Abstract
While antenatal glucocorticoids are widely used to enhance lung function in preterm infants, cellular and molecular mechanisms by which glucocorticoid receptor (GR) signaling influences lung maturation remain poorly understood. Deletion of the glucocorticoid receptor gene (Nr3c1) from fetal pulmonary mesenchymal cells phenocopied defects caused by global Nr3c1 deletion, while lung epithelial- or endothelial-specific Nr3c1 deletion did not impair lung function at birth. We integrated genome-wide gene expression profiling, ATAC-seq, and single cell RNA-seq data in mice in which GR was deleted or activated to identify the cellular and molecular mechanisms by which glucocorticoids control prenatal lung maturation. GR enhanced differentiation of a newly defined proliferative mesenchymal progenitor cell (PMP) into matrix fibroblasts (MFBs), in part by directly activating extracellular matrix-associated target genes, including Fn1, Col16a4, and Eln and by modulating VEGF, JAK-STAT, and WNT signaling. Loss of mesenchymal GR signaling blocked fibroblast progenitor differentiation into mature MFBs, which in turn increased proliferation of SOX9+ alveolar epithelial progenitor cells and inhibited differentiation of mature alveolar type II (AT2) and AT1 cells. GR signaling controls genes required for differentiation of a subset of proliferative mesenchymal progenitors into matrix fibroblasts, in turn, regulating signals controlling AT2/AT1 progenitor cell proliferation and differentiation and identifying cells and processes by which glucocorticoid signaling regulates fetal lung maturation.
Keywords: glucocorticoid signaling, matrix fibroblast, regulatory network systems biology, single cell
INTRODUCTION
Lung morphogenesis and function are critically dependent on complex interactions between endodermally derived epithelial and mesodermally derived mesenchymal cells (34, 67). The fetal lung mesenchyme consists of multiple cell types, including pericytes, smooth muscle, endothelial, and diverse fibroblastic cells, such as matrix fibroblasts, lipofibroblasts, and myofibroblasts. Recent single cell transcriptomic studies identified the heterogeneity of lung mesenchymal cells and their importance in lung morphogenesis (27, 46, 75, 77, 83). Lineage tracing and loss-of-function experiments implicate Wnt2+/Gli1+/Isl1+ mesenchymal cells as multipotent progenitors, contributing to formation of the fetal lung. Mesenchymal progenitors contribute to all layers of the pulmonary vasculature, including airway and vascular smooth muscle cells, endothelial cells, and pericyte-like cells (58). PDGRFα+ fibroblasts enhance self-renewal and differentiation of AT2 epithelial cells, supporting the importance of fibroblast-epithelial cell interactions during normal lung formation and differentiation (2, 11, 25). Recent lineage tracing and RNA-sequencing (RNA-seq) data identified Lgr5+ and Wnt-responsive/PDGFRα+ mesenchymal cell subsets within alveolar niches that influence differentiation of alveolar epithelial cells during repair of the mature lung (11, 46, 82).
Glucocorticoid (GC) signaling has profound effects on perinatal lung morphogenesis and function (3, 12, 26, 31). However, the cellular and molecular mechanisms mediating effects of GC and the potential toxicity of glucocorticoid exposure to the lung and other organs remain unclear. Antenatal treatment of the mother is routinely used to prevent respiratory distress syndrome (RDS) after preterm birth (47). Environmental factors, types of GC agonists, frequency and duration of treatment, and routes of administration influence the effectiveness of antenatal steroids (5, 21, 56). Histologically, antenatal GCs cause thinning of the lung septae and enhance differentiation of alveolar type I (AT1) and AT2 epithelial cells (36). Glucocorticoids have pleotropic effects on pulmonary structure and function, decreasing epithelial and mesenchymal cell proliferation and enhancing surfactant lipid and protein expression and the activity of sodium transporter (ENaC) that contribute to lung function after birth (47, 54, 64). Conditional deletion of Nr3c1 from mesenchymal cells using Twist2Cre or Col1a2Cre recombinase caused respiratory failure at birth, phenocopying findings caused by global Nr3c1 deletion (3, 31, 61). In contrast, epithelial- or endothelial-specific deletion of Nr3c1 did not change lung structure or function, supporting the importance of the pulmonary mesenchyme in GR-mediated lung maturation (3, 31). GC signaling promotes the transition of bronchiolar to alveolar cell fate in peripheral epithelial lung progenitors and controls the timing of alveolar epithelial cell maturation (1, 45, 47). In the present study, we identify molecular and cellular mechanisms by which GR signaling regulates gene expression in a distinct subset of pulmonary mesenchymal cells to control perinatal lung function.
METHODS
Mice and antibodies.
Twist2Cre mice [B6.129X1-Twist2tm1.1(cre)Dor/J, stock no. 008712], Nr3c1fl/fl mice (B6.Cg-Nr3c1tm1.1Jda/J, stock no. 021021), Rosa26 membrane tdTomato/membrane GFP (mT/mG) mice [Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, stock no. 007576] and wild-type C57Bl/6J (stock no. 000664) were purchased from Jax. Animal studies were approved by the Animal Care and Use Committee of the Cincinnati Children’s Research Foundation.
The following antibodies were used for immunostaining:
-
•
GR [Santa Cruz (M-2), cat. no. SC-1004]. Validated by Western blot and for use in immunohistochemistry (IHC), chromatin immunoprecipitation (ChIP), and EMSA assays according to Santa Cruz Biotechnology website that references 129 publications.
-
•
TTF1 (G237, developed in Dr. Jeffrey Whitsett’s laboratory). This guinea pig polyclonal antibody was developed at Cincinnati Children’s Hospital Medical Center (CCHMC) using the same criteria as other antibodies marketed through Seven Hills Bioreagents. It is suitable for Western blots, as well as IHC and immunofluorescence (IF) on human and mouse tissue.
-
•
AGER (R&D, cat no. MAB 1179). Validated by Western blot and for use in IF against mouse and rat protein according to R&D Systems website that includes references to 12 publications. A publication for similar usage to detect AT1 cells [see study by Kato et al. (38a) in which AGAR is similarly used to detect AT1 cells].
-
•
PDPN (DSHB, clone 8.1.1). This monoclonal antibody is reactive against mouse for use in Western blot, IF, IHC, and FACS and has 73 publications listed on the Developmental Studies Hybridoma Bank (DSHB) and CiteAb websites.
-
•
HOPX (Santa Cruz Biotechnologies, FL-73, cat. no. SC-30216). Validated by Western blot and for use in IF against human, mouse, and rat protein according to Santa Cruz Biotechnology (SCBT) website that includes references to four publications. Twenty-three citations have been found for this product on the CiteAb database. Note: this antibody has been discontinued.
-
•
EMCN (R&D Systems, cat no. AF4666). This antibody is reactive to mouse, rat, hamster, cow, dog, human, monkey and hamster and is suitable for Western blotting, IHC-Paraffin, and IHC-Frozen. The website cites seven references including its use on mouse tissue for IF [see study by Havrilak et al. (32a)].
-
•
CD106/VCAM1 (ThermoFisher, cat no. MA5-11447, lot PI1904981). This mouse monoclonal antibody is reactive to human, rat, and mouse and is suitable for ICC, IF, IHC, and flow cytometry. The Thermofisher website lists five references.
-
•
CD106/VCAM1 (R&D Systems, cat. no. AF643). This antibody reacts with mouse and is suitable for Western blot, IHC, and IF. The website sites 18 references, including use on mouse tissue for IF.
-
•
FN1 (Abcam, cat. no. ab2413). This antibody reacts with mouse, rat, hamster, cow, dog, human, African green monkey, Chinese hamster, and Syrian hamster and is suitable for Western blotting and IHC/IF on frozen or paraffin tissue. The website cites 347 references including use on mouse tissue for IF.
-
•
ACTA2 (Sigma Aldrich, cat. no. A5228). This antibody reacts with human, frog, sheep, chicken, goat, bovine, rat, guinea pig, mouse, canine, rabbit, and snake and is suitable for Western blot, IHC, and IF. The website cites over 500 references including its use on mouse tissue for IF.
-
•
FOXM1 (Santa Cruz, cat. no. sc-502 C20). This antibody reacts with mouse, rat, and human, and is suitable for Western blot and IHC. The website cites 39 references including its use on mouse tissue for IF.
Elastin staining protocol.
Paraformaldehyde-fixed, frozen lung sections from embryonic day (E)18.5 mice were rinsed in deionized water for 5 min and incubated in fresh Weigert’s Working Hematoxylin (Poly Scientific R&D Corp., cat. no. s216b) for 10 min. After being washed in water and rinsed in deionized water, sections were placed in Resorcin Fuchsin Working Solution (Poly Scientific R&D Corp., cat. no. s265) overnight. The following day, excess stain was removed by brief dips in 95% ethanol, sections were washed in tap water followed by a quick rinse in deionized water and placed in Van Gieson solution (Poly Scientific R&D Corp., cat. no. s289) for 1 min. Excess stain was removed by a brief water rinse and sections were mounted using Permount mounting medium and coverslipped.
Isolation of primary lung cells for RNA-seq.
Dex- or saline-treated lung mesenchymal cells for bulk RNA-seq Twist2Cre;:Rosa26mT/mG and Rosa26mT/mG mice were time mated and pregnant dams were injected intraperitoneally at E18.5 with 1 mg/kg dexamethasone (DexaJect) or saline as a control. Pups were harvested 2 h postinjection and visually genotyped under a fluorescent microscope on the basis of red only fluorescence (Rosa26mT/mG) or green and red fluorescence (Twist2Cre;Rosa26mT/mG). Lungs were removed, pooled by genotype (n = 6–8 per group), and minced in a petri dish on ice with a razor blade. Minced lung tissue was incubated in 1.5% collagenase (Sigma) in TrypLE (Life Technologies) + 64U DNase I (Sigma) at 37°C for 30 min with occasion tituration. The lung cell suspension was filtered successively through 100-µm, 40-µm, and 20-µm filters, pelleted, and resuspended in 20%FBS in DMEM/HEPES. Cells were sorted on a BD FACSAria II sorter and gated for tdTomato−/GFP+ (Twist2Cre+ mesenchyme) cells. Cells were collected, lysed in RLT+ buffer containing b-mercaptoethanol, and RNA was isolated with the Nucleospin RNA Plus Kit (Macherey-Nagel). RNAs were extracted from mesenchymal cells, and sequencing and alignment were performed in Cincinnati Children’s Hospital Medical Center’s Gene Expression Core.
Single cell isolation and sequencing.
Wild-type C57Bl6/J mice (8 wk old) were time mated, and dams were injected intraperitoneally at E17.5 with 2 mg/kg dexamethasone (DexaJect) or saline as a control. Pups were harvested 24 h after injection. Lungs (n = 6–8 per group) were excised and placed in DMEM. Pooled lung lobes were diced into cubes with a microblade scalpel under a dissecting microscope, placed in digest buffer [2 mg/mL type2 collagenase (Worthington), 1 mg/mL Pronase E (Sigma), 62.5 U/mL DNase I (Sigma), and 5 mM CaCl2 in PBS without calcium and magnesium] at 37°C for 10 min followed by gentle trituration. Digested tissue was passed through a 23-gauge needle, filtered through a 40-µm filter and cells pelleted. Pellets were resuspended in red blood cell lysis buffer for 2 min at room temperature and washed with PBS-0.01% BSA. Cells were resuspended in PBS-0.01% BSA (300 cells/µL) and submitted to the Cincinnati Children’s Hospital Medical Center’s Gene Expression Core for processing for RNA-seq analysis using the Fluidigm C1 platform as previously described (78). Single-cell suspensions were loaded onto a Fluidigm C1 10–17 µchip and captured according to the manufacturer’s protocol. Chambers (96) were microscopically examined and those with single cells were recorded. Cells were lysed for RNA isolation. RNA was dT primed for cDNA synthesis. cDNA products were harvested, diluted, barcoded, and “tagmented” using Illumina Nextera and sequenced on a single lane with Illumina HiSeq2500, generating ~250 million single end 50–100 base pair reads per 96-well plate as described previously (30, 78).
Single cell RNA-seq analysis.
Individual cells were isolated from fetal whole lung and sequenced using C1 Fluidigm platform. Cells with read count <0.5 million and total number of genes expressed <500 were removed from the analysis. Total of 731 individual fetal lung cells passed the QC and used for single cell comparative analysis and pseudotime analysis including 98 (Dex), 142 (saline), and 491 cells obtained from three wild-type fetuses from E16.5, E18.5 and PND1. Cells with cohighly expressed selective markers of two distinct cell types were considered as doublets and were removed from the analysis. Using SINCERA pipeline (30), we identified cell clusters using hierarchical clustering with average linkage and Pearson’s correlation based distance and mapped cell clusters to known cell types based on cell type-specific marker genes. Cell type-specific differentially expressed genes (signature genes) were identified using a two group Welch’s t test (28). Signature genes of each cell type were subject to functional enrichment analysis and pathway analysis using the IPA (Ingenuity) and ToppGene Suite (9).
RNA and ATAC Sequencing of CD106+ mesenchymal cells.
Lungs were isolated from E16.5 (n = 6 total: n = 2 for RNA-seq, n = 4 for ATAC-seq) and E18.5 (n = 4 for ATAC-seq) C57Bl/6J mice and placed in DMEM. Lobes were diced into cubes with a microblade scapel under a dissecting microscope and placed in digest buffer [0.25% trypsin (Gibco) and 62.5 U/mL DNase I (Sigma)] at 37°C for 10 min, followed by gentle trituration at room temperature until completely dissociated. Collagenase type 2 (1 mg/mL, Worthington) was added to the digest buffer for E18.5 lungs. Fetal bovine serum was added after dissociation to inhibit trypsin. Digests were passed through 40-µm filter and pelleted, and red blood cells were lysed for 2 min at room temperature. Cells were resuspended in MACS buffer containing 0.5% BSA (Miltenyi) and blocked with FcR blocking reagent (Miltenyi) at 4°C for 20 min. CD106+ cells were isolated using negative selection followed by positive selection. For negative selection, cells were incubated with biotin-anti-mouse CD45 (clone 30-F11, Biolegend), biotin-anti-mouse CD31 (clone MEC 13.3, BD Biosciences), biotin-anti-mouse CD309 (clone 89B3A5, Biolegend), and biotin-anti-mouse CD144 (clone BV13, Biolegend) at 4°C for 20 min. Cells were pelleted, resuspended in MACS buffer, incubated with anti-biotin microbeads (Miltenyi), and passed over a LS column (Miltenyi) on a QuadroMACS separator. Cells on the column were washed and eluted in MACS buffer, and the flow-through was saved (CD45−/CD31−/CD309− cells). CD106+ cells were then isolated from the CD45−/CD31−/CD309− cell fraction using biotin-anti-mouse CD106 (clone 429, Miltenyi) and anti-biotin microbeads. CD106+ cells were resuspended in DMEM + 10% FBS + 25 mM HEPES in Eppendorf tubes and treated with 100 nM dexamethasone or saline for 4 h at 37°C.
For RNA-seq, cells were collected and lysed in RLT+ buffer containing b-mercaptoethanol and RNA was isolated with the Nucleospin RNA Plus Kit (Macherey-Nagel). Sequencing and alignment were performed in Cincinnati Children’s Hospital Medical Center’s Gene Expression Core.
For ATAC-seq, cells were pelleted and counted and 50,000 cells per sample were prepared for ATAC-seq (8). Tagged and amplified DNA was submitted to Genewiz for sequencing on the Illumina HiSeq platform at a depth of ~1,000,000 reads per sample.
ATAC-seq analysis.
Nextera adapter sequences were removed from the ATAC seq data using Trim Galore (43) and then the paired end FASTQ files were aligned to mm10 reference genome using Bowtie2 with parameters “–X 2000 and –k 1” (44). These parameters allow fragments up to 2 kb to align and only uniquely aligned reads were collected (7). Aligned bam files were sorted using Samtools (23) and duplicates were removed using Picard (v1.89) (http://broadinstitute.github.io/picard). Reads mapped to mitochondrial DNAs were filtered out. MACS2 (84) algorithm was used to call peaks from Dex-treated samples using saline-treated CD106+ as background control. Peaks with more than twofold enrichment to the control samples were selected. Common and unique peaks in E16.5 and E18.5 samples were identified and annotated based on mm10 genome using homer (33). Enriched motifs within Dex-induced differentially accessible peaks were identified from Cis-BP mouse motifs database with control peaks as background using findMotifsGenome.pl wrapper in Homer (33). Functional enrichment analysis on gene sets were performed using ToppGene suite (9).
Bulk RNA-seq analysis on sorted cell populations.
Raw sequenced reads were aligned from FASTQ files to mouse mm10 genome in TopHat2. Quantification was performed with University of California, Santa Cruz (UCSC) reference transcriptome (http://ccb.jhu.edu/software/tophat/index.shtml) using Partek E/M in Partek Flow. Samples were further processed via trimmed mean normalization (22). Differentially expressed genes were identified using Smyth’s moderated t test and Benjamini-Hochberg procedure for adjusted P value (FDR). A gene was considered to be differentially expressed at P ≤ 0.05 (with FDR correction) and expression fold change ≥1.5 was observed in at least one condition. Gene set enrichment and pathway analysis were performed using ToppGene suite (https://toppgene.cchmc.org/) and Ingenuity Pathway Analysis (IPA).
mesGR-knockout microarray analysis.
Whole lung microarray data from mesenchyme-specific GR deletion mice (Col1a2-Cre;Nr3c1fl/fl) at E16.5 and E18.5 (31) were downloaded from Gene Expression Omnibus (GEO) database (GSE30143). Differentially expressed genes in lungs from Col1a2-Cre;Nr3c1fl/fl versus control mice at E16.5 and E18.5 (n = 5 for each condition) were identified using ANOVA with the threshold of corrected P ≤ 0.05 and fold change ≥1.5. Benjamini-Hochberg false discovery rate (FDR) was applied for multiple testing correction. Gene Ontology and Pathway analyses were performed using ToppGene Suite (https://toppgene.cchmc.org/) and Ingenuity Pathway Analysis (IPA; Ingenuity). Promoter sequences (1 kb upstream and 100 bp downstream of the transcription start site) were used to identify potential GR-binding elements (GRE) in the promoter regions of genes using a GR position-weighted matrix (PWM) taken from Genomatix.
Upstream regulator prediction.
Regulatory relationships between transcriptional regulators and the predicted target genes precompiled in the Ingenuity Knowledge Base was used for upstream regulator prediction. The analysis identifies known targets of each transcriptional regulator present in the user's data set and compares their correlation with directional changes in gene expression in the experimental conditions and correlates them with the literature. If expression changes are positively correlated with the literature, transcriptional regulators are defined as in an activated state. For each potential transcriptional regulator, two statistical measures (overlap P value and activation z-score) are computed. The P value is calculated to rank upstream regulators based on significant overlap of differentially expressed genes to the gene targets known to be regulated by the transcriptional regulator. A z-score is used to infer activation states of upstream regulators.
Data availability.
All sequencing data from the present study have been deposited to GEO (https://www.ncbi.nlm.nih.gov/geo/). Specifically, whole lung microarray data from mesenchyme-specific GR deletion mice (Col1a2-Cre;Nr3c1fl/fl) at E16.5 and E18.5 was deposited (GSE30143; Ref. 31). RNA-seq of lung (Twist2Cre;Rosa26mT/mG mice treated with dexamethasone or saline) at E18.5; RNA-seq of CD106+ (CD45−/CD31−/CD309−/CD106+) and CD106− cells; ATAC-seq of CD106+ cells at E16.5 and E18.5; and scRNA-seq of mouse lung (wild-type embryos treated with Dex or saline in vivo and harvested at E18.5) are available at GSE136954. All data from this study can be accessed through Lung Gene Expression Analysis (LGEA) web portal (17, 19), which is freely available at https://research.cchmc.org/pbge/lunggens/GR_signaling.html. R code used for data analysis is available at https://github.com/xu-lab.
RESULTS
Glucocorticoid signaling regulates perinatal lung gene expression.
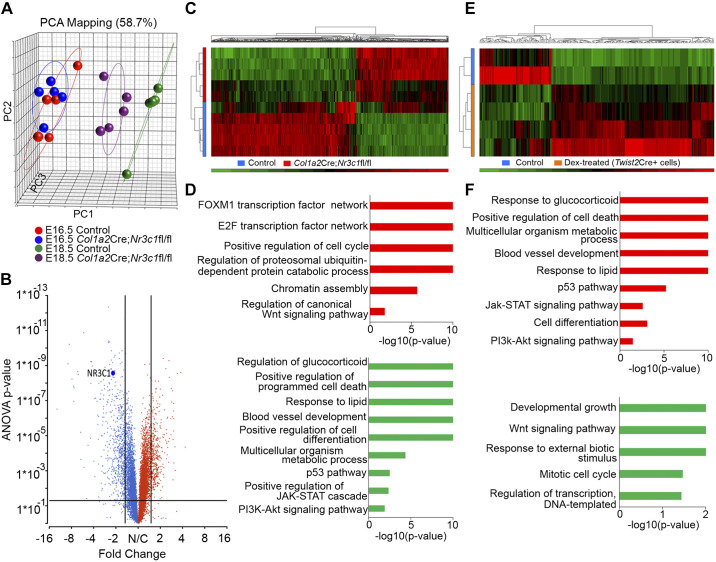
We downloaded and analyzed RNA microarray data from GR-deleted mice (Col1a2Cre,Nr3c1fl/fl) to identify genes and processes regulated by glucocorticoid signaling (31). Since deletion of Nr3c1 causes respiratory failure after birth, pulmonary gene expression was assessed at E16.5 and E18.5 to predict cellular and molecular processes regulated by glucocorticoids in the fetal lung. As shown by principle component analysis (PCA) and differential gene expression analysis (Fig. 1 and Supplemental Tables S1 and S2; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.9794966.v2), there were no major changes in gene expression in Col1a2Cre;Nr3c1fl/fl lungs at E16.5, while clear differences in RNA profiles were observed at E18.5, demonstrating that GC/GR signaling is most active near the time of birth (Fig. 1, A–D).
Fig. 1.
RNA profiling analyses of glucocorticoid receptor (GR) gain- versus loss-of-function of fetal mouse lungs. A: principle component analysis (PCA) of mesenchyme-specific GR knockout (Col1a2Cre;Nr3c1fl/fl) and wild-type mouse lung RNA microarray data (downloaded from GSE30143) from embryonic day (E)16.5 and E18.5 mouse lungs (n = 5 biological replicates were used for each time and each condition). While mRNA profiles were similar in GR-deficient mice at E16.5, deletion of GR from lung mesenchymal cells with Col1a2Cre strongly influenced gene expression and delayed maturation at E18.5. B: volcano plot shows ~800 genes differentially expressed in Col1a2Cre;Nr3c1fl/fl versus control lungs at E18.5 (ANOVA P < 0.05 and fold change >1.5). Red color indicates the genes with increased expression by 1.5-fold and blue indicates the genes with decreased expression by 1.5-fold in GR knockout mice. NR3C1 (GR) is down by 2-fold. C: heatmap of differentially expressed genes from Col1a2Cre;Nr3c1fl/fl (n = 5 lungs, denoted by red bar on the left side of the heatmap) and wild-type (n = 5 lungs, blue bar on the left side of the heatmap) at E18.5. D: enriched bioprocess and pathways induced (red bars) or suppressed (green bars) in E18.5 Col1a2Cre;Nr3c1fl/fl lungs compared with control lungs. E: heatmap of differentially expressed genes in lung mesenchymal cells FACS sorted from E18.5 Twist2Cre;Rosa26mT/mG reporter mice whose dam was injected with 1 mg/kg dexamethasone (n = 4 lungs, orange bar, left side of heatmap) or saline (n = 2 lungs, blue bar, left side of heatmap) 24 h before harvest. Bulk RNA-seq of the isolated cells was used to identify transcripts increased (red) or decreased (green) by dexamethasone treatment. Fold change >1.5 and P < 0.05 are shown. F: enriched bioprocess and pathways increased (red) or decreased (green) by dexamethasone treatment in E18.5 Twist2Cre+ lung mesenchymal cells are shown. Enriched biological functions and pathways in GR gain- vs. loss-of-function of mouse fetal lungs were largely inversely correlated in D and F.
Since mesenchymal expression of Nr3c1 was critical at E18.5, we treated dams with dexamethasone (Dex) and isolated mesenchymal cells at E18.5 to identify genes regulated by glucocorticoid (Fig. 1, E and F). Gene set enrichment analysis was used to compare genes altered by deletion of Nr3c1 or activated by dexamethasone in lung mesenchymal cells at E18.5. Enriched biological functions and pathways identified after deletion (Col1a2Cre;Nr3c1fl/fl) and those regulated by dexamethasone were largely inversely correlated. Genes involved in “response to glucocorticoid and lipids,” “cell differentiation,” “multicellular organism metabolic process,” “blood vessel development,” and “positive regulation of programmed cell death” were suppressed in Col1a2Cre;Nr3c1fl/fl mice and induced by dexamethasone; those involved in “cell cycle” and “cell proliferation” were induced after deletion of Nr3c1 and suppressed by dexamethasone (Fig. 1, D and F). Pathways induced by dexamethasone and suppressed by mesenchymal deletion of Nr3c1 included “P53,” “JAK-STAT,” and “PI3K-AKT” (phosphatidylinositol 3-kinase/protein kinase B) pathways. In contrast, Wnt signaling was activated after deletion of Nr3c1 and suppressed by antenatal dexamethasone (Fig. 1, D and F). Taken together, these data demonstrate that GR signaling controls gene expression in lung mesenchymal cells regulating cell proliferation and differentiation required for perinatal lung function before birth.
Single cell transcriptomic mapping of mouse lung cells during lung sacculation.
To identify pulmonary cell subtypes and associated gene expression patterns during perinatal lung development, we analyzed single cell RNA from mouse lung tissue on E16.5 and E18.5 from Fluidigm C1 RNA data using the SINCERA analytic pipeline (28, 30). At E16.5, nine major lung cell types were identified, including: epithelial, endothelial, myeloid/immune, proliferative mesenchymal progenitor (PMP), myofibroblast (MyoFB), matrix fibroblasts (MFB), pericytes and two intermediate fibroblast cells (IF1 and IF2) (Supplemental Fig. S1A). At E18.5, eight major lung cell types, including AT1 and AT2, ciliated, club, endothelial, myeloid/immune, myofibroblast/smooth muscle, and lipo/matrix fibroblasts were identified (Supplemental Fig. S1B) (17, 28). Using PCA biplots (Supplemental Fig. S1C and S1D), E16.5 epithelial cells were further dissected into four different subtypes: Sox9+ progenitors, Foxa2+ progenitors, pre-AT1 cells, and pre- AT2 cells. Cells were projected by principle components, to increase visibility of epithelial subtypes in different sub-spaces (Supplemental Fig. S1C and S1D). The PMP and SOX9+ clusters present at E16.5 were not detected at E18.5, likely indicating maturation of both of these progenitor populations to more mature fibroblast and mature epithelial cell populations. Signature genes, transcription factors, and surface markers associated with each cell type during lung sacculation were identified and are accessible on the Lung Gene Expression Analysis (LGEA) web-portal (https://research.cchmc.org/pbge/lunggens/mainportal.html) (17, 19). In summary, single cell transcriptome analysis provided a high-resolution blueprint of wild-type mouse lung cells during the saccular stage of lung development.
Genomic effects of GC/GR signaling in epithelial and mesenchymal cells during lung sacculation.
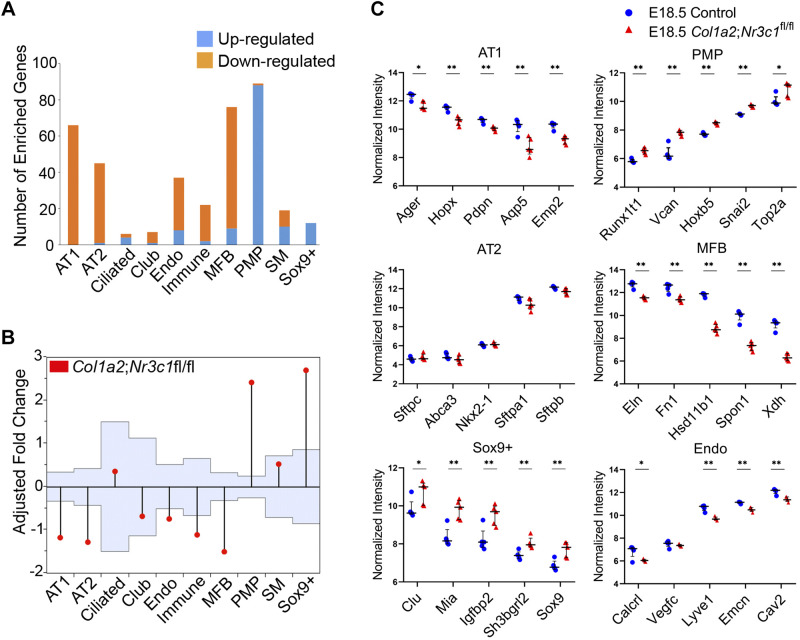
Bulk RNA data from Col1a2Cre;Nr3c1fl/fl lungs were correlated with cell type-specific signature genes identified from single cell RNA data from normal mice at identical embryonic stages, E16.5 and E18.5 (Fig. 2A). At E18.5, mesenchymal deletion of Nr3c1 significantly induced signature genes associated with proliferative mesenchymal progenitors (PMP) and suppressed those selectively expressed in matrix fibroblasts (MFB) to maintain the “stemness” or immaturity status of the mesenchymal progenitor cell type (Fig. 2, A and B). Although GR was abundantly expressed in both matrix fibroblasts and smooth muscle cells at E16.5 and E18.5, deletion of Nr3c1 in the mesenchyme did not influence expression of smooth muscle signature genes (Fig. 2, A and B). In epithelial cell subsets, genes selectively expressed in SOX9+ progenitors were induced and AT1-associated genes were suppressed. Mesenchymal Nr3c1 deletion had moderate effects on AT2 signature genes involved in host defense (e.g., Cela1, C5, Chi3l1, Lcn2, Lyz, Sftpd, Csf2, and Cd36) but did not significantly alter expression of other surfactant protein genes (Fig. 2, A and C). Mice with epithelial- or endothelial-specific Nr3c1 deletion were viable without histological abnormalities (3, 13, 31), supporting the concept that the effects of mesenchymal GR deletion on endothelial and epithelial cells were likely indirect.
Fig. 2.
Glucocorticoid receptor (GR) is required for lung matrix fibroblasts differentiation from specific mesenchymal progenitor cells. Mesenchyme-specific GR knockout (Col1a2Cre;Nr3c1fl/fl) and wild-type lung RNA microarray data from embryonic day (E)16.5 and E18.5 mouse lungs were downloaded from Gene Expression Omnibus (GEO) (GSE30143). Single cell RNA-seq analysis of mouse lung at E16.5 (n = 3) and E18.5 (n = 5) identified cell types and signature genes (28). Lung cell type mapping and cell type-specific signature genes are available in Lung Gene Expression Analysis (LGEA) web portal (19). A: differentially expressed genes in bulk RNA from Col1a2Cre;Nr3c1fl/fl lungs at E18.5 were correlated with cell-specific gene signatures from single cell RNA-seq analysis of wild-type lungs from LGEA to determine the effect of Nr3c1 deletion on individual cell types. The proportion of genes induced (blue bars) or suppressed (orange bars) by mesenchymal GR deletion in each cell type is shown in the bar graph. B: analysis of means (ANOM) was applied to assess effects of mesenchymal Nr3c1 deletion (Col1a2Cre;Nr3c1fl/fl) on different cell types. Light blue area shows 95% upper and lower boundaries of decision limits and values outside of the decision limits are significantly different (P < 0.05) from the overall mean. Vertical lines extend from the boundaries upward or downward, represent the magnitude of the least squares means different from the average. As shown, mesenchymal Nr3c1 deletion significantly induced signature genes in PMP cells and Sox9+ epithelial cells and suppressed genes selectively expressed in MFBs, AT1 and AT2 cells. C: effects of mesenchymal Nr3c1 deletion on representative RNAs in MFBs, PMPs, AT1, AT2, Sox9+ epithelial progenitors, and endo cells. MFB, matrix fibroblasts; PMP, proliferative mesenchymal progenitor cell; AT1, alveolar type 1 cells; AT2, alveolar type 2 cells; PI3K, phosphatidylinositol 3-kinase; SM, smooth muscle. *Student’s t test, P < 0.05; **Student’s t test, P < 0.01.
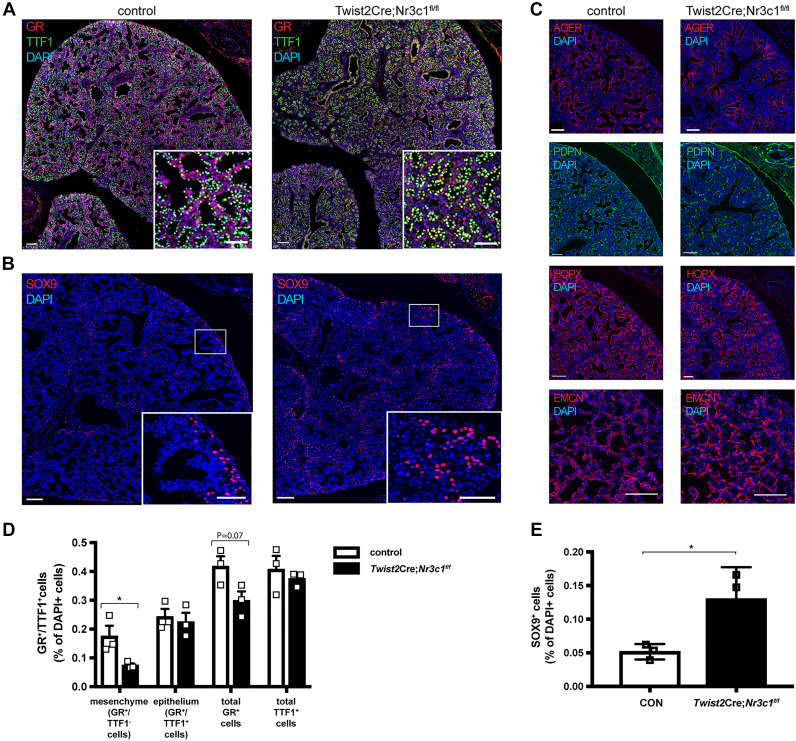
We generated mesenchymal-specific Twist2Cre Nr3c1 knockout mice (Twist2Cre;Nr3c1fl/fl) wherein defects in lung sacculation and perinatal lethality were consistent with findings in Col1a2Cre and Twist2Cre Nr3c1 deleted mice (Supplemental Fig. S2, Fig. 3A, and D) (3, 61). Numbers of SOX9-positive epithelial progenitor cells were increased 2.4-fold in Twist2Cre;Nr3c1fl/fl lungs compared with littermate controls (Fig. 3, B and E). RNAs and immunofluorescence staining of AT1-associated genes were decreased in NR3C1-deficient mice, including Ager, Pdpn and Hopx (Fig. 3C). While RNAs associated with pulmonary endothelial cells were moderately decreased (Fig. 2), patterning of the pulmonary microvasculature was preserved and no clear changes in EMCN staining in GR mesenchymal deletion versus control lungs were observed (Fig. 3C). Collectively, these data demonstrate a selective effect of GR signaling on gene expression in mesenchymal cell subsets (i.e., PMPs and MFBs) that regulate mesenchymal cell proliferation and maturation, which are likely to influence AT1 epithelial cell maturation via paracrine signaling effects on SOX9+ epithelial progenitor cells.
Fig. 3.
Mesenchymal glucocorticoid receptor (GR) deletion increased SOX9+ epithelial cells and decreased alveolar type 1 cells (AT1) cells in embryonic day (E)18.5 Twist2Cre;Nr3c1fl/fl mice. A: representative immunofluorescence staining of GR (red) and the epithelial cell marker TTF-1 (green) control and Twist2Cre:Nr3c1fl/fl mice at E18.5. Note decreased staining of GR in lung mesenchyme (red) in Twist2Cre:Nr3c1fl/fl compared with control littermates (Nr3c1fl/fl is shown; Twist2Cre and Nr3c1fl/fl were used as controls). Sacculation was delayed in the GR-deleted pups. Sections were analyzed from n = 3 mice per group. B: representative lung sections from E18.5 control and Twist2Cre;Nr3c1fl/fl littermates were immunostained for SOX9 (red). Nr3c1fl/fl is shown as control; Twist2Cre and Nr3c1fl/fl were both used as controls. Scale bars = 100 μm (50 μm for insets). Note Increased numbers of SOX9+ epithelial cells in E18.5 Twist2Cre;Nr3c1fl/fl mice. Sections were analyzed from n = 3 mice per group. C: representative immunostaining for AT1 cell markers AGER, PDPN, and HOPX and endothelial cell marker EMCN in E18.5 control and Twist2Cre;Nr3c1fl/fl lungs. Twist2Cre and Nr3c1fl/fl mice were both used as controls. Scale bars = 100 μm. Note decreased staining of AT1 markers AGER, PDPN, and HOPX, while no clear change of EMCN stain. Sections were analyzed from n = 3 mice per group. D: quantitation of GR- and TTF-1-stained cells from E18.5 control (n = 3) and Twist2Cre:Nr3c1fl/fl (n = 3) mice. GR+, TTF-1+, and GR/TTF-1 dual-positive cells were quantitated from n = 3 littermate animals and compared by Student t test. *P < 0.05; means ± SE. GR staining was substantially decreased in lung mesenchyme. E: quantitation of SOX9+ epithelial cells from E18.5 control (n = 3) and Twist2Cre:Nr3c1fl/fl (n = 3) mice. SOX9/TTF1 dual-positive cells were counted from n = 3 littermate animals and compared by Student’s t test. *P < 0.05; means ± SE. Increased SOX9+ epithelial cells were observed in lungs from Twist2Cre:Nr3c1fl/fl mice.
Isolation and characterization of embryonic MFB cells.
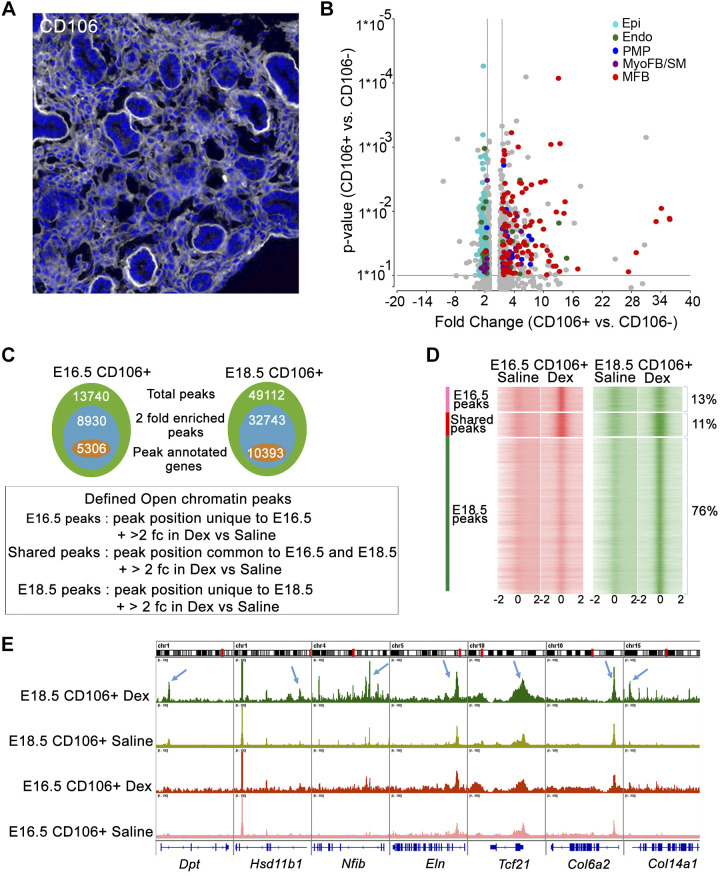
From the single cell RNA analysis, CD106 (VCAM1) was identified as a cell surface marker that was highly enriched in MFB cells. Immunofluorescence staining identified CD106 in mesenchymal rather than epithelial cells in E16.5 mouse lung (Fig. 4A and Supplemental Fig. S3). On the basis of these data, primary lung MFBs were isolated from E16.5 pups using negative selection with CD45/CD31/CD144/CD309 (PTPRC/PECAM1/CDH5/KDR) and positive selection with CD106 antibody. RNA-seq analysis was used to confirm the enrichment of MFBs and to identify differentially expressed genes. The predicted MFB cells (based on scRNA-seq signature gene expression) were highly enriched in the CD106+ cell fraction (Fig. 4B). MFB markers (e.g., Tcf21 and Fn1) were significantly enriched in CD106+ cells; in contrast, CD106− cells were primarily enriched in epithelial cell markers, including Epcam, Abca3 and Cdh1. CD106+ cells also expressed low levels of epithelial markers (Supplemental Fig. S4).
Fig. 4.
Glucocorticoid receptor (GR) activation alters chromatin accessibility in lung matrix fibroblast at embryonic day (E)18.5 A: a subset of lung fibroblasts, matrix fibroblasts (MFBs), were identified by CD106 staining at E16.5 in wild-type mice. Representative image from n = 4 mice analyzed. B: CD106+ cells were isolated from E16.5 control lungs after immune and endothelial cell depletion (CD45/31/144/309) and positive CD106 selection (yield ~1.5 × 10−5 cells per lung). RNA-seq analysis of CD106+ vs CD106− cells (n = 2 isolations/group) demonstrated increased expression of MFB-signature genes in CD106+ cells compared with proliferative mesenchymal progenitor cell (PMP) and myofibroblast (MyoFB). SM, smooth muscle. Volcano plot shows signature genes from PMP (indigo), MyoFB (violet), MFB (red), endothelial (Endo; green), and epithelial (Epi; blue) that were induced more than 1.5-fold in CD106+ cells. Gray color represents genes that were not cell type specific. C: CD106+ cells were isolated from normal mouse fetal lungs at E16.5 (n = 4) and E18.5 (n = 4). Pooled cells were split equally and treated with 100 nM dexamethasone (Dex) or saline for 4 h in vitro and subjected to ATAC-seq. Venn diagrams represent the statistics summary of ATAC-seq peaks detected in CD106+ cells. Total peaks (green), 2-fold enriched peaks by Dex (blue) and 2-fold enriched peaks with annotated genes (orange); 2 fc: 2-fold change. D: ATAC peaks (open chromatin regions) that were unique to E16.5 or E18.5 or shared between each time point in Dex-treated CD106+ cells were identified by MergePeak analysis in Homer. Heatmap displays read density surrounding unique and shared peaks. Open chromatin states (midpoint ± 2 kb of the open region) were identified from saline and Dex-treated CD106+ cells at E16.5 and E18.5, respectively. As shown, 76% of detected open chromatin peaks are unique to Dex-treated CD106+ cells at E18.5. 13% are unique to E16.5 and 11% are shared between 2 times. ATAC-seq analysis is consistent with RNA expression data showing that GC/GR signaling is most active in CD106+ matrix fibroblast cells at E18.5. E: Integrative Genome Viewer (IGV) view shows ATAC-seq peaks for a subset of MFB signature genes in saline and Dex-treated CD106+ cells at E16.5 and E18.5. Blue arrows indicate ATAC-seq peaks induced by Dex near the promoter regions of representative MFB signature genes. Results demonstrated increased chromatin accessibility near loci associated with MFB signature genes by dexamethasone treatment of CD106+ cells at E18.5.
GR alters the chromatin landscape of embryonic CD106+ fibroblasts.
Glucocorticoids influence cell activity primarily by binding to GR to regulate gene transcription. The combinatorial assembly of GR and cofactors bind to chromatin enhancer/repressor elements to influence the magnitude, direction (activation or repression), and cell-specific effects of GR on target genes (37). To identify the effects of GR signaling on chromatin accessibility in MFBs during lung sacculation, ATAC-Seq was performed on primary CD106+ MFBs isolated from E16.5 and E18.5 fetal lung tissue. Cells were treated with dexamethasone for 2 h in vitro before analysis.
Model-based analysis of ChIP-Seq (MACS2) was utilized to identify dexamethasone-induced open chromatin regions at E16.5 (n = 13,740) and E18.5 (n = 49,112) (Fig. 4C). DNA regions enriched more than twofold after dexamethasone were defined as differentially accessible (DA) peaks and selected for further analysis. Unique protein-coding genes (n = 5,306) were associated with 8,930 DA peaks at E16.5, accounting for 11% of total DA peaks; 10,393 unique protein-coding genes were associated with 32,743 DA peaks at E18.5, accounting for 76% of total DA peaks. The remaining peaks (11%) were shared at both E16.5 and E18.5 (Fig. 4, C and D). Numbers and amplitude of DA peaks near MFB signature genes were increased in response to dexamethasone. Many DA peaks associated with MFB signature genes were increased with dexamethasone treatment (Fig. 4E) and decreased after mesenchyme-specific Nr3c1 gene deletion (Supplemental Tables S1 and S2) at E18.5. Genes involved in “regulation of cell differentiation,” “regulation and response to glucocorticoids and lipids,” “extracellular matrix organization,” and “blood vessel development” were highly enriched functions identified by changes in DNA accessibility at both times (Supplemental Fig. S5). Collectively, the ATAC-seq analysis demonstrated that dexamethasone increased chromatin accessibility near loci associated with MFB signature genes, consistent with RNA expression data showing that GC/GR signaling is mostly active at E18.5.
We analyzed the CD106+ MFB ATAC-seq data using Motif enrichment analysis, and literature mining to identify enrichment for canonical GR-binding elements (GREs) and known GR targets among our predicted GR targets. Canonical GRE sites were significantly enriched in dexamethasone induced ATAC peaks at both E16.5 and E18.5 based on Fisher’s exact test (Supplemental Table S3). Known GR target genes were collected by literature mining and were compared with those identified in CD106+ cells after dexamethasone treatment. Known GR target genes were overly represented in genes associated with Dex-induced peaks at both E16.5 (P = 0.0036) and E18.5 (P = 0.0001) in comparison with random chance (Supplemental Table S4). The correlation of annotated peaks mapping to known GR transcriptional targets, and the enrichment of GR binding motifs supported the validity of the ATAC-seq data.
Dexamethasone regulates pulmonary MFB differentiation in late gestation.
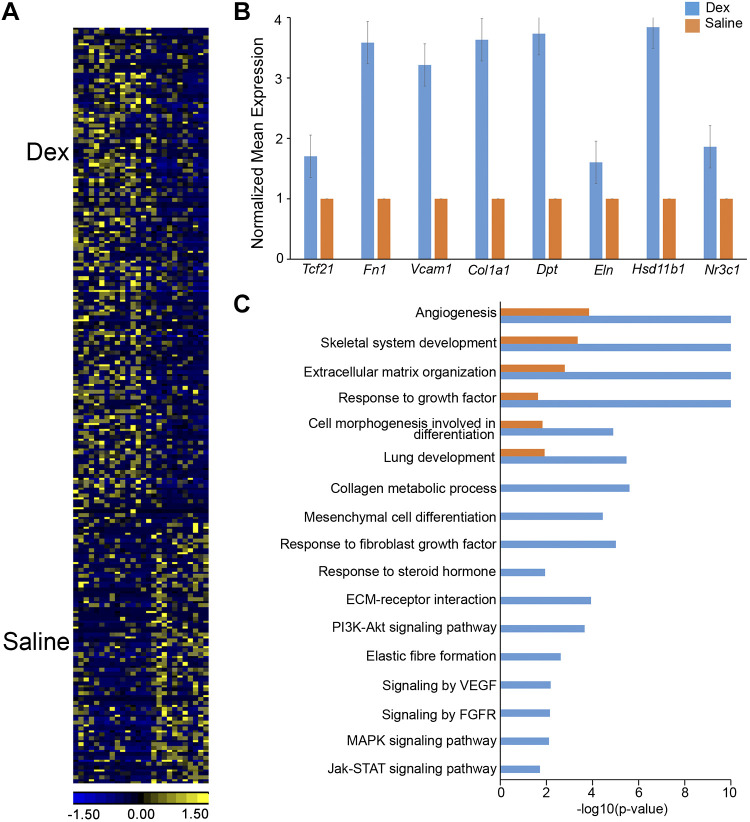
Since RNA expression profiling and chromatin accessibility studies demonstrated that GC/GR signaling in MFBs was most active at E18.5, we performed single cell RNA-seq of mouse lung cells from E18.5 fetuses whose dams were treated with Dex or saline for 24 h to identify GR effects of pulmonary cells before birth. Data were analyzed using the SINCERA pipeline (28, 30) to identify major lung cell types. MFBs were represented by coexpression of signature genes, including Col1a1, Fn1, Eln, Vcam1, and Fgf10. We identified 386 genes that were differentially expressed in CD106+ (VCAM1) MFB cells 24 h after exposure to Dex; 253 RNAs were induced and 133 were suppressed (Fig. 5A). MFB signature genes, including Fn1, Vcam1, Tcf21, Col1a1, Eln, and Hsd11b1, were significantly induced by Dex (Fig. 5B). Genes involved in “mesenchymal cell differentiation,” “response to fibroblast growth factor,” “response to steroid hormone,” “extracellular matrix organization,” “collagen metabolic process,” “elastic fiber formation,” and signaling pathways, including “PI3K-Akt,” “FGF,” “VEGF,” and “JAK-STAT” were induced by Dex (Fig. 5C). Nr3c1 expression was increased 1.8-fold in Dex-treated MFBs (P = 0.024).
Fig. 5.
Dexamethasone induced gene expression and associated bioprocesses in lung matrix fibroblasts. Dams were injected with 2 mg/mL dexamethasone (Dex) or saline in vivo 24 h before euthanize at embryonic day (E)18.5. Single cell RNA was prepared from lungs of embryos (n = 4–6 pooled lungs per condition). Cell type mapping and signature gene identification were performed using SINCERA pipeline (27). Matrix fibroblasts (MFBs) and associated signature genes were used for heatmap and functional enrichment analysis. A: heatmap of differentially expressed genes (P value < 0.01 and fold change ≥ 1.5) in lung MFBs treated with Dex (n = 15 cells) versus saline (n = 11 cells) at E18.5. B: dexamethasone increased expression of MFB signature genes (blue bars) compared with saline (orange bars). Mean expression was normalized to saline control for each gene. All genes passed Student’s t test (P < 0.05, fold change>1.5) C: top enriched bioprocesses and pathways in genes increased by Dex (blue bars) compared with Saline (orange bars) in MFB at E18.5. Hypergeometric test was used for gene set enrichment analysis with significance at P < 0.001. PI3K, phosphatidylinositol 3-kinase; ECM, extracellular matrix.
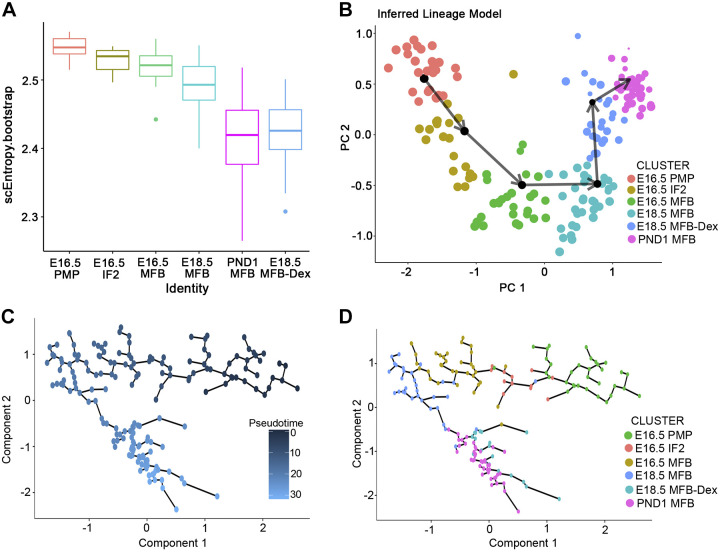
To understand the GC/GR signaling effect on MFB lineage trajectories from E16.5 to postnatal day 1 (PND1), we utilized Single-Cell Lineage Inference Using Cell Expression Similarity and Entropy (SLICE), which we previously developed for single cell pseudotime analysis based on the concept of entropy (27). Mouse lung single cell RNA-seq time course were performed at E16.5, E18.5, and E18.5 with Dex treatment and PND1 using the C1 Fluidigm system. Mesenchymal cells at each time point were selected for trajectory analysis. The decrease in single cell RNA entropy with advancing developmental stages supported a role of GR signaling in MFB differentiation (Fig. 6A). SLICE inferred a lineage model and fibroblast cell transitional path from a proliferative mesenchymal progenitor (PMP) to matrix fibroblast (MFB) at E16.5 (Fig. 6B). Dexamethasone enhanced differentiation of MFBs at E18.5, making E18.5 MFB more similar to those at PND1 in control mice (Fig. 6A). The lineage trajectory predicted by SLICE was consistent with predictions using Monocle 2, a pseudotemporal ordering analysis (62) (Fig. 6, C and D).
Fig. 6.
Dexamethasone induces the differentiation and maturation of lung matrix fibroblast. The prenatal time course of single cell RNA-seq were performed at embryonic day (E)16.5, E18.5, and E18.5 with dexamethasone (Dex) and postnatal day 1 (PND1) using Fluidigm C1 system. Single cell data from non-Dex-treated mouse lungs were obtained from Lung Gene Expression Analysis (LGEA) web portal (17, 19). Single cell data of “E18.5 with Dex” was obtained from the lungs of embryos whose dams were injected with 2 mg/mL Dex in vivo 24 h before euthanize at E18.5. Mesenchymal cells were identified at each time point and used for trajectory analysis. A: single cell entropy was calculated using Single-Cell Lineage Inference Using Cell Expression Similarity and Entropy (SLICE) (27) to predict differentiation states of each mesenchymal cell subtype from E16.5 to PND1. Higher entropy predicts more progenitor-like and less differentiated cell states; lower entropy, more differentiated cell states. Note dexamethasone decreased entropies of matrix fibroblast (MFB) cells to values similar to normal MFB at PND1 in control mice. B: SLICE predicted a MFB differentiation trajectory model from E16.5 to E18.5, to Dex-treated E18.5 and then to PND1 lung MFB cells. Dexamethasone enhanced the differentiation trajectory of MFBs at E18.5 to that near PND1 in control mice. C: the same scRNA-seq data was used to predict lung mesenchymal cell lineage trajectory using Monocle 2 (62) pseudotemporal analysis. D: monocle 2 reconstructed a trajectory model for the mesenchymal progenitor cell (PMP)/MFB lineage, consistent with the trajectory model predicted by SLICE.
A cell-specific GR-dependent regulatory network in MFBs during lung sacculation.
The combinatorial assembly of GR with different cofactors, as well as chromatin landscape and accessibility, influence the magnitude, direction (activation or repression), and cell-specific effects of GR signaling (37). The MFB cell-specific regulatory network, GR cofactors and direct GR target genes in the MFB of the developing lung have not yet been defined. We therefore applied a systems biology approach to integrate data from multiple omics data sets to model the pleotropic effects of GR on lung maturation.
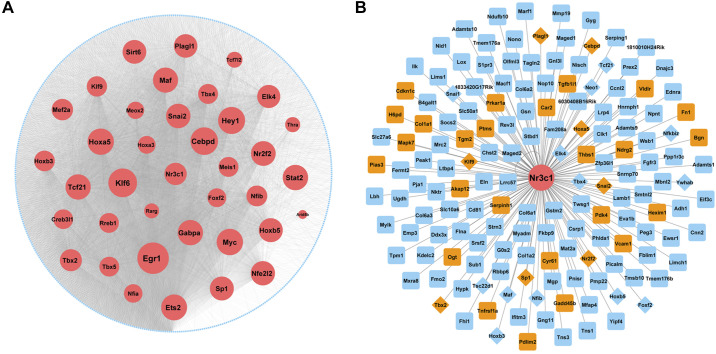
An MFB-specific GR regulatory network was generated using RNA-seq and ATAC-Seq of CD106+ cells (primary CD106+ MFBs) isolated from E18.5 Dex-treated mouse lung. Paired expression and chromatin accessibility (PECA), a statistical model for integrative analysis of matched gene expression and chromatin accessibility data (20) was applied to infer the GR-dependent MFB-specific gene regulatory network (Fig. 7A). The regulatory network was filtered to include only interactions where the transcription factors were abundantly expressed in MFB and target genes were MFB signature genes identified by single cell analysis of E18.5 lung. Egr1, Klf6, Cebpd, Hey1, Hoxa2, Tcf21, Tbx2, and Nr3c1 were among the top ranked transcription factors in the regulatory network (Fig. 7A). Figure 7B depicts a Nr3c1-centered network with predicted cofactors (orange color, diamond nodes) and target genes (blue color, with positive glucocorticoid response elements (GREs defined by ENCODE ChIP-seq-derived regulatory elements) in accessible chromatin regions.
Fig. 7.
Reconstruction of a matrix fibroblast (MFB)-specific glucocorticoid receptor (GR)-dependent transcriptional regulatory network. CD106+ cells were isolated using negative selection (CD45/CD31/CD144/CD309) followed by positive selection of CD106 from mice lung at embryonic day (E)16.5 and E18.5. CD106+ cells were then treated with 100 nM dexamethasone (Dex) or saline for 4 h and ATAC-seq and RNA-seq experiments were performed. A: an MFB-specific GR regulatory network was generated using the RNA-seq and ATAC-seq data from CD106+ cells isolated from E18.5 Dex-treated mouse lung (see above) using paired expression and chromatin accessibility (PECA) (20). The MFB cell-specific regulatory network was constructed using transcription factors (TFs) abundantly expressed in E18.5 MEB and E18.5 MFB signature genes from single cell analysis as target genes (TGs). Node size is proportional to the predicted importance of the TFs in MFB network (red nodes). Edges represent predicted TF-TG interactions in Dex-treated MFB cells. B: GR-centered gene network was constructed using Nr3c1 as TF and its closest (one-hop) neighbors as predicted targets. Potential GR targets were predicted if they have positive GR-binding elements (GREs) in accessible chromatin regions and the corresponding ATAC-seq peaks were induced more than 2-fold by Dex treatment. Orange nodes represent known GR targets from literature mining. Diamond nodes represent transcription factors (potential cofactors of GR).
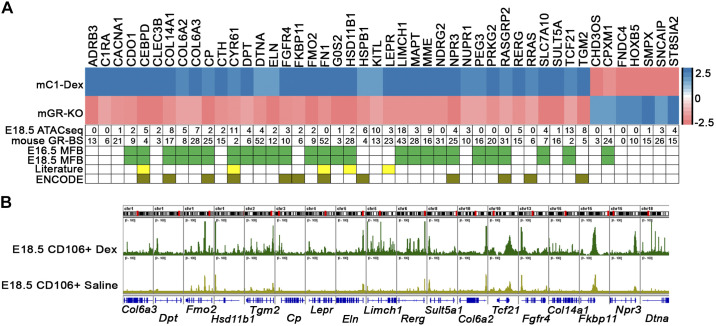
We further narrowed GR target genes via integrative analysis of data from GR gain and loss of function in MFBs at E18.5. Comparative analysis of gene expression profiling of GR gain and loss of function identified 39 genes, which were inversely regulated after mesenchymal Nr3c1 deletion and dexamethasone treatment of MFB cells (Fig. 8A). Genes involved in “extracellular matrix organization,” “negative regulation of cell proliferation,” “positive regulation of cell differentiation,” and “lipid biosynthetic process” were significantly induced by GR activation and suppressed by Nr3c1 deletion. RNA expression profiling data were then interrogated with Dex-dependent differentially accessible chromatin from the ATAC-seq data, promoter GR binging sites analysis, GR ChIP-Seq (from ENCODE) analysis, and GR literature mining (Fig. 8A); 38 of the 39 genes contained one or more putative GREs within −1 kb of the gene’s promoter; 29 of the 39 genes contained Dex-induced ATAC-seq peaks in their promoter regions; and 25 of 39 were identified as MFB signature genes by the single cell analyses of normal lung at E16.5 (https://research.cchmc.org/pbge/lunggens/celltype_E16_p3.html?cid=5) and E18.5 (https://research.cchmc.org/pbge/lunggens/celltype_E18_p3.html?cid=7). GR ChIP-Seq data (downloaded from ENCODE) identified positive GR binding peaks in 12 of these genes (Fig. 8).
Fig. 8.
Integrative analysis of glucocorticoid receptor (GR) regulation of matrix fibroblast cells (MFBs). A: heatmap represents 39 genes that were inversely regulated in mesenchymal deletion of GR (labeled as mGR-KO) and in dexamethasone (Dex)-treated MFBs cells identified by sc-RNA-seq data (labeled as mC1-Dex) at embryonic day (E)18.5. This gene subset was further annotated by 1) number of ATAC-seq peaks that were induced by Dex treatment of isolated CD106+ cells (labeled as E18.5 ATAC-seq); 2) number of putative GR binding motifs located within (−1 kb to +100 bp) of individual gene promoter regions (labeled as mouse GR-BS); 3) MFB signature genes that were identified by scRNA-seq analysis at E16.5 and E18.5 (green boxes); 4) known GR targets were identified from literature mining using IPA knowledge base (yellow boxes); and 5) positive GR chromatin immunoprecipitation (ChIP)-seq peaks identified in ENCODE (column heading: ENCODE). B: Integrative Genome Viewer (Igv) visualization depicting MFB signature genes with ATACseq peaks induced by Dex treatment of CD106+ matrix fibroblast cells at E18.5.
Ingenuity Pathway Analysis (IPA) was used to predict upstream transcriptional regulators mediating the effects of GR on fibroblast differentiation. Predicted upstream regulators or cofactors were filtered on the basis of their expression in MFB cells at E18.5. Using the differentially expressed RNAs identified by GR gain- versus loss-of-function as potential targets, we identified a common group of transcriptional regulators, including Nr3c1, Nr3c2, Ar, Foxm1, Tp53, Ep300, Ctnn1b, and Klf4 as candidate genes regulating GR-dependent genomic responses in MFB (Supplemental Fig. S6).
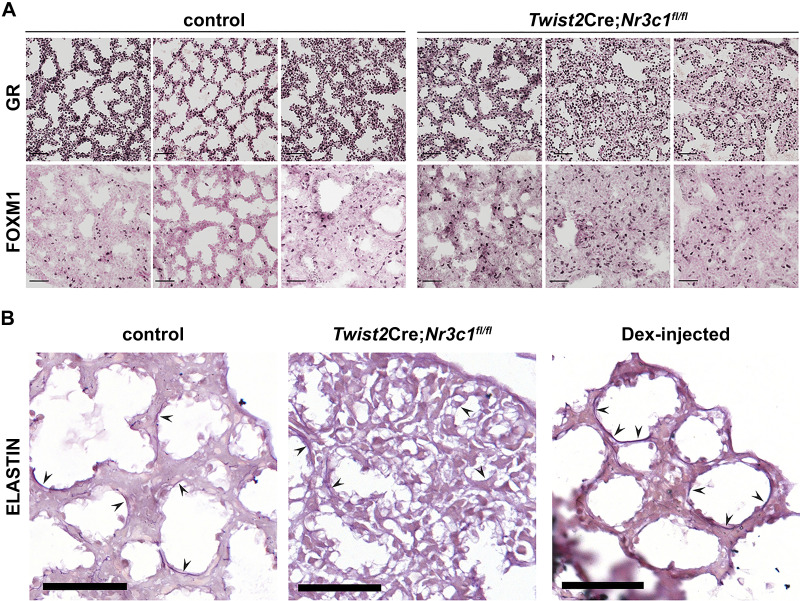
Foxm1 expression and genes in “Foxm1 transcription factor network” (BSID: 137935) were induced in the lung of mesenchymal GR deletion mice at E18.5 (Fig. 1). In addition, the upstream transcriptional regulator analysis predicted Foxm1 as a key regulator of differentially expressed RNAs identified by GR gain- versus loss-of-function (Supplemental Fig. S6). As shown in Fig. 9A, FOXM1 protein content was increased in Twist2Cre;Nr3c1fl/fl lungs, along with decreased mesenchymal GR expression. Expression of genes encoding extracellular matrix components, including Col14a1, Col6a2, Col6a3, Cyr61, Dpt, Fn1, and Eln, were influenced by glucocorticoid signaling in MFBs. As shown in Fig. 9B, elastin content was decreased in the alveolar walls of E18.5 Twist2Cre;Nr3c1fl/fl lungs and increased by dexamethasone. Taken together, these matrix-associated genes likely represent an important subset of direct GR targets that participate in the glucocorticoid dependent maturation of the lung before birth.
Fig. 9.
Dexamethasone increased elastin staining in peripheral saccules of embryonic lung A: embryonic day (E)18.5 lung tissue from control or Twist2Cre;Nr3c1fl/fl mice were stained with glucocorticoid receptor (GR) and FOXM1 antibodies. Each column represents lung tissue from an individual animal. Note decreased GR staining in mesenchyme of Twist2Cre;Nr3c1fl/fl mice and increased FOXM1+ cells compared with controls. Scale bars = 50 µm. B: lung tissues were obtained at E18.5 and stained for elastin. Wild-type mouse dams were injected with saline (left) or 1 mg/mL dexamethasone (Dex; right) in vivo 24 h before euthanize at E18.5. Middle: elastin staining of lung tissue following mesenchymal GR deletion (Twist2Cre;Nr3c1fl/fl). Arrowheads denote elastin staining in developing saccules of the distal lung. Note decreased elastin staining in Twist2Cre;Nr3c1fl/fl lungs and increased staining in the wild-type lung treated with antenatal Dex. Images are representative of n = 3 control lungs; n = 3 Twist2Cre;Nr3c1fl/fl lungs; and n = 7 Dex-injected wild-type lungs. Scale bar = 100 μm.
Potential GR-dependent ligand-receptor signals in MFBs and epithelial cells.
We identified ligands expressed in PMP and MFB lineages and linked them with their known receptors present in both mesenchyme and epithelium to predict autocrine and paracrine signaling pathways related to GR (Supplemental Fig. S7). Ligand-receptor interactions were collected from multiple public knowledge bases including KEGG, DLRP (24), and the FANTOM5 project (63). Paracrine interactions from MFBs to epithelial cells were identified to predict potential GR-dependent signals from MFBs that influence epithelial cell differentiation. We first identified ligands produced by PMP and MFB cells that were changed after mesenchymal deletion of Nr3c1 (Col1a2Cre;GRfl/fl) and/or Dex treatment; we then identified their cognate receptors expressed in both MFB and lung epithelial cells. GC/GR-dependent ligand-receptor interactions were most enriched in extracellular matrix (ECM) components including collagens (Col6A1, Col6A2, Col6A3, and Col13A1), integrins (Itga3, Itga6, Itga8, Itga9, Itgb1, Itgb2, and Itgb6), laminins (Lama4 and Lamb1), fibronectin (Fn1), and elastin (Eln). In general, these ECM genes were suppressed after mesenchymal GR deletion and/or induced by Dex treatment before birth, suggesting their role in prenatal lung maturation (Fig. 9A and Supplemental Fig. S7).
DISCUSSION
Despite the efficacy of antenatal glucocorticoids in preventing respiratory distress in preterm infants, the lung cell subtypes and molecular targets mediating the effects of GR on the perinatal lung remain poorly understood. In the present study, we utilized an integrated bioinformatics approach to identify gene networks controlled by GR signaling in pulmonary mesenchymal cells during lung sacculation. Single cell RNA data were used to predict the identity and differentiation trajectory of lung fibroblasts in late gestation. GR signaling was most active in a subset of fibroblasts, termed proliferative mesenchymal progenitors (PMP) and matrix fibroblasts (MFB), consistent with their essential role in lung maturation before birth. Mesenchymal deletion of Nr3c1 impaired maturation of proliferative mesenchymal progenitors (PMP) into matrix fibroblasts (MFB). Glucocorticoid stimulation of pulmonary MFBs changed chromatin accessibility states associated with increased expression of multiple extracellular matrix (ECM) and ECM-associated genes. GR effects on gene expression and chromatin accessibility were most active at E18.5, consistent with data demonstrating minimal effects of Nr3c1 deletion on lung morphology at E16.5. The observations that Nr3c1 RNA and protein expression are highly correlated during lung development stages and peaked before birth support its important regulatory role in pulmonary maturation (51, 53, 79). While GC/GR signaling primarily influenced gene expression in matrix fibroblasts, deletion of Nr3c1 inhibited maturation of AT1 epithelial cells, likely mediated by paracrine effects of lung mesenchyme on epithelial cell differentiation. Our data are consistent with increased circulating corticosteroid levels in the fetal mouse on E16–17, coincident with the timing of the GR effects on pulmonary maturation (3, 4, 12, 70).
The present study demonstrates that GC/GR signaling primarily influences differentiation of PMPs into MFBs, with the latter playing important roles in synthesis of extracellular matrix and collagen necessary for lung development and survival at birth. Potential GR target genes and associated pathways active in pulmonary matrix fibroblasts (MFBs) were identified through an integrative analysis indicating that “extracellular matrix organization,” “collagen biosynthesis,” and “elastic fiber formation” were highly induced by corticosteroid. Cyr61, Dpt, Eln, Fn1, Vcan, and the collagen family (Col6a2, Col6a3, Col14a1) were selectively expressed in MFBs at E18.5, their expression being induced by Dex and suppressed by Nr3c1 gene deletion. Predicted GRE cis-elements were identified in the promoter regions of these GR responsive genes. Consistent with present findings, fibronectin (Fn1) was identified as a direct GR target gene in breast cancer cells; its expression and extracellular deposition were GR-dependent (66). Expression of dermatopontin (DPT), which interacts with FN1, plays a critical role in both ECM structure and wound healing (38). DPT expression was induced by GR (16). Nr3c1 interacts with Cyr61 RNA to regulate Cyr61 mRNA turnover (35). In the present study, elastin (Eln) gene expression was induced by GC and reduced in Nr3c1-deficient mesenchymal cells, consistent with our data showing that mesenchymal GC/GR signaling regulated Eln gene expression in the perinatal lung (3, 31).
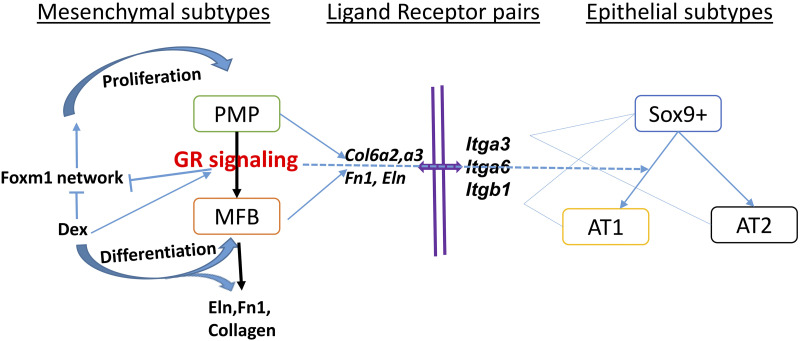
Foxm1 is a master transcriptional activator of cell cycle and cell proliferation and is required for lung morphogenesis (40, 71). Functional interplay between Nr3c1 and Foxm1 have not been reported. In the present study, Foxm1 gene/protein expression and genes in “Foxm1 transcription factor network” were induced in the lung of mesenchymal GR deletion mice at E18.5. Consistent with a role for GC/GR in regulation of Foxm1, we observed increased expression of Foxm1 target genes cyclin B1 (Ccnb1) and cyclin D1 (Ccnd1) after deletion of Nr3c1 in lung mesenchyme (Supplemental Table S2). We speculate that mesenchymal GC/GR signaling restrains PMP cell proliferation and promotes PMP differentiation into mature MFBs by suppressing the Foxm1 network and increasing expression of genes associated with extracellular matrix organization, collagen biosynthesis, and elastic fiber formation (Fig. 10).
Fig. 10.
Schematic model of predicted glucocorticoid receptor (GR) signaling. GR signaling primarily regulates differentiation of mesenchymal progenitor cells (PMPs) into matrix fibroblasts (MFBs), the latter playing crucial roles in synthesis of extracellular matrix and collagen. We propose that glucocorticoid (GC)/GR signaling indirectly regulates lung epithelial cells largely via cell-matrix-cell interactions. The network was generated in Cytoscape v3.7.1. AT1, alveolar type 1 cells; AT2, alveolar type 2 cells; Dex, dexamethasone.
While present data indicate a primary role for GR signaling in pulmonary mesenchymal cells, numbers of SOX9+ epithelial progenitor cells were increased and mature AT1 cell markers were decreased following deletion of Nr3c1 in mesenchymal cells, supporting a role for paracrine signaling from MFBs to influence epithelial cell differentiation. In the present study, deletion of Nr3c1 in mesenchymal cells did not change Sftpa, Sftpb, Sftpc, or lipid-associated gene expression in AT2 cells, findings consistent with previous morphological studies (13). The mechanisms by which GCs enhance perinatal lung function remain unclear. Previous studies demonstrated that the lung gas volumes increase within 24 h after antenatal corticosteroids, preceding changes in surfactant production in sheep and primates (39, 60, 65, 76). These rapid effects of antenatal corticosteroid treatment were likely due to the increase on lung gas volumes and mediated by changes in lung extracellular matrix production, which is consistent with the present data regarding the role of GR on lung mesenchymal cells.
To identify potential GR-dependent paracrine signals from MFBs that influence epithelial cell differentiation, we assessed cell selective expression of ligand-receptor pairs changed after mesenchymal GR deletion and/or Dex treatment. Ligand-receptor interactions regulated by GR consisted primarily of extracellular matrix (ECM) protein interactions (i.e., matrix-cell interaction). For example, present data showed that Itgb1, Itga3, and Itga6 expression was induced in GR mesenchymal deletion and suppressed by Dex treatment. Itgb1, Itga3, and Itga6 are integrins known to regulate lung morphogenesis and alveolarization (14, 32, 41, 42, 59). Furthermore, the present study identified ECM genes, including CRY61, Col6a2, Col6a3, Fn1, and Eln, as direct GR targets in MFB cells (Figs. 4 and 8), known modulators of ECM maturation and function (52).
Combinatorial assembly of GR with a diversity of cofactors and chromatin accessibility states influences the magnitude, direction (activation or repression), and cell-specific effects of GR on target genes (37). The present study predicted cell-specific GR target genes and a GR-dependent regulatory network in MFBs, a distinct mesenchymal cell type defined by single cell RNA analysis (17, 28). We further cross validated transcription factors in the GR dependent MFB network via literature mining by IPA and importantly, many of these have been validated by previous studies. For example, Early Growth Response 1 (EGR1) was predicted as a primary regulator in the Dex induced MFB transcriptional network (Fig. 7A). This zinc-finger protein binds to the Nr3c1 promoter, increasing GR expression via epigenetic reprogramming (histone acetylation and DNA demethylation). Methylation of the EGR1 response element in the Nr3c1 promoter on the other hand, inhibits GR expression (69, 74). EGR1 is a transcriptional target of HOXA5 (40), another predicted key regulator in the network. Loss of Hoxa5 impaired lung alveologenesis, inhibiting alveolar myofibroblast differentiation and causing abnormal elastin deposition (50). Key regulators predicted from present data included CEBPD, MYC, NR2F2 and EP300, which are known to physically interact with and modulate GR-dependent transcription (6, 15, 49). For example, Dex-bound GR recruits EP300 to transcriptional enhancers, including CEBPA and CEBPB, to regulate adipogenesis (57, 68). GR/EP300 complexes regulate transcription in fetal human amnion fibroblasts (73). The GR and CEBPA proteins cooperatively bind the promoter of Hsd11b1, a gene highly induced by Dex in MFBs, to synergistically regulate Hsd11b1 expression after cortisol response (80). Androgen receptor (AR) was also predicted to be a key factor in the network. AR/GR heterodimers bind shared GRE sites to oppose the transcriptional activity of one another (10), representing a factor related to male susceptibility to lung immaturity. With respect to the WNT signaling, GR and beta-catenin (CTNN1B) interact in osteoblastic cells (55) and both GR/CTNN1B and GR/TCF4 complexes promote proliferation and migration of injured astrocytes and glioma cells (72, 81). The specific role of these GR regulators, cofactors, and downstream target genes in MFBs in the regulation of perinatal lung morphogenesis and mesenchymal cell maturation requires further investigation.
In summary, the present study supports the concept that GC/GR signaling regulates cellular processes active before birth to promote differentiation and inhibit proliferation of lung mesenchymal cells. MFB cell-specific, GR-centered transcriptional networks were developed, enabling prediction of upstream regulators of GR, coactivators, and GR gene targets. The present study provides a valuable resource to further elucidate cell-specific transcriptional regulatory circuits controlling intricate patterns of GR dependent gene expression in lung MFB cells; provides new insights of molecular mechanisms underlying context dependent regulation of glucocorticoids; and provides foundation to understand diseases caused by glucocorticoids mediated regulatory dysfunction. A thorough understanding of the mechanisms underlying GR action in specific lung cell types and compartments of the fetal lung, and the timing by which GC/GR signaling regulates cell maturation, will promote the understanding of the pathogenesis of lung diseases associated with preterm birth, providing new potential therapies to prevent and treat RDS in preterm infants.
GRANTS
This work was supported by the Cincinnati Children’s Hospital Medical Center (CCHMC) GAP funding to Y.X and J.P.B.; National Heart, Lung, and Blood Institute (Grants U01HL122642 to J.A.W. and Y.X.; HL131634 to J.P.B.), and a Cincinnati Children’s Research Foundation Endowed Scholar award to M.T.W.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P.B., A.H.J., and Y.X. conceived and designed research; J.P.B., K.B., A.F., J.A.K., and C.S. performed experiments; J.P.B., P.S., D.L., A.W., M.G., Y.D., X.C., M.T.W., and Y.X. analyzed data; J.P.B., P.S., M.G., Y.D., K.B., A.F., C.S., X.C., M.T.W., and Y.X. interpreted results of experiments; J.P.B., P.S., D.L., A.W., Y.D., J.A.K., and Y.X. prepared figures; J.P.B., P.S., J.A.W., and Y.X. drafted manuscript; J.P.B., J.A.K., M.T.W., A.H.J., J.A.W., and Y.X. edited and revised manuscript; J.P.B., P.S., D.L., A.W., M.G., A.F., J.A.K., C.S., X.C., M.T.W., A.H.J., J.A.W., and Y.X. approved final version of manuscript.
REFERENCES
- 1.Alanis DM, Chang DR, Akiyama H, Krasnow MA, Chen J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat Commun 5: 3923, 2014. doi: 10.1038/ncomms4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036, 2013. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bird AD, Choo YL, Hooper SB, McDougall AR, Cole TJ. Mesenchymal glucocorticoid receptor regulates the development of multiple cell layers of the mouse lung. Am J Respir Cell Mol Biol 50: 419–428, 2014. doi: 10.1165/rcmb.2013-0169OC. [DOI] [PubMed] [Google Scholar]
- 4.Bird AD, McDougall AR, Seow B, Hooper SB, Cole TJ. Glucocorticoid regulation of lung development: lessons learned from conditional GR knockout mice. Mol Endocrinol 29: 158–171, 2015. doi: 10.1210/me.2014-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol 32: 76–91, 2001. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 6.Boruk M, Savory JG, Haché RJ. AF-2-dependent potentiation of CCAAT enhancer binding protein beta-mediated transcriptional activation by glucocorticoid receptor. Mol Endocrinol 12: 1749–1763, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10: 1213–1218, 2013. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC‐seq: a method for assaying chromatin accessibility genome‐wide. Curr Protoc Mol Biol 109: 1, 2015. doi: 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37: W305-W311, 2009. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SY, Wang J, Yu G, Liu W, Pearce D. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J Biol Chem 272: 14087–14092, 1997. doi: 10.1074/jbc.272.22.14087. [DOI] [PubMed] [Google Scholar]
- 11.Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145: dev163014, 2018. doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9: 1608–1621, 1995. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 13.Cole TJ, Solomon NM, Van Driel R, Monk JA, Bird D, Richardson SJ, Dilley RJ, Hooper SB. Altered epithelial cell proportions in the fetal lung of glucocorticoid receptor null mice. Am J Respir Cell Mol Biol 30: 613–619, 2004. doi: 10.1165/rcmb.2003-0236OC. [DOI] [PubMed] [Google Scholar]
- 14.De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development 126: 3957–3968, 1999. [DOI] [PubMed] [Google Scholar]
- 15.De Martino MU, Bhattachryya N, Alesci S, Ichijo T, Chrousos GP, Kino T. The glucocorticoid receptor and the orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II interact with and mutually affect each other’s transcriptional activities: implications for intermediary metabolism. Mol Endocrinol 18: 820–833, 2004. doi: 10.1210/me.2003-0341. [DOI] [PubMed] [Google Scholar]
- 16.Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells 24: 1487–1495, 2006. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 17.Du Y, Guo M, Whitsett JA, Xu Y. ‘LungGENS’: a web-based tool for mapping single-cell gene expression in the developing lung. Thorax 70: 1092–1094, 2015. doi: 10.1136/thoraxjnl-2015-207035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, Pryhuber GS, Mariani TJ, Bhattacharya S, Guo M, Potter SS, Dexheimer P, Aronow B, Jobe AH, Whitsett JA, Xu Y. Lung gene expression analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax 72: 481–484, 2017. doi: 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duren Z, Chen X, Jiang R, Wang Y, Wong WH. Modeling gene regulation from paired expression and chromatin accessibility data. Proc Natl Acad Sci USA 114: E4914–E4923, 2017. doi: 10.1073/pnas.1704553114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell PM. Fetal lung development and the influence of glucocorticoids on pulmonary surfactant. J Steroid Biochem 8: 463–470, 1977. doi: 10.1016/0022-4731(77)90248-5. [DOI] [PubMed] [Google Scholar]
- 22.García-Escudero LÁ, Gordaliza A. Robustness properties of k means and trimmed k means. J Am Stat Assoc 94: 956–969, 1999. doi: 10.2307/2670010. [DOI] [Google Scholar]
- 23.Glasgow CG, Avila NA, Lin JP, Stylianou MP, Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest 135: 1293–1300, 2009. doi: 10.1378/chest.08-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graeber TG, Eisenberg D. Bioinformatic identification of potential autocrine signaling loops in cancers from gene expression profiles. Nat Genet 29: 295–300, 2001. doi: 10.1038/ng755. [DOI] [PubMed] [Google Scholar]
- 25.Green J, Endale M, Auer H, Perl AK. Diversity of interstitial lung fibroblasts is regulated by platelet-derived growth factor receptor alpha kinase activity. Am J Respir Cell Mol Biol 54: 532–545, 2016. doi: 10.1165/rcmb.2015-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grier DG, Halliday HL. Effects of glucocorticoids on fetal and neonatal lung development. Treat Respir Med 3: 295–306, 2004. doi: 10.2165/00151829-200403050-00004. [DOI] [PubMed] [Google Scholar]
- 27.Guo M, Bao EL, Wagner M, Whitsett JA, Xu Y. SLICE: determining cell differentiation and lineage based on single cell entropy. Nucleic Acids Res 45: e54, 2017. doi: 10.1093/nar/gkw1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo M, Wang H, Potter SS, Whitsett JA, Xu Y. SINCERA: a pipeline for single-cell RNA-Seq profiling analysis. PLOS Comput Biol 11: e1004575, 2015. doi: 10.1371/journal.pcbi.1004575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo M, Xu Y. Single-cell transcriptome analysis using SINCERA pipeline. Methods Mol Biol 1751: 209–222, 2018. doi: 10.1007/978-1-4939-7710-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habermehl D, Parkitna JR, Kaden S, Brügger B, Wieland F, Gröne HJ, Schütz G. Glucocorticoid activity during lung maturation is essential in mesenchymal and less in alveolar epithelial cells. Mol Endocrinol 25: 1280–1288, 2011. doi: 10.1210/me.2009-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Has C, Spartà G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, Waters A, He Y, Anikster Y, Esser P, Straub BK, Hausser I, Bockenhauer D, Dekel B, Hildebrandt F, Bruckner-Tuderman L, Laube GF. Integrin α3 mutations with kidney, lung, and skin disease. N Engl J Med 366: 1508–1514, 2012. doi: 10.1056/NEJMoa1110813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Havrilak JA, Melton KR, Shannon JM. Endothelial cells are not required for specification of respiratory progenitors. Dev Biol 427: 93–105, 2017. doi: 10.1016/j.ydbio.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589, 2010. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hines EA, Sun X. Tissue crosstalk in lung development. J Cell Biochem 115: 1469–1477, 2014. doi: 10.1002/jcb.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishmael FT, Fang X, Houser KR, Pearce K, Abdelmohsen K, Zhan M, Gorospe M, Stellato C. The human glucocorticoid receptor as an RNA-binding protein: global analysis of glucocorticoid receptor-associated transcripts and identification of a target RNA motif. J Immunol 186: 1189–1198, 2011. doi: 10.4049/jimmunol.1001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jobe A, Ikegami M. Fetal responses to glucocorticoids. In: Endocrinology of the Lung: Development and Surfactant Synthesis, edited by Mendelson C. Totowa, NJ: Humana Press, 2000, p. 45–57. [Google Scholar]
- 37.John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet 43: 264–268, 2011. doi: 10.1038/ng.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato A, Okamoto O, Ishikawa K, Sumiyoshi H, Matsuo N, Yoshioka H, Nomizu M, Shimada T, Fujiwara S. Dermatopontin interacts with fibronectin, promotes fibronectin fibril formation, and enhances cell adhesion. J Biol Chem 286: 14861–14869, 2011. doi: 10.1074/jbc.M110.179762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Kato K, Dieguez-Hurtado R, Park YD, Hog SP, Kato-Azuma S, Adams S, Stehling M, Trappmann B, Wrana JL, Koh GY, Adams RH. Pulmonary pericytes regulate lung morphogenesis. Nat Commun 9: 2448, 2018. doi: 10.1038/s41467-018-04913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kemp MW, Saito M, Schmidt AF, Usuda H, Watanabe S, Sato S, Hanita T, Kumagai Y, Takahashi T, Musk GC, Furfaro L, Stinson L, Fee EL, Eddershaw PJ, Payne MS, Smallwood K, Bridges J, Newnham JP, Jobe AH. The duration of fetal antenatal steroid exposure determines the durability of preterm ovine lung maturation. Am J Obstet Gynecol 222: 183.e1–183.e9, 2020. doi: 10.1016/j.ajog.2019.08.046. [DOI] [PubMed] [Google Scholar]
- 40.Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature. J Biol Chem 280: 22278–22286, 2005. doi: 10.1074/jbc.M500936200. [DOI] [PubMed] [Google Scholar]
- 41.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119: 213–224, 2009. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Krueger F. Trim Galore: A Wrapper Tool Around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ files (Online). https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/, 2015.
- 44.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25, 2009. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laresgoiti U, Nikolić MZ, Rao C, Brady JL, Richardson RV, Batchen EJ, Chapman KE, Rawlins EL. Lung epithelial tip progenitors integrate glucocorticoid- and STAT3-mediated signals to control progeny fate. Development 143: 3686–3699, 2016. doi: 10.1242/dev.134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, Jacks T, Regev A, Kim CF. Anatomically and functionally distinct lung mesenchymal populations marked by lgr5 and lgr6. Cell 170: 1149–1163.e12, 2017. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lignelli E, Palumbo F, Myti D, Morty RE. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 317: L832–L887, 2019. doi: 10.1152/ajplung.00369.2019. [DOI] [PubMed] [Google Scholar]
- 49.Ma T, Copland JA, Brasier AR, Thompson EA. A novel glucocorticoid receptor binding element within the murine c-myc promoter. Mol Endocrinol 14: 1377–1386, 2000. doi: 10.1210/mend.14.9.0524. [DOI] [PubMed] [Google Scholar]
- 50.Mandeville I, Aubin J, LeBlanc M, Lalancette-Hébert M, Janelle MF, Tremblay GM, Jeannotte L. Impact of the loss of Hoxa5 function on lung alveogenesis. Am J Pathol 169: 1312–1327, 2006. doi: 10.2353/ajpath.2006.051333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mariani TJ, Reed JJ, Shapiro SD. Expression profiling of the developing mouse lung: insights into the establishment of the extracellular matrix. Am J Respir Cell Mol Biol 26: 541–548, 2002. doi: 10.1165/ajrcmb.26.5.2001-00080c. [DOI] [PubMed] [Google Scholar]
- 52.Mižíková I, Ruiz-Camp J, Steenbock H, Madurga A, Vadász I, Herold S, Mayer K, Seeger W, Brinckmann J, Morty RE. Collagen and elastin cross-linking is altered during aberrant late lung development associated with hyperoxia. Am J Physiol Lung Cell Mol Physiol 308: L1145–L1158, 2015. doi: 10.1152/ajplung.00039.2015. [DOI] [PubMed] [Google Scholar]
- 53.Moghieb A, Clair G, Mitchell HD, Kitzmiller J, Zink EM, Kim YM, Petyuk V, Shukla A, Moore RJ, Metz TO, Carson J, McDermott JE, Corley RA, Whitsett JA, Ansong C. Time-resolved proteome profiling of normal lung development. Am J Physiol Lung Cell Mol Physiol 315: L11–L24, 2018. doi: 10.1152/ajplung.00316.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mustafa SB, DiGeronimo RJ, Petershack JA, Alcorn JL, Seidner SR. Postnatal glucocorticoids induce α-ENaC formation and regulate glucocorticoid receptors in the preterm rabbit lung. Am J Physiol Lung Cell Mol Physiol 286: L73–L80, 2004. doi: 10.1152/ajplung.00342.2002. [DOI] [PubMed] [Google Scholar]
- 55.Olkku A, Mahonen A. Calreticulin mediated glucocorticoid receptor export is involved in β-catenin translocation and Wnt signalling inhibition in human osteoblastic cells. Bone 44: 555–565, 2009. doi: 10.1016/j.bone.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Olson EB., Jr Role of glucocorticoids in lung maturation. J Anim Sci 49: 225–238, 1979. doi: 10.2527/jas1979.491225x. [DOI] [PubMed] [Google Scholar]
- 57.Park YK, Ge K. Glucocorticoid receptor accelerates, but is dispensable for, adipogenesis. Mol Cell Biol 37: e00260-16, 2017. doi: 10.1128/MCB.00260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, Evans SM, Morrisey EE. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 500: 589–592, 2013. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plosa EJ, Young LR, Gulleman PM, Polosukhin VV, Zaynagetdinov R, Benjamin JT, Im AM, van der Meer R, Gleaves LA, Bulus N, Han W, Prince LS, Blackwell TS, Zent R. Epithelial β1 integrin is required for lung branching morphogenesis and alveolarization. Development 141: 4751–4762, 2014. doi: 10.1242/dev.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polk DH, Ikegami M, Jobe AH, Sly P, Kohan R, Newnham J. Preterm lung function after retreatment with antenatal betamethasone in preterm lambs. Am J Obstet Gynecol 176: 308–315, 1997. doi: 10.1016/S0002-9378(97)70490-3. [DOI] [PubMed] [Google Scholar]
- 61.Prizant H, Sen A, Light A, Cho SN, DeMayo FJ, Lydon JP, Hammes SR. Uterine-specific loss of Tsc2 leads to myometrial tumors in both the uterus and lungs. Mol Endocrinol 27: 1403–1414, 2013. doi: 10.1210/me.2013-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods 14: 979–982, 2017. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramilowski JA, Goldberg T, Harshbarger J, Kloppmann E, Lizio M, Satagopam VP, Itoh M, Kawaji H, Carninci P, Rost B, Forrest AR. A draft network of ligand-receptor-mediated multicellular signalling in human. Nat Commun 6: 7866, 2015. doi: 10.1038/ncomms8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sayegh R, Auerbach SD, Li X, Loftus RW, Husted RF, Stokes JB, Thomas CP. Glucocorticoid induction of epithelial sodium channel expression in lung and renal epithelia occurs via trans-activation of a hormone response element in the 5′-flanking region of the human epithelial sodium channel alpha subunit gene. J Biol Chem 274: 12431–12437, 1999. doi: 10.1074/jbc.274.18.12431. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt AF, Kannan PS, Bridges JP, Filuta A, Lipps D, Kemp M, Miller LA, Kallapur SG, Xu Y, Whitsett JA, Jobe AH. Dosing and formulation of antenatal corticosteroids for fetal lung maturation and gene expression in rhesus macaques. Sci Rep 9: 9039, 2019. doi: 10.1038/s41598-019-45171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorrentino G, Ruggeri N, Zannini A, Ingallina E, Bertolio R, Marotta C, Neri C, Cappuzzello E, Forcato M, Rosato A, Mano M, Bicciato S, Del Sal G. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun 8: 14073, 2017. doi: 10.1038/ncomms14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spooner BS, Wessells NK. Mammalian lung development: interactions in primordium formation and bronchial morphogenesis. J Exp Zool 175: 445–454, 1970. doi: 10.1002/jez.1401750404. [DOI] [PubMed] [Google Scholar]
- 68.Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, Manduchi E, Stoeckert CJ Jr, Lazar MA. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev 24: 1035–1044, 2010. doi: 10.1101/gad.1907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol 26: 139–162, 2005. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Taves MD, Plumb AW, Sandkam BA, Ma C, Van Der Gugten JG, Holmes DT, Close DA, Abraham N, Soma KK. Steroid profiling reveals widespread local regulation of glucocorticoid levels during mouse development. Endocrinology 156: 511–522, 2015. doi: 10.1210/en.2013-1606. [DOI] [PubMed] [Google Scholar]
- 71.Wang IC, Zhang Y, Snyder J, Sutherland MJ, Burhans MS, Shannon JM, Park HJ, Whitsett JA, Kalinichenko VV. Increased expression of FoxM1 transcription factor in respiratory epithelium inhibits lung sacculation and causes Clara cell hyperplasia. Dev Biol 347: 301–314, 2010. doi: 10.1016/j.ydbio.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Q, Lu PH, Shi ZF, Xu YJ, Xiang J, Wang YX, Deng LX, Xie P, Yin Y, Zhang B, Mu HJ, Qiao WZ, Cui H, Zou J. Glucocorticoid receptor beta acts as a co-activator of T-Cell factor 4 and enhances glioma cell proliferation. Mol Neurobiol 52: 1106–1118, 2015. doi: 10.1007/s12035-014-8900-9. [DOI] [PubMed] [Google Scholar]
- 73.Wang W, Guo C, Li W, Li J, Wang W, Myatt L, Sun K. Involvement of GR and p300 in the induction of H6PD by cortisol in human amnion fibroblasts. Endocrinology 153: 5993–6002, 2012. doi: 10.1210/en.2012-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci 27: 1756–1768, 2007. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and regenerating the lung cell by cell. Physiol Rev 99: 513–554, 2019. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Willet KE, Jobe AH, Ikegami M, Newnham J, Brennan S, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res 48: 782–788, 2000. doi: 10.1203/00006450-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 77.Xie T, Wang Y, Deng N, Huang G, Taghavifar F, Geng Y, Liu N, Kulur V, Yao C, Chen P, Liu Z, Stripp B, Tang J, Liang J, Noble PW, Jiang D. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep 22: 3625–3640, 2018. doi: 10.1016/j.celrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, Stripp BR, Whitsett JA. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1: e90558, 2016. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Y, Wang Y, Besnard V, Ikegami M, Wert SE, Heffner C, Murray SA, Donahue LR, Whitsett JA. Transcriptional programs controlling perinatal lung maturation. PLoS One 7: e37046, 2012. doi: 10.1371/journal.pone.0037046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Z, Guo C, Zhu P, Li W, Myatt L, Sun K. Role of glucocorticoid receptor and CCAAT/enhancer-binding protein α in the feed-forward induction of 11β-hydroxysteroid dehydrogenase type 1 expression by cortisol in human amnion fibroblasts. J Endocrinol 195: 241–253, 2007. doi: 10.1677/JOE-07-0303. [DOI] [PubMed] [Google Scholar]
- 81.Yin Y, Zhang X, Li Z, Deng L, Jiao G, Zhang B, Xie P, Mu H, Qiao W, Zou J. Glucocorticoid receptor β regulates injury-mediated astrocyte activation and contributes to glioma pathogenesis via modulation of β-catenin/TCF transcriptional activity. Neurobiol Dis 59: 165–176, 2013. doi: 10.1016/j.nbd.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 82.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, Zhou S, Cantu E, Morrisey EE. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555: 251–255, 2018. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170: 1134–1148.e10, 2017. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137, 2008. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequencing data from the present study have been deposited to GEO (https://www.ncbi.nlm.nih.gov/geo/). Specifically, whole lung microarray data from mesenchyme-specific GR deletion mice (Col1a2-Cre;Nr3c1fl/fl) at E16.5 and E18.5 was deposited (GSE30143; Ref. 31). RNA-seq of lung (Twist2Cre;Rosa26mT/mG mice treated with dexamethasone or saline) at E18.5; RNA-seq of CD106+ (CD45−/CD31−/CD309−/CD106+) and CD106− cells; ATAC-seq of CD106+ cells at E16.5 and E18.5; and scRNA-seq of mouse lung (wild-type embryos treated with Dex or saline in vivo and harvested at E18.5) are available at GSE136954. All data from this study can be accessed through Lung Gene Expression Analysis (LGEA) web portal (17, 19), which is freely available at https://research.cchmc.org/pbge/lunggens/GR_signaling.html. R code used for data analysis is available at https://github.com/xu-lab.