Abstract
Study design
Randomized controlled trial.
Objectives
To study the magnitude of bone loss at forearm in persons with acute spinal cord injury (SCI) & the effect of early administration of Zoledronic acid on its’ prevention.
Settings
Sawai Man Singh Medical College, Jaipur, India.
Methods
Sixty patients with acute SCI were randomized either to receive standard medical and nursing care or Zoledronic acid infusion in combination with standard medical and nursing care. Areal bone mineral density (aBMD) was measured at the forearm (radius + ulna) once patients were medically stable using Dual Energy X-Ray Absorptiometry (DXA) at baseline and at 3, 6 and 12 months.
Results
Significant differences in aBMD was found between the control & Zoledronic acid group at 1/3 forearm (− 0.064; 95% CI − 0.092 to − 0.036, p = 0.001), mid forearm (− 0.059; 95% CI − 0.084 to − 0.034, p = 0.001), UD forearm (− 0.048; 95% CI − 0.097 to 0.001, p = 0.016) and total forearm (− 0.048; 95% CI − 0.088 to − 0.008, p = 0.021) at 1 year in the paraplegic patients with SCI. Similar significant difference was also observed at 1/3 forearm (− 0.046; 95% CI − 0.073 to − 0.019, p = 0.002), mid forearm (− 0.063; 95% CI − 0.088 to − 0.037, p < 0.0001), UD forearm (− 0.084; 95% CI − 0.101 to − 0.067, p < 0.0001) and total forearm (− 0.115; 95% CI − 0.132 to − 0.097, p < 0.0001) respectively at 1 year in the quadriplegic patients with SCI. Significant differences in aBMD between the groups at 6 months post infusion was also observed at these sites in quadriplegic patients. [1/3 forearm − 0.022; 95% CI − 0.039 to − 0.005; p = 0.015, Mid forearm − 0.023; 95% CI − 0.042 to − 0.004; p = 0.019, UD forearm − 0.041; 95% CI − 0.055 to − 0.027; p < 0.0001 and Total forearm − 0.049; 95%CI − 0.062 to − 0.036; p < 0.0001]. Bone loss was reduced in the Zoledronic acid treated group compared to the standard treatment group in both paraplegic and quadriplegic patients.
Conclusion
Single dose of 5mg intravenous Zoledronic acid is an effective treatment in preventing bone loss at the forearm for 12 months following acute spinal cord injury.
Keywords: Bone loss, Osteoporosis, Spinal cord injury (SCI), Zoledronic acid, Bisphosphonate, Dual energy x-ray absorptiometry (DXA)
Introduction
Osteoporosis is well-recognized following of spinal cord injury (SCI). It is defined as skeletal micro-architecture deterioration associated with low bone mass. [1] Osteoporosis following acute SCI is generally considered due to disuse. [2] Severe bone loss results due to hormonal changes, unloading of bones and neural lesion subsequent to SCI [3].
Increased bone resorption due to enhanced osteoclastic activity coupled with decreased osteoblatic activity leads to rapid onset sub-lesional bone loss following immobilization in patients with SCI [4]. Sub-lesional osteoporosis is attributed to non-weight bearing and mechanical stimuli loss, i.e., muscle contraction due to SCI. [ [5], [6] ] Accelerated bone loss consequent to SCI is associated not only with low intensity fragility fractures, but also with increased morbidity, mortality and substantial health care cost [1].
Bisphosphonates inhibit bone resorption resulting in decreased bone loss. [ [4], [7] ] SCI patients in acute phase are nursed in supine position during initial care. Following oral bisphosphonates ingestion, it is advised to remain upright at least for 30 min. It is difficult during acute phase SCI. Intravenous bisphosphonates have, therefore, an advantage over oral bisphosphonates. [7] Zoledronate is a third-generation congener of bisphosphonates, given as yearly infusion, a more potent suppressor of osteoclastic bone resorption.[ [8], [9], [10] ] With this background, we conducted this study to evaluate the magnitude of loss of bone mineral density and its prevention following early administration of Zoledronic acid at forearm (radius + ulna).
Materials and Methods
Patients with acute SCI aged over 18 years, within 3 months from injury with neurological deficits (ASIA Impairment Scale A, B, C), admitted between Feb’ 2013 to Jan’ 2015 in the Department of PM&R were invited to participate in the study. SCI patients with serum calcium less than 8.5 mg/dl, serum 25(OH) vitamin-D3 less than 25 nmol/L, serum creatinine clearance less 30 ml/min) or any history of adverse reaction to bisphosphonates were excluded. Females who were planning to conceive, pregnant or lactating were excluded.
Sixty patients with SCI were included in the study, taking 80% as study power, standard deviation of 9% in aBMD in 12 months, α-error of 0.05 into consideration with a minimum detectable change of percent change in aBMD score of 9. Ethical clearance was obtained from institute ethics committee. Study was registered retrospectively in Clinical trials registry-India (CTRI/2018/03/012408). Written informed consent was taken from the participants.
Participants were randomized either to control group to receive standard medical and nursing care or to treatment group to receive Zoledronic acid infusion (5 mg/100 ml) in combination with standard medical and nursing care according to pre-generated blocked randomization schedule. Neither participants nor investigators were blinded to allocation process. The pre-generated randomized schedule was not accessible to the investigators assigned for screening of participants. Assessor was blinded.
Medically stable patients were subjected to aBMD measurement at forearm (radius + ulna) at baseline in Dept. of Radio-diagnosis, by Hologic QDR-Delphi DXA. Then, follow-up scans were done at 3 months, 6 months and 12 months from the date of injury.
Mean derived for inter- and intra-group difference was not normally distributed although aBMD values were normally distributed around mean. Boot strapping was done to overcome the problem and statistical analyses were done by SPSS version 16.0. Paired t test was used for intra-group and unpaired t test was used for intergroup analyses of mean differences of aBMD. P-value < 0.05 was deemed significant.
Results
Patients with SCI admitted to Dept. of PM&R between Feb’ 2013 and Jan’ 2015 were screened for eligibility. Out of 212 patients screened, 60 participants were eligible for the study.
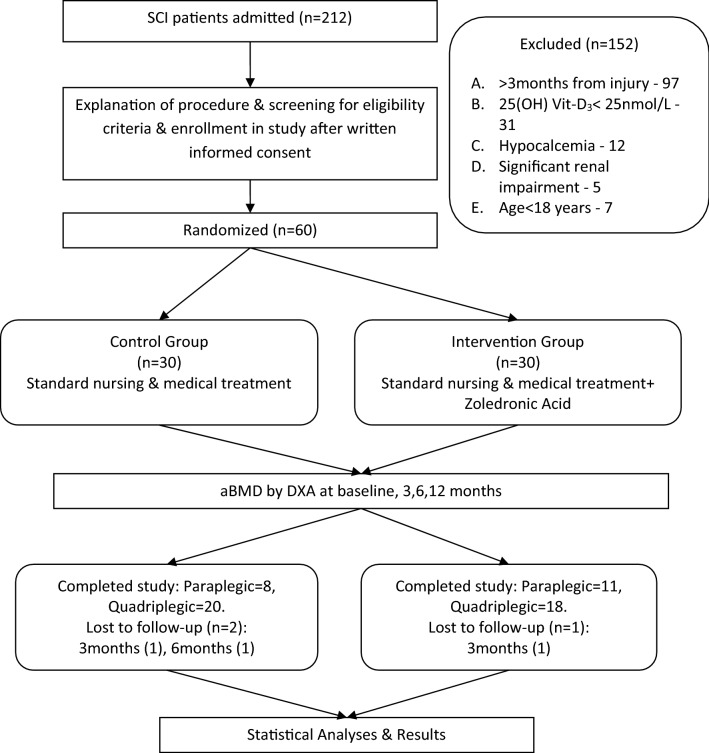
Consenting patients with acute SCI were randomized either to control group to receive standard medical and nursing care or to treatment group to receive zoledronic acid infusion in combination with standard medical and nursing care according to pre-generated blocked randomization schedule (Fig. 1). Both control and zoledronic acid groups on comparison at baseline did not show any significant difference for baseline aBMD, demographic, anthropometric measures and risk factors for low bone mass. (Table 1). Both groups were matched at the baseline. Acute SCI patients admitted within 24 h of injury were infused with methylprednisolone succinate (30 mg/kg bolus over 15 min; 5.4 mg/kg maintenance infusion days 1–3). Enoxaparin (i.m.) was given for 6 weeks for prophylaxis of deep vein thrombosis as a routine treatment.
Fig. 1.
Study flow chart
Table 1.
Baseline Characteristics
| Characteristics | Quadriplegics | Paraplegics | ||
|---|---|---|---|---|
| Control group (n = 20) | Intervention group (n = 18) | Control group (n = 8) | Intervention group (n = 11) | |
| Age (years) | 36.3 ± 14.75 | 38.89 ± 15.05 | 33.7 ± 8.24 | 29.73 ± 8.02 |
| Sex (male:female) | 18:02 | 14:04 | 8:0 | 10:1 |
| Height (cm) | 162.0 ± 8.36 | 161.2 ± 5.51 | 166.40 ± 7.35 | 165.1 ± 9.03 |
| Weight (kg) | 52.60 ± 10.38 | 54.78 ± 8.22 | 54.38 ± 10.50 | 59.64 ± 11.24 |
| Rural:Urban | 5:15 | 3:15 | 7:1 | 6:5 |
| Alcohol intake | 7/20 | 3/18 | 1/8 | 1/11 |
| Smoker | 7/20 | 1/18 | 2/8 | 1/11 |
| Complete(AIS-A)/Incomplete(AIS-B,C) | 7/13 | 6/12 | 7/1 | 10/1 |
| Corticosteroids Given | 17/20 | 15/18 | 5/8 | 5/11 |
| Days to first DXA scan post injury(d) | 28.45 ± 10.47 | 30.06 ± 12.22 | 25.50 ± 11.4 | 23.18 ± 11.34 |
| Days to Zoledronic acid infusion post injury(d) | NA | 30.06 ± 12.22 | NA | 23.18 ± 11.34 |
| Baseline Bone Mineral Density(g/cm2) | ||||
| 1/3 Forearm | 0.747 ± 0.08 | 0.714 ± 0.07 | 0.749 ± 0.02 | 0.732 ± 0.06 |
| Mid Forearm | 0.585 ± 0.07 | 0.566 ± 0.07 | 0.622 ± 0.03 | 0.607 ± 0.06 |
| UD Forearm | 0.458 ± 0.05 | 0.434 ± 0.06 | 0.478 ± 0.05 | 0.465 ± 0.07 |
| Total Forearm | 0.591 ± 0.06 | 0.565 ± 0.06 | 0.609 ± 0.03 | 0.599 ± 0.06 |
Values are mean ± SD unless stated otherwise, AIS - ASIA Impairment Scale, Alcohol Intake ≥ 180 ml/day, 3 times/week
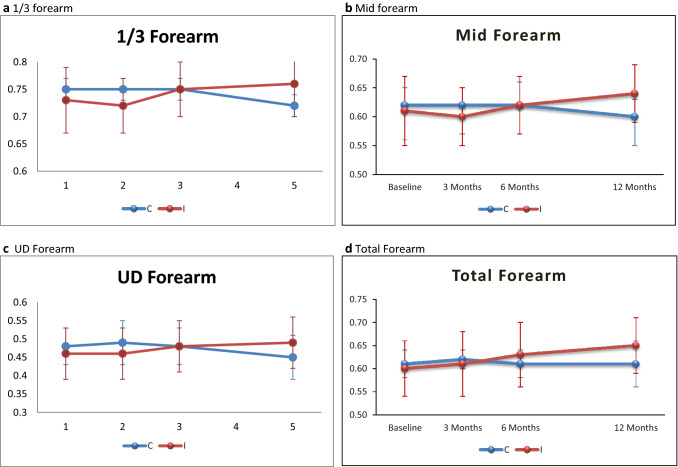
Non-significant difference of mean differences in aBMD between groups in paraplegic SCI patients during the 12-month study period was observed at any of the sites of the forearm except 1/3 region of forearm at 12 months. [3 months: 1/3 forearm – 0.75 ± 0.02 vs. 0.72 ± 0.05, p = 0.192, Mid forearm – 0.62 ± 0.03 vs. 0.6 ± 0.05, p = 0.406, UD forearm – 0.49 ± 0.06 vs. 0.46 ± 0.07, p = 0.337, Total forearm – 0.62 ± 0.02 vs. 0.61 ± 0.07, p = 0.615; 6 months: 1/3 forearm – 0.75 ± 0.02 vs. 0.75 ± 0.05, p = 0.945, Mid forearm – 0.62 ± 0.04 vs. 0.62 ± 0.05, p = 0.815, UD forearm – 0.48 ± 0.05 vs. 0.48 ± 0.07, p = 0.851, Total forearm – 0.61 ± 0.03 vs. 0.63 ± 0.07, p = 0.420; 12 months: 1/3 forearm – 0.72 ± 0.02 vs. 0.76 ± 0.06, p = 0.036, Mid forearm – 0.6 ± 0.03 vs. 0.64 ± 0.05, p = 0.07, UD forearm – 0.45 ± 0.06 vs. 0.49 ± 0.07, p = 0.271, Total forearm – 0.61 ± 0.05 vs. 0.65 ± 0.06, p = 0.179] (Fig. 2).
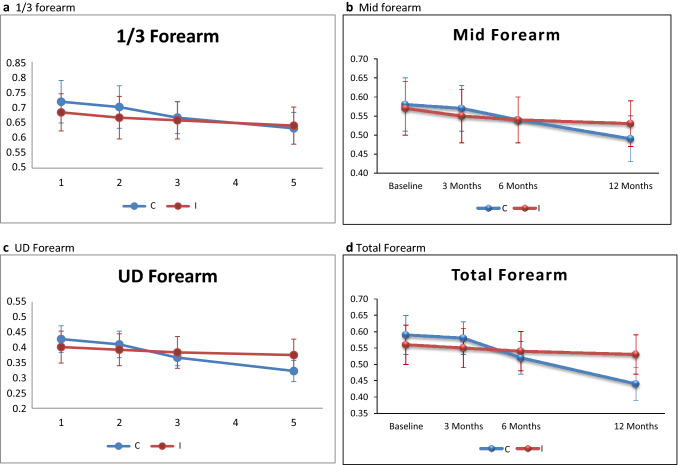
Fig. 2.
Mean BMD ± SD (Quadriplegic). (C = Control group, I = Intervention group)
Similar non-significant difference of mean differences at mid forearm and total forearm at 3 and 6 months between groups in quadriplegic patients. [3 months: 1/3 forearm – 0.73 ± 0.08 vs. 0.69 ± 0.08, p = 0.148, Mid forearm – 0.57 ± 0.06 vs. 0.55 ± 0.07, p = 0.381, UD forearm – 0.44 ± 0.05 vs. 0.42 ± 0.06, p = 0.290, Total forearm – 0.58 ± 0.05 vs. 0.55 ± 0.06, p = 0.197; 6 months: 1/3 forearm – 0.69 ± 0.06 vs. 0.68 ± 0.07, p = 0.627, Mid forearm – 0.54 ± 0.06 vs. 0.54 ± 0.06, p = 0.857, UD forearm – 0.39 ± 0.03 vs. 0.41 ± 0.06, p = 0.290, Total forearm – 0.52 ± 0.05 vs. 0.54 ± 0.06, p = 0.2]. But there was a significant difference at mid forearm (0.49 ± 0.06 vs. 0.53 ± 0.06, p = 0.032), UD forearm – 0.34 ± 0.04 vs. 0.40 ± 0.06, p = 0.001 and total forearm (0.44 ± 0.05 vs. 0.53 ± 0.06, p < 0.001) in this subset of patients at 12 months. This significant difference was conspicuously absent at 1/3 forearm at 12 months (0.65 ± 0.06 vs. 0.66 ± 0.07, p = 0.538). (Figure 3).
Fig. 3.
Mean BMD ± SD (Paraplegics). (C = Control group, I = Intervention group)
Significant differences were observed in intergroup analysis of mean difference of absolute change in aBMD in the paraplegic group at 12 months. Absolute change in aBMD at 6 months was also significant at 1/3 forearm and total forearm, but not at the mid forearm and UD forearm. (Table 3) In the quadriplegic sub-set patients, significant difference in absolute change in aBMD was observed both at 6 and 12 months. (Table 2).
Table 3.
Comparison of mean differences in aBMD at forearm (Paraplegic group) between baseline and follow up(s)
| Site | Control Group (n = 8) | Intervention Group (n = 11) | Mean (between group difference) | 95% CI of Difference | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Change Score | Change score | Lower | Upper | |||||
| Mean | Std. Deviation | Mean | Std. Deviation | |||||
| Baseline and 3 months follow up | ||||||||
| 1/3 Forearm | − 0.001 | 0.011 | − 0.011 | 0.019 | 0.010 | − 0.005 | 0.025 | 0.167 |
| Mid Forearm | − 0.004 | 0.017 | − 0.005 | 0.018 | 0.002 | − 0.016 | 0.019 | 0.846 |
| UD Forearm | 0.014 | 0.019 | − 0.003 | 0.019 | 0.017 | − 0.002 | 0.035 | 0.074 |
| Total Forearm | 0.009 | 0.021 | 0.007 | 0.016 | 0.001 | − 0.016 | 0.019 | 0.885 |
| Baseline and 6 months follow up | ||||||||
| 1/3 Forearm | 0.000 | 0.014 | 0.019 | 0.012 | − 0.019 | − 0.031 | − 0.006 | 0.006 |
| Mid Forearm | − 0.004 | 0.026 | 0.017 | 0.008 | − 0.021 | − 0.043 | 0.001 | 0.061 |
| UD Forearm | 0.004 | 0.021 | 0.011 | 0.012 | − 0.007 | − 0.025 | 0.011 | 0.420 |
| Total Forearm | − 0.004 | 0.025 | 0.027 | 0.011 | − 0.031 | − 0.052 | − 0.010 | 0.009 |
| Baseline and 12 months follow up | ||||||||
| 1/3 Forearm | − 0.031 | 0.033 | 0.033 | 0.011 | − 0.064 | − 0.092 | − 0.036 | 0.001 |
| Mid Forearm | − 0.026 | 0.038 | 0.033 | 0.008 | − 0.059 | − 0.084 | − 0.034 | 0.001 |
| UD Forearm | − 0.024 | 0.059 | 0.024 | 0.012 | − 0.048 | − 0.097 | 0.001 | 0.016 |
| Total Forearm | 0.000 | 0.062 | 0.048 | 0.010 | − 0.048 | − 0.088 | − 0.008 | 0.021 |
Mean and SD of change score of the aBMD at follow-up from baseline of both control & intervention group at mid forearm, total forearm in paraplegic group. Unpaired t test was used to compare intergroup difference in values from baseline between control and intervention groups
Table 2.
Comparison of mean differences in aBMD at forearm (Quadriplegic group) between baseline and follow up(s)
| Site | Control group (n = 20) | Intervention group (n = 18) | Mean (between group difference) | 95% CI of difference | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Change score | Change score | |||||||
| Mean | Std. deviation | Mean | Std. deviation | Lower | Upper | |||
| Baseline and 3 months follow up | ||||||||
| 1/3 Forearm | − 0.017 | 0.032 | − 0.022 | 0.014 | 0.005 | − 0.011 | 0.021 | 0.542 |
| Mid Forearm | − 0.018 | 0.025 | − 0.017 | 0.010 | 0.000 | − 0.013 | 0.012 | 0.939 |
| UD Forearm | − 0.018 | 0.019 | − 0.013 | 0.008 | − 0.006 | − 0.015 | 0.004 | 0.244 |
| Total Forearm | − 0.015 | 0.015 | − 0.013 | 0.006 | − 0.002 | − 0.009 | 0.006 | 0.648 |
| Baseline and 6 months follow up | ||||||||
| 1/3 Forearm | − 0.059 | 0.034 | − 0.037 | 0.016 | − 0.022 | − 0.039 | − 0.005 | 0.015 |
| Mid Forearm | − 0.051 | 0.039 | − 0.028 | 0.011 | − 0.023 | − 0.042 | − 0.004 | 0.019 |
| UD Forearm | − 0.064 | 0.028 | − 0.023 | 0.010 | − 0.041 | − 0.055 | − 0.027 | <0.0001 |
| Total Forearm | − 0.074 | 0.026 | − 0.025 | 0.009 | − 0.049 | − 0.062 | − 0.036 | <0.0001 |
| Baseline and 12 months follow up | ||||||||
| 1/3 Forearm | − 0.095 | 0.055 | − 0.049 | 0.019 | − 0.046 | − 0.073 | − 0.019 | 0.002 |
| Mid Forearm | − 0.100 | 0.052 | − 0.037 | 0.017 | − 0.063 | − 0.088 | − 0.037 | <0.0001 |
| UD Forearm | − 0.119 | 0.034 | − 0.035 | 0.014 | − 0.084 | − 0.101 | − 0.067 | <0.0001 |
| Total Forearm | − 0.154 | 0.033 | − 0.040 | 0.017 | − 0.115 | − 0.132 | − 0.097 | <0.0001 |
Mean and SD of change score of the aBMD at follow-up from baseline of both control & intervention group at mid forearm, total forearm in quadriplegic group. Unpaired t test was used to compare intergroup difference in values from baseline between control and intervention groups
Zoledronic acid was tolerated well and no patient had any significant adverse reaction to the infusion.
Discussion
Early and rapid decrease in aBMD at forearm following acute SCI was demonstrated. About 12.9% at 1/3 forearm, 16.7% at mid forearm, 25.7% at UD forearm and 26.1% at total forearm, aBMD reduction was observed over 12 months in quadriplegic patients. Although bone loss in acute SCI is mainly sub-lesional, maintenance of optimal aBMD in the upper limb is essential for rehabilitation of patients with SCI for ambulation, transfer and other activities of daily living. aBMD levels of forearm never reached the baseline in any group during the study period despite the Zoledronic acid infusion to the intervention group. Paraplegic patients in the intervention group also had significant decrease in absolute change of aBMD at 12 months demonstrating a supra-lesional bone loss possibly due to decreased loading, limited mobility and decreased adherence to the rehabilitation program with passage of time.
Frey-Rindova et al. [11] reported a prospective study in 29 acute SCI patients to demonstrate significant decrease in BMD by peripheral quantitative computed tomography at radius and ulna (both trabecular and cortical bone) after 6–12 months from injury. (Radius—19%, p < 0.05; Ulna—6%, p < 0.05 at 6 months and Radius—28%, p < 0.05; Ulna—15%, p < 0.05 at 12 months after SCI). On the contrary, BMD change of neither trabecular nor cortical bone of forearm was significant.
Gilchrist et al. in a study on 31 acute SCI patients reported non-significant preservation of aBMD at total body arms (p = 0.197) following weekly administration of oral alendronate (70 mg/week). [12] But, Schnitzer et al. found significant reduction in sub-lesional bone mineral loss in SCI patients following zoledronic acid infusion over a period of 24 months in comparison to placebo. [13] In both the studies, a note on difference of BMD between paraplegic and quadriplegic group and supra-lesional BMD was not illustrated.
Issues such as nursing care of patients with SCI in upright posture following oral bisphosphonates and incidences of dysphagia in cervical SCI were potentially avoided with zoledronic acid infusion. Also, compliance was ensured with single dose of zoledronate for 12 months. [14] Zoledronate infusion reduced bone loss at forearm and was tolerated well without any significant documented complications. aBMD level never reached the baseline in any group during the study period, despite the zoledronate infusion to the treatment group indicating that single infusion of zoledronic acid was not sufficient to bring back the pre-injury levels of aBMD in these patients necessitating the requirement of a second or third yearly infusion of zoledronic acid or by repeating infusions at shorter intervals.
Conclusion
Zoledronate infusion effectively reduces the bone loss at the forearm in patients with acute SCI for 12 months. But there is ongoing bone resorption due to non-weight bearing of bones and further treatment may be needed. Maintenance of optimum aBMD is required not only for prevention of bone loss or fragility fractures but also for maintenance of bone strength parameters for rehabilitation of patients with SCI for ambulation, transfer and other activities of daily living. Studies with larger sample size and longer follow-up period to assess the magnitude of decrease in bone resorption are recommended.
Limitations
Effect of rehabilitation program on maintenance of aBMD (viz. use of walking aids, assistive devices; time of start, intensity, frequency and duration of therapy programs) was not evaluated separately.
Compliance with Ethical Standards
Conflict of interest
The authors do not declare any conflicts of interests.
Ethical standard statement
Institute ethical committee clearnce was taken for the study. Also the study was registered in Clinical Trials Registry-India (CTRI/2018/03/012408).
Informed consent
Written informed consent was taken from all the study participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sunil Goenka, Email: dr_goenka@yahoo.com.
Satyaranjan Sethi, Email: drsatya.1979@gmail.com.
References
- 1.Sheng-Dan J, Li-Yang D, Lei-Sheng J. Osteoporosis after spinal cord injury. Osteoporosis International. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 2.Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I. Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. Journal of Rehabilitation Research and Development. 2000;37:225–233. [PubMed] [Google Scholar]
- 3.Sheng-Dan J, Lei-Sheng J, Li-Yang D. Mechanisms of osteoporosis in spinal cord injury. Clinical Endocrinology. 2006;65:555–565. doi: 10.1111/j.1365-2265.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro J, Smith B, Beck T, Ballard P, Dapthary M, BrintzenhofeSzoc K, et al. Treatment with Zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcified Tissue International. 2007;80:316–322. doi: 10.1007/s00223-007-9012-6. [DOI] [PubMed] [Google Scholar]
- 5.Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporosis International. 2005;16:263–272. doi: 10.1007/s00198-004-1665-7. [DOI] [PubMed] [Google Scholar]
- 6.Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. Journal of Bone and Mineral Research. 2004;19:48–55. doi: 10.1359/jbmr.0301208. [DOI] [PubMed] [Google Scholar]
- 7.Bubbear JS, Gall A, Middleton FR, Ferguson-Pell M, Swaminathan R, Keen RW. Early treatment with Zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporosis International. 2011;22:271–279. doi: 10.1007/s00198-010-1221-6. [DOI] [PubMed] [Google Scholar]
- 8.Green JR, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. Journal of Bone and Mineral Research. 1994;9:745–751. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- 9.Jonathan RG, Michael JR. Pharmacologic profile of Zoledronic acid: a highly potent inhibitor of bone resorption. Drug Development Research. 2002;55:210–224. doi: 10.1002/ddr.10071. [DOI] [Google Scholar]
- 10.Widler L, Jaeggi K, Glatt M, Müller K, Bachmann R, Bisping M, et al. Highly potent geminal bisphosphonates from pamidronate disodium (Aredia) to Zoledronic acid (Zometa) Journal of Medicinal Chemistry. 2002;45(17):3721–3738. doi: 10.1021/jm020819i. [DOI] [PubMed] [Google Scholar]
- 11.Frey-Rindova P, De Bruin ED, Stussi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- 12.Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: A randomized, double-blind, placebo-controlled study. Journal of Clinical Endocrinology and Metabolism. 2007;92:1385–1390. doi: 10.1210/jc.2006-2013. [DOI] [PubMed] [Google Scholar]
- 13.Schnitzer TJ, Kim K, Marks J, Yeasted R, Simonian N, Chen D. Zoledronic acid treatment after acute spinal cord injury: Results of a randomized, placebo controlled pilot trial. PM R. 2016;8:833–843. doi: 10.1016/j.pmrj.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, Black D, Adachi J, Shea B, Tugwell P, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis II Meta-analysis of alendronate for the treatment of postmenopausal women. Endocrine Reviews. 2002;23:508–516. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]