Abstract
Background
In the era of increasing drug resistance in pulmonary tuberculosis (TB), it is prudent to assess causes of poor response to anti tubercular therapy (ATT) and drug sensitivity pattern (DSP) in osteoarticular TB.
Materials and Methods
As a part of Bombay Orthopaedic society’s research project, members were asked to refer non responders to ATT to our institute. Cases were enrolled from October 2010 to March 2014. Deep tissue samples were obtained in all but five cases and subjected to a battery of tests including histopathology (HPE) and TB culture and sensitivity. The DSP was compared with the study performed by the principle author from 2004 to 2007 and published in 2009.
Results
39 male and 50 female patients with a mean age of 24.85 years (2–66) were included and classified in four groups after results. (1) Culture and HPE positive-36. 24 had MDR and three XDR TB. Primary resistance to even second line drugs and deterioration of DSP since last study was noted, (2) culture negative and HPE positive-21. The cause of poor response was surgical in more than half cases, (3) non representative samples or lost to follow-up-15, (4) TB mimics-16.
Conclusion
There is increasing incidence of primary resistance to second line drugs, primary resistance in children and worsening of resistance patterns as compared to older studies. ATT initiation is a fateful decision and every attempt should be made to rule out TB mimics and establish DSP before initiation.
Keywords: Extra pulmonary tuberculosis, Multidrug-resistant tuberculosis, Osteoarticular tuberculosis, Empirical antitubercular therapy, Treatment failure, Drug resistance, Tissue biopsy
Introduction
Indian tryst with tuberculosis (TB) has gone from reaching the precipice of success to a state where it has the dubious distinction of being named the “global hub” of TB [1, 2]. Way back in 1955, Dr. B. Mukopadhaya asserted in his Hunterian Lecture delivered at the Royal college of Surgeons of England that “the routine use of anti-tuberculous drugs Streptomycin, PAS and INH has had—strikingly beneficial effects”. He ended his lecture by stating “The struggle against tuberculous disease has been hard, long and costly, but today we appear to be on the threshold of success” [3].
However, the HIV epidemic that hit the world and India changed the scenario with a dramatic resurgence of TB and drug-resistant (DR) TB as was highlighted by various authors [4, 5].
The current situation can be judged by the report of first National Anti-Tuberculosis Drug Resistance survey conducted by the Indian Government in collaboration with the World Health Organization (WHO) and the United States Agency for International Development (USAID). This showed that close to 23% of new cases have resistance to any drug, with multi drug-resistant (MDR) TB detected in 3% [6].
The orthopaedic community, however has continued to delve in empirical anti tubercular therapy (ATT) with the belief that diagnosis of osteoarticular (OA) TB is clinicoradiological (CR) in endemic areas like India and the tissue culture yield is poor. This thought process gives no consideration to tackle the challenge of DR TB as per drug sensitivity pattern (DSP) and is reflected in studies as recent as 2019 [7–9].
Thus, it is prudent to assess the current position, and changing trends as regards TB in general and OA TB in particular.
This study analyses culture yield, DSP and changes there in as well as incidence of false positive cases in Mumbai since 2004. The clinical cases were sent by members of Bombay Orthopedic Society. The study was sponsored by Bombay orthopedic society (BOS), P. D. Hinduja Hospital and a Philanthropic senior orthopedic surgeon.
Materials and Methods
A prospective study of non responders in OA TB was sponsored by BOS. This study was conducted at our institute and approved by the institutional review. Patients were enrolled from October 2010 to March 2014 and later followed up for a minimum of 2 years after completion of their ATT.
The DSP was compared with the study performed for BOS by the principle author from 2004 to 2007 and published in 2009.
Inclusion Criteria
Over a 4 years period patients who failed to respond to ATT for at least 3 months (non respondents) were prospectively included. “Non-response” was defined based on clinical and radiological criteria (Table 1). The average duration of ATT was 9.32 months (3–60) before presentation.
Table 1.
Criteria for non-response to anti tubercular therapy for osteoarticular TB
|
ATT for a period of at least 3 months, compliant to treatment but has at least one or more of the following: Persistent pain, or increased pain Persistent or increased swelling, or development of new swelling Development of new lesion (clinical or radiological) Persistent discharging sinus or development of new sinus Neurological deterioration or development of new neurological deficits |
| Development of non-healing ulcer |
Orthopaedic surgeons across Mumbai were asked to direct such patients to our institution. The patients were thoroughly assessed clinically and imaging studies were done in indicated cases. Tissue samples were procured either under image guidance or by open exploration and biopsy in all but those cases where the diagnosis was obviously non TB. Image guided biopsies were always done with wide bore or J needle. Fine needle aspiration biopsy (FNAC) was never used, knowing that the culture yield would be poor. Samples were sent to our microbiology and pathology department. Granulation tissue and/ or pus were sent for smear, aerobic, anaerobic, fungal and TB cultures in a specified sterile leak proof container with no or minimal additive (normal saline). TB cultures were done by Mycobacterium Growth Indicator Tube (MGIT) as well as conventional Lowenstein Jenson Medium (L. J. medium). For histopathological examination, granulation tissue, sinus tract and in selected cases bone samples were collected in sterile container with formalin.
All laboratory tests and image guided biopsies were done at our institute. However the patients were referred back to the primary surgeon if they needed surgery. In such a scenario they were given appropriate containers and all the laboratory tests were done at our institute.
Drug sensitivity testing (DST) for four primary first-line and 9 s line ATT drugs was performed using the MGIT 960 system for all positive cultures [10] (Table 2).
Table 2.
List of 1st and 2nd line drugs used for DSP
| First line drugs | Isoniazid, Rifampicin, Pyrazinamide, Ethambutol. Streptomycin |
| Second line drugs | Capreomycin, Amikacin, Kanamycin, PAS, Ethionamide, Clofazamine, Moxifloxacin, Ofloxacin, Linezolid |
Results
Over a 4 years period, 89 patients were included (39 male and 50 female). Mean age was 24.85 (2–66 years). Mean duration of ATT was 9.32 months (3–60 months). Details of this cohort are depicted in Table 3. None of the patients was HIV positive.
Table 3.
Baseline data of non-responders (n = 89)
| M:F: 37:52 |
|
Mean age: 25.7 Unifocal/ multifocal (patients): 52/37 Family history of TB: 29 (32.6%) ATT without tissue diagnosis: 72 (80.9%) ATT after biopsy: 17 (19.1%) ATT after DST: 8/17 (47.1%) 2nd line ATT: 14/89 (15.7%) New lesion on therapy 21/89 (23.6%) Site of lesions: spine-48, hip-7, knee-7, others-76 |
Two patients were lost to follow-up prior to obtaining a tissue biopsy. Of the remaining 87 patients, three were not subjected to biopsy as non TB diagnosis was obvious based on thorough clinical examination and repeat imaging studies. Of the remaining 84 patients, 44 had an open biopsy (52.4%) and 40 (47.6%) had image guided biopsy (71 at our institute, and 13 in outside institutes).
Based on the investigatory reports, patients were classified into the following four groups:
Definite TB: Those with definite evidence of TB (culture and histopathology positive).
Probable TB: Those who were culture negative but had histopathological evidence of caseating epithelioid granulomas with multinuclear giant cells, with or without demonstration of acid fast bacilli.
Possible TB: CR suspicion but no microbiological or histological confirmation. All had non representative sample.
Non-TB.
Group 1: Definite Tuberculosis (n = 36) (40.5%)
Three of the 36 patients (8.3%) were sensitive to all 13 ATT drugs but did not respond to treatment. The cause of non-response was an underlying sequestrum. Once this was surgically taken care of, all three responded adequately to the original drug therapy.
33/36 (91.7%) patients were found to be resistant to at least one ATT drug. 24 patients (66.7%) were found to have MDR TB defined as tuberculosis resistant to at least isoniazid (INH) and rifampicin (RMP).
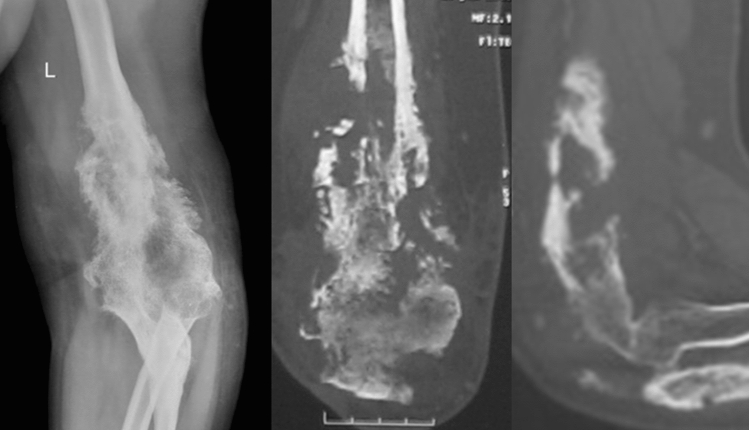
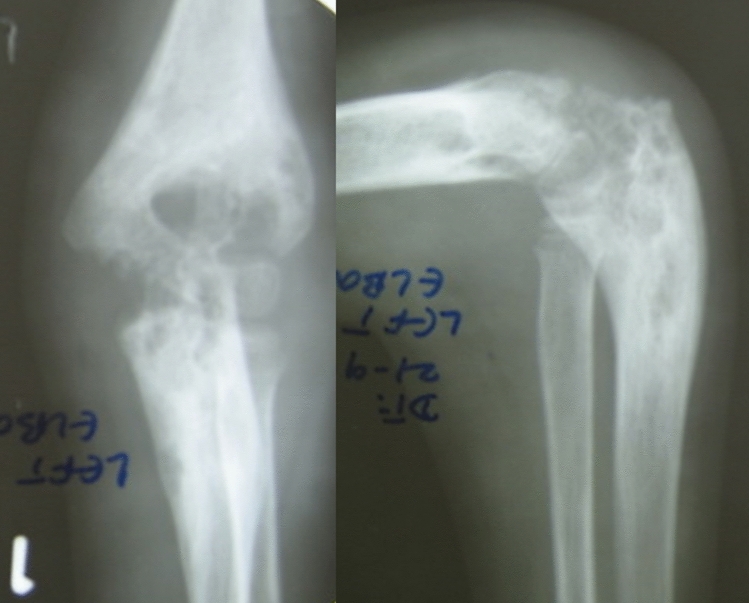
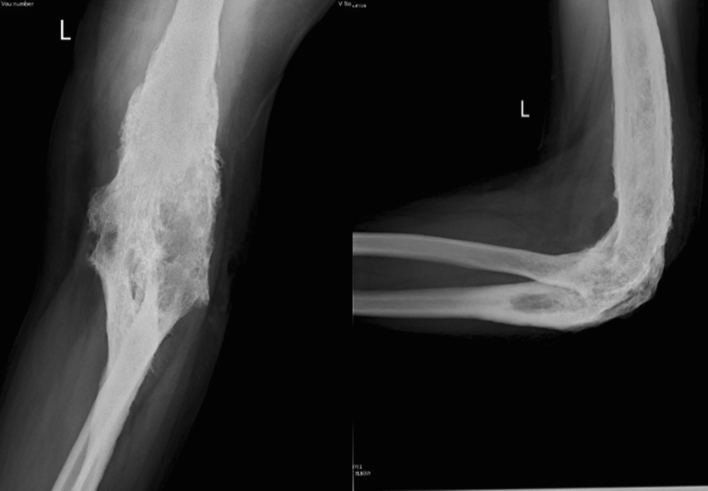
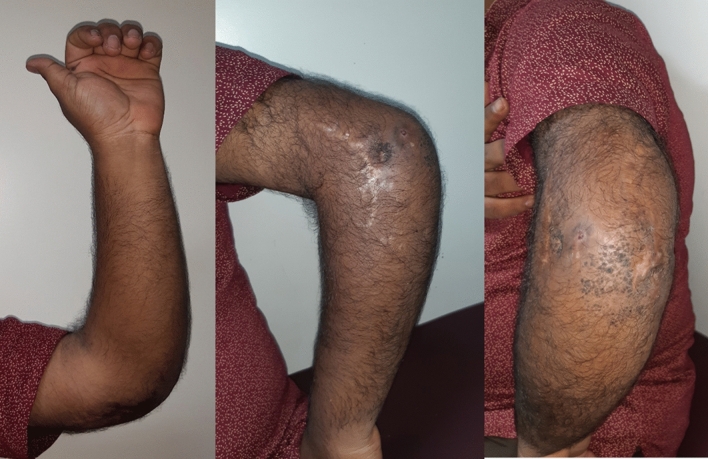
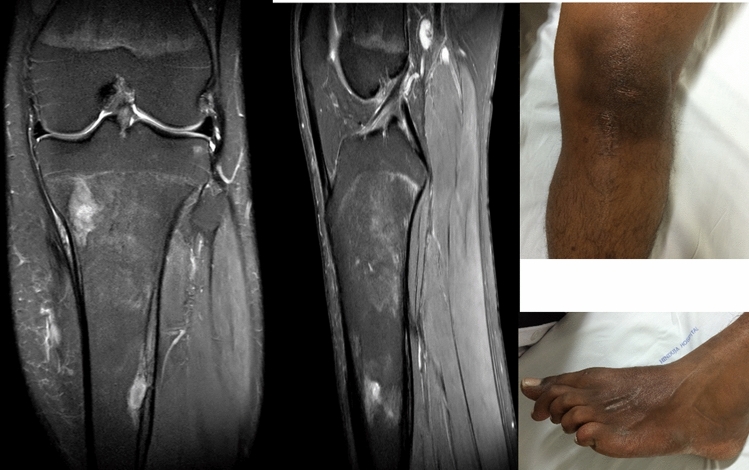
Illustration 1: A 23 years old male presented with a discharging sinus from a fused left elbow joint in 2012 (Fig. 1). He was initially started on empirical 1st line ATT based on histopathology in 1996 (Fig. 2) and continued for 60 months. He had multiple recurrences in the elbow in 1998, 2001, 2002, 2006, 2007, 2009 and 2012. As cultures were negative in spite of two debridements, ATT was modified to Rifampicin, Isoniazid, Ethambutol, levofloxacin, Ethionamide, Cycloserine and clarithromycin based on presumptive resistance. We operated him in 2012 as a part of project, and deep tissue cultures grew a MDR strain resistant to all the 1st line drugs. ATT was appropriately modified (Moxifloxacin, Clofazamine, PAS, Ethionamide and Cycloserine) and continued for 2 years. He had complete remission at 5 years follow-up after discontinuation of ATT (Figs. 3, 4).
Fig. 1.
Fused left elbow secondary to tuberculous arthritis
Fig. 2.
Left elbow destruction in 1996
Fig. 3.
Healed Osteomyelitis left elbow at 60 months follow-up
Fig. 4.
Healed soft tissue at 60 months follow-up
3 patients (8.3%) had extensively drug-resistant TB (XDR-TB), defined as resistance to Rifampicin and Isoniazid as well as to any member of the quinolone family and at least one of the following second-line anti-TB injectable drugs: kanamycin, capreomycin or amikacin as defined by world health organisation (WHO).
Apart from the defined resistance patterns, 17 patients (47.2%) had resistance to 6 or more drugs (range 6–12).
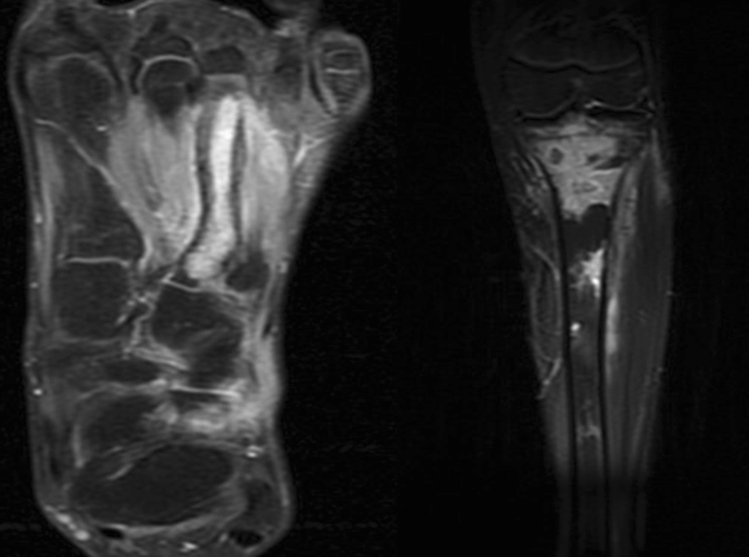
Illustration 2: One from this group was a 17 years male who presented with pain and swelling over left foot and proximal part of leg, with a discharging sinus on the foot for the last 6 months. He was treated for pulmonary kochs 2 years ago. He developed diplopia 2 months after stopping ATT and was restarted on first line ATT with a diagnosis of cerebro-pontine and meningeal TB. He then developed symptoms in the foot and leg, and presented to us (Fig. 5). We proceeded with deep tissue biopsy which grew a MDR strain resistant to Ofloxacin and Moxifloxacin in addition to all first line drugs. He had an excellent response after starting 2nd line ATT (Kanamycin, Ethionamide, PAS, Clofazamine, and Clarithromycin) and had a CR remission at 25 months after cessation of ATT (Fig. 6).
Fig. 5.
Osteomyelitis left 3rd metatarsal and proximal tibia
Fig. 6.
Clinico-radiological remission at 25 months follow-up
We separately analysed patients who were on ATT for between 3 and 5 months to assess the resistance pattern as they could be considered as cases of “primary Resistance”.
Out of 22 cases, 9 (40.9%) were resistant to ATT. Findings are tabulated in Table 4.
Table 4.
Patients who were on ATT between 3 and 5 months
| Project no. | Age | Duration of ATT | Resistance |
|---|---|---|---|
| 2 | 38 | 4 | S, H, R, E |
| 3 | 14 | 4 | S, H, E, EHO |
| 8 | 9 | 4 | PZA |
| 17 | 9 | 4 | |
| 22 | 1 | 4 | S, H, R, E, PZA, EHO, PAS, MOXI |
| 25 | 4 | 4 | |
| 27 | 28 | 4 | |
| 32 | 34 | 3 | |
| 33 | 6 | 3 | S, H, R, E, EHO |
| 41 | 28 | 4 | S, H, R, E, PZA |
| 44 | 23 | 3 | S, H, R, E, PZA, OFLOX, MOXI |
| 49 | 28 | 4 | |
| 50 | 27 | 4 | |
| 55 | 37 | 4 | |
| 65 | 26 | 4 | |
| 67 | 66 | 4 | |
| 70 | 17 | 4 | S, H, R, E, PZA, EHO, OFLX, MOXI |
| 74 | 29 | 4 | |
| 75 | 35 | 4 | S, H, R, E |
| 78 | 28 | 4 | |
| 87 | 24 | 4 |
Of the 75 cases who were on 1st line ATT prior to presentation, 9 (12%) had resistance to 2nd line drugs as well. This could possibly be primary resistance to the 2nd line drugs as they were never treated with them.
Group 2: Probable Tuberculosis (n = 21) (23.6%)
In 11 out of the 21 cases (52.4%), we attributed the cause of non-response to a surgical cause and these patients were subjected to operative management (surgical debulking, abscess drainage, sequestrectomy, open or arthroscopic synovectomy, and scraping of osteomyelitis) in an attempt to decrease the disease burden and avoid local complications. 6 out of the 11 cases (54.5%) treated surgically responded to their initial first line ATT post operatively and were continued on the same, while the remaining 5 cases did not show significant improvement and were referred to an infectious disease specialist and were started in empirical second line ATT to which they responded and thus continued on this therapy.
Of the remaining ten patients, one patient was on appropriate ATT but the non-response (increasing pain) was due to hypovitaminosis D. Treatment of this led to significant improvement with continuation of first line ATT.
The cause of non-response could not be ascertained in nine patients and all were empirically treated with second line ATT. Four responded adequately while three continued to deteriorate in spite of such modifications and surgical treatment and two were lost to follow-up.
Group 3: Possible TB (n = 13) (14.6%)
In 13 out of the 89 patients no confirmatory tissue diagnosis was established even after biopsy. We advised repeat biopsy, however they were lost to follow-up.
Group 4: The Non-TB Mimics (n = 16) (18%)
In 16 patients the “non-response” was due to wrong diagnosis of TB. 13 had a biopsy from target site to confirm the diagnosis. The remaining three patients were treated appropriately on the basis of blood investigations and imaging suggestive of the non-tubercular diagnosis. There were three rheumatoid arthritis, two infections from other organisms (one non-tubercular mycobacteria and one fungal infection), two stress reaction of bone and one each of seronegative arthropathy, stress fracture of proximal femur, organised hematoma of dorsal spine, reactive synovitis, spinal metastasis from breast carcinoma, benign periosteal chondroma, osteoid osteoma, osteoblastoma and sequelae of perthes.
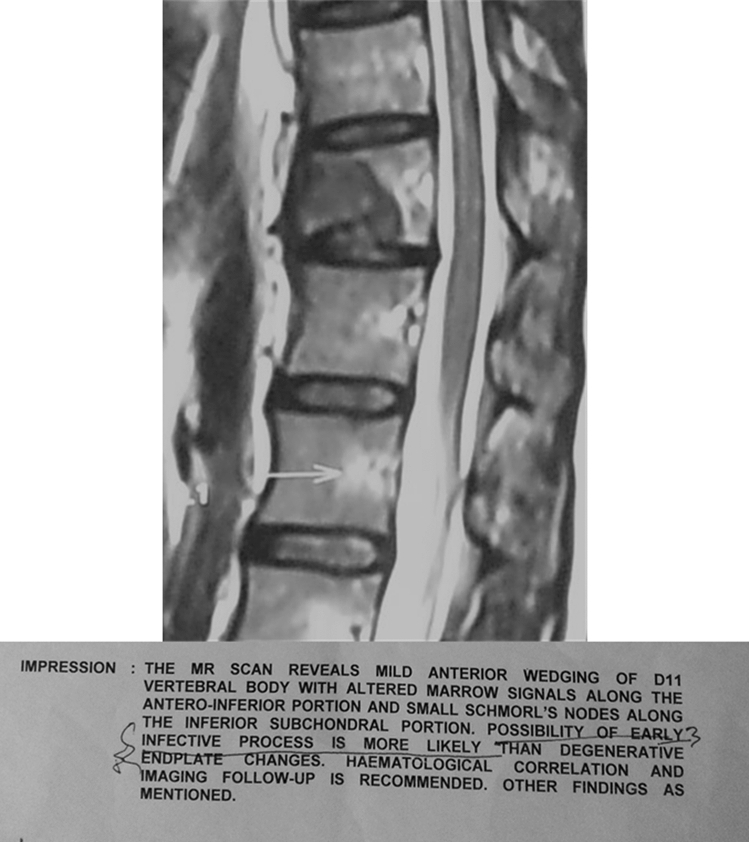
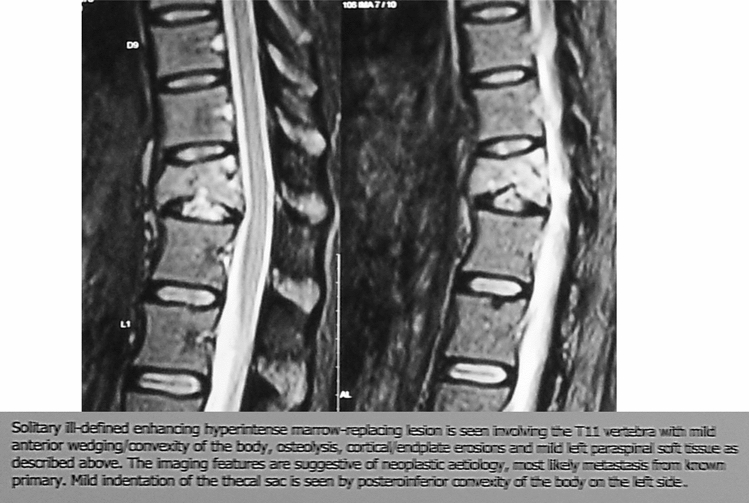
Illustration 3: A 37 years old female presented to us with increasing pain in the dorso- lumbar region for over 6 months. She was Clinico Radiologically diagnosed as Koch’s D11 vertebra and was started on empirical 1st line ATT (Fig. 7). On presentation, she gave a history of carcinoma of the breast treated 3 years prior. A repeat MRI, revealed a similar lesion in the D11 vertebrae, which was however reported to be most likely a metastasis, further confirmed on CT guided biopsy. ATT was stopped and further managed by oncologists and spine surgeons (Fig. 8).
Fig. 7.
Collapse of D11 vertebra with suspected infective lesion
Fig. 8.
Collapse of D11 vertebra with suspected metastatic lesion
All patients with a definitive or probable diagnosis of TB were referred to either a chest physician or an infectious disease specialist for advice about ATT and modification of ATT and were closely monitored for improvement following modification in therapy. They were all followed up for a minimum of 2 years after their inclusion in the study. The fourth group was referred back to the treating orthopaedic surgeons for their appropriate management.
Overall, 52 patients (58.4%) achieved healed status, with 4 failures (4.5%), 16 wrong diagnosis (18%) and 17 lost to follow-up (19.1%).
Discussion
The National strategic planning for TB 2017–25 has a set goal to achieve a rapid decline in burden of TB, morbidity and mortality while working towards elimination of TB in India by 2025–2030.
This goal needs to be viewed in light of conclusions by, Law et al. [9] who estimated from a dynamic modelling study that if TB management practices across sectors in India remain unchanged over next 20 years, there will be a 275% increase in risk of MDR TB infection and an estimated 85% of these will be primary MDR [11]. Udwadia et al. described one of the first cases of totally drug-resistant TB and impressed on the need for ideal diagnostic and therapeutic protocols [12].
One of the main reasons for this drastic deterioration in sensitivity pattern is the HIV epidemic which hit the India and the world as suggested by Maniar et al. in their hallmark study on 8640 HIV patients over 6 years with 93.5% having TB (42% pulmonary, 43.5% extra pulmonary, 14.5% disseminated) [4, 5]. 482/1154 sputum positive had resistance. The emergence of MDR and XDR TB especially in megacities like Mumbai, could possibly be related to the HIV pandemic [11, 13]. However, surprisingly, none of the patients in our study were HIV positive.
Studies by Pawar et al. and Mohan et al. have shown increasing incidence of MDR and XDR OA TB mirroring similar patterns seen in pulmonary TB [14, 15].
Thus there is a need to procure deep tissue samples to evaluate DSP and avoid empirical ATT [13, 16–18].
Yet, many recent publications from Indian Authors as well as the guidelines published by Ministry of Health and Family Welfare and World health Organisation in 2016 still show reliance on CR diagnosis and empirical TB citing poor culture yields [7–9, 19].
The concerns about poor culture yield was addressed by our group as a part of first project [20], wherein our culture yield was around 50%. Our current study also highlights that appropriate sampling can improve culture yield, reserving empirical ATT for culture negative cases but with a very strong CR suspicion.
Our study has also shown a large number of patients with varied drug resistance patterns so empirical initiation of second line treatment will carry significant risk.
One of the most frightening outcome of our study was the comparison to the previous study by the same author [20] of drug sensitivity pattern. We found that resistance to Pyrazinamide, Ethambutol, Streptomycin, Ethionamide, Ofloxacin and Moxifloxacin has significantly increased (Table 5). We also noted emergence of resistance to Kanamycin, Amikacin and Capreomycin, not seen in the earlier study. Most of the patients with wide spectrum of resistance were young suggesting that these were cases of primary resistance.
Table 5.
Comparison of drug resistance patterns
| Percentage of resistance of individual drugs in drug resistant cases | ||
|---|---|---|
| Anti-tuberculosis drug | Agashe et al. [20] | Present study |
| First line drugs | ||
| Isoniazid | 100 | 100 |
| Rifampicin | 80 | 87 |
| Pyrazinamide | 0 | 80.6 |
| Ethambutol | 40 | 77.4 |
| Streptomycin | 73.3 | 90.3 |
| Second line drugs | ||
| Kanamycin | 6 | 9.6 |
| Ethionamide | 20 | 45.16 |
| PAS | 6 | 9.6 |
| Ofloxacin | 20 | 48.3 |
| Moxifloxacin | 0 | 48.3 |
| Amikacin | 0 | 6.4 |
| Clofazimine | 0 | 0 |
| Capreomycin | 0 | 6.4 |
The numbers in bold indicate increase in Percentage of resistance of individual drugs in drug resistant cases in the new study as compared to the old
9 cases had resistance to 2nd line drugs in spite of never being exposed to any of them. This coupled with persistently high resistance to Isoniazid and Rifampicin, presents an increasingly common scenario where empirical ATT without procuring tissues for microbiology in treatment naïve will result in treatment failures and emergence of drug resistance.
Another very important aspect of our study was the inclusion of patients as “Non Respondents” if they were not improving at 3 months. Generally by convention patients not responding at 5 months are considered “Non Respondents” or clinically refractory [21]. We felt since most patients start responding well by 3 months, poor response at this stage needs to be investigated.
Out of 21 such patients, 9 were found to be resistant, 8 of them being MDR. Most of them were in paediatric age group. Getting culture studies before initiating ATT helped these patients as they probably had primary resistance.
Lastly and more importantly, there was a sub group in our study wrongly labelled as TB and started on empirical ATT, which was discontinued on accurately diagnosing the original pathology; similar to our older study where patients with multiple myeloma, ankylosing spondylitis and other such pathologies were wrongly labelled and treated for TB [20].
The argument that procuring tissue biopsy is not always practical across the country, must be weighed against the consequences of unknowingly increasing the burden of MDR and XDR TB. A combined effort by the government, health ministry, WHO, National and International NGO’s, public and private health sector is essential ensure facilities are made available right up to the grass root level all across the country and then to lay down stringent protocols for management of suspected cases of OA TB.
Our study has some limitations. A study with a larger cohort would have a stronger impact. Quite a few patients were lost to follow-up for varied reasons, a worrisome trend seen all across the country. Lastly this was conducted in Mumbai which is considered to be a major HIV hub with impact on Tuberculosis. Situation in other areas of the country may be a little different.
Conclusion
It is apparent from our study that the medical community including Orthopaedic surgeons are be dealing with
Increasing incidence of Primary resistance even to second line drugs.
Worsening of resistance patterns as compared to older studies.
Development of Primary resistance in children.
Thus starting ATT based solely on establishing the diagnosis, be it on CR findings or histopathology, may lead to a poor response. Good culture yield is possible if proper guidelines are followed. Empirical treatment for OA TB is to be avoided except in cases where all tests have been unfruitful and the surgeon strongly suspects OA TB.
This drastic change of mind- set and efforts in this direction are needed at every level from medical community to laboratories and the Government. The increase in cost initially will surely be compensated by the decrease in morbidity, prolonged illness and possibly relapse. Unless there is a 180 turn at every level the goal of elimination of TB by 2025–30 will remain a distant dream!
We hope that we are already not too late in this endeavour.
Acknowledgements
The authors would like to acknowledge the Bombay Orthopaedic Society (BOS) and a senior Orthopaedic surgeon (who wishes to remain unnamed) for their generous financial contribution for this project.
Author contributions
All authors have contributed to the study conception, design, methodology, material preparation, data collection, formal analysis and investigations. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Contributor Information
Vikas M. Agashe, Email: agashfam@gmail.com
Camilla Rodrigues, Email: dr_crodrigues@hindujahospital.com.
Rajiv Soman, Email: rajeev.soman@yahoo.com.
Anjali Shetty, Email: anjalishettyuk@yahoo.co.in.
R. B. Deshpande, Email: atharnas@yahoo.com
Kanchan Ajbani, Email: k.ajbani03@gmail.com.
Jitendra Pingle, Email: drjitendrapimple@yahoo.com.
Mandar Agashe, Email: mandarortho@gmail.com.
Hitendra Patil, Email: hitendrapatil@kem.edu.
Sagar Raghuwanshi, Email: sagarraghuwanshi7@gmail.com.
Manit Gundavda, Email: manit.gundavda@gmail.com.
Raju Gite, Email: raju.gite@gmail.com.
Mithun Jakkan, Email: mithunjakkan@gmail.com.
Amit Mishra, Email: dramitm@gmail.com.
Aditya Menon, Email: docmenon83@gmail.com.
References
- 1.TB India 2014. Ministry of Health and Family Welfare. https://tbcindia.gov.in/showfile.php?lid=3142.
- 2.2011–2015 WHO-Stop TB Plan. https://www.stoptb.org/assets/documents/global/plan/tb_globalplantostoptb2011-2015.pdf.
- 3.Mukopadhaya B. The role of excisional surgery in the treatment of bone and joint tuberculosis. Annals of Royal College of Surgeons of England [Internet] 1955;18(5):288–313. [PMC free article] [PubMed] [Google Scholar]
- 4.Hodkinson B, Osman N, Botha-Scheepers S. HIV infection and osteoarticular tuberculosis: strange bedfellows. Case Reports in Rheumatology [Internet] 2016;2016:5718423. doi: 10.1155/2016/5718423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maniar J, Kamath R, Mandalia S, Shah K, Maniar A. HIV and tuberculosis: Partners in crime. Indian Journal of Dermatology, Venereology and Leprology [Internet] 2006;72(4):276. doi: 10.4103/0378-6323.26723. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health and Family Welfare Government of India. National anti-TB drug resistance survey.pdf. 2016. p. 36. https://www.stoptb.org/assets/documents/global/plan/tb_globalplantostoptb2011-2015.pdf.
- 7.Mittal S, Jain A, Chakraborti K, Aggarwal A, Upreti L, Bhayana H. Evaluation of healed status in tuberculosis of spine by fluorodeoxyglucose-positron emission tomography/computed tomography and contrast magnetic resonance imaging abstract. Indian Journal of Orthopaedicx. 2019;53(1):160–168. doi: 10.4103/ortho.IJOrtho_224_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad S, Wakhlu A, Misra R, Aggarwal A, Lawrence A, Gupta R, et al. Features of extra-spinal musculoskeletal tuberculosis: A retrospective study from an North Indian Tertiary Care Institute. Indian Journal of Rheumatology [Internet] 2017;12(3):146. doi: 10.4103/injr.injr_38_17. [DOI] [Google Scholar]
- 9.Kumar Gogia K, Gupta S. Osteoarticular tuberculosis—a study associated with socio demographic factors. Annals of International Medical and Dental Research [Internet] 2016;2(6):12–17. [Google Scholar]
- 10.Morcillo N, Imperiale B, Di Giulio B. Evaluation of MGIT 960 and the colorimetric-based method for tuberculosis drug susceptibility testing. International Journal of Tuberculosis and Lung Disease [Internet] 2010;14(9):1169–1175. [PubMed] [Google Scholar]
- 11.Law S, Piatek AS, Vincent C, Oxlade O, Menzies D. Emergence of drug resistance in patients with tuberculosis cared for by the Indian health-care system: A dynamic modelling study. Lancet Public Health [Internet] 2017;2(1):e47–55. doi: 10.1016/S2468-2667(16)30035-4. [DOI] [PubMed] [Google Scholar]
- 12.Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. Totally drug-resistant tuberculosis in India. Clinical Infectious Diseases [Internet] 2012;54(4):579–581. doi: 10.1093/cid/cir889. [DOI] [PubMed] [Google Scholar]
- 13.Mistry N, Tolani M, Osrin D. Drug-resistant tuberculosis in Mumbai, India: An agenda for operations research. Operations Research for Health Care [Internet] 2012;1(2–3):45–53. doi: 10.1016/j.orhc.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawar UM, Kundnani V, Agashe V, Nene A, Nene A. Multidrug-resistant tuberculosis of the spine—is it the beginning of the end? Spine Phila Pa (1976) [Internet] 2009;34(22):E806–E810. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]
- 15.Mohan K, Rawall S, Pawar UM, Sadani M, Premik S, et al. Drug resistance patterns in 111 cases of drug-resistant tuberculosis spine. European Spine Journal [Internet] 2013;22(4):S647–S652. doi: 10.1007/s00586-012-2154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain A. Tuberculosis of spine: Research evidence to treatment guidelines. Indian Journal of Orthopaedics [Internet] 2016;50(1):3. doi: 10.4103/0019-5413.173518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arathi N, Ahmad F, Huda N. Osteoarticular tuberculosis—a three years’ retrospective study. Journal of Clinical and Diagnostic Research [Internet] 2013;7(10):2189–2192. doi: 10.7860/JCDR/2013/6859.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pai M, Temesgen Z. Mind the gap: Time to address implementation gaps in tuberculosis diagnosis and treatment. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases [Internet] 2017;6:14–15. doi: 10.1016/j.jctube.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Index TB Guidelines. Guidelines on extra pulmonary tuberculosis for India. https://tbcindia.gov.in/showfile.php?lid=3245.
- 20.Agashe V, Shenai S, Mohrir G, Deshmukh M, Bhaduri A, Deshpande R, et al. Osteoarticular tuberculosis—diagnostic solutions in a disease endemic region. Journal of Infection in Developing Countries. 2009;3(7):511–516. doi: 10.3855/jidc.469. [DOI] [PubMed] [Google Scholar]
- 21.Rockwood N, du Bruyn E, Morris T, Wilkinson RJ. Assessment of treatment response in tuberculosis, expert review of respiratory medicine. England: Taylor and Francis Ltd; 2016. pp. 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]