Watch a video presentation of this article
Watch an interview with the author
Abbreviations
- ALT
alanine aminotransferase
- APRI
AST/platelet ratio index
- AST
aspartate aminotransferase
- CK‐18
cytokeratin 18
- CT
computerized tomography
- FIB‐4
Fibrosis‐4
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- N/A
information not available
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- NHANES
National Health and Nutrition Examination Survey
- SNP
single‐nucleotide polymorphism
- T2D
type 2 diabetes
Nonalcoholic fatty liver disease (NAFLD) is defined as a spectrum of diseases, including simple steatosis to nonalcoholic steatohepatitis (NASH), with or without fibrosis and cirrhosis, that occurs in the absence of significant alcohol consumption and after exclusion of other etiologies of liver disease. 1 About 25% of the world’s population is known to have some form of NAFLD. The incidence of NAFLD has continued to increase worldwide, with the parallel rise of obesity and metabolic syndrome, particularly in western countries. 1 , 2 NASH has now emerged as one of the leading causes of chronic liver disease, cirrhosis, and hepatocellular carcinoma (HCC). 3
There is a significant racial disparity in the prevalence of NAFLD in the United States, with a disproportionately higher predilection for Hispanics/Latinos. 4 In a recent systematic review and meta‐analysis, Rich et al. 5 showed significant racial disparities in NAFLD prevalence and severity in the United States, with the highest proportion in Hispanics and the lowest burden in blacks. Recent epidemiological study showed that the mortality rates for NAFLD‐cirrhosis and HCC have increased in non‐Hispanic whites followed by Hispanics and non‐Hispanic blacks. 6 In this review, we focused on the racial differences in the diagnosis and prognosis of NAFLD.
Racial Disparities in the Prevalence of NAFLD/NASH
NASH is estimated to affect 3% to 5% of the global population. Estes et al. 7 recently reported that in the United States, NAFLD is likely to increase from 83.1 million (2015) to 100.9 million by 2030, and NASH from 16.52 million (2015) to 27 million.
A meta‐analysis in 2018 composed of 34 studies reported that NAFLD prevalence was highest in Hispanics, intermediate in whites, and lowest in blacks. There was a wide variation in NAFLD prevalence rates among studies, ranging from 6.6% to 46.0%. 5 Among patients with NAFLD, risk for progression to NASH was higher in Hispanics (relative risk, 1.09; 95% confidence interval, 0.98–1.21) and lower in blacks (relative risk, 0.72; 95% confidence interval, 0.60–0.87) than whites. However, the proportion of patients with significant fibrosis did not significantly differ among racial groups. Prevalence appeared to depend on the method of NAFLD diagnosis, with the highest prevalence reported in studies using ultrasound or magnetic resonance imaging (MRI).
In a meta‐analysis done in 2005 that included 742 patients with chronic liver disease, Hispanics (28%) had higher prevalence rate of NAFLD compared with Asians (18%), African Americans (3%), and other races (6%). 8 Wagenknecht et al. 9 used computerized tomography (CT) to assess liver/spleen density ratio and abdominal fat distribution to establish that prevalence rates in Hispanic Americans and African Americans were 23% and 10%, respectively. Trico et al. 10 used MRI for quantifying hepatic fat and demonstrated the prevalence rates of NAFLD were 42.9% in whites, 15.7% in blacks, and 59.6% in Hispanics. Mohanty et al. 11 retrospectively examined liver biopsies of 238 patients and showed higher rates of Mallory bodies in Hispanics, hepatic ballooning in Asians, and lower degree of steatosis in blacks compared with whites. Although most studies agree that prevalence of NAFLD is lowest in blacks and highest in Hispanics, some demonstrate variability in the prevalence of NAFLD when comparing whites with Hispanics. The reason for this variation may be related to geographical and diagnostic methodological differences. Table 1 summarizes the findings of these prevalence studies.
Table 1.
Studies Demonstrating Disparities in NAFLD Prevalence
| Author/Year | Study Design | Method of NAFLD Diagnosis | NAFLD Prevalence, n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Overall | White | Black | Hispanic | Asian | Other | |||
| Weston et al. (2005) 8 | Cross‐sectional | Imaging (ultrasound, CT) | 159 (21.4) | 71 (N/A) | 5 (N/A) | 45 (N/A) | 28 (N/A) | 10 (N/A) |
| Laboratory tests | ||||||||
| Biopsy | ||||||||
| Lazo et al. (2013) 40 | Cross‐sectional | Imaging (ultrasound) | 2366 (19) | 421 (17.8) | 319 (13.5) | 570 (24.1) | N/A | N/A |
| Wagenknecht et al. (2009) 9 | Cross‐sectional | Imaging (CT) Laboratory tests | 224 (19.6) | N/A | 22 (10) | 54 (24) | N/A | N/A |
| Glucose tolerance | ||||||||
| Trico et al. (2018) 10 | Cohort | Imaging (MRI) Genotyping | 209 (41.6) | 82 (42.9) | 21 (15.7) | 106 (59.6) | N/A | N/A |
| Glucose tolerance | ||||||||
| Patel et al. (2018) 41 | Retrospective | Biopsy | 76 (19.0) | 55 (N/A) | 9 (N/A) | 4 (N/A) | 2 (N/A) | 6 (N/A) |
| Case‐control | ||||||||
| Mohanty et al. (2009) 11 | Cross‐sectional | Imaging | 238 (34.8) | 154 (N/A) | 36 (N/A) | 32 (N/A) | 16 (N/A) | N/A |
| Laboratory tests | ||||||||
| Biopsy | ||||||||
| Birerdinc et al. (2012) 42 | Cross‐sectional | Laboratory tests (aminotransferases) | 1782 (9.6) | 1247 (14.0) | 131 (6.4) | 314 (14.0) | N/A | N/A |
| Browning et al. (2004) 43 | Cross‐sectional | Imaging (MRI) | 687 (30.7) | 242 (33.0) | 265 (24.0) | 180 (44.9) | N/A | N/A |
| Kim et al. (2013) 44 | Cross‐sectional | Imaging (ultrasound) | 94 (28.3) | 47 (36.2) | 47 (23.4) | N/A | N/A | N/A |
| Loomba et al. (2015) 45 | Cross‐sectional | Imaging (MRI) | 26 (21.7) | 18 (19.1) | N/A | 5 (27.8) | N/A | N/A |
| North et al. (2012) 46 | Cross‐sectional | Imaging (CT) | 181 (6.6) | 159 (7.2) | 22 (4.3) | N/A | N/A | N/A |
| Reddy et al. (2013) 47 | Cross‐sectional | ICD‐9 codes | 32,347 (10.7) | 20536 (11.8) | 2798 (5.8) | 4135 (11.4) | N/A | N/A |
| Tison et al. (2015) 48 | Cross‐sectional | Imaging (CT) | 706 (17.3) | 229 (15.2) | 138 (11.2) | 259 (27.1) | N/A | N/A |
| Williams et al. (2011) 49 | Cross‐sectional | Imaging (ultrasound) | 151 (46.0) | 91 (44.4) | 13 (35.1) | 42 (58.3) | N/A | N/A |
| Biopsy | ||||||||
| Younossi et al. (2012) 50 | Cross‐sectional | Imaging (ultrasound) | 2492 (21.5) | 1885 (12.2) | 211 (6.6) | 190 (12.6) | N/A | N/A |
| Laboratory tests (aminotransferases) | ||||||||
Disparities in the Distribution of Metabolic Risk Factors and NAFLD
Obesity, type 2 diabetes (T2D), and hyperlipidemia are major risk factors for NAFLD as seen in Fig. 1. T2D was shown to increase risk for NAFLD and NASH in whites compared with blacks. 12 Distribution of metabolic factors and susceptibility to metabolic factors varies between races. However, despite similar prevalence of T2D and obesity among blacks compared with whites, the latter group has significantly higher risk for NAFLD. 13 , 14 Browning et al. 12 compared biopsy‐proved NAFLD and its progression between black and white patients with severe obesity stratified by the presence or absence of T2D. Whites were significantly more likely than blacks to have NAFLD, NASH, and advanced fibrosis. T2D was associated with increased odds of NAFLD, NASH, and advanced fibrosis in whites only (P < 0.05). Also, a higher proportion of blacks than whites with T2D were free of NAFLD (58% versus 22%; P < 0.01).
Fig 1.
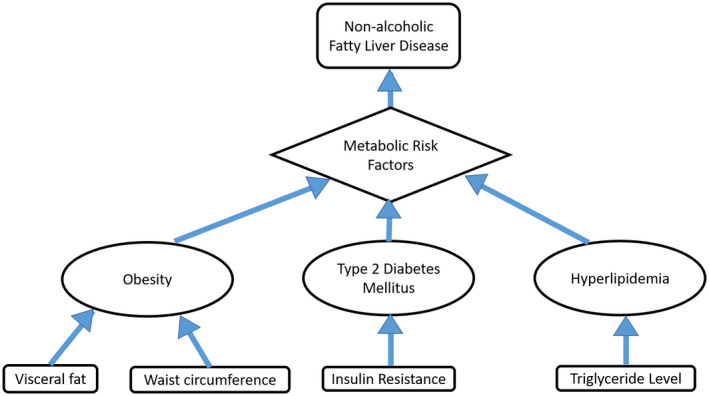
Metabolic risk factors for NAFLD. Among others, these risk factors, such as obesity related to visceral fat and waist circumference, insulin resistance as a result of T2D, and hyperlipidemia related to elevated triglyceride levels, have been shown to differ among races.
As per National Health and Nutrition Examination Survey (NHANES) data, NAFLD risk, body mass index, and waist circumference were lower in Asian Americans compared with non‐Hispanic whites. 15 Bril et al. 16 compared African American patients with Caucasian patients and reported a lower intrahepatic triglyceride content and the presence of NAFLD in African Americans (25.0% versus 51.9%), but the prevalence rate of NASH was not different between ethnicities (57.1% vs. 73.3%; P = 0.12). Moreover, they showed similar severity in each of the individual histological parameters (inflammation, ballooning, and fibrosis).
Disparities in Lifestyle and Sociodemographic Factors
Lifestyle and sociodemographic factors play an important role in the disparities of NAFLD. In a San Antonio heart study, Mexican Americans consumed increased carbohydrates and saturated fats compared with Anglo‐Americans, but there was no impact of socioeconomic status among different ethnicities on consumption of atherogenic diet. 17 Although many genetic factors play an important role in the pathophysiology of NAFLD, physical inactivity, excessive sugar, and decreased vegetable consumption can influence the association of genetic factors and NAFLD. 18 Davis et al. 19 showed that Hispanic children carrying the GG genotype of the PNPLA3 gene are susceptible to increased hepatic fat when dietary carbohydrate intake was high. Having health insurance or doing shift work did not have any association with increased risk for NAFLD. 20
Disparities Secondary to Underdiagnosis and Reliability of Diagnostic Markers in Different Ethnicities
Several scoring systems have been developed to detect hepatic fibrosis and progression of NAFLD, including alanine aminotransferase/aspartate aminotransferase (AST/ALT) ratio, NAFLD fibrosis score (NFS), Fibrosis‐4 (FIB‐4) index, and AST/platelet ratio index (APRI). De Silva et al. 21 noted that noninvasive markers, such as NFS, APRI, FIB‐4, and AST/ALT ratio, were less sensitive at detecting advanced fibrosis in South Asian compared with white patients. In a study involving a Chinese population, diagnostic value of miR‐34a for NASH was superior to that of ALT, FIB‐4, and APRI in the diagnosis of fibrosis in NASH. 22 Pan et al. 23 noticed that intermediate NFS and APRI scores were associated with hepatic steatosis in Hispanics, but FIB‐4 scores were not as outlined in Fig. 2. As per Alazawi et al., 24 a significant Bangladeshi population had elevated liver function tests, but 88.4% of patients did not have coded liver diagnosis, demonstrating significant underdiagnosis in that country.
Fig 2.

Disparities in the reliability of diagnostic markers among ethnicities.
Genetic Disparities
Several studies noticed genetic disparities in patients with NAFLD. An allele in Patatin‐like phospholipase domain‐containing protein 3 (PNPLA3) was found to be strongly associated with increased risk for NAFLD and was most commonly seen in Hispanics. In a study by Kallwitz et al., 25 9342 participants with NAFLD demonstrated PNPLA3 G frequency was different among Hispanics, with the highest proportion in Mexicans (52%) and the lowest in Dominicans (23%). Also, Hispanics with American ancestry had increased risk for NAFLD, whereas those with African and European ancestry were inversely associated with NAFLD. 25 As per Carrasco et al., 26 lysophospholipase like 1 (LYPLAL1), Protein Phosphatase 1 Regulatory Subunit 3B (PPP1R3B), and Glucokinase Regulator (GCKR) are also associated with increased NAFLD in Mexicans. Membrane Bound O‐Acyltransferase Domain Containing 7 (MBOAT7) plays an important role in development of NAFLD in Europeans by increasing hepatic fat accumulation. 27 A meta‐analysis including 12 case‐controlled studies showed that the single‐nucleotide polymorphism (SNP) rs738409 G allele increases risk for NAFLD in Asian populations. 28 As summarized in Fig. 3, the earlier genetic variations are influenced by ethnicity and affect the susceptibility of different races to NAFLD.
Fig 3.
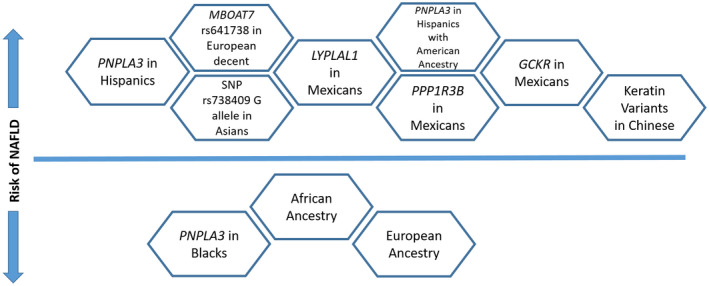
Genetic and racial influences on NAFLD risk. Factors above the horizontal line represent an increased risk for NALFD, whereas factors below the horizontal line reduce the risk for NALFD. Data are from Kallwitz et al., 25 Larrieta‐Carrasco et al., 26 Li et al., 51 Mancina et al., 27 Zhang et al., 28 and Romeo et al. 52
Disparities in Prognosis of Different Ethnicities
NASH‐Related Cirrhosis and HCC in Different Races
A systematic review done in south Texas and another large, retrospective analysis of patients undergoing liver transplant showed Hispanics experienced greatest burden of NAFLD‐related HCC compared with other ethnicities. 29 , 30 Non‐US‐born Hispanics are at lower risk for HCC compared with US‐born Hispanics, whereas foreign‐born Asians are at increased risk for HCC compared with US‐born Asians. 31 Hispanic and white patients were likely to be diagnosed with cirrhosis at an age younger than 40 years compared with African American patients. 32
Significance of Racial Disparities in Patients Undergoing Liver Transplantation
Couto et al. 33 noted that patients of Hispanic ethnicity are at increased risk for needing liver transplant for NASH‐related HCC. As per the United Network for Organ Sharing database, African Americans had lower post–liver transplant survival compared with non‐Hispanic whites. 34 Ha et al. 35 noticed that Caucasians are at increased risk for liver cirrhosis, but after 88 months of follow‐up, Asians, Hispanics, and Caucasians are equally at increased risk for cirrhosis, HCC, hepatic decompensation, and all‐cause mortality.
NASH‐Related Morbidity and All‐Cause Mortality in Different Ethnicities
As per NHANES III data published in 2008, NAFLD had higher overall mortality and liver‐related mortality than the general US population. 36 Younossi and colleagues 37 reported significantly higher rates of all‐cause mortality among blacks than whites in the NAFLD cohort. Yet another study showed there was no difference in all‐cause mortality in different ethnicities. Patients with NAFLD are at increased risk for cardiovascular mortality and are closely associated with the presence of metabolic factors, which vary in different ethnicities. 38 NAFLD is strongly associated with increased stroke risk in white patients compared with black patients. 39 A recent meta‐analysis included six studies and noted that racial disparities overall played a smaller role in NAFLD prognosis compared with prevalence. 5
Conclusion
There are notable racial disparities in multiple aspects of NAFLD, including its prevalence, severity, genetic predisposition, and overall prognosis. NAFLD prevalence was lowest in African Americans and highest in Hispanics in the majority of studies. PNPLA3 gene is strongly associated with NAFLD and mostly seen in Hispanics. Concerning prognosis, limited studies among various racial subsets in NAFLD do not always corroborate one another. In conclusion, studies are urgently needed to identify determinants of NAFLD disparities and design appropriate intervention strategies to reduce racial disparities to improve NAFLD‐related morbidity and mortality.
Potential conflict of interest: Nothing to report.
References
- 1. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 3. Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748‐755.e743. [DOI] [PubMed] [Google Scholar]
- 4. Iqbal U, Perumpail BJ, Akhtar D, et al. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines (Basel) 2019;6:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rich NE, Oji S, Mufti AR, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2018;16:198‐210.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim D, Li AA, Perumpail BJ, et al. Changing trends in etiology‐based and ethnicity‐based annual mortality rates of cirrhosis and hepatocellular carcinoma in the United States. Hepatology 2019;69:1064‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology 2005;41:372‐379. [DOI] [PubMed] [Google Scholar]
- 9. Wagenknecht LE, Scherzinger AL, Stamm ER, et al. Correlates and heritability of nonalcoholic fatty liver disease in a minority cohort. Obesity (Silver Spring) 2009;17:1240‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trico D, Caprio S, Rosaria Umano G, et al. Metabolic features of nonalcoholic fatty liver (NAFL) in obese adolescents: Findings from a multiethnic cohort. Hepatology 2018;68:1376‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohanty SR, Troy TN, Huo D, et al. Influence of ethnicity on histological differences in non‐alcoholic fatty liver disease. J Hepatol 2009;50:797‐804. [DOI] [PubMed] [Google Scholar]
- 12. Browning MG, Khoraki J, DeAntonio JH, et al. Protective effect of black relative to white race against non‐alcoholic fatty liver disease in patients with severe obesity, independent of type 2 diabetes. Int J Obes (Lond) 2018;42:926‐929. [DOI] [PubMed] [Google Scholar]
- 13. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011‐2012. JAMA 2014;311:806‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988‐2012. JAMA 2015;314:1021‐1029. [DOI] [PubMed] [Google Scholar]
- 15. Golabi P, Paik J, Hwang JP, et al. Prevalence and outcomes of non‐alcoholic fatty liver disease (NAFLD) among Asian American adults in the United States. Liver Int 2019;39:748‐757. [DOI] [PubMed] [Google Scholar]
- 16. Bril F, Cusi K. Response to comment on Bril et al. Clinical and histologic characterization of nonalcoholic steatohepatitis in African American patients. Diabetes Care 2018;41:187‐192. Diabetes Care 2018;41:e137‐e138. [DOI] [PubMed] [Google Scholar]
- 17. Haffner SM, Knapp JA, Hazuda HP, et al. Dietary intakes of macronutrients among Mexican Americans and Anglo Americans: The San Antonio heart study. Am J Clin Nutr 1985;42:1266‐1275. [DOI] [PubMed] [Google Scholar]
- 18. Wang S, Song J, Shang X, et al. Physical activity and sedentary behavior can modulate the effect of the PNPLA3 variant on childhood NAFLD: A case‐control study in a Chinese population. BMC Med Genet 2016;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis JN, Le KA, Walker RW, et al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr 2010;92:1522‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balakrishnan M, El‐Serag HB, Kanwal F, et al. Shiftwork is not associated with increased risk of NAFLD: Findings from the national health and nutrition examination survey. Dig Dis Sci 2017;62:526‐533. [DOI] [PubMed] [Google Scholar]
- 21. De Silva S, Li W, Kemos P, et al. Non‐invasive markers of liver fibrosis in fatty liver disease are unreliable in people of South Asian descent. Frontline Gastroenterol 2018;9:115‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu XL, Pan Q, Zhang RN, et al. Disease‐specific miR‐34a as diagnostic marker of non‐alcoholic steatohepatitis in a Chinese population. World J Gastroenterol 2016;22:9844‐9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan JJ, Fisher‐Hoch SP, Chen C, et al. Burden of nonalcoholic fatty liver disease and advanced fibrosis in a Texas Hispanic community cohort. World J Hepatol 2015;7:1586‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alazawi W, Mathur R, Abeysekera K, et al. Ethnicity and the diagnosis gap in liver disease: A population‐based study. Br J Gen Pract 2014;64:e694‐e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kallwitz ER, Tayo BO, Kuniholm MH, et al. American ancestry is a risk factor for suspected nonalcoholic fatty liver disease in Hispanic/Latino adults. Clin Gastroenterol Hepatol 2019;17:2301‐2309. [DOI] [PubMed] [Google Scholar]
- 26. Larrieta‐Carrasco E, Flores YN, Macias‐Kauffer LR, et al. Genetic variants in COL13A1, ADIPOQ and SAMM50, in addition to the PNPLA3 gene, confer susceptibility to elevated transaminase levels in an admixed Mexican population. Exp Mol Pathol 2018;104:50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mancina RM, Dongiovanni P, Petta S, et al. The MBOAT7‐TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology 2016;150:1219‐1230.e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang L, You W, Zhang H, et al. PNPLA3 polymorphisms (rs738409) and non‐alcoholic fatty liver disease risk and related phenotypes: A meta‐analysis. J Gastroenterol Hepatol 2015;30:821‐829. [DOI] [PubMed] [Google Scholar]
- 29. Ha J, Chaudhri A, Avirineni A, Pan JJ. Burden of hepatocellular carcinoma among hispanics in South Texas: A systematic review. Biomark Res 2017;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zarrinpar A, Faltermeier CM, Agopian VG, et al. Metabolic factors affecting hepatocellular carcinoma in steatohepatitis. Liver Int 2019;39:531‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang ET, Yang J, Alfaro‐Velcamp T, et al. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev 2010;19:3106‐3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sajja KC, Mohan DP, Rockey DC. Age and ethnicity in cirrhosis. J Investig Med 2014;62:920‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Couto CA, Gelape CL, Calmet F, et al. Effect of ethnicity on liver transplant for hepatocellular carcinoma. Exp Clin Transplant 2013;11:339‐345. [DOI] [PubMed] [Google Scholar]
- 34. Wong RJ, Ahmed A. Combination of racial/ethnic and etiology/disease‐specific factors is associated with lower survival following liver transplantation in African Americans: An analysis from UNOS/OPTN database. Clin Transplant 2014;28:755‐761. [DOI] [PubMed] [Google Scholar]
- 35. Ha NB, Trinh S, Le RH, et al. 950 disease presentation and natural history of non‐alcoholic fatty liver disease (NAFLD) in an ethnically diverse US patient population: A long‐term follow‐up study. Gastroenterology 2016;150:s1055. [Google Scholar]
- 36. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver‐related mortality in non‐alcoholic fatty liver disease. J Hepatol 2008;49:608‐612. [DOI] [PubMed] [Google Scholar]
- 37. Younossi ZM, Otgonsuren M, Venkatesan C, et al. In patients with non‐alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism 2013;62:352‐360. [DOI] [PubMed] [Google Scholar]
- 38. Stahl EP, Dhindsa DS, Lee SK, et al. Nonalcoholic fatty liver disease and the heart: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019;73:948‐963. [DOI] [PubMed] [Google Scholar]
- 39. Alexander KS, Zakai NA, Lidofsky SD, et al. Non‐alcoholic fatty liver disease, liver biomarkers and stroke risk: The Reasons for Geographic and Racial Differences in Stroke cohort. PLoS One 2018;13:e0194153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988‐1994. Am J Epidemiol 2013;178:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy‐proven advanced non‐alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther 2018;47:268‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Birerdinc A, Stepanova M, Pawloski L, et al. Caffeine is protective in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2012;35:76‐82. [DOI] [PubMed] [Google Scholar]
- 43. Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004;40:1387‐1395. [DOI] [PubMed] [Google Scholar]
- 44. Kim C, Harlow SD, Karvonen‐Gutierrez CA, et al. Racial/ethnic differences in hepatic steatosis in a population‐based cohort of post‐menopausal women: The Michigan Study of Women’s Health Across the Nation. Diabet Med 2013;30:1433‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loomba R, Schork N, Chen CH, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 2015;149:1784‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. North KE, Graff M, Franceschini N, et al. Sex and race differences in the prevalence of fatty liver disease as measured by computed tomography liver attenuation in European American and African American participants of the NHLBI family heart study. Eur J Gastroenterol Hepatol 2012;24:9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reddy SK, Zhan M, Alexander HR, et al. Nonalcoholic fatty liver disease is associated with benign gastrointestinal disorders. World J Gastroenterol 2013;19:8301‐8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tison GH, Blaha MJ, Nasir K, et al. Relation of anthropometric obesity and computed tomography measured nonalcoholic fatty liver disease (from the Multiethnic Study of Atherosclerosis). Am J Cardiol 2015;116:541‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011;140:124‐131. [DOI] [PubMed] [Google Scholar]
- 50. Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319‐327. [DOI] [PubMed] [Google Scholar]
- 51. Li R, Liao XH, Ye JZ, et al. Association of keratin 8/18 variants with non‐alcoholic fatty liver disease and insulin resistance in Chinese patients: A case‐control study. World J Gastroenterol 2017;23:4047‐4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]


