Abstract
Introduction
Furcation involvement (FI) in multi-rooted teeth is challenging for proper oral hygiene, clinical treatment, and leads to poor prognosis. Traditional treatment modalities often result in sacrificing periodontal bone. Multiple regenerative approaches have been attempted to treat furcation defects, but complete regeneration of the periodontal apparatus in grade III furcation has not been reported. Platelet rich fibrin (PRF) shows great potential in enhancing tissue regeneration, angiogenesis, and prevention of infection. This case report introduces a treatment combining allogenic bone grafts with PRF to treat mandibular grade III furcation lesions with a one-year follow-up.
Case presentation
Two patients presented with grade III FIs of the mandibular first molars, with intrabony defects requiring guided tooth regeneration (GTR). PRF was collected from each patient to serve as biologics, by mixing with allogenic bone graft, and packed into the furcation and intrabony defects. The PRF membranes were also used for space maintenance. The twelve-month postoperative follow-up demonstrated quicker tissue healing, significant pocket reduction, clinical attachment gain, as well as radiographic bone fill in both cases.
Conclusion
Successful periodontal regeneration of grade III furcation defects can be achieved by using PRF in combination with bone allograft.
Keywords: Platelet-rich fibrin (PRF), Grade III furcation, Guided tissue regeneration (GTR), Allograft, Intrabony defect
1. Introduction
Furcation involvement (FI) represents a formidable problem in periodontal therapy. This is due to the anatomic complexity and irregularity,1 which increases susceptibility to bacterial retention and impedes accessibility for oral hygiene and periodontal debridement.
The FI classifications are based on the extension and degree of a horizontal/vertical defect into the furcation area of a multi-rooted tooth.2,3 Grade III furcation is a through-and-through lesion. Teeth with a grade III FI is one of the most difficult periodontal lesions to treat and have a poorer prognosis.4 They require more extensive resective therapy such as tunneling, root amputation or hemisection for elimination of the lesion and proper infection control.1 Recently, multiple regenerative attempts have been used to close the furcation defects, including bone grafts, guided tissue regeneration (GTR), application of growth factors, and enamel matrix derivatives, and bone morphogenetic proteins.5 Variable clinical outcomes have been reported with these techniques. But complete regeneration of the periodontal attachment apparatus, including bone, periodontal ligament, and cementum in grade III furcation, has not been reported.
Platelet rich fibrin (PRF), developed by Choukroun et al. is a second-generation platelet concentrate of autologous fibrin matrix enriched with leukocytes, platelets and growth factors from an anticoagulant-free blood harvest. It has been reported to facilitate tissue regeneration, angiogenesis, and prevention of infection.6, 7, 8 The combination of PRF with bone graft suggests a better promise in furcation resolution.9,10 The present case report describes the treatment of grade III furcation lesions using allografts and PRF with one-year follow-up.
2. Materials and methods
2.1. Clinical presentation
Case 1
A healthy, 56-year-old, Caucasian female presented with a chief complaint of being unable to chew efficiently and desiring dental implants to replace her missing teeth. She reported a non-contributory medical and social history. At the baseline examination, the patient presented partially edentulism with teeth #s 2–5, 14, 30 and 31 being extracted due to non-restorability. Grade III and grade II FIs were found on tooth #19 and #18 respectively, with #19 having pocket depths ranging from 3 to 7 mm (Table 1). Radiographic examination and clinical presentation were shown in Fig. 2.
Case 2
A healthy, 34-year-old, Indian American female was referred after complaining of persistent tooth sensitivity in the posterior lower right sextant for over a year. Comprehensive examination revealed fair oral hygiene, Gingival index of 38%, and generalized probing depths of 2–4 mm. The right mandibular first molar (tooth #30), had vertical bone loss with a 9–11 mm probing depth at the distal, grade III FI, and Class I mobility (Table 1). Radiographic examination and clinical presentation were shown in Fig. 3.
Table 1.
Baseline and 12-month clinical measurements of tooth #19 and tooth #30 from the patients in Case 1 and 2, respectively.
|
Case 1 (Tooth #19) |
Case 2 (Tooth #30) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Surgical |
12-month PO |
Pre-Surgical |
12-month PO |
||||||||||
| M | MB/ML | D | M | MB/ML | D | M | MB/ML | D | M | MB/ML | D | ||
| Buccal | PPD (mm) | 3 | 6 | 5 | 3 | 4 | 4 | 4 | 7 | 10 | 4 | 5 | 5 |
| CAL (mm) | 0 | 3 | 2 | 0 | 1 | 1 | 0 | 4 | 7 | 0 | 1 | 2 | |
| Recession (mm) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| BOP | ++ | ||||||||||||
| Furcation | III | I | III | I | |||||||||
| Lingual | PPD (mm) | 3 | 7 | 5 | 3 | 4 | 3 | 4 | 9 | 9 | 4 | 5 | 4 |
| CAL (mm) | 0 | 4 | 2 | 0 | 2 | 1 | 0 | 6 | 6 | 0 | 2 | 2 | |
| Recession (mm) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| BOP | ++ | +++ | + | ||||||||||
| Furcation | III | I | III | I | |||||||||
| Mobility | N/A | N/A | II | N/A | |||||||||
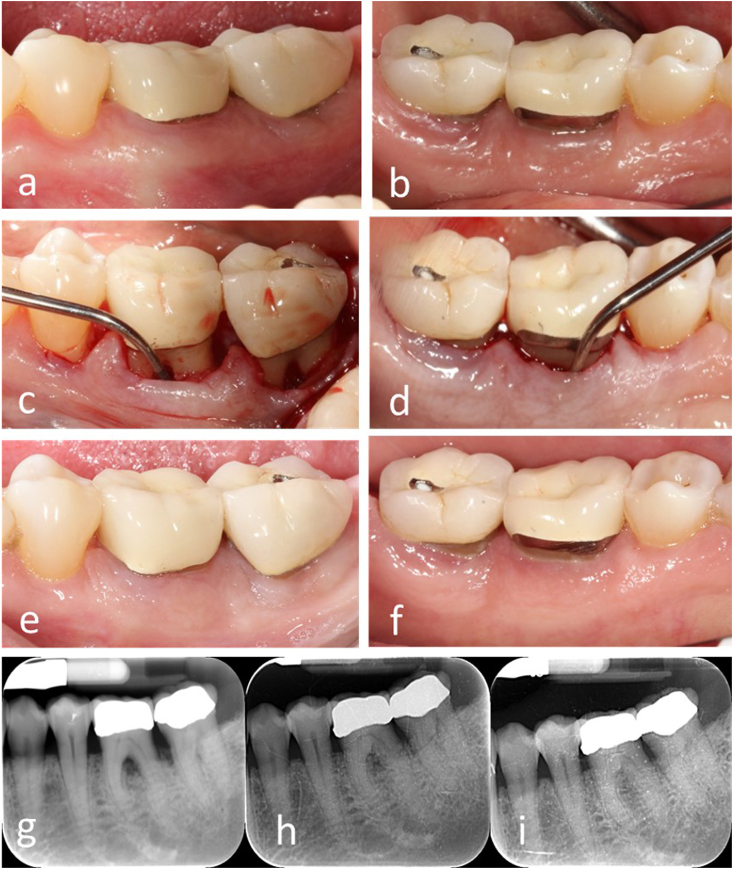
Fig. 2.
Case 1. a-b), Pre-operative intraoral view of the mandibular left posterior sextant. c-d), Buccal and lingual grade III furcation were defected using Naber's probe to show deep infrabony bone loss. e-f), 1-year post-operative intraoral photograph. g-i), Pre-operative and 6-month, 1-year post-operative periapical radiographs.
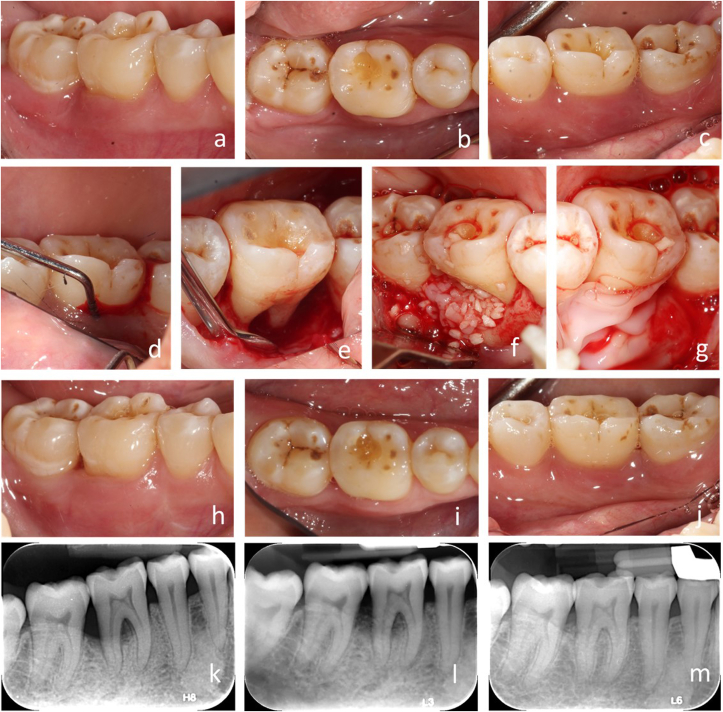
Fig. 3.
Case 2. a-c), Pre-operative intraoral view of the mandibular right posterior sextant. d), Pre-operative intraoral photograph, lingual view of furcation defect showing ML deep pocket and grade III furcation involvement of tooth #30. e) Clinical lingual view showing horizontal and vertical bone loss of tooth #30. f), Application of grafting material to the buccal surface of bony defect. g), Application of harvested PRF membrane to cover the grafted defect. h-j) 1-year post-operative intraoral photographs, facial, occlusal and lingual view. k), Periapical radiograph showing status of #30 bone defect pre-operatively. l-m), Periapical radiograph showing the status of #30 bone defects before surgery, 6-month and 1-year post-operatively.
2.2. Case management
Both patients received oral hygiene instruction, scaling and root planning, selective grinding and occlusal adjustment to reduce occlusal trauma. All possible treatment options were presented to both patients before surgery. Informed consents for treatment were obtained.
To prepare PRF, 4 tubes of 10 ml fresh intravenous blood were drawn from the anticubital fossa of each patient. Samples were immediately centrifuged at 3000 rpm (approximately 400 g) for 10 min using the PRF centrifuge (Boca Dental Supply, FL, USA). Each PRF clot was obtained by removing the red blood cells at the bottom and acellular plasma at the top.7 The PRF clots were prepared as membrane or chopped to mix with bone graft materials. PRF exudate was collected for hydration of bone graft.
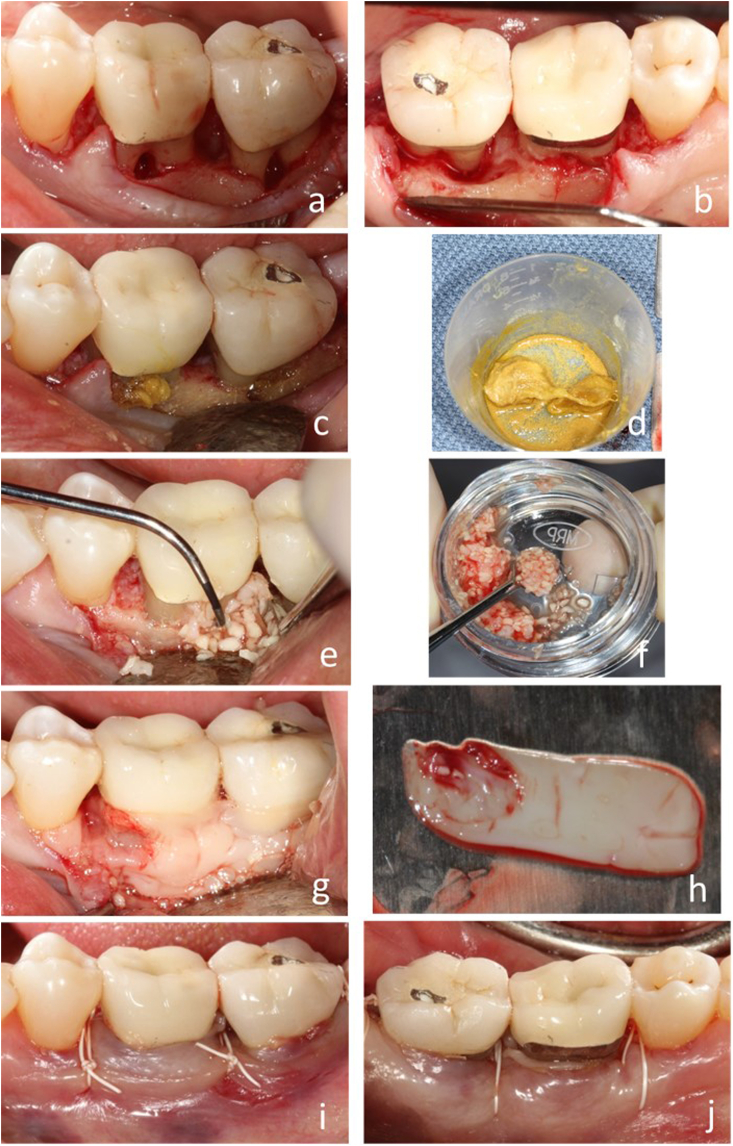
The surgical procedures were demonstrated in Fig. 1. During the surgery, full thickness flaps were elevated with sulcular incisions one tooth mesial and distal to the surgical sites under local anesthesia (Fig. 1, a-b). Granulation tissue was removed, and thorough scaling and root planning were performed. The roots of the affected teeth were treated with 100 mg/ml of Tetracycline hydrochloride (Fig. 1, c-d). Allogenic demineralized cortical particulate bone graft (OraGRAFT, LifeNet Health, VA, USA) was hydrated with PRF exudate. Chopped PRF and bone graft mixtures were packed into the furcation area and intrabony defect (Fig. 1, e-f). The grafts were covered with PRF membrane wrapping the roots (Fig. 1, g-h). Horizontal mattress sutures (4-O non-resorbable PTFE suture from Osteogenics Biomedical, TX, USA) were used to coronally advance the flap and multiple single interrupted sutures used for primary closure (Fig. 1i–j).
Fig. 1.
Surgical procedure. a-b), Sulcular incision exposed the furcation defect showing horizontal and vertical bone loss of involved teeth. c-d) Decontamination of root with Tetracycline. Harvested PRFs (h) were mixed with particulate allogenic bone (f) grafted in the defect (e), and PRF membrane covered the site (g). i-j), Buccal and lingual views of coronal advancement of flap and suturing.
Post-operative instructions were given verbally and in writing. Amoxicillin, chlorhexidine, and ibuprofen were prescribed. The patients were instructed to rinse with chlorhexidine for 4 weeks after surgery with no brushing or flossing at the surgical site. The sutures were removed at 2-week post-operative appointment. After 4 weeks, brushing and flossing resumed, and the patients returned to their regular periodontal maintenance routine.
3. Results
The post-operative follow-up revealed normal healing. Patients were recalled for routine periodontal maintenance therapy and reported no symptoms and easy access of cleaning. Periodontal examination and radiographs were taken at 6 and 12-months after surgery (Fig. 2, Fig. 3l-3m). These examinations revealed significant improvement of periodontal probing depth, clinical attachment loss, and FI defects. (Fig. 2e–f, 3h-i & Table 1). Compared to the pre-surgical examination, radiographic changes were observed with evident gain of bone height, and the furcation areas were filled with bone showing lamina dura lining the intra-radicular areas (Figs. 2h & 3l-m).
4. Discussion
To our knowledge, this is the first case report of successful treatment of mandibular grade III furcations using PRF and allogenic bone graft. In these two cases, we showed significant closure of the furcation defects, reduction in PD, and gain in clinical attachment at the 1-year postoperative follow-up. Radiographically, fill of the bony defects increased in bone intensity, and continuous interradicular lamina dura lining the root furca, were observed. These changes might have been a result of true periodontal regeneration by means of new attachment, or a long junctional epithelium between the newly regenerated tissues and the root surface.5 These results are consistent with other clinical and radiological findings using PRF in the treatment of a mandibular grade II furcation.10
PRF is constructed by dense fibrin matrix containing concentrated leukocytes, platelets and circulating stem cells. They generate and progressively release various growth factors such as TGF- β1 (transforming growth factor-1β), PDGF-BB (platelet-derived growth factor-BB), VEGF (vascular endothelial growth factor), and thrombospondin-1 etc.6 These factors can promote cell migration, osteogenic differentiation, angiogenesis to enhance wound healing and periodontal regeneration.8 PRF has been reported to be an effective modality of therapy in the regenerative treatment of intrabony defects in chronic periodontitis patients. A combination of PRF with allografts were used in the reconstruction of atrophic maxilla, periodontal infrabony defects, extraction socket preservations, and simultaneous implant placement.11 In this study, the mixing of chopped PRF with the graft materials is theorized to stimulate local bone formation from inside of the graft by recruiting osteogenic stem cells and secretion of growth factors. In addition, the PRF membrane created a natural barrier to exclude epithelial cell down growth but without interfering soft tissue healing for 1–2 weeks.8 Therefore, by properly taking advantage of these properties of PRF, we could achieve better tissue regeneration and wound healing.
Proper case selection is important for a desirable outcome of periodontal regeneration. In order to maintain the space for periodontal tissue regeneration, the clinician must consider the defect location, residual bone height, the geometrical architecture of the defect (1-wall, 2-wall, circumferential), and apply proper surgical skills.
5. Conclusion
This case report showed successful treatment of mandibular grade III furcation utilizing PRF and bone allograft. It suggested that the application of PRF with bone allograft may enhance periodontal regeneration and wound healing. Further large clinical trials and histologic investigations are needed to evaluate regenerative outcome to treat periodontal tissues.
Declaration of competing interest
The authors declare no conflicts of interest.
References
- 1.Carnevale G., Pontoriero R., Hurzeler M.B. Management of furcation involvement. Periodontol 2000. 1995;9:69–89. doi: 10.1111/j.1600-0757.1995.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 2.Hamp S.E., Nyman S., Lindhe J. Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol. 1975;2:126–135. doi: 10.1111/j.1600-051x.1975.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 3.Tarnow D., Fletcher P. Classification of the vertical component of furcation involvement. J Periodontol. 1984;55:283–284. doi: 10.1902/jop.1984.55.5.283. [DOI] [PubMed] [Google Scholar]
- 4.McGuire M.K., Nunn M.E. Prognosis versus actual outcome. II. The effectiveness of clinical parameters in developing an accurate prognosis. J Periodontol. 1996;67:658–665. doi: 10.1902/jop.1996.67.7.658. [DOI] [PubMed] [Google Scholar]
- 5.Sanz M., Jepsen K., Eickholz P., Jepsen S. Clinical concepts for regenerative therapy in furcations. Periodontol 2000. 2015;68:308–332. doi: 10.1111/prd.12081. [DOI] [PubMed] [Google Scholar]
- 6.Dohan D.M., Choukroun J., Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Dohan D.M., Choukroun J., Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Choukroun J., Diss A., Simonpieri A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Lekovic V., Camargo P.M., Weinlaender M., Vasilic N., Aleksic Z., Kenney E.B. Effectiveness of a combination of platelet-rich plasma, bovine porous bone mineral and guided tissue regeneration in the treatment of mandibular grade II molar furcations in humans. J Clin Periodontol. 2003;30:746–751. doi: 10.1034/j.1600-051x.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A., Pradeep A.R. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: a randomized clinical trial. J Periodontol. 2011;82:1396–1403. doi: 10.1902/jop.2011.100731. [DOI] [PubMed] [Google Scholar]
- 11.Simonpieri A., Del Corso M., Vervelle A. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharmaceut Biotechnol. 2012;13:1231–1256. doi: 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]