Abstract
Free-living amoeba (FLA) such as Acanthamoeba, Naegleria, Balamuthia, and Vermamoeba have been identified from both natural and human-made environments such as Hot springs and spa. Naegleria fowleri causes Primary Amoebic Meningoencephalitis (PAM), while Acanthamoeba and Balamuthia cause chronic granulomatous encephalitis. Acanthamoeba also can cause cutaneous lesions and Amoebic Keratitis (AK) that is associated with contact lens use or corneal trauma. FLA are known to serve as host of and vehicles for diverse intracellular organisms. This study aimed was to identify the presence of FLA in the hot springs and beaches of the Caspian Sea in Ramsar tourist town located in the northern part of Iran. Water samples were collected in sterile bottles and were transferred to the laboratory. One litre of each sample passed through the nitrocellulose membrane filter. Each filter insert was then placed in non-nutrient agar plates already seeded with lawn culture of Escherichia coli. Positive samples were analyzed by morphological keys and Polymerase chain reaction (PCR) using 18S rDNA gene and ITS region to identify amoeba isolates. A total of 81 water sampled were tasted. After identified using the morphological key and PCR assay, 54 (66.6%) of the samples were positive for FLA. Ten of the samples were identified as Acanthamoeba (belong to T3, T4, and T5 genotypes), three as Vermamoeba vermiformis, four as Naegleria (3 N.australiensis and 1 N.grubery). Only one sample was positive Vahlkampfia. The presence of thermotolerant FLA in the Hot springs and beaches of the Caspian Sea as places for recreational purposes or wellness may be a potential health risk.
Keywords: Free-living amoeba, Ramsar, Hot spring, Caspian Sea
1. Introduction
Free-living amoebae (FLA) are protozoa widely distributed throughout the world that can survive and replicate in the environment without a host. FLA are present in a large variety of natural habitats and human-made ecosystems, such as rivers, lakes, swimming pools, Hot springs and spa (Martinez, 1985; Teixeira et al., 2009). Among the many genera of FLA amoebae in nature, only four genera have an association with human and animal's disease, Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri and Sappinia diploidea. N.fowleri and Several species of Acanthamoeba can cause central nervous system disability called primary amoebic meningoencephalitis (PAM) and granulomatous amoebic encephalitis (GAE), respectively. PAM is a devastating infection of the brain caused by the N. fowleri and cases have been reported from several countries (Abrahams-Sandí et al., 2015). Until now, 47 different Naegleria spp. are described. Species of the same genus, N. australiensis, and N. italica are natural pathogens of laboratory mice, rats, and rabbits. We don't have any information about another genus of Neglaria (De Jonckheere, 2014). Among the many genera of FLA that exist in nature, Naegleria spp., Acanthamoeba spp., Vermamoeba vermiformis and Balamuthia mandrillaris isolated from hot springs (Latifi et al., 2017; Niyyati et al., 2016; Latifi et al., 2016). Acanthamoeba is a prevalent genus of FLA in recreational waters also can cause cutaneous lesions and Amoebic Keratitis (AK) that is associated with contact lens use or corneal trauma. (Schuster and Visvesvara, 2004; Visvesvara et al., 2007). AK infection of the eye that typically occurs in healthy persons and can result in permanent visual impairment or blindness (Visvesvara et al., 2007). Risk factors such as a history of trauma, contact lens wear, swimming or hot spring use with contact lenses inserted, were documented (Mathers et al., 1998; Kaji et al., 2005). The current molecular classification divides Acanthamoeba spp. Into 21 genotypes (T1–T21), based on nucleotide sequence variations in the 18S rRNA gene (Corsaro et al., 2017). FLA such as Acanthamoeba spp. and Vermamoeba vermiformis are known to serve as host of and vehicles for diverse intracellular organisms such as Mimivirus, legionella pneumophila and others endosymbionts (Siddiqui and Khan, 2012; Pagnier et al., 2015). Hot spring water as natural treatment options for Musculoskeletal Pain and skin diseases in the world (Hao et al., 2011; Ozçelik et al., 2000; Yazdi et al., 2015). Ahmad et al., 2011; Dyková et al., 1999). Therefore, it is crucial to rapidly detect thermotolerant FLA in hot springs and similar recreational water environments where people swim and bathe because of their possible impact on human health. This study aimed to identify the presence of FLA in the hot springs and beaches of the Caspian Sea in Ramsar tourist town located in the northern part of Iran.
2. Material and methods
2.1. Sampling
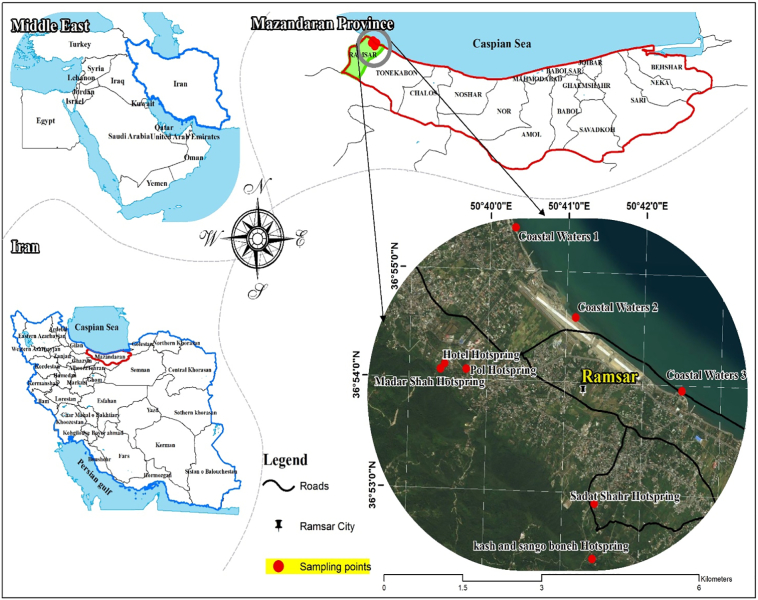
The samples described in the current study were collected from the five hot springs and closest recreational beaches to them in July 2018 (Fig. 1). Nine samples were collected from the surface of the water (<10 cm below), each with a sterilized 1.5-l bottle. A total of 13.5 l of water was collected from each beach and each hot spring. All bottles were transferred to Parasite Laboratory, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran for FLA identification. Temperature and pH values of hot springs and beaches were measured using a thermometer and digital pH meter, respectively.
Fig. 1.
Location of hot springs and beaches close to the hot springs in the Ramsar tourist city, in northern Iran.
2.2. Filtration and cultivation
One litre of each sample was passed through a cellulose nitrate membrane filter (Sartoriu,pore size 0.45 μm), using a vacuum pump. The filters were immediately placed on 1.5% non-nutrient agar (NNA) medium. To enrich the cultural media, we added some heat-killed Escherichia coli K19. The plates were sealed with parafilm and then incubated at 35 °C for 30 days. Each sample was examined daily by the inverted microscopy to detect trophozoites or cysts of FLA (Yousuf et al., 2017).
2.3. Microscopic examination and cloning
Amoebae were morphologically identified according to taxonomic criteria (Page, 1988). Positive samples were purified to exclude fungi and bacterial populations. Only a few cysts were transferred to the fresh medium, and they were followed for several weeks.
2.4. DNA extraction and amplification
Finally, FLA colonies washed by PBS thoroughly; the suspended were centrifuged at 1000g for 10 min. Sediments were used for DNA extraction was performed using the MagNA Pure LC DNA Isolation Kit I (Roche Germany) without modification according to the manufacturers' instructions contained in the kit inserts. Amplification of FLA DNA was performed using the 18S rDNA gene and ITS region to identify amoeba isolates. Five sets of primers were used to detect various FLA, including Acanthamoeba spp. (primers: JDP1 5′-GGCCCAGATCGTTTACCGTGAA-3′ and JDP2 5′-TCTCACAAGCTGCTAGGGAGTCA3′ (Schroeder et al., 2001), Vahlkampfiids (ITS1,2 primers: F5’-GAACCTGCGTAGGGATCATTT-3′ and R 5’TTTCTTTTCCTCCCCTTATTA-3) (Pélandakis and Pernin, 2002) and Vermamoebae primers (NA1, 2 primers: NA1 5′-GCTCCAATAGCG TATATT AA-3′ and NA2 5′-AGAAAGAGCTATCAATCTGT-3′) (Lasjerdi et al., 2011), Balamuthia mandrillaris (Balspec16S, 5-CGCATGTATGAAGAAGACCA-3 and Balspec16Sr, 5-TTACCTATATAATTGTCGATACCA −3) (Booton et al., 2004). The PCR reaction was performed using Red master mix (Denmark), and 25 μl of the master mix were combined with DNA (10 ng), specific primer pairs and distilled water. The cycling condition was set as a pre-denaturation step for 3 min at 94 °C, followed by 35 repetitions at 94 °C for 35 s, annealing steps were at 56 °C, 56 °C, 56 °C and 58 °C for one min (for Acanthamoeba, Vahlkampfiids, Balamuthia and Vermamoeba, respectively), and an extension step at 72 °C for one min. The PCR products were visualized on a 1.5% agarose gel containing ethidium bromide and a 100 bp DNA ladder (Sina gen, Iran).
2.5. DNA sequencing
In addition to morphological and microscopic identifications, all positive samples in this study were also identified using the molecular method with specific primers for Acanthamoeba spp. Vahlkampfidea, Vermamoeba, Naegleria fowleri and Balamuthia mandrillaris. Because of the similarity of the results each site, some of these samples were selected and sent for sequencing. Purified PCR products were sequenced with an automatic sequencer by Bioneer (Daejeon, South Korea). The genes were blasted by BLAST (https://www.ncbi.nlm.nih.gov/Blast). Sequences obtained in this study were submitted to the GenBank database.
2.6. Temperature tolerance
Thermotolerance tests were performed to assay the pathogenicity of the positive isolates. For the thermotolerance test, trophozoites and cysts of positive FLA isolates were separately inoculated onto NNA medium (In the form of triplicate). Then each plate was incubated at three different temperatures (30, 37 and 44 °C). The density of amoebal growth on the plates was recorded during the7 days of incubation. (John and Howard, 1996) (Table 3). The growth of amoebas on plates was observed daily using an inverted microscope. Also, one of the plates was scraped daily and the number of amoebas was counted using Neobar slides.
Table 3.
Thermo tolerance test of the isolated Vahlkampfids, Acanthamoenba spp. and Vermamoeba sp.
| Code | Type of amoeba | 30°C | 37°C | 44°C |
|---|---|---|---|---|
| AL1 | Acanthamoeba (T4genotype) | + | + | - |
| AL2 | Acanthamoeba (T4genotype) | + | + | + |
| AL3 | Acanthamoeba (T4genotype) | + | + | + |
| AL4 | Acanthamoeba (T4genotype) | + | + | + |
| AL5 | Acanthamoeba (T4genotype) | + | + | + |
| AL6 | Acanthamoeba (T4genotype) | + | + | - |
| AL7 | Acanthamoeba (T4genotype) | + | + | + |
| AL8 | Acanthamoeba (T3genotype) | + | + | - |
| AL9 | Acanthamoeba (T5genotype) | + | + | - |
| AL10 | Acanthamoeba (T5genotype) | + | + | - |
| AL | N.australiensis | + | + | + |
| AL | N.australiensis | + | + | + |
| AL | N.grubery | + | + | + |
| AL | Vahlkampfia | + | + | - |
| ALV1 | Vermamoeba vermiformis | + | + | + |
| ALV2 | Vermamoeba vermiformis | + | + | + |
| ALV3 | Vermamoeba vermiformis | + | + | + |
3. Results
A total of 81 water samples were identified using morphological keys and PCR assay. After three days to 1 month of incubation, 54 (66.6%) of the 81 total water samples were positive for FLA (Fig. 2). Eighteen samples with sharp bands were selected from every hot spring and every beach and sent for sequencing analysis. Of the isolated amoebae, ten were identified as Acanthamoeba (belong to T3, T4, and T5 genotypes), three as Vermamoeba vermiformis, four as Naegleria (3 N.australiensis and 1 N.grubery). Only one sample was positive Vahlkampfia. The BLAST analysis of the sequences presented a high percentage of identity (98%–100%) and query coverage (91%– 99%) in comparison with the deposited genes in the GenBank database. Acanthamoeba spp. were the most common amoebae in the surveyed water samples. Acanthamoeba genotype T4 (isolates in 3 samples) have 100% homology with Acanthamoeba castellanii, Acanthamoeba genotype T5 (isolates in 1 sample) have98% homology with Acanthamoeba lenticulata and Acanthamoeba genotype T3 (isolates in 1 sample) have 98% homology with Acanthamoeba griffin. The Morphological survey and PCR assay failed to show any positive results for Balamuthia mandrillaris and N.fowleri. Table 1, Table 2 show the distribution rate of FLA in various water sources in the present study. The temperature and pH of the surveyed water are shown in Table 1. A total of eleven out of 17 were able to grow at high temperatures. The results of the thermotolerance assays are summarized in Table 3.
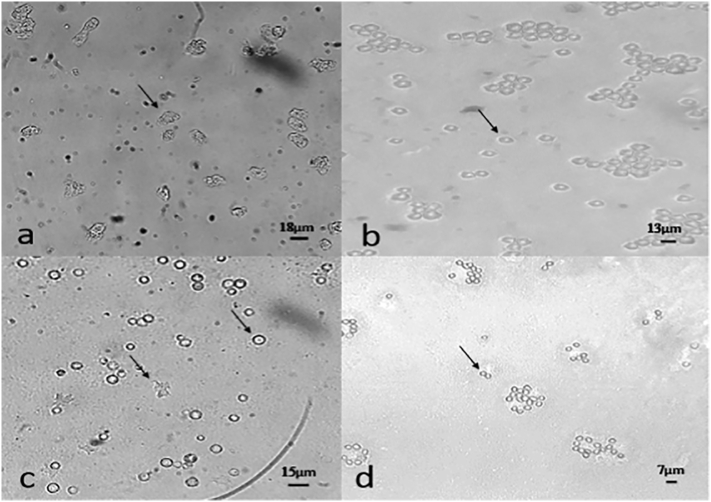
Fig. 2.
Free-living amoeba in NNA (magnification 20x) A) The trophozoites of Acanthamoeba spp. b) The Cysts of Acanthamoeba spp. c) The trophozoites and Cysts of Vahlkampfiids. d) The cysts of Vermamoeba sp.
Table 1.
Location and description of hot springs and data regarding isolated free-living amoeba from hot springs and coast of the Caspian Sea.
| Name of Hotspring And Sampling Site | Number of Samples | Number positive samples | Mixed Acanthamoeba spp. and Vahlkampfiids | Mixed Acanthamoeba spp. and Vermamoeba sp. | Acanthamoeba spp. | pH | Temperature °C |
|---|---|---|---|---|---|---|---|
| POL | 3/3 | 3/3 | 3 | 0 | 0 | 7/8 | 40 |
| Mother | 3/3 | 3/3 | 3 | 0 | 0 | 7/7 | 39 |
| Hotel | 3/3 | 2/3 | 2 | 0 | 0 | 7/9 | 44 |
| Sadat shahr (katalom) | 3/3 | 3/3 | 2 | 0 | 1 | 7/7 | 39/5 |
| Sang o Boneh | 3/3 | 2/3 | 0 | 2 | 0 | 7/5 | 38 |
| Kash | 3/3 | 2/3 | 1 | 1 | 0 | 7/5 | 38 |
| Coast 1 | 3/3 | 2/3 | 0 | 2 | 0 | 7 | 29 |
| Coast 2 | 3/3 | 0/3 | 0 | 0 | 0 | 7 | 29 |
| Coast 3 | 3/3 | 1/3 | 0 | 0 | 1 | 7 | 29 |
| Total | 27 | 18 (66.6%) | 11 | 5 | 2 | - | - |
Table 2.
Molecular data regarding isolated free-living amoeba from hot springs and coast of the Caspian Sea.
| Name of Hotspring And Sampling Site | Isolate code | Morphology | PCR (JDP1,2) | PCR (ITS1, 2) | PCR for N. fowleri | PCR (NA1,2) | Sequencing | Accession Number |
|---|---|---|---|---|---|---|---|---|
| Madar | AL1 | Acanthamoeba spp. | + | - | - | - | T4genotype | MH938694 |
| AL10 | Acanthamoeba spp. | + | - | - | - | T5genotype | MH938703 | |
| AL | Vahlkampfids | - | + | - | - | N.australiensis | MK034875 | |
| Pol | AL2 | Acanthamoeba spp. | + | - | - | - | T4genotype | MH938695 |
| Vahlkampfids | - | + | - | - | N.australiensis | MK034876 | ||
| Vahlkampfids | - | + | - | - | Vahlkampfia | MK034879 | ||
| Hotel | AL3 | Acanthamoeba spp. | + | - | - | - | T4genotype | MH938696 |
| AL | Vahlkampfids | - | + | - | - | N.grubery | MK034878 | |
| Sadat shahr (Katalom) | AL4 | Acanthamoeba spp. | + | - | - | - | T4genotype | MH938697 |
| AL | Vahlkampfids | - | + | - | - | N.australiensis | MK034877 | |
| Sang o Boneh | AL5 | Acanthamoeba spp. | + | - | - | - | T4genotype | MH938698 |
| AL9 | Acanthamoeba spp. | + | - | - | - | T5genotype | MH938702 | |
| AL1 | Vermamoeba sp. | - | - | - | + | Vermamoeba vermiformis | MH899918 | |
| Kash | AL6 | Acanthamoeba spp. | + | - | - | - | T4genotype | MH938699 |
| AL2 | Vermamoeba sp. | - | - | - | + | Vermamoeba vermiformis | MH899919 | |
| Coast 1 | AL7 | Acanthamoeba spp. | + | - | - | - | T4genotype | MH938700 |
| AL3 | Vermamoeba sp. | - | - | - | + | Vermamoeba vermiformis | MH899920 | |
| Coast 3 | AL8 |
Acanthamoeba spp. |
+ | - | - | - | T3genotype | MH938701 |
4. Discussion
Hot springs and spa are used for recreational purposes or wellness applications (Giampaoli and Romano Spica, 2014; Van Tubergen and van der Linden, 2002; Routh et al., 1996). The increasing the use of the sea for recreation has led to major concern regarding health hazards to both local and tourist populations (Fewtrell and Kay, 2015). The presence of potential pathogen free-living amoeba in hot springs and Seawater has been confirmed in most parts of the world (Huang and Hsu, 2010; Ozçelik et al., 2012; Booton et al., 2004; Latifi et al., 2017; Latifi et al., 2016; Badirzadeh et al., 2011; Niyyati et al., 2016). Previous reports have linked N. fowleri infections in the USA to water exposure in warm-weather states, particularly among young males (Visvesvara and Stehr-Green, 1990). N. fowleri has been frequently detected in hot spring water samples, and three case reports have been associated with the same hot spring (Sheehan et al., 2003; Seidel, 1985). Exposure to Acanthamoeba species is common due to its ubiquitous nature. In some cases, the source has been proven to be tap water, which is colonized with Acanthamoeba species, subsequently contaminating contact lenses, which serve as vectors (Kilvington et al., 2004). The major risk factors for developing AK comprise epithelial microtrauma, contact lens overuse, improper contact lens maintenance, contact lens wear in contaminated water such as the swimming pool, and exposure to contaminated water (Hammersmith, 2006). They are resistant to killing by freezing, desiccation, and chlorination commonly used in municipal water supplies, swimming pools, and hot tubs. (Khan, 2009). Ramsar is one the counties of Mazandaran province on the shore of Caspian Sea in northern Iran with beautiful sights and exquisite natural attractions, is considered as one of the top regions in the country for nature tourism and it attracts a great number of travellers and tourists every year. Ramsar is a popular sea resort and has hottest hot springs for therapeutic and recreational purposes. In this study for the first time, the Caspian coasts were examined for the presence of pathogenic amoebae. After morphological and molecular surveys, waters of these coasts were contaminated with Acanthamoeba genotype T4, T3, and Vermamoeba vermiformis. Hot springs are also contaminated with Acanthamoeba genotype T4, T5, N. australiensis, N. grubery, Vahlkampfia, and Vermamoeba vermiformis. The survey was conducted in the summer of 2018. another similar study was conducted by Latifi et al. (Autumn 2017) to identify Naegleria spp. and Balamuthia mandrillaris in all hot springs of Mazandaran province. Balamuthia mandrillaris was found in the Ramsar Hotel and Bridge hot springs (Latifi et al., 2017; Latifi et al., 2016). But in the present study, Balamuthia mandrillaris was not found in hot springs. About Naegleria spp., the bridge Hotsprings was still contaminated with N. australiensis, But in this current study, N. australiensis was isolated instead of N. fultoni from the Sadatshahr Hotspring (Katalom). In the present study, several hot springs that negative in the previous study, was positive for the presence of FLA. Due to fluctuations in temperature in sampling seasons. During this study, for the first time, Vahlkampfia was identified from the bridge hot springs of Ramsar city. Niyyti reported Vahlkampfia and Acanthamoeba genotype T3 of the cultured corneal epithelial cells and contact lenses of patients with contact lens-related AK (Niyyati et al., 2010). cytopathogenicity of Acanthamoeba, Vahlkampfia, and Vermamoeba sp. have been proven on keratocytes. Kinner et al. showed that these amoeba ability to produce a cytopathic effect on keratocytes was similar in magnitude and mechanism to that of the known pathogen Acanthamoeba castellanii (Kinnear, 2003). In other studies on the corneal scrape, specimens were obtained from patients with keratitis. These specimens were studied using electron microscopy and prepared pure axenic cultures. They showed the presence of Acanthamoeba, Hartmannella, and Vahlkampfia trophozoites and cysts. Some appeared smaller and morphologically distinct from Acanthamoeba and were identified as Vahlkampfia. (Aitken et al., 1996; Alexandrakis et al., 1998). In the present study, N. australiensis were isolated from three hot springs. N. fowleri is the only known human pathogen of the 30 Naegleria species that have been identified but, the pathogenesis of N. australiensis has been confirmed in laboratory animals (Latifi et al., 2017; Latifi et al., 2018). Other results of this study showed the presence of Acanthamoeba castellanii, this amoeba causes Granulomatous Acanthamoeba Encephalitis (GAE) and Acanthamoeba keratitis (AK) in animals and humans, with a healthy immune system (Sheng et al., 2009; Noorjahan, 2010). The other isolate was Acanthamoeba lenticulata Acanthamoeba lenticulata. This amoeba reported from a fatal case of disseminated acanthamebiasis caused by Acanthamoeba lenticulata (genotype T5) in a 39-year-old heart transplant recipient (Barete et al., 2007). It is also isolated from patients with keratitis (Van Zyl et al., 2013). The other isolate was Acanthamoeba griffini. Some cases of keratitis have been reported by Acanthamoeba griffin (Heredero-Bermejo et al., 2015; Ledee et al., 1996). This amoeba is also isolated from the ocean sediments (Liu et al., 2006). Vermamoeba vermiformis was one of the other amoebae identified in our study. Vermamoeba vermiformis can act as hosts to several different microorganisms that may coexist simultaneously (Delafont et al., 2018; Slimani et al., 2013). Vermamoeba vermiformis has been isolated from a corneal biopsy sample in a contact lens wearer alone and combination with Acanthamoeba species (Aitken et al., 1996; Inoue et al., 1998; Lorenzo-Morales et al., 2007; Abedkhojasteh et al., 2013). The results of the thermotolerance tests showed that most FLA in this study were thermotolerant. These are indirect factors related to pathogenicity (Khan, 2001). This feature enables the amoeba to resist normal body temperature or even fever in the host. Moreover, the growth of amoebae at temperatures above 40 °C is directly correlated to their ability to produce cellular damage in vitro (Griffin, 1972; Walochnik et al., 2000). The presence of thermotolerant FLA in the Hot springs and beaches of the Caspian Sea as places for recreational purposes or wellness may be a potential health risk. So it is necessary to the water of hot springs as an environment for the growth of pathogen free-living amoeba, filter and clean before use. Warning signs shall be installed next to these hot springs to inform people about the dangers of these FLA. Also before getting into these hot springs, use of swimming glasses and nosepieces.
References
- Abedkhojasteh H., Niyyati M., Rahimi F., Heidari M., Farnia S., Rezaeian M. First report of Hartmannella keratitis in a cosmetic soft contact lens wearer in Iran. Iran. J. Parasitol. 2013;8(3):481–485. [PMC free article] [PubMed] [Google Scholar]
- Abrahams-Sandí E., Retana-Moreira L., Castro-Castillo A., Reyes-Batlle M., Lorenzo-Morales J. Fatal meningoencephalitis in child and isolation of Naegleria fowleri from hot springs in Costa Rica. Emerg. Infect. Dis. 2015;21(2):382–384. doi: 10.3201/eid2102.141576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A.F., Andrew P.W., Kilvington S. Development of a nested PCR for environmental detection of the pathogenic free-living amoeba Balamuthia mandrillaris. J. Eukaryot. Microbiol. 2011;58(3):269–271. doi: 10.1111/j.1550-7408.2011.00541.x. [DOI] [PubMed] [Google Scholar]
- Aitken D., Hay J., Kinnear F.B., Kirkness C.M., Lee W.R., Seal D.V. Amebic keratitis in a wearer of disposable contact lenses due to a mixed Vahlkampfia and Hartmannella infection. Ophthalmology. 1996;103(3):485–494. doi: 10.1016/s0161-6420(96)30667-2. [DOI] [PubMed] [Google Scholar]
- Alexandrakis G., Miller D., Huang A.J. Amebic keratitis due to Vahlkampfia infection following corneal trauma. Arch. Ophthalmol. 1998;116(7):950–951. [PubMed] [Google Scholar]
- Badirzadeh A., Niyyati M., Babaei Z., Amini H., Badirzadeh H., Rezaeian M. Isolation of free-living amoebae from Sarein hot springs in Ardebil province, Iran. Iran. J. Parasitol. 2011;6(2):1–8. [PMC free article] [PubMed] [Google Scholar]
- Barete S., Combes A., de Jonckheere J.F., Datry A., Varnous S., Martinez V., Ptacek S.G., Caumes E., Capron F., Francès C., Gibert C., Chosidow O. Fatal disseminated Acanthamoeba lenticulata infection in a heart transplant patient. Emerg. Infect. Dis. 2007;13(5):736–738. doi: 10.3201/eid1305.061347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booton G.C., Rogerson A., Bonilla T.D., Seal D.V., Kelly D.J., Beattie T.K., Tomlinson A., Lares-Villa F., Fuerst P.A., Byers T.J. Molecular and physiological evaluation of subtropical environmental isolates of Acanthamoeba spp., causal agent of Acanthamoeba keratitis. J Eukaryot Microbiol. Apr. 2004;51(2):192–200. doi: 10.1111/j.1550-7408.2004.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Corsaro D., Köhsler M., Di Filippo M.M., Venditti D., Mon DiCave D., Berrilli F., Walochnik J. Update on Acanthamoeba jacobsi genotype T15, including full-length 18S rDNA molecular phylogeny. Parasitol. Res. 2017;116(4):1273–1284. doi: 10.1007/s00436-017-5406-1. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J.F. What do we know by now about the genus Naegleria? Exp. Parasitol. 2014;145:S2–S9. doi: 10.1016/j.exppara.2014.07.011. Suppl. [DOI] [PubMed] [Google Scholar]
- Delafont V., Rodier M.H., Maisonneuve E., Cateau E. Vermamoeba vermiformis: a free-living amoeba of interest. Microb. Ecol. 2018;76(4):991–1001. doi: 10.1007/s00248-018-1199-8. [DOI] [PubMed] [Google Scholar]
- Dyková I., Lom J., Schroeder-Diedrich J.M., Booton G.C., Byers T.J. Acanthamoeba strains isolated from organs of freshwater fishes. J. Parasitol. 1999;85(6):1106–1113. [PubMed] [Google Scholar]
- Fewtrell L., Kay D. Recreational water and infection: a review of recent findings. Curr Environ Health Rep. 2015;2(1):85–94. doi: 10.1007/s40572-014-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampaoli S., Romano Spica V. Health and safety in recreational waters. Bull. World Health Organ. 2014;92(2):79. doi: 10.2471/BLT.13.126391. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.L. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science. 1972;178(4063):869–887. doi: 10.1126/science.178.4063.869. [DOI] [PubMed] [Google Scholar]
- Hammersmith K.M. Diagnosis and management of Acanthamoeba keratitis. Curr. Opin. Ophthalmol. 2006;17(4):327–331. doi: 10.1097/01.icu.0000233949.56229.7d. [DOI] [PubMed] [Google Scholar]
- Hao W., Wang X., Xiang Y., Gu Li A.M., Li M., Zhang X. History of hot spring bath treatment in China. Zhonghua Yi Shi Za Zhi. 2011;41(4):235–239. [PubMed] [Google Scholar]
- Heredero-Bermejo I., Criado-Fornelio A., De Fuentes I., Soliveri J., Copa-Patiño J.L., Pérez-Serrano J. Characterization of a human-pathogenic Acanthamoeba griffini isolated from a contact lens-wearing keratitis patient in Spain. Parasitology. 2015;142(2):363–373. doi: 10.1017/S0031182014001140. [DOI] [PubMed] [Google Scholar]
- Huang S.W., Hsu B.M. Isolation and identification of Acanthamoeba from Taiwan spring recreation areas using culture enrichment combined with PCR. Acta Trop. 2010;115(3):282–287. doi: 10.1016/j.actatropica.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Inoue T., Asari S., Tahara K., Hayashi K., Kiritoshi A., Shimomura Y. Acanthamoeba keratitis with symbiosis of Hartmannella ameba. Am J. Ophthalmol. 1998;125(5):721–723. doi: 10.1016/s0002-9394(98)00026-9. [DOI] [PubMed] [Google Scholar]
- John D.T., Howard M.J. Techniques for isolating thermotolerant and pathogenic freeliving amebae. Folia Parasitol (Praha) 1996;43(4):267–271. [PubMed] [Google Scholar]
- Kaji Y., Hu B., Kawana K., Oshika T. Swimming with soft contact lenses: danger of acanthamoeba keratitis. Lancet Infect. Dis. 2005;5(6):392. doi: 10.1016/S1473-3099(05)70143-2. [DOI] [PubMed] [Google Scholar]
- Khan N.A. Pathogenicity, morphology, and differentiation of Acanthamoeba. Curr. Microbiol. 2001;43(6):391–395. doi: 10.1007/s002840010325. [DOI] [PubMed] [Google Scholar]
- Khan N.A. 1st edn. Caister Academic Press; 2009. Acanthamoeba, Biology and Pathogenesis. (Norwich, UK Kilvington S, Gray T, Dart J, et al. Acanthamoeba keratitis: The role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci 2004; 45: 165-9) [Google Scholar]
- Kilvington S., Gray T., Dart J., Morlet N., Beeching JR., Frazer DG., Matheson M. Acanthamoeba keratitis: the role of domestic tap contamination in the United Kingdom. Invest. Ophthalmol. Vis. Sci. 2004;45(1):165–169. doi: 10.1167/iovs.03-0559. [DOI] [PubMed] [Google Scholar]
- Kinnear F.B. Cytopathogenicity of acanthamoeba, vahlkampfia and hartmannella: quantative & qualitative in vitro studies on keratocytes. J. Inf. Secur. 2003;46(4):228–237. doi: 10.1053/jinf.2002.1116. [DOI] [PubMed] [Google Scholar]
- Lasjerdi Z., Niyyati M., Haghighi A., Shahabi S., Biderouni F.T., Taghipour N., Eftekhar M., Nazemalhosseini Mojarad E. Potentially pathogenic free-living amoebae isolated from hospital wards with immunodeficient patients in Tehran, Iran. Parasitol. Res. 2011;109(3):575–580. doi: 10.1007/s00436-011-2288-5. [DOI] [PubMed] [Google Scholar]
- Latifi, A, R., Niyyati, M., Lorenzo-Morales, J., Haghighi, A., Seyyed Tabaei, S.J., Lasjerdi, Z., 2016. Presence of Balamuthia mandrillaris in hot springs from Mazandaran province, northern Iran. Epidemiol. Infect. 144 (11): 2456–61. [DOI] [PMC free article] [PubMed]
- Latifi A.R., Niyyati M., Lorenzo-Morales J., Haghighi A., Tabaei S.J., Lasjerdi Z., Azargashb Occurrence of Naegleria species in therapeutic geothermal water sources, Northern Iran. Acta Parasitol. 2017;62(1):104–109. doi: 10.1515/ap-2017-0012. 1. [DOI] [PubMed] [Google Scholar]
- Latifi A., Niyyati M., Seyyed Tabaei S.J., Tahvildar Biderouni F., Haghighi A., Lasjerdi Z. An experimental model of primary amoebic Meningoence phalitis due to Naegleria australiensis in Iran. Iran. J. Parasitol. 2018;13(3):369–372. [PMC free article] [PubMed] [Google Scholar]
- Ledee D.R., Hay J., Byers T.J., Seal D.V., Kirkness C.M. Acanthamoeba griffini. Molecular characterization of a new corneal pathogen. Invest. Ophthalmol. Vis. Sci. 1996;37(4):544–550. [PubMed] [Google Scholar]
- Liu H., Ha Y.R., Lee S.T., Hong Y.C., Kong H.H., Chung D.I. Genetic diversity of Acanthamoeba isolated from ocean sediments. Korean J Parasitol. 2006;44(2):117–125. doi: 10.3347/kjp.2006.44.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Morales J., Martínez-Carretero E., Batista N., Álvarez-Marín J., Bahaya Y., Walochnik J., Valladares B. Early diagnosis of amoebic keratitis due to a mixed infection with Acanthamoeba and Hartmannella. Parasitol. Res. 2007;102(1):167–169. doi: 10.1007/s00436-007-0754-x. [DOI] [PubMed] [Google Scholar]
- Martinez A.J. CRC Press Inc; Boca Raton, FL: 1985. Free-Living Amebas: Natural History Prevention, Diagnosis, Pathology, and Treatment of Disease. [Google Scholar]
- Mathers W.D., Sutphin J.E., Lane J.A., Folberg R. Correlation between surface water contamination with amoeba and the onset of symptoms and diagnosis of amoeba-like keratitis. Br. J. Ophthalmol. 1998;82:1143–1146. doi: 10.1136/bjo.82.10.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyyati M., Lorenzo-Morales J., Rezaie S., Rahimi F., Martín-Navarro C.M., Mohebali M., Maghsood A.H., Farnia S., Valladares B., Rezaeian M. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp. Parasitol. 2010;126(1):89–90. doi: 10.1016/j.exppara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Niyyati M., Saberi R., Latifi A., Lasjerdi Z. Distribution of Acanthamoeba genotypes isolated from recreational and therapeutic geothermal water sources in southwestern Iran. Environ Health Insights. 2016;10:69–74. doi: 10.4137/EHI.S38349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorjahan P. Pathogenesis of Acanthamoeba keratitis. Ocul Surf. 2010;8(2):70–79. doi: 10.1016/s1542-0124(12)70071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozçelik S., Polat H.H., Akyol M., Yalçin A.N., Ozçelik D., Marufihah M. Kangal hot spring with fish and psoriasis treatment. J. Dermatol. 2000;27(6):386–390. doi: 10.1111/j.1346-8138.2000.tb02188.x. [DOI] [PubMed] [Google Scholar]
- Ozçelik S., Coşkun K.A., Yünlü O., Alim A., Malatyalı E. The prevalence, isolation and morphotyping of potentially pathogenic free-living amoebae from tap water and environmental water sources in Sivas. Turkiye Parazitol Derg. 2012;36(4):198–203. doi: 10.5152/tpd.2012.48. [DOI] [PubMed] [Google Scholar]
- Pagnier I., Valles C., Raoult D., La Scola B. Isolation of Vermamoeba vermiformis and associated bacteria in hospital water. Microb. Pathog. 2015;80:14–20. doi: 10.1016/j.micpath.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Pélandakis M., Pernin P. Use of multiplex PCR and PCR restriction enzyme analysis for detection and exploration of the variability in the free-living amoeba Naegleria in the environment. Appl. Environ. Microbiol. 2002;68(4):2061–2065. doi: 10.1128/AEM.68.4.2061-2065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh H.B., Bhowmik K.R., Parish L.C., Witkowski J.A. Balneology, mineral water, and spas in historical perspective. Clin. Dermatol. 1996;14:551–554. doi: 10.1016/s0738-081x(96)00083-1. [DOI] [PubMed] [Google Scholar]
- Schroeder J.M., Booton G.C., Hay J., Niszl I.A., Seal D.V., Markus M.B., Fuerst P.A., Byers T.J. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001;39(5):1903–1911. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster F.L., Visvesvara G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004;34(9):1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Seidel J. Primary amebic meningoencephalitis. Pediatr. Clin. N. Am. 1985;32:881–892. doi: 10.1016/s0031-3955(16)34860-x. [DOI] [PubMed] [Google Scholar]
- Sheehan K.B., Fagg J.A., Ferris M.J., Henson J.M. PCR detection and analysis of the free-living amoeba Naegleria in hot springs in Yellowstone and Grand Teton National Parks. Appl. Environ. Microbiol. 2003;69(10):5914–5918. doi: 10.1128/AEM.69.10.5914-5918.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W.H., Hung C.C., Huang H.H., Liang S.Y., Cheng Y.J., Ji D.D., Chang S.C. First case of granulomatous amebic encephalitis caused by Acanthamoeba castellanii in Taiwan. Am J Trop Med Hyg. 2009;81(2):277–279. [PubMed] [Google Scholar]
- Siddiqui R., Khan N.A. Biology and pathogenesis of Acanthamoeba. Parasit. Vectors. 2012;10(5):6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimani M., Pagnier I., Raoult D., La Scola B. Amoebae as battlefields for bacteria, giant viruses, and virophages. J. Virol. 2013;87(8):4783–4785. doi: 10.1128/JVI.02948-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L.H., Rocha S., Pinto R.M., Caseiro M.M., Costa S.O. Prevalence of potentially pathogenic free-living amoebae from Acanthamoeba and Naegleria genera in non-hospital, public, internal environments from the city of Santos, Brazil. Braz. J. Infect. Dis. 2009;13(6):395–397. doi: 10.1590/s1413-86702009000600001. [DOI] [PubMed] [Google Scholar]
- Van Tubergen A., van der Linden S. A brief history of spa therapy. Ann. Rheum. Dis. 2002;61:273–275. doi: 10.1136/ard.61.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zyl L.M., Andrew N., Chehade M., Sadlon T.A., Badenoch P.R. Acanthamoeba lenticulata keratitis in a hard contact lens wearer. Clin. Exp. Ophthalmol. 2013;41(8):810–812. doi: 10.1111/ceo.12104. [DOI] [PubMed] [Google Scholar]
- Visvesvara G.S., Stehr-Green J.K. Epidemiology of free-living ameba infections. J Protozool. 1990;37(4):25S–33S. doi: 10.1111/j.1550-7408.1990.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Visvesvara G.S., Moura H., Schuster F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- Walochnik J., Obwaller A., Aspöck H. Correlations between morphological, molecular biological and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Am Soc Microbiol. 2000;66(10):4408–4413. doi: 10.1128/aem.66.10.4408-4413.2000. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi M., Taheri M., Navi P. Environmental geochemistry and sources of natural arsenic in the Kharaqan hot springs, Qazvin, Iran. Environ. Earth Sci. 2015;73:5395–5404. [Google Scholar]
- Yousuf F.A., Siddiqui R., Khan N.A. Presence of rotavirus and free-living amoebae in the water supplies of Karachi, Pakistan. Rev. Inst. Med. Trop. Sao Paulo. 2017;1(59):e32. doi: 10.1590/S1678-9946201759032. [DOI] [PMC free article] [PubMed] [Google Scholar]