Abstract
Because elevated hemodynamic pulsatility could be mechanical stress against the brain, the dampening function of central and cerebral arteries is crucial. Regular endurance exercise training favorably restores the deteriorated dampening function of the aorta and carotid arteries in older populations, yet its effect on cerebrovascular dampening function remains unknown. To address this question, we compared cerebrovascular impedance, a frequency-domain relationship of the cerebral pressure and flow, in 21 middle-aged masters athletes who have been engaged in endurance training and races for >10 yr (MA, 53 ± 4 yr) with sedentary 21 age-matched (MS, 53 ± 5 yr) and 21 young (YS, 29 ± 6 yr) individuals. Using transfer function analysis, cerebrovascular impedance was computed from the simultaneously recorded carotid artery pressure (CAP, via applanation tonometry) and middle cerebral artery blood flow velocity (CBFV, via transcranial Doppler). In the frequency range of 0.78–3.12 Hz, coherence between pulsatile changes in CAP and CBFV was higher than 0.90 in all groups. All subjects exhibited the highest impedance modulus in the range of the first harmonic oscillations (0.78–1.56 Hz) mainly originating from cardiac ejection. Impedance modulus in this range was significantly lower in the MA than MS groups (0.88 ± 0.24 vs. 1.15 ± 0.29 mmHg·s/cm, P = 0.011) and equivalent to the YS (0.92 ± 0.30 mmHg·s/cm). Among middle-aged subjects, higher impedance modulus was correlated with lower mean CBFV (r = −0.776, P < 0.001) and cerebral cortical perfusion evaluated by MRI (r = −0.371, P = 0.015). These results suggest that middle-aged endurance athletes exhibited the significantly lower modulus of cerebrovascular impedance, which is associated with higher CBFV and cerebral cortical perfusion.
NEW & NOTEWORTHY Impedance modulus in the range of first harmonic oscillations (0.78–1.56 Hz), which reflects heart rate at rest, was lower in middle-aged endurance athletes than in age-matched sedentary peers and was similar to young individuals. Prolonged endurance training is associated with the improved cerebrovascular dampening function in middle-aged adults. Lower cerebrovascular impedance modulus may contribute to maintaining brain perfusion in midlife.
Keywords: arterial spin labeling, cerebral blood flow, cerebrovascular impedance, endurance training, transcranial Doppler
INTRODUCTION
Regular physical exercise is associated with better brain health such as improved cognitive performance (13) and reduced risk of cerebrovascular incidence in older adults (23). However, the current evidence has been inconsistent as to whether regular aerobic exercise or higher cardiorespiratory fitness can attenuate the age-related reduction in cerebrovascular function. Previously, we conducted a series of studies that compared lifelong old endurance athletes with sedentary old and young individuals using the well-accepted measures of cerebrovascular function (2, 39, 47). In these studies, we found that cortical cerebral blood flow (CCBF) measured by arterial spin labeling (ASL) using magnetic resonance imaging (MRI) was similar between the old athlete and sedentary groups and showed the age-related reductions in both of the old groups (39). The dynamic cerebral autoregulation (dCA) measured by transcranial Doppler (TCD) during repeated sit-stand maneuvers showed similar transfer function gain and phase between the old athlete and sedentary groups (2). Additionally, we reported from a different sample of healthy participants that these dCA functions are not significantly affected by aging (46). Moreover, cerebral vasomotor reactivity to carbon dioxide (CVMR) measured by TCD and blood-oxygen level-dependent imaging using MRI showed either no difference between the old athlete and sedentary groups (47) or were lower in the athletes (39), as consistently reported from a recent study from different research group (18). Besides, the aging effects on CVMR have been inconsistent such that older adults showed lower (5) and higher CVMR (40) than young adults, and these inconsistencies are likely to be related to the lack of standardized protocol for CVMR assessment. Therefore, these previous findings collectively suggest that regular aerobic exercise may only have limited impact on the conventional measures of cerebrovascular function (e.g., global perfusion, dCA, CVMR) in aging humans.
Cerebrovascular impedance represents the cushioning ability or the Windkessel function of cerebral vasculature to dampen arterial pulsatility (48). The obvious feature of “blood flow in arteries” is that it is pulsatile, which originates from cardiac ejections (28). Recently, the pathophysiological importance of pulsatile hemodynamics (of flow as well as pressure) has increasingly been recognized (29). To date, it is well known that central elastic arteries (e.g., aorta and carotid artery) attenuate blood pressure and flow pulsatility by expanding and recoiling with cyclic cardiac ejections, which smoothen blood flow in the capillaries and may protect the brain that is continuously and passively perfused with a high volume of the blood due to low vascular resistance. Moreover, a recent study suggests that intracranial arteries and arterioles also have the ability to buffer hemodynamic pulsations (42). The Windkessel function of vasculature is partly determined by the smooth muscle tone, which is often evaluated by vascular resistance or resistance index (=mean arterial pressure/mean blood flow volume or velocity). However, these indices assume the pressure-flow relationship under the steady (nonoscillatory) condition. Currently, the limited number of studies tried to quantify the pulsatile pressure-flow relationships (e.g., compliance) using TCD and ASL using MRI (15). However, these methods may be insufficient for describing pressure-flow relationships in a system where pressure at any given instant depends not only on flow at the same instant but also on flow at previous instants (28). In this context, impedance (or “input impedance”) may be suitable for quantifying pulsatile pressure-flow relationships as an index of compliance and Windkessel function. We previously reported that modulus of cerebrovascular impedance in the frequency range of 0.78–4 Hz is higher in the elderly than in the young individuals (48), which at least in part suggests that cerebrovascular compliance is lower in older adults.
Developing the preventive strategies against age-related vascular dysfunction is of pathophysiological importance because it may contribute to the reduced risks of cerebrovascular and neurological diseases in late life. Currently, it is well established that regular endurance exercise can restore the impaired compliance of large conduit arteries (17, 34, 35, 37). Therefore, it is reasonable to speculate that regular endurance exercise also has a similar beneficial effect for improving cerebral arterial compliance (15). However, such an effect remains unclear in middle-aged and older individuals. Therefore, the present study aimed to examine the association between endurance exercise training and cerebrovascular impedance in middle-aged adults. We hypothesized that middle-aged endurance athletes would have a lower modulus of cerebrovascular impedance than the age-matched sedentary peers.
METHODS
Subjects.
We recruited 21 middle-aged masters athletes (MA, 46–61 yr, mean age of 53 ± 4 yr, 10 women) who have been engaging in endurance training and competitions. To investigate the associations with age and exercise training status, we randomly selected 21 middle-aged sedentary (MS, 45–63 yr, mean age of 53 ± 5 yr, 10 women) and 21 young sedentary adults (YS, 22–38 yr, mean age of 29 ± 6 yr, 11 women) from the cohort reported by previous studies (45, 46), while age and sex were matched across three groups. Masters athletes were participating in more than 10 yr of regular endurance training for competing in events (e.g., marathon, triathlon, ironman race). On average, they have been training for 22.9 ± 9.6 yr with a recent history of 10.8 ± 4.7 h of exercise per week. Sedentary subjects were not participating in any structured exercise or physical activity program for the past 2 yr.
To focus on the impact of regular endurance training, we used the following exclusion criteria and tried to minimize the confounding effects of cardio- and cerebrovascular disease risk factors: body mass index (BMI) > 40 kg/m2, smoking, the presence of ischemic or structural heart disease screened by 12-lead electrocardiogram (ECG) and echocardiography, carotid artery atherosclerotic plaque or stenosis with > 50% occlusion evaluated by ultrasound image, use of antidiabetic drugs or fasting blood glucose > 6.9 mmol/L, active alcohol or drug abuse, brain damage or trauma, and the presence or history of cerebrovascular (e.g., stroke), neurological, psychiatric, or inflammatory diseases. Pregnant or breastfeeding women were also excluded. This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas and was performed following the guidelines of the Declaration of Helsinki and Belmont Report. Written informed consent was signed by all subjects for study participation.
Study protocol and measurements.
Each subject underwent 1) vascular measurements, 2) MRI scanning, and 3) cardiorespiratory fitness evaluation on separate days. Subjects were instructed to abstain from caffeinated beverages, alcohol, and vigorous exercise for at least 24 h before vascular and MRI sessions. On the day of cardiorespiratory fitness evaluation, subjects were allowed to have a light meal a couple of hours before the test. All data were measured in an ambient temperature-controlled laboratory (≈ 22°C).
Vascular measurements were performed in the supine position after quiet supine resting for ≥10 min. Heart rate was measured by ECG (via the three-lead system, Hewlett-Packard). Cerebrovascular impedance was calculated from the simultaneously recorded changes in carotid arterial pressure (CAP) and cerebral blood flow velocity (CBFV) at the right middle cerebral artery (MCA), as previously reported (48). The MCA was chosen because it is an extension of the carotid artery, and the distance between the two measurement sites is relatively short (~10 cm). The CAP waveform was recorded with applanation tonometry and calibrated offline using the following procedures (20, 22). First, brachial cuff systolic and diastolic blood pressures (SBP and DBP) were measured in triplicate from the right upper arm (Suntech Medical Instruments 4240). Next, brachial artery pressure waveform was measured via applanation tonometry (SphygmoCor 8.0; AtCor Medical) and used to calculate mean arterial pressure (MAP) based on its time-averaged integral. Subsequently, CAP waveform was calibrated to the brachial DBP and MAP. CBFV was measured at the right middle cerebral artery via transcranial Doppler (TCD) ultrasonography (Multi-Dop X2; DWL). End-tidal CO2 (ETCO2, via a nasal cannula using capnography, Capnogard; Novametrix) was also simultaneously measured. All signals were stored on a computer using a commercial software package for data acquisition (AcqKnowledge 4.2, Biopac Systems, Inc.) with a sampling frequency of 1 kHz.
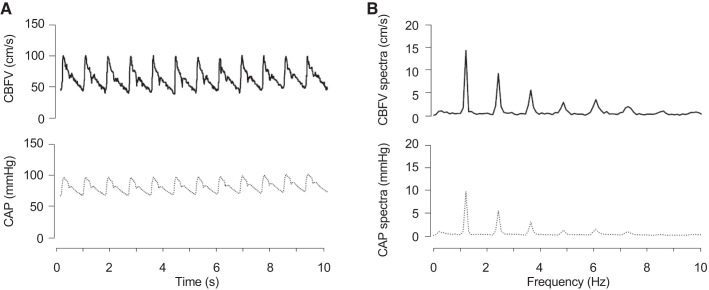
Cerebrovascular impedance was calculated as we previously reported (48). In brief, harmonic components of CAP and CBFV were extracted by the Fourier analysis and then transfer function analysis was performed (Fig. 1) (7). For the latter analysis, changes in CBFV and CAP were used as “input” and “output” signals, respectively. Auto-spectra and cross-spectra of CBFV and CAP were estimated using the Welch algorithm (44). Time series of CBFV and CAP waveforms were resampled at 100 Hz, and the mean values of time series were removed. Then, these data were subdivided into 256-point segments (2.56 s), with a 50% overlap for spectral estimation. To reduce the potential effects of including fractional cardiac cycles in these data segments on spectral estimation, each data segment was multiplied by a Hamming window before the periodogram estimation and averaged (26). This process resulted in a spectral resolution of 0.39 Hz for impedance estimation. The modulus of cerebrovascular impedance quantifies the magnitude relationship between changes in CBFV and CAP. The coherence function represents the strength of the linear relationship between CBFV and CAP, with 1 being perfect linearity. Because the first harmonic oscillations of CBFV and CAP mainly originating from cardiac ejection were the highest in the range of 0.78–1.56 Hz (Fig. 1), averaged impedance modulus in this range was obtained as the value corresponding to the first harmonic oscillations (Z1) for group comparisons. In addition to these impedance measures, cerebrovascular resistance index (CVRi = mean CAP/mean CBFV) and cerebral cortical vascular resistance [CCVR = mean CAP/mean cerebral cortical perfusion (CCP), see below] were obtained as time-domain indices of vascular tone.
Fig. 1.
A representative data set showing time series of cerebral blood flow velocity (CBFV) and carotid arterial pressure (CAP) (A) and spectra of CBFV and CAP (B).
Cerebral cortical perfusion (CCP) was measured by two-dimensional (2-D) pseudo-continuous arterial spin labeling (PCASL) using a 3-Tesla MRI scanner (Philips Medical System, Best, The Netherlands). The PCASL data were acquired with multislice single-shot 2-D echoplanar imaging (EPI) sequence (label duration = 1,650 ms, postlabel delay = 1,525 ms, time of repetition = 4,260 ms, echo time = 14 ms, EPI factor = 35 ms, voxel size = 3 × 3 × 5 mm, field of view = 240 × 240 × 145 mm, slice number = 29). A total of 40 pairs of control and labeled images were acquired to average and increase the signal-to-noise ratio. Additionally, a three-dimensional magnetization-prepared rapid acquisition gradient echo (MPRAGE) image was collected using the following parameters: echo time/time of repetition = 3.7/8.1 ms, flip angle = 12°, field of view = 256 × 256 mm, number of slices = 160 (no gap), resolution = 1 × 1 × 1 mm3, and sensitivity encoding (SENSE) factor = 2. The PCASL and MPRAGE data were processed by the FMRIB Software Library (FSL version 5.0, Oxford University, UK; https://www.fmrib.ox.ac.uk/fsl) and the FreeSurfer software (version 6.0, Boston, MA; https://surfer.nmr.mgh.harvard.edu/), respectively. CCP was calculated according to the recommended procedure of PCASL processing (3). After preprocessing steps, individual perfusion maps were registered to their native MPRAGE space and then CCP data were extracted from the cerebral cortex segmented by the FreeSurfer’s cross-sectional analysis pipeline (i.e., recon-all).
Maximal oxygen uptake (V̇o2max), an index of cardiorespiratory fitness, was measured by a modified Astrand–Saltin protocol using a treadmill (4, 6). The treadmill grade was increased by 2% every 2 min until exhaustion while subjects walked, jogged, or ran at a fixed speed. The speeds were selected based on the individual subject’s cardiorespiratory fitness level, which was assessed via a submaximal exercise test conducted before V̇o2max testing. The submaximal protocol was conducted to find a treadmill speed for V̇o2max testing based on the individual subject’s fitness level. During the protocol, treadmill speed was increased in a stepwise fashion until the subject’s heart rate reached 40–50 beats/min below the age-predicted maximal heart rate (i.e., 220 − age) while V̇o2, carbon dioxide production, and respiratory exchange ratio were continuously monitored. Exercise blood pressure, 12-lead ECG, and heart rate were monitored continuously by a registered nurse or a board-certified cardiologist. The criteria to confirm that V̇o2max was achieved included an increase in V̇o2 <150 mL despite increasing work rate of 2% grade, a respiratory exchange ratio ≥1.1, and heart rate within 5 beats/min of age-predicted maximal values. In all cases, at least two of these criteria were achieved, confirming the identification of V̇o2max based on the American College of Sports Medicine guidelines (31). V̇o2 was measured during the second minute of each stage using the Douglas bag method (12). Gas fractions were analyzed by mass spectrometry (Marquette MGA 1100), and ventilator volume was measured by a Tissot spirometer. Mass spectrometry and gas sampling system were calibrated before each test to ensure measurement accuracy and reliability. V̇o2max was defined as the highest V̇o2 measured from a >30-s Douglas bag during the last stage of testing.
Statistical analysis.
One-way analysis of variance (ANOVA) was used for comparing continuous variables among the three groups. For cerebrovascular impedance indices (e.g., modulus and coherence), mixed ANOVA was applied to evaluate the interaction effect of group (between-subject effect) and frequency (within-subject effect). Additionally, analysis of covariance (ANCOVA) with age, sex, and BMI as covariates was performed for comparisons of Z1, CVRi, and CCVR. The Bonferroni post hoc test was applied when statistical significance was observed for the main or interaction effect. The Pearson’s product-moment correlation analysis was used to determine the relationship between the time- and frequency-domain hemodynamic variables. All statistical analyses were performed using Statistica 64 version 12 (DELL Inc., Oklahoma City, OK). Data are presented as means and SD unless otherwise stated. The significance level was set a priori to P < 0.05.
RESULTS
The MS group showed greater body weight (P = 0.03 vs. YS; P = 0.07 vs. MA) and BMI (P < 0.001 vs. YS; P < 0.001 vs. MA) than the other groups (Table 1). As expected, MA participants had significantly higher V̇o2max than the other groups (P < 0.001 for both), and the YS showed higher V̇o2max than the MS group (P < 0.001).
Table 1.
Subject characteristics
| MA | MS | YS | P Value | |
|---|---|---|---|---|
| n (men/women) | 21 (11/10) | 21 (11/10) | 21 (10/11) | |
| Age, yr | 53.4 ± 4.4 | 52.9 ± 4.5 | 29.2 ± 5.6*† | <0.001 |
| Height, cm | 173.1 ± 7.7 | 170.3 ± 15.1 | 171.2 ± 9.7 | 0.716 |
| Weight, kg | 72.3 ± 11.5 | 82.0 ± 17.2* | 70.8 ± 14.9† | 0.020 |
| BMI, kg/m2 | 24.0 ± 3.1 | 28.4 ± 3.9* | 23.9 ± 3.5† | <0.001 |
| V̇o2max, mL·kg−1·min−1 | 43.0 ± 5.4 | 27.2 ± 4.6* | 34.7 ± 6.3*† | <0.001 |
Values are means ± SD. BMI, body mass index; MA, masters athlete; MS, middle-aged sedentary; YS, young sedentary; V̇o2max, maximal oxygen uptake.
P < 0.05 vs. MA;
P < 0.05 vs. MS.
Table 2 summarizes the results of cerebral and systemic hemodynamic variables. Heart rate was significantly lower in MA than the other groups (P = 0.001 vs. MS; P < 0.001 vs. YS). The brachial SBP and pulse pressure (PP) were similar across groups, whereas MS group had higher brachial MAP (P = 0.002) and DBP (P = 0.003) than the YS group. Carotid SBP was significantly higher in the MS compared with YS groups (P = 0.004), whereas no difference in carotid PP was observed among groups. Systolic CBFVs were significantly higher in MA and YS than in MS groups (P = 0.004 and P < 0.001, respectively). Systolic and pulsatile CBFVs were significantly higher in MA (P = 0.004 and P < 0.001, respectively) and YS (P = 0.01 and P = 0.02, respectively) than in MS groups. Mean CBFV was significantly higher in YS than MS group (P = 0.04), whereas diastolic CBFVs were similar among groups. CCP was lower in the MS compared with YS group (P = 0.03), although it was not different between the YS and MA groups (P = 0.22).
Table 2.
Cerebral and systemic hemodynamic variables
| MA | MS | YS | P Value | |
|---|---|---|---|---|
| Heart rate, beats/min | 52 ± 9 | 62 ± 8* | 64 ± 8* | <0.001 |
| Brachial SBP, mmHg | 112 ± 11 | 114 ± 12 | 107 ± 10 | 0.140 |
| Brachial MAP, mmHg | 86 ± 8 | 90 ± 9 | 81 ± 7† | 0.003 |
| Brachial DBP, mmHg | 70 ± 7 | 73 ± 7 | 66 ± 6† | 0.004 |
| Brachial PP, mmHg | 42 ± 7 | 41 ± 7 | 42 ± 8 | 0.905 |
| Carotid SBP, mmHg | 107 ± 11 | 114 ± 13 | 102 ± 12† | 0.006 |
| Carotid PP, mmHg | 37 ± 7 | 41 ± 9 | 36 ± 8 | 0.112 |
| Systolic CBFV, cm/s | 96.8 ± 26.3 | 77.1 ± 13.2* | 94.8 ± 16.3† | 0.003 |
| Mean CBFV, cm/s | 62.9 ± 16.4 | 52.9 ± 9.9 | 63.6 ± 13.9† | 0.023 |
| Diastolic CBFV, cm/s | 39.3 ± 10.2 | 34.5 ± 6.9 | 41.6 ± 11.1 | 0.055 |
| Pulsatile CBFV, cm/s | 57.5 ± 17.4 | 42.6 ± 8.2* | 53.2 ± 9.7† | <0.001 |
| CCP, mL·100 g−1·min−1 | 48.6 ± 8.2 | 46.6 ± 7.1 | 53.4 ± 9.9† | 0.036 |
| ETCO2, mmHg | 37.0 ± 4.1 | 37.8 ± 3.4 | 38.1 ± 4.5 | 0.688 |
Values are means ± SD. CBFV, cerebral blood flow velocity; CCP, cerebral cortical perfusion; DBP, diastolic blood pressure; ETCO2, end-tidal CO2; MA, masters athlete; MAP, mean arterial pressure; MS, middle-aged sedentary; PP, pulse pressure; SBP, systolic blood pressure; YS, young sedentary.
P < 0.05 vs. MA;
P < 0.05 vs. MS.
Figure 2 presents the frequency plots of cerebrovascular impedance modulus and coherence. In this study, we focused on the frequency range from 0.78–3.12 Hz because average coherence was greater than 0.90 in all groups (MA, 0.962 ± 0.04; MS, 0.973 ± 0.02; YS, 0.974 ± 0.02). In this frequency range, impedance modulus showed significant effects of group (P = 0.002) and frequency (P < 0.001) without interaction effect (P = 0.209). The between-group post hoc comparisons further showed that MA and YS have significantly higher modulus than the YS groups (P = 0.003 and P = 0.02, respectively). The within-subject frequency comparisons exhibited the highest impedance modulus around the first heart rate harmonic (0.78–1.56 Hz).
Fig. 2.
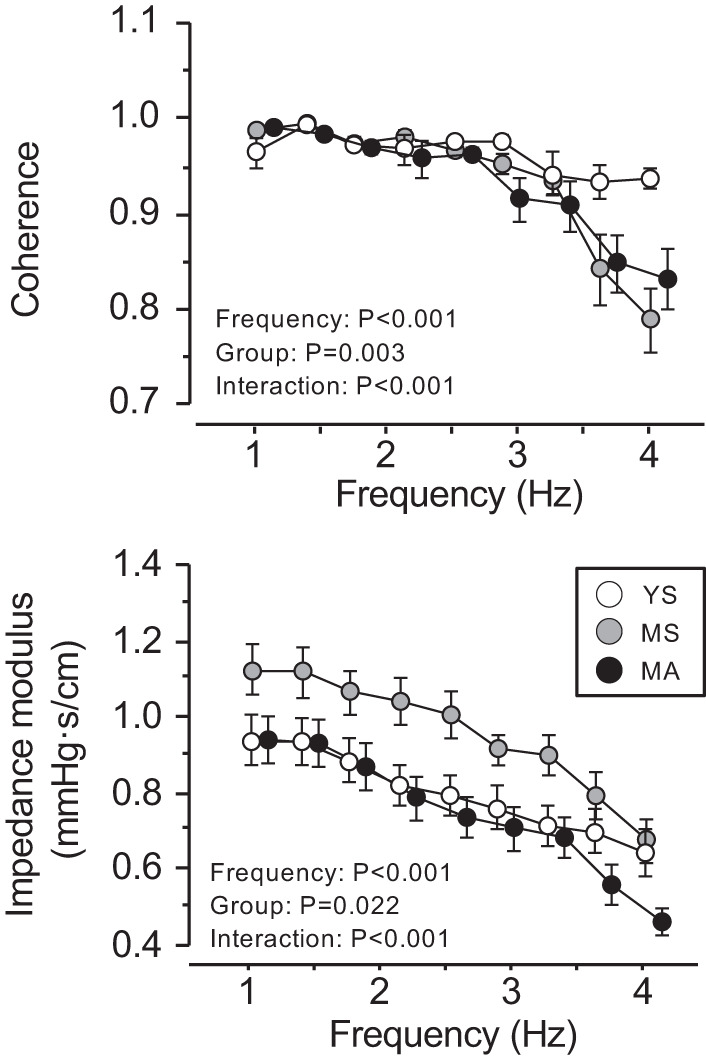
Group-averaged frequency plots of cerebrovascular impedance modulus and coherence. Data are means ± SE. YS, MS, and MA indicate young sedentary (10 men, 11 women), middle-aged sedentary (11 men, 10 women), and masters athletes (11 men, 10 women), respectively.
Figure 3 shows group comparisons of CVRi, CCVR, and impedance modulus at the first heart rate harmonic oscillation (Z1). These indices in the MS group were significantly higher than those in YS group. CVRi and CCVR in the MA group were not different from those in the MS group, whereas Z1 was significantly lower in MA than MS groups (0.88 ± 0.24 vs. 1.15 ± 0.29 mmHg·s/cm, P = 0.011) and equivalent to the YS (0.92 ± 0.30 mmHg·s/cm). After adjustment for age, sex, and BMI, Z1 and CVRi in the MA group was significantly lower than in the MS group (P = 0.002 and P = 0.035, respectively) and equivalent to the YS, whereas group difference in CCVR was no longer significant (P = 0.555).
Fig. 3.
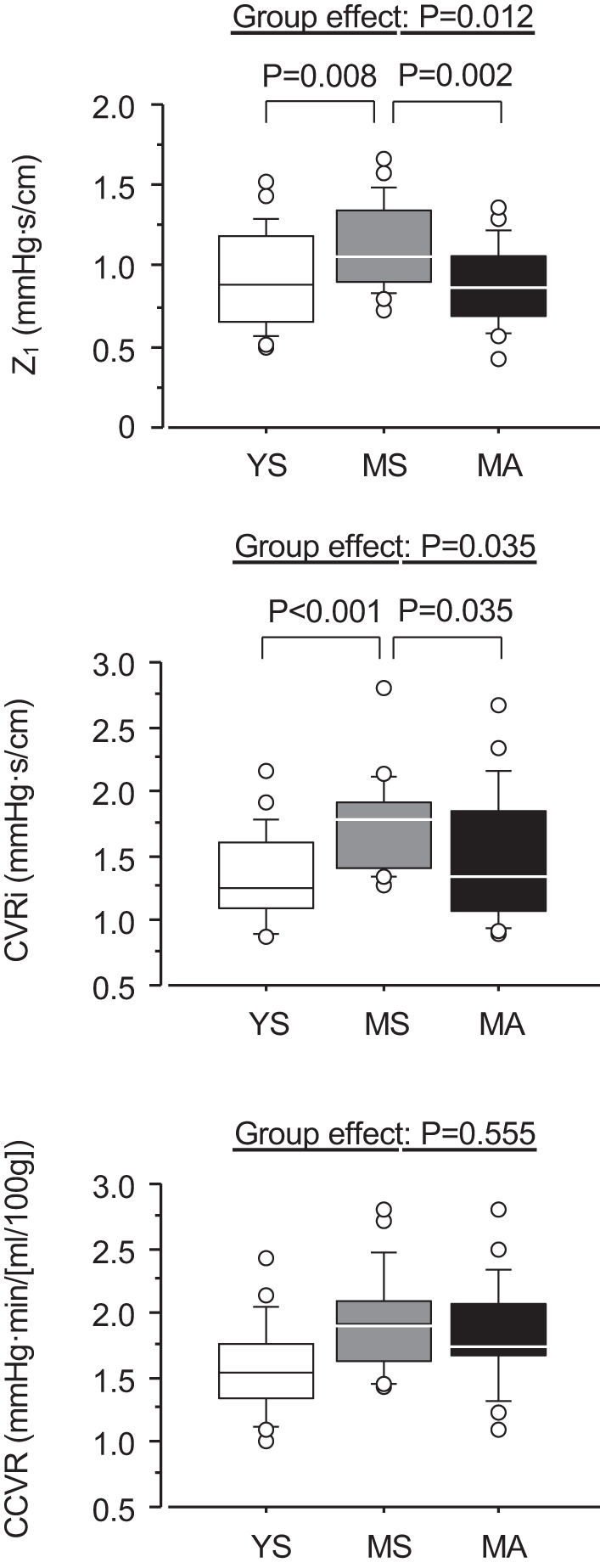
Comparisons of cerebrovascular hemodynamics. YS, MS, and MA indicate young sedentary (10 men, 11 women), middle-aged sedentary (11 men, 10 women), and masters athletes (11 men, 10 women), respectively. P values are results of analysis of covariance with age, sex, and body mass index as covariates and Bonferroni test. CCVR, cerebral-cortex vascular resistance; CVRi, cerebrovascular resistance index; Z1, cerebrovascular impedance modulus at the first harmonics.
Figure 4 presents the relations of Z1 with CBFV and CCP. Z1 was negatively correlated with mean (r = −0.775, P < 0.001), systolic (r = −0.772, P < 0.001), diastolic (r = −0.666, P < 0.001), and pulsatile (r = −0.704, P < 0.001) CBFV as well as CCP (r = −0.326, P = 0.009).
Fig. 4.
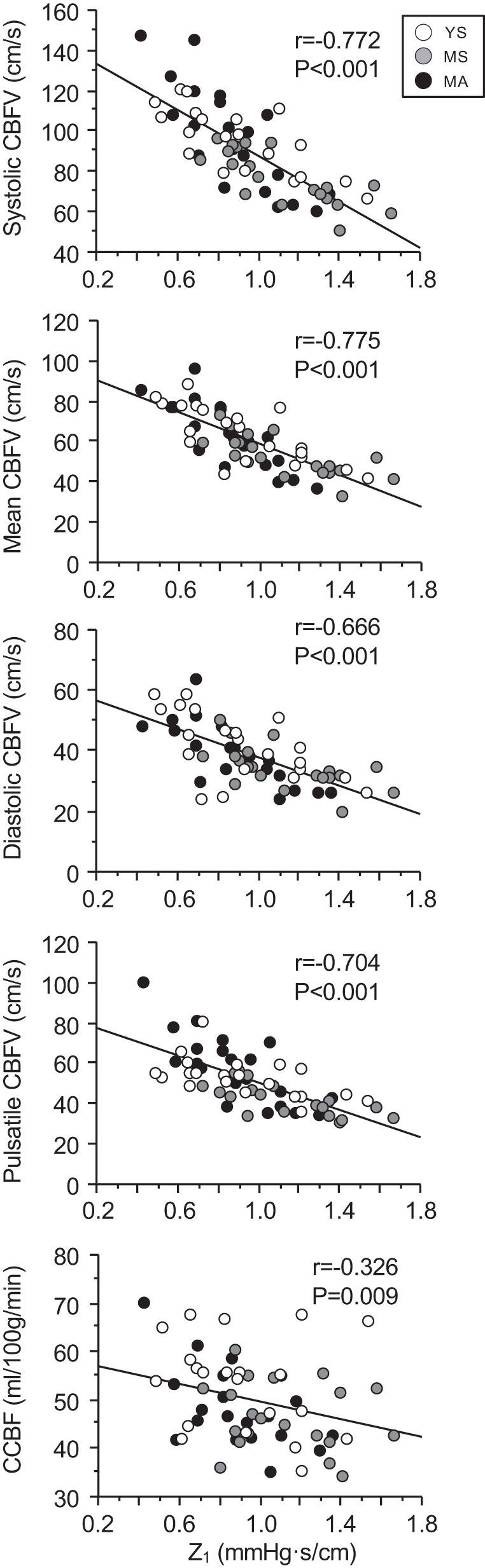
Correlations between cerebrovascular impedance modulus at the first heart rate harmonic (Z1) and cerebral blood flow velocity (CBFV) and cerebral cortical perfusion (CCP). YS, MS, and MA indicate young sedentary (10 men, 11 women), middle-aged sedentary (11 men, 10 women), and masters athletes (11 men, 10 women), respectively.
DISCUSSION
The primary findings of the present study are twofold. First, in middle-aged athletes who have been training for at least 10 yr of endurance exercise, Z1 was lower than the age-matched sedentary adults and equivalent to young sedentary individuals. Second, the lower impedance modulus was negatively correlated with higher levels of CBFV and CCP. These results suggest that prolonged endurance training is associated with the improved cerebrovascular dampening function, which may contribute to higher brain perfusion in the endurance-trained middle-aged adults.
We previously demonstrated the feasibility of assessing cerebrovascular impedance, a frequency-domain pressure-flow relationship, by noninvasive CBFV recording from the MCA (via transcranial Doppler) and the carotid arterial pressure (via applanation tonometry) (48). Fourier analysis indicated that the first harmonic of CBFV and CAP corresponding to the heart rate at rest has the highest value (48). The following transfer function analysis showed that coherence between pulsatile changes in CAP and CBFV was also highest in the frequency range of 0.78–1.56 Hz. These results support the validity of using the transfer function method and demonstrate a strong linear relationship between pulsatile changes in CBFV and arterial pressure at the first heart rate harmonic.
The findings of this study provide several important implications. First, MS participants have significantly higher Z1 than the YS group by ~20%. We previously reported that when compared with young adults, impedance modulus at the range of 0.78–2 Hz is higher by 38% in the elderly aged 70 yr (48). Thus, the current finding fills the missing age range (i.e., middle age). The cerebrovascular impedance modulus quantifies the magnitude relationship between pulsatile changes in CBFV and CAP. Because the largest energy of signals was contained at the first harmonic (<1.56 Hz), which is associated with the heart rate frequency at rest, elevated Z1 may reflect deterioration of buffering ability of the cerebral vasculature against pulsatility. Stiffening of the intracranial arteries may start during early life as central elastic arteries (e.g., aorta and carotid arteries) lose their elasticity (36, 37). The deterioration of the cerebrovascular buffering ability could facilitate penetration of pulsatile blood pressure and flow into the cerebral microvasculature (29), which consequently may lead to brain structural and functional abnormalities (11, 27, 29, 30). In late life, central arterial compliance can be modified through regular endurance training. Therefore, consistent with the central arteries, our findings suggest similar benefits of regular endurance training for increasing cerebrovascular compliance in middle-aged adults.
Second and more importantly, MA exhibited significantly lower Z1 than the MS group, and the average value was similar to that of the YS group. A recent study demonstrated that higher cardiorespiratory fitness is associated with greater cerebral arterial compliance in early adulthood (15), yet the beneficial effect on cerebrovascular compliance has been unclear in middle-aged and older populations. Thus, this is one of the first studies suggesting that endurance training may mitigate the age-related increase in cerebrovascular impedance modulus (i.e., improved cerebrovascular compliance). In this study, we also observed that MA participants have higher systolic and pulsatile CBFV than the MS group, which is likely to reflect greater stroke volume at rest in the MA group. In the literature, the excessive elevation of CBF pulsatility has been shown to correlate with cerebral microvascular damage (9, 27). So, does our finding indicate that higher CBFV pulsatility in MA participants is unfavorable training-induced adaptation? In this regard, we (38) have previously shown that when analyzing the correlation of white matter hyperintensity volume (i.e., an MRI marker of cerebral small vessel disease) with systolic and diastolic CBFV separately (10), the correlation was stronger with diastolic CBFV. These results suggest that repetitive cerebral hypoperfusion during diastole might pathophsysiologically be more important than greater pulsatility per se. In this context, our MA group showed a similar level of diastolic CBFV to the YS group as shown in Table 2. Besides, lower Z1 was correlated with higher systolic and pulsatile CBFV (Fig. 4). Therefore, these findings collectively suggest that MA group has an increased transmission of cardiovascular pulsatility into the brain without the reduction of diastolic CBFV, and such hemodynamic adaptation may partly be supported by higher cerebrovascular compliance.
Third, age-related reduction of cerebral perfusion might be an early risk factor for late-life neurodegenerative disorders such as Alzheimer’s disease (24). Conversely, aerobic exercise training may attenuate age-related cognitive decline and reduce the future risk of dementia (33). Furthermore, emerging evidence suggests that exercise-related improvement in cognitive function is correlated with changes in cerebrovascular regulation (16). Nevertheless, the current evidence is still limited as to the exact cerebrovascular mechanism by which aerobic exercise training improves brain health in the aged humans. In this regard, our previous studies that compared old endurance athletes and sedentary adults did not clearly demonstrate the exercise-related benefits for cerebrovascular function, such as global perfusion, dCA, and CVMR (2, 39, 47). On the other hand, the current findings that lower Z1 is associated with higher mean CBFV and CCP among middle-aged individuals potentially suggest that the lowering effects of endurance training on Z1 may partly contribute to maintaining brain perfusion in midlife. Our findings could further support and expand the notion that exercise is good for the brain health. To confirm our findings, the future trials using exercise training intervention are needed to address whether exercise-related reduction of cerebrovascular impedance can increase the perfusion and have neuroprotective benefits, particularly in the high-risk population with brain hypoperfusion (i.e., older populations and patients with cognitive impairment).
We can only speculate the mechanism by which regular endurance exercise improves cerebrovascular impedance or compliance in the present study. Intracranial arterial compliance may be determined by the intrinsic structural (i.e., the composition of elastin and collagen) and functional component, such as the vasoconstrictor tone exerted by its smooth muscle cells, as well as myogenic factor. The accommodative ability of microvasculature (i.e., size of vascular bed and endothelium-dependent vasodilatory function) may also be involved. Availability of nitric oxide declines with advancing age, which may lead to unfavorable alterations of vascular function, including vasoconstriction, increase in arterial blood pressure, and development of atherosclerosis in not only peripheral but also the cerebral arteries (19). Regular exercise could maintain the cerebrovascular endothelial function and contribute to an optimal regulation of cerebral blood flow (8).
Limitations.
Several study limitations should be mentioned. First, change in CBFV measured by TCD could be representative of change in flow with the assumption that the insonated arterial diameter remains constant. Although large fluctuations in MAP and ETCO2 have been shown to change the diameter of MCA (25, 43), we think that the physiological range of variation in MAP and ETCO2 during the resting condition will likely have a minor effect on the diameter. Second, “vascular impedance” usually refers to vascular input impedance, which quantifies the pressure-flow relationship at the input site of a given vascular bed; therefore, changes in pressure and blood flow should be measured simultaneously and at the same site. However, because of difficulty assessing pressure at the MCA, we used the carotid arterial pressure, which provided the highest amplitude signal as a surrogate for cerebral arterial pressure. Additionally, we measured CBFV at the MCA, a direct extension of the internal carotid artery, as we previously reported (48). Third, we did not collect menopausal status or control menstrual cycle phase in female participants. Therefore, we cannot rule out a possibility that our results are potentially confounded by the effect of sex hormones. To partially address this issue, we carefully but also randomly matched the age and sex of participants in each group using the cohort reported by our previous studies (45, 46). Also, because the influences of sex on systemic and cerebral circulation as well as arterial stiffness have been well perceived (1, 21, 45), we used ANCOVA to confirm that lower Z1 in the MA versus MS groups is not confounded by sex. Based on the literature, the impact of the menstrual cycle phase on cerebral hemodynamic regulation (i.e., mean CBFV, CVRi, and cerebral autoregulation) has also been reported to be small (14). Finally, because of the cross-sectional nature of the current study design, the potential influence of genetic and/or other lifestyle factors cannot be ruled out. Additionally, correlational and regression analyses cannot infer causal relations of measurements. However, the investigation on masters athletes may be one of the few methods to determine the long-term influence of exercise training on physiological adaptations (32, 41). Future studies need to confirm whether the age-related increase in cerebrovascular impedance in middle-aged and elderly populations could be prevented and recovered by chronic endurance exercise training.
In conclusion, middle-aged masters athletes who have been training for at least 10 yr of endurance exercise exhibited the significantly lower modulus of cerebrovascular impedance, which is associated with higher CBFV and cerebral cortical perfusion. These results support the concept that regular physical activity benefits cognitive functioning during early and late periods of the lifespan from the aspects of maintaining the ability to buffer cerebral hemodynamic pulsations as well as maintaining brain perfusion.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants K99-HL-133449 (T. Tarumi) and R01-HL-102457 (to R. Zhang), and Japan Society for the Promotion of Science (JSPS) KAKENHI Grant No. 16KK0011 (to J. Sugawara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T. Tarumi and R.Z. conceived and designed research; T. Tarumi, T. Tomoto, and J.R. performed experiments; J.S., T. Tarumi, T. Tomoto, and J.R. analyzed data; J.S., T. Tarumi, T. Tomoto, and R.Z. interpreted results of experiments; J.S. prepared figures; J.S. drafted manuscript; J.S., T. Tarumi, T. Tomoto, and R.Z. edited and revised manuscript; J.S., T. Tomoto, J.R., R.Z., and T. Tarumi approved final version of manuscript.
REFERENCES
- 1.Aanerud J, Borghammer P, Rodell A, Jónsdottir KY, Gjedde A. Sex differences of human cortical blood flow and energy metabolism. J Cereb Blood Flow Metab 37: 2433–2440, 2017. doi: 10.1177/0271678X16668536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aengevaeren VL, Claassen JA, Levine BD, Zhang R. Cardiac baroreflex function and dynamic cerebral autoregulation in elderly Masters athletes. J Appl Physiol (1985) 114: 195–202, 2013. doi: 10.1152/japplphysiol.00402.2012. [DOI] [PubMed] [Google Scholar]
- 3.Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73: 102–116, 2015. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Åstrand PO, Saltin B. Oxygen uptake during the first minutes of heavy muscular exercise. J Appl Physiol 16: 971–976, 1961. doi: 10.1152/jappl.1961.16.6.971. [DOI] [PubMed] [Google Scholar]
- 5.Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44: 3235–3238, 2013. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 6.Balke B, Nagle FJ, Daniels J. Altitude and maximum performance in work and sports activity. JAMA 194: 646–649, 1965. doi: 10.1001/jama.1965.03090190068016. [DOI] [PubMed] [Google Scholar]
- 7.Bendat J, Piersol G. Engineering Application of Correlations and Spectral Analysis. New York: Wiley-Interscience, 1980. [Google Scholar]
- 8.Bolduc V, Thorin-Trescases N, Thorin E. Endothelium-dependent control of cerebrovascular functions through age: exercise for healthy cerebrovascular aging. Am J Physiol Heart Circ Physiol 305: H620–H633, 2013. doi: 10.1152/ajpheart.00624.2012. [DOI] [PubMed] [Google Scholar]
- 9.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol 74: 1237–1263, 2019. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341: c3666, 2010. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J, Mitchell GF, Bots ML, Sigurdsson S, Harris TB, Garcia M, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik study. Arterioscler Thromb Vasc Biol 35: 1889–1895, 2015. doi: 10.1161/ATVBAHA.115.305451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas CG. A method for determining the total respiratory exchange in man. J Physiol 42: 17–23, 1911. [Google Scholar]
- 13.Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE; 2018 Physical Activity Guidelines Advisory Committee . Physical activity, cognition, and brain outcomes: a review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc 51: 1242–1251, 2019. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favre ME, Serrador JM. Sex differences in cerebral autoregulation are unaffected by menstrual cycle phase in young, healthy women. Am J Physiol Heart Circ Physiol 316: H920–H933, 2019. doi: 10.1152/ajpheart.00474.2018. [DOI] [PubMed] [Google Scholar]
- 15.Furby HV, Warnert EA, Marley CJ, Bailey DM, Wise RG. Cardiorespiratory fitness is associated with increased middle cerebral arterial compliance and decreased cerebral blood flow in young healthy adults: A pulsed ASL MRI study. J Cereb Blood Flow Metab 2019: 271678X19865449, 2019. doi: 10.1177/0271678X19865449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guadagni V, Drogos LL, Tyndall AV, Davenport MH, Anderson TJ, Eskes GA, Longman RS, Hill MD, Hogan DB, Poulin MJ. Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology 94: e2245–e2257, 2020. doi: 10.1212/WNL.0000000000009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa N, Fujie S, Horii N, Uchida M, Kurihara T, Sanada K, Hamaoka T, Iemitsu M. Aerobic exercise training-induced changes in serum C1q/TNF-related protein levels are associated with reduced arterial stiffness in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 314: R94–R101, 2018. doi: 10.1152/ajpregu.00212.2017. [DOI] [PubMed] [Google Scholar]
- 18.Intzandt B, Beck EN, Silveira CR. The effects of exercise on cognition and gait in Parkinson’s disease: A scoping review. Neurosci Biobehav Rev 95: 136–169, 2018. doi: 10.1016/j.neubiorev.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Katusic ZS, Austin SA. Endothelial nitric oxide: protector of a healthy mind. Eur Heart J 35: 888–894, 2014. doi: 10.1093/eurheartj/eht544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly R, Hayword C, Ganis J, Daley J, Avolio A, O’Rourke M. Noninvasive registration of the arterial pressure pulse waveform using high-fidelity applanation tonometry. J Vascul Med Biol 3: 142–149, 1989. [Google Scholar]
- 21.Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Drapeau A, Smirl JD, Bailey DM, Brassard P. Dynamic cerebral autoregulation is attenuated in young fit women. Physiol Rep 7: e13984, 2019. doi: 10.14814/phy2.13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 23.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke 34: 2475–2481, 2003. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 24.Leeuwis AE, Benedictus MR, Kuijer JPA, Binnewijzend MA, Hooghiemstra AM, Verfaillie SCJ, Koene T, Scheltens P, Barkhof F, Prins ND, van der Flier WM. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer’s disease. Alzheimers Dement 13: 531–540, 2017. doi: 10.1016/j.jalz.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Lewis NC, Smith KJ, Bain AR, Wildfong KW, Numan T, Ainslie PN. Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (Lond) 129: 169–178, 2015. doi: 10.1042/CS20140751. [DOI] [PubMed] [Google Scholar]
- 26.Marple S., Jr Digital Spectral Analysis with Applications. Englewood Cliffs, NJ: Prentice Hall, 1987. [Google Scholar]
- 27.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols WW, McDonald DA. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. London: Hodder Arnold, 2011. [Google Scholar]
- 29.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46: 200–204, 2005. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 30.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47: 2256–2261, 2016. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pescatello LS; American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health, 2014, p. xxiv. [Google Scholar]
- 32.Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM Jr, Levine BD. The effect of lifelong exercise frequency on arterial stiffness. J Physiol 596: 2783–2795, 2018. doi: 10.1113/JP275301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med 72: 239–252, 2010. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S, Tanaka H. Reduction in α-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol 135: 346–352, 2009. doi: 10.1016/j.ijcard.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugawara J, Otsuki T, Tanabe T, Hayashi K, Maeda S, Matsuda M. Physical activity duration, intensity, and arterial stiffening in postmenopausal women. Am J Hypertens 19: 1032–1036, 2006. doi: 10.1016/j.amjhyper.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Sugawara J, Tomoto T, Tanaka H. Heart-to-brachium pulse wave velocity as a measure of proximal aortic stiffness: MRI and longitudinal studies. Am J Hypertens 32: 146–154, 2019. doi: 10.1093/ajh/hpy166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 38.Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 34: 971–978, 2014. [Erratum in J Cereb Blood Flow Metab 34: 1255, 2014]. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 38: 1177–1183, 2013. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomoto T, Riley J, Turner M, Zhang R, Tarumi T. Cerebral vasomotor reactivity during hypo- and hypercapnia across the adult lifespan. J Cereb Blood Flow Metab 40: 600–610, 2020. doi: 10.1177/0271678X19828327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Huang H, Zhang R. White matter integrity in physically fit older adults. Neuroimage 82: 510–516, 2013. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzeng YC, MacRae BA, Ainslie PN, Chan GS. Fundamental relationships between blood pressure and cerebral blood flow in humans. J Appl Physiol (1985) 117: 1037–1048, 2014. doi: 10.1152/japplphysiol.00366.2014. [DOI] [PubMed] [Google Scholar]
- 43.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 44.Welch P. The use of fast fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio and Electroacoustics 15: 70–73, 1967. doi: 10.1109/TAU.1967.1161901. [DOI] [Google Scholar]
- 45.Xing CY, Tarumi T, Liu J, Zhang Y, Turner M, Riley J, Tinajero CD, Yuan LJ, Zhang R. Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab 37: 2848–2856, 2017. doi: 10.1177/0271678X16676826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing CY, Tarumi T, Meijers RL, Turner M, Repshas J, Xiong L, Ding K, Vongpatanasin W, Yuan LJ, Zhang R. Arterial pressure, heart rate, and cerebral hemodynamics across the adult life span. Hypertension 69: 712–720, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu YS, Tarumi T, Tseng BY, Palmer DM, Levine BD, Zhang R. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and Masters athletes. J Cereb Blood Flow Metab 33: 1190–1196, 2013. doi: 10.1038/jcbfm.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu YS, Tseng BY, Shibata S, Levine BD, Zhang R. Increases in cerebrovascular impedance in older adults. J Appl Physiol (1985) 111: 376–381, 2011. doi: 10.1152/japplphysiol.01418.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]