Oxyntic atrophy, the loss of gastric parietal cells, is a critical precursor of metaplasia in the stomach. In the case of humans, Helicobacter pylori infection induces loss of parietal cells from the corpus of the stomach over years, while 6–12 months of Helicobacter infection is required to induce oxyntic atrophy in rodents.1 In mice, acute models of parietal cell loss have helped distinguish key steps in the induction of chief cell reprogramming into metaplasia. These studies initially used the drug DMP-777, which was demonstrated to function as a parietal cell secretory membrane protonophore using assay of H/K-ATPase–dependent proton gradients in isolated rabbit tubulovesicles.2 DMP-777 did not directly inhibit the proton pump activity. Treatment with DMP-777 caused rapid parietal cell loss in both mice and rats over 3 days, leading to development of spasmolytic polypeptide-expressing metaplasia.2,3 Pretreatment of rats with proton pump inhibitors blunted the ability of DMP-777 to induce oxyntic atrophy.4 Similar findings were seen for a number of molecular cousins of DMP-777, including L635.5 While DMP-777 induces oxyntic atrophy without a prominent immune response, because of its coordinate action as a neutrophil elastase inhibitor, L635 lacks this action against elastase and elicits parietal cell loss and an exuberant immune response.5
In more recent years, other studies have demonstrated the ability of tamoxifen administration (especially intraperitoneal doses of 5 mg or greater) to also induce parietal cell loss.6 As with DMP-777, pretreatment of mice with proton pump inhibitors ameliorated the effects of tamoxifen to induce parietal cell loss.6 However, no studies have directly assayed the effects of tamoxifen as a parietal cell protonophore. We have therefore sought to compare the effects of tamoxifen with those of DMP-777 and L635 on acid sequestration in parietal cell tubulovesicles.
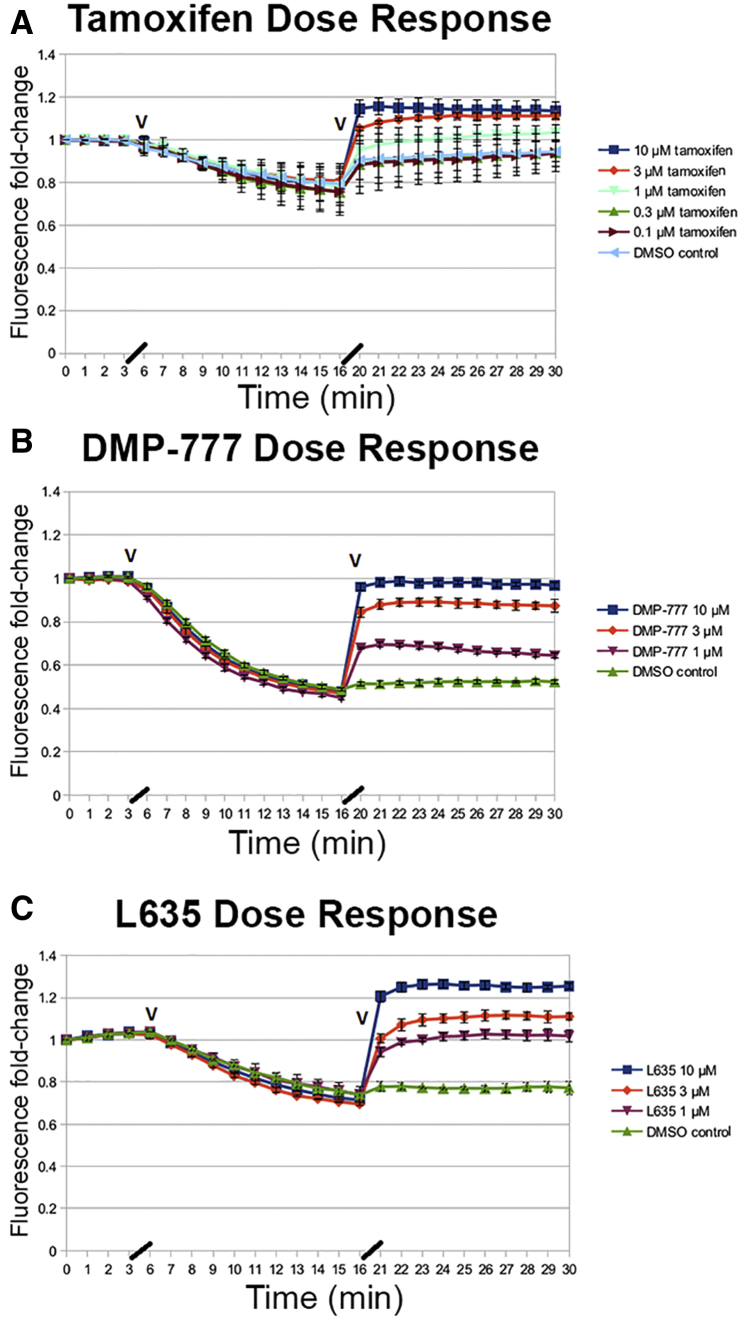
Parietal cell tubulovesicles were isolated from rabbit stomach mucosa using standard protocols and tubulovesicles layering above 20% sucrose in gradient centrifugation were utilized as the tightest tubulovesicle membranes (see supplementary methods).7 The fluorescence of acridine orange was assayed in tubulovesicles using induction of pumping in the presence of adenosine triphosphate and valinomycin. Activation of acid pumping into tubulovesicles causes a rapid quenching of acridine orange fluorescence indicative of pumping of acid into the lumen of tubulovesicles (Figure 1). After the establishment of the proton gradient in tubulovesicles, tamoxifen, DMP-777, or L635 were added in concentrations from 0.1 to 10 μM and effects on the quenching of acridine orange were assayed (Figure 1). At concentrations from 1 to 10 μM, all 3 drugs caused a rapid decrease in acridine orange fluorescence, indicative of disruption of the proton gradient. These findings suggested that all 3 drugs act similarly as protonophores in parietal cell acid secretory membranes.
Figure 1.
Assay of acridine orange fluorescence in isolated tubulovesicles treated with tamoxifen, DMP-777, or L635. Isolated rabbit gastric tubulovesicles were incubated with acridine orange, and initiation of the proton pumping gradient was activated with addition of valinomycin and adenosine triphosphate (first black arrowhead). Control (no addition), vehicle solutions, or drugs were then added at the indicated concentrations after 15 minutes (second black arrowhead) and dequenching was analyzed for 20 min. The traces (± SD) represent representative experiments from 4 separate analyses. (A) Tamoxifen response. (B) DMP-777 response. (C) L635 response. (D) Comparison of tamoxifen, DMP-777, and L635 response at 10 μM. Note that dimethyl sulfoxide (DMSO) vehicle control samples showed a small baseline adjustment likely due to the time required for addition of drugs.
The results in these studies confirm that tamoxifen does act as a parietal cell protonophore, which can lead to back wash of acid into secreting parietal cells, the likely cause of parietal cell death following administration of high-dose tamoxifen. The potency of tamoxifen as a parietal cell protonophore is similar to that both DMP-777 and L635. It should be noted that a number of hydrophobic drugs with properties of weak bases may have parietal cell protonophore activities. KN-93, which has been utilized as an inhibitor of calmodulin-dependent protein kinase II, has a strong protonophore effect on parietal cells.8 Nevertheless, it should be noted that these protonophore actions require extremely high doses of drug that are usually greater than an order of magnitude higher than doses used for treating patients.6
Nevertheless, because tamoxifen-inducible Cre recombinase strains are commonly used, especially to track stem cell activity, it is critical to recognize that tamoxifen can kill parietal cells, triggering adaptive, nonhomeostatic patterns of proliferation in the stomach. A recent report by Samuelson et al9 has highlighted the dose dependence of tamoxifen action in the stomach. That report noted a gap in the knowledge of the precise mechanism of tamoxifen action related to oxyntic atrophy leading us to perform the present studies. It is notable that the effects of tamoxifen to induce parietal cell loss, as well as the recovery from tamoxifen, were not dependent on gastrin. Similar results were previously observed with DMP-777 administration.3 Thus, cholinergic stimulation in these animals may be adequate to maintain sufficient acid secretion and lead to acute parietal cell loss. While tamoxifen, DMP-777, and L635 all possess a parietal cell protonophore capacity, it remains unclear as to whether their off-target effects may differ. It is clear that these 3 models of acute oxyntic atrophy all differ in the range of inflammatory reactions they incite. Given the recent evidence for the roles of immune cells in promoting the induction and progression of metaplasia,10 each of these models needs to be evaluated in detail, and in comparison with more chronic forms of oxyntic atrophy.
Footnotes
CRediT Authorship Contributions Elizabeth Manning (Data curation: Lead; Formal analysis: Equal; Methodology: Lead; Writing – review & editing: Supporting) Lapierre A. Lapierre (Data curation: Equal; Formal analysis: Equal; Methodology: Equal; Writing – review & editing: Supporting) Jason C. Mills (Conceptualization: Equal; Writing – review & editing: Supporting)James R. Goldenring, MD, PhD (Conceptualization: Lead; Methodology: Equal; Project administration: Lead; Supervision: Lead; Writing – original draft: Lead)
Conflicts of interest The authors disclose no conflicts
Funding These studies were supported by Department of Veterans Affairs Merit Review Award Grant No. IBX000930 (to James R. Goldenring); National Institutes of Health Grant Nos. RO1 DK071590 (to James R. Goldenring), RO1 DK101332 (to James R. Goldenring), R01 DK094989 (to Jason C. Mills), RO1 DK105129 (to Jason C. Mills), and RO1 DK110406 (to Jason C. Mills); and core resources of the Vanderbilt Digestive Disease Center (National Institutes of HealthP30 DK058404).
Supplementary Materials and Methods
Materials
DMP-777 was a gift of DuPont-Merck Corporation (North Billerica, MA). L635 was synthesized in the Vanderbilt Chemical Biology Shared Resource and was validated as a single enantiomeric compound of >99.5% purity. Tamoxifen was obtained from Sigma-Aldrich (St. Louis, MO). All other compounds were obtained from Sigma-Aldrich.
Preparation of Rabbit Gastric Tubulovesicles
Rabbit tubulovesicles were prepared from homogenized gastric mucosa by differential centrifugation followed by sucrose gradient velocity sedimentation, as previously described.1 Tubulovesicle membranes that floated on the 20% sucrose cushion were utilized as high-resistance tubulovesicles.
Assay of Acridine Orange Accumulation Into Tubulovesicles
Assay master mix was composed of 1.2-μM acridine orange/290-μM adenosine triphosphate/24 μg/mL tubulovesicles from a freshly thawed aliquot in acridine orange diluent (150-mM sucrose/75-mM KCl/250-μM MgSO4/5-mM piperazine-N,N′-bis(2-ethanesulfonic acid)/5-mM Tris, pH 7). Valinomycin was diluted to 20 μM in acridine orange diluent from a 0.9-mM stock in dimethyl sulfoxide. Control and sample assay reagents were diluted as described in Supplementary Table 1.
Master mix (85 μL) was added to wells in a 96-well microtiter plate in quadruplicate for each control or sample. The plate was read (time point 1) using a BioTek Synergy 4 (BioTek, Winooski, VT) plate reader at 493-nm excitation/530-nm emission once per minute for 3 minutes at ambient temperature, then ejected. Valinomycin (5 μL of 20-μM solution) was added to each well to a final concentration of 1 μM (time point 2), then the plate was shaken for 5 seconds and read for 10 more minutes and ejected. Control and sample reagents diluted in acridine orange diluent (10 μL each) were added to the wells to the final concentrations described in Supplementary Table 1 (time point 3). The total volume in each well after time point 3 was 100 μL. The plate was shaken for 5 seconds and read for at least 15 more minutes.
Data Analysis
Raw absorbance data for each well were converted to fold change by dividing all absorbance readings by the first absorbance reading using Google Sheets. Fold-change means and standard deviations for each control and sample across the quadruplicates were also calculated. Figures were made using Open Office Calc (Apache Software Foundation, Forest Hill, MD).
Supplementary Table 1.
Formulation of Test Compounds
| Control or sample name (reagent added at time point 3) | Control or sample details | Reagent storage stock | Reagent solution prepared for assay | Final reagent concentration in assay well |
|---|---|---|---|---|
| Diluent only | Negative control for assay | Acridine orange diluent | ||
| DMSO | Negative control for DMP-777, L635, and tamoxifen | 2.5% DMSO in acridine orange diluent | 0.25% | |
| DMP-777 | Positive control | 10 mM in DMSO | 100 μM in acridine orange diluent | 10, 3, or 1 μM |
| L635 | Positive control | 11 mM in DMSO | 100 μM in acridine orange diluent | 10, 3, or 1 μM |
| Tamoxifen | Sample | 4 mM in DMSO | 100 μM in acridine orange diluent | 10, 3, 1, 0.3, or 0.1 μM |
DMSO, dimethyl sulfoxide.
References
- 1.Fox J.G. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 2.Goldenring J.R. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 3.Nomura S. Am J Physiol Gastrointest Liver Physiol. 2004;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa M. Dig Dis Sci. 2006;51:431–439. doi: 10.1007/s10620-006-3151-x. [DOI] [PubMed] [Google Scholar]
- 5.Nam K.T. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh W.J. Gastroenterology. 2012;142:21–24.e7. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crothers J.M., Jr. Am J Physiol. 1993;265:G231–G241. doi: 10.1152/ajpgi.1993.265.2.G231. [DOI] [PubMed] [Google Scholar]
- 8.Mamiya N. Biochem Biophys Res Commun. 1993;195:608–615. doi: 10.1006/bbrc.1993.2089. [DOI] [PubMed] [Google Scholar]
- 9.Keeley T.M. Cell Mol Gastroenterol Hepatol. 2019;8:365–367. doi: 10.1016/j.jcmgh.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen C.P. Gastroenterology. 2014;146:1727–1738. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference
- 1.Lapierre L.A. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1249–G1262. doi: 10.1152/ajpgi.00505.2006. [DOI] [PubMed] [Google Scholar]