Abstract
Skeletal muscle atrophy continues to be a serious consequence of many diseases and conditions for which there is no treatment. Our understanding of the mechanisms regulating skeletal muscle mass has improved considerably over the past two decades. For many years it was known that skeletal muscle atrophy resulted from an imbalance between protein synthesis and protein breakdown, with the net balance shifting toward protein breakdown. However, the molecular and cellular mechanisms underlying the increased breakdown of myofibrils was unknown. Over the past two decades, numerous reports have identified novel genes and signaling pathways that are upregulated and activated in response to stimuli such as disuse, inflammation, metabolic stress, starvation and others that induce muscle atrophy. This review summarizes the discovery efforts performed in the identification of several pathways involved in the regulation of skeletal muscle mass: the mammalian target of rapamycin (mTORC1) and the ubiquitin proteasome pathway and the E3 ligases, MuRF1 and MAFbx. While muscle atrophy is a common outcome of many diseases, it is doubtful that a single gene or pathway initiates or mediates the breakdown of myofibrils. Interestingly, however, is the observation that upregulation of the E3 ligases, MuRF1 and MAFbx, is a common feature of many divergent atrophy conditions. The challenge for the field of muscle biology is to understand how all of the various molecules, transcription factors, and signaling pathways interact to produce muscle atrophy and to identify the critical factors for intervention.
Keywords: MAFbx, mTORC1, MuRF1, protein synthesis, ubiquitin proteasome pathway
INTRODUCTION
Skeletal muscle is a complex and dynamic tissue that comprises ~40% of body weight and performs critical functions related to movement (power output and sensory feedback), metabolism (substrate utilization, storage, and supply) and thermogenesis. Skeletal muscle mass has been shown to be predictive of longevity in older adults and is a critical variable in predicting mortality as a consequence of diseases such as cancer, type II diabetes, and cardiovascular disease (75, 80). The regulation of skeletal muscle mass is multifactorial integrating signals from hormones, growth factors, cytokines, nutrients, load and activity to regulate multiple intersecting pathways that control the balance between protein synthesis and protein degradation (63). Furthermore, skeletal muscle is a multicellular tissue that relies on an intact vascular system and motor/sensory innervation to maintain its size and function. The fact that skeletal muscle interacts with and integrates signals from multiple systems (neural, endocrine, cardiovascular) and organs (liver, adipose, gut) within the body complicates the efforts to identify the key regulators of skeletal muscle mass.
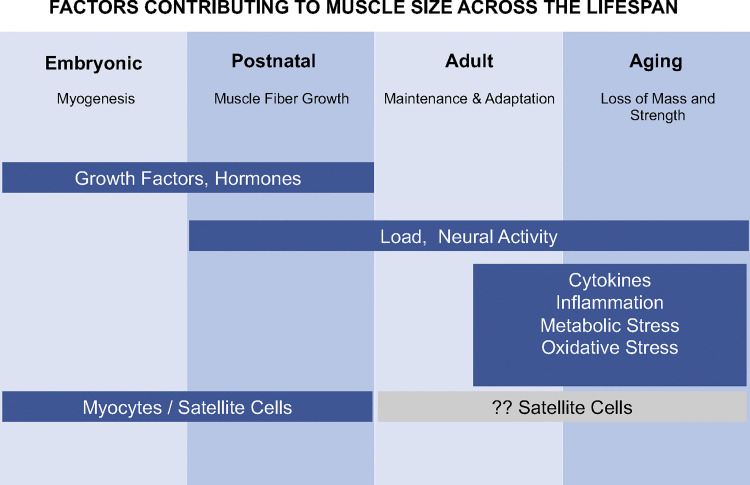
Muscle size is a very plastic characteristic of limb muscles, changing over the life span with different signals playing critical regulatory roles at each life stage (Fig. 1). Alterations in muscle mass can occur as a consequence of changes in both the number and/or size of individual muscle fibers depending on the life stage. The number of fibers in a muscle is established during embryonic and fetal development and is dependent on the proliferation, differentiation and fusion of myoblasts to form myofibers (37, 51). The number of muscle fibers in a muscle remains constant in healthy muscles throughout most of the life span, with decreases occurring during advanced aging (51). In contrast, changes in fiber size can occur throughout the life span. Increases in both the length and cross-sectional area of individual fibers occur before and during puberty and are dependent on satellite cell proliferation and differentiation (67, 82). The predominant change that occurs postpuberty is to fiber cross-sectional area, resulting in both increases (i.e., hypertrophy) and decreases (i.e., atrophy) in size. The role of satellite cells in adaptive hypertrophy of adult muscle is still debated, but recent evidence suggests that adaptive muscle hypertrophy can occur without satellite cells and the addition of myonuclei to individual muscle fibers (9, 24, 54).
Fig. 1.
Regulation of skeletal muscle mass throughout the life span. This diagram illustrates some of the critical factors that regulate skeletal muscle size at different life stages. At each life stage skeletal muscle undergoes specific changes that are regulated by different critical factors. During the embryonic stage growth factors, hormones and myocytes/satellite cells play a critical role in the formation and innervation of muscle fibers. During the postnatal stage, external loading and increased neural activation become important factors along with growth factors, hormones, and satellite cells in regulating increases in the length and cross-sectional area of muscle fibers. As adults, maintaining muscle mass and adapting to changes in external loading and neural activation become the dominant focus. With advancing age, decreases in external loading and activity, as well as, inflammation, increasing levels of cytokines, oxidative stress, and metabolic stress can lead to a decrease in muscle mass and strength.
Our understanding of the factors and pathways that regulate both hypertrophy and atrophy of skeletal muscle has increased greatly over the past two decades. This review is a summary of the Adolph Distinguished Lecture given by me at the 2019 Experimental Biology meeting. The work presented in this review reflects a journey that started for me as a graduate student interested in understanding skeletal muscle plasticity and in particular how force is generated and regulated in skeletal muscle to control movement. Over the course of my career my research became more focused on undercovering the mechanisms regulating skeletal muscle size, especially under conditions leading to the loss of muscle mass and function. In this review, I will highlight research conducted since the late 1990s that has contributed to a greater understanding of the mechanisms underlying skeletal muscle atrophy.
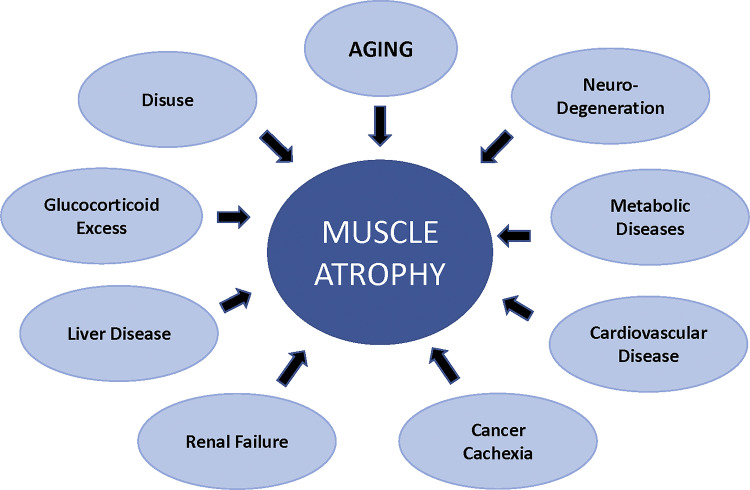
In the 1990s our understanding of the cellular and molecular mechanisms regulating skeletal muscle was relatively limited. It was known that loss of muscle mass occurred under a variety of diseases and conditions such as immobilization, spinal cord injury, aging and others as shown in Fig. 2. In animal models, muscle atrophy could be induced by decreases in neural activity, decreases in external loading, increases in glucocorticoids, increases in inflammatory cytokines and nutrient deficiency (30, 52, 66). The induction of muscle atrophy was related to an imbalance between protein synthesis and degradation with the net balance being shifted toward protein breakdown. There were data to suggest that both decreases in protein synthesis and increases in protein degradation contributed to muscle loss, and that the relative contribution of each process to muscle loss depended on the conditions (3, 28, 29, 43, 56, 62); however, unknown at the time was the identity of the signaling pathways responsible for the changes in protein synthesis and degradation.
Fig. 2.
Skeletal muscle atrophy is prevalent in many diseases. Skeletal muscle atrophy is a serious consequence of the diseases and conditions listed in this diagram.
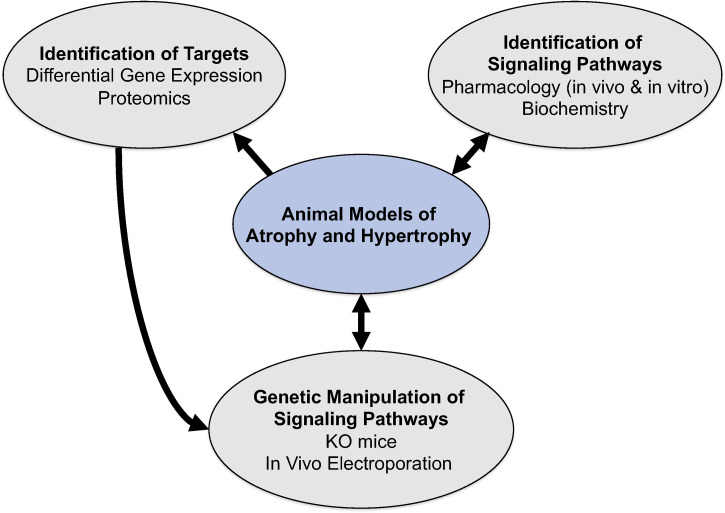
In the late 1990s, while at Regeneron Pharmaceuticals, we initiated a discovery program aimed at determining the signaling pathways regulating the loss of skeletal muscle mass. To approach this complex problem, we developed a multi-disciplinary muscle research program diagrammed in Fig. 3. At the center of the program was the establishment of rodent models of muscle atrophy and hypertrophy, from which skeletal muscles were obtained at multiple time points postexperimental manipulation and then used for the identification of signaling pathways and differentially regulated genes and proteins. A variety of pharmacological agents were also used to induce atrophy (e.g., dexamethasone, cytokines) and hypertrophy (e.g., clenbuterol) or block specific enzymes and signaling pathways (e.g., rapamycin). Finally, emerging technologies, such as mouse genetic engineering and in vivo electroporation, were utilized to overexpress or delete specific genes in skeletal muscle. These approaches allowed for the discovery of novel pathways involved in the regulation of skeletal muscle mass.
Fig. 3.
Building a multidisciplinary muscle research program. A multidisciplinary research program was developed to discover novel mechanisms regulating skeletal muscle size. At the center of the program was the establishment of animal models of skeletal muscle atrophy (denervation, nerve crush-reinnervation, joint immobilization, hindlimb suspension, glucocorticoid excess) and hypertrophy (functional overload, reloading following disuse). Muscle tissues taken at different time points were used in the identification of novel gene and protein targets using differential gene expression and proteomics approaches. Muscle samples were also used to identify signaling pathways that were altered in response to stimuli that induced atrophy and hypertrophy. Furthermore, pharmacological agents, such as clenbuterol, IGF1, rapamycin, glucocorticoids, were utilized both in vivo and in vitro to manipulate selective pathways to induce changes in muscle size. Finally, technologies such as in vivo electroporation and mouse genetic engineering were utilized to modify gene expression in skeletal muscle.
In the following sections, a summary of work that led to the discovery of novel signaling pathways involved in the regulation of skeletal muscle mass is presented. It begins with a description of the experiments that identified mTORC1 activation as a critical regulator of skeletal muscle hypertrophy in adult mammals; and continues with a description of the experimental approaches taken to identify novel signaling pathways involved in the loss of skeletal muscle mass. Details are provided regarding the discovery of two skeletal muscle-specific E3 ubiquitin ligases, MuRF1 and MAFbx/Atrogin1, followed by highlights of published findings related to the transcriptional regulation of MuRF1 and MAFbx and their expression patterns during different forms of atrophy, as well as, the use of mice with global deletions of either MuRF1 or MAFbx to identify the potential mechanisms of action of these E3 ligase in the regulation of skeletal muscle mass. The final sections discuss the link between MuRF1 and MAFbx expression and proteasome activity, and the continuing search for MuRF1 and MAFbx substrates. The review concludes by highlighting some of the important questions that remain to be answered.
THE IDENTIFICATION OF mTORC1 AS A MAJOR REGULATOR OF SKELETAL MUSCLE SIZE IN MAMMALS
Skeletal muscle is highly responsive to changes in external loading, increasing in size in response to enhanced loading and decreasing in size in response to reduced loading (66). It had been shown in both human and rodent muscle, that the rate of protein synthesis is modified in response to changes in external loading. Following an acute bout of resistance exercise in humans (87) or simulated resistance exercise in rats (84), protein synthesis increases; whereas, the early response to conditions that decrease loading, such as immobilization or bedrest, is a decrease in protein synthesis (25, 28). While robust changes in protein synthesis in response to alterations in loading had been demonstrated, the cellular mechanisms responsible for the changes were unknown. Some possible candidates at the time included Insulin-like Growth Factor 1 (IGF-1), calcineurin, and the PI(3)K/Akt signaling pathway. The case for IGF-1 as a major mediator of growth and protein synthesis in skeletal muscle was supported by reports that IGF-1 induces an increase in myotube size in vitro (65, 77), IGF-1 is elevated in vivo following acute high-load contractions (1), and transgenic overexpression of IGF-1 in mice results in enhanced muscle size (2, 18). Another pathway that had been linked to growth in model organisms and mice was the PI(3)K/Akt/mTOR pathway. Genetic manipulation in Drosophila of PI(3)K, PKB/Akt and S6K1 (p70s6k) revealed that deletion of components of this pathway resulted in smaller cells, but not fewer cells, suggesting that the PI(3)K/Akt/mTOR pathway played a major role in the regulation of cell size (57, 81). Further support for this pathway came from the deletion of S6K1 in mice which led to lower body weights and organ growth relative to wild-type (WT) littermates (72). Finally, data were emerging that the PI(3)K/Akt/mTOR pathway may play a role in skeletal muscle growth in response to resistance exercise. Baar and Esser demonstrated that following high resistance exercise training in rats, phosphorylation of S6K1 (p70s6k) increased in those muscles experiencing an increase in loading resulting in hypertrophy (4). Given that S6K1 is downstream of mTORC1, these data suggested that activation of mTORC1 and its downstream targets were important for muscle growth.
Given what was known at the time, we asked the following questions: Is the PI(3)k/Akt/mTOR pathway regulated in vivo during hypertrophy or atrophy, and what happens to growth or atrophy when this pathway is inhibited? To address the first question, we used synergist ablation, a model of functional overload, to determine whether components of the PI(3)k/Akt/mTORC1 pathways were activated in a muscle undergoing hypertrophy. We found that following synergist ablation, all components of the PI3k/Akt/mTORC1 were activated in the plantaris muscle and that activation of this pathway was an early response (12). Phosphorylation and total amount of Akt/PKB increased early leading to activation of mTORC1 as measured by (1) an increase in phosphorylation of mTORC1 at Ser2448 (64), (2) an increase in the phosphorylation and activity of S6K1/p70s6k (12), and (3) a decrease in the amount of 4EBP1/PHAS-I bound to eIF4E coupled with an increase in the amount of eIF4E bound to eIF4G (64). These data were the first to show that activation of the Akt/mTORC1 pathway was associated with skeletal muscle growth in vivo. Subsequent studies have shown that this pathway is activated in human muscle in response to resistance exercise and associated with an increase in protein synthesis (21, 22, 34, 58). The question remained as to whether activation of this pathway was necessary for skeletal muscle growth. To address this question, adult rats were given rapamycin to inhibit mTORC1 and its downstream targets, without affecting activation of Akt or inhibition of GSK3. Rapamycin treatment for a duration of up to 14 days prevented 95% of hypertrophy of both slow and fast fibers in the rat plantaris muscle following functional overload (12), revealing that activation of mTORC1 was necessary to achieve adaptive hypertrophy of adult skeletal muscle. Activation of the Akt/mTORC1 pathways was also shown to decrease in response to hindlimb unloading, which induced atrophy, and to increase in response to reloading, which stimulated muscle regrowth (12). Interestingly, rapamycin treatment given upon return of weight-bearing locomotion following hindlimb unloading significantly inhibited growth of the soleus, plantaris and medial gastrocnemius muscles; however, the inhibition was not as great as that seen in the functional overload model. These data suggest that muscle regrowth following a period of atrophy is not identical to muscle growth in response to increased loading associated with resistance exercise in healthy muscle. The differences between the two growth models, i.e., functional overload and unloading/reloading, have not been explored in detail. The increase in Akt/mTORC1 activation in the functional overload model was presumed to be in response to an increase in IGF-1 signaling since IGF-1 had been shown to activate the PI3k/Akt/mTORC1 pathway in myotubes and induce hypertrophy (65), and transgenic overexpression of IGF1 in embryonic mice resulted in larger muscles (18). However, a subsequent study by Spangenburg et al. (74) revealed that activation of the IGF1 receptor was not required for the phosphorylation of Akt and S6K1/p70S6K and the induction of muscle hypertrophy in response to functional overload.
In summary, many studies have now shown that activation of mTORC1 and increases in protein synthesis are critical for achieving muscle hypertrophy in response to increased loading in adult mammals. This increase in protein synthesis and stimulation of muscle fiber growth does not appear to be dependent on an increase in satellite cell proliferation and the addition of myonuclei to the muscle fiber (40, 54). Furthermore, suppression of mTORC1 and decreased protein synthesis can contribute to the loss of muscle mass, especially under conditions of disuse. These results might suggest that activating the mTORC1 pathway would be a good strategy for treating muscle atrophy. In fact, it has been shown that overexpression of Akt in muscle can lead to hypertrophy and prevent muscle atrophy (12, 49). However, chronic activation of mTORC1 has been shown to be deleterious to muscle, leading to muscle atrophy (35). Closer inspection of how mTORC1 is activated in muscle shows that in resting adult muscle mTORC1 is at a relatively low activation state. Activation of mTORC1 occurs periodically as the result of increased loading or amino acid ingestion (19, 33). In healthy adult muscle, mTORC1 is rarely in a chronically activated state, and short-term rapamycin treatment in adult animals does not affect muscle mass (12). Furthermore, a recent study showed that deletion of raptor and suppression of mTORC1 activity in resting adult mice for 5 mo had no effect on muscle mass (36). An exception is muscle in aged animals where mTORC1 activation is chronically elevated under resting conditions and shows reduced activation in response to anabolic stimuli. Chronic activation of mTORC1 is thought to contribute to the loss of mass and function with age, and a recent study suggests that partial suppression of mTORC1 in older animals could be beneficial (41). It should be noted that mTORC1 is also activated in muscle upon denervation, a condition that causes muscle atrophy (29). Interestingly, inhibition of mTORC1 with rapamycin during denervation results in an attenuation of muscle atrophy (44). The mechanisms underlying the beneficial effects of rapamycin during denervation and possibly aging remain unclear and require further investigation.
IDENTIFICATION OF MUSCLE ATROPHY PATHWAY
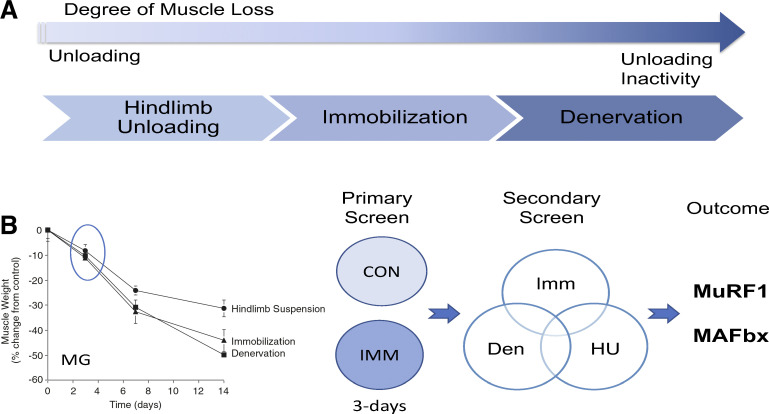
The loss of muscle mass occurs as the result of many conditions and diseases, as shown in Fig. 2. To identify novel pathways responsible for muscle atrophy, we initially focused our discovery efforts on models of disuse atrophy. Disuse atrophy occurs as the result of decreases in external loading, as well as, decreases in neural activation of muscle, and ranges in the degree of muscle loss; being less severe in bedrest (modeled as hindlimb suspension in animals) and most severe following denervation (Fig. 4). Examination of the extent of atrophy (% change in muscle mass) of the rat medial gastrocnemius over 14 days in three models of disuse (hindlimb unloading, ankle joint immobilization, denervation) shows that the rate of loss over the first 3 days was similar across the three models, whereas after 7 days the rate of loss diverges across the models (Fig. 4). In an effort to discover potential early triggers of muscle atrophy, a differential expression analysis (GeneTag method) was performed on control and 3-day immobilized rat medial gastrocnemius muscle (11) (Fig. 4). In the primary screen we identified genes that were differentially regulated by threefold in the immobilized muscle. A secondary screen was then performed to identify the subset of genes that were also differentially regulated following denervation and hindlimb suspension, in addition to immobilization. The secondary screen identified two genes that were upregulated in all disuse atrophy models tested, as well as, dexamethasone and interleukin-1 induced atrophy. Both genes were shown to be E3 ubiquitin ligases: one gene was a RING finger protein previously identified in the heart as MURF1 (Trim63) and the other gene was a novel protein that contained an F-box domain and was shown to be a member of the Skp-Cullin1-F-box (SCF) protein family of E3 ubiquitin ligases. We named this novel protein MAFbx for Muscle Atrophy F-box (FBX032). This same protein was also identified by Goldberg and colleagues in the mouse gastrocnemius muscle following starvation using an Affymetrix microarray and called Atrogin-1 (32). Since their initial identification in 2001, my laboratory has been studying the role of MuRF1 and MAFbx in the regulation of skeletal muscle mass by examining their expression patterns under a variety of atrophy and hypertrophy conditions, their transcriptional regulation, and the impact of their deletion on the loss of muscle mass in different atrophy conditions.
Fig. 4.
Discovery of MuRF1 and MAFbx in skeletal muscle. A: animal models of disuse atrophy range in severity from those that primarily produce decreased external loading of the muscles, such as hindlimb unloading to those that produce both a decrease in external loading and a complete absence of neural activity such as denervation or spinal cord injury. Joint immobilization is a model that produces decreased external loading and a reduction in neural activity depending on the degree to which the joint is immobilized. B: loss of mass of the rat medial gastrocnemius muscle (MG) was compared in three models of disuse atrophy (hindlimb suspension, ankle joint immobilization, and denervation). To identify a potential common trigger of muscle atrophy, 3 days postimmobilization was chosen as the time point and model to perform a differential gene expression analysis (GeneTag, Applied Bioscience). In the primary screen, all those genes that were differentially regulated 3-fold were identified. A secondary screen was utilized to identify those genes that were similarly regulated in three disuse models (immobilization, hindlimb unloading, and denervation). The secondary screen consisted of using Northern blots to analyze gene expression in the medial gastrocnemius muscle over a time course of atrophy (0, 1, 3, 7 days) for each of the disuse models. From the secondary analysis, two genes (MuRF1 and MAFbx) were identified that were similarly upregulated in all atrophy models examined.
MuRF1 and MAFbx Are Excellent Markers of Muscle Atrophy
MuRF1 and MAFbx demonstrate many characteristics that make them excellent markers of muscle atrophy. First, both genes are selectively expressed in muscle tissue (skeletal, cardiac and smooth muscle), and in resting skeletal muscle these genes are expressed at relatively low levels (11). Second, the expression of both genes rapidly increases in response to a variety of stressors including unloading, decreased neural activity, elevated glucocorticoids, elevated cytokines, increased oxidative stress, and malnutrition (10, 11, 32, 61). The expression patterns of both genes vary depending on the atrophy stimulus. For example, in response to unloading and denervation, expression of both MuRF1 and MAFbx increases rapidly within 48 h reaching a peak around 7–10 days and then gradually declining to baseline by 14 days. Recently, it was confirmed using mass spectrometry that MuRF1 protein expression in mouse muscle changed over the same time course as the mRNA following denervation (50). Validation of protein expression, especially of MuRF1, has been difficult because of the lack of specificity of commercially available antibodies. We have tested the majority of commercially available MuRF1 antibodies, and found positive staining with the MuRF1 antibody when used on lysates from muscles taken from MuRF1 KO mice. Moreover, in these KO tissues we often observe a positive band at the same molecular weight as predicted for MuRF1 (8). Our data suggest that the majority of commercial antibodies for MuRF1 are nonspecific and should not be used to quantify MuRF1 protein expression in muscle tissue.
The expression patterns of both MuRF1 and MAFbx in response to elevated glucocorticoids and cytokines differ compared with disuse; rising rapidly to a peak within days and then maintaining an elevated level for as long as the signal (glucocorticoid or cytokine levels) is present (5). Interestingly, under increased loading conditions (such as reloading following unloading, reinnervation following nerve injury, and functional overload) the expression of MuRF1 and MAFbx is suppressed below baseline (6, 7). While MuRF1 and MAFbx expression are suppressed at 7 days following functional overload, the expression of both genes is transiently increased at 1 day following the synergist ablation surgery (6). This same pattern of elevated expression is seen immediately following an acute bout of intensive eccentric contractions in humans (53, 86). The mechanism underlying the suppression of these genes in response to increased loading is unknown. One possible mechanism could be a change in the redox state of the muscle upon loading. In summary, MuRF1 and MAFbx are excellent markers of muscle atrophy due to the fact that in all atrophy models tested to date expression of MuRF1 and MAFbx has been shown to increase at some time point during the course of the disease.
MuRF1 and MAFbx Are Regulated By Multiple Transcription Factors
Examination of the expression patterns of MuRF1 and MAFbx under different atrophy conditions has revealed two interesting observations: 1) both genes are upregulated together under most atrophy conditions and 2) both genes are strongly induced by the synthetic glucocorticoid, dexamethasone. Based on these observations, we were interested in understanding the transcriptional regulation of these genes under different atrophy conditions. We started our investigation into the transcriptional regulation of MuRF1 and MAFbx by first examining the proximal promoter regions, 5,000 bp upstream of the transcriptional start site, of both genes for putative transcription factor binding sites. Interestingly, we found that the proximal region of the MuRF1 promoter, but not the MAFbx promoter, contained a perfect palindromic glucocorticoid response element (GRE) (78). We also found that the proximal promoter region of each gene contained several consensus Class O forkhead (FOXO) binding sites (FBE), capable of binding FOXO1, FOXO3a, and FOXO4; all of which are expressed in skeletal muscle. This finding was particularly interesting since several reports had shown that activated FOXO1 (76) could increase MuRF1 transcription in myotubes, while activated FOXO3a could increase MAFbx expression in vitro and in vivo (68). Our studies revealed for the first time that dexamethasone could directly activate the MuRF1 promoter, but not the MAFbx promoter (78). Furthermore, we provided additional evidence for direct activation of the MuRF1 and MAFbx promoters by the FOXO transcription factors. Our studies, however, revealed that while the promoters of both MuRF1 and MAFbx could be activated by all of the FOXO transcriptions factors, MAFbx was much more responsive than MuRF1. Moreover, the promoters, were not equally activated by all of the FOXO transcription factors. For example, activation of the MAFbx promoter was greatest in response to FOXO3a and least in response to FOXO1. In contrast, the MuRF1 promoter was similarly activated by FOXO1 and FOXO3a. Interestingly, we found a synergistic activation of the MuRF1 promoter only by FOXO1 and the activated glucocorticoid receptor (78). The differential response of MuRF1 and MAFbx to the various FOXO transcription factors is important because many studies treat all of the FOXO transcription factors as interchangeable. FOXO3a and FOXO1 are the predominate FOXO transcription expressed in skeletal muscle. Many published reports utilize either FOXO1 or FOXO3a in their studies, and then assume that the results apply to all FOXO transcriptional factors. A study by Milan et al. illustrates the differential gene regulation of MuRF1 and MAFbx in vivo by FOXO1 and FOXO3a (55). In their study, Milan et al. measured expression of MuRF1 and MAFbx following denervation in WT mice and mice with a muscle-specific deletion of FOXO1 or FOXO1/3a/4. In response to denervation, the expression of both FOXO1 and FOXO3a increases, as does the expression of MuRF1 and MAFbx. Deletion of only FOXO1a in muscle significantly suppressed the activation of MuRF1, but not MAFbx. In contrast, deletion of all three FOXOs in muscle resulted in a significant suppression of both MuRF1 and MAFbx. Interestingly, MuRF1 and MAFbx still increased in response to denervation even in the absence of the FOXO transcription factors. This activation is likely not related to an activated glucocorticoid receptor since we showed that upregulation of both MuRF1 and MAFbx following denervation was similar in WT and muscle-specific GR knockout mice (79). These data do illustrate that transcriptional control of MuRF1 and MAFbx is complex, and the literature shows that multiple transcription factors can activate both genes. In addition to the glucocorticoid receptor and FOXO1/FOXO3a, the following transcription factors have been shown to activate MuRF1 and MAFbx: NF-κB (15, 39, 85), KLF15 (73), C/EBP β (88, 89) and Smad3 (13).
Deletion of MuRF1, but not MAFbx, Results in Sparing of Muscle Mass
The substrates targeted for ubiquitination by MuRF1 and MAFbx remain poorly defined, and thus the mechanisms by which upregulation of MuRF1 and/or MAFbx contribute to muscle atrophy remain poorly understood. The generation of mice with a global deletion of either MuRF1 or MAFbx has assisted us in understanding the role of these E3 ligases in the regulation of skeletal muscle mass (11). The phenotype of the knockout mice has been previously described (10). In brief, both knockout strains develop normally and show no phenotype until later in life (~16–18 mo) when the MAFbx KO mice die prematurely of congestive heart failure. The MuRF1 KO mice have a normal life span and have preserved muscle mass, but not function, with age (38). Muscle atrophy has been examined in both knockout (KO) strains and the results have revealed that, in general, deletion of MuRF1 leads to better functional muscle sparing than deletion of MAFbx. Deletion of MuRF1 leads to functional sparing of muscle mass following denervation (31), hindlimb unloading (48), exogenous glucocorticoid treatment (5), and acute lung injury (26). In contrast, deletion of MAFbx only spares muscle mass following denervation, however, the sparing is not functional. Histological analysis of denervated muscle from MAFbx KO mice after 14 days reveals an increase in vacuole-containing fibers and an increase in fibrosis (31). Interestingly, muscle sparing in the MuRF1 KO mice following denervation occurs even though MAFbx expression is higher than measured in WT denervated muscle and is sustained at a higher level for a longer period of time as compared with WT mice (31). In general, our muscle atrophy studies in the KO mice have revealed that suppression of MAFbx is not required for sparing of muscle mass, however, suppression of MuRF1 is necessary to achieve muscle sparing. The one atrophy model where muscle atrophy is not affected by deletion of either MuRF1 or MAFbx is nutritional deprivation (5).
Increases in MuRF1 and MAFbx Expression are not Always Linked to Increases in Proteasome Activity
MuRF1 and MAFbx are E3 ubiquitin ligases that are expressed at low levels in resting muscle and increase rapidly in response to stimuli that induce muscle atrophy. Given these facts, it has been hypothesized that increases in MuRF1 and MAFbx expression lead to muscle atrophy by increasing proteasome activity and protein degradation. In our atrophy models, we have examined the relationship between the expression levels of MuRF1 and MAFbx and proteasome activity. Proteasome activity is measured using in vitro assays in which the activity of the specific proteasome subunits (β1, β2, and β5) is determined using specific proteasome inhibitors and substrates in the presence (26S proteasome) or absence (20S proteasome) of ATP (31). Following denervation, the expression of both MuRF1 and MAFbx significantly increases by 3 days and then decreases to baseline by 14 days. In comparison, at 3 days of denervation, proteasome activity is elevated for only a couple of subunits (26S β1 and β2), whereas, at 14 days proteasome activity is significantly elevated in all subunits (26S (β1, β2, β5, and 20S β1, β2, β5) (31). In MuRF1 KO mice, at 3 days of denervation, proteasome activity is elevated in the 26S β2 subunit only, while at 14 days of denervation, proteasome activity is significantly elevated in all subunits. Interestingly, the increase in proteasome subunit activity is significantly higher in the KO mice compared with WT mice. The finding that proteasome activity increased to a greater extent in the MuRF1 KO mice than the WT mice was unexpected since higher proteasome activity has generally been linked to an increase in muscle atrophy (56), and a report by Cohen et al. suggested that proteasome activity was decreased in the MuRF1 KO mice (16).
An elevation in proteasome activity in the MuRF1 KO mice was also found in response to aging. Examination of old (24 mo) WT and MuRF1 KO male mice revealed that muscle mass was maintained in the MuRF1 KO mice with age, while it decreased in WT mice (38). Measurement of proteasome activity revealed a decrease in the activity of all of the proteasome subunits as a consequence of aging in WT mice. In contrast, old MuRF1 KO mice had higher proteasome subunit activities compared with WT. These data suggest that an elevated proteasome activity is protective under some conditions, contributing to an increase in protein quality control and a decrease in cellular stress. The mechanism(s) by which a deletion of MuRF1 leads to an increase in proteasome activity are unclear and are under study.
The use of MuRF1 and MAFbx expression as markers of increased proteasome activity, and by extension protein degradation, is widespread in the literature. As mentioned previously, MuRF1 and MAFbx expression are excellent markers of muscle atrophy; however, an increase in their expression levels does not always coincide with an increase in proteasome activity. In the case of denervation, the increase in proteasome activity occurs as the expression levels of MuRF1 and MAFbx are decreasing back to baseline. In another disuse model, hindlimb suspension, expression of MuRF1 and MAFbx increases in the soleus, medial gastrocnemius and tibialis anterior muscles with unloading, peaking at around 7 days and returning to baseline by 14 days (8). In comparison, proteasome activity increases only in the soleus and rises continuously over 14 days of unloading. Another example of the disconnect between MuRF1 and MAFbx expression and proteasome activity is functional overload. In response to functional overload, MuRF1 and MAFbx expression significantly increases at day 1 following the synergist ablation surgery and decreases to baseline by 3 days followed by suppression below baseline (6). In contrast, proteasome activity increases immediately following surgery and continues to rise until around day 7 post functional overload, returning to baseline levels by 14 days. These data highlight the fact that elevated proteasome activity is not always a sign of atrophy, but is also required for remodeling and growth. These data also strongly suggest that MuRF1 and MAFbx expression should not be used as substitute markers for proteasome activity or protein degradation.
Identification of MuRF1 and MAFbx Substrates
MuRF1 and MAFbx were first identified as atrophy-associated E3 ligases expressed selectively in muscle in 2001 (11, 32), however, much is still unknown regarding how their upregulation contributes to the loss of skeletal muscle mass. We have compared the response of WT and MuRF1 KO mice to denervation and dexamethasone treatment and found that while muscle mass is spared in the KO mice under both conditions, the mechanisms of action appear to be very different (5, 31). For example, following dexamethasone treatment, muscle sparing in the MuRF1 KO mice appears to be related to the suppression of FOXO1 gene expression and the maintenance of protein synthesis (5). In comparison, following denervation, muscle sparing in the MuRF1 KO mice is related to the suppression of genes associated with inactivity (such as HDAC4 and neuromuscular junction associated genes) and a paradoxical increase in the ubiquitin proteasome pathway (31).
These results could suggest that different sets of substrates are targeted for ubiquitination by MuRF1 under these divergent atrophy conditions. What mechanisms could accomplish differential targeting of substrates under different atrophy conditions? One possibility is that MuRF1 (an E3 ligase) pairs with different E2 ligases under different atrophy conditions. It has been shown that the specific E2-E3 pairing can alter the type of ubiquitin chains that are added to a substrate (42, 60). It is also possible that different E2-E3 pairings affect the specific substrates that are targeted. Furthermore, it is known that different proteins can bind to E3 ligases and possibly alter their localization within the cell and substrates (83). One possibility is that in response to different stressor, MuRF1 has different binding partners which modify its cellular localization and potential substrates. While the literature suggests that the primary substrates for MuRF1 are proteins associated with the thick myofilament (16), this list is most likely incomplete and may not be accurate since most of the data have been collected in vitro using E2 ligases that may not associate with MuRF1 in vivo. Clearly, more investigation is needed to identify the in vivo substrates of MuRF1 and MAFbx under different atrophy conditions. Identification of the substrates will provide further understanding of the mechanisms by which MuRF1 and MAFbx regulate skeletal muscle size.
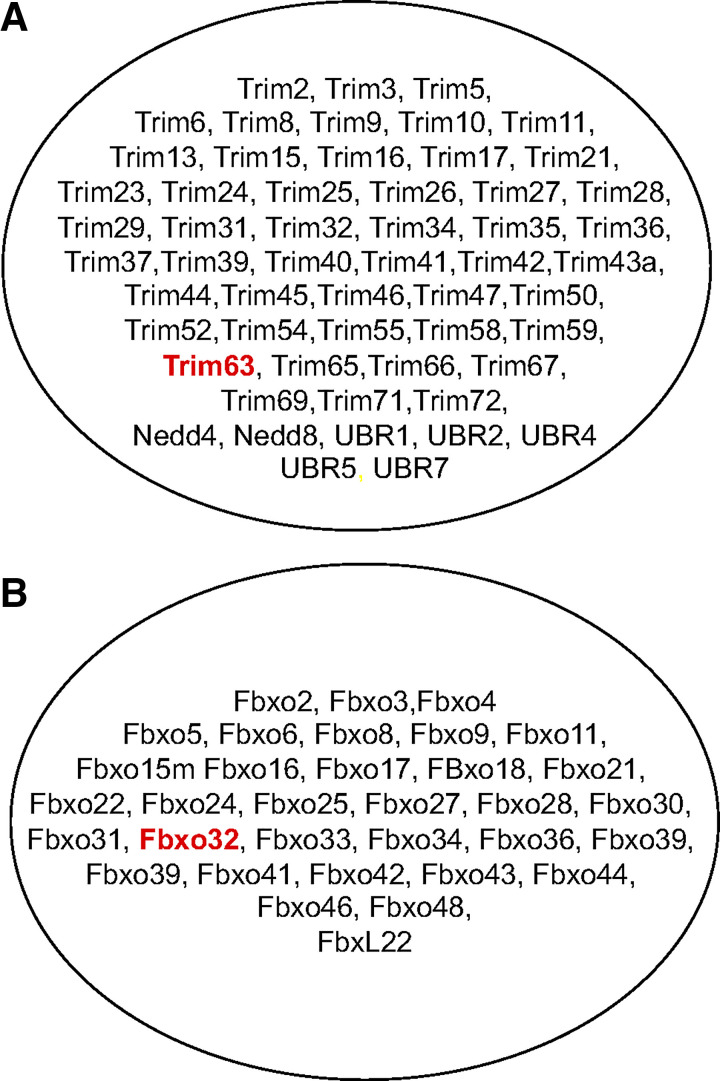
MuRF1 and MAFbx represent just two E3 ligases that are expressed in skeletal muscle (see Fig. 5). To date, only a handful of E3 ligases have been investigated in skeletal muscle under baseline or atrophy-inducing conditions. In addition to MuRF1 and MAFbx, the E3 ligases that have received the most attention, and been shown to play some role in atrophy, include Fbx30 (MUSA (70),), Trim 32 (17, 46, 47), and Nedd4 (45, 59). A major challenge for the field has been identifying the substrates targeted for ubiquitination by an individual E3 ligase and then understanding the effect of ubiquitination on the protein. While ubiquitination of a protein can target a protein for degradation by the proteasome, it is can also serve other functions such as altering the activity of the protein, altering the cellular localization of the protein, modifying protein-protein interactions and regulating transcriptional activity (20).
Fig. 5.
E3 Ubiquitin ligases expressed in skeletal muscle. There are over 600 E3 ligases in the human genome and they can be broadly classified into two types: RING finger E3s and the cullin-RING E3 ligase (CRL) superfamily. MuRF1 belongs to the Ring finger E3 family and MAFbx belongs to the cullin-RING E3 superfamily. MuRF1 (Trim63) and MAFbx (Fbxo32) represent just two of the many E3 ligases expressed in skeletal muscle. The majority of RING finger E3s (A) and cullin-RING E3s (B) expressed in skeletal muscle have not been studied in any detail.
SKELETAL MUSCLE ATROPHY IS A COMPLEX PROCESS
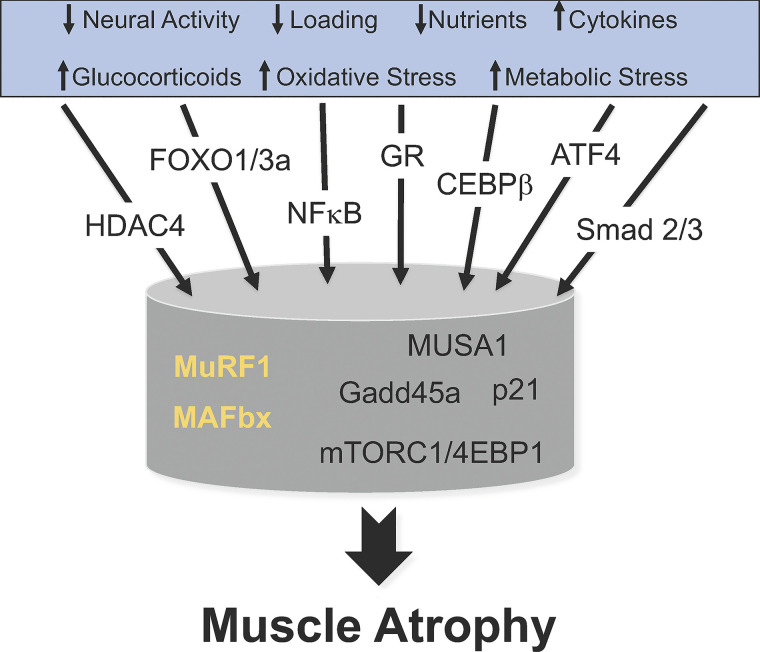
The loss of skeletal muscle mass is the final common outcome of many diseases and conditions. Over the past twenty years, a number of proteins and pathways have been identified as playing a role in the atrophy process (10, 23, 69, 71) (see Fig. 6). The E3 ubiquitin ligases, MuRF1 and MAFbx, are particularly interesting because they are relatively muscle specific, are expressed at low levels under resting conditions, and are upregulated under all forms of muscle atrophy. It is noteworthy that MuRF1 and MAFbx are upregulated under divergent atrophy-inducing conditions such as disuse, inflammation, metabolic stress, oxidative stress, and excess glucocorticoids; which is not true for all atrophy associated genes. For example, HDAC4 and Gadd45a are strongly upregulated in response to denervation and disuse, but not induced in response to glucocorticoid treatment (14, 27). There continues to be an ongoing search to determine whether there is a common set of genes responsible for muscle atrophy. To answer the question, investigations should examine multiple divergent atrophy models at multiple time points during the course of atrophy. It is possible that different stimuli such as decreased loading, inactivity, inflammation, metabolic stress or oxidative stress activate an array of initial factors (e.g., activated GR, FOXOs, ATF4, NF-κB, HDAC4, CEBPβ, Smad1/2) that activate some common pathways (e.g., MuRF1 and MAFbx) and some divergent pathways (e.g., increased Gadd45a and p21 expression, decreased mTORC1 activation, etc.) all leading to the activation of a common final program to increase the disassembly and degradation of myofibrils leading to a decrease in muscle fiber size and muscle atrophy. If this model is correct, then a single drug would likely not be able to treat all forms of atrophy. Furthermore, the timing of the treatment will be important. For example, following disuse and denervation-induced atrophy MuRF1 increases rapidly and then returns to baseline by 14 days. Therefore, the use of a MuRF1 inhibitor after 14 days may not be appropriate. However, deletion of MuRF1 continues to result in muscle sparing after 14 days of disuse or denervation, suggesting that MuRF1 upregulation initiates specific signaling cascades that initiate and sustain muscle atrophy. Thus, the critical missing data are the identification and verification of the in vivo substrates for MuRF1 and other E3 ligases which will provide insights into the mechanisms by which they regulate skeletal muscle mass.
Fig. 6.
Multiple pathways lead to muscle atrophy. This diagram illustrates that skeletal muscle atrophy is initiated by a variety of signals as listed in the blue box. These divergent signals activate a variety of transcription factors leading to the upregulation of a variety of genes and alterations in signaling pathways. The E3 ligases MuRF1 and MAFbx are highlighted since they are two genes that are upregulated in response to divergent signals such as disuse, inflammation, oxidative stress, and starvation. Other genes such as MUSA1, Gadd45a, and p21 appear to upregulate in response to specific signals. Suppression of the mTORC1 pathways and protein synthesis is a common response to many atrophy conditions; an exception being denervation. All of these pathways lead to a common outcome, the loss of muscle mass, i.e., muscle atrophy. A major challenge is to understand how these various pathways intersect to induce muscle atrophy.
CONCLUSIONS
Skeletal muscle atrophy continues to be a serious consequence of many diseases and conditions for which there is no treatment. The development of treatments relies on an understanding of the molecules and pathways involved in the atrophy process. Over the past two decades considerable progress has been made in our understanding of the molecular and cellular mechanisms underlying the loss of muscle mass. The data suggest that while the loss of muscle mass is a common outcome in many diseases, the pathways leading to the outcome may vary depending on the initiating signal, i.e., unloading, inactivity, elevated cytokines or malnutrition. Interestingly, the upregulation of the E3 ligases MuRF1 and MAFbx seems to be a common early event in many divergent atrophy conditions, e.g., inflammation vs disuse vs starvation. However, it is clear that upregulation of MuRF1 and MAFbx is not the complete picture since deletion of these genes does not result in the complete sparing of muscle mass. The challenge for the field of muscle biology is to determine the “network” of pathways involved in muscle atrophy and to understand how all of the various molecules, transcription factors and signaling pathways interact to produce muscle atrophy. Identification of the critical nodes that when suppressed result in a reduction or blockage of the atrophy process will be necessary for the successful development of new treatments.
GRANTS
This work was supported by grants awarded to S.C.B. by National Institutes of Health DK075801 AR070031 and the Veterans Administration 1I0RX000673.
DISCLOSURES
Dr. Bodine holds equity in Emmyon, Inc., and serves on the Scientific Advisory Board.
AUTHOR CONTRIBUTIONS
S.C.B. conceived and designed research, prepared figures, drafted, edited and revised, and approved final version of manuscript.
ACKNOWLEDGMENTS
Throughout my career, I have been fortunate to have worked with great colleagues at the University of California, Los Angeles; University of California, San Diego; Regeneron Pharmaceuticals; University of California, Davis; and most recently the University of Iowa. I would like to thank the many colleagues who have contributed to this research.
REFERENCES
- 1.Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol (1985) 81: 2509–2516, 1996. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- 2.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol (1985) 84: 1716–1722, 1998. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- 3.Arnold J, Campbell IT, Samuels TA, Devlin JC, Green CJ, Hipkin LJ, MacDonald IA, Scrimgeour CM, Smith K, Rennie MJ. Increased whole body protein breakdown predominates over increased whole body protein synthesis in multiple organ failure. Clin Sci (Lond) 84: 655–661, 1993. doi: 10.1042/cs0840655. [DOI] [PubMed] [Google Scholar]
- 4.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 5.Baehr LM, Furlow JD, Bodine SC. Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J Physiol 589: 4759–4776, 2011. doi: 10.1113/jphysiol.2011.212845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baehr LM, Tunzi M, Bodine SC. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol 5: 69, 2014. doi: 10.3389/fphys.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8: 127–146, 2016. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baehr LM, West DWD, Marshall AG, Marcotte GR, Baar K, Bodine SC. Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J Appl Physiol (1985) 122: 1336–1350, 2017. doi: 10.1152/japplphysiol.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaauw B, Reggiani C. The role of satellite cells in muscle hypertrophy. J Muscle Res Cell Motil 35: 3–10, 2014. doi: 10.1007/s10974-014-9376-y. [DOI] [PubMed] [Google Scholar]
- 10.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab 307: E469–E484, 2014. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodine SC, Latres E, Baumhueter S, Lai VK-M, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan Z-Q, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 12.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 13.Bollinger LM, Witczak CA, Houmard JA, Brault JJ. SMAD3 augments FoxO3-induced MuRF-1 promoter activity in a DNA-binding-dependent manner. Am J Physiol Cell Physiol 307: C278–C287, 2014. doi: 10.1152/ajpcell.00391.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bongers KS, Fox DK, Ebert SM, Kunkel SD, Dyle MC, Bullard SA, Dierdorff JM, Adams CM. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab 305: E907–E915, 2013. doi: 10.1152/ajpendo.00380.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185: 1083–1095, 2009. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Zhai B, Gygi SP, Goldberg AL. Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. J Cell Biol 198: 575–589, 2012. doi: 10.1083/jcb.201110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270: 12109–12116, 1995. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 19.Condon KJ, Sabatini DM. Nutrient regulation of mTORC1 at a glance. J Cell Sci 132: jcs222570, 2019. doi: 10.1242/jcs.222570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem 78: 399–434, 2009. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 21.Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 199: 71–81, 2010. doi: 10.1111/j.1748-1716.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebert SM, Al-Zougbi A, Bodine SC, Adams CM. Skeletal muscle atrophy: discovery of mechanisms and potential therapies. Physiology (Bethesda) 34: 232–239, 2019. doi: 10.1152/physiol.00003.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egner IM, Bruusgaard JC, Gundersen K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 143: 2898–2906, 2016. doi: 10.1242/dev.134411. [DOI] [PubMed] [Google Scholar]
- 25.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 26.Files DC, D’Alessio FR, Johnston LF, Kesari P, Aggarwal NR, Garibaldi BT, Mock JR, Simmers JL, DeGorordo A, Murdoch J, Willis MS, Patterson C, Tankersley CG, Messi ML, Liu C, Delbono O, Furlow JD, Bodine SC, Cohn RD, King LS, Crow MT. A critical role for muscle ring finger-1 in acute lung injury-associated skeletal muscle wasting. Am J Respir Crit Care Med 185: 825–834, 2012. doi: 10.1164/rccm.201106-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furlow JD, Watson ML, Waddell DS, Neff ES, Baehr LM, Ross AP, Bodine SC. Altered gene expression patterns in muscle ring finger 1 null mice during denervation- and dexamethasone-induced muscle atrophy. Physiol Genomics 45: 1168–1185, 2013. doi: 10.1152/physiolgenomics.00022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson JN, Smith K, Rennie MJ. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet 332: 767–770, 1988. doi: 10.1016/S0140-6736(88)92417-8. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg AL. Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem 244: 3223–3229, 1969. [PubMed] [Google Scholar]
- 30.Goldberg AL, Goodman HM. Relationship between cortisone and muscle work in determining muscle size. J Physiol 200: 667–675, 1969. doi: 10.1113/jphysiol.1969.sp008715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes AV, Waddell DS, Siu R, Stein M, Dewey S, Furlow JD, Bodine SC. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB J 26: 2986–2999, 2012. doi: 10.1096/fj.12-204495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman CA. Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. J Appl Physiol (1985) 127: 581–590, 2019. doi: 10.1152/japplphysiol.01011.2018. [DOI] [PubMed] [Google Scholar]
- 34.Gundermann DM, Walker DK, Reidy PT, Borack MS, Dickinson JM, Volpi E, Rasmussen BB. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 306: E1198–E1204, 2014. doi: 10.1152/ajpendo.00600.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guridi M, Kupr B, Romanino K, Lin S, Falcetta D, Tintignac L, Rüegg MA. Alterations to mTORC1 signaling in the skeletal muscle differentially affect whole-body metabolism. Skelet Muscle 6: 13, 2016. doi: 10.1186/s13395-016-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ham AS, Chojnowska K, Tintignac LA, Lin S, Schmidt A, Ham DJ, Sinnreich M, Rüegg MA. mTORC1 signalling is not essential for the maintenance of muscle mass and function in adult sedentary mice. J Cachexia Sarcopenia Muscle 11: 259–273, 2020. doi: 10.1002/jcsm.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes SM, Schiaffino S. Control of muscle fibre size: a crucial factor in ageing. Acta Physiol Scand 167: 307–312, 1999. doi: 10.1046/j.1365-201x.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 38.Hwee DT, Baehr LM, Philp A, Baar K, Bodine SC. Maintenance of muscle mass and load-induced growth in Muscle RING Finger 1 null mice with age. Aging Cell 13: 92–101, 2014. doi: 10.1111/acel.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackman RW, Wu C-L, Kandarian SC. The ChIP-seq-defined networks of Bcl-3 gene binding support its required role in skeletal muscle atrophy. PLoS One 7: e51478, 2012. doi: 10.1371/journal.pone.0051478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol 303: C854–C861, 2012. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseph GA, Wang SX, Jacobs CE, Zhou W, Kimble GC, Tse HW, Eash JK, Shavlakadze T, Glass DJ. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol Cell Biol 39: e00141-19, 2019. doi: 10.1128/MCB.00141-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HT, Kim KP, Lledias F, Kisselev AF, Scaglione KM, Skowyra D, Gygi SP, Goldberg AL. Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J Biol Chem 282: 17375–17386, 2007. doi: 10.1074/jbc.M609659200. [DOI] [PubMed] [Google Scholar]
- 43.Kimball SR, Jefferson LS. Mechanisms of translational control in liver and skeletal muscle. Biochimie 76: 729–736, 1994. doi: 10.1016/0300-9084(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 44.Kline WO, Panaro FJ, Yang H, Bodine SC. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J Appl Physiol (1985) 102: 740–747, 2007. doi: 10.1152/japplphysiol.00873.2006. [DOI] [PubMed] [Google Scholar]
- 45.Koncarevic A, Jackman RW, Kandarian SC. The ubiquitin-protein ligase Nedd4 targets Notch1 in skeletal muscle and distinguishes the subset of atrophies caused by reduced muscle tension. FASEB J 21: 427–437, 2007. doi: 10.1096/fj.06-6665com. [DOI] [PubMed] [Google Scholar]
- 46.Kudryashova E, Kudryashov D, Kramerova I, Spencer MJ. Trim32 is a ubiquitin ligase mutated in limb girdle muscular dystrophy type 2H that binds to skeletal muscle myosin and ubiquitinates actin. J Mol Biol 354: 413–424, 2005. doi: 10.1016/j.jmb.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 47.Kudryashova E, Wu J, Havton LA, Spencer MJ. Deficiency of the E3 ubiquitin ligase TRIM32 in mice leads to a myopathy with a neurogenic component. Hum Mol Genet 18: 1353–1367, 2009. doi: 10.1093/hmg/ddp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labeit S, Kohl CH, Witt CC, Labeit D, Jung J, Granzier H. Modulation of muscle atrophy, fatigue and MLC phosphorylation by MuRF1 as indicated by hindlimb suspension studies on MuRF1-KO mice. J Biomed Biotechnol 2010: 693741, 2010. doi: 10.1155/2010/693741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24: 9295–9304, 2004. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang F, Aravamudhan S, Nolte H, Türk C, Hölper S, Müller S, Günther S, Blaauw B, Braun T, Krüger M. Dynamic changes in the mouse skeletal muscle proteome during denervation-induced atrophy. Dis Model Mech 10: 881–896, 2017. doi: 10.1242/dmm.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99: 427–511, 2019. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li JB, Goldberg AL. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol 231: 441–448, 1976. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- 53.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 103: 1744–1751, 2007. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 54.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milan G, Romanello V, Pescatore F, Armani A, Paik J-H, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 6: 6670, 2015. doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905, 1996. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 57.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science 285: 2126–2129, 1999. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 58.Moore DR, Atherton PJ, Rennie MJ, Tarnopolsky MA, Phillips SM. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol (Oxf) 201: 365–372, 2011. doi: 10.1111/j.1748-1716.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- 59.Nagpal P, Plant PJ, Correa J, Bain A, Takeda M, Kawabe H, Rotin D, Bain JR, Batt JA. The ubiquitin ligase Nedd4-1 participates in denervation-induced skeletal muscle atrophy in mice. PLoS One 7: e46427, 2012. doi: 10.1371/journal.pone.0046427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Napolitano LM, Jaffray EG, Hay RT, Meroni G. Functional interactions between ubiquitin E2 enzymes and TRIM proteins. Biochem J 434: 309–319, 2011. doi: 10.1042/BJ20101487. [DOI] [PubMed] [Google Scholar]
- 61.Powers SK, Morton AB, Ahn B, Smuder AJ. Redox control of skeletal muscle atrophy. Free Radic Biol Med 98: 208–217, 2016. doi: 10.1016/j.freeradbiomed.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rennie MJ, Edwards RH, Emery PW, Halliday D, Lundholm K, Millward DJ. Depressed protein synthesis is the dominant characteristic of muscle wasting and cachexia. Clin Physiol 3: 387–398, 1983. doi: 10.1111/j.1475-097X.1983.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 63.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- 64.Reynolds TH IV, Bodine SC, Lawrence JC Jr. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277: 17657–17662, 2002. doi: 10.1074/jbc.M201142200. [DOI] [PubMed] [Google Scholar]
- 65.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009–1013, 2001. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 66.Roy RR, Baldwin KM, Edgerton VR. The plasticity of skeletal muscle: effects of neuromuscular activity. Exerc Sport Sci Rev 19: 269–312, 1991. doi: 10.1249/00003677-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Rudar M, Fiorotto ML, Davis TA. Regulation of muscle growth in early postnatal life in a swine model. Annu Rev Anim Biosci 7: 309–335, 2019. doi: 10.1146/annurev-animal-020518-115130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sartori R, Gregorevic P, Sandri M. TGFβ and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab 25: 464–471, 2014. 10.1016/j.tem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Sartori R, Schirwis E, Blaauw B, Bortolanza S, Zhao J, Enzo E, Stantzou A, Mouisel E, Toniolo L, Ferry A, Stricker S, Goldberg AL, Dupont S, Piccolo S, Amthor H, Sandri M. BMP signaling controls muscle mass. Nat Genet 45: 1309–1318, 2013. doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- 71.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280: 4294–4314, 2013. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 72.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J 17: 6649–6659, 1998. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S, Nakae J, Tagata Y, Nishitani S, Takehana K, Sano M, Fukuda K, Suematsu M, Morimoto C, Tanaka H. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab 13: 170–182, 2011. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol 586: 283–291, 2008. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 127: 547–553, 2014. doi: 10.1016/j.amjmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 77.Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol Cell Physiol 260: C475–C484, 1991. doi: 10.1152/ajpcell.1991.260.3.C475. [DOI] [PubMed] [Google Scholar]
- 78.Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab 295: E785–E797, 2008. doi: 10.1152/ajpendo.00646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watson ML, Baehr LM, Reichardt HM, Tuckermann JP, Bodine SC, Furlow JD. A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. Am J Physiol Endocrinol Metab 302: E1210–E1220, 2012. doi: 10.1152/ajpendo.00512.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, Beishuizen A. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care 18: R12, 2014. doi: 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weinkove D, Leevers SJ. The genetic control of organ growth: insights from Drosophila. Curr Opin Genet Dev 10: 75–80, 2000. doi: 10.1016/S0959-437X(99)00042-8. [DOI] [PubMed] [Google Scholar]
- 82.White RB, Biérinx AS, Gnocchi VF, Zammit PS. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol 10: 21, 2010. doi: 10.1186/1471-213X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams FP, Haubrich K, Perez-Borrajero C, Hennig J. Emerging RNA-binding roles in the TRIM family of ubiquitin ligases. Biol Chem 400: 1443–1464, 2019. doi: 10.1515/hsz-2019-0158. [DOI] [PubMed] [Google Scholar]
- 84.Wong TS, Booth FW. Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol (1985) 69: 1718–1724, 1990. doi: 10.1152/jappl.1990.69.5.1718. [DOI] [PubMed] [Google Scholar]
- 85.Wu CL, Kandarian SC, Jackman RW. Identification of genes that elicit disuse muscle atrophy via the transcription factors p50 and Bcl-3. PLoS One 6: e16171, 2011. doi: 10.1371/journal.pone.0016171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Y, Jemiolo B, Trappe S. Proteolytic mRNA expression in response to acute resistance exercise in human single skeletal muscle fibers. J Appl Physiol (1985) 101: 1442–1450, 2006. doi: 10.1152/japplphysiol.00438.2006. [DOI] [PubMed] [Google Scholar]
- 87.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab 265: E210–E214, 1993. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- 88.Zhang G, Jin B, Li YP. C/EBPβ mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. EMBO J 30: 4323–4335, 2011. doi: 10.1038/emboj.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang G, Li Y-P. p38β MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPβ. Skelet Muscle 2: 20, 2012. doi: 10.1186/2044-5040-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]