l-NAME sites (Supplemental Table S1).
At combined lidocaine plus l-NAME-treated sites, plateau values averaged 20 ± 11% CVCmax (P < 0.0001 vs. control, 0.1040 vs. lidocaine, and > 0.999 vs. l-NAME) for normotensive non-Hispanic whites, 34 ± 18% CVCmax (P = 0.0041 vs. control, P = 0.9551 vs. lidocaine, P > 0.9999 vs. l-NAME) for prehypertensive non-Hispanic whites, 17 ± 7% CVCmax (P = 0.0076 vs. control, 0.0160 vs. lidocaine, P > 0.9999 vs. l-NAME) for normotensive non-Hispanic blacks, and 26 ± 16% CVCmax (P = 0.1157 vs. control, 0.9750 vs. lidocaine and > 0.9999 vs. l-NAME) for prehypertensive non-Hispanic blacks. There were no observed between-group differences for combined lidocaine plus l-NAME sites (Supplemental Table S1).
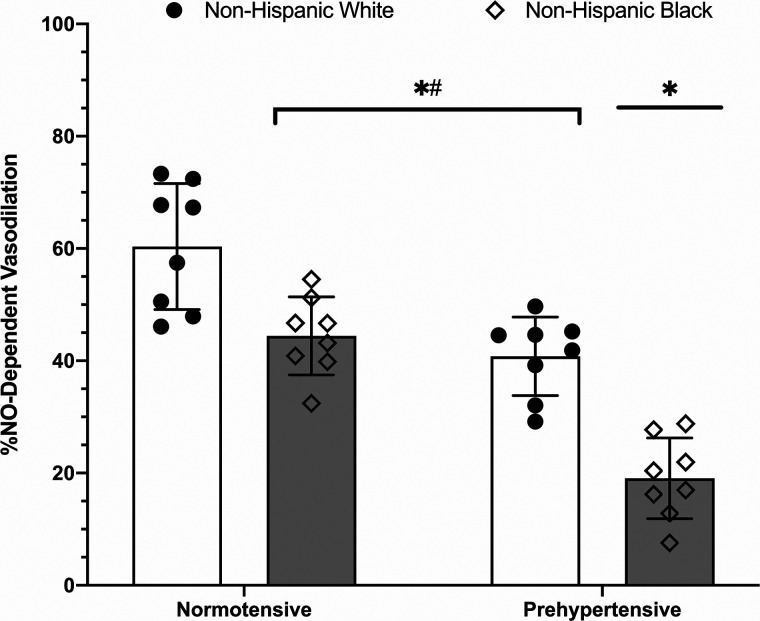
The percent contribution of NO to the plateau, an estimate of microvascular endothelial NO-dependent vasodilation, is shown in Fig. 5. As there were no observed differences (P > 0.9999 for all comparisons of NO-dependent vasodilation between control and lidocaine sites) in the percent contribution of NO between the control and lidocaine-treated sites between or within groups, percent NO data are shown for the control sites only for clarity and brevity. The percent contribution of NO to the plateau was observed to be reduced in prehypertensive non-Hispanic whites (41 ± 7%; P = 0.0003), normotensive non-Hispanic blacks (44 ± 7%; P = 0.0034), and prehypertensive non-Hispanic blacks (19 ± 7%; P < 0.0001) relative to normotensive non-Hispanic whites (60 ± 11%). Observed differences also occurred between normotensive and prehypertensive non-Hispanic blacks (P < 0.0001) and between prehypertensive non-Hispanic blacks and non-Hispanic whites (P < 0.0001). There were no observed differences between normotensive non-Hispanic blacks and prehypertensive non-Hispanic whites (P = 0.8183).
Fig. 5.
Percent contribution of nitric oxide (NO) to the initial peak. Bars depict means ± SD (n = 8 participants per group; n = 32 total) with individual responses overlaid on the group data. The percent NO contribution to the initial peak was reduced in normotensive [5 women (W)/3 men (M)] and prehypertensive (4W/4M) non-Hispanic blacks and prehypertensive non-Hispanic whites (3W/5M) relative to normotensive non-Hispanic whites (5W/3M). Non-Hispanic white, open bars, circles; non-Hispanic black, gray bars, diamonds. *Significant at P < 0.05 vs. normotensive non-Hispanic white, #significant at P < 0.05 vs. prehypertensive non-Hispanic black. Comparisons assessed via two-way ANOVA.
DISCUSSION
The major findings of this study are that cutaneous sensory nerve-mediated vasodilation and cutaneous endothelial NO-dependent vasodilation are reduced in normotensive non-Hispanic blacks and prehypertensive non-Hispanic whites and non-Hispanic blacks relative to normotensive non-Hispanic whites.
Baseline and maximal cutaneous vascular conductance.
We did not observe any significant differences in baseline or maximal cutaneous vascular conductance, which is consistent with some (30, 35, 36), but not all (45), previous studies of cutaneous vascular control in non-Hispanic blacks and whites. Although this is the first study to directly investigate mechanisms of cutaneous vascular control in prehypertensive individuals, previous data from individuals with overt hypertension indicate that baseline and maximal cutaneous vascular conductance sometimes, but more frequently do not, differ compared with normotensive participants (5, 15, 16, 49). This collectively suggests that basal regulation of cutaneous microvascular function and the ability of the cutaneous microvasculature to vasodilate appear to be minimally affected by race and/or blood pressure status.
Cutaneous sensory nerve-mediated vasodilation.
To our knowledge, this is the first study to directly investigate the sensory nerve contribution to local heating in prehypertensive non-Hispanic whites as well as normotensive and prehypertensive non-Hispanic blacks. Type 2 diabetes and chemotherapy are widely known to be risk factors for developing sensory nerve dysfunction (i.e., diabetic sensory neuropathy and chemotherapy-induced neuropathy, respectively), but some evidence suggests race and blood pressure may also be risk factors for developing sensory nerve dysfunction (11, 17, 21, 51). Additionally, we recently observed that the temperature threshold for cutaneous sensory nerve activation is shifted to a higher temperature in non-Hispanic blacks; however, we were unable to assess the magnitude of sensory nerve-mediated vasodilation in our previous study (52). A recent study by Patik et al. (45) found that the magnitude of the initial peak response was reduced in non-Hispanic blacks relative to non-Hispanic whites; however, determination of the contribution of sensory nerves to the initial peak was not assessed in that particular study. The magnitude of the initial peak responses in the four groups in our study were similar to previous studies (10, 45, 49), but we were unable to detect any significant differences between groups, possibly due to what appears to be greater variability in the initial peak response in our current study. It is also possible that redundant mechanisms compensated for reduced sensory nerve-mediated mechanisms; however, it is unclear what potential redundant mechanisms may be most prominent as there is very little data focused on specifically understanding the mechanisms of the initial peak response beyond those related to sensory nerve function and activation [i.e., transient receptor potential-vanilloid (TRPV)-1 channels and neurokinin-1 receptor activation]. Although the magnitude of the initial peak was not different between groups, there was minimal effect of lidocaine in normotensive non-Hispanic blacks and prehypertensive non-Hispanic blacks and non-Hispanic whites, which resulted in a reduction of the calculated sensory nerve contribution.
The initial peak is largely mediated by cutaneous sensory nerves and TRPV-1 channels (42, 55). Application of topical lidocaine resulted in reduction of the initial peak response only in normotensive non-Hispanic whites; however, there was a fairly large Δmeans for the prehypertensive non-Hispanic whites. Interestingly, lidocaine application did not attenuate the initial peak in the non-Hispanic black groups, which therefore resulted in a substantial reduction in the calculated contribution of sensory nerves to the initial peak in both non-Hispanic black groups (Fig. 2A). This suggests there is minimal contribution of cutaneous sensory nerve-mediated vasodilation to the initial peak response in both normotensive and prehypertensive non-Hispanic blacks. Contrary to our hypothesis, we also observed a diminished sensory nerve contribution in prehypertensive non-Hispanic whites, suggesting subclinical increases in blood pressure begin to exert detrimental effects on cutaneous sensory nerve-mediated mechanisms in non-Hispanic whites. Whether TRPV-1 channel function is altered in non-Hispanic blacks or those with prehypertension is unknown.
The contribution of endothelial NO-dependent and -independent contributions to the initial peak is less robust than the contribution of sensory nerve mechanisms. In the present study, we observed that NO contributed ~50% to the initial peak in normotensive non-Hispanic whites, which is similar to that reported by Choi et al. (10), from which we derived the local heating protocol in the present study. Similar to the calculated contribution of sensory nerves to the initial peak, we also observed a reduced NO component to the initial peak in prehypertensive non-Hispanic whites and both non-Hispanic black groups (Fig. 2B). Overt hypertension is known to adversely affect cutaneous vasodilation through dysregulation of the NO system (5, 15, 20), but these previous studies did not directly address the influence of blood pressure on the initial peak. The present data extend findings from previous studies in overt hypertension to suggest reduced NO signaling in populations with subclinical increases in blood pressure.
We only investigated the contribution of NO to the initial peak but, as reported by Brunt et al. (6), there appears to be a significant EDHF component. Whether this pathway is altered in non-Hispanic blacks or prehypertension is unclear; however, data from Ozkor et al. (44) demonstrated preserved EDHF vasodilation in non-Hispanic blacks, and this may be a mechanism responsible for the portion of the initial peak not attributable to either sensory nerves or NO.
Although we did not directly investigate mechanisms underlying the reduced sensory nerve and NO contributions to the initial peak, recent data from Patik et al. (45) found that inhibition of xanthine oxidase and NADPH oxidase improved the initial peak response in young, healthy non-Hispanic black individuals, suggesting that oxidative stress and, potentially, reduced bioavailable NO, may be a contributing factor. There are also data to suggest that adequate levels of bioavailable NO are necessary for neuropeptide release (e.g., CGRP, substance P) from sensory nerves (25, 26, 28, 54). We previously demonstrated that pretreatment of the skin with substance P, with and without NO synthase inhibition, significantly alters the initial peak. Whether altered neuropeptide release concomitant with reduced bioavailable NO is a plausible mechanism to explain the findings observed in prehypertensives and non-Hispanic blacks is unknown and requires direct experimental evidence.
NO-dependent plateau.
The plateau response to local heating is highly dependent on an intact endothelium, with endothelial-derived NO accounting for 60–80% of the plateau response (10, 32–34, 42). As such, this phase of the local heating response allows for direct quantification of endothelial-dependent vasodilation in vivo. Previous data demonstrated that bioavailable endothelial NO in the cutaneous microvasculature is reduced in overt hypertension as well as in young healthy non-Hispanic blacks (15, 20, 30, 35, 36, 45). The data from the present study extend these previous findings to demonstrate reduced endothelial-dependent NO vasodilation in not only normotensive non-Hispanic blacks, but also non-Hispanic whites and blacks with subclinical increases in blood pressure.
Recent mechanistic data from the Brothers laboratory (30, 35, 36, 45) provide strong support for increased oxidative stress, derived largely from xanthine oxidase and NADPH oxidase, contributing to reduced cutaneous NO-dependent vasodilation in young healthy non-Hispanic blacks. These data from the Brothers laboratory are consistent with data in hypertensive humans that indicate excessive NO production from inducible NO synthase (iNOS) in the pro-oxidant environment that exists in hypertension (15, 49). Based on these previous findings, it is likely that reduced cutaneous NO-dependent vasodilation observed in both non-Hispanic black groups and in prehypertensive non-Hispanic whites stems from heightened oxidative stress and, possibly, upregulation of iNOS but the role of iNOS in prehypertension and/or non-Hispanic blacks is not known.
Although oxidative stress appears to collectively reduce cutaneous NO-dependent vasodilation in non-Hispanic black individuals, recent data from Patik et al. (45) suggest there may be sex-related differences contributing to this response where oxidative stress is more predominant in non-Hispanic black men versus women. We observed a reduction in cutaneous NO-dependent vasodilation in both non-Hispanic black men and women, but we were not adequately powered to determine if the reduction in NO-dependent vasodilation was different between non-Hispanic black men and women.
Both endothelial-dependent and -independent vasodilation is reduced in the forearm (assessed via plethysmography) and the brachial artery (assessed via ultrasound) of non-Hispanic blacks (7, 8, 43, 44). It is thus possible the reduced NO-dependent plateau, as well as the initial peak, is due to reduced maximal vasodilation, which would result in a reduced calculated %CVCmax. Similar to previous studies (15, 16, 20, 30, 35, 36, 45, 49), we did not observe any differences in maximal CVC between groups, suggesting the present data are not a mathematical artifact. Because nitroprusside is an endothelium-independent vasodilator, these data further suggest that microvascular smooth muscle function is not affected by subclinical increases in blood pressure or by race. The different findings for smooth muscle function may be due to structural and functional differences between the cutaneous microvasculature and the brachial artery or between the cutaneous and skeletal muscle microvasculature.
In the present study, we did not observe any differences in %CVCmax for the plateau phase of local heating, which is in contrast to previous studies (30, 35, 36, 45). It is possible that these observed differences are due to differences in participant recruitment and enrollment. Previous studies from the Brothers laboratory recruited and enrolled African American and Caucasian American participants, which may also include individuals with Latinx/Hispanic ancestry. In our study, we specifically excluded any participants with Latinx/Hispanic ancestry. Whether there is a more substantial reduction in microvascular function in African Americans with Latinx/Hispanic ancestry (e.g., Panamanian, Colombian) compared with non-Hispanic blacks remains speculative. In this context, it is also possible there was partial compensation for reduced bioavailable NO by other vasodilators. Ozkor et al. (44) observed that EDHFs can partially compensate for reduced NO in non-Hispanic blacks. In individuals with subclinical cardiovascular disease, such as prehypertension, redundant mechanisms (e.g., EDHF, prostaglandins, or K+ channels) may be able to completely compensate for reduced NO-dependent vasodilation, thereby minimizing any differences in cutaneous vascular conductance. Conversely, in overt cardiovascular disease, there may be incomplete compensation by redundant mechanisms that manifests as reduced cutaneous vascular conductance. It is difficult to speculate as to which redundant mechanism(s) may be more important and/or exert the largest effect as there is currently not enough data in the literature in clinical populations. There are not enough studies using multiple pharmacological blockade via microdialysis in clinical populations to draw on. Furthermore, studies from healthy participants that have performed multiple blockades are difficult to extrapolate to (sub)clinical populations as mechanisms may change in the transition from health to (sub)clinical conditions. Empirical data using multiple blockades are clearly an important area for future research.
Limitations.
There are a few limitations to this study that warrant consideration. First, blood pressure status was only assessed three times before enrollment in the study and during the study itself rather than with 24-h ambulatory measurements. We thus only captured a small window of each participants’ daily blood pressure; however, all participants enrolled were well within the limits defining the categories of normotensive and prehypertensive.
Second, we recruited participants who self-identified as either non-Hispanic black or white as genotyping participants was well beyond the scope of this study; however, we did exclude potential participants who reported multiracial/ethnic backgrounds as well as those with Hispanic backgrounds. Because we relied on self-identification rather than objective genotyping, we cannot rule out the possibility that some of the participants may be from multiracial/ethnic backgrounds and some participants may also be of Hispanic descent.
Third, we did not perform blood analyses for lipids or glucose and, as such, we cannot rule out that some of our participants had dyslipidemia, hypercholesterolemia, or were prediabetic (no participants reported being type 1 or 2 diabetic). Inasmuch as the magnitude of responses in all groups was similar to previous studies where lipids and glucose were analyzed, it appears unlikely that our findings are the result of additional subclinical states.
Finally, although there was no statistical significance, lidocaine treatment appeared to reduce the plateau response to local heating. The plateau phase is largely mediated by endothelial NO-dependent mechanisms, and previous studies demonstrated that sensory nerve inhibition does not affect the plateau (23, 42). Although the magnitude of the mean response at lidocaine sites was lower than that at control sites, there was a large amount of variability at both control and lidocaine sites. Additionally, our pilot studies indicated that 5% lidocaine significantly inhibited the initial peak response without affecting the plateau phase; however, we cannot rule out the possibility that 5% lidocaine did have some direct effect on the plateau phase and that there may be an interaction between NO and sensory nerves during the plateau.
Conclusion.
In the present study, we provide evidence to suggest that both cutaneous sensory nerve-mediated and endothelial NO-dependent vasodilation are reduced in non-Hispanic blacks relative to non-Hispanic whites. We additionally found that sensory nerve impairments and microvascular endothelial dysfunction begin to manifest themselves with subclinical increases in blood pressure in both non-Hispanic whites and blacks. There is a plethora of data demonstrating robust sensory nerve-mediated and NO-dependent cutaneous vasodilation in young, healthy (most likely non-Hispanic white) individuals (6, 10, 22, 23, 32–34, 42, 55–57) as well as reduced cutaneous microvascular vasodilation in healthy non-Hispanic black individuals (30, 35, 36, 45) and in those with overt hypertension (5, 15, 16, 49). Our data confirm previous findings of reduced cutaneous vasodilation in healthy non-Hispanic blacks and also show that transition from normotensive to prehypertensive status elicits reductions in cutaneous vascular function. These data suggest sensory nerve and microvascular endothelial dysfunction are a continuum and may begin to develop before the development of overt CVD.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-141205 (to B. J. Wong). D. C. Walker was supported by National Institutes of Health Administrative Research Supplement to Promote Diversity in Health-Related Research (3R01HL141205-01S1) and assisted with this project as part of her Honors College thesis at Georgia State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.J.W., J.S.O., and A.A.Q. conceived and designed research; B.J.W., C.G.T., J.T.M., and D.C.W. performed experiments; B.J.W., D.C.W., Y.S., and M.J.H. analyzed data; B.J.W. interpreted results of experiments; B.J.W. prepared figures; B.J.W. drafted manuscript; B.J.W., C.G.T., J.T.M., D.C.W., Y.S., M.J.H., J.S.O., and A.A.Q. edited and revised manuscript; B.J.W., C.G.T., J.T.M., D.C.W., Y.S., M.J.H., J.S.O., and A.A.Q. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all of the participants for willingness to take part in this study and Joseph Hwang for assistance with data collection.
REFERENCES
- 1.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg 42: 574–581, 2005. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa TC, Kaur J, Stephens BY, Akins JD, Keller DM, Brothers RM, Fadel PJ. Attenuated forearm vascular conductance responses to rhythmic handgrip in young African-American compared with Caucasian-American men. Am J Physiol Heart Circ Physiol 315: H1316–H1321, 2018. doi: 10.1152/ajpheart.00387.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, , et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2019 update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 4.Brothers RM, Fadel PJ, Keller DM. Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am J Physiol Heart Circ Physiol 317: H777–H789, 2019. doi: 10.1152/ajpheart.00126.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruning RS, Kenney WL, Alexander LM. Altered skin flowmotion in hypertensive humans. Microvasc Res 97: 81–87, 2015. doi: 10.1016/j.mvr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 40: 754–760, 2002. doi: 10.1016/S0735-1097(02)02015-6. [DOI] [PubMed] [Google Scholar]
- 8.Cardillo C, Kilcoyne CM, Cannon RO III, Panza JA. Racial differences in nitric oxide-mediated vasodilator response to mental stress in the forearm circulation. Hypertension 31: 1235–1239, 1998. doi: 10.1161/01.HYP.31.6.1235. [DOI] [PubMed] [Google Scholar]
- 9.Carter SJ, Hodges GJ. Sensory and sympathetic nerve contributions to the cutaneous vasodilator response from a noxious heat stimulus. Exp Physiol 96: 1208–1217, 2011. doi: 10.1113/expphysiol.2011.059907. [DOI] [PubMed] [Google Scholar]
- 10.Choi PJ, Brunt VE, Fujii N, Minson CT. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol (1985) 117: 277–283, 2014. doi: 10.1152/japplphysiol.01397.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen JA, Jeffers BW, Faldut D, Marcoux M, Schrier RW. Risks for sensorimotor peripheral neuropathy and autonomic neuropathy in non-insulin-dependent diabetes mellitus (NIDDM). Muscle Nerve 21: 72–80, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 12.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens 26: 56–63, 2012. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 13.Craighead DH, Alexander LM. Topical menthol increases cutaneous blood flow. Microvasc Res 107: 39–45, 2016. doi: 10.1016/j.mvr.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craighead DH, McCartney NB, Tumlinson JH, Alexander LM. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc Res 110: 43–47, 2017. doi: 10.1016/j.mvr.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craighead DH, Smith CJ, Alexander LM. Blood pressure normalization via pharmacotherapy improves cutaneous microvascular function through NO-dependent and NO-independent mechanisms. Microcirculation 24: e12382, 2017. doi: 10.1111/micc.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craighead DH, Wang H, Santhanam L, Alexander LM. Acute lysyl oxidase inhibition alters microvascular function in normotensive but not hypertensive men and women. Am J Physiol Heart Circ Physiol 314: H424–H433, 2018. doi: 10.1152/ajpheart.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emdin CA, Anderson SG, Woodward M, Rahimi K. Usual blood pressure and risk of new-onset diabetes: evidence from 4.1 million adults and a meta-analysis of prospective studies. J Am Coll Cardiol 66: 1552–1562, 2015. doi: 10.1016/j.jacc.2015.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol 95: 946–954, 2010. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- 19.Greaney JL, Dillon GA, Saunders EF, Alexander LM. Peripheral microvascular serotoninergic signaling is dysregulated in young adults with major depressive disorder. J Appl Physiol (1985) 128: 100–107, 2020. doi: 10.1152/japplphysiol.00603.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greaney JL, Kutz JL, Shank SW, Jandu S, Santhanam L, Alexander LM. Impaired hydrogen sulfide-mediated vasodilation contributes to microvascular endothelial dysfunction in hypertensive adults. Hypertension 69: 902–909, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hébert HL, Veluchamy A, Torrance N, Smith BH. Risk factors for neuropathic pain in diabetes mellitus. Pain 158: 560–568, 2017. doi: 10.1097/j.pain.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodges GJ, Chiu C, Kosiba WA, Zhao K, Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol (1985) 106: 1112–1118, 2009. doi: 10.1152/japplphysiol.91508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodges GJ, Del Pozzi AT, McGarr GW, Mallette MM, Cheung SS. The contribution of sensory nerves to cutaneous vasodilatation of the forearm and leg to local skin heating. Eur J Appl Physiol 115: 2091–2098, 2015. doi: 10.1007/s00421-015-3188-7. [DOI] [PubMed] [Google Scholar]
- 24.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 25.Holzer P, Jocic M. Cutaneous vasodilatation induced by nitric oxide-evoked stimulation of afferent nerves in the rat. Br J Pharmacol 112: 1181–1187, 1994. doi: 10.1111/j.1476-5381.1994.tb13208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holzer P, Wachter C, Heinemann A, Jocic M, Lippe IT, Herbert MK. Sensory nerves, nitric oxide and NANC vasodilatation. Arch Int Pharmacodyn Ther 329: 67–79, 1995. [PubMed] [Google Scholar]
- 27.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2006. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes SR, Brain SD. Nitric oxide-dependent release of vasodilator quantities of calcitonin gene-related peptide from capsaicin-sensitive nerves in rabbit skin. Br J Pharmacol 111: 425–430, 1994. doi: 10.1111/j.1476-5381.1994.tb14752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurr C, Kim K, Harrison ML, Brothers RM. Attenuated cerebral vasodilatory capacity in response to hypercapnia in college-aged African Americans. Exp Physiol 100: 35–43, 2015. doi: 10.1113/expphysiol.2014.082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurr C, Patik JC, Kim K, Christmas KM, Brothers RM. Tempol augments the blunted cutaneous microvascular thermal reactivity in healthy young African Americans. Exp Physiol 103: 343–349, 2018. doi: 10.1113/EP086776. [DOI] [PubMed] [Google Scholar]
- 31.Keen JT, Levitt EL, Hodges GJ, Wong BJ. Short-term dietary nitrate supplementation augments cutaneous vasodilatation and reduces mean arterial pressure in healthy humans. Microvasc Res 98: 48–53, 2015. doi: 10.1016/j.mvr.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Kellogg DL Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol (1985) 86: 1185–1190, 1999. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 33.Kellogg DL Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellogg DL Jr, Zhao JL, Wu Y, Johnson JM. Antagonism of soluble guanylyl cyclase attenuates cutaneous vasodilation during whole body heat stress and local warming in humans. J Appl Physiol (1985) 110: 1406–1413, 2011. doi: 10.1152/japplphysiol.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K, Brothers RM. Acute consumption of flavanol-rich cocoa beverage improves attenuated cutaneous microvascular function in healthy young African Americans. Microvasc Res 128: 103931, 2020. doi: 10.1016/j.mvr.2019.103931. [DOI] [PubMed] [Google Scholar]
- 36.Kim K, Hurr C, Patik JC, Matthew Brothers R. Attenuated cutaneous microvascular function in healthy young African Americans: Role of intradermal l-arginine supplementation. Microvasc Res 118: 1–6, 2018. doi: 10.1016/j.mvr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Kruger A, Stewart J, Sahityani R, O’Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006. doi: 10.1038/sj.ki.5001511. [DOI] [PubMed] [Google Scholar]
- 38.Levitt EL, Keen JT, Wong BJ. Augmented reflex cutaneous vasodilatation following short-term dietary nitrate supplementation in humans. Exp Physiol 100: 708–718, 2015. doi: 10.1113/EP085061. [DOI] [PubMed] [Google Scholar]
- 39.Magerl W, Treede RD. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol 497: 837–848, 1996. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mata-Greenwood E, Chen DB. Racial differences in nitric oxide-dependent vasorelaxation. Reprod Sci 15: 9–25, 2008. doi: 10.1177/1933719107312160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol (1985) 109: 1239–1246, 2010. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 43.Neuman RB, Hayek SS, Poole JC, Rahman A, Menon V, Kavtaradze N, Polhemus D, Veledar E, Lefer DJ, Quyyumi AA. Nitric oxide contributes to vasomotor tone in hypertensive African Americans treated with nebivolol and metoprolol. J Clin Hypertens (Greenwich) 18: 223–231, 2016. doi: 10.1111/jch.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozkor MA, Rahman AM, Murrow JR, Kavtaradze N, Lin J, Manatunga A, Hayek S, Quyyumi AA. Differences in vascular nitric oxide and endothelium-derived hyperpolarizing factor bioavailability in blacks and whites. Arterioscler Thromb Vasc Biol 34: 1320–1327, 2014. doi: 10.1161/ATVBAHA.113.303136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patik JC, Curtis BM, Nasirian A, Vranish JR, Fadel PJ, Brothers RM. Sex differences in the mechanisms mediating blunted cutaneous microvascular function in young black men and women. Am J Physiol Heart Circ Physiol 315: H1063–H1071, 2018. doi: 10.1152/ajpheart.00142.2018. [DOI] [PubMed] [Google Scholar]
- 46.Selassie A, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension 58: 579–587, 2011. doi: 10.1161/HYPERTENSIONAHA.111.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen J, Poole JC, Topel ML, Bidulescu A, Morris AA, Patel RS, Binongo JG, Dunbar SB, Phillips L, Vaccarino V, Gibbons GH, Quyyumi AA. Subclinical vascular dysfunction associated with metabolic syndrome in African Americans and whites. J Clin Endocrinol Metab 100: 4231–4239, 2015. doi: 10.1210/jc.2014-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith CJ, Craighead DH, Alexander LM. Effects of vehicle microdialysis solutions on cutaneous vascular responses to local heating. J Appl Physiol (1985) 123: 1461–1467, 2017. doi: 10.1152/japplphysiol.00498.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. Increased angiotensin ii sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension 70: 382–389, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH; EURODIAB Prospective Complications Study Group . Vascular risk factors and diabetic neuropathy. N Engl J Med 352: 341–350, 2005. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 52.Turner CG, Miller JT, Otis JS, Hayat MJ, Quyyumi AA, Wong BJ. Cutaneous sensory nerve-mediated microvascular vasodilation in normotensive and prehypertensive non-Hispanic Blacks and Whites. Physiol Rep 8: e14437, 2020. doi: 10.14814/phy2.14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whelton P, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 71: e13–e115, 2017. [DOI] [PubMed] [Google Scholar]
- 54.Wong BJ. Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 304: R651–R656, 2013. doi: 10.1152/ajpregu.00464.2012. [DOI] [PubMed] [Google Scholar]
- 55.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 588: 4317–4326, 2010. doi: 10.1113/jphysiol.2010.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong BJ, Minson CT. Altered thermal hyperaemia in human skin by prior desensitization of neurokinin-1 receptors. Exp Physiol 96: 599–609, 2011. doi: 10.1113/expphysiol.2011.057356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong BJ, Williams SJ, Minson CT. Minimal role for H1 and H2 histamine receptors in cutaneous thermal hyperemia to local heating in humans. J Appl Physiol (1985) 100: 535–540, 2006. doi: 10.1152/japplphysiol.00902.2005. [DOI] [PubMed] [Google Scholar]