Abstract
Background
Osteoarthritis (OA) of the knee is one of the leading causes of disability characterized by degeneration of hyaline cartilage combined with reparative processes. Its strong association with metabolic syndrome is postulated to be due to both mechanical and biochemical factors. Our study aims to study differential effect of metabolic risk factors on cartilage degeneration and regeneration at biomarker level.
Design
After screening 281 patients presenting with knee pain, 41 patients who met the selection criteria were included and were divided into metabolic (MetS) OA and non-metabolic (Non-MetS) OA phenotypes using National Cholesterol Education Programme—Adult Treatment Panel—III (NCEP-ATP-III) criteria for metabolic syndrome. Serum Cartilage Oligomeric Matrix Protein (COMP) and Procollagen type IIA N terminal Propeptide (PIIANP) levels were used as tools to assess cartilage degeneration and regeneration, respectively.
Results
22 among 41 patients (53.66%) had metabolic syndrome. Covariates like age, gender, Kellgren Lawrence (KL) grades were comparable in both groups. MetS-OA group showed significant increase in serum COMP levels (p = 0.03) with no significant effect on serum PIIANP levels (p = 0.46). Hypertriglyceridemia showed independent association with both cartilage anabolism (p = 0.03) and catabolism (p = 0.03).
Conclusion
Metabolic syndrome, though has no effect on cartilage regeneration tends to shift cartilage homeostasis towards degeneration with hypertriglyceridemia showing significant independent effect on cartilage metabolism.
Keywords: Osteoarthritis, Biomarker, Metabolic syndrome, Cartilage metabolism, COMP, PIIANP
Introduction
Metabolic syndrome (MetS) is a group of disorders that includes hyperglycemia, dyslipidemia (increase triglyceride or reduced HDL/increased LDL), central obesity and hypertension. Presence of any three out of these five parameters is defined as MetS as per NCEP ATP-III guideline [1]. In context to the musculoskeletal disorder, the osteoarthritis (OA) of the knee is particularly found to have strong association. OA of the knee joint is the most prevailing rheumatological condition significantly affecting the activity of daily living in > 40 year old population. It is characterized by pain, swelling, deformity and stiffness of joint. In general, it is believed that OA happens because of only degeneration of the hyaline cartilage, however the pathological pathway includes combined degenerative and reparative responses of the articular cartilage [2, 3]. Which is controlled by environmental, metabolic and mechanical factors.
Earlier it was believed that patients with MetS are prone for OA of knee solely because of the mechanical factor related to the obesity. However, involvement of the non-weight bearing joints raised concern to explore reasons other than mechanical factor [4]. It was identified in multiple studies that metabolic syndrome is known to have multidimensional influence on the OA of knee joint e.g.; increased degeneration of articular cartilage [4, 5], higher pain score [5] and early onset of disease [3]. It has been hypothesized that proinflammatory state and oxidative stress induced by MetS considered as important trigger for OA. However, study focused to look at the response of the articular cartilage at the molecular level using biomarkers in cases of OA knee with MetS and without MetS has never been evaluated.
The primary objective of our study was to compare the cartilage degeneration and regeneration in cases with OA knee with and without MetS. Our secondary objective was to study the differences in subjective severity of disease and the proinflammatory state in these two phenotypes.
Materials and Methods
It is prospective observational study to find the clinical, serum biomarker-based differences in cases with early OA of knee with or without MetS. Institutional ethics committee approval was taken for the study. All the cases with the knee pain without any history of trauma/surgery and without involvement of any other joint were screened for OA knee using clinical and radiological examination methods. During the study period of 1 year, a total of 281 patients presenting with knee pain to a tertiary care Centre in north India were screened. Patients aged more than 40 years with early OA knee (KL gd 1/2) with complaints of knee pain that increases with walking, running, going up/downstairs, present for greater than 6 weeks or recurring knee pain with stiffness after period of inactivity were included. Patients with multiple joint arthritis, advanced arthritis (KL gd 3/4), arthritis secondary to trauma, previous surgery, inflammatory arthritis, with patellofemoral or tibiofemoral instability, suspected meniscus injury were excluded. A total of 41 cases meeting these selection criteria (Table 1) were included in the study after thorough clinical and radiological examination. The selected study population was divided into two phenotypes based on NCEP ATP III criteria after relevant investigations (Table 2).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
1. Knee pain increasing with walking, running, climbing up or going downstairs 2. Duration > 6 weeks or recurring knee pain 3. Stiffness after a period of inactivity 4. Age > 40 years 5. Early OA knee KL grade 1/2 |
1. Knee pain with suspected meniscus injury, patellofemoral/tibiofemoral instability 2. Inflammatory arthritis, multiple joint pains 3. Post traumatic/post surgical knee pain 4. Severe OA knee KL grade 3/4 |
Table 2.
Modified NCEP-ATP3 criteria for metabolic syndrome
| Parameter | Men | Women |
|---|---|---|
| Abdominal obesity | >/= 90 cm | >/= 80 cm |
| Blood pressure | > 130/85 mm Hg | > 130/85 mm Hg |
| Fasting blood sugar | > 100 mg/dl | > 100 mg/dl |
| HDL | < 40 mg/dl | < 50 mg/dl |
| Triglyceride | >/= 150 mg/dl | >/= 150 mg/dl |
*Presence of 3 of the above 5 is called metabolized syndrome
In all the selected cases the activity of daily living and severity of disease were assessed clinically with WOMAC (western Ontario and MC masters university osteoarthritis index) knee score. Serum hsCRP was used as marker for circulatory proinflammatory state, response of hyaline cartilage was analysed using serum COMP and serum PIIANP, at presentation and 6 months of follow up. Conservative management was applied in all the patients to alleviate symptoms according to OARSI (Osteoarthritis research society international) guidelines except for intraarticular steroid use during period of observation.
Statistical Analysis
Collected data were subjected to statistical analysis using SPSS v20 software. The descriptive statistics for two groups (e.g. mean, standard deviation) were calculated for all parameters (i.e. WOMAC, COMP, PIIANP, hs CRP, etc.). Age and KL grade were compared between the groups using the t test. The absolute values at presentation and after 6 months and also difference in COMP, PIIANP, hs-crp and WOMAC after 6 months were compared using Student t test for significance.
Results
Among the 41 patients included in the study 22 patients (53.66%) had MetS and the 19 without MetS. (As per modified NCEP-ATP III criteria). Among 22 patients with MetsOA 17 were females and 5 were males. Average age of patients in MetS-OA group was 54.3 years while it was 57 years in non-MetS OA group. There was no significant difference in covariates age (p = 0.07) and KL grading (p = 0.55) between the two groups (Table 3).
Table 3.
Age, gender, KL grade distribution in two groups
| Age | Gender | KL grade | |||
|---|---|---|---|---|---|
| Male | Female | 1 | 2 | ||
| Mets-OA | 54.3 | 5 | 17 | 25 | 19 |
| Non Mets OA | 57 | 6 | 13 | 25 | 13 |
Among the 41 patients included in study 90.24% of the study population had high waist circumference, 58.54% had low HDL, 48.78% had high triglyceride, 43.9% were diabetic and 41.46% had hypertension (Fig. 1).
Fig. 1.
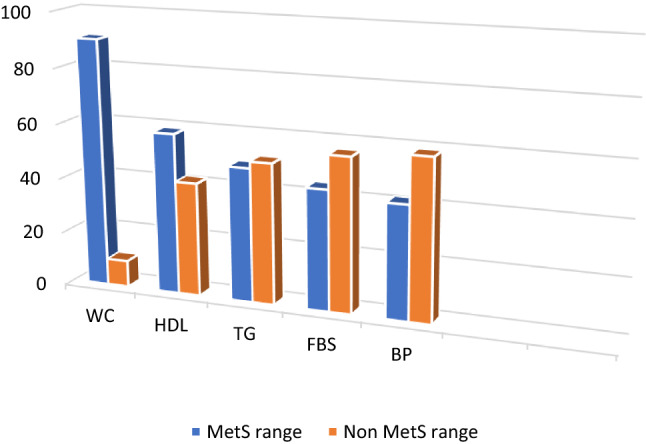
Bar graph showing percentage distribution of MetS parameters in the study population (n = 41)
Among 19 patients Non-MetS OA, 4 patients had at least one among five abnormal metabolic parameter, 15 patients had two among five abnormal metabolic parameters. Among 22 patients with metabolic syndrome, 10 patients had three abnormal metabolic parameters, while 8 patients had four and 4 had all the five abnormal parameters.
The difference in the serum levels of cartilage metabolism markers and inflammatory markers over 6 months of study period was calculated (Table 4). There was a significant increase in the levels of serum COMP over 6 months in MetS-OA group compared to non-MetS OA group (p = 0.03). On the other hand, changes in serum PIIANP levels (p = 0.46) and serum hsCRP (p = 0.27) levels were not statistically different between the two groups. On testing the correlation of each marker with the WOMAC knee score, only serum COMP level showed positive correlation (Pearson’s correlation = 0.615). Changes in WOMAC score over 6 months period was significantly more in the MetS OA group (p = 0.04) (Table 5). Among metabolic markers, hypertriglyceridemia showed significant positive association with increase in serum COMP (p = 0.03) and PIIANP (p = 0.03) levels.
Table 4.
Difference in serum comp, piianp and hs-crp levels over 6 months in two groups
| ∆comp | SD | P value | ∆piianp | SD | P value | ∆hs-crp | SD | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Mets OA | + 32.2 | 126.3 | 0.03 | + 1.36 | 5.64 | 0.46 | + 0.25 | 1.29 | 0.27 |
| Non Mets OA | − 53.8 | 121.7 | + 2.67 | 1.2 | − 0.11 | 0.87 |
∆comp: difference in COMP levels over 6 months
∆piianp: differences in PIIANP levels over 6 months
∆hs-crp: difference in hs-CRP levels over 6 months
Table 5.
Difference in WOMAC scores in two groups over 6 months
| WOMAC | ∆WOMAC | SD | P value | ||
|---|---|---|---|---|---|
| Day 1 | 6 months | ||||
| Mets OA | 34.1 | 36.8 | + 2.7 | 4.39 | 0.04 |
| Non Mets OA | 28.8 | 27.5 | − 0.7 | 3.82 | |
∆womac: difference in WOMAC scores over 6 months
Discussion
Every component of the MetS is either directly or indirectly related to lifestyle and habit. Being a developing country, Indian population is going through phase of transition in the lifestyle, which is reflected as the rising incidence of MetS [5], so is the incidence osteoarthritis [6]. In our study, the prevalence of metabolic syndrome in early cases of knee osteoarthritis was 53.66% which is comparable to the study by Xie et al. and LeClanche et al. [3, 7]. Other important factor to consider here is that the rest 46.33% of patients in our study also had at least one of the abnormal metabolic parameter further highlighting the role of metabolic abnormalities in the disease pathogenesis.
Symptomatic OA knee in association with MetS is related to mechanical effect on the joint because of overweight and also alteration in synovial and serological milieu leading to early and symptomatic degeneration of the joint [3, 4, 8]. However, the radiological grade and age of the patients were comparable in both the group. The possible reason could be strict inclusion criteria to select patient with grade I and II OA only. Many patients with early onset OA were excluded because of higher severity of disease. Several authors have emphasized upon contribution of MetS for more severity of OA at lower age compare to non-MetS [3, 9, 10]. In our study, clinical severity of OA knee was more severe in Mets OA knee compared to Non-Mets OA knee. WOMAC scores at presentation (p value = 0.03) and at 6 months (p = 0.0004) were significantly higher in MetS group. Furthermore, the increase in WOMAC score was also significantly higher (p value = 0.04) in MetS OA group.
Systemic raised level of proinflammatory marker is one of the common pathway responsible for MetS and OA [11]. The adipokines, proinflammatory markers released by white adipose tissue play crucial role to trigger systemic inflammatory response [12–15]. On the contrary, in our study the systemic inflammatory marker levels were not significantly different in both the groups (p = 0.27). We found it to be related to comparable distribution of the central obesity in both the groups (MetS-20 out of 22 patients vs non-MetS-17 out of 19 patients).
In our study we have used serum level of COMP as marker of cartilage degeneration and PIIANP as marker of reparative process as proposed by OARSI [16–18]. These biomarkers belong to BPD categories of BIPEDS classification (Burden of disease, Investigative, prognostic, efficacy of intervention, diagnostic)of biomarkers. These biomarkers have been proposed by OARSI group for inclusion in DMOADS (Disease modifying osteoarthritis drugs) studies. However, the standardization of the value has not been done based on age, race, ethnicity and disease severity, which limits the clinical utility of single value [19, 20]. To overcome this limitation we have looked at the trend of changes in biomarker value over 6 months. Serial measurement of cartilage metabolism markers showed significant increase in the serum levels of COMP in MetS-OA group compared to non-MetS OA group. However, serum PIIANP levels were comparable in both the groups. This indicates predominant effect of MetS on increased rate of degeneration without any significant effect on reparative process, hence shifting the cartilage homeostasis towards degeneration. Our finding is corroborative to the observation of Goldring et al. [21].
MetS increases the risk of OA in younger population by > 5 time. But each component of MetS does predict risk for development of OA [5]: however, in our study only hypertriglyceridemia has shown significant negative effect on cartilage biomarkers. On the other hand, diabetes mellitus and hypertension also showed some association with increase in COMP levels but was not statistically significant. We could not compare the independent effects of obesity on cartilage metabolism due to lack of a strong control group, as only four patients in the study population were not obese.
The interpretation of results of our study has got limitation because of small sample size, short follow up and biomarker measured in serum only. However, Darweesh et al. have found positive correlation between synovial and serum COMP levels and the WOMAC index [22]. This correlation of synovial and serum levels of COMP obviates the need for invasive step to harvest the synovial fluid from painful joint.
To summarize, metabolic syndrome has high prevalence in patients with osteoarthritis of knee. Presence of MetS worsens the clinical presentation in OA knee and favours relatively rapid clinical deterioration and disease progression. MetS shifts the balance of cartilage homeostasis leading to degradation of cartilage and shows the dose–response relation to the degeneration. Besides these the current study is food for thought for extending the clinical/laboratory evaluation of patients visiting to orthopaedic OPD and comprehensive care of these sub-group of patients. In future we will work on to extend the pilot work into a study with larger sample size and will study the correlation of therapeutic intervention for metabolic syndrome to the clinical outcome and cartilage metabolism.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rajath Siddaramanna Onkarappa, Email: drrajathso@gmail.com.
Devendra Kumar Chauhan, Email: drdevnim@gmail.com.
Biman Saikia, Email: bimansaikia@hotmail.com.
Rajendra Kumar Kanojia, Email: drkanojia_rajendra@yahoo.co.in.
References
- 1.Misra A, Choubey P, Makkar BM, et al. Consensus statement for diagnosis of obesity, abdominal obesity and metabolic syndrome for Asian Indians and recommendation for physical activity, medical and surgical management. The Journal of the Association of Physicians of India. 2009;57:163–170. [PubMed] [Google Scholar]
- 2.Loeser R, Goldring S, Scanzello C, Goldring M. Osteoarthritis: A disease of the joint as an organ. Arthritis and Rheumatism. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Clanche S, Bonnefont RD, Sari-Ali E, Rannou F, Borderie D. Inter-relations between osteoarthritis and metabolic syndrome: A common link? Biochimie. 2015;121:238–252. doi: 10.1016/j.biochi.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Dong N, Gao YH, Liu B, Zhao CW, Yang C, Li SQ, Liu JG, Qi X. Differential expression of adipokines in knee osteoarthritis patients with and without metaboli syndrome. International Orthopaedics. 2018;42(6):1283–1289. doi: 10.1007/s00264-018-3761-x. [DOI] [PubMed] [Google Scholar]
- 5.Pan F, Tian J, Mattap SM, Cicuttini F, Jones G. Association between metabolic syndrome and knee structural change on MRI: A 10.7-year follow-up study. Rheumatology (Oxford) 2020;59(1):185–193. doi: 10.1093/rheumatology/kez266. [DOI] [PubMed] [Google Scholar]
- 6.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 7.Xie DX, Wie J, Zeng C, et al. Association between metabolic syndrome and knee osteoarthritis: A cross sectional study. BMC Musculoskeletal Disorders. 2017;18(1):533. doi: 10.1186/s12891-017-1890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afifi AEMA, Shaat RM, Gharbia OM, et al. Osteoarthritis of knee joint in metabolic syndrome. Clinical Rheumatology. 2018;37:2855. doi: 10.1007/s10067-018-4201-4. [DOI] [PubMed] [Google Scholar]
- 9.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgraduate Medicine. 2009;121(6):9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 10.Chadha R. Revealed aspect of metabolic osteoarthritis. Journal of Orthopaedics. 2016;13(4):347–351. doi: 10.1016/j.jor.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis and Cartilage. 2015;23(11):1955–1965. doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Staikos C, Ververidis A, Droso G, Manolopoulos VG, Varettas DA, Tavridou A. The association of adipokine levels in plasma and synovial fluid with the severity of knee osteoarthritis. Rheumatology (Oxford) 2013;52(6):1077–1083. doi: 10.1093/rheumatology/kes422. [DOI] [PubMed] [Google Scholar]
- 13.Bas S, Finckh A, Puskas GJ, Suva D, Hoffmeyer P, Gabay C, Lübbeke A. Adipokines correlate with pain in lower limb osteoarthritis: different associations in hip and knee. International Orthopaedics. 2014;38:2577–2583. doi: 10.1007/s00264-014-2416-9. [DOI] [PubMed] [Google Scholar]
- 14.Sowers MR, Karvonen-Guterrez CA. The evolving role of obesity in knee osteoarthritis. Current Opinion in Rheumatology. 2010;22(5):533–537. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 16.Daghestani HN, Jordan JM, Renner JB, Doherty M, Wilson AG, Kraus VB. Serum N-propeptide of collagen IIA (PIIANP) as a marker of radiographic osteoarthritis burden. PLoS One. 2017;12(12):e0190251. doi: 10.1371/journal.pone.0190251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma P, Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: A novel diagnostic and prognostic biomarker. Journal of Orthopaedic Research. 2013;31:999–1006. doi: 10.1002/jor.22324. [DOI] [PubMed] [Google Scholar]
- 18.Van Spil WE, DeGroot J, Lems W, Oostveen J, Lafeber F. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis and Cartilage. 2010;18:605–612. doi: 10.1016/j.joca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Saberi Hosnijeh F, Bierma-Zeinstra SM, Bay-Jensen AC. Osteoarthritis year in review 2018: Biomarkers (biochemical markers) Osteoarthritis Cartilage. 2019;27(3):412–423. doi: 10.1016/j.joca.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Felson DT. The current and future status of biomarkers in osteoarthritis. Journal of Rheumatology. 2014;41(5):834–836. doi: 10.3899/jrheum.140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Current Rheumatology Reports. 2013;15(11):375. doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darweesh H, Abbass D, Kadah R, Rashad A, El Basel M, Nasr A. Serum and synovial cartilage oligomeric matrix protein in patients with rheumatoid arthritis and osteoarthritis. Indian Journal of Rheumatology. 2010;5:112–117. doi: 10.1016/S0973-3698(10)60556-0. [DOI] [Google Scholar]


