Abstract
Purpose
d-Dimer estimation has been proposed as a reliable biomarker in prosthetic joint infections. Its role in non-prosthetic orthopaedic implant infections has, however, not been studied. The objectives of this study were to assess the levels of plasma d-Dimer in non-prosthetic orthopaedic implant infection. The diagnostic efficiency of d-dimer on orthopaedic implant-related infection was evaluated.
Methods
The study was designed as a cross-sectional comparative study. Patients who presented with orthopaedic implant-related infection as diagnosed by modified MSIS criteria were allocated to case group (n = 49) and patients who underwent surgical procedures with orthopaedic implants with no evidence of infection at 6 weeks postoperatively were allocated to the control group (n = 48). Serum d-Dimer levels were assessed quantitatively using immunoturbidimetric assays in both groups and compared between both groups.
Results
The mean (± SD) value of serum d-Dimer in case group was 0.64 (± 0.45) μg/ml and control group was 0.77 (± 0.47) μg/ml. No significant difference was found in serum d-Dimer levels between cases and control groups (p value = 0.183). The diagnostic accuracy of d-dimer in orthopaedic implant-related infection also could not be demonstrated.
Conclusion
The findings of d-dimer as a marker for the diagnosis of prosthetic joint infections cannot be extrapolated to non-prosthetic orthopaedic implant infection.
Electronic supplementary material
The online version of this article (10.1007/s43465-020-00120-8) contains supplementary material, which is available to authorized users.
Keywords: d-dimer, Biomarkers, Implant associated infection, Internal fixation
Introduction
Infection, systemic or localised triggers an inflammatory response which results in the release of acute-phase reactants [1]. The measurement of these proteins has been traditionally used as biomarkers of the infective process [1]. Recent research has shown that along with the inflammatory response, the activation of coagulation–fibrinolytic response is an important component in the first line for defence against infection [2]. d-dimer is a degradation product of fibrin and its estimation is routinely used in the evaluation of venous thrombosis [3]. Elevated levels of d-dimer are also encountered in infection due to close interaction of the inflammatory and coagulation pathways [4]. d-dimer levels have been used to diagnosis and prognostication of infection [5–7]. The role of d-dimer in orthopaedic infection has been largely limited to prosthetic joint infection [8, 9]. The role of d-dimer as a marker of prosthetic joint infection (PJI) is promising [4]. Recently, elevated d-dimer levels in infected non-unions have been reported [10].
Orthopaedic implant-related infection (OIRI) remains a difficult problem to diagnose and treat for orthopaedic surgeons worldwide [11]. Slow-growing organisms, muted host defence mechanisms, and culture negativity, render infection associated with implants and prosthetic joints extremely difficult to diagnose and treat [4]. One of the principal causes of failure in devising a treatment strategy for OIRI is the lack of a definitive diagnostic test. The outcomes of the studies on d-dimer in PJI motivated us to investigate its behaviour in non-prosthetic orthopaedic implant infection. Non-prosthetic orthopaedic implant infection essentially refers to infection associated with fracture fixation implants. Though diagnostic criteria have been suggested for fracture-related infection, diagnosis remains difficult [12–14]. The levels of d-dimer in fracture fixation device-related infection have not been studied before.
This study aims to assess and compare the levels of d-dimer between diagnosed non-prosthetic orthopaedic implant infection cases and those with orthopaedic implant surgery without infection (controls). The diagnostic accuracy of d-dimer in such orthopaedic implant infection was also verified.
Materials and Methods
This descriptive cross-sectional comparative study was conducted from September 2017 to July 2019 in a tertiary care centre meant for the treatment of orthopaedic trauma. The study was approved by the institutional review board. Patients of 18 years or more presenting to the outpatient department with OIRI were included in the study and termed as cases. In this study, the term OIRI was limited to infection associated with non-prosthetic orthopaedic implants used for fracture fixation. Patients who underwent fracture surgery with orthopaedic implant, without any evidence of infection after 6 weeks of operation served as controls. Recent diagnostic criteria for Prosthetic Joint Infections (PJI) by the Musculoskeletal Infection Society (MSIS) were modified and taken as the diagnostic criteria for the OIRI for the study [14]. OIRI was said to be present if at least one of the following criteria was present:
-
i.
Visible purulence of a pre-operative aspirate or intraoperative peri-implant tissue.
-
ii.
Presence of a sinus tract communicating with the implant.
-
iii.
Microbial growth of the same organism in two or more cultures from pre-operative implant aspirate, intraoperative periprosthetic tissue or sonication fluid of the removed implant.
-
iv.
Detection of acute inflammation in tissue histopathology.
Patients with diagnosis or a history of venous thromboembolism, joint injury (involving the joint scheduled for surgery) within the last 2 weeks, remote infection, recent operation (within 2 weeks), cancer, pregnancy, on oral anticoagulants and American society of anaesthesiologists (ASA) category III or more, were excluded from the study [15]. After due consent, samples were collected for the estimation of blood ESR, C-reactive protein, white blood cell (WBC) counts, and serum d-dimer levels on presentation in cases and 6 weeks post-operative in controls.
Blood ESR was estimated by the Westergren method.
Fluorescence flow cytometry was used to measure total and differential WBC and count.
CRP estimation was done by the latex agglutination test.
Samples for serum d-dimer were collected in a tube containing sodium citrate as an anticoagulant. The collected blood samples were immediately centrifuged at 2500 rpm for 30 min and the plasma was separated and stored in a deep freezer at a temperature of − 80 °C for not more than 6 months from the date of collection. The serum d-dimer estimation was done using latex agglutination assay (Suyog Diagnostics Pvt. Ltd, Mumbai, India) based on immunoturbidimetric technique. The results were reported on qualitative (positive and negative) as well as quantitative scale (µg/ml). Values of d-dimer suggested by Zhang et al. were considered as the reference for this study [16]. A plasma d-dimer value of 0.5 µg/ml or above was recorded as positive for the presence of infection in cases and controls. d-dimer is normally present in human plasma. Normal d-dimer levels are typically below 0.5 µg/ml [16]. All controls were followed up for 1 year after operation for the presence of infection. The organisms causing infection were also identified.
Statistical Analysis
The sample size was estimated using the statistical formula for comparing two independent means with a minimum expected difference in mean level in serum d-dimer as 50 with a standard deviation of 75 at a 5% level of significance with 90% power [17].The distribution of categorical data of clinical features, causative organism profile, socio-demographic data, and comorbidities was expressed as frequency and percentages. The continuous data such as age, serum d-dimer levels, duration since the operation were expressed as mean with SD or median with range. The comparison of the level of d-dimer between the groups was carried out using independent student’s t test. The comparison of the d-dimer between the categorical variables mentioned above was carried out using independent student’s t test. All statistical analysis was carried out a 5% level of significance and p value < 0.05 was considered as significant. The receiver operating characteristic (ROC) curve was plotted for assessing the diagnostic accuracy for plasma d-dimer in cases.
Results
A total of 97 participants were recruited to the study. There were 49 cases and 48 controls. The cases and controls were age and gender matched. There were 42 males and 7 females in cases; and 35 males and 13 females in the control group. None of the patients in the control group reported infection. All infection pertained to fracture fixation implant-related infection only. Staphylococcus aureus was the most commonly isolated organism (Table1).
Table 1.
Organisms identified with infected cases
| Cultured organism | N (%) |
|---|---|
| Staphylococcus Aureus | 14 (28.6) |
| Acinetobacter baumannii | 8 (16.3) |
| Pseudomonas species | 5 (10.2) |
| MRSA | 4 (8.2) |
| Klebsiella pneumonia | 3 (6.1) |
| Enterobacter Species | 3 (6.1) |
| Staphylococcus aureus with Pseudomonas aeruginosa | 2 (4.1) |
| Enterococcus | 2 (4.1) |
| Staphylococcus aureus with beta hemolytic streptococci | 2 (4.1) |
| No growth | 2 (4.1) |
| Morganella morgani | 1 (2) |
| Beta haemolytic streptococci | 1 (2) |
| Escherichia coli | 1 (2) |
| Beta haemolytic streptococci with klebsiella pneumonia | 1 (2) |
There was a difference in mean ESR between cases 65.16 (± 31.72) mm/1st hour and controls 32.63 (± 23.90) significant mm/1st hour (p ≤ 0.001). Values of measured CRP did not follow normal distribution. The median (IQR) CRP value was 0.60 (1.20) mg/L in cases and 0.0 (0.60) mg/L in controls. The mean (± SD) WBC count between cases and controls was 9387.55 (± 3420.67) cells/µl and 9782.73 (± 3121.36) cells/µl. There was a statistically significant difference in CRP levels in cases and controls. (p ≤ 0.001). The difference in WBC counts between cases and controls was not significant. (p = 0.554).
The mean plasma d-dimer levels were 0.64 (± 0.45) µg/ml in cases and 0.77 (± 0.47) µg/ml in controls, and the difference between them was not significant (p = 0.183).
The area under the curve (AUC) was found to be < 0.5 (0.412). The ROC curve did not demonstrate diagnostic accuracy for d-dimer (Fig. 1).
Fig. 1.
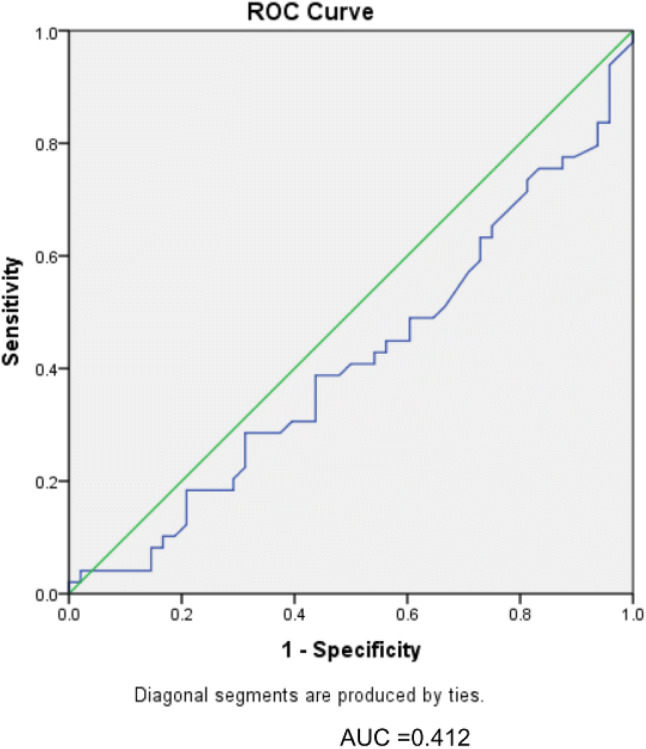
Receiver operating characteristic (ROC) curve assessing diagnostic accuracy of plasma d-dimer in orthopaedic implant-related infections
Discussion
We did not find a difference in d-dimer levels between non-prosthetic orthopaedic implant-associated infection and those without infection. Further, the diagnostic accuracy of d-dimer was not demonstrated in cases plotted on the ROC curve.
Plasma d-dimer levels follow a consistent pattern of rising and fall in the postoperative period [8]. Elevated levels beyond 6 weeks suggest the activation of the coagulation–fibrinolytic system as a part of the defence mechanism against infection. This observation was the basis of d-dimer estimations in controls at 6 weeks in this study. The pattern of ESR and CRP levels in our study mirror with published literature [11]. The rapid rise and fall of d-dimer levels make it more suitable than ESR or CRP levels in the diagnosis of early post-operative infection [18].
We believe though prosthetic joint infection and infection associated with fixation implants are clubbed together as OIRI, they behave differently. The inflamed synovium in PJI secretes a huge amount of fibrin, which when degraded leads to the formation of d-dimer. This leaks from the synovial fluid into the systemic circulation, where it can be estimated as a plasma marker for PJI [4]. The role of the synovial membrane in d-dimer levels has been studied [4]. All the cases in our study involved infections with extra-articular fracture fixations. It is postulated that since the synovium is not involved in extra-articular fracture fixations, d-dimer levels were not different between cases and controls. Fibrinolysis within a joint has protective action as hemarthrosis is known to cause articular cartilage damage. This process may be limited to the joint and unlikely to be triggered when the bone sustains the infection.
Literature has distinguished between plasma and serum estimation of d-dimer in the diagnosis of infection [19]. We used plasma d-dimer estimations in our study. Semi-quantitative (whole blood agglutination and ELISA/ELFA), and quantitative (latex agglutination by immunoturbidimetric) methods for estimation of d-dimer have been described [3]. We used the quantitative method of investigating d-dimer levels in our study.
To the best of our knowledge, ours is the first study that investigated the role of d-dimer in extra-articular orthopaedic infections with an implant in situ. Confounding variables that could have led to an elevation of plasma d-dimer have been excluded in our study. Tranexamic acid, fluoroquinolones, and macrolides have been reported to cause a decrease in levels of plasma d-dimer [20]. These agents were not used in either cases or controls in this study.
The study achieved its target sample size, yet did not find any difference between OIRI and controls.
There were a few limitations to our study. Our study did not do pre-operative and serial d-dimer assessments which could have diagnosed post-operative infection on day 2. However, the normal progression of plasma d-dimer levels has been investigated by different studies [8]. No study has reported the levels of plasma d-dimer in the early postoperative period (day 2) among patients that later turned out to be a post-operative infection. We followed our control group for 1 year and none developed a post-operative infection.
Conclusion
We conclude that the findings of d-dimer as a marker for the diagnosis of early, late and occult prosthetic joint infections cannot be extrapolated to extra-articular implant-related orthopaedic infections. The plasma d-dimer levels have no diagnostic accuracy in diagnosing orthopaedic implant-related infection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This study was funded by an intra-mural grant from the Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The study was done in accordance with the ethical standards of the institutional research committee (Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India, Project number: JIP/IEC/2017/0256) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
Patients signed informed consent regarding publishing their data and photographs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. New England Journal of Medicine. 1999;340:448. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 2.Antoniak S. The coagulation system in host defense. Research and Practice in Thrombosis and Haemostasis. 2018;2(3):549–557. doi: 10.1002/rth2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: d-dimer. Journal of the American College of Cardiology. 2017;70(19):2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum d-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. The Journal of Bone and Joint Surgery. 2017;99(17):1419–1427. doi: 10.2106/JBJS.16.01395. [DOI] [PubMed] [Google Scholar]
- 5.Goebel PJ, Williams JB, Gerhardt RT. A pilot study of the performance characteristics of the d-dimer in presumed sepsis. The Western Journal of Emergency Medicine. 2010;2:7. [PMC free article] [PubMed] [Google Scholar]
- 6.Hao L, Wang N. Changes in plasma thrombomodulin and d-dimer levels and their clinical significance in neonates with sepsis. Chinese Journal of Contemporary Pediatrics. 2013;15(10):841–844. [PubMed] [Google Scholar]
- 7.Kaya B, Sana B, Eris C, Karabulut K, Bat O, Kutanis R. The diagnostic value of d-dimer, procalcitonin and CRP in acute appendicitis. International Journal of Medical Sciences. 2012;9(10):909–915. doi: 10.7150/ijms.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YS, Lee YK, Han SB, Nam CH, Parvizi J, Koo KH. Natural progress of D-dimer following total joint arthroplasty: A baseline for the diagnosis of the early postoperative infection. Journal of Orthopaedic Surgery and Research. 2018;13:36. doi: 10.1186/s13018-018-0730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alijanipour P, Bakhshi H, Parvizi J. Diagnosis of periprosthetic joint infection: The threshold for serological markers. Clinical Orthopaedics and Related Research. 2013;471(10):3186–3195. doi: 10.1007/s11999-013-3070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Zheng C, Wen S, Wang J, Zhang Z, Qiu X, Chen Y. Usefulness of serum d-dimer for preoperative diagnosis of infected nonunion after open reduction and internal fixation. Infection and Drug Resistance. 2019;1(12):1827–1831. doi: 10.2147/IDR.S213099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvand A, Rezapoor M, Parvizi J. The role of biomarkers for the diagnosis of implant-related infections in orthopaedics and trauma. Advances in Experimental Medicine and Biology. 2017;971:69–79. doi: 10.1007/5584_2017_11. [DOI] [PubMed] [Google Scholar]
- 12.Morgenstern M, Kühl R, Eckardt H, Acklin Y, Stanic B, Garcia M, et al. Diagnostic challenges and future perspectives in fracture-related infection. Injury. 2018;49:S83–90. doi: 10.1016/S0020-1383(18)30310-3. [DOI] [PubMed] [Google Scholar]
- 13.Metsemakers WJ, Morgenstern M, McNally MA, Moriarty TF, McFadyen I, Scarborough M, et al. Fracture-related infection: A consensus on definition from an international expert group. Injury. 2018;49(3):505–510. doi: 10.1016/j.injury.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Tschudin-Sutter S, Frei R, Dangel M, Jakob M, Balmelli C, Schaefer DJ, et al. Validation of a treatment algorithm for orthopaedic implant-related infections with device-retention—results from a prospective observational cohort study. Clinical Microbiology and Infection. 2016;22(5):457.e1–457.e9. doi: 10.1016/j.cmi.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Bytniewski P, Macha W, Romanowski L, Wiśniewski W, Kosowski K. The dynamics of d-dimer level fluctuation in patients after the cemented and cementless total hip and total knee replacement. Journal of Orthopaedic Surgery and Research. 2014;9:89. doi: 10.1186/s13018-014-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Li M. A call for standardization and age adjusted d-dimer cut-off value. Journal of Blood Disorders Symptoms and Treatments. 2017;1:2. [Google Scholar]
- 17.Rodelo JR, De la Rosa G, Valencia ML, Ospina S, Arango CM, Gómez CI, et al. d-dimer is a significant prognostic factor in patients with suspected infection and sepsis. American Journal of Emergency Medicine. 2012;30(9):1991–1999. doi: 10.1016/j.ajem.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Xiong L, Li S, Dai M. Comparison of d-dimer with CRP and ESR for diagnosis of periprosthetic joint infection. Journal of Orthopaedics Surgery and Research. 2019;14:240. doi: 10.1186/s13018-019-1282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Shao H-Y, Hao L-B, Yu B-Z, Qu P-F, Zhou Y-X, et al. Plasma fibrinogen exhibits better performance than plasma d-dimer in the diagnosis of periprosthetic joint infection: A multicenter retrospective study. Journal of Bone and Joint Surgery. 2019;101(7):613–619. doi: 10.2106/JBJS.18.00624. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Xie J, Huang Q, Lei Y, Zhang S, Pei F. Plasma fibrin degradation product and D-dimer are of limited value for diagnosing periprosthetic joint infection. Journal of Arthroplasty. 2019;34(10):2454–2460. doi: 10.1016/j.arth.2019.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


