Abstract
Background
The axillary reverse mapping (ARM) technique, identify and preserve arm nodes during sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND), was developed to prevent breast-cancer related lymphedema (BCRL) remains controversial.
Methods
A comprehensive search of Medline Ovid, Pubmed, Web of Science and the Cochrane CENTRAL databases was conducted from the inception till January 2020. The key word including “breast cancer”, “axillary reverse mapping”, and “lymphedema”. Stata 15.1 software was used for the meta-analysis.
Results
As a result, twenty-nine related studies involving 4954 patients met our inclusion criteria. The pooled overall estimate lymphedema incidence was 7% (95% CI 4%–11%, I2 = 90.35%, P < 0.05), with SLNB showed a relatively lower pooled incidence of lymphedema (2%, 95% CI 1%–3%), I2 = 26.06%, P = 0.23) than that of ALND (14%, 95% CI 5%–26%, I2 = 93.28%, P < 0.05) or SLNB and ALND combined (11%, 95% CI 1%–30%). The ARM preservation during ALND procedure could significantly reduce upper extremity lymphedema in contrast with ARM resection (OR = 0.27, 95% CI 0.20–0.36, I2 = 31%, P = 0.161). Intriguingly, the result favored ALND-ARM over standard-ALND in preventing lymphedema occurrence (OR = 0.21, 95% CI 0.14–0.31, I2 = 43%, P = 0.153). The risk of metastases in the ARM-nodes was not significantly lower in the patients who had received neoadjuvant chemotherapy, as compared to those without neoadjuvant treatment (OR = 1.20, 95% CI 0.74–1.94, I2 = 49.4%, P = 0.095).
Conclusions
ARM was found to significantly reduce the incidence of BCRL. The selection of patients for this procedure should be based on their axillary nodal status. Preoperative neoadjuvant chemotherapy has no significant impact on the ARM lymph node metastasis rate.
Keywords: Breast cancer, Axillary reverse mapping, Lymphedema, Meta-analysis
Abbreviations: ARM, axillary reverse mapping; SNL, sentinel lymph node; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; BCRL, breast cancer-related lymphedema; AHRQ, Agency for Healthcare Research and Quality; NAC, neoadjuvant chemotherapy; CI, confidence interval; OR, odds ratio
Highlights
-
•
ARM is a simple and feasible technique that effectively reduces the incidence of BCRL.
-
•
Performing ARM technique for breast cancer patients should be considered carefully on account of its oncological safety.
-
•
Preoperative NAC is not correlated with less metastatic involvement in the ARM lymph nodes.
1. Introduction
Breast cancer is the most frequently diagnosed malignancy in most countries worldwide and is a leading cause of cancer-related death among women. Breast cancer-related lymphedema (BCRL) is a common complication after breast cancer surgery and/or radiation therapy; it negatively impacts the comfort, function, and quality of life of most breast cancer survivors [1]. Its occurrence is mainly due to the unnecessary sacrifice of the lymphatics of the arm. Nowadays, sentinel lymph node biopsy (SLNB) is widely used to avoid unnecessary axillary lymph node dissection (ALND) in clinically node-negative patients (cN0) [2]. As such, SLNB has reduced – but not eliminated – surgical complications in patients who forgo ALND. The morbidity caused by SLNB remains significant, with lymphedema rates ranging from 0% to 13% [3]. Recently published data from the Z0011 trial cast doubt on the necessity of ALND for patients with sentinel lymph node (SLN) metastases; however, ALND is still indicated for a substantial proportion of patients [4], especially those with clinically node-positive axilla (CP–N+).
In 2007, Thompson et al. developed axillary reverse mapping (ARM) to map and preserve arm lymphatic drainage during axillary surgery [5]. This technique enables differentiating the lymphatic drainages of the breast from those of the arm by using a blue dye, fluorescence, or radioisotope to visualize the lymphatic channels of the upper extremity. The combination of SLNB and/or ALND with ARM can reduce the incidences of arm lymphedema compared to standard procedures alone; nevertheless, the rates of successful ARM node identification as well as the incidences of lymphedema after the ARM procedure vary among previously performed studies [3,[6], [7], [8]].
As previous studies found that the lymphatic drainage system of the upper extremity is not entirely independent from that of the breast, the preservation of any crossover SLN-ARM nodes, which results in the lymph nodes of the SLN and arm maintaining shared lymphatic channels, can maintain the risk of metastases [[9], [10], [11]]. Because ARM is performed for patients who undergo ALND for lymph node metastases that are confirmed by SLNB or preoperative cytology, both the therapeutic benefit of preserving the ARM nodes as well as the unclear oncological safety of doing so should be seriously considered. In recent years, there has been an increasing trend towards performing either SLNB or targeted axillary dissection following neoadjuvant chemotherapy (NAC) for patients with clinically node-positive breast cancer. However, high false-negative rates of more than 20% have been reported [1].
Given the diversity of the data in the literature, there is no consensus regarding the clinical application of ARM for the prevention of BCRL. Hence, we performed a systematic review and meta-analysis to uncover the available evidence regarding the clinical utility, feasibility, and oncological safety of this novel technique as related to preventing BCRL.
2. Materials and methods
2.1. Literature search
We searched Medline Ovid, Pubmed, Web of Science, and the Cochrane CENTRAL to identify relevant studies. The following search terms were applied in various combinations: “breast cancer,” “breast carcinoma,” “breast neoplasm,” “axillary reverse mapping,” and “lymphedema.” We also conducted manual searches of the reference lists of the extracted articles to identify additional relevant publications. The final search was performed in January 2020.
2.2. Eligibility criteria
Publications that met the following criteria were included: (1) described a randomized controlled trial or prospective non-randomized study, (2) comprised a full-text article published in English, (3) described patients diagnosed with breast cancer who underwent ARM procedures during SLNB and/or ALND, (4) described patients with no prior axillary surgery except needle biopsy or concurrent SLNB, (5) patients were followed for lymphedema for more than three months, (6) described patients with no history of lymphedema in either arm, and (7) patient outcomes of interest were reported.
The following papers were excluded: (1) systematic reviews, meta-analyses, case reports, case series, conference abstracts, retrospective studies, protocols, unpublished studies, letters, editorials, or commentary; (2) duplicate studies; (3) those with missing or incomplete primary data, and (4) those describing low-quality studies. All assessments of extracted publications were performed independently in a non-blinded, standardized manner by two reviewers (W.A.W. and J.P.). Disagreements between reviewers were resolved by consensus. If no agreement was reached, a third reviewer (Y.H.H.) who was unaware of prior determinations arbitrated.
2.3. Data extraction and quality assessment
Eligible studies were further divided into two different outcomes: primary and secondary. The primary outcome was the clinical utility of ARM on BCRL (i.e., the incidences of lymphedema post-ARM SLNB and/or ALND, in the ARM-preserved vs. ARM-resected groups, and in the ALND-ARM vs. standard-ALND groups). Secondary outcomes included the feasibility of ARM (SLNB and ALND identification rates) and oncological safety of ARM (i.e., the SLN-ARM node crossover rate, ARM node metastatic rate, relationship between axillary status and ARM metastasis, and association between preoperative NAC and the metastasis rate within resected ARM nodes). To better determine the oncological safety of the ARM procedure, we further classified metastases within the resected ARM nodes into two groups according to their primary clinical axillary status. The first group (SLN+) comprised patients with micro- or macro-metastatic lymph node involvement in the SLN who were advised to undergo complementary ALND. The second group (CP–N+) comprised patients who had preoperative diagnoses of lymph node metastasis (confirmed via fine-needle aspiration cytology) and were scheduled for ALND. Additionally, we compared the rates of metastases between patients with pN0–1 and pN2–3 stage breast cancer. More importantly, we calculated the odd ratios (ORs) of lymphedema incidence, ALND-ARM vs. standard-ALND lymphedema incidence, preoperative NAC and non-NAC ARM node metastatic rate, and pN0–1 vs. pN2–3 stage ARM nodes metastatic rate in the ARM-preserved vs. ARM-resected groups. To determine the rates of SLNB, ALND identification, SLN-ARM crossover, and ARM node metastasis, individuals were further divided into subgroups according to the ARM mapping method used (blue dye, fluorescence, or radioisotope) and continent of origin (Asia, America, Europe, Africa, or Australia). Two independent reviewers (W.A.W. and J.P.) extracted the data, with discrepancies and disagreements resolved by discussion. The extracted data included the first author, study design, country, continent, procedures, mapping method, identification rate of ARM nodes or lymphatics, SLN-ARM crossover rate, rate of ARM node metastasis, lymphedema incidence, and lymphedema follow-up duration. When multiple publications were identified that reported the same populations and outcomes, only the most representative and comprehensive study was included for further meta-analysis to avoid data duplication. Quality assessment was conducted using the 11-item checklist recommended by the Agency for Healthcare Research and Quality (AHRQ) to evaluate the quality of individual cohort studies [12]. Meanwhile, we assessed the risk of bias for each randomized controlled trial using The Cochrane Collaboration tool [13].
2.4. Statistical analysis
After checking for consistency, data analysis was performed using Stata 15.1 (StataCorp, College Station, TX USA, 2018). For non-comparative binary outcomes, the pooled estimates were generated using the “metaprop” command. The 95% confidence interval (CI) was estimated using the Wilson score method and calculated using the DerSimonian-Laird random-effects model with Freeman-Tukey double arcsine transformation. Testing for heterogeneity among the selected studies was performed primarily via Cochran Q and I2 statistics. I2 statistics of 25–50%, 50–75%, and >75% were considered mild, moderate, and severe heterogeneity, respectively. If heterogeneity was greater than 50%, the pooled estimate and 95% CI were calculated by using a random-effects model. Dichotomous variables were analyzed using the Mantel-Haenszel method and are expressed as ORs. Moreover, subgroup and meta-regression analyses were used to explore any potential heterogeneity. A two-tailed P-value of less than 0.05 denoted statistical significance. Publication bias (P < 0.05 was considered significant) was assessed by visual estimation of a funnel pot, Egger’s test, and Begg’s test.
3. Results
3.1. Search results and included trials
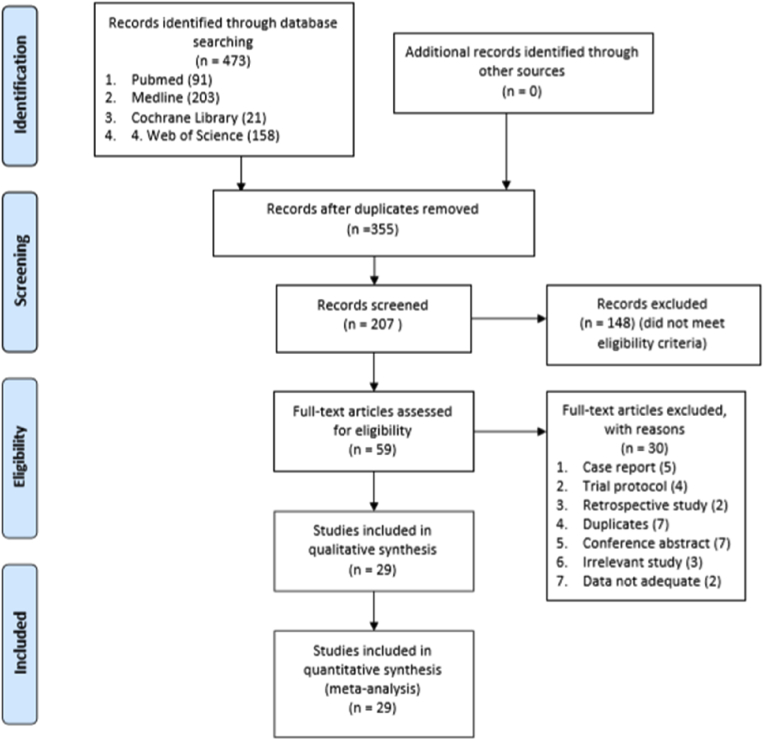
Twenty-nine articles published between January 2007 and January 2020 describing studies of 4954 patients were included in this meta-analysis [1,2,[6], [7], [8], [9],11,[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]]. These included four randomized controlled trials [1,[20], [21], [22]] and 25 prospective non-randomized studies [2,[6], [7], [8], [9],11,[14], [15], [16], [17], [18], [19],[23], [24], [25]]. Four randomized controlled trials were at low risk of bias according to the Cochrane Collaboration’s tool(Table S2). The risk of bias assessment of 25 prospective non-randomized studies revealed AHRQ quality scores ranging from 4 to 8 points, suggesting that the qualities of the eligible articles was moderate-to-high(Table S1). The results of the database selection process are shown in Fig. 1, whereas the characteristics of the patients examined in the selected articles are shown in Table 1.
Fig. 1.
Flow diagram of studies selection.
Table 1.
Main characteristics of eligible studies.
| First Author | Study Design | Country | Procedures (N) | Mapping Material | Identification Rate of ARM Nodes or Lymphatics | SLN-ARM Crossover Rate | Metastatic Rate of ARM Nodes | Lymphedema Incidence | Follow-Up Duration | Study Quality | Study Period |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nos et al. (2007) [ 27] | Prospective | France | ALND alone (21) | Blue dye | ALND: 71%(15/21) | NA | 0%(0/10) | NA | NA | Moderate quality | November 2004–February 2005 |
| Nos et al. (2008) [28] | Prospective | France | ALND alone (23) | Blue dye + radioisotope | ALND: 91%(21/23) | NA | 14%(3/21) | NA | NA | Moderate quality | July 2006–March 2008 |
| Casabona et al. (2009) [23] | Prospective | Italy | SLNB with or without ALND (72); ALND + SLNB (9) |
Blue dye | SLND: 38%(27/72) ALND: 89%(8/9) |
0%(0/72) | 0%(0/3) | NA | 9 months | Moderate quality | January 2007–July 2008 |
| Ponzone et al. (2009) [29] | Prospective | Italy | ALND alone (49) | Blue dye | ALND: 55%(27/49) | NA | 11%(3/27) | NA | NA | Moderate quality | June 2007–December 2008 |
| Bedrosian et al. (2010) [30] | Prospective | USA | ALND alone (30) | Blue dye | ALND: 50%(15/30) | NA | 13%(2/15) | NA | NA | Moderate quality | May 2008–January 2009 |
| Deng et al. (2011) [9] | Prospective | China | SLNB alone (69) | Blue dye | NA | 28%(19/69) | 9%(6/69) | NA | NA | Moderate quality | October 2009–August 2010 |
| Han et al. (2012) [14] | Prospective | Korea | SLNB with or without ALND (97); ALND with SLNB (83) |
Blue dye | SLND: 71%(10/14) ALND: 84%(70/83) |
7%(7/97) | 12%(2/17) | SLNB: 0%(0/14) SLNB + ALND: 1%(1/83) |
9.6 months | Moderate quality | January 2009–October 2010 |
| Rubio et al. (2012) [25] | Prospective | Spain | SLNB with ALND (15); ALND with or without SLNB (36) |
Blue dye | ALND: 83%(30/36) | 14%(2/14) | 13%(4/30) | NA | 20 months | Moderate quality | July 2009–May 2010 |
| Connor et al. (2013) [11] | Prospective | USA | SLNB alone (155); ALND with or without SLNB (57) |
Blue dye | SLND: 47%(73/155) ALND: 72(41/57) |
11%(22/197) SLNB:18/155 ALND:4/42 |
8%(3/37) | SLNB: 4%(6/137) | 12 months | Moderate quality | December 2009–02/2012 |
| Gennaro et al. (2013) [16] | Prospective | Italy | ALND (15); selective axillary dissection (45) | Radioisotope | ALND: 75%(45/60) | NA | NA | ALND: 15%(9/60) | 16 months | Moderate quality | June 2009–February 2012 |
| Tausch et al. (2013) [8] | Prospective | Switzerland | ALND alone (143) | Blue dye + radioisotope | ALND: 78%(112/143) | NA | 15%(17/115) | ALND: 31%(35/114) | 19 months | Moderate quality | April 2009–April 2012 |
| Ikeda et al. (2014) [17] | Prospective | Japan | ALND with or without SLNB (98) | Fluorescence | ALND: 82%(80/98) | NA | 22%(17/76) | ALND: 28%(13/47) SLNB + ALND: 22%(11/51) |
24 months | High quality | January 2010–December 2012 |
| Khandelwal et al. (2014) [31] | Prospective | India | ALND alone (51) | Blue dye | ALND: 88%(45/51) | NA | 27%(12/45) | NA | NA | Moderate quality | May 2011–May 2013 |
| Kuusk et al. (2014) [15] | Prospective | Canada | SLNB alone (37); ALND alone (15) |
Blue dye | SLND: 19%(7/37) ALND: 47%(7/15) |
8%(4/52) SLNB:5%(2/37) ALND:2/15 |
7%(1/15) | SLNB + ALND: 2%(1/47) | 24 months | Moderate quality | July 2010–November 2012 |
| Ochoa et al. (2014) [18] | Prospective | USA | SLNB alone (237); ALND with or without SLNB (123) |
Blue dye | SLND: 34%(80/237) ALND: 76%(93/123) |
4%(15/348) | 19%(5/27) | SLNB: 2%(4/237) SLNB + ALND: 2% (3/123) |
12 months | Moderate quality | 05/2006–10/2011 |
| Sakurai et al. (2014) [19] | Prospective | Japan | SLNB alone (321) | Blue dye + fluorescence | SLND: 32%(120/372) | 21%(77/372) | NA | SLNB: 2%(5/321) | 12 months | Moderate quality | 08/2009–07/2012 |
| Schunemann et al. (2014) [7] | Prospective | Brazil | ALND alone (45) | Blue dye | ALND: 89%(40/45) | NA | 25%(10/40) | NA | NA | Moderate quality | 01/2010–10/2012 |
| Beek et al. (2015) [32] | Prospective | Netherlands | ALND alone (112) | Blue dye | ALND: 88%(98/112) | NA | 20%(20/98) | NA | NA | High quality | 10/2009–11/2013 |
| Yue et al. (2015) [20] | RCT | China | ALND alone (127); ALND + ARM (138) |
Blue dye + radioisotope | ALND: 93%(129/138) | NA | 9%(11/129) | ALND: 21%(51/245) | 20 months | High quality | 01/2012–03/2014 |
| Gandhi et al. (2016) [33] | Prospective | India | ALND alone (50) | Radioisotope | ALND: 94%(47/50) | NA | 11%(5/47) | NA | NA | Moderate quality | 04/2012–08/2012 |
| Ngui et al. (2016) [26] | Prospective | Australia | ALND alone (87) | Blue dye | ALND: 77%(67/87) | 9%(3/32) | 27%(18/67) | NA | NA | Moderate quality | 06/2012–12/2014 |
| Noguchi et al. (2016) [2] | Prospective | Japan | SLNB with or without ALND(292); ALND with SLNB (48) |
Blue dye + fluorescence | SLND: 31% (90/292) ALND: 86%(36/42) |
27%(77/286) | 21%(19/90) | SLNB: 1%(2/244) | 45 months | Moderate quality | 06/2012–12/2014 |
| Nos et al. (2016) [34] | Prospective | France | ALND alone (172) | Radioisotope | ALND: 100%(172/172) | NA | 31%(54/172) | SLNB + ALND: 27% | 34 months | Moderate quality | 12/2009–12/2012 |
| Kumar et al. (2017) [35] | Prospective | India | ALND alone (20) | Blue dye | ALND: 75%(15/20) | NA | 13%(2/15) | NA | NA | Moderate quality | 05/2014–07/2015 |
| Tummel et al. (2017) [6] | Prospective | USA | SLND alone (472), ALND with or without SLNB (213) | Blue dye + radioisotope | SLND: 29%(138/472) ALND: 72%(153/213) |
4%(18/472) | 8%(7/74) | SLNB: 3%(12/350) ALND: 21%(33/154) |
26 months | Moderate quality | 06/2007–12/2013 |
| Faisal et al. (2019) [22] | RCT | Egypt | ALND alone (24); ALND + ARM (24) | Blue dye | ALND: 83%(20/24) | NA | 0%(0/4) | ALND: 10%(5/48) | 6 months | High quality | 06/2017–01/2018 |
| Ma et al. (2019) [24] | Prospective | China | ALND with SLNB (44) | Blue dye + fluorescence | SLNB: 29/44(66%) ALND: 96%(44/46) |
11%(5/44) | 16%(7/44) | NA | NA | Moderate quality | 02/2017–10/2017 |
| Yuan et al. (2019) [21] | RCT | China | ALND alone (665); ALND + ARM (689) | Blue dye + fluorescence | ALND: 81%(558/689) | NA | 7%(38/558) | ALND: 10%(117/1191) | 37 months | High quality | 02/2013–10/2017 |
| Beek et al. (2020) [1] | RCT | Netherlands | ALND alone (46); ALND + ARM (48) | Blue dye | ALND: 78%(73/94) | NA | 3%(1/35) | ALND: 27% (18/66) | 24 months | High quality | 06/2013–08/2016 |
RCT randomized controlled trial; SLN sentinel lymph node; SLNB sentinel lymph node biopsy; ARM axillary reverse mapping; ALND axillary lymph node dissection; NA not applicable.
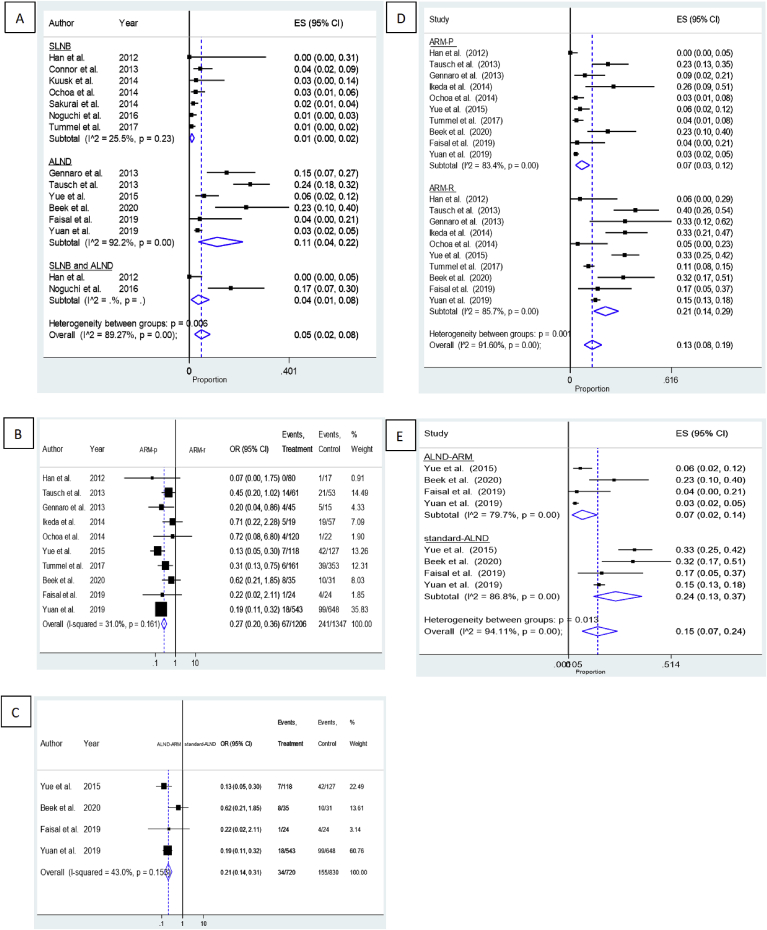
3.2. Primary outcome: clinical utility of ARM in preventing BCRL
Fig. 2 shows the forest plots for the clinical utility of ARM for patients with BCRL. Fourteen studies investigated the incidence of upper extremity lymphedema after post-ARM SLNB and/or ALND [1,2,6,8,11,[14], [15], [16], [17], [18], [19], [20], [21], [22]]. The overall lymphedema incidence was 7% (95% CI 4%–11%, I2 = 90.35%, P < 0.05). On subgroup analysis according to the type of procedure, SLNB showed a relatively lower incidence of lymphedema (2%, 95% CI 1%–3%), I2 = 26.06%, P = 0.23) than did ALND (14%, 95% CI 5%–26%, I2 = 93.28%, P < 0.05) or SLNB and ALND combined (11%, 95% CI 1%–30%).
Fig. 2.
Forest plots of the clinical utility of ARM on BCRL. (A) the pooled incidence rate of lymphedema; (B) OR for ARM-preserved vs. ARM-resected group lymphedema incidence; (C) OR for ALND-ARM vs. standard-ALND lymphedema incidence.
Of the 29 studies identified, 10 reported the incidence of upper extremity lymphedema in patients who underwent ALND with or without preserving the ARM lymph nodes and/or lymphatics [1,6,8,14,[16], [17], [18],[20], [21], [22]]. Five studies used the water displacement method to evaluate the incidence of upper extremity lymphedema [1,6,8,18,21], nine measured the arm circumference [11,[14], [15], [16], [17],19,20,22,23], and three relied on patients’ subjective complaints or questionnaire surveys [1,6,21]. The pooled estimates showed that patients for whom the ARM nodes and/or lymphatics were preserved during the ALND procedure had significantly reduced upper extremity lymphedema when compared to those in whom these tissues were resected (OR = 0.27, 95% CI 0.20–0.36, I2 = 31%, P = 0.161).
Four randomized controlled trials encompassing 1550 patients compared the incidence of lymphedema between those who underwent ALND-ARM and those who underwent standard ALND [1,[20], [21], [22]]; the follow-up duration ranged from 6 to 37 months. The pooled data indicated that the incidence of lymphedema was significantly reduced with ARM, with patients undergoing ALND-ARM experiencing more favorable results than those who underwent standard ALND in terms of preventing lymphedema occurrence (OR = 0.21, 95% CI 0.14–0.31, I2 = 43%, P = 0.153).
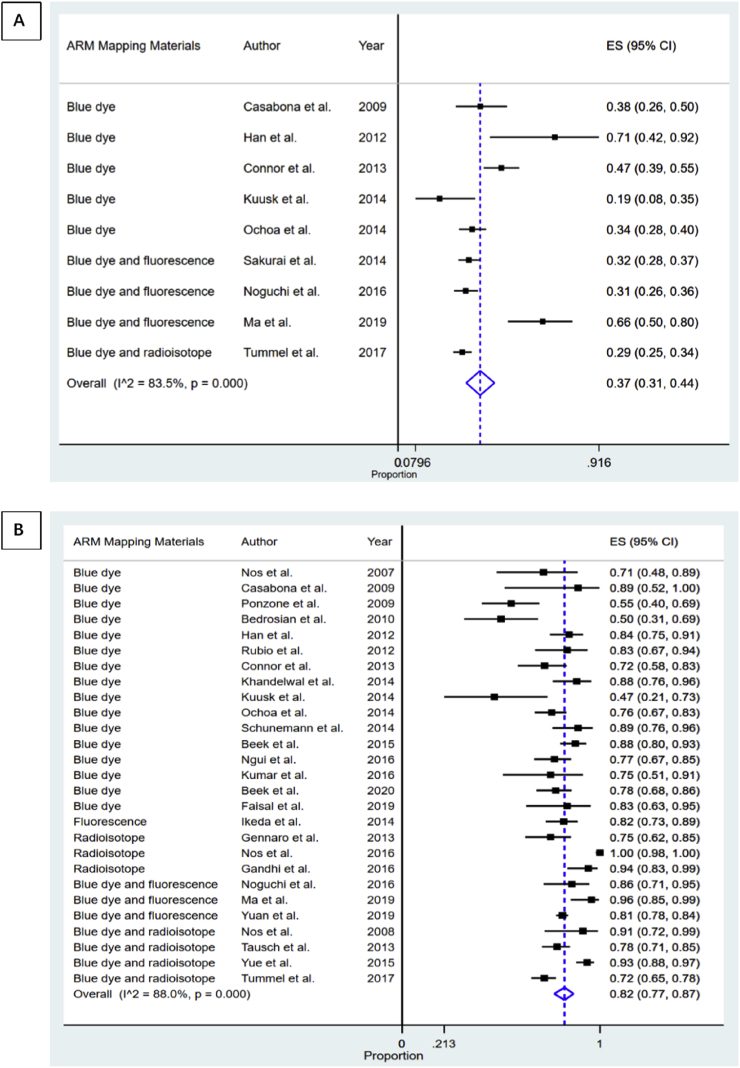
3.3. Secondary outcome: feasibility and oncological safety of ARM
Fig. 3 shows forest plots depicting the feasibility of the ARM technique. Nine studies observed the identification rate of ARM lymph nodes and/or lymphatics in the axilla during SLNB [2,6,11,14,15,18,19,23,24]. The aggregate results showed that the identification rate of ARM lymph nodes and/or lymphatics in the SLNB field was 37% (95% CI 31%–44%, I2 = 83.5%, P < 0.05). Subgroup analyses according to ARM mapping methods and the patients’ continents of origin were performed (Table 2 and Figs. S1–2 in the Supplementary Materials). Notably, the pooled identification rate remained similar in these stratified analyses, with statistically significant heterogeneity across all subgroups; the meta-regression analysis revealed no obvious source for the heterogeneity (P = 0.124). There was no evidence of publication bias as indicated by funnel plot analysis, Egger’s test (P-value for bias = 0.181), and Begg’s test (P-value for bias = 0.175).
Fig. 3.
Forest plot of ARM feasibility. (A) the ARM identification rate during SLNB; (B) the ARM identification rate during ALND.
Table 2.
Subgroup results for ARM feasibility.
| Stratification criterion | Number of studies | Pooled results (95%CI) | I2 | P - value for difference | |
|---|---|---|---|---|---|
| SLNB identification rate | Overall | 9 | 37%(31%–44%) | 83.5% | <0.05 |
| Mapping materials: | |||||
| - Blue dye | 5 | 39%(28%–50%)_ | 79.6% | <0.05 | |
| - Blue dye and fluorescence | 3 | 40%(28%–54%) | – | – | |
| - Blue dye and radioisotope | 1 | 29%(25%–34%) | – | – | |
| Geographical region: | |||||
| - Asia | 4 | 45%(32%–59%) | 89.3% | <0.05 | |
| - America | 4 | 33%(24%–42%) | 84.7% | <0.05 | |
| - Europe |
1 |
38%(26%–50%) |
– |
– |
|
| Stratification criterion | Number of studies | Pooled results (95%CI) | I2 | P - value for difference | |
| ALND identification rate | Overall | 27 | 82%(77%–87%) | 88% | <0.05 |
| Mapping materials: | |||||
| - Blue dye | 16 | 77%(71%–82%) | 69.1% | <0.05 | |
| - Fluorescence | 1 | 82%(73%–89%) | – | – | |
| - Radioisotope | 3 | 93%(71%–100%) | – | – | |
| - Blue dye and fluorescence | 3 | 87%(77%–95%) | – | – | |
| - Blue dye and radioisotope | 4 | 84%(71%–94%) | 90.5% | <0.05 | |
| Geographical region: | |||||
| - Asia | 9 | 87%(83%–92%) | 72.5% | <0.05 | |
| - America | 6 | 71%(62%–80%) | 73.5% | <0.05 | |
| - Europe | 10 | 83%(71%–93%) | 92.9% | <0.05 | |
| - Australia | 1 | 77%(67%–85%) | – | – | |
| - Africa | 1 | 83%(63%–95%) | – | – | |
Based on data from 27 publications [1,2,[6], [7], [8],11,[14], [15], [16], [17], [18],[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]], the overall pooled estimate of the rate of identification of axillar ARM lymph nodes and/or lymphatics in the ALND field was 82% (95% CI 77%–87%, I2 = 88%, P < 0.05) (Fig. 3). This pooled rate remained similar on stratified analyses, with statistically significant heterogeneity across all subgroups (Table 2 and Figs. S3–4 in the Supplementary Materials). The sample size coefficient was statistically significant on meta-regression analysis (P = 0.007), indicating that the number of enrolled patients may influence the ALND identification rate.
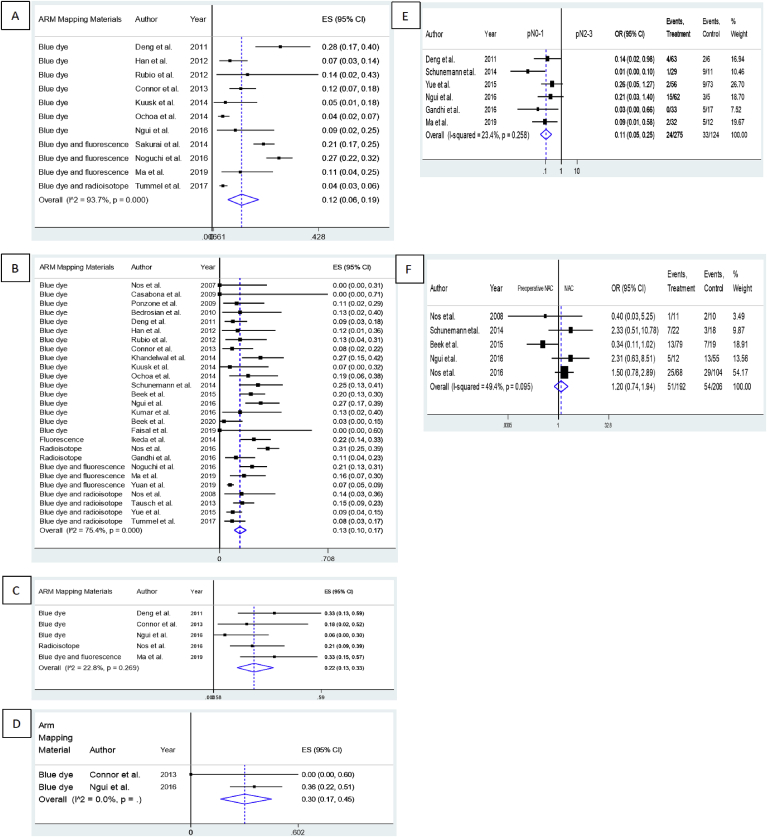
Eleven studies investigated the crossover rate between the SLN and ARM lymph nodes [2,6,9,11,14,15,18,19,[24], [25], [26]]; the overall pooled crossover rate was 12% (95% CI 6%–19%, I2 = 93.7%, P < 0.05) (Fig. 4A). Studies using blue dye, blue dye with fluorescence, and blue dye with a radioisotope showed pooled crossover rates of 10% (95% CI 5%–17%), 21% (95% CI 15%–28%), and 4% (95% CI 3%–6%), respectively. Asian, American, European, and Australian studies revealed pooled crossover estimates of 19% (95% CI 12%–26%), 6% (95% CI 3%–9%), 14% (95% CI 2%–43%), and 9% (95% CI 2%–25%), respectively. All subgroup analyses still showed significant heterogeneity (Table 3 and Figs. S5–6 in the Supplementary Materials). The coefficient was not statistically significant for sample size on further meta-regression analysis (P = 0.461). Heterogeneity among the studies was not significant for either analysis as assessed using funnel plots, Egger’s test (P-value for bias = 0.448), or Begg’s test (P-value for bias = 0.938).
Fig. 4.
Forest plot of ARM oncological safety. (A) the pooled identification rate of the SLN-ARM crossover rate during SLNB; (B) the pooled identification rate of the overall resected ARM metastatic rate; (C) the pooled resected ARM metastatic rate in the SLN+patient group; (D) the pooled resected ARM metastatic rate in the CP-N+patient group; (E) OR for the association between axillary status and the risk of ARM metastasis; (F) OR for the association between preoperative NAC and the risk of ARM metastasis.
Table 3.
Subgroup results for ARM oncological safety.
| Stratification criterion | Number of studies | Pooled results (95%CI) | I2 | P - value for difference | |
|---|---|---|---|---|---|
| SLN-ARM crossover rate | Overall | 11 | 12%(6%–19%) | 93.7% | <0.05 |
| Mapping materials: | |||||
| - Blue dye | 7 | 10%(5%–17%) | 81.1% | <0.05 | |
| - Blue dye and fluorescence | 3 | 21%(15%–28%) | – | – | |
| - Blue dye and radioisotope | 1 | 4%(3%–6%) | – | – | |
| Geographical region: | |||||
| - Asia | 5 | 19%(12%–26%) | 84% | <0.05 | |
| - America | 4 | 6%(3%–9%) | 71.6% | 0.01 | |
| - Europe | 1 | 14%(2%–43%) | – | – | |
| - Australia |
1 |
9%(2%–25%) |
– |
– |
|
| Stratification criterion | Number of studies | Pooled results (95%CI) | I2 | P - value for difference | |
| Overall resected ARM metastatic rate | Overall | 27 | 13%(10%–17%) | 75.4% | <0.05 |
| Mapping materials: | |||||
| - Blue dye | 17 | 13%(9%–18%) | 42.8% | 0.03 | |
| - Fluorescence | 1 | 22%(14%–33%) | – | – | |
| - Radioisotope | 2 | 26%(21%–32%) | – | – | |
| - Blue dye and fluorescence | 3 | 13%(4%–26%) | – | – | |
| - Blue dye and radioisotope | 4 | 10%(7%–14%) | 8.4% | 0.35 | |
| Geographical region: | |||||
| - Asia | 10 | 14%(9%–19%) | 75.9% | <0.05 | |
| - America | 6 | 12%(7%–19%) | 32.9% | 0.19 | |
| - Europe | 9 | 13%(6%–21%) | 73.6% | <0.05 | |
| - Australia | 1 | 27%(17%–39%) | – | – | |
| - Africa |
1 |
0%(0%–60%) |
– |
– |
|
| Stratification criterion | Number of studies | Pooled results (95%CI) | I2 | P - value for difference | |
| SLN+ARM metastatic rate | Overall | 5 | 22%(13%–33%) | 22.8% | 0.269 |
| Mapping materials | |||||
| - Blue dye | 3 | 18%(4%–37%) | – | – | |
| - Radioisotope | 1 | 21%(9%–39%) | – | – | |
| - Blue dye and fluorescence | 1 | 33%(15%–57%) | – | – | |
| Geographical region: | |||||
| - Asia | 2 | 33%(19%–49%) | – | – | |
| - America | 1 | 18%(2%–52%) | – | – | |
| - Europe | 1 | 21%(9%–39%) | – | – | |
| - Australia |
1 |
6%(0%–30%) |
– |
– |
|
| Stratification criterion | Number of studies | Pooled results (95%CI) | I2 | P - value for difference | |
| CP-N+ARM metastatic rate | Overall | 2 | 30%(17%–45%) | – | – |
Twenty-seven publications reported the rate of metastatic involvement in resected ARM lymph nodes [1,2,[6], [7], [8], [9],11,14,15,17,18,[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]]. The pooled overall ARM node metastasis rate was 13% (95% CI 10%–17%, I2 = 75.4%, P < 0.05) (Fig. 4B). Further stratification by the ARM mapping methods and patients’ continents of origin are shown in Table 3 and Figs. S7–8 in the Supplementary Materials. Heterogeneity remained significant in the Asian and European study subgroups. On meta-regression analysis, the sample size coefficient was not statistically significant (P = 0.283). No publication bias was detected by funnel plot, Egger’s test (P-value for bias = 0.196), or Begg’s test (P-value for bias = 0.471).
Several studies investigated the SLN+ axillary status ARM metastatic rate [9,11,24,26,34]. Regardless of the presence of additional metastases in other lymph nodes extracted during ALND, our pooled ARM node metastasis rate within the SLN+ patient group was 22% (95% CI 13%–33%, I2 = 22.8%, P = 0.269) (Fig. 4C). Furthermore, subgroup analysis according to ARM method and geographical region still showed significant heterogeneity (Table 3 and Figs. S9–10 in the Supplementary Materials). Based on two studies [11,26] comprising 49 patients, the overall pooled CP-N+ ARM metastasis rate was 30% (95% CI 17%–45%, I2 = 0.0%) (Fig. 4D).
Six studies [7,9,20,24,26,33] compared the rates of metastasis between patients with stages pN0–1 vs. pN2–3 disease and found that the former had a significantly lower risk of ARM metastasis than the latter (OR = 0.11, 95% CI 0.05–0.25, I2 = 23.4%, P = 0.258) (Fig. 4E). Moreover, the pooled results of five studies [7,26,28,32,34] did not show a significant correlation between NAC and ARM nodes (OR = 1.20, 95% CI 0.74–1.94, I2 = 49.4%, P = 0.095) (Fig. 4F). This indicated that preoperative NAC did not necessarily reduce ARM node metastases.
4. Discussion
Treatment of the axilla in patients with breast cancer has recently been a subject of debate, especially with respect to post-axillary surgery-induced BCRL. The ability of ARM to reduce the BCRL rate has been investigated since its introduction over a decade ago by Thompson et al. [5] and Nos et al. [27] Hitherto, many surgeons view the lack of evidence regarding the clinical utility, feasibility, and oncological safety of this novel technique as an obstacle to its use. Our study aimed to shed light on this matter.
ARM is a minimally invasive technique that can be readily added to SLNB and/or ALND and can significantly reduce the incidence of BCRL, which reportedly varies according to measurement technique, length of follow-up, time to measurement, use of whole breast radiation, and extent of surgery [36]. Complications that are frequently associated with BCRL include restricted ipsilateral shoulder mobility in approximately 16.7% of women treated for breast cancer [37]. Breast cancer survivors with lymphedema might also experience different degrees of physical and emotional disability that could affect the quality of their everyday lives [38]. A recent meta-analysis of BCRL incidence in patients with unilateral breast cancer estimated that patients who receive standard ALND have a lymphedema incidence four times higher than those who receive standard SLNB (19.9% [95% CI: 13.5–28.2] vs. 5.6% [95% CI: 6.1–7.9]) [39]. Our meta-analysis revealed that the incidences of lymphedema in the ALND + ARM and SLNB + ARM groups were 14% and 2%, respectively, while the overall post-ARM lymphedema incidence was 7%. Our findings demonstrated that the standard procedure combined with ARM node preservation results in a lower incidence of lymphedema. The obviously higher incidence of lymphedema after ALND + ARM than after SLNB + ARM could be explained by the greater disruptions in lymphatic channels during ALND because the majority of the lymphatics that drain the arm may be located deep within the SLN field [40]. Extensive dissection during ALND may cause accidental damage, thereby compromising upper extremity lymphatic drainage. Additionally, a meta-analysis of four randomized controlled trials that compared the incidence of lymphedema between patients undergoing ARM and non-ARM procedures suggested that ALND in combination with ARM results in fewer upper extremity lymphedema events than does ALND alone [1,[20], [21], [22]]. These data indicate that using the ARM technique to identify and avoid the transection of ipsilateral arm lymphatics can minimize the risk of BCRL but not eliminate it entirely.
Our findings were consistent with those reported in a systematic review by Beek et al. [41], in which ARM nodes were identified in 47–100% and 19–71% of patients who underwent ALND and SLNB, respectively. The significantly higher identification rate in patients who underwent ALND can be largely explained by the greater surgical exposure achieved during this procedure and the differences in the anatomical locations of SLNs and ARM nodes [23,29]. Moreover, the wide ranges of these identification rates are possibly associated with variations in the definitions of a successful ARM procedure, intervals between the blue dye injection and the start of the surgery, types and/or volumes of mapping materials, and surgeons’ experiences between the reported studies [3,5,19,29].
The oncological safety of ARM is a priority for patients undergoing this procedure. Particular attention should be paid to crossover between SLN-ARM nodes to enable the preservation of the arm-draining lymph nodes. Our present analysis revealed an overall SLN-ARM node crossover rate of 12%; most included studies found similar rates of metastasis in the ARM nodes and SLNs, indicating that crossover is a significant contributing factor to metastases in the lymph nodes of the upper limb. As a proof of concept, data based on the analysis of the 27 studies [1,2,[6], [7], [8],11,[14], [15], [16], [17], [18],[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]] revealed that the pooled metastatic rate of ARM nodes was 13%. As such, ARM nodes are indeed less likely to contain metastases than other axillary lymph nodes.
There appears to be significant variability in patient selection when investigating the oncologic safety of the ARM procedure. The pooled ARM metastatic rate in the SLN+ group was 22%, which was not significantly lower than that in the CP-N+ group (30%). This lack of significance may be owing to the small number of patients included in our study; as such, larger studies are required to determine whether ARM is safe for these patients. As other have reported, we found that the risk of ARM node positivity correlates with the burden of axillary disease. Corroborating data from a previously published systematic review [41], our meta-analysis revealed an association between the number of lymph nodes involved in the axilla and the presence of metastases in nodes detected using ARM. Patients with pN0–1 stage had a significantly lower risk of ARM metastases than did those with pN2–3 stage. Furthermore, patients with extensive axillary involvement had an increased risk of metastasis to the ARM nodes; therefore, such patients are not the best candidates for the ARM procedure.
There are two possible explanations for the metastatic involvement of arm-draining lymph nodes [42]. First, it could be a consequence of the natural progression of the disease, as the breast tumor’s growth may alter the pattern of lymphatic flow between the arm and breast, causing the upper limb’s lymph nodes to be compromised. Second, the lymph nodes of the breast and arm typically converge into the infraclavicular nodes in Berg’s level III; anatomical variations in the crossover between the breast and arm lymph nodes may also exist in Berg’s level I or II [43]. Some studies have shown an inverse relationship between NAC use and ARM node positivity. Moreover, it is thought that NAC reduces the metastatic disease burden in the axilla [44]. In our study, the risk of metastases in the ARM nodes was not significantly lower in patients who had received NAC when compared to those who had not. Our findings are consistent with those of the American College of Surgeons Oncology Group Z107122 Alliance trial [45] and SENTinel NeoAdjuvant23 [46] trials, which found that NAC resulted in high false-negative rates (12.6% in the former and 18.5% in the latter). If there is a heavier disease burden in the axilla, then it follows that lymphatic “back up” would lead to the ARM nodes becoming involved. This supports the notion that the application of ARM to prevent lymphedema is feasible for patients with clinically node-negative breast cancer.
There were some limitations of our study. First, some clinical variables that may be related to the occurrence of BCRL after SLNB or ALND (such as age, body mass index, postoperative radiotherapy, a history of wound infection or lymphangitis, the duration of axillary drainage, and residual lymph node disease after NAC) were not adjusted for in most studies. Second, the definition of lymphedema was not consistent between studies. High levels of variability were observed in the measurement techniques as well as the follow-up durations for patients with upper extremity lymphedema. To address these limitations, additional well-designed randomized controlled trials are warranted to provide more convincing evidence. While ARM may be a promising method for decreasing the occurrence of lymphedema during SLND or ALND, we must recognize that the drainage pathway from the upper limb and ipsilateral breast in a minority of patients is through the common lymphatic channel. Therefore, preserving these ARM lymph nodes may increase the risk of cancer recurrence, especially in clinically node-positive patients [47]. As such, investigating the role of microsurgical lymphaticovenous bypass in reducing the risk of BCRL when combined with the ARM procedure is warranted.
In summary, ARM is a simple and feasible technique that effectively reduces the incidence of BCRL. However, preserving lymph nodes and/or lymphatics using the ARM technique should be considered carefully in clinical practice on account of its oncological safety. It is oncologically unacceptable to perform ARM in a heavily burdened axilla (pN2–3 stage breast cancer) owing to the increased risk of ARM node metastasis. Furthermore, preoperative NAC is not correlated with less metastatic involvement in the ARM lymph nodes.
Sources of funding
This research was supported by the National Natural Science Foundation of China (81101180; 81071282).
Acknowledgements
We would like to give special thanks, admiration and respect to Dr. JiaYe Liu for his kind help, guidance and valuable support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.08.007.
Contributor Information
Junjie Chen, Email: cjjemail@163.com.
Ying Cen, Email: cenying0141@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Beek M.A., Gobardhan P.D., Klompenhouwer E.G. A patient- and assessor-blinded randomized controlled trial of axillary reverse mapping (ARM) in patients with early breast cancer. Eur J Surg Oncol. 2020;46(1):59–64. doi: 10.1016/j.ejso.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi M., Ohno Y., Morioka E. Feasibility study of axillary reverse mapping for patients with clinically node-negative breast cancer. Eur J Surg Oncol. 2016;42(5):650–656. doi: 10.1016/j.ejso.2016.02.244. [DOI] [PubMed] [Google Scholar]
- 3.Boneti C., Korourian S., Diaz Z. Scientific Impact Award: axillary reverse mapping (ARM) to identify and protect lymphatics draining the arm during axillary lymphadenectomy. Am J Surg. 2009;198(4):482–487. doi: 10.1016/j.amjsurg.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano A.E., Hunt K.K., Ballman K.V. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. J Am Med Assoc. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson M., Korourian S., Henry-Tillman R. Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14(6):1890–1895. doi: 10.1245/s10434-007-9412-x. [DOI] [PubMed] [Google Scholar]
- 6.Tummel E., Ochoa D., Korourian S. Does axillary reverse mapping prevent lymphedema after lymphadenectomy? Ann Surg. 2017;265(5):987–992. doi: 10.1097/SLA.0000000000001778. [DOI] [PubMed] [Google Scholar]
- 7.Schunemann E., Jr., Doria M.T., Silvestre J.B. Prospective study evaluating oncological safety of axillary reverse mapping. Ann Surg Oncol. 2014;21(7):2197–2202. doi: 10.1245/s10434-014-3626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tausch C., Baege A., Dietrich D. Can axillary reverse mapping avoid lymphedema in node positive breast cancer patients? Eur J Surg Oncol. 2013;39(8):880–886. doi: 10.1016/j.ejso.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Deng H., Chen L., Jia W. Safety study of axillary reverse mapping in the surgical treatment for breast cancer patients. J Canc Res Clin Oncol. 2011;137(12):1869–1874. doi: 10.1007/s00432-011-1064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noguchi M., Noguchi M., Nakano Y. Axillary reverse mapping using a fluorescence imaging system in breast cancer. J Surg Oncol. 2012;105(3):229–234. doi: 10.1002/jso.22094. [DOI] [PubMed] [Google Scholar]
- 11.Connor C., McGinness M., Mammen J. Axillary reverse mapping: a prospective study in women with clinically node negative and node positive breast cancer. Ann Surg Oncol. 2013;20(10):3303–3307. doi: 10.1245/s10434-013-3113-4. [DOI] [PubMed] [Google Scholar]
- 12.Hu J., Dong Y., Chen X. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatr. 2015;61:78–89. doi: 10.1016/j.comppsych.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J.W., Seo Y.J., Choi J.E., Kang S.H. The efficacy of arm node preserving surgery using axillary reverse mapping for preventing lymphedema in patients with breast cancer. Journal of Breast Cancer. 2012;15(1):91–97. doi: 10.4048/jbc.2012.15.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuusk U., Seyednejad N., McKevitt E.C. Axillary reverse mapping in breast cancer: a Canadian experience. J Surg Oncol. 2014;110(7):791–795. doi: 10.1002/jso.23720. [DOI] [PubMed] [Google Scholar]
- 16.Gennaro M., MacCauro M., Sigari C. Selective axillary dissection after axillary reverse mapping to prevent breast-cancer-related lymphoedema. Eur J Surg Oncol. 2013;39(12):1341–1345. doi: 10.1016/j.ejso.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda K., Ogawa Y., Kajino C. The influence of axillary reverse mapping related factors on lymphedema in breast cancer patients. Eur J Surg Oncol. 2014;40(7):818–823. doi: 10.1016/j.ejso.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Ochoa D., Korourian S., Boneti C. Axillary reverse mapping: five-year experience. Surgery (United States) 2014;156(5):1261–1268. doi: 10.1016/j.surg.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakurai T., Endo M., Shimizu K. Axillary reverse mapping using fluorescence imaging is useful for identifying the risk group of postoperative lymphedema in breast cancer patients undergoing sentinel node biopsies. J Surg Oncol. 2014;109(6):612–615. doi: 10.1002/jso.23528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue T., Zhuang D., Zhou P. A prospective study to assess the feasibility of axillary reverse mapping and evaluate its effect on preventing lymphedema in breast cancer patients. Clin Breast Canc. 2015;15(4):301-306. doi: 10.1016/j.clbc.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Q., Wu G., Xiao S.-Y. Identification and preservation of arm lymphatic system in axillary dissection for breast cancer to reduce arm lymphedema events: a randomized clinical trial. Ann Surg Oncol. 2019;26(11):3446–3454. doi: 10.1245/s10434-019-07569-4. [DOI] [PubMed] [Google Scholar]
- 22.Faisal M., Sayed M.G., Antonious K. Prevention of lymphedema via axillary reverse mapping for arm lymph-node preservation following breast cancer surgery: a randomized controlled trial. Patient Saf Surg. 2019;13(35):35. doi: 10.1186/s13037-019-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casabona F., Bogliolo S., Menada M.V. Feasibility of axillary reverse mapping during sentinel lymph node biopsy in breast cancer patients. Ann Surg Oncol. 2009;16(9):2459–2463. doi: 10.1245/s10434-009-0554-x. [DOI] [PubMed] [Google Scholar]
- 24.Ma X., Wen S., Liu B. Relationship between upper extremity lymphatic drainage and sentinel lymph nodes in patients with breast cancer. Journal of Oncology. 2019;2019 doi: 10.1155/2019/8637895. (no pagination)(8637895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio I.T., Cebrecos I., Peg V. Extensive nodal involvement increases the positivity of blue nodes in the axillary reverse mapping procedure in patients with breast cancer. J Surg Oncol. 2012;106(1):89–93. doi: 10.1002/jso.23048. [DOI] [PubMed] [Google Scholar]
- 26.Ngui N.K., French J., Kilby C.J. Axillary reverse mapping in patients with breast cancer: is it oncologically safe? J Surg Oncol. 2016;113(7):726–731. doi: 10.1002/jso.24231. [DOI] [PubMed] [Google Scholar]
- 27.Nos C., Lesieur B., Clough K.B., Lecuru F. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol. 2007;14(9):2490–2496. doi: 10.1245/s10434-007-9450-4. [DOI] [PubMed] [Google Scholar]
- 28.Nos C., Kaufmann G., Clough K.B. Combined axillary reverse mapping (ARM) technique for breast cancer patients requiring axillary dissection. Ann Surg Oncol. 2008;15(9):2550–2555. doi: 10.1245/s10434-008-0030-z. [DOI] [PubMed] [Google Scholar]
- 29.Ponzone R., Cont N.T., Maggiorotto F. Extensive nodal disease may impair axillary reverse mapping in patients with breast cancer. J Clin Oncol. 2009;27(33):5547–5551. doi: 10.1200/JCO.2009.22.1846. [DOI] [PubMed] [Google Scholar]
- 30.Bedrosian I., Babiera G.V., Mittendorf E.A. A phase I study to assess the feasibility and oncologic safety of axillary reverse mapping in breast cancer patients. Cancer. 2010;116(11):2543–2548. doi: 10.1002/cncr.25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khandelwal R., Poovamma C.U., Shilpy C. Axillary reverse mapping: is it feasible in locally advanced breast cancer patients? Breast Dis. 2014;34(4):151–155. doi: 10.3233/BD-140371. [DOI] [PubMed] [Google Scholar]
- 32.Beek M.A., Gobardhan P.D., Klompenhouwer E.G. Axillary reverse mapping (ARM) in clinically node positive breast cancer patients. Eur J Surg Oncol. 2015;41(1):59–63. doi: 10.1016/j.ejso.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi S., Satish C., Sundaram P. Feasibility study of axillary reverse mapping lymphoscintigraphy in carcinoma breast: a concept toward preventing lymphedema. Indian J Nucl Med. 2016;31(1):9–13. doi: 10.4103/0972-3919.172341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nos C., Clough K.B., Bonnier P. Upper outer boundaries of the axillary dissection. Result of the SENTIBRAS protocol: multicentric protocol using axillary reverse mapping in breast cancer patients requiring axillary dissection. Eur J Surg Oncol. 2016;42(12):1827–1833. doi: 10.1016/j.ejso.2016.07.138. [DOI] [PubMed] [Google Scholar]
- 35.Kumar K.S., Hemanth G.N., Panjwani P.K. Feasibility of axillary reverse mapping and clinicopathological features predicting ARM node metastasis in breast cancer-a pilot study. Indian Journal of Surgical Oncology. 2017;8(2):119–122. doi: 10.1007/s13193-016-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foldi M., Foldi E. “Foldi’s textbook of lymphology for physicians and lymphedema therapists. Mosby Elsevier; ” Maryland Heights, MO: 2006. [Google Scholar]
- 37.Kanda M.H., da Costa Vieira R.A., Lima J. Late locoregional complications associated with adjuvant radiotherapy in the treatment of breast cancer: systematic review and meta-analysis. J Surg Oncol. 2020;121(5):766–776. doi: 10.1002/jso.25820. [DOI] [PubMed] [Google Scholar]
- 38.Stamatakos M., Stefanaki C., Kontzoglou K. Lymphedema and breast cancer: a review of the literature. Breast Cancer. 2011;18(3):174–180. doi: 10.1007/s12282-010-0246-1. [DOI] [PubMed] [Google Scholar]
- 39.DiSipio T., Rye S., Newman B., Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 40.Seyednejad N., Kuusk U., Wiseman S.M. Axillary reverse lymphatic mapping in breast cancer surgery: a comprehensive review. Expert Rev Anticancer Ther. 2014;14(7):771–781. doi: 10.1586/14737140.2014.896209. [DOI] [PubMed] [Google Scholar]
- 41.Beek M.A., Gobardhan P.D., Schoenmaeckers E.J. Axillary reverse mapping in axillary surgery for breast cancer: an update of the current status. Breast Canc Res Treat. 2016;158(3):421–432. doi: 10.1007/s10549-016-3920-y. [DOI] [PubMed] [Google Scholar]
- 42.Noguchi M., Yokoi M., Nakano Y. Axillary reverse mapping for breast cancer. Breast Canc Res Treat. 2010;119:529–535. doi: 10.1007/s10549-009-0578-8. [DOI] [PubMed] [Google Scholar]
- 43.Hama Y., Koyama Y., Urano Y. Simultaneous two-color spectral fluorescence lymphangiography with near infrared quantum dots to map two lymphatic flows from the breast and the upper extremity. Breast Canc Res Treat. 2007;103(1):23–28. doi: 10.1007/s10549-006-9347-0. [DOI] [PubMed] [Google Scholar]
- 44.Fisher B., Brown A., Mamounas E. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: fingdings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 45.Boughey J.C., Suman V.J., Mittendorf E.A. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. J Am Med Assoc. 2013;310(14):1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuehn T., Bauerfeind I., Fehm T. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 47.Shao X., Sun B., Shen Y. Axillary reverse mapping (ARM): where to go. Breast Cancer. 2019;26(1):1–10. doi: 10.1007/s12282-018-0886-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.