Abstract
Objectives
This study investigated the relationship between body mass index (BMI) and metabolic syndrome on sperm DNA fragmentation (SDF) in males from infertile couples.
Methods
This cross-sectional study was performed from September 2018 to September 2019 at the Hue Center for Reproductive Endocrinology and Infertility (HUECREI), Vietnam. The study included men from couples with at least one year of infertility, who were subjected to semen analysis and SDF assay (Halosperm). We also performed a 2-h oral glucose tolerance test and measured lipidemia. Metabolic syndrome (MetS) was defined based on the NHLBI/AHA-ATP III guidelines.
Results
The mean age of the patients was 35.26 ± 5.87 years and 53.8% of them had a BMI ≥23.0 kg/m2. The DNA fragmentation index was significantly associated with overweight (p = 0.024). Men without MetS had a higher rate of big halos and a lower rate of small halos, no halos, and degraded semen compared to that in men with MetS, but the differences were not significant (p > 0.05). By performing multivariable analysis, we found that the SDF value was significantly different among the two groups with either overweight or normal weight.
Conclusion
In males from infertile couples with a relatively young mean age, BMI can be an independent indicator for SDF. MetS thus has a significant role in the development of sperm DNA fragmentation, at least in overweight individuals; it should thus be assessed under the scope of BMI, for better/earlier detection of increased SDF.
Keywords: Body mass index, Metabolic syndrome, Male infertility, DNA fragmentation
Highlights
-
•
Sperm DNA fragmentation recently appears to be a good marker for male reproductive potential.
-
•
BMI can be an independent indicator for increasing SDF.
-
•
MetS has a significant role in the development of SDF, at least in overweight individuals.
1. Introduction
Infertility affects 10–15% of couples in reproductive age worldwide [1]. The male factor is present in 20–50% of these couples, either independently or in combination with the female factor [2]. In recent years, emerging evidence of the effect of sperm DNA integrity on the reproductive outcome and the development of sperm DNA fragmentation (SDF) assays has opened a new clinical approach in the field. Higher levels of SDF can be found in infertile couples, irrespective of other semen parameters [3]. Furthermore, high SDF results are associated with a longer time to achieve a natural pregnancy compared to low SDF [4]. Thus, it is now clear that SDF plays an important role in predicting male reproductive outcomes.
Several external, post-testicular, and intra-testicular factors have been correlated with increased levels of male sperm DNA damage (e.g., diabetes, varicocele, spinal cord injury, cancer and chemotherapy, infections, age, lifestyle, and high temperature) [5]. The effect of metabolic syndrome (MetS) on semen quality, particularly on sperm DNA integrity, is supported by limited data. Pearce et al. were the first to suggest a link between MetS-induced obesity, increased intestinal permeability, and endotoxin exposure, and oxidative stress-mediated sperm DNA damage [6].
MetS, is defined by several guidelines. The National Cholesterol Education Program’s Adult Treatment Panel III (ATP III), includes the following parameters: increased waist circumference, high triglyceride levels, decreased HDL, high blood pressure, and elevated plasma glucose [7]. The new International Diabetes Federation definition includes central obesity (high WC), plus any two of the following four factors: raised triglycerides, reduced HDL cholesterol, raised blood pressure, and raised fasting plasma glucose [8]. At present, insulin resistance is not required for diagnosing MetS [9]. Because MetS is a cluster of different disorders and not a single disease, multiple concurrent definitions have been introduced. Notably, the requirement of central obesity plays a key feature in defining MetS. MetS might affect male semen parameters [10,11] and induce low testosterone levels [10]. Obesity and overweight can lead to hypogonadism, impaired spermatogenesis, increased scrotal temperatures, and increased sperm DNA damage. Moreover, dyslipidemia can increase oxidative stress in the testicular microenvironment and ductal system, which could further decrease fertility [12]. Futher studies are thus needed to elucidate the relationship between MetS and male infertility. This study aimed to determine the impact of body mass index (BMI) and MetS on SDF in male partners from infertile couples.
2. Methods
2.1. Study design
This cross-sectional study was performed at the Hue Center for Reproductive Endocrinology and Infertility (HUECREI), Hue University Hospital, Vietnam from September 2018 to September 2019. Men from infertile couples (at least 1 year of unsuccessful conception) were recruited. The sample size was calculated for the rate estimate investigation: . With the prevalence of abnormal DFI in men from infertile couples p = 8.8% [13], Δ = 0.04, α = 0.05, and , the minimum simple size was estimated to be 193 men. A total of 290 men were enrolled as the study population during the recruitment period.
The selection criteria in this study include men who were diagnosed with infertility according to WHO standards, eligible for the halosperm test and having enough required information regarding anthropometry and biochemical assays. Patients with acute systemic diseases, acute urinary tract infection, hepatic function disorders, malignant diseases, retrograde ejaculation, or azoospermia were excluded from the study. We recorded the following general characteristics for all patients: age, geography, education, occupation, clinical history, and physical examinations such as infertility type, infertility duration, a history of mumps, a history of surgery of the reproductive urinary tract, and chronic diseases such as hypertension, diabetes, osteoarthritis, cardiac or pulmonary disorders, and so on. The components of metabolic syndrome including waist circumference, blood pressure, fasting plasma glucose, and blood lipid were tested and the ATP III criteria were used to diagnose patients with MetS. Semen parameters were determined and the results of SDF assay by were obtained using the Halo sperm test. The study was approved by the Hue University of Medicine and Pharmacy Ethics Committee with approval number H2019/436.
2.2. Anthropometry
We measured the height and weight of all patients. Body mass index (BMI) was calculated as weight (kilograms) divided by the square of height (in meters). According to the Asian classifications for BMI, patients were categorized as obese (≥25 kg/m2), overweight (23.0–24.9 kg/m2), normal (18.5–22.9 kg/m2), and underweight (<18.5 kg/m2). Hip circumference was measured at the level of the pubic symphysis. Waist circumference (WC) was defined by measuring the perimeter at the level of the umbilicus, recorded at the end of the expiration. Abdominal obesity was determined as a WC ≥ 90 cm and a waist-to-hip ratio (WHR) of 0.9. Blood pressure (BP) was measured in the sitting position after a 5-min rest.
2.3. Biochemical assays
Fasting glucose levels, oral glucose tolerance test (OGTT) results, total cholesterol, triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were obtained using a Roche/Hitachi Cobas system (Module COBAS 4000/6000, Roche Diagnostics, Indianapolis, IN, USA).
2.4. Definition of metabolic syndrome
Metabolic syndrome was diagnosed by following the National Heart, Lung, and Blood Institute/American Heart Association (NHLBI/AHA) ATP III guidelines [7]. When a subject fulfilled at least three of the following five criteria, MetS was diagnosed: (1) WC ≥ 90 cm, (2) TG ≥ 1.7 mmol/L, (3) HDL-C < 1.03 mmol/L, (4) BP ≥ 130/85 mmHg, and (5) fasting glucose ≥ 5.6 mmol/L.
2.5. Semen analyses
Semen quality was evaluated according to the WHO 2010 standards [14]. After 3–5 days of ejaculatory abstinence, the semen sample was collected by masturbation. The samples were analyzed within 1 h after collection, after liquefaction. The following parameters were evaluated: color, volume, pH, liquefaction time, total count, concentration, progressive motility, morphology, and leukocytes. According to the WHO 2010 criteria, patients were classified as having normal semen parameters if the volume was ≥1.5 ml; the progressive motility rate was ≥32%; the sperm concentration was ≥15 million spermatozoa per ml; and the sperm morphology was ≥4% [14].
2.6. Sperm DNA fragmentation assay
The Halosperm kit from Halotech DNA, S.L (Spain) was used to evaluate SDF [15]. After being collected in clean containers, samples were diluted to a concentration of 5–10 million spermatozoa/ml. A total of 25 μL of the semen sample was added to an agarose Eppendorf and mixed. The cell suspension from the agarose Eppendorf (SCS) was then placed onto the treated side of a microscopy slide and covered with a glass coverslip. The slide was then placed on a cold surface and stored in a refrigerator at 4 °C for 5 min. After removing the slide cover, the slide was immersed immediately into a previously prepared DA solution (80 μL of HCL in 10 μL distilled water) in a horizontal position and incubated for 7 min. Next, the slide was incubated for 25 min in another tray containing 10 ml of tempered lysis solution (LS). The slide was then rinsed to remove the LS and consecutively placed in a tray with 70% ethanol (2 min), 90% ethanol (2 min), and 100% ethanol (2 min). The slide was then left to dry and observed under a fluorescence microscope. Spermatozoa with fragmented DNA showed halos of dispersed DNA, which were classified as small (sh) or no halo (wh), whereas sperm nuclei with intact DNA either presented a large (bh) or medium (mh) halo. Fragmented spermatozoa were also classified as degraded (d). Fernandez’s criteria were used to classify the different halo sizes [15]. Each slide was screened for 500 sperms and the total of small halo, no halo, degenerated sperm was scored as the DNA fragmentation index (DFI) for each patient.
2.7. Statistical analysis
All analyses were performed using SPSS software, version 20.0 (IBM Co., Armonk, NY, USA). Independent-samples Student’s t-test was used to compare continuous variables among the different groups, while categorical variables were evaluated with the chi-square test. Multivariable analysis was performed to test the association of anthropometric data with DFI in patients with MetS. Results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs) and 2-sided P-values. A P value lower than 0.05 indicated statistical significance.
3. Results
In our cohort, the mean age was 35.26 ± 5.87 years as shown in Table 1. Most men had primary infertility (64.5%) and had never smoked before (60.7%). The mean infertility duration was 4.14 ± 2.87 years. Approximately, one third of the men (31.4%) had a BMI ≥ of 25.0 kg/m2. Moreover, the rate of infertile men not having a history of chronic diseases was 82.8%, while 47.2% did not consume alcohol regularly.
Table 1.
Baseline characteristic of male in infertile couples.
| Characteristics | Number | Percent (%) |
|---|---|---|
| Age (years) | ||
| Mean (IQR): 35.26 ± 5.87 (24–55) | ||
| <35 | 145 | 50.0 |
| ≥35 |
145 |
50.0 |
| Infertile types | ||
| Primary Infertility | 187 | 64.5 |
| Secondary Infertility |
103 |
35.5 |
| Infertile durations (years) | ||
| Mean (range): 4.14 ± 2.87 (1–17) | ||
| <3 | 100 | 34.5 |
| ≥3 |
190 |
65.5 |
| Body mass index (kg/m2) | ||
| Mean ± SD: 23.54 ± 2.97 | ||
| <18.5 | 5 | 1.7 |
| 18.5–<23.0 | 129 | 44.5 |
| 23–<25.0 | 65 | 22.4 |
| ≥25.0 |
91 |
31.4 |
| Chronic diseases | ||
| Yes | 50 | 17.2 |
| No | 240 | 82.8 |
| Alcohol consumption | 137 | 47.2 |
| Smoking | 114 | 39.3 |
It has been shown in Table 2 that the DFI had significant positive relation with BMI (p = 0.024). The incidence of men with DFI <30 in the normal weight group (defined as a BMI < 23) was 81.34%, whereas this value in the overweight group (BMI ≥ 23) was just 69.87%. A total of 30.13% of overweight men had a DFI ≥30, whereas only 18.66% of normal-weight men presented this condition. However, we found no significant relationship between DFI and metabolic syndrome (p = 0.208).
Table 2.
Association between DNA fragmentation, metabolic syndrome and body mass index.
| Factors | Total (n = 290) | DFI ≥ 30 | DFI < 30 | P value∗ |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| <23 | 134 (46.2) | 25 (18.7) | 109 (81.3) | 0.024 |
| ≥23 |
156 (53.8) |
47 (30.1) |
109 (69.9) |
|
| Metabolic syndrome | ||||
| Yes | 65 (22.4) | 20 (30.8) | 45 (69.2) | 0.208 |
| No | 225 (77.6) | 52 (23.1) | 173 (76.9) | |
BMI: Body Mass Index; DFI: DNA fragmentation index; ∗P – bivariate (not adjusted).
Table 3 shows the metabolic indicators profile, sperm parameters, and SDF data. The sample mean waist circumference was 83.41 ± 8.24 cm. Triglyceride, HDL, and fasting glucose levels were 2.44 ± 1.63 mmol/L, 1.24 ± 0.40 mmol/L, and 5.60 ± 1.08 mmol/L, respectively. Regarding semen parameters, the mean semen concentration, rate of progressive motility, and the percentage of normal morphology sperm were 32.55 ± 13.69 million/ml, 30.85 ± 13.21%, and 4.03 ± 2.42%, respectively. Regarding SDF, the big, medium, and small halo values were 34.15 ± 18.86%, 43.23 ± 17.89%, and 7.93 ± 8.48%, respectively, while the degraded sperm percentage and the mean DFI value were 4.52 ± 3.54% and 22.60 ± 17.68%, respectively. The table shows that the overweight patients have worse results of metabolic indicators and sperm DNA fragmentation (waist, DBP, triglycerides, HDL, fasting glucose, degraded sperm, and DFI fragmentation; p < 0.05).
Table 3.
Profile of metabolic indicators, sperm parameters and sperm DNA fragmentation.
| Variables | BMI < 23 (n = 134) |
BMI ≥ 23 (n = 156) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | MS (n = 16) | Non-MS (n = 118) | p1 | Total | MS (n = 49) | Non-MS (n = 107) | p2 | p3 | |
| Metabolic indicators | |||||||||
| Waist | 78.75 ± 5.97 | 81.69 ± 6.47 | 78.36 ± 5.82 | 0.044 | 87.40 ± 7.82 | 92.45 ± 7.03 | 85.09 ± 7.06 | <0.001 | <0.001 |
| SBP (mmHg) | 113.84 ± 8.47 | 119.06 ± 12.94 | 113.14 ± 7.48 | 0.124 | 115.48 ± 12.07 | 120.82 ± 15.76 | 113.04 ± 9.03 | 0.0004 | 0.189 |
| DBP (mmHg) | 71.72 ± 5.95 | 75.0 ± 9.66 | 71.27 ± 5.16 | 0.116 | 73.40 ± 7.99 | 76.53 ± 10.11 | 71.96 ± 6.36 | 0.001 | 0.046 |
| Triglyceride (mmol/L) | 1.99 ± 1.57 | 3.97 ± 3.16 | 1.72 ± 0.96 | <0.001 | 2.82 ± 1.60 | 3.77 ± 1.57 | 2.39 ± 1.41 | <0.001 | <0.001 |
| HDL (mmol/L) | 1.30 ± 0.37 | 1.04 ± 0.27 | 1.34 ± 0.37 | 0.0002 | 1.19 ± 0.41 | 1.03 ± 0.23 | 1.26 ± 0.46 | 0.0001 | 0.002 |
| Fasting glucoses (mmol/L) |
5.46 ± 0.84 |
6.17 ± 0.42 |
5.36 ± 0.84 |
<0.001 |
5.73 ± 1.24 |
6.39 ± 1.64 |
5.42 ± 0.86 |
<0.001 |
0.034 |
| Sperm quality | |||||||||
| Concentration (mil/ml) | 32.28 ± 12.95 | 30.0 ± 13.56 | 32.59 ± 12.89 | 0.365 | 32.78 ± 14.33 | 32.24 ± 15.93 | 33.020 ± 13.61 | 0.792 | 0.761 |
| Progressive motility (%) | 30.75 ± 13.01 | 29.0 ± 13.54 | 30.99 ± 12.98 | 0.861 | 30.93 ± 13.43 | 30.18 ± 13.82 | 31.27 ± 13.30 | 0.714 | 0.910 |
| Viability (%) | 78.28 ± 10.35 | 79.88 ± 6.66 | 78.07 ± 10.76 | 0.481 | 77.82 ± 10.84 | 76.82 ± 12.43 | 78.28 ± 10.11 | 0.962 | 0.712 |
| Normal morphology (%) | 4.15 ± 2.49 | 3.81 ± 2.23 | 4.19 ± 2.53 | 0.775 | 3.93 ± 2.36 | 3.84 ± 2.50 | 3.98 ± 2.30 | 0.605 | 0.455 |
| Abnormal head (%) | 85.59 ± 4.81 | 85.94 ± 5.37 | 85.54 ± 4.75 | 0.764 | 85.53 ± 4.94 | 85.49 ± 5.54 | 85.55 ± 4.66 | 0.688 | 0.920 |
| Abnormal tail (%) |
61.90 ± 10.74 |
64.06 ± 8.20 |
61.60 ± 11.04 |
0.385 |
62.12 ± 10.62 |
60.45 ± 10.20 |
62.89 ± 10.76 |
0.160 |
0.857 |
| Sperm DNA fragmentation (%) | |||||||||
| Big halo | 36.26 ± 19.45 | 39.31 ± 22.2 | 35.84 ± 19.10 | 0.7315 | 32.33 ± 18.19 | 28.22 ± 19.16 | 34.21 ± 17.51 | 0.047 | 0.084 |
| Medium halo | 42.78 ± 18.57 | 42.19 ± 18.80 | 42.86 ± 18.61 | 0.9754 | 43.62 ± 17.34 | 43.44 ± 19.93 | 43.70 ± 16.11 | 0.992 | 0.564 |
| Small halo | 7.50 ± 8.92 | 6.65 ± 3.51 | 7.62 ± 9.42 | 0.249 | 8.29 ± 8.11 | 10.62 ± 10.09 | 7.23 ± 6.80 | 0.0155 | 0.112 |
| Without halo | 7.45 ± 9.49 | 5.88 ± 4.37 | 7.66 ± 9.98 | 0.997 | 8.10 ± 8.46 | 9.98 ± 11.67 | 7.23 ± 6.37 | 0.430 | 0.094 |
| Degradation |
6.01 ± 5.13 |
5.98 ± 5.04 |
6.02 ± 5.16 |
0.959 |
7.66 ± 7.69 |
7.74 ± 6.90 |
7.63 ± 8.06 |
0.808 |
0.038 |
| DFI fragmentation | 20.92 ± 17.92 | 18.5 ± 11.58 | 21.29 ± 18.61 | 0.561 | 24.05 ± 17.40 | 28.34 ± 21.91 | 22.09 ± 14.59 | 0.037 | 0.023 |
DPB: diastolic blood pressure; FSH: follicle-stimulating hormone; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SBP: systolic blood pressure; WC: waist circumference; WHR: waist-to-hip ratio. Data are presented as mean ± SD or number (%). ∗Comparison was performed between men with and without MS using the independent-samples t-test and Chi-square test.
The semen analysis data in men without MetS seemed to be better than that in men with MetS (Table 4); however, the observed differences were not significant (p > 0.05). Regarding SDF, patients without MetS had a higher rate of big halos compared with that in patients with MetS (35.07 ± 18.34 vs. 30.95 ± 20.38, p = 0.122). Further, the rates of small halos, no halos, degraded semen, and DFI in the group of men with MetS was higher than those in the group without MetS (25.92 ± 20.24 vs. 21.65 ± 16.80, 9.64 ± 9.07 vs. 7.43 ± 8.26, 8.97 ± 10.48 vs. 7.45 ± 8.44, and 7.30 ± 6.50 vs. 6.78 ± 6.73, respectively). However, the difference was also not significant for these parameters (p > 0.05). Table 5 presents the effects of metabolic syndrome on DNA fragmentation based on a multivariable analysis, adjustment with age, infertility type, and infertility duration in each body mass index group. The sperm DNA fragmentation index was significantly higher in the MetS group than the non-MetS group [Coef. 6.89 (95%CI: 1.01-12.78)] in overweight individuals. However, this difference was not significant in normal-weight patients (p = 0.376).
Table 4.
The association between sperm parameters and metabolic syndrome.
| Variables | Metabolic syndrome (n = 65) | Non – metabolic syndrome (n = 225) | P value∗ |
|---|---|---|---|
| Sperm quality | |||
| Concentration (mil/ml) | 31.69 ± 15.31 | 32.80 ± 13.21 | 0.568 |
| Progressive motility (%) | 29.89 ± 13.66 | 31.12 ± 13.10 | 0.509 |
| Viability (%) | 77.57 ± 11.32 | 78.17 ± 10.43 | 0.689 |
| Normal morphology (%) | 3.83 ± 2.42 | 4.09 ± 2.42 | 0.442 |
| Abnormal head (%) | 85.6 ± 5.46 | 85.55 ± 4.70 | 0.938 |
| Abnormal tail (%) |
61.34 ± 9.81 |
62.21 ± 10.90 |
0.561 |
| Sperm DNA fragmentation (%) | |||
| Big halo | 30.95 ± 20.38 | 35.07 ± 18.34 | 0.122 |
| Medium halo | 43.13 ± 19.52 | 43.26 ± 17.43 | 0.958 |
| Small halo | 9.64 ± 9.07 | 7.43 ± 8.26 | 0.064 |
| Without halo | 8.97 ± 10.48 | 7.45 ± 8.44 | 0.229 |
| Degradation | 7.30 ± 6.50 | 6.78 ± 6.73 | 0.580 |
| DFI fragmentation | 25.92 ± 20.24 | 21.65 ± 16.80 | 0.086 |
DFI: DNA fragmentation index; P – bivariate (not adjustment).
Table 5.
Multivariate analysis of DNA fragmentation and metabolic syndrome group.
| Variable |
BMI <23 |
BMI ≥23 |
||||
|---|---|---|---|---|---|---|
| Coef. |
95% CI |
p |
Coef. |
95% CI |
p |
|
| Age (years) | 0.40 | −0.19-0.99 | 0.184 | −0.235 | −0.73-0.26 | 0.349 |
| Metabolic syndrome | ||||||
| None | Ref | ref | ||||
| Yes |
−4.49 |
−14.50-5.52 |
0.376 |
6.89 |
1.01–12.78 |
0.022 |
| Infertile type | ||||||
| Primary | ref | ref | ||||
| Secondary |
−0.51 |
−7.46-6.43 |
0.884 |
−2.14 |
0.54–1.83 |
0.990 |
| Infertile duration (years) | −1.22 | −1.13-0.89 | 0.811 | −0.91 | −2.08-0.27 | 0.131 |
BMI: Body Mass Index.
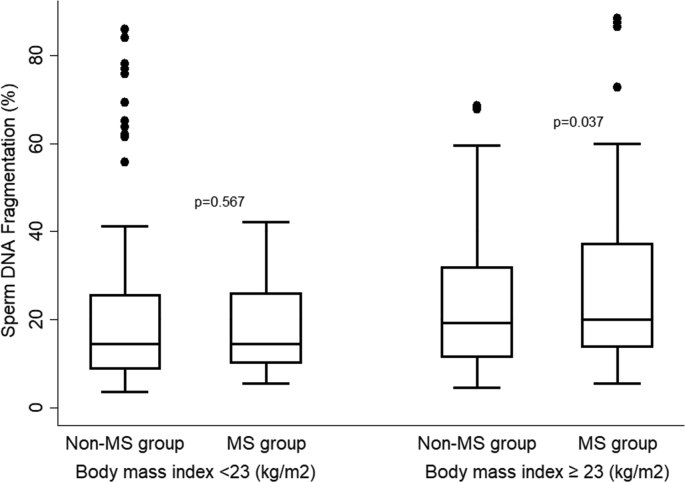
The data from Fig. 1 indicate that overweight patients with metabolic syndrome have a higher level of sperm DNA fragmentation (DFI %) (p = 0.037), however, this trend was not observed in normal weight patients with metabolic syndrome (p = 0.567).
Fig. 1.
Bivariate analysis between SDF and metabolic syndrome by BMI.
4. Discussion
Although studies in the past decade have confirmed a relationship between MetS and lower levels of total testosterone, sex hormone-binding globulin, and dehydroepiandrosterone sulfate (DHEA-S) [16], the impact of MetS or detailed components such as dyslipidemia, obesity, and glucose tolerance on the quality of semen, especially SDF, remains unclear. This study aimed to investigate the relationship between body mass index (BMI) and metabolic syndrome on sperm DNA fragmentation (SDF). Our data from males in infertile couples with relatively young mean age suggested that BMI but not MetS is correlated with an increased SDF. Insulin resistance and diabetes seem to have a negative effect on sperm quantity and quality. Reportedly, diabetic patients have lower semen volumes compared to those in control patients [16]. Moreover, recent studies have reported that diabetes can lead to erectile dysfunction and ejaculatory dysfunction [17,18]. A prospective cohort study conducted in 2014 found that increase in free cholesterol and phospholipid levels was associated with a decrease in sperm head size and the proportion of sperms with an intact acrosome [19].
It has been suggested that an excess of adipose tissue leads to the conversion of testosterone to estrogen, consequently inhibiting the hypothalamic-pituitary-gonadal axis, which has a negative effect on spermatogenesis [12]. An increased BMI correlates with lower sperm concentration and pregnancy rates in assisted reproductive technology (ART) cycles [20]. In fact, high testosterone levels in obese men result in increased conversion of androgens to estrogen, thus causing oxidative stress and dysregulation of the pituitary-hypothalamus, which may contribute to increase sperm DNA damage [21]. Thus, there was a correlation between WC and low total sperm count as well as low sperm concentration [21], A meta-analysis in 2013 of 21 studies found that the OR for azoospermia/oligospermia in overweight, obese, and morbidly obese men was 1.11, 1.28, and 2.04, respectively [22]. Furthermore, suprapubic lipectomy in infertile men showed potential to improve semen quality and pregnancy outcomes [23]. Our study found no significant difference in semen parameters related to BMI, this may result from a younger mean age and a lower BMI in the study population with a mean BMI of 23.54 ± 2.97 kg/m2. Stone et al. concluded a negative effect on semen quality and successful pregnancy rate following intercourse declines in men older than 34 years of age [24]. Importantly, our data showed that overweight patients have worse results of sperm DNA fragmentation with significant differences. These data impressively confirmed the impact of BMI on sperm quality using a better indicator of male fertility.
By examining the semen parameters of patients who met the criteria for MetS, a previous study showed that men who met more than three MetS criteria had a lower percentage of sperm with normal morphology [25]. Similarly, Ventimiglia et al. also showed that patients with MetS had a lower semen volume, sperm concentration, normal sperm morphology, and total progressive motility values compared to those of control patients [10].
However, our study is not in agreement with those studies, as we also found that the semen parameter differences between the MetS group and the non-MetS group were not significant. The discrepancy in these studies might be explained by the different cohorts studied in terms of ethnicity, age, and other baseline characteristics. The older age of the patients might have contributed to the incidence of MetS [26], whereas our study population was quite young with a mean age of 35.26 ± 5.87. Moreover, the waist circumference threshold of the Asian population criteria applied in this study was 90 cm, but not 102 cm as in other studies based on international criteria. Notably, we found that the SDF value was significantly different between the group with and without MetS (p = 0.037) in overweight patients. This main finding can suggest the contribution of MetS on sperm quality in some ways, even though the difference is still insignificant in normal weight patients (p = 0.567). Obesity was supposed to affect male fertility by reducing sperm quality as well as by changing the physical and molecular structure of germ cells in the testes and affecting the maturation of sperm cells [21]. Therefore, MetS should not be assessed alone but should be considered in the context of BMI as a better/earlier indicator of sperm quality assessed by both semen analysis and SDF. Recent studies have reported that dyslipidemia has negative effects on male reproductive capacity, by increasing the levels of reactive oxygen species at the molecular level. A study in 2010 using a neutral comet assay to assess sperm DNA integrity concluded that obese men, but not overweight men, had higher levels of DNA damage [27]. Moreover, Kort et al. performed a sperm structure chromatin assay and found that overweight and obese men had significantly higher rates of sperm with fragmented DNA compared with those in men from the normal group [28]. Another study, measuring 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels to evaluate seminal oxidative stress, reported that SDF was positively associated with all measures of adiposity (BMI, body fat, and waist circumference) [6]. While the latest studies have mainly focused on the relationship between SDF and obesity, data evaluating the effect of MetS on DNA fragmentation are still limited. Our study presents preliminary data to assess the impact of MetS on reproductive capacity, by measuring DNA fragmentation levels. Additional studies are needed to confirm our results on different populations using various assays.
A significant difference in SDF between the groups with and without MetS in overweight patients, but not in normal weight patients as revealed by our data, suggests a potential effect of MetS on sperm DNA fragmentation. Potentially the negative correlation of each phenotype of MetS with Sperm DNA fragmentation might have resulted from the insufficient power of the study or the limited number of individuals recruited. Furthermore, different studies have shown that DNA fragmentation evaluated by the sperm chromatin dispersion (SCD) technique, such as the Halosperm assay used in this study, is a good parameter for predicting the reproductive capacity in men [13,29]. However, there is currently no unified classification matrix among the different SCD assays available. While the diameter of the halo is a continuous variable, different halo dimension categories are discrete variables. Thus, SCD assays are not sufficient to propose a gold standard at present. This is a relative limitation of the present study.
In conclusion, we found that in males from infertile couples with relatively young mean age, BMI can be an independent indicator for increasing SDF. The MetS has a significant role in the development of sperm DNA fragmentation, at least in overweight individuals and should thus be assessed under the scope of BMI, for better/earlier detection of increased SDF. Further studies with men of advanced age and with a larger sample size should be carried out to accurately determine the impact of MetS on sperm quality.
Ethics approval and consent to participate
This study was approved by the Hue University of Medicine and Pharmacy Ethics Committee with approval number H2019/436.
Availability of data and material
The dataset used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Minh Tam Le: Supervision, Conceptualization, Methodology, Formal analysis, Writing, Original draft preparation, Reviewing and Editing, All authors contributed to the interpretation of the data and approved the final manuscript. Dac Nguyen Nguyen: Conceptualization, Methodology, Formal analysis, Writing, Original draft preparation, Reviewing and Editing, All authors contributed to the interpretation of the data and approved the final manuscript. Dinh Duong Le: Data curation, All authors contributed to the interpretation of the data and approved the final manuscript. Nhu Quynh Thi Tran: Data curation, All authors contributed to the interpretation of the data and approved the final manuscript.
Declaration of competing interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
This work was supported by a research grant from Vietnam Ministry of Education and Training (grant number DHH2018-04-78). The grantor had no influence in the content of the publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100054c
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mascarenhas M.N., Flaxman S.R., Boerma T., Vanderpoel S., Stevens G.A. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12) doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigman M., Jarow J. Male infertility. In: Walsh, editor. Campbell’s urology. eighth ed. Saunders; Philadelphia: 2002. pp. 1475–1531. [Google Scholar]
- 3.Saleh R.A., Agarwal A., Nelson D.R., Nada E.A., El-Tonsy M.H., Alvarez J.G. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78:313–318. doi: 10.1016/s0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 4.Spanò M., Bonde J.P., Hjøllund H.I., Kolstad H.A., Cordelli E., Leter G. Sperm chromatin damage impairs human fertility. The Danish first pregnancy planner study team. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 5.Pourmasumi S., Sabeti P., Rahiminia T., Mangoli E., Tabibnejad N., Reza Talebi A. The etiologies of sperm DNA abnormalities in male infertility: an assessment and review. Int J Reprod Biomed. 2017;15(6):331–344. [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce K.L., Hill A., Tremellen K.P. Obesity related metabolic endotoxemia is associated with oxidative stress and impaired sperm DNA integrity. Basic Clin Androl. 2019;29(1):6. doi: 10.1186/s12610-019-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A. Diagnosis and management of the metabolic syndrome: an American Heart association/national Heart, Lung, and blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Alberti G., Zimmet P., Shaw J., Grundy S.M. The IDF consensus worldwide de nition of the metabolic syndromeMetabolic syndrome. 2006. https://www.idf.org/component/attachments/attachments.html?id=705&task=download Available at:
- 9.Einhorn D., Reaven G.M., Cobin R.H., Ford E., Ganda O.P., Handelsman Y. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 10.Ventimiglia E., Capogrosso P., Colicchia M., Boeri L., Serino A., Castagna G. Metabolic syndrome in white European men presenting for secondary couple’s infertility: investigation of the clinical and reproductive burden. Int J Androl. 2016;4(5):944–951. doi: 10.1111/andr.12232. [DOI] [PubMed] [Google Scholar]
- 11.Elsamanoudy A.Z., Abdalla H.A., Hassanien M., Gaballah M.A. Spermatozoal cell death-inducing DNA fragmentation factor-α-like effector A (CIDEA) gene expression and DNA fragmentation in infertile men with metabolic syndrome and normal seminogram. Diabetol Metab Syndrome. 2016;8(1):1–10. doi: 10.1186/s13098-016-0192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasturi S.S., Tannir J., Brannigan R.E. The metabolic syndrome and male infertility. J Androl. 2008;29(3):251–259. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- 13.Borges E., Jr., Zanetti B.F., Setti A.S., Braga D.P.A.F., Provenza R.R., Iaconelli A., Jr. Sperm DNA fragmentation is correlated with poor embryo development, lower implantation rate, and higher miscarriage rate in reproductive cycles of non-male factor infertility. Fertil Steril. 2019;112(3):483–490. doi: 10.1016/j.fertnstert.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 15.Fernández J.L., Muriel L., Goyanes V., Segrelles E., Gosalvez J., Enciso M. Halosperm is an easy, available, and cost-effective alternative for determining sperm DNA fragmentation. Fertil Steril. 2005;84:860. doi: 10.1016/j.fertnstert.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Agbaje I.M., Rogers D.A., McVicar C.M., McClure N., Atkinson A.B., Mallidis C. Insulin dependent diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22(7) doi: 10.1093/humrep/dem077. 1871e7. [DOI] [PubMed] [Google Scholar]
- 17.Ponholzer A., Temml C., Mock K., Marszalek M., Obermayr R., Madersbacher S. Prevalence and risk factors for erectile dysfunction in 2869 men using a validated questionnaire. Eur Urol. 2005;47(1):80e5. doi: 10.1016/j.eururo.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Fedder J., Kaspersen M.D., Brandslund I., Højgaard A. Retrograde ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Int J Androl. 2013;1(4):602e6. doi: 10.1111/j.2047-2927.2013.00083.x. [DOI] [PubMed] [Google Scholar]
- 19.Schisterman E.F., Mumford S.L., Chen Z., Browne R.W., Boyd Barr D., Kim S. Lipid concentrations and semen quality: the LIFE study. Int J Androl. 2014;2(3) doi: 10.1111/j.2047-2927.2014.00198.x. 408e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakos H.W., Henshaw R.C., Mitchell M., Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril. 2011;95(5):1700e4. doi: 10.1016/j.fertnstert.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg M.L., Kim S., Chen Z., Sundaram R., Schisterman E.F., Buck Louis G.M. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29(2) doi: 10.1093/humrep/det428. 193e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sermondade N., Faure C., Fezeu L., Shayeb A.G., Bonde J.P., Jensen T.K. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19(3) doi: 10.1093/humupd/dms050. 221e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafik A., Olfat S. Lipectomy in the treatment of scrotal lipomatosis. Br J Urol. 1981;53(1):55e61. doi: 10.1111/j.1464-410x.1981.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 24.Stone B.A., Alex A., Werlin L.B., Marrs R.P. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100:952–958. doi: 10.1016/j.fertnstert.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 25.Lotti F., Corona G., Degli Innocenti S., Filimberti E., Scognamiglio V., Vignozzi L. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Int J Androl. 2013;1(2) doi: 10.1111/j.2047-2927.2012.00031.x. 229e39. [DOI] [PubMed] [Google Scholar]
- 26.Scuteri A., Laurent S., Cucca F. The metabolic syndrome across Europe – different clusters of risk factors. Eur J Cardiovasc Prev Rehabil. 2015;22(4):486–491. doi: 10.1177/2047487314525529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavarro J.E., Toth T.L., Wright D.L., Meeker J.D., Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93(7):2222–2231. doi: 10.1016/j.fertnstert.2009.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kort H.I., Massey J.B., Elsner C.W., Mitchell-Leef D., Shapiro D.B., Witt M.A. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–452. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 29.Desai N., Abdel Hafez F., Goldberg E., Chase R., Karode M. Evaluation of sperm DNA fragmentation using the Halo sperm kit. Fertil Steril. 2009;92:S139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author upon reasonable request.