Abstract
Few patients with bacteremia from a nonpulmonary source develop acute respiratory distress syndrome (ARDS). However, the mechanisms that protect the lung from injury in bacteremia have not been identified. We simulated bacteremia by adding Streptococcus pneumoniae to the perfusate of the ex vivo perfused human lung model. In contrast to a pneumonia model in which bacteria were instilled into the distal air spaces of one lobe, injection of high doses of S. pneumoniae into the perfusate was not associated with alveolar epithelial injury as demonstrated by low protein permeability of the alveolar epithelium, intact alveolar fluid clearance, and the absence of alveolar edema. Unexpectedly, the ex vivo human lung rapidly cleared large quantities of S. pneumoniae even though the perfusate had very few intravascular phagocytes and lacked immunoglobulins or complement. The bacteria were cleared in part by the small number of neutrophils in the perfusate, alveolar macrophages in the airspaces, and probably by interstitial pathways. Together, these findings identify one mechanism by which the lung and the alveolar epithelium are protected from injury in bacteremia.
Keywords: acute lung injury, ARDS, epithelial injury, sepsis, shock
INTRODUCTION
Acute respiratory distress syndrome (ARDS) develops in approximately 7% of patients who present to the emergency department with sepsis (8, 10). However, ARDS is much more common in patients with sepsis from pneumonia than patients with sepsis from a nonpulmonary source (33, 38). Bacteremia is a frequent clinical problem, but nonpulmonary sepsis without shock leads to acute lung injury and ARDS in only 1.4% of cases (8). These findings suggest that the lung may be protected from injury caused by nonpulmonary bacteremia.
Experimental and clinical studies have demonstrated that alveolar epithelial injury is critical to the pathogenesis of both ARDS and noncardiogenic pulmonary edema (5, 30, 31, 35, 36, 41). Our prior studies in the ex vivo perfused human lung have shown that intra-alveolar instillation of bacteria, as a model of pneumonia, leads to alveolar epithelial and endothelial injury. This manifests as an increase in protein permeability across both the endothelial and epithelial barriers, and by impaired alveolar fluid clearance (25, 27). Animal studies suggest that the lungs may play an important role in the clearance of bacteremia (4, 6). However, the effect of bacteremia on the alveolar epithelium has not been studied in a clinically relevant human lung model, and there is no information on clearance of circulating bacteria by the human lung in the absence of the reticuloendothelial system of the liver and spleen. Furthermore, the role of circulating blood in the clearance of bacteria by the isolated human lung has not been studied.
Therefore, these studies were designed to address the following questions. First, does high-dose intravenous bacteremia in the isolated perfused human lung produce physiologic and functional injury to the alveolar epithelium? Second, does the isolated perfused human lung have the endogenous capacity to clear bacteremia? And third, what mechanisms contribute to the clearance of bacterermia by the isolated perfused lung?
MATERIALS AND METHODS
Study approval.
Explicit approval for the use of donor lungs for research was sought from each donor’s family by Donor Network West.
Ex vivo perfused lung preparation.
The ex vivo human lung preparation was reviewed recently (32). In brief, donor lungs that had been rejected for transplantation were received from Donor Network West. Lungs may be rejected for a wide range of reasons, including a mismatch in sex, race, size, or geography between the donor and available recipients, donor age or smoking history, radiographic or bronchoscopic findings that suggest atelectasis, edema or infection, or other elements of the donor history or clinical course (3, 23). All available lungs that could technically be perfused (i.e., no lacerations to the pleura or hilar vessels and pulmonary artery of at least 1 cm in length) were perfused. The right and left lungs were separated, and one lung was selected for these studies based on gross appearance. A cannula was placed in the pulmonary artery and secured with a purse-string suture. The main bronchus was cannulated with a standard no. 7.5 endotracheal tube. The lung preparation was weighed and suspended in a custom-made acrylic chamber (Fig. 1). The chamber was partially submerged in a water bath, and the base of the chamber served as a reservoir for the perfusate solution. The temperature of the perfusate at the pulmonary vein was maintained at 37°C. The lung was perfused with two liters of DME-H21 medium containing 5% bovine serum albumin (BSA) at 37°C with a roller pump (Terumo Sarns, Tokyo, Japan). The pulmonary veins were not cannulated, allowing the perfusate to drain passively from the pulmonary veins into the reservoir at the base of the acrylic chamber from where it was recycled through the pump. The pulmonary arterial pressure was measured via a pulmonary arterial catheter (Cook, Bloomington, IN) placed in the circuit at the level of the left atrium. The pump flow rate (usually ~0.2 L/min) was adjusted to maintain a pulmonary arterial pressure of 10–12 mmHg (Biopac, Santa Barbara, CA) to prevent hydrostatic pulmonary edema. Once the preparation reached 36°C, continuous positive airway pressure of 8 cmH2O was applied using room air. In some experiments, 100 mL of fresh whole blood were added to the perfusate 1 h after the target experimental condition was reached, since prior authors have demonstrated that a small amount of blood in the circuit was required to generate a robust immunologic response to the administration of bacterial components in the airspaces (24). Fresh whole blood in these experiments was supplied in the morning of each experiment from three investigators (J.T.R., N.N., M.A.M.), based on availability of the investigator. Perfusate samples were collected from a pulmonary artery catheter.
Fig. 1.
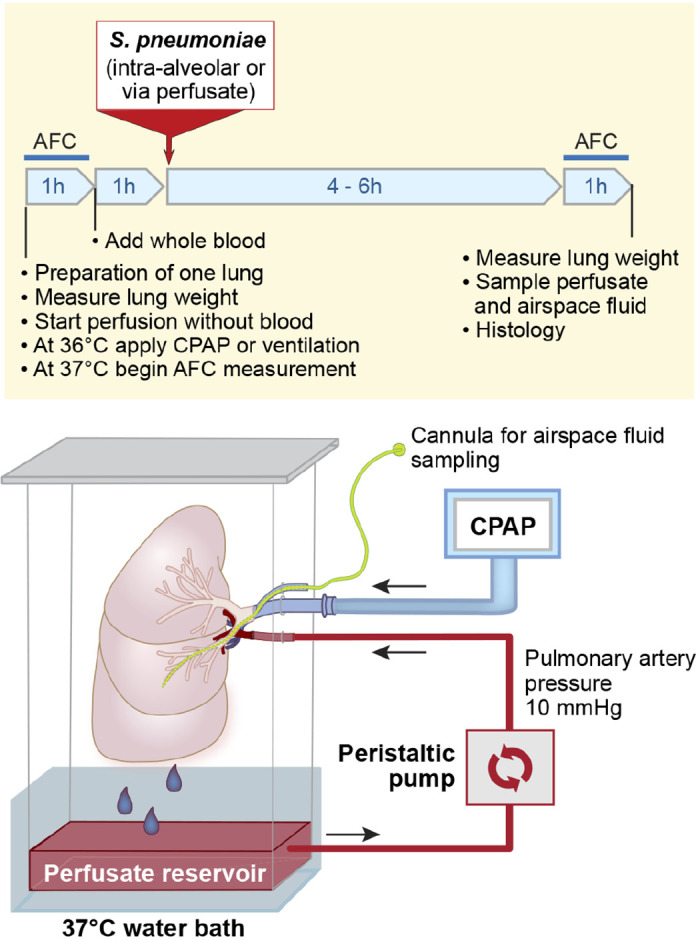
Schematic diagram of the ex vivo perfused human lung. Human donor lungs rejected for transplantation are perfused with 5% BSA in DME-H21 high-glucose medium at 37°C, via a cannula sewn into the pulmonary artery. In some experiments, 100 mL of fresh whole blood is added to the perfusate. The perfusion rate is adjusted to 0.2 L/min via a roller pump, to maintain a pulmonary arterial pressure of 10 mmHg. Perfusate drains passively from the pulmonary veins into a reservoir at the bottom of the chamber. The main bronchus is cannulated with an endotracheal tube, and the lung is inflated using room air with continuous positive airway pressure of 8 cmH2O. A second cannula is inserted through a side port in the endobronchial tube and passed into the distal airspaces of the desired lobe, to allow sampling of the alveolar fluid. Perfusate was sampled from a pulmonary artery catheter. S. pneumoniae, Streptococcus pneumoniae; AFC, alveolar fluid clearance; CPAP, continuous positive airway pressure.
Preparation, labeling, and administration of Streptococcus pneumoniae.
Streptococcus pneumoniae serotype 19F (49619; ATCC, Manassas, VA) was grown in brain-heart broth (Becton-Dickinson, Sparks MD), harvested at midlog phase, centrifuged, and resuspended in PBS. The dose (1010 bacteria) was determined by weight-based adjustment from a murine model of severe pneumonia and sepsis developed in our laboratory (11, 12). Bacteria were either added to the perfusate (intravenous exposure, a model of bacteremia) or instilled into the distal airspaces (a model of pneumonia) 2 h after target temperature was reached. In some experiments, bacteria were labeled with carboxyfluorescein succinimidyl ester (CFSE, CellTrace CFSE Cell Proliferation Kit; Invitrogen, Eugene, OR; see Ref. 34) before its addition to the perfusate. Of note, CFSE addition did not alter bacterial growth kinetics.
Measurement of pulmonary edema.
Pulmonary edema was estimated by measuring the change in weight of the perfused lung between the beginning and the end of the experiment (hours 0 and 6 were chosen as accurate weight measurement required disconnecting the lung from the perfusion circuit). The relationship between pulmonary edema and weight gain in this ex vivo human lung preparation has been recently reviewed; lung weight gain after experimental ex vivo perfusion is a good proxy for pulmonary edema (32).
Measurement of alveolar fluid clearance.
After equilibration of the lung on the circuit, a catheter (PE-240 tubing; BD) was inserted through a side port in the endobronchial tube and advanced into the desired lobe. One hundred milliliters of 37°C 5% BSA in normal saline was instilled via the catheter into the distal airspaces. Fluid samples were obtained at 5 and 35 min, and the total protein in each sample was measured by refractometry. Alveolar fluid clearance (AFC) was calculated using the following formula: AFC (%/h) = 2(1 – Ci/Cf), where Ci is the protein concentration of the 5-min sample and Cf is the protein concentration of the 35-min sample (9, 28). AFC was measured after equilibration of the lung on the circuit, and at 5 h (to allow full AFC measurement before the end of the experiment at 6 h).
Measurement of IgM in alveolar fluid.
The measurement of IgM in alveolar fluid has been used for the assessment of endothelial and epithelial protein permeability in patients with ARDS, as published in this journal (16). IgM concentration in airspace fluid was measured by ELISA (Abcam, Cambridge, UK). Because a known volume of alveolar fluid was present at the start of the experiment (100 mL of 5% albumin), and the rate of alveolar fluid clearance was measured, the mass of IgM in the airspace at each time point was calculated to allow comparison between experiments. IgM concentration at 4 h was chosen for comparison, since preliminary experiments showed peak concentrations of IgM in the airspace at this time point.
Perfusate cell surface protein staining for flow cytometry.
Perfusate samples were collected before and 3 h after the addition of intravenous CFSE-labeled S. pneumoniae. Samples were centrifuged at 1,500 rpm for 15 min and resuspended in phosphate-buffered saline (PBS). A cocktail of surface protein monoclonal antibodies for CD45 APC-EF780 (eBioscience), CD14 PE (BD Bioscience), CD66b Alexa Fluor 647 (BD Bioscience), and CD3 V500 (BD Bioscience) was added. After 20 min, 1 mL of red blood cell lysis buffer (BD FACS Lysing Solution; BD Biosciences) was added for 20 min, followed by two wash steps (PBS, 2% fetal bovine serum, 0.1% sodium azide). All incubations were performed at room temperature in the dark. Samples were read on a BD LSRII flow cytometer, and data were analyzed using FlowJo software (Treestar, Ashland, OR).
Monocyte phagocytosis assay.
Whole blood (10 mL) was collected from healthy donors and diluted with 10 ml D-PBS. This mixture was then overlaid on 15 mL of Ficoll-Paque Plus (BD Biosciences) density gradient and centrifuged at 800 g for 30 min. The middle layer, containing mononuclear cells, was collected, washed two times with D-PBS, resuspended using RPMI 1640 + 10% FBS, and placed in a 24-well plate (5 × 105 cells in 500 μL/well). S. pneumoniae (50 µL) in D-PBS [20 million colony-forming units (CFU), preparation described above] or sterile D-PBS were added to wells containing monocytes, or to control wells containing media with no human cells. The multiplicity of infection was 40. After 2 h of incubation at 37°C and 5% CO2, the supernatant media underwent 10-fold serial dilutions in D-PBS and was plated on blood agar plates. The plates were then incubated overnight at 37°C and 5% CO2, before counting the number of CFUs per plate and calculating the total CFU per well.
Statistics.
Results are expressed as means ± SD if the data were normally distributed and as the median and interquartile range if the data were not normally distributed. Comparisons between two groups were made using the unpaired t test, paired t test, Mann-Whitney test, Chi square test, or Fisher’s exact test as appropriate. Statistical analysis was performed with Prism8 (GraphPad, San Diego, CA).
RESULTS
Clinical characteristics of lung donors.
We studied 99 human lungs from 99 organ donors whose lungs were rejected for transplantation. Donor median age was 45 yr, 68% of the donors were male, and 30% of deaths were attributed to trauma. The majority of lungs were procured from brain-dead donors (91%), with the remainder of lungs donated after cardiac death (9%). The median ischemia time was 23 h. There were no significant differences in sex, age, -to- ratio, ischemia time, trauma, cardiopulmonary resuscitation administration, or donation after cardiac death in the control relative to the intervention groups (Table 1).
Table 1.
Clinical characteristics of human lung donors
| Control (n = 40) | Intravenous pneumococcus (n = 42) | Airspace pneumococcus (n = 17) | P | |
|---|---|---|---|---|
| Male, n (%) | 26 (65) | 29 (69) | 12 (71) | 0.2 |
| Age, yr | 41 (35–51) | 47 (32–58) | 48 (36–58) | 0.6 |
| P/F ratio* | 262 (207–337) | 329 (239–404) | 254 (124–409) | 0.1 |
| Ischemia time, h† | 22 (10–31) | 25 (10–31) | 19 (9–30) | 0.9 |
| Trauma, n (%) | 11 (28) | 13 (31) | 6 (35) | 0.4 |
| CPR, n (%) | 23 (58) | 22 (52) | 11 (65) | 0.8 |
| DCD donors, n (%)‡ | 4 (10) | 4 (10) | 1 (6) |
There were a total of n = 99 donors.
-to-ratio (P/F) calculated from challenge gas with 100% when available, or standard gas when unavailable.
Ischemia time is the time from aortic cross-clamp of donor (or time of loss of cardiac function in DCD donors) to the time at which the lung is perfused in the ex vivo preparation.
DCD donors, donors after cardiac death, are donors without cardiac function at the time of organ procurement. In contrast, the majority of organ donors in the United States are “brain dead” donors who lack brainstem function but who have cardiac function at the time of organ procurement.
CPR, cardiopulmonary resuscitation. Comparisons via Kruskal-Wallis or Chi square tests.
Lung fluid and protein balance.
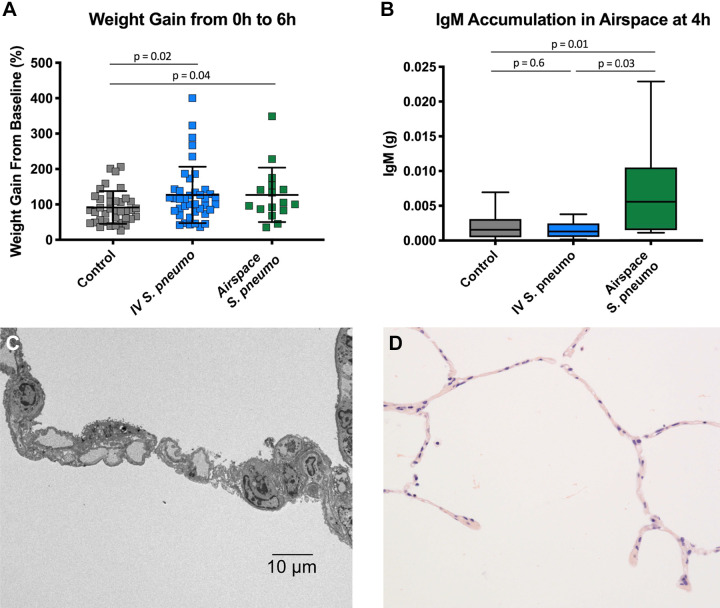
The majority of the lungs (81%) had normal alveolar fluid clearance at baseline (>10%/h), as in our prior publications (9). Control lungs without bacteria had a modest weight gain over a 6-h experiment (Fig. 2A). Lungs exposed to intravenous or airspace S. pneumoniae showed a comparable increase in weight gain over 6 h that was significantly greater relative to the weight gain observed in control lungs.
Fig. 2.
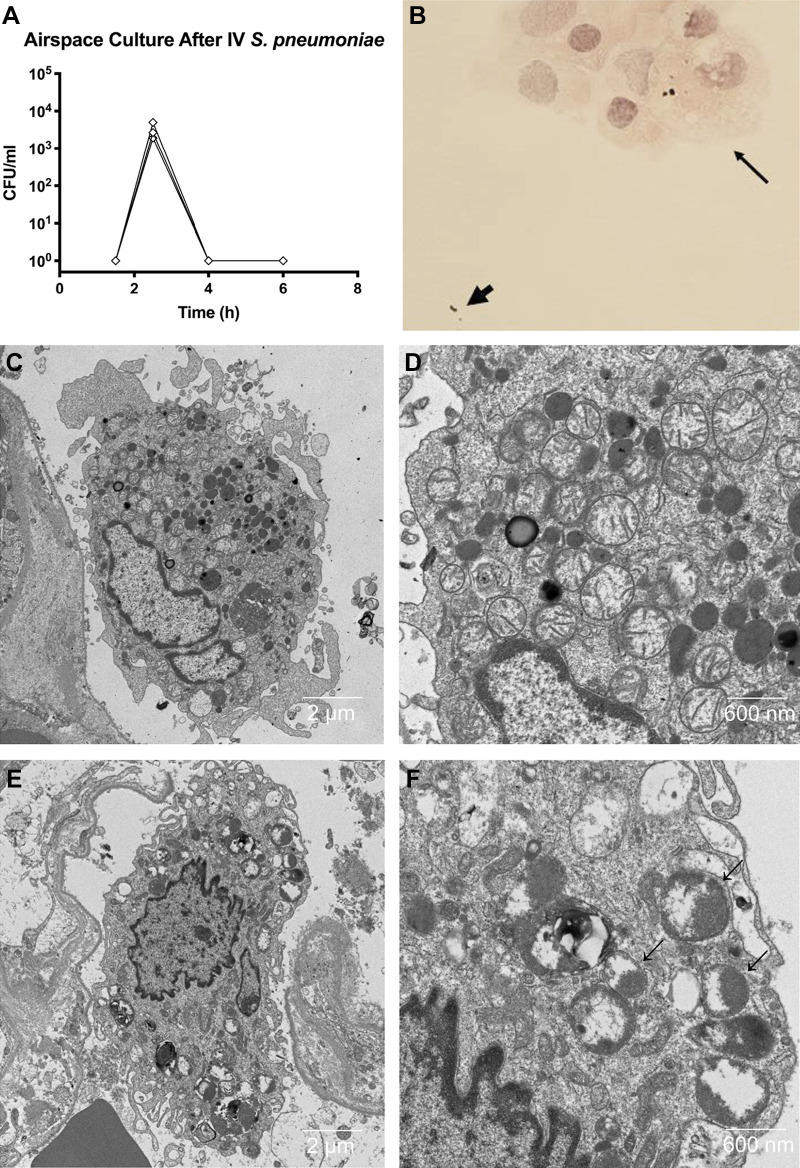
Measures of endothelial and epithelial injury. A: comparison of percent weight gain over the 6-h experiment demonstrated significant weight gain in both the iv (n = 40) and airspace Streptococcus pneumoniae (n = 16) groups, showing that the introduction of bacteria into either compartment produces endothelial injury (control n = 38). Analysis includes lungs with and without exogenous blood. Comparison via unpaired t test. B: comparison of the quantity of IgM in the airspace fluid at 4 h demonstrated that only lungs with airspace S. pneumoniae (n = 10) accumulated significant IgM in the airspace fluid relative to controls (controls n = 13, iv n = 9). This analysis includes only lungs with blood added to the perfusate, since this is the source of IgM in the preparation. Comparison via unpaired t test. C: representative transmission electron micrograph 4 h after addition of iv bacteria do not show ultrastructural changes to the epithelium despite rapid translocation of bacteria across the epithelium and into the airspaces. D: representative light micrograph 2 h after the addition of iv S. pneumoniae, demonstrating intact alveolar septae without alveolar edema.
Intravenous S. pneumoniae did not alter alveolar epithelial permeability.
The predominant source of IgM in the ex vivo human lung preparation was the fresh whole blood added to the perfusate. IgM cannot cross the intact alveolar epithelium because of molecular size (970 kDa), making it a good marker of increased epithelial permeability to protein (29). There was no difference in IgM accumulation in the airspaces of the intravenous S. pneumoniae lungs compared with control lungs (Fig. 2B). In contrast, lungs exposed to airspace S. pneumoniae accumulated a significantly greater quantity of IgM than control lungs. Consistent with these data, lung ultrastructural studies showed no injury to the alveolar epithelium after intravenous S. pneumoniae (Fig. 2C). Furthermore, there was no evidence of alveolar edema in the histology sections from the intravenous S. pneumoniae studies (Fig. 2D).
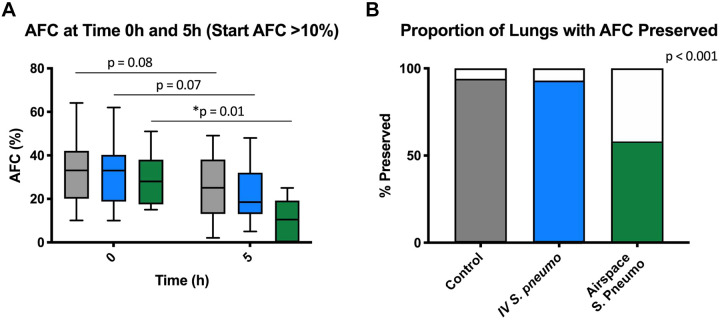
Intravenous S. pneumoniae did not impair alveolar fluid clearance.
There was no statistically significant difference in the rate of alveolar fluid clearance (AFC) between 1 and 5 h in lungs exposed to intravenous S. pneumoniae or the control lungs (Fig. 3A). In contrast, lungs exposed to airspace S. pneumoniae showed a significant decrease in AFC between 0 and 5 h. Because baseline AFC in human lungs can be variable (9), we carried out an additional analysis comparing the proportion of lungs in each experimental group in which a normal AFC was preserved (i.e., AFC ≥10% at 1 and 5 h). According to this analysis, most lungs in the control and in the intravenous S. pneumoniae groups had preserved AFC while a majority of the lungs in the airspace S. pneumoniae group had compromised AFC (P < 0.001, Fig. 3B).
Fig. 3.
Measures of alveolar fluid clearance (AFC). A: comparison of AFC at 0 and 5 h demonstrated a significant drop in AFC in lungs that were infected with airspace Streptococcus pneumoniae (n = 12) but not iv S. pneumoniae (iv n = 30, controls n = 31). Comparison via paired t test. B: comparison of the proportion of lungs in each group with AFC preserved throughout experiment (i.e., proportion of lungs in which AFC at 1 and 5 h both ≥10%). Comparison via Chi square test.
Intravenous S. pneumoniae was rapidly cleared by the ex vivo lung.
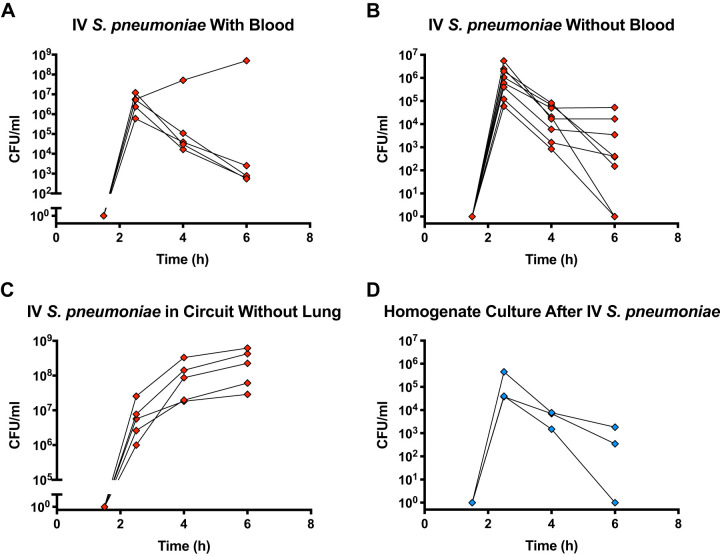
Cultures of the perfusate before and after the addition of 1010 S. pneumoniae to the perfusate in the presence of a lung demonstrated a rise in colony-forming units (CFU) 30 min after the addition of bacteria, followed by a 10,000-fold reduction over 3.5 h (Fig. 4A). This marked reduction in bacterial burden over time was observed in 12 of 13 experiments. The lung that lacked a reduction in bacterial growth came from a donor after cardiac death who had severe ARDS (/ ratio 78 mmHg) and likely had significant injury before the experiment.
Fig. 4.
Cultures of perfusate after addition of iv Streptococcus pneumoniae. A: cultures of perfusate after the addition of iv S. pneumoniae in experiments with a lung and with 100 mL of fresh whole blood added to the perfusate (n = 5). B: cultures of perfusate in experiments without added blood (n = 8). C: cultures of perfusate after addition of iv S. pneumoniae in experiments with a standard perfusate, circuit, and with addition of 100 mL of fresh whole blood, but without a lung (n = 5). D: cultures of lung homogenate after iv S. pneumoniae (n = 3).
To determine whether bacteria were cleared by phagocytes in the blood added to the perfusate, we repeated the experiment without adding blood to the perfusate and observed the same pattern of bacterial clearance as in experiments with exogenous blood added to the perfusate (Fig. 4B). To confirm that the bacteria were not simply adhering to the plastic perfusion circuit, we administered S. pneumoniae to the perfusion circuit with added blood but in the absence of a lung and observed robust bacterial growth in the perfusate (Fig. 4C). Therefore, the decrease in bacteria was not explained by the effect of the perfusate, the perfusion circuit, or the addition of fresh exogenous blood to the circuit. Furthermore, cultures of lung homogenate after the addition of intravenous S. pneumoniae corroborated the observation of bacterial clearance in the perfusate (Fig. 4D). Thus, the decrease in bacteria could not be attributed to the accumulation or sequestration of live bacteria in the microcirculation or the parenchyma of the lung.
A total of 70% of lung donors received antibiotics in the donor management period, either to treat an existing infection or as surgical prophylaxis during organ procurement. However, as part of the procurement protocol, lungs were flushed with a low-potassium dextran solution, and, upon perfusion of the ex vivo lung, any residual antibiotic was further diluted with 2 liters of perfusate solution (32). Moreover, donors of 6 of the 13 lungs with a significant drop in bacterial burden had not received antibiotics. Furthermore, the donor of the one lung with robust bacterial growth was treated with an antibiotic (cefazolin). Therefore, donor antibiotics did not appear to have an effect on the results of our experiments.
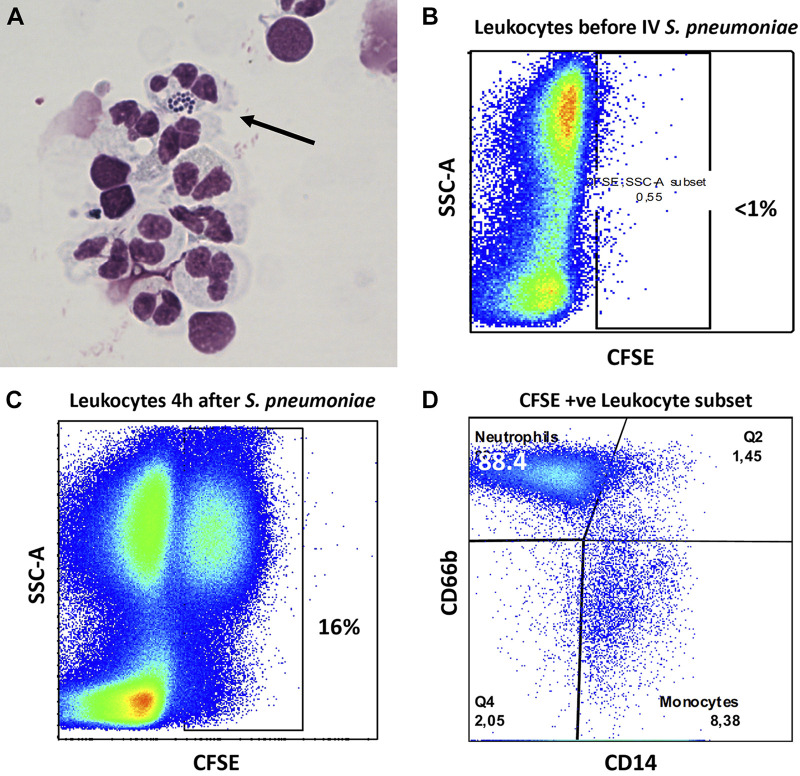
Neutrophils in the perfusate contributed to the clearance of S. pneumoniae.
In experiments without exogenous blood, cytospin of the perfusate 4 h after the addition of intravenous S. pneumoniae demonstrated the presence of some neutrophils that phagocytosed S. pneumoniae (Fig. 5A). These findings were supported by flow cytometry analysis of the perfusate 4 h after addition of intravenous CFSE-labeled S. pneumoniae. Approximately 16% of donor-derived leukocytes in the perfusate phagocytosed S. pneumoniae. Of these cells, approximately 90% were neutrophils and 8% were monocytes (Fig. 5, B–D). Notably, these findings suggest that either neutrophils remained in the vascular compartment after it was flushed during organ procurement or that neutrophils migrated into the vascular from the interstitial compartment after perfusion. However, the absolute neutrophil count of the perfusate in experiments without exogenous blood was only 20 cells/μL.
Fig. 5.
Bacterial clearance from the vascular compartment. A: cytospin of perfusate following iv Streptococcus pneumoniae in an experiment without added blood, including a circulating neutrophil containing multiple S. pneumoniae (thin arrow). B: flow cytometry of perfusate samples before the addition of carboxyfluorescein succinimidyl ester (CFSE)-labeled S. pneumoniae. C: flow cytometry of perfusate samples after the addition of CFSE-labeled S. pneumoniae. D: flow cytometry of the CFSE-positive leukocyte population (CD66b +ve cells represent neutrophils, CD14 +ve cells represent monocytes).
Alveolar macrophages in the airspaces contributed to the clearance of S. pneumoniae.
Cultures of airspace fluid after the addition of intravenous S. pneumoniae to the ex vivo perfused human lung preparation demonstrated that bacteria translocated from the perfusate to the airspaces within 30 min (Fig. 6A). Similar to the pattern observed in the perfusate, bacterial numbers in the airspace fluid declined over time. Cytospin of alveolar fluid samples demonstrated that alveolar macrophages are involved in phagocytosis of S. pneumoniae (Fig. 6B). Transmission electron microscopy similarly demonstrated that bacteria in the airspace were phagocytosed by alveolar macrophages (Fig. 6, C–F). In addition, in vitro studies demonstrated that alveolar macrophages isolated from donor lungs impaired growth of S. pneumoniae (Fig. 7).
Fig. 6.
Bacterial clearance from the airspaces. A: cultures of alveolar fluid after the addition of iv Streptococcus pneumoniae demonstrating the rapid translocation of bacterial from the vascular compartment to the airspaces (n = 3, same experiments as Fig. 4B). B: cytospin of alveolar fluid 30 min after the addition of iv S. pneumoniae in an experiment without added blood, showing bacteria free in the alveolar fluid (thick arrow) and bacteria that have been phagocytosed by an alveolar macrophage (thin arrow). C and D: representative transmission electron microscopy (TEM) in control lung at 4 h showing an alveolar macrophage without bacteria. E and F: representative TEM 4 h after addition of iv bacteria showing an alveolar macrophage containing multiple S. pneumoniae bacteria (arrows). CFU, colony-forming units.
Fig. 7.
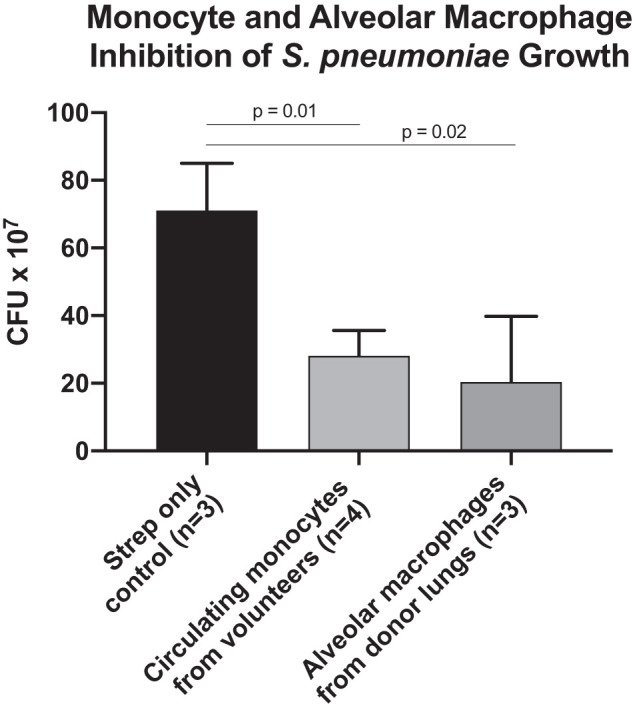
Monocytes and alveolar macrophages inhibit Streptococcus pneumoniae growth. Cultures of 2 × 107 S. pneumoniae incubated for 2 h in culture media alone (control), with 5 × 105 monocytes isolated from the blood of healthy volunteers, or with 5 × 105 alveolar macrophages isolated from donor human lungs. Comparisons using Mann-Whitney test. CFU, colony-forming units.
DISCUSSION
Several experimental and clinical studies have established that injury to the alveolar epithelium is a critical factor in the development of alveolar edema and ARDS (30). The overall objective of these experiments was to study the effects of high doses of a clinically relevant pathogen (S. pneumoniae) on the function of the alveolar epithelium in the human lung, using a model of bacteremia compared with a model of pneumonia. The primary findings can be summarized as follows. A high dose of intravenous S. pneumoniae caused a modest increase in lung weight consistent with endothelial injury, but, in contrast to the pneumonia model, bacteremia did not injure the alveolar epithelium. In addition, the bacteria were rapidly cleared from the intravascular compartment even in the absence of immunoglobulins, complement, and phagocytic immune cells. Some bacteria translocated to the alveoli but were rapidly cleared by alveolar macrophages.
Why was the alveolar epithelium resistant to injury from a high dose of intravascular bacteria? Under normal conditions, alveolar epithelial barriers are much tighter than the adjacent lung endothelium, restricting the passage of large- and small-molecular-weight molecules (13). The tight alveolar epithelium protects the air spaces against alveolar flooding, and both type 1 and type 2 alveolar epithelial cells remove excess alveolar fluid by vectorial ion transport (30). Prior sheep studies demonstrated that the alveolar epithelium is resistant to injury from intravenous and/or intra-alveolar lipopolysaccharide administration (39) and to intravenous Pseudomonas aeruginosa (2). However, instillation of Escherichia coli endotoxin or live bacteria directly in the distal air spaces of the ex vivo perfused human lung produces alveolar epithelial injury with an increase in protein permeability and a reduction in alveolar fluid clearance (24, 25). In this study, we reproduced the finding of alveolar epithelial injury with high-dose intra-alveolar S. pneumoniae. However, in contrast to the pneumonia model, we found that the alveolar epithelium in the human lung was not injured from high-dose intravenous S. pneumoniae. This may be partly explained by the comparatively high concentration of bacteria present at the luminal side of the epithelium in the pneumonia model. At this concentration, secreted bacterial factors such as pneumolysin may directly damage the epithelium, or bacteria may activate alveolar macrophages to release factors such as pro-oxidants that might injure the epithelium. Despite the translocation of S. pneumoniae from the vascular compartment into the airspaces, there was no evidence of ultrastructural damage to the epithelium, enhanced permeability to protein, or impaired alveolar fluid clearance (Figs. 2C and 3). This may in part be explained by the rapid phagocytosis and clearance of these comparatively lower concentrations of S. pneumoniae.
The most unexpected finding of this study was the endogenous capacity of the ex vivo perfused human lung to rapidly and efficiently clear a large intravenous inoculum of a clinically relevant gram-positive pathogen, S. pneumoniae. The clearance of circulating bacteria is classically attributed to resident mononuclear cells in the liver and spleen (14, 19, 21). The lung has been thought to play a minor role, in part because of the lack of pulmonary intravascular macrophages (40). However, some investigators have reported novel mechanisms of bacterial clearance in the mouse lung. These include the sequestration of bacteria in the pulmonary microvasculature (15), the recruitment and activation of intravascular neutrophils, and the actions of a recently described population of resident extravascular neutrophils in the lung parenchyma (22, 40). In addition, Lefrançais et al. (26) recently described the important role of neutrophil extracellular traps in bacterial clearance in the lung. These experiments provide the first evidence of the endogenous ability of the human lung to clear large quantities of bacteria administered into the vascular compartment. The rapid bacterial clearance may in part explain the lack of alveolar epithelial injury in spite of the very large inoculum.
In these studies, what was the contribution of intravascular neutrophils to the rapid clearance of the S. pneumoniae? The number of neutrophils in the vascular compartment in the lungs perfused without blood was very low (20 cells/μL). Neutropenia is most frequently defined as an absolute neutrophil count of <500 cells/μL. Therefore, the residual neutrophils in the perfusate represent ~4% of neutrophils present in a neutropenic host and 1% of total neutrophils present in an immunocompetent host. The data suggest that neutrophils in the perfusate contribute to the clearance of some of the S. pneumoniae after intravenous infection. However, given the low number of neutrophils in the vascular compartment of the ex vivo human lung model, this mechanism does not appear to be sufficient to explain the clearance of such a large bacterial inoculum. Last, it is possible that some bacterial clearance occurs by neutrophils that are known to be adherent to the vascular endothelium (7, 17, 18), although we could not address their contribution in these studies.
Substantial fractions of the bacteria translocated from the vascular compartment into both the interstitium and the alveoli. How were these bacteria cleared from these two compartments? Some of the bacteria that translocated to the interstitial space may have been cleared by lung lymphatics, although we could not study this pathway in the isolated human lung. We did not observe bacterial phagocytosis by interstitial macrophages or neutrophils, possibly because of the sampling limitations associated with transmission electron microscopy, but it is likely that these populations of alveolar interstitial macrophages may also contribute to some bacterial clearance (20). In addition, some of the bacteria translocated to the distal airspaces, where alveolar macrophages participated in phagocytosis and clearance (Figs. 6, B–F, and 7). Notably, phagocytosis was not substantially dependent on opsonization, as has been described (1).
The ex vivo perfused human lung preparation is valuable for its clinical relevance because it permits the study of the human lung in isolation, without the effects of the liver or spleen (32). Similar to prior reports, although all lungs in this study had been rejected for transplantation, the majority was in good condition (9, 37). Upon reperfusion after an average of 23 h of ischemia, the lungs were biologically active, and the majority (81%) had normal alveolar fluid clearance (>10%/h).
There are some limitations to this study. Although we used a high dose of a clinically relevant pathogen, S. pneumoniae, we did not evaluate other pathogenic gram-positive organisms such as S. aureus or important gram-negatives such as E. coli. Therefore, the rapid bacterial clearance observed could be specific to S. pneumoniae. Furthermore, although we administered 100 mL of fresh blood intravenously in some of the experiments, the relative leukopenia of the ex vivo human lung preparation may alter some of the mechanisms important in endothelial and epithelial injury (30). Our experiments indicate that it is unlikely that a soluble factor in the perfusate plays a major role in bacterial clearance because the concentration of soluble factors in the circuit after perfusion is extremely low. However, we did not directly address the possibility that a soluble factor could be rapidly released from the alveolar microcirculation or by resident immune cells that could contribute to bacterial clearance.
Conclusions.
The human alveolar epithelium is resistant to injury from high-dose S. pneumoniae bacteremia. In addition, the human lung has a remarkable endogenous capacity to clear large quantities of bacteria from the vascular and extravascular compartments. Bacterial clearance is multifactorial and in part is dependent on circulating neutrophils, alveolar macrophages, and likely on lung lymphatics and interstitial macrophages. The rapid clearance of bacteria by the lung provides one mechanism for the resistance of the alveolar epithelium to bacteremia.
GRANTS
J.T.R. is supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant T32DK007573-23. N.N. is supported by the Société Française d’Anesthésie-Réanimation (Paris, France), the Association Chirurgicale pour le Développement et l’Amélioration des Techniques de Dépistage de Traitement des Maladies Cardiovasculaires (Paris, France) and the Journées Rennaises d’Anesthésie-Réanimation association (Rennes, France). A.L. is supported by the Canadian Institutes of Health Research Banting Postdoctoral Fellowship and the University of Toronto Clinician Scientist Training Program. R.L.Z. is supported by NIH National Heart, Lung, and Blood Institute (NHLBI) Grant R01HL131608. M.A.M. is supported in part by NIH NHLBI Grants HL51856, HL134828, and HL126456.
DISCLOSURES
Conflict of interest statement: No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.T.R., N.N., J.E.G., and M.A.M. conceived and designed research; J.T.R., N.N., A.L., R.L.Z., R.Y.M., E.M., and C.L. performed experiments; J.T.R., N.N., A.L., R.L.Z., R.Y.M., E.M., and C.L. analyzed data; J.T.R., N.N., A.L., R.L.Z., R.Y.M., C.L., J.E.G., and M.A.M. interpreted results of experiments; J.T.R., N.N., and R.Y.M. prepared figures; J.T.R. and N.N. drafted manuscript; J.T.R., N.N., A.L., R.L.Z., R.Y.M., J.E.G., and M.A.M. edited and revised manuscript; J.T.R., N.N., A.L., R.L.Z., R.Y.M., E.M., C.L., J.E.G., and M.A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Robin Kunkel for assistance with the electron microscopy.
The primary results of these studies were presented in abstract form at the annual Shock Society meeting in San Diego in June 2019.
REFERENCES
- 1.Belchamber KBR, Singh R, Batista CM, Whyte MK, Dockrell DH, Kilty I, Robinson MJ, Wedzicha JA, Barnes PJ, Donnelly LE; COPD-MAP Consortium . Defective bacterial phagocytosis is associated with dysfunctional mitochondria in COPD macrophages. Eur Respir J 54: 1802244, 2019. doi: 10.1183/13993003.02244-2018. [DOI] [PubMed] [Google Scholar]
- 2.Brigham KL, Woolverton WC, Blake LH, Staub NC. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest 54: 792–804, 1974. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaney J, Suzuki Y, Cantu E III, van Berkel V. Lung donor selection criteria. J Thorac Dis 6: 1032–1038, 2014. doi: 10.3978/j.issn.2072-1439.2014.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crocker SH, Lowery BD, Eddy DO, Wismar BL, Buesching WJ, Obenauf RN. Pulmonary clearance of blood-borne bacteria. Surg Gynecol Obstet 153: 845–851, 1981. [PubMed] [Google Scholar]
- 5.Cui H, Xie N, Banerjee S, Ge J, Guo S, Liu G. Impairment of fatty acid oxidation in alveolar epithelial cells mediates acute lung injury. Am J Respir Cell Mol Biol 60: 167–178, 2019. doi: 10.1165/rcmb.2018-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehring DJ, Fader RC, Traber LD, Traber DL. Cardiopulmonary changes occurring with pulmonary intravascular clearance of live bacteria in sheep. Circ Shock 29: 245–256, 1989. [PubMed] [Google Scholar]
- 7.Doerschuk CM, Beyers N, Coxson HO, Wiggs B, Hogg JC. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol (1985) 74: 3040–3045, 1993. doi: 10.1152/jappl.1993.74.6.3040. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson ND, Frutos-Vivar F, Esteban A, Gordo F, Honrubia T, Peñuelas O, Algora A, García G, Bustos A, Rodríguez I. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit Care 11: R96, 2007. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol 293: L52–L59, 2007. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H III, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Malinchoc M; U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS) . Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 183: 462–470, 2011. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotts JE, Bernard O, Chun L, Croze RH, Ross JT, Nesseler N, Wu X, Abbott J, Fang X, Calfee CS, Matthay MA. Clinically relevant model of pneumococcal pneumonia, ARDS, and nonpulmonary organ dysfunction in mice. Am J Physiol Lung Cell Mol Physiol 317: L717–L736, 2019. doi: 10.1152/ajplung.00132.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotts JE, Chun L, Abbott J, Fang X, Takasaka N, Nishimura SL, Springer ML, Schick SF, Calfee CS, Matthay MA. Cigarette smoke exposure worsens acute lung injury in antibiotic-treated bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 315: L25–L40, 2018. doi: 10.1152/ajplung.00405.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillot L, Nathan N, Tabary O, Thouvenin G, Le Rouzic P, Corvol H, Amselem S, Clement A. Alveolar epithelial cells: master regulators of lung homeostasis. Int J Biochem Cell Biol 45: 2568–2573, 2013. doi: 10.1016/j.biocel.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Halpern BN. The role and function of the reticulo-endothelial system in immunological processes. J Pharm Pharmacol 11: 321–338, 1959. doi: 10.1111/j.2042-7158.1959.tb12560.x. [DOI] [PubMed] [Google Scholar]
- 15.Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124: 915–927, 2006. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickson CM, Abbott J, Zhuo H, Liu KD, Calfee CS, Matthay MA; NHLBI ARDS Network . Higher mini-BAL total protein concentration in early ARDS predicts faster resolution of lung injury measured by more ventilator-free days. Am J Physiol Lung Cell Mol Physiol 312: L579–L585, 2017. doi: 10.1152/ajplung.00381.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogg JC. Neutrophil kinetics and lung injury. Physiol Rev 67: 1249–1295, 1987. doi: 10.1152/physrev.1987.67.4.1249. [DOI] [PubMed] [Google Scholar]
- 18.Hogg JC, Walker BA. Polymorphonuclear leucocyte traffic in lung inflammation. Thorax 50: 819–820, 1995. doi: 10.1136/thx.50.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol 18: 49–53, 2006. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Hume PS, Gibbings SL, Jakubzick CV, Tuder RM, Curran-Everett D, Henson PM, Smith BJ, Janssen WJ. Localization of macrophages in the human lung via design-based stereology. Am J Respir Crit Care Med 201: 1209–1217, 2020. doi: 10.1164/rccm.201911-2105OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins SJ, Hume DA. Homeostasis in the mononuclear phagocyte system. Trends Immunol 35: 358–367, 2014. doi: 10.1016/j.it.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci USA 107: 18073–18078, 2010. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kukreja J, Chen J, Brzezinski M. Redefining marginality: donor lung criteria. Curr Opin Organ Transplant 25: 280–284, 2020. doi: 10.1097/MOT.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 24.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 187: 751–760, 2013. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 3: e98178, 2018. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu A, Park JH, Zhang X, Sugita S, Naito Y, Lee JH, Kato H, Hao Q, Matthay MA, Lee JW. Therapeutic effects of hyaluronic acid in bacterial pneumonia in ex vivo perfused human lungs. Am J Respir Crit Care Med 200: 1234–1245, 2019. doi: 10.1164/rccm.201812-2296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthay MA. Resolution of pulmonary edema. Thirty years of progress. Am J Respir Crit Care Med 189: 1301–1308, 2014. doi: 10.1164/rccm.201403-0535OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthay MA, Landolt CC, Staub NC. Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol 53: 96–104, 1982. doi: 10.1152/jappl.1982.53.1.96. [DOI] [PubMed] [Google Scholar]
- 30.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers 5: 18, 2019. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel BV, Wilson MR, O’Dea KP, Takata M. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol 190: 4274–4282, 2013. doi: 10.4049/jimmunol.1202437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross JT, Nesseler N, Lee JW, Ware LB, Matthay MA. The ex vivo human lung: research value for translational science. JCI Insight 4: e128833, 2019. doi: 10.1172/jci.insight.128833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 34.Vander Top EA, Perry GA, Gentry-Nielsen MJ. A novel flow cytometric assay for measurement of in vivo pulmonary neutrophil phagocytosis. BMC Microbiol 6: 61, 2006. doi: 10.1186/1471-2180-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 36.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 37.Ware LB, Wang Y, Fang X, Warnock M, Sakuma T, Hall TS, Matthay M. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet 360: 619–620, 2002. doi: 10.1016/S0140-6736(02)09774-X. [DOI] [PubMed] [Google Scholar]
- 38.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network . Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575, 2006. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 39.Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest 88: 864–875, 1991. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yipp BG, Kim JH, Lima R, Zbytnuik LD, Petri B, Swanlund N, Ho M, Szeto VG, Tak T, Koenderman L, Pickkers P, Tool ATJ, Kuijpers TW, van den Berg TK, Looney MR, Krummel MF, Kubes P. The lung is a host defense niche for immediate neutrophil-mediated vascular protection. Sci Immunol 2: 2, 2017. doi: 10.1126/sciimmunol.aam8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeyed YF, Bastarache JA, Matthay MA, Ware LB. The severity of shock is associated with impaired rates of net alveolar fluid clearance in clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol 303: L550–L555, 2012. doi: 10.1152/ajplung.00190.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]