Abstract
Caspase-3 and -7 are executioner caspases whose enzymatic activity is necessary to complete apoptotic cell death. Here, we questioned whether endothelial cell infection leads to caspase-3/7-mediated cell death. Pulmonary microvascular endothelial cells (PMVECs) were infected with Pseudomonas aeruginosa (PA103). PA103 caused cell swelling with a granular appearance, paralleled by intracellular caspase-3/7 activation and cell death. In contrast, PMVEC infection with ExoY+ (PA103 ΔexoUexoT::Tc pUCPexoY) caused cell rounding, but it did not activate intracellular caspase-3/7 and it did not cause cell death. However, ExoY+ led to a time-dependent accumulation of active caspase-7, but not caspase-3, in the supernatant, independent of apoptosis. To study the function of extracellular caspase-7, caspase-7- and caspase-3-deficient PMVECs were generated using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology. Caspase-7 activity was significantly reduced in supernatants from infected caspase-7-deficient cells but was unchanged in supernatants from infected caspase-3 deficient cells, indicating an uncoupling in the mechanism of activation of these two enzymes. Because ExoY+ leads to the release of heat stable amyloid cytotoxins that are responsible for transmissible cytotoxicity, we next questioned whether caspase-7 contributes to the severity of this process. Supernatants obtained from infected caspase-7-deficient cells displayed significantly reduced transmissible cytotoxicity when compared with supernatants from infected wild-type controls, illustrating an essential role for caspase-7 in promoting the potency of transmissible cytotoxicity. Thus, we report a mechanism whereby ExoY+ infection induces active caspase-7 accumulation in the extracellular space, independent of both caspase-3 and cell death, where it modulates ExoY+-induced transmissible cytotoxicity.
Keywords: amyloid, caspase-3, Pseudomonas aeruginosa, tau, transmissible cytotoxicity
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen and the most common cause of hospital-acquired pneumonia (10, 44). Virulence of this organism is largely determined by the presence of a type 3 secretion system (T3SS), which introduces exoenzymes directly into the host cell cytoplasm to evade immune detection (14). Four T3SS effectors have been described, including ExoS, ExoT, ExoU, and ExoY (14). The ExoY gene is expressed in 89% of clinically isolated P. aeruginosa strains (13). Once injected into the host cell cytosol, ExoY acts as a nucleotidyl cyclase that requires actin as a host cell cofactor for enzymatic activity (7, 31, 45). ExoY generates canonical (i.e., cAMP and cGMP) and noncanonical (i.e., cUMP and cCMP) cyclic nucleotides that activate at least protein kinases A and G, resulting in tau hyperphosphorylation (6, 29, 31, 35). Hyperphosphorylated tau prevents microtubule assembly, leading to cytoskeletal rearrangement, which in turn results in cell rounding and subsequent endothelial gap formation (5). In vivo, ExoY-induced hyperpermeability contributes to lung exudative edema and hemorrhage in both animal models of infection and critically ill patients suffering from nosocomial pneumonia (20, 35).
Nosocomial pneumonia survivors have high rates of morbidity and mortality, although the underlying mechanism(s) for this phenomenon remains unknown (41). Stroke, arrhythmias, renal dysfunction, and deficits in learning and memory have all been described in representative patient cohorts (32). Our group has recently found that bacteria responsible for nosocomial pneumonias, including P. aeruginosa, elicit endothelial cell generation and release of cytotoxic protein complexes that are capable of self-propagation as a proteinopathy associated with end-organ dysfunction (25). ExoY intoxication of endothelial cells drives the production and release of amyloid-β complexes and hyperphosphorylated tau species (4, 28). These amyloids and/or tau complexes exert cytotoxic effects on naïve PMVECs independently of bacteria (4, 28). However, mechanisms that modulate the potency of ExoY-induced transmissible cytotoxicity remain unknown.
During P. aeruginosa-induced pneumonia, bacterial virulence factors induce caspase-mediated apoptosis, which in turn contributes to endothelial permeability (15, 23). However, it is unknown whether ExoY-intoxication of endothelial cells leads to caspase-mediated apoptosis. Moreover, nonapoptotic functions of caspases are under investigation. Of note is that tau, which is released from endothelial cells upon ExoY-intoxication, is a known substrate of caspase-3, and its function and turnover are regulated by caspase-3 (9, 17, 27, 34). However, it remains unknown whether infection-induced activated caspases influence amyloid function during infection or recovery from infection. Here, we sought to determine whether P. aeruginosa ExoY (ExoY+) infection of PMVECs leads to caspase-3/7 activation, caspase-3/7-dependent apoptosis, and/or caspase-3/7-dependent transmissible cytotoxicity.
MATERIALS AND METHODS
Cell culture.
Rat pulmonary microvascular endothelial cells (PMVECs) were isolated from the distal lung parenchyma, as previously described (18). PMVECs were isolated from adult male Sprague-Dawley rats (Rattus norvegicus), selected, and expanded based on their morphology and then screened by flow cytometry for expression of typical endothelial cell markers, including eNOS, VE-cadherin, and PECAM-1. Cells exhibited LDL uptake and were shown to form networks on Matrigel. To discriminate between macro- and microvascular cell phenotypes, PMVECs were screened for Helix pomatia, Griffonia simplicifolia, and Glycine max lectin binding. PMVECs did not recognize Helix pomatia but recognized Griffonia simplicifolia and Glycine max. Cells were shown to be mycobacterium-free by nuclear staining (cat. no. 10 236 276 001; Roche).
PMVECs for this study were obtained through the University of South Alabama Center for Lung Biology cell culture core. All knockout cell lines originated from a single cell line derived from a single donor, which represents a limitation of the study. However, in this study, the parental cell line is used as a control for clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-dependent deletion of caspases-3 and -7. The present studies were designed to mechanistically address the importance of these executioner caspases in the endothelial cell response to infection.
PMVECs were cultured in Dulbecco’s modified Eagle’s medium, high glucose (DMEM), supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. PMVECs were seeded to grow to confluence overnight in multiwell dishes for infection. Prior to infection, PMVECs were washed in phosphate-buffered saline (PBS). Infections were performed in Hanks’ balanced salt solution (HBSS) at 37°C, 5% CO2, for ≤6 h.
Bacterial preparation.
Pseudomonas aeruginosa strain PA103, expressing exoenzyme U and exoenzyme T, and an isogenic mutant, PA103, lacking exoenzyme U and exoenzyme T but expressing exoenzyme Y (PA103 ΔexoUexoT::Tc pUCPexoY; ExoY+) (45), were obtained from Dr. Dara Frank at the Medical College of Wisconsin. Frozen stock solutions were stored at −80°C. Aliquots were grown overnight on Vogel-Bonner agar (PA103) supplemented with 400 µg/mL carbenicillin (ExoY+). Bacteria were suspended in PBS and diluted into HBSS for infection at a multiplicity of infection of 20 bacteria to 1 cell (MOI 20:1).
Microscopy.
Infected PMVECs were imaged using a Nikon Eclipse (TE2000-S) inverted microscope equipped with an environmental chamber. Time-lapse images were taken with a ×50 objective at a frame rate of one frame per 2 min. Images were converted into a movie using ImageJ software.
CRISPR/Cas9 knockout of caspase-7 and caspase-3.
Caspase-7- and caspase-3-deficient PMVECs were generated by transiently transfecting PMVECs with pX330-U6-Chimeric_BB-CBh-hSpCas9 (Addgene 42230), pExodus CMV.Trex2 (Addgene 40210), pEF1αEGFP, and two constructs expressing guide RNAs targeting either TCTCGAAGTCCATACGGTAC and GGTCCCGGGTGGTACTGACG or TCTGGGCAGCTGTGTACTCA and TCTCGAAGTCCATACGGTAC for caspase-7 or TTTTGGAACGAACGGACCTG and TCTTCAGAGGCGACTACTGC for caspase-3. PMVEC populations were seeded in a six-well dish to 70–80% confluency after 18 h. Cells were washed in 1× PBS before transfection with plasmids using Xfect Transfection Reagent (631317; Takara) in DMEM. DMEM with Xfect polymer was removed and replaced with fresh DMEM 18 h posttransfection. Twenty-four hours later, GFP-positive cells were single-cell sorted into four 96-well plates using the BD FACS Aria cell sorter. After 3 wk, colonies were expanded into 24-well dishes. They were screened for putative deletions by PCR using primers flanking the target site (ACTCAGACAGGCTCCTACATAC and ATTCCTTGGTTCAGCCACTTAC for caspase-7; TCCGATGAGAAAGCCAGATAC and GTGGAAGCGTAAGGTGAATAAA for caspase-3) and primers within the target site (CCTCTATGTGCCCCGTCA or GTGCCCACTTACCTGTACC for caspase-7; TGTGGACCTGAAAAAACTAACTAG and TTCTTCAGAGGCGACTACTG for caspase-3). For caspase-7, wild-type clones were expected to yield both a 790-bp and a small product (either 276 or 241 bp), whereas putative caspase-7 knockout (KO) clones were expected to yield only a 790-bp product. For caspase-3, wild-type clones were expected to yield both a 523-bp and a small product (either 313 bp or 288 bp), and putative caspase-3 KO clones were expected to yield only a 523-bp product. Putative knockout clones were expanded in six-well plates for Western blot screening and Sanger sequencing and maintained in 150-mm dishes. Knockout cell lines are available to qualified investigators upon request.
Gene editing verification by Sanger sequencing.
Targeted genomic locus was amplified with Platinum SuperFI Green DNA polymerase (12357010; Invitrogen), ligated into pBluescriptII SK+ plasmid, and the resulting ligation mixture was electroporated into E. coli GeneHogs (Invitrogen) and plated on LB plates supplemented with ampicillin, Xgal, and IPTG (200 μg/mL, 40 μg/mL, and 1 mM, respectively). The inserts in the resulting white colonies were amplified by PCR, and the PCR products were purified from unincorporated primers and dNTPs by treating with exonuclease I and shrimp alkaline phosphatase and submitted for Sanger sequencing (Eurofins Genomics). DNA sequences were aligned using the ClustalX2 multiple alignment tool, and sequences were translated using the ExPASy translation tool.
Annexin V/propidium iodide staining.
Annexin V (AV) and propidium iodide (PI) staining was performed using the Dead Cell Apoptosis Kit (Invitrogen; V13242) according to manufacturer’s instructions. In brief, PMVECs were washed in 1× PBS twice by centrifugation, resuspended in Annexin binding buffer, and incubated with FITC AV and PI protected from light for 15 min at room temperature. PMVECs were analyzed by flow cytometry (BD FACS Aria).
LDH cytotoxicity assay.
A lactate dehydrogenase (LDH) cytotoxicity assay was performed using the CyQUANT LDH Cytotoxicity Assay Kit (C20300; ThermoFisher) according to the manufacturer’s instructions. In brief, 50 µL of cell culture supernatant was transferred to a 96-well dish. Fifty µL of reaction reagent was mixed into each well, and the plate was incubated for 30 min at room temperature protected from light. Fifty microliters of stop solution was added to each well. Absorbance was read at 490 nm and 680 nm using the ID5 spectrometer.
Detection of intracellular active caspase-3/7, caspase-8, or caspase-9 by FLICA.
PMVECs were loaded with 0.5× FAM-DEVD-FMK (caspase-3/7), FAM-LETD-FMK (caspase-8), or FAM-LEHD-FMK (caspase-9) FLICA reagent (94, 99, or 912, respectively; Immunochemistry) for the last 3 h of the infection in 12-well dishes, as previously described (33). At the time of collection, supernatants were discarded, and cells were rinsed in 1 mL of wash buffer (Immunochemistry) and subsequently washed twice in wash buffer by centrifugation (600 g). After the last wash, cell pellets were resuspended in 750 μL of wash buffer and transferred to flow cytometry tubes containing 250 μL of 10% formalin. PMVECs were analyzed on a BD FACS Canto flow cytometer.
Detection of extracellular caspase-3/7 activity by fluorescence.
Supernatants from infected PMVECs in 100-mm dishes were analyzed for caspase-3/7 activity using a caspase-3 activity assay kit (5723; Cell Signaling Technology) according to the manufacturer’s instructions. Briefly, a 25-µL supernatant was mixed with 200 µL of substrate solution containing the caspase-3/7 substrate Ac-DEVD-AMC in a 96-well plate and incubated at 37°C protected from light for 1 h. Fluorescence was analyzed on a SpectraMax iD5 Multi-Mode microplate reader with excitation at 380 nm and emission at 450 nm. Supernatant protein concentration was determined by Pierce BCA Protein Assay Kit (23250; ThermoFisher Scientific).
Detection of cleaved caspase-3/7 by Western blot.
Following infection, cell culture supernatants and cell lysates were analyzed separately. Proteins in the supernatant were precipitated in 10% trichloroacetic acid (TCA) overnight on ice at 4°C. TCA precipitates were collected by centrifugation at 21,000 g for 40 min at 4°C. Protein pellets were washed in 1 mL of 100% ice-cold ethanol. Samples were centrifugated at 21,000 g for 20 min. Protein pellets were resuspended into 30 μL of 1× Laemmli SDS-sample buffer (BP-111R; Boston Bioproducts). Samples were heated at 95°C for 5 min. Samples were either immediately resolved by SDS-PAGE or stored at −80°C. Cells were lysed in lysis buffer (1× PBS, 1% Triton X-100, 0.5% SDS, 1× protease inhibitor cocktail). Protein concentration was determined using the Pierce BCA Protein Assay Kit (23250; ThermoFisher Scientific). Protein from cell lysates or supernatants were resolved by SDS-PAGE on 4–12% Bis-Tris polyacrylamide gels and transferred onto a 0.2-µm nitrocellulose membrane. Membranes were cut to appropriate size and blocked in 5% milk in Tris-buffered saline containing 1% Tween 20 (TBST) at room temperature. Membranes were incubated with a primary antibody (caspase-3 antibody, 1:1,000, Novus; NB100-56708 lot C-4, Santa Cruz Biotechnology; sc-7272 or rabbit polyclonal antibody CST 9662S, mouse caspase-7 antibody 1:2000, Novus; NB100-56529 lot 061200–2; rabbit caspase-7 antibody 1:1,000, Cell Signaling Technology; 9492S lot 6, cleaved caspase-7 D198 antibody; Cell Signaling Technology, 9491T lot 11) overnight at 4°C, and secondary antibody (goat anti mouse 1:5000 or goat anti rabbit 1:5,000) for 1 h at room temperature. Membranes were developed using SuperSignal West Femto Maximum Sensitivity substrate (34095; ThermoFisher Scientific). Membranes were stripped using stripping buffer (BP98; Boston Bioproducts) and reprobed using a GAPDH antibody (2118, 1:1,000; Cell Signaling Technology) for 1 h at room temperature and a secondary antibody (goat anti rabbit 1:5,000) for 1 h at room temperature.
Statistics.
GraphPad Prism version 7 was used for all analyses. Data are reported as means ± SE. One-way ANOVA or two-way ANOVA was used as indicated and Tukey’s post hoc test applied as necessary. Differences with a P value of <0.05 were considered significant.
RESULTS
ExoY+ infection of PMVECs leads to cell rounding independent of caspase-dependent cell death.
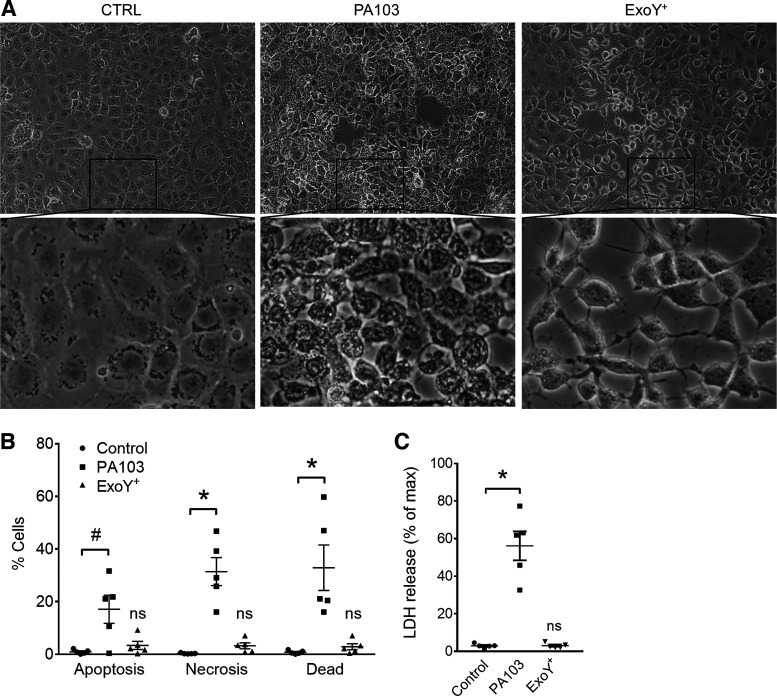
P. aeruginosa utilizes a T3SS to directly intoxicate the host cell cytosol with exoenzymes. Exoenzymes U (ExoU) and Y (ExoY) elicit endothelial gap formation (28, 31). ExoU-induced gap formation is accompanied by rapid lytic endothelial cell death (2). However, it is not known whether ExoY induces endothelial cell death. To address this question, PMVEC monolayers were infected with P. aeruginosa strains expressing exoenzyme U and T (PA103) or ExoY (ExoY+). Cell morphology was assessed by microscopy, and cell death was evaluated by AV/PI staining and LDH release into the culture medium. PA103 infection of PMVECs resulted in cells with a granular appearance after 4 h (Fig. 1A). In stark contrast, ExoY+ infection led to cell rounding but no granular appearance, which started around 4 h and worsened until 6 h (Fig. 1A). The time course for PA103- (Supplemental Video S1; available online at https://doi.org/10.6084/m9.figshare.12132699.v1) and ExoY+-induced (Supplemental Video S2; available online at https://doi.org/10.6084/m9.figshare.12137427.v1) changes in cell morphology is resolved by time-lapse movies. PA103 infection led to AV-only, PI-only, and double-positive staining after 4 h, suggesting both apoptotic (AV) and necrotic (PI) cell death mechanisms. However, ExoY+ infection did not result in significant staining with either cell death indicator after 6 h of infection, when cell rounding is most evident (Fig. 1B and Supplemental Figure S1; https://doi.org/10.6084/m9.figshare.12137511). Additionally, PA103 infection resulted in significant cell lysis, as evidenced by LDH release into the culture medium, whereas ExoY+ infection did not (Fig. 1C). These data indicate that ExoY+-induced cell rounding occurs independent of cell death at the time course tested.
Fig. 1.
Pseudomonas aeruginosa exoenzyme Y (ExoY) causes pulmonary microvascular endothelial cell (PMVEC) rounding independent of cell death. A: PMVECs were treated with vehicle control (CTRL; left) or inoculated with P. aeruginosa strain PA103 (middle) or ExoY+ (right). Monolayer images were taken at 4 h (PA103) or 6 h (CTRL and ExoY+) postinoculation. B: infected PMVECs were stained with both annexin V (AV) and propidium iodide (PI). Cell death was analyzed by flow cytometry. “Apoptosis” refers to cells that stained positive for AV alone, “necrosis” refers to cells that stained positive for PI alone, and “dead” refers to cells that stained positive for both AV and PI. Each data point represents 1 experiment performed in duplicate. Data were analyzed by 2-way ANOVA with Tukey’s multiple-comparison test. Interaction not significant (P = 0.1615), row factor (cell death type) not significant (P = 0.2359), column factor (infection condition) significant, P < 0.0001. C: cell culture supernatants from infected PMVECs were analyzed for lactate dehydrogenase (LDH) release. Each data point represents 1 experiment performed in duplicate. Data were analyzed by ordinary 1-way ANOVA with Bonferroni’s multiple-comparison test. ANOVA, P < 0.0001. #P = 0.0158; *P < 0.0001. NS, not significantly different from control, n = 5.
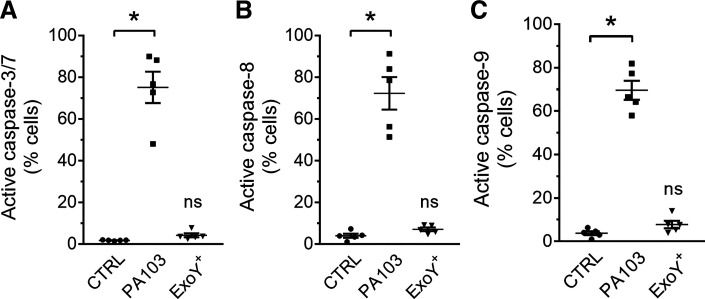
Apoptosis is a form of programmed cell death that is governed by caspases, a large family of cysteine proteases. Caspase-8 is an initiator caspase in the extrinsic apoptosis pathway, caspase-9 is an initiator caspase in the intrinsic apoptosis pathway, and caspases-3 and -7 are executioner caspases of apoptosis (1, 16, 30). We sought to determine whether ExoY+ infection of PMVECs elicits activation of caspase-8, caspase-9, or caspase-3/7 using fluorescently labeled active caspase-8, -9, or -3/7 probes FAM-LETD-FMK, FAM-LEHD-FMK, or FAM-DEVD-FMK, respectively. These membrane-permeable caspase substrates irreversibly bind to active intracellular caspases and are retained inside the cell after vigorous washing (33). ExoY+ did not activate intracellular caspase-8, caspase-9, or caspase-3/7 6 h postinfection. In contrast, PA103 elicited a very strong caspase-8, caspase-9, and caspase-3/7 response 4 h postinfection (Fig. 2 and Supplemental Fig. S2; available online at https://doi.org/10.6084/m9.figshare.12137529). These data support the results shown in Fig. 1 and collectively indicate that ExoY+-induced PMVEC rounding occurs independent of caspase-dependent cell death.
Fig. 2.
Pseudomonas aeruginosa exoenzyme Y (ExoY) does not induce intracellular caspase-3/7 activation in pulmonary microvascular endothelial cells (PMVECs). PMVECs were treated with vehicle control or inoculated with PA103 or ExoY+. PMVECs were loaded with fluorescently labeled active caspase-3/7 substrate FAM-DEVD-FMK (FLICA; A), caspase-8 substrate FAM-LETD-FMK (B), or caspase-9 substrate FAM-LEHD-FMK (C), for the last 3 h of infection. Intracellular active caspase was assessed by flow cytometry 4 h (PA103) or 6 h [control (CTRL) and ExoY+] postinoculation. Each data point represents 1 experiment performed in duplicate. Data were analyzed by ordinary 1-way ANOVA with Bonferroni’s multiple-comparison test. ANOVA, P < 0.0001. *P < 0.0001. NS, not significantly different from control, n = 5.
ExoY+ infection of PMVECs leads to accumulation of extracellular active caspase-7 independent of caspase-3.
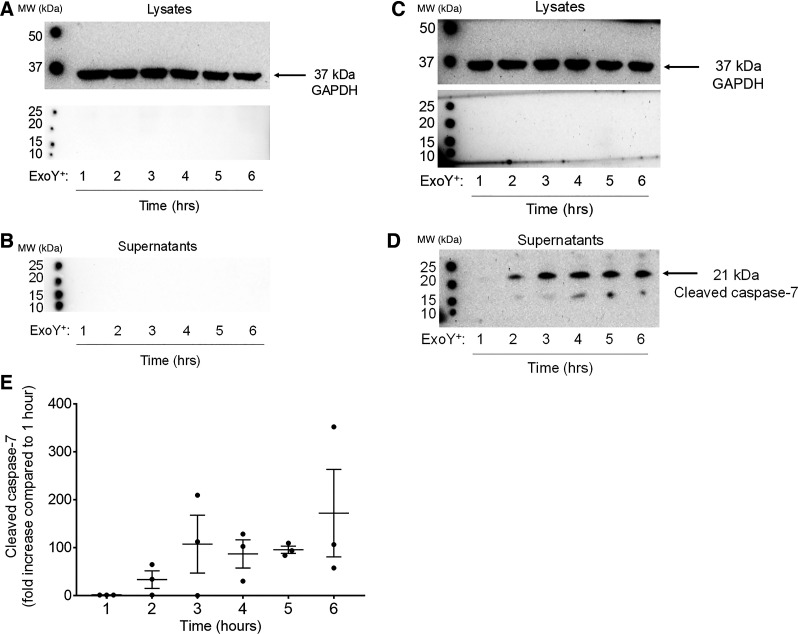
Because FAM-DEVD-FMK does not discriminate between caspases-3 and -7 and only detects intracellular caspases, we assessed whether ExoY+ infection caused cleavage of caspase-3 and/or caspase-7 by immunoblotting PMVEC lysates and culture supernatants separately. ExoY+ infection of PMVECs did not result in detection of cleaved caspase-3 in either cell lysates or supernatants (Fig. 3, A and B). These data are consistent with the FAM-DEVD-FMK data shown in Fig. 2 and suggest that ExoY+ infection of PMVECs does not lead to activation of caspase-3.
Fig. 3.
Pseudomonas aeruginosa exoenzyme Y (ExoY) induces time-dependent release of cleaved caspase-7. Pulmonary microvascular endothelial cells (PMVECs) were inoculated with ExoY+ for ≤6 h. A–D: every hour, cell lysates (A and C) and culture supernatants (B and D) were collected separately and analyzed for cleaved caspase-3 (A and B) or cleaved caspase-7 (C and D). E: densitometry of cleaved caspase-7 bands is represented as fold increase compared with 1 h postinfection. MW, molecular weight.
Similarly, ExoY+ infection did not consistently generate caspase-7 cleavage products in PMVEC lysates, supporting the results obtained with FAM-DEVD-FMK (Fig. 3C). However, in one of five experiments, a lower 17-kDa caspase-7-immunoreactive band was detected in the lysates at 1 and 2 h after ExoY+ infection (data not shown). Importantly, cleaved caspase-7 (21kDa) was consistently detected in the culture supernatant in a time-dependent manner (Fig. 3D). Cleaved extracellular caspase-7 was detected as early as 2 h postinfection, which is before cell rounding and independent of cell death (Fig. 3D). Densitometry of the cleaved caspase-7 band revealed a maximal increase in secreted caspase-7 by 3 h that was sustained throughout the 6-h time course (Fig. 3E).
To study the activity and function of extracellular cleaved caspase-7, we generated caspase-3- (C3KO) and caspase-7 (C7KO)-deficient PMVECs using CRISPR/Cas9 technology. First, PMVECs were single-cell cloned to ensure that the cell culture would be genetically homogenous. Then, PMVECs were transfected with two guide RNA sequences (5297-1 and 5297-2 for caspase-7 and 5153-1 and 5153-2 for caspase-3; see materials and methods).
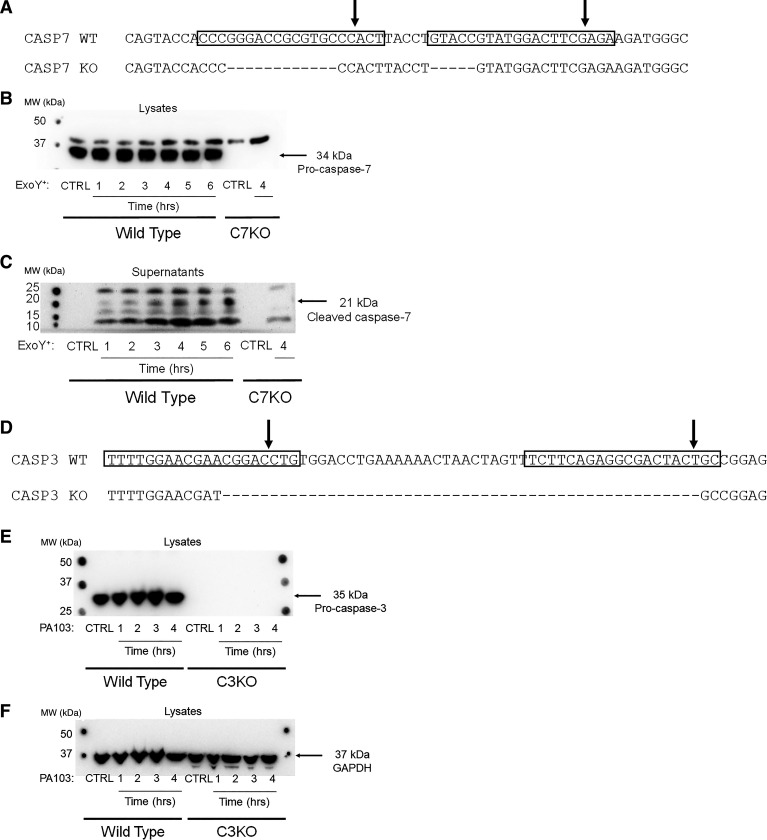
After single-cell sorting of potential caspase-7-deficient cells, 71 clones were expanded and screened for deletions by PCR. Out of 71 clones, 14 clones showed potential deletions and were sequenced. However, all of these clones demonstrated frame shift deletions that did not result in a premature stop codon and, therefore, were not predicted to result in loss of protein. Therefore, we generated two new guide RNA sequences (5406-5 and 5406-6) that were transfected in combination with the previously discussed gRNA sequence 5297-1. A new set of WT single-cell-cloned PMVECs were transfected with these constructs. After single-cell sorting, 121 clones were expanded and screened for potential deletions by PCR. Out of 121 clones, eight clones showed potential deletions and were sequenced. Out of eight sequenced clones, one clone presented with a deletion of 17 base pairs, resulting in a premature stop codon, and a truncated caspase-7 protein at Lys69 (Fig. 4A). This truncated caspase-7 protein was predicted to be deficient of the active site at Cys186. Immunoblotting of WT and C7KO lysates confirmed absence of pro-caspase-7 protein (Fig. 4B). Importantly, immunoblotting of C7KO culture supernatants after ExoY+ infection showed an absence of the 21-kDa cleaved caspase-7 fragment (Fig. 4C).
Fig. 4.
Caspase-7- (C3KO) and caspase-3-deficient (C3KO) pulmonary microvascular endothelial cells (PMVECs) were generated using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9. A: wild-type (WT) and C7KO nucleotide sequence indicates a 17-base pair deletion. Rectangles indicate gRNA sequence, and arrows point at predicted Cas9 cut sites. B: WT and C7KO PMVECs were treated with vehicle control (CTRL) or infected with exoenzyme Y+ (ExoY+) for ≤6 h (WT) or 4 h (C7KO). Cell lysates were analyzed by immunoblot for presence of pro-caspase-7 using rabbit polyclonal caspase-7 antibody. C7KO PMVEC lysates lack the 34-kDa pro-caspase-7 band. C: cell culture supernatants were analyzed by immunoblot for cleaved caspase-7 using cleaved caspase-7 antibody. The 21-kDa cleaved caspase-7 band was not detected in C7KO PMVEC supernatants after 4 h of ExoY+ infection. D: WT and C3KO nucleotide sequence indicates a 50-base pair deletion. Rectangles indicate gRNA sequence, and arrows point at predicted Cas9 cut sites. E: WT and C3KO PMVECs were treated with vehicle control (CTRL) or infected with PA103 for 4 h. Cell lysates were analyzed by immunoblot for presence of pro-caspase-3 using rabbit polyclonal caspase-3 antibody. C3KO PMVEC lysates lack the 35-kDa pro-caspase-3 band. F: membrane was stripped and reprobed with GAPDH as loading control. MW, molecular weight.
After single-cell sorting of potential caspase-3-deficient cells, 76 clones were expanded and screened for deletions by PCR. Out of 76 clones, eight clones showed potential deletions and were sequenced. All eight clones presented with large deletions, resulting in premature stop codons. One clone presented with a deletion of 50 base pairs, resulting in an amino acid sequence that differed from WT starting at Asn131, and was truncated at Asp192 (Fig. 4D). This truncated caspase-3 protein was predicted to be deficient in the active site at Cys163. Immunoblotting of WT and C3KO lysates confirmed absence of pro-caspase-3 protein (Fig. 4, E and F).
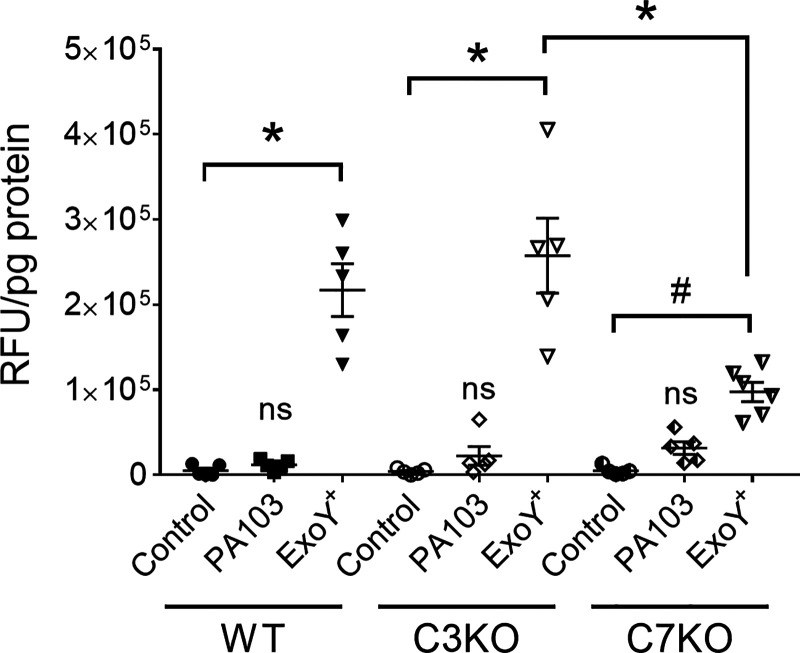
To determine whether the extracellular cleaved caspase-7 is active, WT, C3KO, and C7KO PMVECs were infected with ExoY+, supernatant protein concentrations were normalized, and supernatants were analyzed for caspase-3/7 enzyme activity using a caspase-3/7 substrate Ac-DEVD-AMC. First, PA103 infection did not result in extracellular caspase-3/7 activity, indicating that PA103 elicits intracellular but not extracellular caspase activation (Figs. 2A and Fig. 5). Second, ExoY+ infection of WT PMVECs resulted in substantial extracellular enzyme activity, indicating that the secreted cleaved caspase-7 observed in Fig. 3D was active (Fig. 5). This activity was significantly reduced in C7KO PMVECs, indicating that the signal from WT PMVECs represents caspase-7 activity specifically and confirming immunoblot results seen in Fig. 3D and Fig. 4C. Importantly, caspase-7 activity was detected in supernatants from ExoY+-infected C3KO PMVECs, indicating that secretion of active caspase-7 occurs independently of caspase-3 (Fig. 5). These data demonstrate that ExoY+ infection leads to the accumulation of extracellular active caspase-7 independent of caspase-3 and cell death, consistent with the emerging appreciation that caspase-3/7 can fulfill nonapoptotic cellular functions (38). It remains unclear as to whether caspase-7 is activated inside the cell before its release or outside the cell upon its release.
Fig. 5.
Pseudomonas aeruginosa exoenzyme Y (ExoY) induces extracellular active caspase-7 independent of caspase-3. Wild-type (WT), caspase-3-deficient (C3KO), and caspase-7-deficient (C7KO) pulmonary microvascular endothelial cells (PMVECs) were infected with either PA103 (4 h on WT and C3KO, 2.5 h on C7KO) or ExoY+ (6 h on WT and C3KO, 4 h on C7KO). Caspase-3/7 activity was assessed in supernatants. RFU, relative fluorescent units. Data were analyzed by 2-way ANOVA with Tukey’s multiple-comparison test. Interaction was significant (P = 0.0004), row factor (infection condition) was significant (P < 0.0001), and column factor (cell type) was significant (P = 0.0122). *P < 0.0001; #P = 0.0023, NS, not significantly different from control, n = 5. Each symbol represents a single experiment performed in duplicate.
Genetic deletion of caspase-7 in PMVECs does not protect against primary infection but alleviates transmissible cytotoxicity.
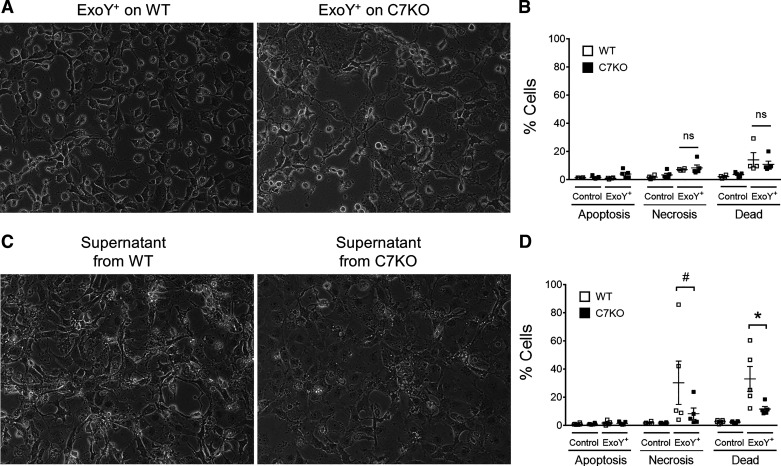
Our group has previously established that primary ExoY+ infection of PMVECs elicits the release of high-molecular-weight tau that is sufficient to cause PMVEC gap formation independent of bacteria (28). To assess whether caspase-7 contributes to the potency of this ExoY+-induced transmissible cytotoxicity, supernatants were generated by infecting WT and C7KO PMVECs with ExoY+ for 6 and 4 h, respectively. Although ExoY+ infection did not cause cell death in C7KO cells, as evidenced by AV/PI staining, substantial cell rounding was observed at 4 h postinfection (Fig. 6, A and B). Therefore, the primary infection on C7KO was terminated at 4 h, when cell rounding was observed to be similar to WT PMVECs at 6 h. Supernatants were filter sterilized, boiled for 30 min at 100°C, and transferred to naïve wild-type PMVECs for 16 h. Bacteria are not present in this supernatant, and heat-labile proteins are inactivated by boiling. Cell morphology was assessed by microscopy, and cell death was evaluated by AV/PI staining. Supernatant from ExoY+-infected C7KO PMVECs caused decreased cell death on naïve WT PMVECs compared with supernatant from ExoY+-infected WT PMVECs (Fig. 6, C and D), suggesting that caspase-7 contributes to the severity of transmissible cytotoxicity. Collectively, our study indicates that ExoY+ infection of PMVECs results in the accumulation of extracellular active caspase-7, independent of caspase-3 and apoptosis, where it contributes to the severity of transmissible cytotoxicity.
Fig. 6.
Deletion of caspase-7 protects naïve cells from transmissible cytotoxicity. A: wild-type (WT) and caspase-7-deficient (C7KO) pulmonary microvascular endothelial cells (PMVECs) were infected with exoenzyme Y+ (ExoY+) (MOI 20:1). C7KO PMVECs presented with gap at 4 h as opposed to WT PMVECs at 6 h. B: infected WT and C7KO PMVECs were stained with annexin V (AV) and propidium iodide (PI) and analyzed for cell death by flow cytometry. There was no significant difference in cell death between WT and C7KO PMVECs. C: supernatants from ExoY+-infected WT or C7KO PMVECs were filter sterilized, boiled at 100°C for 30 min, and transferred to naïve WT PMVECs for 16 h. Gap formation in cells treated with supernatants from ExoY+-infected C7KO PMVECs was less severe compared with cells treated with supernatants from ExoY+-infected WT PMVECs. D: naïve PMVECs treated with cytotoxic supernatants were stained with AV and PI and analyzed for cell death by flow cytometry. ExoY+-derived supernatant from C7KO PMVECs caused significantly less cell death compared with ExoY+-derived supernatant from WT PMVECs. Data were analyzed by 2-way ANOVA with Tukey’s multiple-comparison test. Interaction was significant (P = 0.0253), row factor (cell death type) was significant (P = 0.0038), and column factor (supernatant origin) was significant, P < 0.0001. *P = 0.0192 and #P = 0.0154; n = 5.
DISCUSSION
Herein, we describe a noncanonical role for caspase-7 in the modulation of ExoY+-induced endothelial transmissible cytotoxicity. First, we report that ExoY+ infection of PMVECs leads to caspase-7 activation independent of cell death. Whereas vascular cell death has been reported in a mouse-model of ExoY+ infection after 24 h (20), our study indicates that the acute ExoY+-induced endothelial gap formation occurs independent of cell death, consistent with the idea that ExoY functions as an edema factor (35). Second, we found that ExoY+ induces the accumulation of active caspase-7 outside the cell independent of caspase-3. Third, we present evidence that extracellular caspase-7 contributes to generation of or modulates the potency of a transmissible endothelial cytotoxin. Transmissible cytotoxicity is due to endothelium-derived oligomeric tau and amyloid-β (4, 28). These endothelium-derived amyloids contribute to end-organ dysfunction in the aftermath of critical illness due their cytotoxic effect on naïve endothelial cells and brain tissue (25). The regulatory mechanism of endothelial amyloid production and/or potency remains unknown. Amyloids are substrates of caspases; specifically, caspase-3 is known to cleave tau (9, 17, 27, 34). Therefore, it is attractive to hypothesize that extracellular caspase-7 cleaves oligomeric tau and/or amyloid-β during primary infection and adjusts their transmissible cytotoxic potency.
Caspase-3 and -7 are widely recognized as executioner caspases. Caspase-mediated apoptotic cell death occurs through two well-established signaling pathways. The extrinsic, or death-receptor mediated, apoptosis pathway is initiated following ligand binding to the CD95 or Fas receptor (21, 37). The Fas receptor then recruits the adaptor protein fas-associated death domain (FADD), which in turn recruits pro-caspase-8 to form the death-inducing signaling complex (DISC) (19). Pro-caspase-8 is then proteolytically cleaved, and active caspase-8 is released as the central regulatory caspase in the extrinsic apoptotic cell death pathway (16, 30). Caspase-8, an initiator caspase, cleaves effector caspases-3 and -7, which in turn cleave multiple cellular targets, including cytoskeletal components. Cytoskeletal cleavage results in the loss of cellular structure producing shrinkage, both of which are physiological features of cells undergoing apoptosis (16).
During the intrinsic, or mitochondria-mediated, apoptosis pathway, intracellular danger signals initiate BH3-BCL2-BAX/BAK signaling, resulting in mitochondrial membrane permeability and the release of cytochrome c (39). Cytochrome c-mediated recruitment of pro-caspase-9 into apoptotic protease-activating factor (APAF) results in the formation of the apoptosome, with similar function to DISC (1). Pro-capsase-9 is proteolytically cleaved to active caspase-9, an initiator caspase that cleaves effector caspases-3 and -7. It is noteworthy that with both extrinsic and intrinsic apoptosis pathways, caspases-3 and -7 are considered to have redundant functions.
Our studies revealed that PA103 leads to the activation of both caspases-8 and -9, indicating that PMVECs sense the bacteria through the intrinsic and extrinsic pathways. In stark contrast, ExoY+ was not perceived by either of these pathways, as neither caspase-8 nor caspase-9 were activated. ExoY+ is a genetically engineered mutant of PA103 that lacks exoenzymes U and T but expresses ExoY (see materials and methods). The T3SS itself does not cause endothelial cell death during acute infection (3). Our studies suggest that the intracellular presence of ExoY, which can bind to F-actin and nonenzymatically impair its branching (7, 26), and ExoY’s nucleotidyl cyclase activity, which accounts for microtubule breakdown (6, 29), do not initiate DISC or apoptosome formation and/or impair activation of caspases-8 and -9. Despite this escape from detection by the apoptotic machinery, ExoY+ promoted the accumulation of active caspase-7 outside of the cell. The mechanisms that are responsible for the escape from detection by extrinsic (i.e., caspase-8) and intrinsic (i.e., caspase-9) apoptosis pathways, and stimulation of caspase-7- but not caspase-3-activation, are important future areas of study.
It is also not clear where caspase-7 activation occurs. Specifically, whether ExoY+ infection induces caspase-7 activation inside the cell before its release, or whether caspase-7 activation occurs outside the cell after it is released, has not been resolved. We detected neither the 21-kDa cleaved caspase-7 nor the FLICA-positive caspase-3/7 activity inside the cell. Together with the absence of intracellular caspase-8 or caspase-9 activity, the simplest interpretation is that ExoY+ causes the release of pro-caspase-7 into the extracellular milieu, where it is metabolized into an active form. Future studies will focus on the site of caspase-7 activation following ExoY+ infection, specifically, whether and how pro-caspase-7 could be activated once it is released from the cell.
Executioner caspases-3 and -7 not only share sequence homology but also substrate specificity. Proteomic analysis has revealed that both caspases target the four-amino acid sequence DEVD, supporting the assertion that caspases-3 and -7 are, in fact, redundant (36, 40). However, detailed biochemical analyses reveal that differences in substrate specificity, affinity, and selectivity exist between these enzymes, consistent with their nonredundant roles (11, 43). The significance of these nonredundant roles is largely unexplored. Our studies contribute to this emerging field, as ExoY+ infection of PMVECs specifically elicited caspase-7 and not caspase-3 activation.
Nonapoptotic functions of caspases are under investigation. Caspase-3 plays a nonapoptotic function in endothelial permeability (38). Caspase-7, but not caspase-3, has been shown to play a role in the inflammasome/caspase-1 signaling pathway (22). In studies germane to our work on transmissible cytotoxicity, caspase-3, but not caspase-7, was found to cleave γ-secretase-activating protein, thereby directing γ-secretase to cleave APP into an amyloid (8). In our infection model, active caspase-7 was resolved outside the cell independent of endothelial cell death or caspase-3. Whereas PA103 infection of PMVECs led to intracellular caspase-3/7 activation and corresponding cell death, ExoY+ infection resulted in time-dependent accumulation of extracellular active caspase-7 but not caspase-3. Importantly, extracellular active caspase-7 was detected in the absence of any cell death markers. These data suggest a nonapoptotic role for extracellular caspase-7 during ExoY+ infection, which led us to explore its putative role in transmissible cytotoxicity. Few studies investigate the presence of caspase-7 outside of cells. In the few instances where caspase-7 has been measured in the circulation, it is reported as a marker of cell death (12, 24, 42). Our studies suggest that this is not necessarily the case.
ExoY+ infection of PMVECs leads to time-dependent release of cytotoxic oligomeric tau (4, 25, 28). Oligomeric tau has been resolved at multiple different molecular weights, depending upon the antibodies used for detection and, in our studies, dependent upon the bacterial strain/mutant tested. Nonetheless, high-molecular-weight (i.e., >100 kDa), ≈75-kDa, and smaller-fragment (i.e., <25 kDa) tau species are commonly resolved using TOC1 and T22 antibodies (4, 25, 28). Mechanisms by which ExoY+ intoxication leads to the generation of cytotoxic tau fragments during infection are unknown. Tau is a known substrate of caspase-3 (9), and caspase-3-cleaved tau contributes to development of neurofibrillary tangles in Alzheimer’s disease (17, 27). Because our studies incriminate caspase-7 in transmissible cytotoxicity, and because transmissible cytotoxicity is dependent upon oligomeric tau, it is possible that active caspase-7 cleaves oligomeric tau into cytotoxic fragments. Future studies are necessary to identify whether and where ExoY+-induced extracellular caspase-7 targets and cleaves tau and to rigorously assess the important structural elements of relevant cytotoxic tau fragments.
In conclusion, we report that ExoY+ infection of PMVECs elicits accumulation of an extracellular form of active caspase-7 that contributes to the severity of transmissible cytotoxicity. These findings offer novel insight into stimuli capable of promoting noncanonical functions of caspase-7, and they provide rare examples of nonredundancy among these so-called executioner caspases. We report this unique regulation of caspase-7 secondary to production of cyclic nucleotide monophosphate signaling during an infection. At present, we are unaware of how this signaling event coordinates caspase-7 activation/release independent from caspase-3 or how it differs from the PA103-induced activation of both caspase-3 and -7 that corresponds with apoptosis. Because this study was designed to mechanistically address the importance of executioner caspases in the endothelial cell response to infection, the endothelial cell sample size is small and represents a limitation of the work. Indeed, in future studies it will be important to determine whether active caspase-7 is present in biological fluids, i.e., bronchoalveolar lavage fluid, blood, and cerebrospinal fluid, during the course of P. aeruginosa infection and how active caspase-7 contributes to amyloid cytotoxicity in this in vivo setting.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-66299 and HL-60024 awarded to T.S.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.R., M.A., and T.S. conceived and designed research; P.R., N.K., V.P., D.S., and S.S.P. performed experiments; P.R., D.T., M.A., and T.S. analyzed data; P.R., M.A., and T.S. interpreted results of experiments; P.R. and S.S.P. prepared figures; P.R. and T.S. drafted manuscript; P.R., M.A., D.W.F., and T.S. edited and revised manuscript; P.R., N.K., V.P., D.S., S.S.P., D.T., M.A., D.W.F., and T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Linn Ayers and the Center for Lung Biology Cell Culture Core for providing PMVECs used in these studies. We thank Dara Frank for providing bacterial strains.
REFERENCES
- 1.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell 9: 423–432, 2002. doi: 10.1016/S1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez DF, Housley N, Koloteva A, Zhou C, O’Donnell K, Audia JP. Caspase-1 activation protects lung endothelial barrier function during infection-induced stress. Am J Respir Cell Mol Biol 55: 500–510, 2016. doi: 10.1165/rcmb.2015-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audia JP, Lindsey AS, Housley NA, Ochoa CR, Zhou C, Toba M, Oka M, Annamdevula NS, Fitzgerald MS, Frank DW, Alvarez DF. In the absence of effector proteins, the Pseudomonas aeruginosa type three secretion system needle tip complex contributes to lung injury and systemic inflammatory responses. PLoS One 8: e81792, 2013. doi: 10.1371/journal.pone.0081792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balczon R, Morrow KA, Zhou C, Edmonds B, Alexeyev M, Pittet JF, Wagener BM, Moser SA, Leavesley S, Zha X, Frank DW, Stevens T. Pseudomonas aeruginosa infection liberates transmissible, cytotoxic prion amyloids. FASEB J 31: 2785–2796, 2017. doi: 10.1096/fj.201601042RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balczon R, Prasain N, Ochoa C, Prater J, Zhu B, Alexeyev M, Sayner S, Frank DW, Stevens T. Pseudomonas aeruginosa exotoxin Y-mediated tau hyperphosphorylation impairs microtubule assembly in pulmonary microvascular endothelial cells. PLoS One 8: e74343, 2013. doi: 10.1371/journal.pone.0074343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckert U, Wolter S, Hartwig C, Bähre H, Kaever V, Ladant D, Frank DW, Seifert R. ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation. Biochem Biophys Res Commun 450: 870–874, 2014. doi: 10.1016/j.bbrc.2014.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belyy A, Raoux-Barbot D, Saveanu C, Namane A, Ogryzko V, Worpenberg L, David V, Henriot V, Fellous S, Merrifield C, Assayag E, Ladant D, Renault L, Mechold U. Actin activates Pseudomonas aeruginosa ExoY nucleotidyl cyclase toxin and ExoY-like effector domains from MARTX toxins. Nat Commun 7: 13582, 2016. doi: 10.1038/ncomms13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu J, Li JG, Joshi YB, Giannopoulos PF, Hoffman NE, Madesh M, Praticò D. Gamma secretase-activating protein is a substrate for caspase-3: implications for Alzheimer’s disease. Biol Psychiatry 77: 720–728, 2015. doi: 10.1016/j.biopsych.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwag BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis 8: 162–172, 2001. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 10.Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV Jr. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 109: 1019–1029, 1996. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 11.Demon D, Van Damme P, Vanden Berghe T, Deceuninck A, Van Durme J, Verspurten J, Helsens K, Impens F, Wejda M, Schymkowitz J, Rousseau F, Madder A, Vandekerckhove J, Declercq W, Gevaert K, Vandenabeele P. Proteome-wide substrate analysis indicates substrate exclusion as a mechanism to generate caspase-7 versus caspase-3 specificity. Mol Cell Proteomics 8: 2700–2714, 2009. doi: 10.1074/mcp.M900310-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Ansary A, Al-Ayadhi L. Neuroinflammation in autism spectrum disorders. J Neuroinflammation 9: 265, 2012. doi: 10.1186/1742-2094-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147: 2659–2669, 2001. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 14.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7: 654–665, 2009. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Dunne WM, Swanson PE, Davis CG, Tinsley KW, Chang KC, Buchman TG, Karl IE. Role of apoptosis in Pseudomonas aeruginosa pneumonia. Science 294: 1783, 2001. doi: 10.1126/science.294.5548.1783a. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med 361: 1570–1583, 2009. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Choi H, Lee W, Park H, Kam TI, Hong SH, Nah J, Jung S, Shin B, Lee H, Choi TY, Choo H, Kim KK, Choi SY, Kayed R, Jung YK. Caspase-cleaved tau exhibits rapid memory impairment associated with tau oligomers in a transgenic mouse model. Neurobiol Dis 87: 19–28, 2016. doi: 10.1016/j.nbd.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 18.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 14: 5579–5588, 1995. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloth C, Schirmer B, Munder A, Stelzer T, Rothschuh J, Seifert R. The role of Pseudomonas aeruginosa ExoY in an acute mouse lung infection model. Toxins (Basel) 10: 185, 2018. doi: 10.3390/toxins10050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krammer PH. CD95's deadly mission in the immune system. Nature 407: 789–795, 2000. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 22.Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Núñez G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics 7: 2350–2363, 2008. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Berre R, Faure K, Fauvel H, Viget NB, Ader F, Prangère T, Thomas AM, Leroy X, Pittet JF, Marchetti P, Guery BP. Apoptosis inhibition in P. aeruginosa-induced lung injury influences lung fluid balance. Intensive Care Med 30: 1204–1211, 2004. doi: 10.1007/s00134-004-2165-0. [DOI] [PubMed] [Google Scholar]
- 24.Lin CH, Chen M, Sun MC. Circulating apoptotic factors in patients with acute cerebral infarction. Clin Biochem 43: 761–763, 2010. doi: 10.1016/j.clinbiochem.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Lin MT, Balczon R, Pittet JF, Wagener BM, Moser SA, Morrow KA, Voth S, Francis CM, Leavesley S, Bell J, Alvarez DF, Stevens T. Nosocomial pneumonia elicits an endothelial proteinopathy: evidence for a source of neurotoxic amyloids in critically ill patients. Am J Respir Crit Care Med 198: 1575–1578, 2018. doi: 10.1164/rccm.201801-0060LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancl JM, Suarez C, Liang WG, Kovar DR, Tang WJ. Pseudomonas aeruginosa exoenzyme Y directly bundles actin filaments. J Biol Chem 295: 3506–3517, 2020. doi: 10.1074/jbc.RA119.012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Means JC, Gerdes BC, Kaja S, Sumien N, Payne AJ, Stark DA, Borden PK, Price JL, Koulen P. Caspase-3-dependent proteolytic cleavage of tau causes neurofibrillary tangles and results in cognitive impairment during normal aging. Neurochem Res 41: 2278–2288, 2016. doi: 10.1007/s11064-016-1942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow KA, Ochoa CD, Balczon R, Zhou C, Cauthen L, Alexeyev M, Schmalzer KM, Frank DW, Stevens T. Pseudomonas aeruginosa exoenzymes U and Y induce a transmissible endothelial proteinopathy. Am J Physiol Lung Cell Mol Physiol 310: L337–L353, 2016. doi: 10.1152/ajplung.00103.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow KA, Seifert R, Kaever V, Britain AL, Sayner SL, Ochoa CD, Cioffi EA, Frank DW, Rich TC, Stevens T. Heterogeneity of pulmonary endothelial cyclic nucleotide response to Pseudomonas aeruginosa ExoY infection. Am J Physiol Lung Cell Mol Physiol 309: L1199–L1207, 2015. doi: 10.1152/ajplung.00165.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell 85: 817–827, 1996. doi: 10.1016/S0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 31.Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J Biol Chem 287: 25407–25418, 2012. doi: 10.1074/jbc.M111.301440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rello J, Jubert P, Vallés J, Artigas A, Rué M, Niederman MS. Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis 23: 973–978, 1996. doi: 10.1093/clinids/23.5.973. [DOI] [PubMed] [Google Scholar]
- 33.Renema P, Hardy KS, Housley N, Dunbar G, Annamdevula N, Britain A, Spadafora D, Leavesley S, Rich T, Audia JP, Alvarez DF. cAMP signaling primes lung endothelial cells to activate caspase-1 during Pseudomonas aeruginosa infection. Am J Physiol Lung Cell Mol Physiol 318: L1074–L1083, 2020. doi: 10.1152/ajplung.00185.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis 11: 341–354, 2002. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 35.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95: 196–203, 2004. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 36.Schilling O, Overall CM. Proteome-derived, database-searchable peptide libraries for identifying protease cleavage sites. Nat Biotechnol 26: 685–694, 2008. doi: 10.1038/nbt1408. [DOI] [PubMed] [Google Scholar]
- 37.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 14: 6136–6147, 1995. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suresh K, Carino K, Johnston L, Servinsky L, Machamer CE, Kolb TM, Lam H, Dudek SM, An SS, Rane MJ, Shimoda LA, Damarla M. A nonapoptotic endothelial barrier-protective role for caspase-3. Am J Physiol Lung Cell Mol Physiol 316: L1118–L1126, 2019. doi: 10.1152/ajplung.00487.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11: 621–632, 2010. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 40.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272: 17907–17911, 1997. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 41.Tumbarello M, De Pascale G, Trecarichi EM, Spanu T, Antonicelli F, Maviglia R, Pennisi MA, Bello G, Antonelli M. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med 39: 682–692, 2013. doi: 10.1007/s00134-013-2828-9. [DOI] [PubMed] [Google Scholar]
- 42.Uyanikoglu H, Sabuncu T, Dursun H, Sezen H, Aksoy N. Circulating levels of apoptotic markers and oxidative stress parameters in women with polycystic ovary syndrome: a case-controlled descriptive study. Biomarkers 22: 643–647, 2017. doi: 10.1080/1354750X.2016.1265004. [DOI] [PubMed] [Google Scholar]
- 43.Walsh JG, Cullen SP, Sheridan C, Lüthi AU, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci USA 105: 12815–12819, 2008. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber DJ, Rutala WA, Sickbert-Bennett EE, Samsa GP, Brown V, Niederman MS. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol 28: 825–831, 2007. doi: 10.1086/518460. [DOI] [PubMed] [Google Scholar]
- 45.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 95: 13899–13904, 1998. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]