Abstract
Smaller airways increase resistance and the propensity toward turbulent airflow, both of which are thought to be mechanisms behind greater resistive and total work of breathing (Wb) in females. Previous research examining the effect of airway size on the Wb between the sexes is limited by the inability to experimentally manipulate airway size. Heliox (21% oxygen, balance helium) is less dense than room air, which reduces turbulent airflow and airway resistance. The purpose of our study was to utilize heliox inspiration in women to provide a stimulus physiologically similar to increasing airway size. We hypothesized that when breathing heliox women would have a Wb similar to men breathing room air. Eighteen healthy young subjects (n = 9 women, 9 men) completed two maximal exercise tests on a cycle ergometer over 2 days. Subjects breathed room air for one test and heliox for the other. Wb was assessed with an esophageal balloon catheter. During the room air trial, when ventilations were >65 L/min, women had a significantly greater Wb compared with men (P < 0.05). The greater Wb in women was due to greater resistance to turbulent flow. For both sexes, breathing heliox resulted in increased expiratory flow (+132 ± 18% of room air), an elimination of expiratory flow limitation, and a reduction in Wb (69 ± 12% of room air) (all P < 0.05). When the women were breathing heliox, Wb was not different from that in the men breathing room air. Our findings support the idea that the smaller conducting airways in females are responsible for a greater total and resistive Wb.
NEW & NOTEWORTHY When healthy young women breathe heliox gas during exercise, their work of breathing is not different from men breathing room air. Heliox inspiration reduces airway resistance and promotes laminar flow, which is a physiologically similar effect of increasing airway size. Our findings provide experimental evidence that smaller airways in women are responsible for the greater work of breathing during exercise.
Keywords: airway resistance, flow limitation, mechanical ventilatory constraints, sex differences, ventilation
INTRODUCTION
The variability in lung versus airway size between individuals is referred to as dysanapsis and is thought to explain differences in maximal flow-volume envelopes (17). Females appear to have a greater degree of dysanapsis compared with males, but this observation is based on indirect methods (12, 24). The concept of sex differences in dysanapsis was recently confirmed based on studies using quantitative computed tomography imaging. Specifically, the luminal area of the conducting airways is greater in males than in females, which has been shown in groups of lung size-matched ex-smokers (33), healthy height-matched adults (10), and healthy pediatric patients (31). Airway luminal area is a major determinant of turbulent airflow and resistance. Therefore, if females have smaller conducting airways it would be expected that they also have a greater work of breathing (Wb). Accordingly, for a given ventilation (V̇e), females have been shown to have a higher Wb (12, 21, 35). When the Wb is partitioned into viscoelastic and resistive components, the resistive Wb is greater in females than in males, whereas the viscoelastic Wb is similar between the sexes (12, 20, 25). These findings are supported by the aforementioned sexual dimorphism of the conducting airways as well as by studies showing that lung tissue elasticity is similar in men and women (7). Accordingly, our working hypothesis is that during exercise the smaller conducting airways in females increase the propensity toward turbulent airflow, thereby increasing airway resistance and, by association, the Wb at ventilations above ~50–60 L/min (13).
An important limitation of the aforementioned studies examining the Wb is the lack of experimental manipulations of the proposed causative mechanism, airway size. Unfortunately, acutely manipulating airway size in healthy individuals has technical shortcomings. For example, although it is possible to modify airway size with broncho-provocative agents, the associated change in airway diameter is variable between individuals and not consistent within the lung (8, 9, 32). If only a portion of the airways experienced considerable bronchoconstriction, there would be areas of highly turbulent airflow coupled with unaffected areas. Alternatively, if airway resistance could be uniformly manipulated throughout the lungs, this might serve as a more consistent, and physiologically relevant, surrogate for altered airway size. A method that results in more uniformly altered airway resistance would be to change the mechanical properties of inspired air with gases with different resistances to flow. Relative to atmospheric air, heliox (HE; ~21% O2, 79% helium) is a gas mixture in which nitrogen has been replaced with helium as the inert backing gas. Since helium is considerably less dense than nitrogen (0.17 vs. 1.17 g/m3 at 20°C and 1 atm for He and nitrogen, respectively), HE has a greater propensity towards laminar flow and thus can be used to acutely reduce airway resistance, and subsequently Wb, with minimal impact on the viscoelastic component. Heliox has been used previously in men to lower the Wb and reduce mechanical ventilatory constraints during exercise (1, 15). However, any studies comparing the sexes have only used single exercise stages (11, 27) or only permit comparisons at high ventilations (37), none of which allows for the complete assessment of how reducing airway resistance impacts the Wb throughout a range of ventilations.
Accordingly, the purpose of this study was to utilize HE to reduce airway resistance, which provides a physiological stimulus analogous to increasing airway size. By simulating increased airway size in women, we are eliminating the proposed cause for the difference in Wb between the sexes. We hypothesized that while breathing HE women would have a Wb during exercise similar to men breathing room air.
METHODS
Ethical approval.
The study was approved by the Office of Research ethics review board at the University of Waterloo, which adheres to the Declaration of Helsinki (approval number: 40823). All subjects were informed of the experimental procedures and potential risks involved and provided written informed consent.
Subjects.
Eighteen healthy young subjects (n = 9 women, n = 9 men) were recruited to participate in two testing sessions. Subjects were excluded if they had a history of smoking, had symptoms of cardiovascular, respiratory, or metabolic diseases, or were taking medication that would influence the response to exercise. Women were tested at random points in their menstrual cycle based on the findings that menstrual cycle phase has no effect on exercise ventilation (22).
Experimental overview.
Subjects completed 2 days of testing separated by at least 48 h. Both days involved a maximal exercise test on a cycle ergometer. On one day subjects respired compressed room air (RA), and for the other they inspired HE (20–21% O2: balance helium). Both gas mixtures were humidified and delivered in an identical manner. Subjects were blinded to the gas mixture they were breathing, and the order was randomized. Before exercise, an esophageal balloon catheter was placed to quantify the Wb.
Incremental exercise testing.
After a self-selected warm-up, subjects completed an incremental exercise test to volitional fatigue on an electronically braked cycle ergometer (Monark, LC7TT). Male and female subjects started the exercise test at 80 W and 40 W, respectively, with the workload increasing in a stepwise fashion by 20 W every 2 min until volitional fatigue. Different starting workloads were utilized to ensure that the groups exercised for a similar duration. The test was terminated when subjects could no longer maintain a cadence of 60 rpm despite verbal encouragement. At the 1.5 min mark of each stage, subjects were prompted to perform an inspiratory capacity maneuver (18) and were subsequently asked to rate their perceived breathlessness with the modified Borg scale (5).
Flow, pressure, and volume.
Ventilatory and mixed expired variables were collected with a customized metabolic cart. Subjects breathed through low-resistance, large-bore tubing connected to a nonrebreathing valve (2700B; Hans Rudolph, Kansas City, MO). Inspired and expired flow were collected with two independent, calibrated pneumotachometers (model 3813; Hans Rudolph). Mouth pressure was measured via a port in the mouthpiece connected to a calibrated differential pressure transducer (DP15-32; Validyne Engineering, Northridge, CA). The distal end of an esophageal balloon catheter (see below) was connected to a separate differential pressure transducer. Both pressure transducers were open to room air on the atmospheric side without an additional length of tubing. Mixed expired oxygen and carbon dioxide were measured with calibrated gas analyzers (S-3-A/I and CD-3Am, respectively; Applied Electrochemistry, Bastrop, TX). The experimental setup was identical between days except for the following modifications done during the HE trials: 1) to prevent excessive moisture buildup, the expired pneumotach was heated to 43°C and a low-resistance filter was placed in line immediately prior to the pneumotachometer; 2) pneumotachometers were calibrated with HE gas rather than RA; and 3) to account for the effect of helium on the infrared signal used by the CO2 analyzer, both gas analyzers were calibrated with a gas mixture containing 16% O2, 4% CO2 and balance helium, rather than N2.
Esophageal pressure measurement.
Before each incremental exercise test, a topical anesthetic was applied to the subject’s nares and nasal conchae (Xilocaine, lidocaine hydrochloride) before passing an esophageal balloon-tipped catheter (no. 47-9005; Cooper Surgical) through the nose and into the stomach. Subjects were asked to perform a brief Valsalva maneuver while the catheter was open to the atmosphere to empty the balloon, and then 1 mL of air was inserted into the balloon with a glass syringe. The balloon was then positioned in the lower third of the esophagus to measure esophageal pressure. The validity of the esophageal balloon position was assessed with a dynamic occlusion test (4) before it was secured in place with tape.
Maximal expiratory flow-volume curves, operating lung volumes, and expiratory flow limitation.
To determine the maximal expiratory flow-volume curves, subjects performed a series of maximal exhalations from total lung capacity to residual volume at varying respiratory efforts. The subject was coached on the different efforts by the experimenter, who ensured a range of expiratory flow. Between four and six maneuvers were performed both before and after exercise to account for the effects of thoracic gas compression and exercise-induced bronchodilation (19). Subjects were allowed to take several tidal breaths between each expiratory maneuver.
To determine operational lung volumes, subjects performed inspiratory capacity maneuvers during each stage. Before each inspiratory capacity maneuver, esophageal pressure was monitored to ensure consistent end-expiratory and peak inspiratory pressure as previously recommended (18). If either pressure deviated during the inspiratory capacity maneuver, the subject was asked to perform a second one.
To determine the presence of expiratory flow limitation, 8–10 tidal breaths were placed within the maximal expiratory flow-volume curve to determine the degree of overlap between the two. Lung volume was determined from the inspiratory capacity maneuver and forced vital capacity as described below. The percentage of limitation was calculated by dividing the volume of the breath that overlapped the curve by the total tidal volume. If a subject had a value > 5% they were considered flow limited.
Data analysis.
Raw signals were recorded at 200 Hz with a 16-channel analog-to-digital data acquisition system (PowerLab/16SP model ML 795; ADInstruments, Colorado Springs, CO) and stored on a personal computer for analysis. The pneumotachograph temperature was accounted for off-line when calculating expired flow during helium trials. Metabolic volumes [e.g., oxygen uptake (V̇o2)] are expressed in STPD, whereas other volumes are in BTPS. For inspiratory capacity analysis, approximately 10 breaths before the inspiratory capacity maneuver were selected to correct for any pneumotachometer drift. Expiratory reserve volume was calculated by subtracting the inspiratory capacity volume from the forced vital capacity, and end-inspiratory lung volume was calculated as the sum of tidal volume and expiratory reserve volume. Approximately 10 tidal breaths before an inspiratory capacity maneuver were ensemble-averaged and placed within the maximal expiratory flow-volume curve according to the measured expiratory reserve volume. The Wb was determined by integrating composite average transpulmonary pressure-volume loops. The total Wb was also divided into inspiratory elastic, inspiratory resistive, and total expiratory components based on respiratory system compliance along with lung volumes at end inspiration and expiration (12) We then modeled the total Wb based on the equation below (21, 28):
where Wb is the work of breathing, a is the constant describing resistance to turbulent airflow, b is the constant for resistance to laminar airflow and viscous resistance offered by lung tissue, and V̇e is ventilation. We fitted the above equation to each subject’s raw Wb-V̇e curve for both RA and HE to generate constants a and b for each subject on each condition. Pooling the constants then allowed for statistical comparisons and generation of a composite of Wb-V̇e curves for each sex and condition. The breakpoint at which the HE Wb increased out of proportion to the RA Wb was defined as the V̇e when there was at least 10% difference in Wb that was sustained for greater V̇e. The breakpoint was also visually confirmed for each subject.
Statistical analysis.
Descriptive characteristics were compared with unpaired Student’s t tests. Maximal exercise variables were compared with a 2 [sex (male, female)] × 2 [gas (RA, HE)] repeated-measures analysis of variance. To compare the occurrence of expiratory flow limitation, we grouped the sexes together to examine the effect of HE. Thereafter, a Fisher’s exact test was used to determine any differences in the occurrence of expiratory flow limitation at maximal exercise. The Wb was compared with unpaired Student’s t tests at 1 L/min ventilation intervals with a Bonferroni correction for multiple comparisons. Statistical significance was set at P < 0.05. All data are presented as means ± SD.
RESULTS
Subjects.
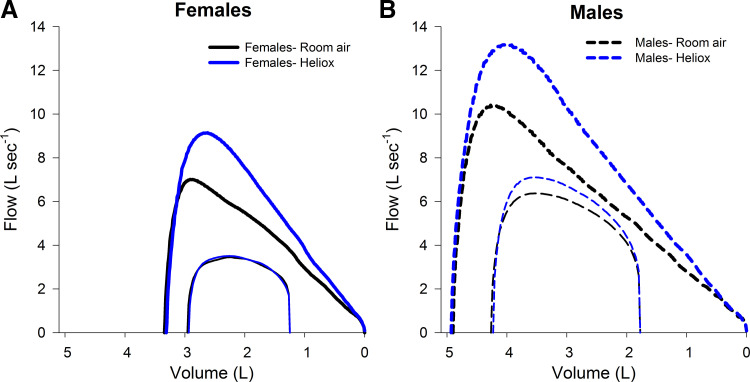
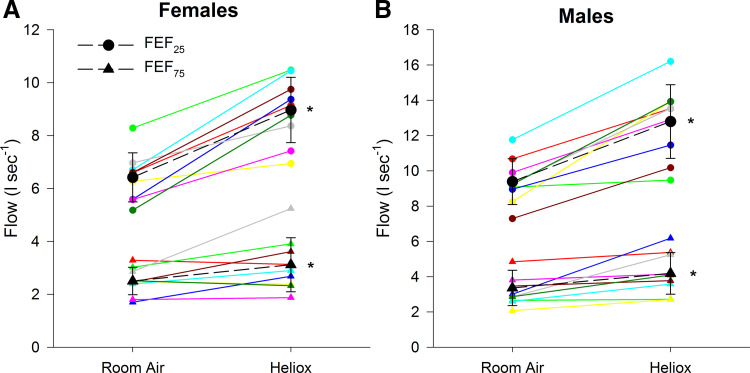
Male subjects were significantly taller (175 ± 4 vs. 164 ± 3 cm) and had a greater mass (78 ± 8 vs. 63 ± 12 kg), but there were no differences in age (23 ± 3 vs. 23 ± 2 yr) or body mass index (25.6 ± 3.0 vs. 23.3 ± 4.5 kg/m2) for male and female subjects, respectively. Male subjects had greater volumes and expiratory flows than female subjects for both gases (Table 1). Forced vital capacity was not different between RA and HE for male or female subjects (P = 0.50) (Table 1, Fig. 1). Compared with RA, HE resulted in increased maximal expiratory flows in both sexes, which was most evident at higher lung volumes (Figs. 1 and 2, Table 1). The relative increase in expiratory flow was not different between the sexes (P > 0.05). Forced expiration time was significantly higher when breathing RA for male (1.69 ± 0.79 vs. 1.36 ± 0.56, P < 0.05) and female (1.57 ± 0.85 vs. 1.24 ± 0.61, P < 0.05) subjects compared with HE.
Table 1.
Lung volume and peak and expiratory flows breathing room air and heliox
| Female |
Male |
HE as % RA |
||||
|---|---|---|---|---|---|---|
| RA | HE | RA | HE | F | M | |
| FVC, L | 3.34 ± 0.25 | 3.31 ± 0.23 | 4.90 ± 0.90† | 4.93 ± 0.78† | 99 ± 6 | 100 ± 5 |
| PEF, L/s | 7.10 ± 0.73 | 9.25 ± 1.14* | 10.65 ± 1.44† | 13.50 ± 2.37*† | 130 ± 11 | 127 ± 17 |
| FEF25, L/s | 6.42 ± 0.93 | 8.97 ± 1.23* | 9.38 ± 1.30† | 12.80 ± 2.09*† | 141 ± 21 | 137 ± 18 |
| FEF50, L/s | 5.16 ± 0.79 | 6.86 ± 1.20* | 6.82 ± 1.05† | 9.20 ± 1.34*† | 133 ± 14 | 136 ± 15 |
| FEF75, L/s | 2.50 ± 0.52 | 3.11 ± 1.02* | 3.36 ± 1.00† | 4.19 ± 1.19*† | 125 ± 32 | 128 ± 32 |
Values are means ± SD. All subjects (n = 18, n = 9 women) are represented in the table. F, female; FEF25, forced expiratory flow at 25% forced vital capacity (FVC); FEF50, forced expiratory flow at 50% FVC; FEF75, forced expiratory flow at 75% FVC; HE, heliox; M, male; PEF, peak expiratory flow; RA, room air.
Significantly different from RA within sex;
significantly different from female subjects with the same inspirate, P < 0.05.
Fig. 1.
Composite average maximal expiratory flow-volume curves along with expiratory tidal flow-volume loop at maximal exercise in female (A) and male (B) subjects. Composite averaging of each curve is done in with respect to volume and variance at discrete points can be found in Table 1. Zero on the x-axis represents residual volume. x- and y-axis scales are identical in A and B.
Fig. 2.
Forced expiratory flow (FEF) at 25% and 75% of forced vital capacity for female (A) and male (B) subjects breathing room air and heliox. Each color represents a single subject within each sex. *Significantly different from room air within sex.
Maximal exercise.
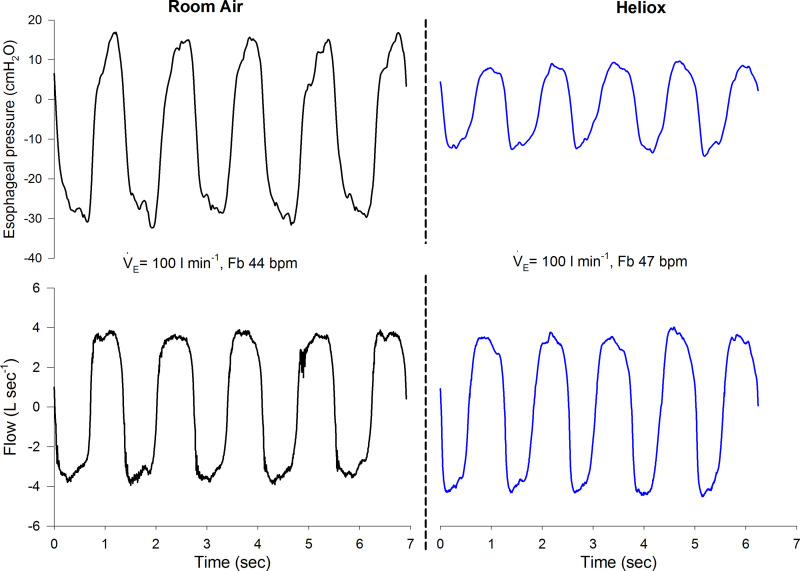
Maximal exercise data for the RA and HE trials are presented in Table 2. As expected, male subjects had greater absolute tidal volume, V̇e, oxygen uptake, and Wb. The main effects of HE compared with RA were increased breathing frequency and a lower Wb. There were no significant interaction effects. A representative trace for esophageal pressure and flow for a single female subject at maximal exercise is shown in Fig. 3, where the pattern of flow is similar between conditions but the magnitude of esophageal pressure swings is reduced during HE. There were no significant effects of condition on expiratory reserve volume or end-inspiratory lung volume (Table 2). During exercise, approximately half of the male and female subjects developed expiratory flow limitation during RA trial, and in all but one case expiratory flow limitation was abolished during the HE trial (Table 2). There was no main effect of sex or inspirate on the dyspnea rating (Table 2).
Table 2.
Maximal exercise values
| Female |
Male |
P Values |
||||||
|---|---|---|---|---|---|---|---|---|
| RA | HE | RA | HE | Sex | Gas | Inter | ||
| Work rate, W | 178 ± 31 | 176 ± 26 | 253 ± 20 | 256 ± 19 | <0.001 | 0.99 | 0.44 | |
| Heart rate, beats/min | 184 ± 7 | 183 ± 9 | 195 ± 5 | 192 ± 8 | 0.01 | 0.19 | 0.55 | |
| Vt, L | 1.90 ± 0.18 | 1.86 ± 0.17 | 2.68 ± 0.51 | 2.55 ± 0.4 | <0.001 | 0.28 | 0.31 | |
| Vt/FVC, % | 57.4 ± 7.8 | 57.1 ± 7.1 | 56.7 ± 8.3 | 53.3 ± 4.6 | 0.49 | 0.15 | 0.25 | |
| fB, breaths/min | 50 ± 5 | 54 ± 7 | 57 ± 9 | 62 ± 13 | 0.09 | 0.01 | 0.55 | |
| V̇e, L/min | 95 ± 17 | 100 ± 14 | 153 ± 11 | 158 ± 16 | <0.001 | 0.09 | 0.86 | |
| VTe/Te | 2.9 ± 0.7 | 2.9 ± 0.5 | 5.0 ± 0.4 | 4.9 ± 0.5 | <0.001 | 0.50 | 0.56 | |
| VTi/Ti | 3.4 ± 0.6 | 3.7 ± 0.5 | 5.2 ± 0.4 | 5.5 ± 0.8 | <0.001 | 0.01 | 0.66 | |
| Ti/Ttot | 46 ± 3 | 44 ± 3 | 49 ± 2 | 47 ± 3 | 0.01 | 0.007 | 0.76 | |
| Wb, J/min | 206 ± 77 | 132 ± 44 | 339 ± 55 | 245 ± 63 | <0.001 | <0.001 | 0.39 | |
| Resistive Wb, % total Insp work | 48 ± 7 | 40 ± 9 | 42 ± 6 | 37 ± 8 | 0.18 | <0.001 | 0.38 | |
| V̇o2, L/min | 2.45 ± 0.33 | 2.52 ± 0.3 | 3.62 ± 0.32 | 3.70 ± 0.31 | <0.001 | 0.1 | 0.86 | |
| V̇o2, mL·kg−1·min−1 | 40 ± 9 | 41 ± 8 | 48 ± 9 | 49 ± 7 | <0.001 | 0.1 | 0.86 | |
| V̇co2, L/min | 2.77 ± 0.33 | 2.68 ± 0.3 | 4.10 ± 0.40 | 3.92 ± 0.36 | <0.001 | 0.02 | 0.39 | |
| RER | 1.13 ± 0.05 | 1.07 ± 0.03 | 1.13 ± 0.02 | 1.06 ± 0.03 | 0.80 | <0.001 | 0.79 | |
| V̇e/V̇o2 | 39 ± 5 | 39 ± 4 | 42 ± 4 | 43 ± 4 | 0.06 | 0.57 | 0.54 | |
| V̇e/V̇co2 | 35 ± 4 | 37 ± 3 | 37 ± 6 | 41 ± 4 | 0.04 | <0.001 | 0.36 | |
| Dyspnea (Borg) | 5.7 ± 1.5 | 4.9 ± 1.2 | 7.2 ± 2.6 | 6.2 ± 1.9 | 0.07 | 0.06 | 0.81 | |
| ERV, % FVC | 37 ± 4 | 34 ± 3 | 36 ± 8 | 36 ± 6 | 0.67 | 0.39 | 0.17 | |
| EILV, % FVC | 89 ± 8 | 85 ± 8 | 87 ± 6 | 86 ± 6 | 0.97 | 0.13 | 0.32 | |
| Expiratory flow limited, n | 4/9 | 0/9 | 5/9 | 1/9 | 0.007† | |||
Values are means ± SD. All subjects (n = 18, n = 9 women) are represented in the table. EILV, end-inspiratory lung volume; ERV, expiratory reserve volume; fB, breathing frequency; FVC, forced vital capacity; HE, heliox; Inter, interaction; RA, room air; RER, respiratory exchange ratio; V̇co2, carbon dioxide output; V̇e, minute ventilation; V̇o2, oxygen uptake; Vt, tidal volume; Wb, work of breathing; % total Insp work, percentage of work on inspiration; Te, expiratory time; Ti, inspiratory time; Ttot, total respiratory time; VTe, expiratory tidal volume; VTi, inspiratory tidal volume.
Male and female subjects grouped to test the effect of heliox specifically.
Fig. 3.
Raw esophageal pressure and flow from a single female subject during maximal exercise breathing room air (left) and heliox (right). The increase in breathing frequency (fB) but similar ventilation (V̇e) is consistent with the average group findings in Table 1. bpm, Breaths per minute.
Work of breathing.
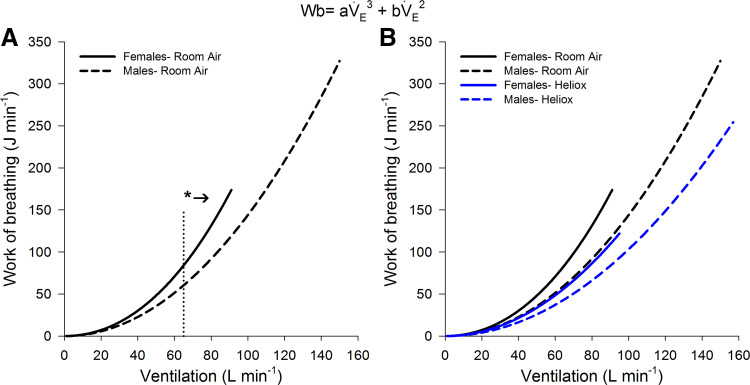
At maximal exercise, HE resulted in a significantly lower Wb in both sexes (Table 2). Although male subjects had a greater absolute Wb and V̇e at maximal exercise than female subjects, the relative decrease in Wb with HE was not different between the sexes (66 ± 13% vs. 72 ± 11% of RA for female and male subjects, respectively, P = 0.28). The reduction in Wb with HE was principally due to reductions in resistive work (Table 2). The relationship between the Wb and V̇e is shown in Fig. 4. When breathing RA, female subjects had a significantly greater Wb than male subjects for a given V̇e above ~65 L/min. The sex difference in Wb during the RA trial was due to a higher constant a (turbulent resistance) rather than constant b (viscous resistance) (Table 3). When RA and HE were compared for female subjects, the Wb was significantly lower after ~55 L/min, and this was also due to a lower constant a (Table 3). When female subjects breathing HE were compared with male subjects breathing RA, there was no difference in the V̇e vs. Wb relationship (Fig. 4). When breathing HE, male subjects also had a significant reduction in the Wb compared with their RA trial. At low V̇e the Wb was similar between RA and HE, but for each subject a characteristic breakpoint was noted where the RA Wb increased out of proportion to that of the HE. Figure 5 displays this breakpoint for a single subject. For female subjects, this breakpoint occurred at a significantly lower absolute V̇e (59 ± 5 L/min; range 52–65 L/min) compared with male subjects (99 ± 16 L/min; range 75–125 L/min) (P < 0.001).
Fig. 4.
The relationship between work of breathing (Wb) and minute ventilation (V̇e) for male and female subjects. A: the averaged response between the sexes while breathing room air. B: the individual responses for male and female subjects breathing room air and heliox. For each, the relationship was modeled on the following equation for each subject: Wb = aV̇e3 + bV̇e2, where a and b are constants describing resistance to turbulent airflow and resistance to laminar airflow and viscous resistance, respectively. *Significantly greater in female subjects, P < 0.001.
Table 3.
Work of breathing model constants and regression coefficient
| Female |
Male |
|||
|---|---|---|---|---|
| RA | HE | RA | HE | |
| a (mean) | 3.9 × 10−5 | 7.2 × 10−7* | 2.6 × 10−6† | 4.4 × 10−13 |
| a (SD) | 3.6 × 10−5 | 2.2 × 10−7 | 7.0 × 10−6 | 2.6 × 10−13 |
| b (mean) | 1.8 × 10−2 | 1.4 × 10−2 | 1.4 × 10−2 | 1.0 × 10−2* |
| b (SD) | 6.5 × 10−3 | 3.3 × 10−3 | 1.1 × 10−3 | 1.2 × 10−3 |
| R2 | 0.995 ± 0.004 | 0.959 ± 0.03** | 0.981 ± 0.014 | 0.935 ± 0.073** |
All subjects (n = 18, n = 9 women) are represented in the table. HE, heliox; RA, room air.
Significantly different compared with RA within sex;
significantly different from female subjects on RA;
significant main effect of gas, P < 0.05.
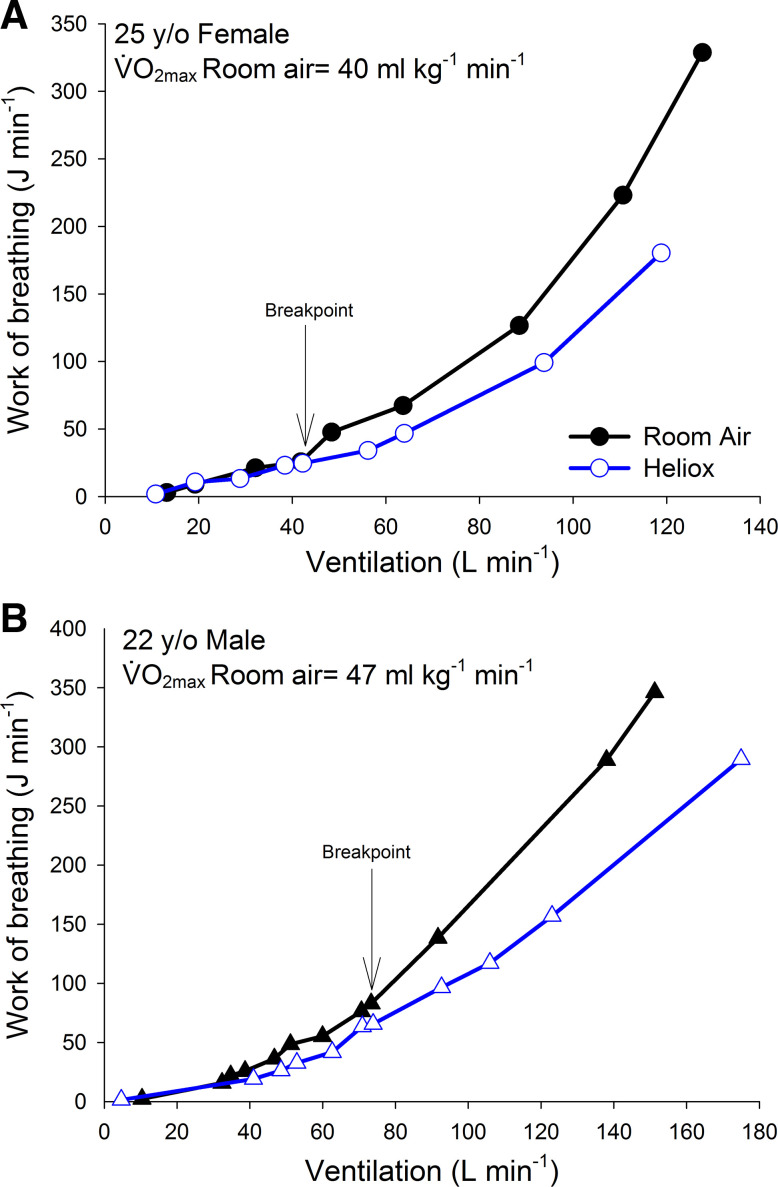
Fig. 5.
The relationship between work of breathing and minute ventilation for a single female subject (A, circles) and a single male subject (B, triangles) . The arrow indicates where the breakpoint is where the work of breathing on room air rises out of proportion to that of heliox. V̇o2max, maximum oxygen uptake.
DISCUSSION
Major findings.
We used HE gas to reduce airway resistance in healthy young men and women, which would provide a physiological stimulus that is analogous to increasing airway caliber. Our major finding is that breathing HE gas eliminates sex differences in Wb during exercise, which we attribute to the effect of helium on airway resistance and the propensity toward laminar flow. Overall, our findings further support the hypothesis that smaller conducting airways in healthy females are responsible for the greater Wb observed in females during exercise.
Work of breathing.
It has been hypothesized that smaller conducting airways in females is the causative factor leading to the greater resistive and total Wb for a given V̇e in females (13). We found that when breathing RA female subjects had a significantly higher Wb at a V̇e of >65 L/min (Fig. 4), which is in keeping with previous studies (12, 21, 26). Sex differences in conducting airway size (10, 33) and Wb during exercise (12, 21, 25, 35) have been repeatedly shown in separate studies; however, no previous study has experimentally manipulated airway size to confirm the mechanistic basis of sex differences in Wb. Although smaller airway cross-sectional area is thought to be the root cause, ultimately it is airway resistance that is the physiological mechanism underlying sex differences in Wb. Therefore, we utilized HE gas to lower airway resistance uniformly to provide a stimulus physiologically analogous to altered airway caliber and determine whether this impacted sex differences in the V̇e-Wb relationship during exercise. HE lowered the Wb in both sexes (Fig. 4), and the difference was apparent when V̇e was >55 L/min in female subjects and >90 L/min in male subjects. That the Wb was similar at lower V̇e is consistent with HE effect, where, because of the lower density of helium compared with nitrogen and the propensity toward laminar flow, resistance is reduced. However, helium is also ~10% more viscous than nitrogen, and this will actually increase resistance during laminar flow. The above two aspects may explain why at lower V̇e HE had a negligible effect on Wb, whereas when V̇e was relatively high, and flow was likely turbulent, HE had a greater effect on Wb. Although we note that Wb was reduced at maximal exercise in both sexes, this is not always the case (1, 2). The difference is likely due to our subjects having no change in V̇e at maximal exercise, whereas others noted an increase with HE (1–3). We also found that the reduction of Wb was more prominent on inspiration rather than expiration, which further supports our hypothesis of smaller conducting airways in females. If increased resistance due to expiratory flow limitation (EFL) was the cause of the Wb, we would have observed HE reducing the expiratory work considerably more.
It is important to emphasize that if the Wb is compared between males and females at a relative intensity the sex differences are no longer present. The issue of whether to compare the sexes in absolute or relative terms is an ongoing dilemma (13). The purpose of the present study was to determine whether sex differences in Wb are eliminated when airflow resistance is reduced with HE (our physiological equivalent of increasing airway size). If we made comparisons between the sexes at relative intensities, the respiratory flows would be significantly different owing to the different V̇e. As resistance and turbulence are dependent of flow, this would make our results difficult to interpret. Rather, we have compared the sexes at absolute V̇e, where flows will be more similar.
When comparing female subjects breathing HE and male subjects breathing RA, we found that the Wb was nearly identical (Fig. 4). We interpret this to further support our hypothesis that airway resistance, which is directly influenced by airway caliber, is the mechanism behind a greater Wb during exercise in females relative to males. Our previous cross-sectional work indicates that the difference in Wb between the sexes is due to resistive, and not viscoelastic, work (12, 14, 20, 25). This also confirms others who have found that static recoil (as an indicator of lung elasticity) is not different between the sexes (7, 26). Our present modeling results also confirm this in that constant a (representing turbulent resistance) was greater in female subjects breathing RA and constant b (representing viscous resistance) was not different (Table 3).
When the raw Wb and V̇e data were inspected on an individual basis, a characteristic inflection occurred in all subjects, which gives the curve the exponential rise. The inflection point is presumably where flow in the large conducting airways begins to transition from laminar to turbulent, thereby increasing airway resistance and Wb. Evidence for this arises from a noticeable “breakpoint” in the RA and HE V̇e-Wb curve that occurred in all subjects (Fig. 5). Before the breakpoint, the Wb at a given V̇e is nearly identical between the RA and HE trials; however, after the breakpoint, Wb with RA rises out of proportion to that of the HE curve. The breakpoint occurred at a significantly higher V̇e in male subjects compared with female subjects (99 ± 16 L/min vs. 59 ± 5 L/min), presumably because their airways are larger. In female subjects, the breakpoint occurred at a similar V̇e as the point where sex differences in Wb become evident (i.e., at a V̇e of ~55–65 L/min). Furthermore, we noted variability in the V̇e at which the breakpoint occurs in both sexes. This is consistent with our previous finding that although on average women have smaller conducting airways, there is considerable variability and overlap between the sexes (10).
Maximal expiratory flow-volume curves, operational lung volume, and flow limitation.
The average maximal expiratory flow-volume curves for each group are shown in Fig. 1. During RA, male subjects had significantly larger volumes and flows compared with female subjects. The larger volumes are due to a greater number of alveoli along with increased thoracic cavity size (34), whereas the greater flows in men are attributed to larger airways (13). Breathing HE had no impact on forced vital capacity for either sex, with RA and HE values being nearly identical (Fig. 1). This finding is expected, as there is no physiological rationale for helium to acutely alter total lung capacity or residual volume in young, healthy subjects. Instead, breathing HE increased expiratory flows for both sexes (Table 1). Although the absolute maximal expiratory flows were greater in male than female subjects, the relative increase in flows with HE was ~35% in both sexes, which is in keeping with previous studies (6, 11, 23, 37). In both sexes, the effect of HE was most evident at higher lung volumes. Because of the characteristic shape of the maximal expiratory flow-volume curve, flows at lower lung volumes are considerably less than at higher lung volumes. It follows that at a low lung volume maximal expiratory flows are more likely to be laminar, and are viscous dependent, at which point the HE effect of increasing the propensity toward laminar flow would be moot. Conversely, at a higher lung volume breathing RA normally results in turbulent flow, but HE would be able to reduce the propensity toward turbulent flow by reducing the Reynolds number.
When breathing RA, ~50% of male and female subjects developed expiratory flow limitation during exercise (Table 2). The lack of an effect of sex on the proportion of individuals who develop expiratory flow limitation during exercise is in agreement with our previous work in healthy, young men and women of normative cardiorespiratory fitness (12, 26). Breathing HE eliminated expiratory flow limitation originally present during the RA trial in all but one subject. In the latter case, the subject demonstrated severe EFL during the RA trial (>80% overlap between their tidal and maximal expiratory flow-volume curves) and only minimal EFL during HE (<10% overlap). Therefore, while still categorized as having EFL, the subject’s mechanical ventilatory constraints would have been minimized. HE eliminating EFL in virtually all subjects is consistent with previous findings (11, 27).
We found no effect of HE on operational lung volumes (Table 2), which is similar to others who tested only women during maximal tests (23). Others, however, have found that expiratory reserve volume decreases with HE (1, 2). The discrepancy likely is the result of differing rates and severity of expiratory flow limitation during exercise. If severe flow limitation is present during RA exercise, it is likely the individual will increase their expiratory reserve volume in order to access greater expiratory flows, although this effect is likely variable between individuals (29).
Although breathing frequency was significantly higher during the HE trial than the RA trial, there was no significant change in V̇e (Table 2). Previous work has shown that only subjects who develop EFL on RA increase their V̇e when breathing HE (11, 23). As only half our subjects developed EFL, it is unsurprising that V̇e did not systematically change. Others have shown a more variable change in V̇e (1, 3), but they studied older individuals and clinical populations where the prevalence of EFL and mechanical constraints are likely greater than our present cohort. The HE trial also resulted in no significant change in either V̇o2 or peak work rate (Table 2).
Dyspnea.
At peak exercise, dyspnea was statistically similar between gas conditions and did not differ based on sex (Table 2). The lack of a significant change in dyspnea with HE, despite a lower Wb and the elimination of EFL, can likely be explained by the negligible effect of acute changes in mechanical ventilatory constraints on the perception of dyspnea in healthy subjects. Indeed, we recently noted that physiologically relevant manipulations of mechanical ventilatory constraints during constant-load exercise at a moderate intensity had no impact on dyspnea in healthy older men and women (27). Similarly, others have used HE during exercise and found that dyspnea was not different than during exercise with RA when V̇e was similar and the Wb was presumed to be lower (16). The fact that we did not observe an effect of sex on dyspnea at peak exercise is also in keeping with previous work, in which healthy young men and women had a similar perception of dyspnea at peak exercise (13). However, it should be noted that in both cases the effect of gas and the effect of sex did approach statistical significance, and these data should be interpreted with caution given that our study was not powered to detect a significant difference in the perception of dyspnea between groups or conditions. It is also possible that only those who had EFL would have a decrease in dyspnea with HE. Unfortunately, this too is underpowered to accurately determine for the present study but warrants further investigation.
Methodological considerations.
When interpreting the findings of the present study, there are important methodological considerations that merit comment. First, although some have shown that exercise performance is improved with HE (30, 37, 38), it is important to note that our study was not designed to address this question. Our subjects were not endurance trained, and our exercise task was intended to allow for the collection of a complete range of V̇e rather than address exercise performance. However, we did note a small but significant decrease in carbon dioxide output and respiratory exchange ratio. Second, the effect of helium is flow dependent and therefore male subjects, because of higher absolute flows, may have gained more of an effect from HE inspiration. However, our main objective was to compare the sexes at similar V̇e when expired flows would be similar. The relative reduction in Wb for the HE trial was also not different between the sexes. Third, although we sought to have a uniform reduction in airway resistance throughout the lung, HE would have minimal effect in small airways because of the low airflow velocity, yet there is currently no evidence suggesting that the luminal area of the small airways is different between young healthy men and women. Fourth, like all mathematical models, our model to describe the Wb has a series of assumptions and limitations, especially as to what physiological aspect each component represents. However, regardless of any sex-based comparisons, that HE breathing reduced the constant representing resistance to turbulence in every subject provides further support of the model’s robustness. Finally, we did not match the external apparatus resistance between trials. Again, because of the flow-dependent nature of HE for lowering resistance, higher flow (likely generated by male subjects) would have a greater benefit in terms of external resistance minimization. However, at the level of V̇e where we noticed sex differences in Wb (i.e., ~60 L/min), it is unlikely the resistance offered by the external apparatus would have a large impact on Wb. This is because the cross-sectional area of the tubing (i.e., ~962 mm2) is considerably larger compared with that of the trachea (10).
Conclusions.
When airway resistance and airflow turbulence are minimized via HE inspiration in healthy young female subjects, their Wb is not different from male subjects respiring RA. Our finding provides experimental evidence that further supports the notion that smaller conducting airways are the mechanism responsible for sex differences in the V̇e-Wb relationship during exercise.
GRANTS
This study was supported by the Natural Science and Engineering Council of Canada (RGPIN-2019-04615) and an infrastructure grant from the Canada Foundation for Innovation (38432).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.B.D. conceived and designed research; L.M.M., E.A.G., J.S.C., A.Y., and P.B.D. performed experiments; L.M.M., E.A.G., J.S.C., A.Y., Y.M.-S., and P.B.D. analyzed data; L.M.M., E.A.G., J.S.C., A.Y., Y.M.-S., and P.B.D. interpreted results of experiments; L.M.M., E.A.G., J.S.C., A.Y., Y.M.-S., and P.B.D. prepared figures; L.M.M., E.A.G., J.S.C., A.Y., Y.M.-S., and P.B.D. drafted manuscript; L.M.M., E.A.G., J.S.C., A.Y., Y.M.-S., and P.B.D. edited and revised manuscript; L.M.M., E.A.G., J.S.C., A.Y., Y.M.-S., and P.B.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for enthusiastic participation.
REFERENCES
- 1.Babb TG. Ventilatory response to exercise in subjects breathing CO2 or HeO2. J Appl Physiol (1985) 82: 746–754, 1997. doi: 10.1152/jappl.1997.82.3.746. [DOI] [PubMed] [Google Scholar]
- 2.Babb TG. Ventilation and respiratory mechanics during exercise in younger subjects breathing CO2 or HeO2. Respir Physiol 109: 15–28, 1997. doi: 10.1016/S0034-5687(97)84026-1. [DOI] [PubMed] [Google Scholar]
- 3.Babb TG. Breathing He-O2 increases ventilation but does not decrease the work of breathing during exercise. Am J Respir Crit Care Med 163: 1128–1134, 2001. doi: 10.1164/ajrccm.163.5.9908025. [DOI] [PubMed] [Google Scholar]
- 4.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788–791, 1982. [DOI] [PubMed] [Google Scholar]
- 5.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Butcher SJ, Jones RL, Mayne JR, Hartley TC, Petersen SR. Impaired exercise ventilatory mechanics with the self-contained breathing apparatus are improved with heliox. Eur J Appl Physiol 101: 659–669, 2007. doi: 10.1007/s00421-007-0541-5. [DOI] [PubMed] [Google Scholar]
- 7.Colebatch HJ, Greaves IA, Ng CK. Exponential analysis of elastic recoil and aging in healthy males and females. J Appl Physiol 47: 683–691, 1979. doi: 10.1152/jappl.1979.47.4.683. [DOI] [PubMed] [Google Scholar]
- 8.de Lange EE, Altes TA, Patrie JT, Battiston JJ, Juersivich AP, Mugler JP 3rd, Platts-Mills TA. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology 250: 567–575, 2009. doi: 10.1148/radiol.2502080188. [DOI] [PubMed] [Google Scholar]
- 9.de Lange EE, Altes TA, Patrie JT, Gaare JD, Knake JJ, Mugler JP 3rd, Platts-Mills TA. Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest 130: 1055–1062, 2006. doi: 10.1378/chest.130.4.1055. [DOI] [PubMed] [Google Scholar]
- 10.Dominelli PB, Ripoll JG, Cross TJ, Baker SE, Wiggins CC, Welch BT, Joyner MJ. Sex differences in large conducting airway anatomy. J Appl Physiol (1985) 125: 960–965, 2018. doi: 10.1152/japplphysiol.00440.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominelli PB, Foster GE, Dominelli GS, Henderson WR, Koehle MS, McKenzie DC, Sheel AW. Exercise-induced arterial hypoxaemia and the mechanics of breathing in healthy young women. J Physiol 591: 3017–3034, 2013. doi: 10.1113/jphysiol.2013.252767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominelli PB, Molgat-Seon Y, Bingham D, Swartz PM, Road JD, Foster GE, Sheel AW. Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol (1985) 119: 1105–1113, 2015. doi: 10.1152/japplphysiol.00409.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominelli PB, Molgat-Seon Y, Sheel AW. Sex differences in the pulmonary system influence the integrative response to exercise. Exerc Sport Sci Rev 47: 142–150, 2019. doi: 10.1249/JES.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 14.Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Romer LM, Sheel AW. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 593: 1965–1979, 2015. doi: 10.1113/jphysiol.2014.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eves ND, Petersen SR, Haykowsky MJ, Wong EY, Jones RL. Helium-hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 174: 763–771, 2006. doi: 10.1164/rccm.200509-1533OC. [DOI] [PubMed] [Google Scholar]
- 16.Eves ND, Petersen SR, Jones RL. Submaximal exercise with self-contained breathing apparatus: the effects of hyperoxia and inspired gas density. Aviat Space Environ Med 74: 1040–1047, 2003. [PubMed] [Google Scholar]
- 17.Green M, Mead J, Turner JM. Variability of maximum expiratory flow-volume curves. J Appl Physiol 37: 67–74, 1974. doi: 10.1152/jappl.1974.37.1.67. [DOI] [PubMed] [Google Scholar]
- 18.Guenette JA, Chin RC, Cory JM, Webb KA, O’Donnell DE. Inspiratory capacity during exercise: measurement, analysis, and interpretation. Pulm Med 2013: 956081, 2013. doi: 10.1155/2013/956081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenette JA, Dominelli PB, Reeve SS, Durkin CM, Eves ND, Sheel AW. Effect of thoracic gas compression and bronchodilation on the assessment of expiratory flow limitation during exercise in healthy humans. Respir Physiol Neurobiol 170: 279–286, 2010. doi: 10.1016/j.resp.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Guenette JA, Querido JS, Eves ND, Chua R, Sheel AW. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am J Physiol Regul Integr Comp Physiol 297: R166–R175, 2009. doi: 10.1152/ajpregu.00078.2009. [DOI] [PubMed] [Google Scholar]
- 21.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 581: 1309–1322, 2007. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacNutt MJ, De Souza MJ, Tomczak SE, Homer JL, Sheel AW. Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J Appl Physiol (1985) 112: 737–747, 2012. doi: 10.1152/japplphysiol.00727.2011. [DOI] [PubMed] [Google Scholar]
- 23.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol (1985) 84: 1872–1881, 1998. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- 24.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121: 339–342, 1980. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- 25.Molgat-Seon Y, Dominelli PB, Guenette JA, Sheel AW. Modelling the effects of age and sex on the resistive and viscoelastic components of the work of breathing during exercise. Exp Physiol 104: 1737–1745, 2019. doi: 10.1113/EP087956. [DOI] [PubMed] [Google Scholar]
- 26.Molgat-Seon Y, Dominelli PB, Ramsook AH, Schaeffer MR, Molgat Sereacki S, Foster GE, Romer LM, Road JD, Guenette JA, Sheel AW. The effects of age and sex on mechanical ventilatory constraint and dyspnea during exercise in healthy humans. J Appl Physiol (1985) 124: 1092–1106, 2018. doi: 10.1152/japplphysiol.00608.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molgat-Seon Y, Ramsook AH, Peters CM, Schaeffer MR, Dominelli PB, Romer LM, Road JD, Guenette JA, Sheel AW. Manipulation of mechanical ventilatory constraint during moderate intensity exercise does not influence dyspnoea in healthy older men and women. J Physiol 597: 1383–1399, 2019. doi: 10.1113/JP277476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol 2: 592–607, 1950. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- 29.Pellegrino R, Brusasco V, Rodarte JR, Babb TG. Expiratory flow limitation and regulation of end-expiratory lung volume during exercise. J Appl Physiol (1985) 74: 2552–2558, 1993. doi: 10.1152/jappl.1993.74.5.2552. [DOI] [PubMed] [Google Scholar]
- 30.Powers SK, Jacques M, Richard R, Beadle RE. Effects of breathing a normoxic He-O2 gas mixture on exercise tolerance and V̇O2max. Int J Sports Med 07: 217–221, 1986. doi: 10.1055/s-2008-1025762. [DOI] [PubMed] [Google Scholar]
- 31.Ripoll JG, Guo W, Andersen KJ, Baker SE, Wiggins CC, Shepherd JR, Carter RE, Welch BT, Joyner MJ, Dominelli PB. Sex differences in paediatric airway anatomy. Exp Physiol 105: 721–731, 2020. doi: 10.1113/EP088370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samee S, Altes T, Powers P, de Lange EE, Knight-Scott J, Rakes G, Mugler JP 3rd, Ciambotti JM, Alford BA, Brookeman JR, Platts-Mills TA. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol 111: 1205–1211, 2003. doi: 10.1067/mai.2003.1544. [DOI] [PubMed] [Google Scholar]
- 33.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol (1985) 107: 1622–1628, 2009. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres-Tamayo N, García-Martínez D, Lois Zlolniski S, Torres-Sánchez I, García-Río F, Bastir M. 3D analysis of sexual dimorphism in size, shape and breathing kinematics of human lungs. J Anat 232: 227–237, 2018. doi: 10.1111/joa.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanke T, Formanek D, Schenz G, Popp W, Gatol H, Zwick H. Mechanical load on the ventilatory muscles during an incremental cycle ergometer test. Eur Respir J 4: 385–392, 1991. [PubMed] [Google Scholar]
- 37.Wilkie SS, Dominelli PB, Sporer BC, Koehle MS, Sheel AW. Heliox breathing equally influences respiratory mechanics and cycling performance in trained males and females. J Appl Physiol (1985) 118: 255–264, 2015. doi: 10.1152/japplphysiol.00400.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson GD, Welch HG. Effects of varying concentrations of N2/O2 and He/O2 on exercise tolerance in man. Med Sci Sports Exerc 12: 380–384, 1980. doi: 10.1249/00005768-198025000-00015. [DOI] [PubMed] [Google Scholar]