Abstract
Aim
The aim of this study was to evaluate the feasibility of heparinised saline as flushing media for frequency-domain optical coherence tomography (FD-OCT) image acquisition during percutaneous coronary intervention (PCI) optimisation.
Methods and results
Twenty-seven patients undergoing FD-OCT–guided PCI were enrolled. Heparinised saline was injected into the coronary during FD-OCT image acquisition. A total of 118 runs were analysed for image quality and diagnostic value. FD-OCT runs were categorised as follows: good runs (GRs), clinically usable runs (CURs) and clinically not usable runs (NURs); GRs and CURs were combined as clinically effective runs (ERs). Saline FD-OCT enabled visualisation of all possible coronary lesions. Of the 118 runs analysed, 61%, 27.1%, 11.9% and 88.1% were GRs, CURs, NURs and ERs, respectively. Sixty-one percent of total runs were left coronary system (LCS) and 39% were right coronary system (RCS) runs. Among LCS runs, 55.6%, 30.6%, 13.8% and 86.2% were GRs, CURs, NURs and ERs, respectively. Among RCS runs, 69.6%, 21.7%, 8.7% and 91.3% were GRs, CURs, NURs and ERs, respectively.
Conclusion
This is the first study to demonstrate the technical feasibility of isolated saline FD-OCT for PCI optimisation.
Keywords: Optical coherence tomography, Percutaneous coronary intervention, Saline, Contrast-induced nephropathy
1. Introduction
Frequency-domain optical coherence tomography (FD-OCT) imaging is an emerging and useful tool for percutaneous coronary intervention (PCI) optimisation.1 This coronary imaging modality is safe with good reproducibility.2,3,4 It uses near-infrared light for image acquisition of the coronary vessel wall with a high resolution of up to 10 μm.5 The high-resolution images obtained by FD-OCT allow study of (1) coronary anatomy and pathology, including the vessel wall, vessel size and lumen; (2) plaque characteristics; (3) coronary erosion or plaque rupture as a mechanism of acute coronary syndrome; (4) coronary dissections or recanalised thrombus; (5) stent placement and (6) mechanism of in-stent restenosis.6,7
Despite the various benefits of FD-OCT, its clinical applicability is limited by contrast-induced nephropathy (CIN), which is acute renal failure caused by exposure to iodine-based contrast media, used as the flushing agent for clearance of blood during image acquisition.8,9 The risk factors for developing CIN include pre-existing reduced renal function, age >75 years, heart failure, diabetes mellitus and female gender.8
Efforts are ongoing to find a contrast-saving alternative approach for coronary FD-OCT. Low-molecular-weight dextran is one of the options that have been explored as an alternative to contrast for coronary FD-OCT.10,11,12 Studies that compared low-molecular-weight dextran with iodine-based contrast media for coronary OCT image acquisition revealed no significant difference regarding image quality between the two methods.10,11,12 However, the use of dextran has been found to be associated with nephrotoxicity and anaphylactoid reactions.13,14 A colloid solution containing starch known as Voluven is another flushing solution that has been compared with iodine-based contrast in an experimental setting. The results showed that a 50:50 mixture of contrast and Voluven may be an efficient alternative to the contrast flushing solution alone.15
First-generation OCT or time-domain OCT (TD-OCT) uses balloon occlusion proximal to the area of coronary interest, along with injection of noncontrast agents, such as saline or Ringer's lactate solution for blood clearance during image acquisition. This technique is feasible and yields acceptable results,16 but coronary occlusion during TD-OCT imaging coupled with its slow pullback rate may result in an increased risk of ischaemia.17 This disadvantage with TD-OCT is overcome by FD-OCT imaging, which can be performed without balloon occlusion and with a faster image acquisition time.17 However, iodinated contrast media are preferred over noncontrast flushing solutions, such as saline or Ringer's lactate for nonocclusion techniques, as high viscosity solutions may be advantageous for complete blood clearance.16
The use of saline as flushing media for FD-OCT image acquisition has been studied in few preclinical,18 peripheral intervention19,20 and human carotid artery imaging studies.21 There is a need to further understand the feasibility of saline as flushing media for coronary FD-OCT imaging so as to optimise the imaging technique and avoid CIN as a result of use of contrast flushing solution. Hence, we conducted the present study to examine the feasibility of heparinised saline as flushing media for FD-OCT image acquisition during PCI. Furthermore, we also compared the image quality and diagnostic value of saline-mediated FD-OCT versus contrast-mediated FD-OCT in one case.
2. Methods
This was a prospective observational study conducted at the Department of Cardiology, Base Hospital Delhi Cantt, New Delhi, India. The study protocol was reviewed and approved by the hospital's ethics committee. The informed consent form was obtained from all patients before enrolment in the study.
Patients aged ≥18 years, undergoing FD-OCT–guided PCI for chronic stable angina or acute coronary syndrome (except patients with ST elevation myocardial infarction) and willing to participate in this study were enrolled into the study.
Imaging was performed using an FD-OCT intravascular catheter system (ILUMIEN OPTIS imaging System; St. Jude Medical, Minneapolis, MN, USA). The imaging catheter used was Dragonfly Duo (St. Jude Medical). Heparinised saline was injected into the coronary system for blood clearance during OCT image acquisition. Manual flushing was performed using the 30-mL Luer lock syringe. The average amount of saline used for blood clearance in the right coronary system (RCS) was 15 mL and the left coronary system (LCS) was 18–20 mL. The guiding catheter was properly engaged before image acquisition. Before every OCT run, 100 mcg of intracoronary nitroglycerine (NTG) was given to avoid catheter- or saline-induced coronary spasm. Electrocardiographic and haemodynamic changes were observed during pullback. A pullback length of 54/75 mm was taken for each run at the speed of 25 mm/s. Runs were carried out, both before and after PCI, by a single operator using heparinised saline as flushing media. All saline OCT runs were analysed for image quality and diagnostic value, based on specific parameters, by the study investigator experienced in reviewing OCT images.
The OCT runs were divided into three categories: good runs (GRs), clinically usable runs (CURs) and clinically not usable runs (NURs). GRs were characterised by visualisation of the following: (1) all three layers of the coronary vessel wall more than 270° of circumference throughout the length of the run; (2) no blood swirl in the run and (3) detailed coronary lesion and stent characteristics. CURs were characterised by the following: (1) up to 270° vessel wall visualisation; (2) visible blood in the run, but not hampering the coronary lesion characteristics and minimum lumen area; (3) a visible proximal and distal reference landing zone and (4) visualisation of stent strut apposition, stent edge dissection and plaque prolapse. NURs were those in which no diagnostic information could be obtained, largely because of poor clearance of blood. GRs and CURs were further combined as clinically effective runs (ERs) for PCI optimisation.
Statistical analysis was carried out using SPSS version 24.0. The categorical variables were expressed as percentages, and continuous variables were expressed as mean ± standard deviation. The chi-square test was used to compare the categorical variables. A p-value less than 0.05 was considered as significant.
3. Results
A total of 27 patients undergoing FD-OCT were enrolled, and 118 runs (51 runs before PCI and 67 runs after PCI) runs were analysed. There were no haemodynamic changes observed during saline OCT. Transient electrocardiographic changes were observed, which were found to be normal; there was no incidence of ventricular tachycardia or cardiac arrhythmia during saline flush.
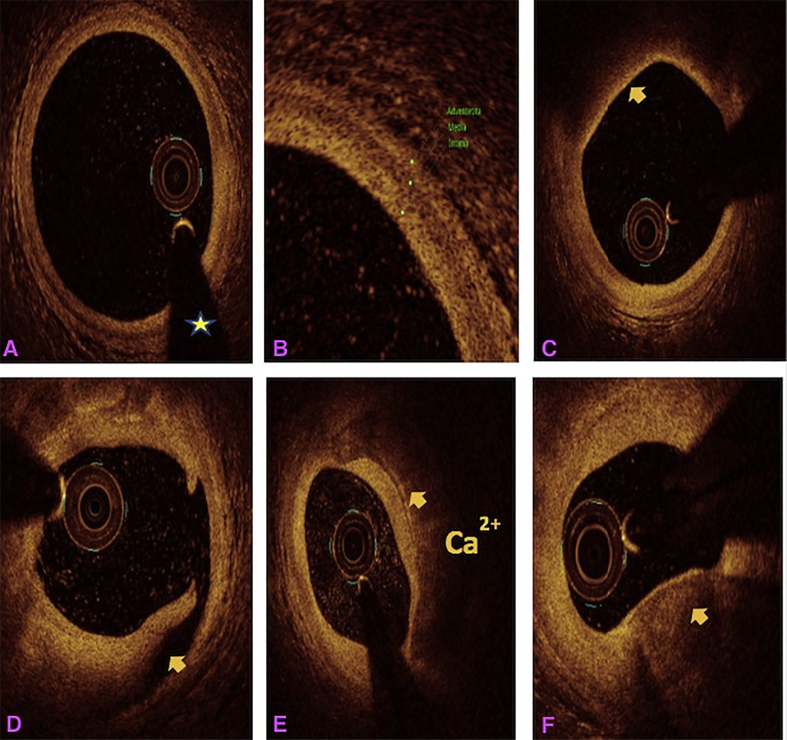
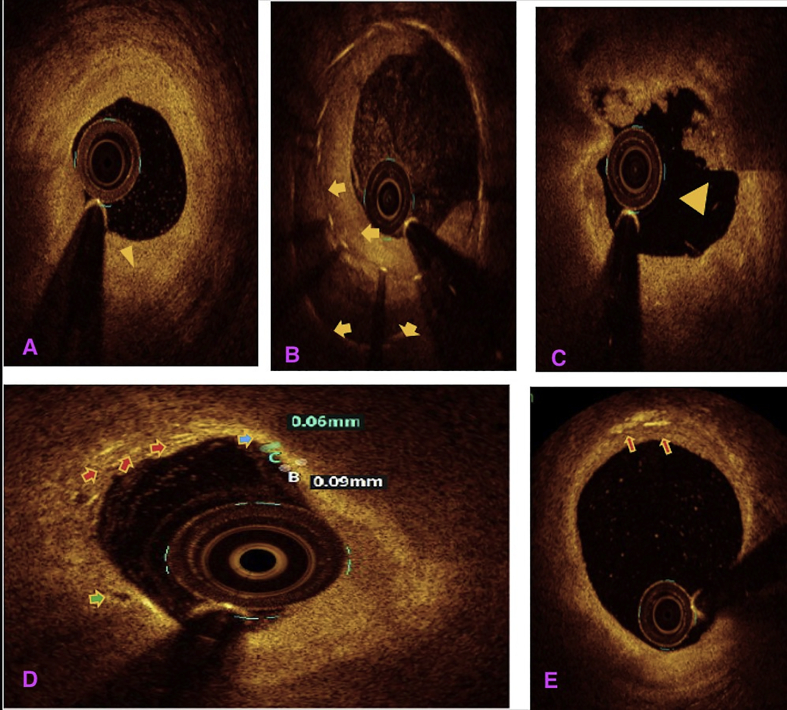
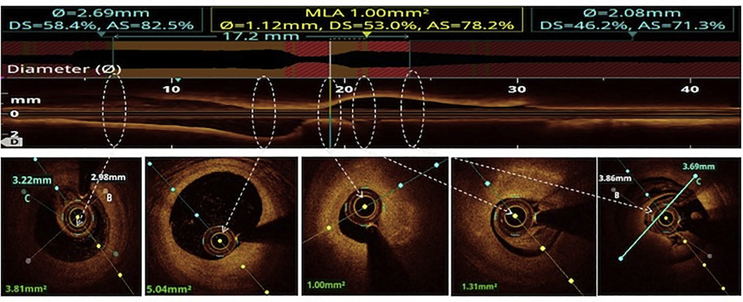
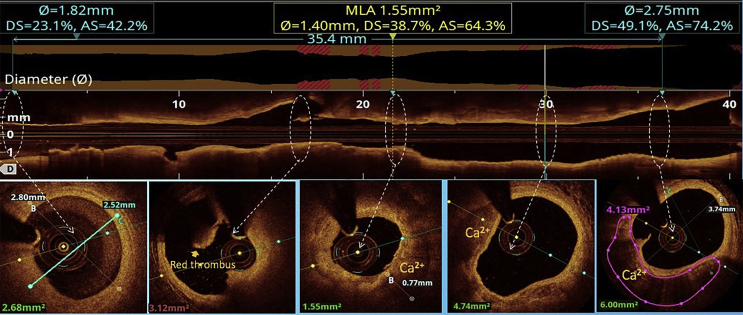
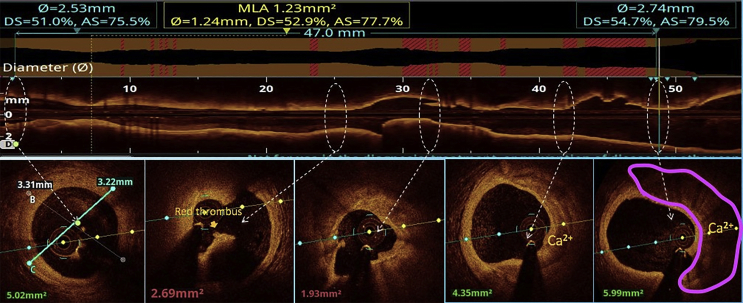
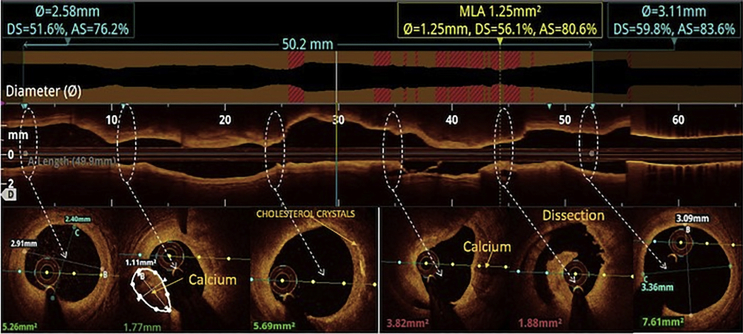
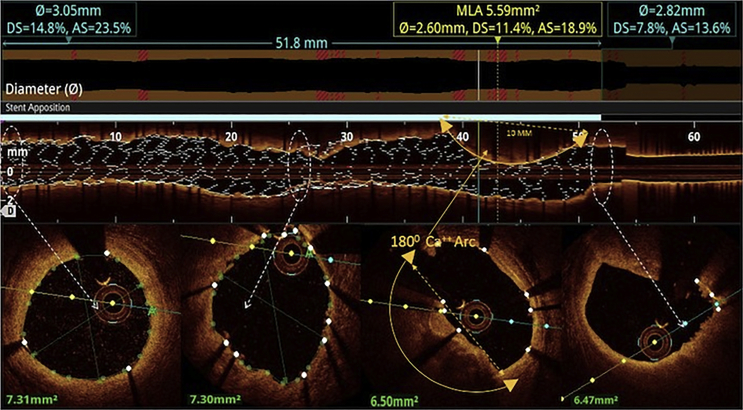
All the three layers of the coronaries were well visualised by saline OCT (Fig. 1A and B) along with guidewire effect. Vulnerable plaque characterised by thin-cap fibroatheroma (TCFA) with a large lipid core was clearly visualised (Fig. 1C). All coronary lesions including coronary dissections (Fig. 1D), more than 180° calcified arc (Fig. 1E), calcified nodules (Fig. 1F), fibrotic plaques (Fig. 2A), in-stent restenosis (Fig. 2B) and red thrombus (Fig. 2C) were visualised clearly. Active coronary lesions characterised by TCFA (Fig. 2D), subintimal macrophages (Fig. 2D) and cholesterol crystals (Fig. 2E) were clearly appreciated. Supplementary Fig. 1 (video 1) and 2 (video 2) show pre- and post-PCI saline FD-OCT L-mode and cross-sectional frames of the right coronary artery, respectively. Malapposition, plaque prolapse and rendered stent view were clearly appreciated, as shown in Supplementary Fig. 2. Both saline and contrast-mediated FD-OCT were performed in one patient. Supplementary Fig. 3 (video 3) and 4 (video 4) show pre-PCI left anterior descending (LAD) artery runs with saline and contrast, respectively, with the OCT catheter at the same position in this case. There was no difference in the image quality and measured vessel parameters. Supplementary Figs. 5 and 6 show pre- and post-PCI LAD artery saline OCT runs, respectively. Supplementary Fig. 1 (video 1) and 3 (video 3) were analysed as CURs, and Supplementary Fig. 2 (video 2), 5 and 6 were analysed as GRs.
Fig. 1.
Saline FD-OCT frames: (A) normal coronary with guidewire effect (shown by a star), (B) all three layers of the normal coronary, (C) TCFA (arrow) with the large lipid core, (D) arrow showing coronary dissection, (E) arrow showing a circumferential calcified lesion and (F) arrow showing a calcium nodule. FD-OCT = frequency-domain optical coherence tomography; TCFA = thin-cap fibroatheroma.
Fig. 2.
Saline FD-OCT frames: (A) fibrotic lesion (shown by an arrow head), (B) in-stent restenosis with arrows showing two layers of stent struts, (C) arrow head showing red thrombus, (D) blue arrow showing TCFA with a cap thickness of 0.06 mm, red arrows showing intimal macrophages in the lesion and green arrow showing vasa vasorum and (E) red arrow showing intimal cholesterol crystals. FD-OCT = frequency-domain optical coherence tomography; TCFA = thin-cap fibroatheroma.
The following are the supplementary data related to this article:
Saline FD-OCT run of the right coronary artery before angioplasty. This is an example of a CUR, in which blood swirl is present but the proximal landing zone, the distal landing zone and lesion morphology are visible. FD-OCT = frequency-domain optical coherence tomography; CUR = clinically usable run.
Saline FD-OCT run of the right coronary artery after angioplasty. This is an example of a GR, in which the proximal landing zone, distal landing zone of the stent, stent strut malapposition, plaque prolapse and rendered stent view are clearly visible, as in Supplementary Fig. 4. FD-OCT = frequency-domain optical coherence tomography; GR = good run.
Pre-PCI left anterior artery descending artery saline FD-OCT run, showing an calcium arc up to 180°, small intimal dissection in multiple frames and red thrombus. This is an example of a CUR. FD-OCT = frequency-domain optical coherence tomography; PCI = percutaneous coronary intervention; CUR = clinically usable run.
Contrast FD-OCT run of the left anterior descending artery in same patient, as shown in video 3 with the imaging catheter at the same position. FD-OCT = frequency-domain optical coherence tomography.
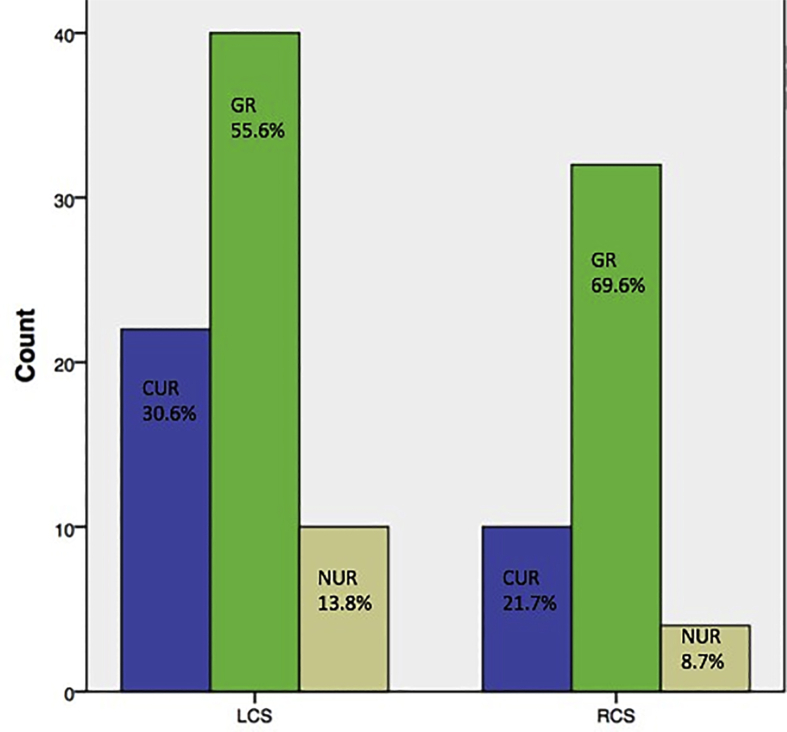
Of the 118 runs analysed, 61% (72) were GRs, 27.1% (32) were CURs and 11.9% (14) were NURs. ERs for PCI optimisation were 88.1% (104). Of the 118 runs, 61% (72) were LCS runs and 39% (46) were RCS runs. The distribution of GRs, CURs and NURs among LCS and RCS runs has been shown in Fig. 3. There was no statistically significant difference between GRs, CURs and NURs among LCS runs and the corresponding RCS runs. ERs were seen in 86.2% of the LCS runs and 91.3% of the RCS runs. There was no significant difference in ERs between LCS and RCS runs.
Fig. 3.
Bar diagram showing image quality-wise distribution of saline FD-OCT runs in the left coronary system and right coronary system. FD-OCT = frequency-domain optical coherence tomography. GR = good run; CUR = clinically usable run; NUR = clinically not usable run; LCS = left coronary system; RCS = right coronary system.
4. Discussion
FD-OCT imaging is an emerging tool for PCI optimisation.1 This technology has been used extensively in coronary imaging to augment conventional coronary angiography. It provides detailed coronary lesion morphology with near-light microscope resolution. It has many important clinical applications such as detection of TCFA that is at a high risk of rupture22,23 and detection of stent strut malapposition that is associated with late coronary stent thrombosis.24 Furthermore, OCT has been shown to detect these findings with higher sensitivity than intravascular ultrasound (IVUS); the acute malapposition rates detected with FD-OCT and IVUS have been found to be 96.2% and 42.3%, respectively.25
The clinical utility of FD-OCT is limited by the use of iodine-based contrast as flushing media for image acquisition owing to the risk of CIN.8 Few small studies and case reports have used low-molecular-weight dextran as an alternative to contrast for coronary OCT image acquisition.10,11,12 These studies showed no difference in image quality between dextran and contrast and proposed dextran as an alternative to contrast. However, dextran use is limited by its cost, availability, risk of nephrotoxicity and anaphylactoid reaction.13,14 Heparinised saline is a safe, cheap and commonly used flushing solution during coronary intervention. Preclinical studies have used saline as flushing media during OCT.18 Saline has also been used as an alternative to contrast for FD-OCT during peripheral interventions.19,20
The present study used heparinised saline for coronary FD-OCT image acquisition during PCI optimisation. This is the first study in which isolated heparinised saline was used for coronary FD-OCT. Simard et al18 showed that OCT with saline results in smaller area and diameter values when than contrast-mediated OCT during intravascular imaging using OCT in rabbits. In our study, intracoronary NTG was given before each saline OCT run to prevent OCT catheter– and saline-induced spasm. The use of saline for FD-OCT in our study was not associated with any haemodynamic changes. Although there were transient electrocardiographic changes, there was no incidence of cardiac arrhythmia or ventricular tachycardia. A recent case series that assessed the feasibility of the use of FD-OCT using iodinated contrast diluted with heparinized normal saline also reported no haemodynamic or electrocardiographic changes or any other complications.26
All the coronary lesions, including vulnerable plaque (i.e., TCFA), coronary dissections, calcified lesions, fibrotic lesions, in-stent restenosis, red thrombus, coronary vasa vasorum and active coronary lesions with macrophages, were seen with saline OCT (Fig. 1, Fig. 2). Post-PCI saline OCT clearly showed stent apposition, proximal landing zone, distal landing zone, edge dissection, plaque prolapse and rendered stent view (Supplementary Fig. 2). In a single case, in which both saline and contrast were used, saline-mediated OCT showed no difference in image quality and diagnostic value compared with contrast-mediated OCT (Supplementary Figs. 3 and 4).
In the present study, 61% of total runs were GRs. Another 27.1% of total runs were CURs as they had all the information required for PCI optimisation. About 88.1% of saline OCT runs were ERs for PCI optimisation. Studies have shown similar success rates with contrast OCT image acquisition (93%).27 Although about 12% of the runs analysed were NURs, saline FD-OCT with about 88% ERs may be a feasible and better alternative in patients who may benefit from avoidance of contrast.
Saline OCT was effective in both the RCS and LCS in our study, and the difference in the percentage of ERs between the RCS and LCS was not statistically significant. There was no adverse event observed during and after saline OCT. The only prerequisite for good saline OCT image was a properly engaged coronary catheter for clearance of blood. This study showed that saline can be used as a contrast-saving flushing medium for coronary FD-OCT with good success rate (88.1%). This reduces the extra contrast load required for coronary imaging during OCT-guided PCI optimisation, thereby preventing the risk of CIN.
The limitations of this study include a lack of comparison of image quality and diagnostic value of saline OCT images with that of contrast OCT images in all cases. This could be done in only one case that showed no difference in outcomes between the two techniques.
5. Conclusion
This is the first study to demonstrate the technical feasibility of coronary FD-OCT using isolated saline as a flushing medium. Both saline and contrast were used in one case, and the image quality and diagnostic value of saline OCT were found to be similar to those of contrast OCT in this case. Saline OCT is safe and can be used for PCI optimisation. Thus, an obligatory extra contrast load and associated risk of CIN can be avoided during OCT-guided PCI optimisation.
6. Impact on daily practice
The use of contrast as flushing media for image acquisition during coronary FD-OCT for optimisation of PCI procedures is limited by CIN. This is the first study to demonstrate the technical feasibility of coronary saline FD-OCT; 88.1% of runs were found to be clinically effective and usable. Saline FD-OCT could be a potential contrast-saving alternative for coronary PCI optimisation. Large-scale studies in future comparing the efficacy and safety of contrast-based FD-OCT and saline FD-OCT for PCI optimisation are needed to further confirm these findings.
Funding
None declared.
Declaration of competing interest
All authors have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2020.03.013.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Saline FD-OCT L-mode and cross-sectional frames of right coronary artery pre-PCI. FD-OCT = frequency-domain optical coherence tomography; PCI = percutaneous coronary intervention.
Saline FD-OCT–rendered stent view and cross-sectional frames of the right coronary artery after stent placement. Rendered stent view shows red stent struts that are malapposed and the apposition indicator above the stent. Cross-sectional frames at various levels show malapposed stent struts with white thrombus. FD-OCT = frequency-domain optical coherence tomography.
Supplementary Fig. 3.
Saline FD-OCT L-mode and cross-sectional frames of the left anterior descending artery. L-mode shows the calcified coronary artery, and cross-sectional frames show red thrombus and 180° calcium arc. FD-OCT = frequency-domain optical coherence tomography.
Supplementary Fig. 4.
Contrast FD-OCT L-mode and cross-sectional frames of the left anterior descending artery in the same patient shown in Supplementary Fig. 5 with the imaging catheter at the same position. L-mode shows the calcified coronary artery, and cross-sectional frames show that red thrombus and 180° calcium arc are the same as that of the saline run. FD-OCT = frequency-domain optical coherence tomography.
Supplementary Fig. 5.
Saline FD-OCT L-mode and cross-sectional frames of the left anterior descending artery before angioplasty. L-mode shows the calcified coronary artery, and cross-sectional frames show calcified lesion at various levels, cholesterol crystals and dissection. FD-OCT = frequency-domain optical coherence tomography.
Supplementary Fig. 6.
Saline FD-OCT L-mode and cross-sectional frames of the left anterior descending artery after angioplasty. L-mode shows rendered stent view with the apposition indicator above it; yellow arrow depicts a poorly expanded stent due to the underlying calcium arc. Calcium arc and poor stent expansion can be appreciated in cross-sectional frames. FD-OCT= frequency-domain optical coherence tomography.
References
- 1.Tearney G.J., Regar E., Akasaka T. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 2.Imola F., Mallus M.T., Ramazzotti V. Safety and feasibility of frequency domain optical coherence tomography to guide decision making in percutaneous coronary intervention. EuroIntervention. 2010;6:575–581. doi: 10.4244/EIJV6I5A97. [DOI] [PubMed] [Google Scholar]
- 3.Barlis P., Gonzalo N., Di Mario C. A multicentre evaluation of the safety of intracoronary optical coherence tomography. EuroIntervention. 2009;5:90–95. doi: 10.4244/eijv5i1a14. [DOI] [PubMed] [Google Scholar]
- 4.Fedele S., Biondi-Zoccai G., Kwiatkowski P. Reproducibility of coronary optical coherence tomography for lumen and length measurements in humans (The CLI-VAR [Centro per la Lotta contro l'Infarto- VARiability] study) Am J Cardiol. 2012;110:1106–1112. doi: 10.1016/j.amjcard.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Suter M.J., Nadkarni S.K., Weisz G. Intravascular optical imaging technology for investigating the coronary artery. JACC Cardiovasc Imaging. 2011;4:1022–1039. doi: 10.1016/j.jcmg.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prati F., Regar E., Mintz G.S. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 7.Vijayvergiya R., Krishnappa D., Kasinadhuni G. Coronary dissection or a recanalized thrombus? Optical coherence tomography has the answer. IHJ Cardiovasc Case Rep (CVCR) 2018;2:6–8. [Google Scholar]
- 8.Morcos S.K. Prevention of contrast media-induced nephrotoxicity after angiographic procedures. J Vasc Intervent Radiol. 2005;16:13–23. doi: 10.1097/01.RVI.0000145224.02920.C2. [DOI] [PubMed] [Google Scholar]
- 9.Au T.H., Bruckner A., Mohiuddin S.M., Hilleman D.E. The prevention of contrast- induced nephropathy. Ann Pharmacother. 2014;48:1332–1342. doi: 10.1177/1060028014541996. [DOI] [PubMed] [Google Scholar]
- 10.Vijayvergiya R., Ratheesh K.J., Gupta A. Low molecular weight dextran: an alternative to radiographic contrast agent for optical coherence tomography imaging. IHJ Cardiovasc Case Rep (CVCR) 2017;1:10–11. [Google Scholar]
- 11.Ozaki Y., Kitabata H., Tsujioka H. Comparison of contrast media and low-molecular-weight dextran for frequency-domain optical coherence tomography. Circ J. 2012;76:922–927. doi: 10.1253/circj.cj-11-1122. [DOI] [PubMed] [Google Scholar]
- 12.Frick K., Michael T.T., Alomar M. Low molecular weight dextran provides similar optical coherence tomography coronary imaging compared to radiographic contrast media. Cathet Cardiovasc Interv. 2014;84:727–731. doi: 10.1002/ccd.25092. [DOI] [PubMed] [Google Scholar]
- 13.Data J.L., Nies A.S. Drugs five years later: dextran 40. Ann Intern Med. 1974;81:500–504. doi: 10.7326/0003-4819-81-4-500. [DOI] [PubMed] [Google Scholar]
- 14.Seeliger E., Flemming B., Wronski T. Viscosity of contrast media perturbs renal hemodynamics. J Am Soc Nephrol. 2007;18:2912–2920. doi: 10.1681/ASN.2006111216. [DOI] [PubMed] [Google Scholar]
- 15.Hamdan R., Ghostine S., Amabile N. Optimisation of new generation endo-coronary Fourier domain optical coherence tomography. Eur J Cardiovasc Med. 2013;2:8. [Google Scholar]
- 16.Bezerra H.G., Costa M.A., Guagliumi G. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon J.H., Vito D.L., Moses J.W. Feasibility and safety of the second-generation, frequency domain optical coherence tomography (FD-OCT): a multicenter study. J Invasive Cardiol. 2012;24:206–209. [PubMed] [Google Scholar]
- 18.Simard T., Motazedian P., Ramirez F. Pre-clinical comparison of saline and contrast for intravascular imaging using optical coherence tomography. Can J Cardiol. 2017;33:S188. [Google Scholar]
- 19.Kendrick D.E., Allemang M.T., Gosling A.F. Dextran or saline can replace contrast for intravascular optical coherence tomography in lower extremity arteries. J Endovasc Ther. 2016;23:723–730. doi: 10.1177/1526602816657392. [DOI] [PubMed] [Google Scholar]
- 20.Secco G.G., Grattoni C., Parisi R. Saline vs contrast infusion during optical coherence tomography imaging of peripheral percutaneous intervention. Int J Cardiol. 2014;172:246–248. doi: 10.1016/j.ijcard.2013.12.288. [DOI] [PubMed] [Google Scholar]
- 21.Given C.A., Attizzani G.F., Jones M.R. Frequency-domain optical coherence tomography assessment of human carotid atherosclerosis using saline flush for blood clearance without balloon occlusion. AJNR Am J Neuroradiol. 2013;34:1414–1418. doi: 10.3174/ajnr.A3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawada T., Shite J., Garcia-Garcia H.M. Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur Heart J. 2008;29:1136–1146. doi: 10.1093/eurheartj/ehn132. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka A., Imanishi T., Kitabata H. Distribution and frequency of thin-capped fibroatheromas and ruptured plaques in the entire culprit coronary artery in patients with acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol. 2008;102:975–979. doi: 10.1016/j.amjcard.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 24.Hassan A.K., Bergheanu S.C., Stijnen T. Late stent malapposition risk is higher after drug-eluting stent com- pared with bare-metal stent implantation and associates with late stent thrombosis. Eur Heart J. 2010;31:1172–1180. doi: 10.1093/eurheartj/ehn553. [DOI] [PubMed] [Google Scholar]
- 25.Bezerra H.G., Attizzani G.F., Sirbu V. Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention. JACC Cardiovasc Interv. 2013;6:228–236. doi: 10.1016/j.jcin.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Varga Z., Rajpurohit N., Li S. Frequency domain-optical coherence tomography of coronary arteries using a diluted iodinated contrast-saline mix with 5-Fr guide catheters. Cureus. 2019;11 doi: 10.7759/cureus.4892. e4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prati F., Cera M., Ramazzotti V. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention. 2007;3:365–370. doi: 10.4244/eijv3i3a66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Saline FD-OCT run of the right coronary artery before angioplasty. This is an example of a CUR, in which blood swirl is present but the proximal landing zone, the distal landing zone and lesion morphology are visible. FD-OCT = frequency-domain optical coherence tomography; CUR = clinically usable run.
Saline FD-OCT run of the right coronary artery after angioplasty. This is an example of a GR, in which the proximal landing zone, distal landing zone of the stent, stent strut malapposition, plaque prolapse and rendered stent view are clearly visible, as in Supplementary Fig. 4. FD-OCT = frequency-domain optical coherence tomography; GR = good run.
Pre-PCI left anterior artery descending artery saline FD-OCT run, showing an calcium arc up to 180°, small intimal dissection in multiple frames and red thrombus. This is an example of a CUR. FD-OCT = frequency-domain optical coherence tomography; PCI = percutaneous coronary intervention; CUR = clinically usable run.
Contrast FD-OCT run of the left anterior descending artery in same patient, as shown in video 3 with the imaging catheter at the same position. FD-OCT = frequency-domain optical coherence tomography.
Saline FD-OCT–rendered stent view and cross-sectional frames of the right coronary artery after stent placement. Rendered stent view shows red stent struts that are malapposed and the apposition indicator above the stent. Cross-sectional frames at various levels show malapposed stent struts with white thrombus. FD-OCT = frequency-domain optical coherence tomography.