Abstract
Background & Aims
Colonic musculature contain smooth muscle cells (SMC), interstitial cells of Cajal (ICC), and platelet-derived growth factor receptor α+ cells (PDGFRα+ cells), which are electrically coupled and operate together as the SIP syncytium. PDGFRα+ cells have enriched expression of small conductance Ca2+-activated K+ (SK) channels. Purinergic enteric neural input activates SK channels in PDGFRα+ cells, hyperpolarizes SMC, and inhibits colonic contractions. Recently we discovered that PDGFRα+ cells in mouse colon have enriched expression of α1A adrenoceptors (ARs), which coupled to activation of SK channels and inhibited colonic motility, and α1A ARs were principal targets for sympathetic regulation of colonic motility. Here we investigated whether PDGFRα+ cells in human colon express α1A ARs and share the roles as targets for sympathetic regulation of colonic motility.
Methods
Isometric tension recording, intracellular recording, and Ca2+ imaging were performed on muscles of the human colon. Responses to α1 ARs agonists or electric field stimulation with AR antagonists and neuroleptic reagents were studied.
Results
Exogenous or endogenous norepinephrine released from nerve fibers inhibited colonic contractions through binding to α1A ARs or enhanced colonic contractions by acting on α1D ARs. Inhibitory responses were blocked by apamin, an antagonist of SK channels. Phenylephrine, α1 AR agonists, or norepinephrine increased intracellular [Ca2+] in PDGFRα+ cells, but not in ICC, and hyperpolarized SMCs by binding to α1 ARs expressed by PDGFRα+ cells.
Conclusions
Human colonic contractions are inhibited by α1A ARs expressed in PDGFRα+ cells and activated by α1D ARs expressed in SMC.
Keywords: PDGFRα+ Cells, Colonic Motility, Sympathetic Nervous System, α1 Adrenoceptor, SIP Syncytium
Abbreviations used in this paper: Ach, acetylcholine; ADP, adenosine diphosphate; ARs, adrenoceptors; AUC, area under the curve; CM, circular muscle; EFS, electrical field stimulation; Epi, epinephrine; FBD, functional bowel disorders; ICC, interstitial cells of Cajal; L-NNA, N-nitro-L-arginine methyl ester hydrochloride; NE, norepinephrine; PDGFRα+ cells, platelet-derived growth factor receptor α+ cells; PE, phenylephrine; SK channels, small conductance Ca2+-activated K+ channels; SMC, smooth muscle cells; SPCs, spontaneous phasic contractions; TPM, transcripts per kilobase million; TTX, tetrodotoxin
Graphical abstract
Summary.
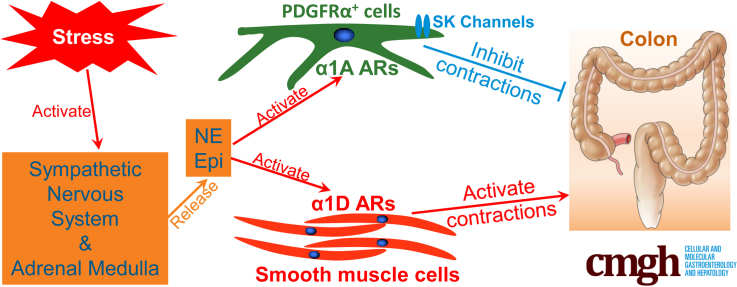
Norepinephrine inhibits colonic contractions by α1A ARs expressed in PDGFRα+ cells and stimulates contractions by α1D ARs expressed in SMC. The dual effects of norepinephrine may be the physiological background underlying diverse responses of colonic motility to stressful occurrences.
Colonic musculature is composed of 3 types of cells, smooth muscle cells (SMC), interstitial cells of Cajal (ICC), and platelet-derived growth factor receptor α+ cells (PDGFRα+ cells), which are electrically coupled and operate together as a minimal motor unit known as the SIP syncytium.1, 2, 3 ICC and PDGFRα+ cells are interstitial cells located between intrinsic and extrinsic nerves and SMC, have similar distributions, and form distinct networks in all layers of the tunica muscularis (eg, circular muscle, myenteric plexus, and longitudinal muscle).4, 5, 6 The interstitial cells wrap around nerve fibers and myenteric ganglia in mouse and human colons, express receptors for enteric neurotransmitters, and perform neurotransduction to assist in the coordination of colonic contractions.2,3,5,6 PDGFRα+ cells have enriched expression of small conductance Ca2+-activated K+ (SK) channels, through which hyperpolarization responses are generated when intracellular [Ca2+] increases.5,7 The hyperpolarization responses developed in PDGFRα+ cells are conveyed to SMC via gap junctions, and the hyperpolarization of SMC reduces the open probability of voltage-dependent (L-type) Ca2+ channels and inhibits contractions of SMC.8 Purinergic signaling, which is one of major enteric inhibitory neurotransductions in the gut and a dominant inhibitory neural signaling in the distal colon, uses mechanisms expressed by PDGFRα+ cells to provide inhibitory regulation of colonic motility.5,7, 8, 9, 10
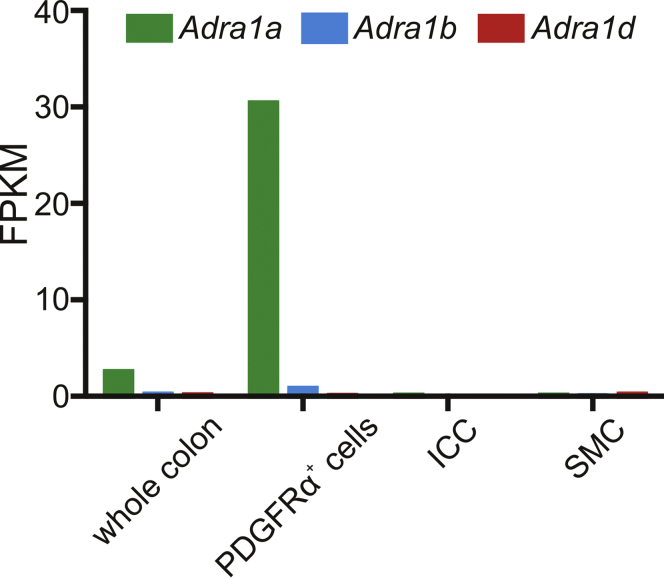
Analysis of transcriptomes of each type of murine SIP cell11, 12, 13 showed that α1 adrenoceptors (ARs), especially α1A ARs, were expressed exclusively by PDGFRα+ cells (Figure 1). Therefore, we investigated the functional roles of α1A ARs in PDGFRα+ cells and discovered that binding of α1A AR agonists activated SK channels, hyperpolarized PDGFRα+ cells, and inhibited colonic motility.14 Our data suggested that this novel post-synaptic signaling pathway was the principal mechanism of sympathetic regulation of colonic motility in mice, which contrasted with the long-held dogma that inhibition of cholinergic enteric motor neurons via α2 ARs was the dominant mechanism of sympathetic neural regulation.14, 15, 16, 17, 18 Human colon also displays an abundance of PDGFRα+ cells, with distributions of these cells and enriched expression of SK channels similar to the mouse colon.7 Therefore, we hypothesized that PDGFRα+ cells in the human colon might also express α1A ARs, have coupling between α1A ARs and activation of SK channels, and share a similar role in sympathetic regulation of colonic motility as in the mouse. Such a hypothesis may mean that PDGFRα+ cells are responsible for inhibition of colonic motility under the stress by which sympathetic nervous system is activated.19 This pathway might be a promising target for treating functional bowel disorders (FBD), especially irritable bowel syndrome with predominant constipation and functional constipation.20, 21, 22, 23, 24, 25
Figure 1.
Bar graph depicting expression profile of the genes of adrenergic receptor α1 (AR α1) family created from transcriptome data of SIP syncytium of mouse colon that we published in 2015–2017.11, 12, 13 Fragments per kilobase of transcript per million (FPKM) of AR α1A, α1B, and α1D are 30.41, 0.80, and 0.07 in PDGFRα+ cells, 0.11, 0.00, and 0.00 in ICC, and 0.13, 0.06, and 0.21 in SMC, respectively.
In this study we recorded contractile activity from human colonic muscles, measured electrical responses using intracellular electrical recording, and monitored intracellular Ca2+ transients by using cell permeable, fluorescent Ca2+ indicators and video imaging. Our results show that α1A ARs are expressed by PDGFRα+ cells, and α1D ARs are expressed by SMCs. Norepinephrine (NE) elicits inhibitory effects via α1A ARs and excitatory effects via α1D ARs on human colonic contractions. These novel mechanisms may help to explain the variable responses of colonic motility to the stress.
Results
Norepinephrine Modulates Spontaneous Phasic Contractions of Colonic Circular Muscle via α1 Adrenoceptors
No specific antibodies against α1 ARs appear to be available,26 so expression of these receptors in human colon was determined by interrogation of published transcriptome data. Transcripts per kilobase million (TPM) of α1 ARs, ADRA1A (α1A), ADRA1B (α1B), and ADRA1D (α1D) were 0.6, 0.5 and 0.3, respectively, (www.proteinatlas.org)27; thus, all subtypes of α1 ARs appear to be expressed in human colon.
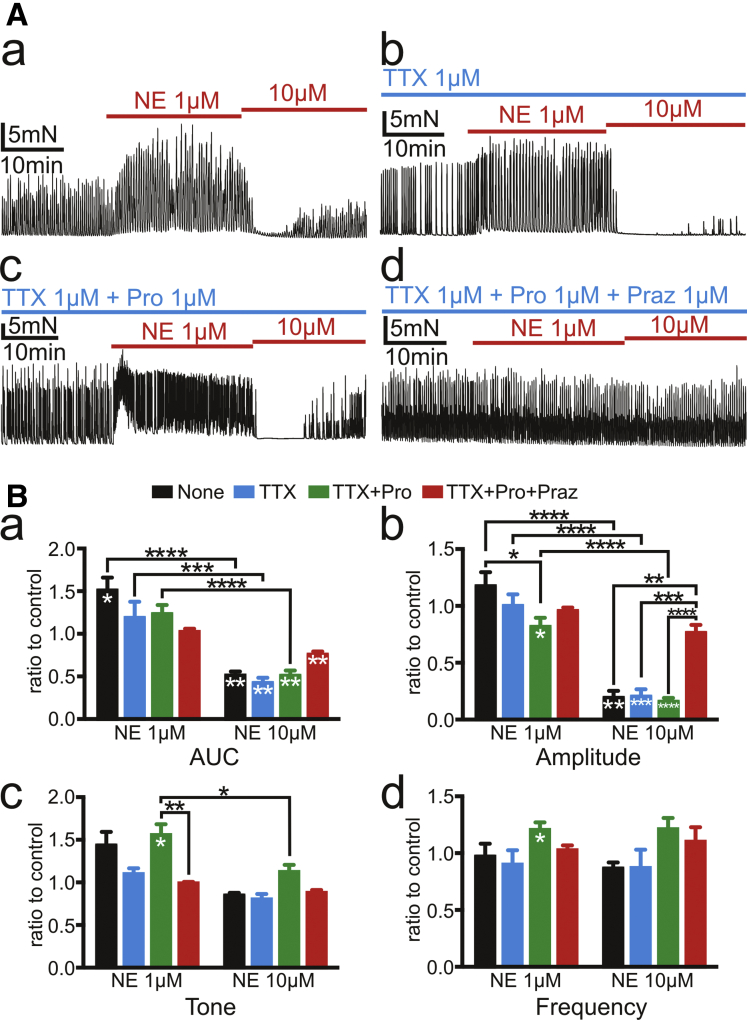
The effects of exogenous NE (1 and 10 μmol/L) on spontaneous phasic contractions (SPCs) of circular muscle (CM) strips of human sigmoid colon were investigated. NE (1 μmol/L) increased the amplitude of SPCs with an increase in the basal tone resulting in increased area under the curve (AUC) (n = 4; Figure 2Aa and black bars in Figure 2Ba–c). To eliminate enteric neural influence induced by NE on SPCs, tetrodotoxin (TTX) (1 μmol/L), a neurotoxin, was applied, and action potentials of all neural fibers were blocked. TTX (1 μmol/L) did not affect responses to NE (1 μmol/L) significantly (n = 5; Figure 2Ab and black and blue bars in Figure 2Ba), suggesting that neurotransmission was not significantly involved in the effects of NE (1 μmol/L), even though α2 ARs expressed by enteric motor neurons have been thought to affect the release of enteric motor neurotransmitters.28
Figure 2.
Tension recordings of CM strips of human sigmoid colon. (A) NE 1 μmol/L activated SPCs and NE 10 μmol/L inhibited them (a) regardless of the presence of TTX 1 μmol/L (b). Propranolol 1 μmol/L (Pro) enhanced the elevation of tone by NE but reduced the increments of amplitude of contractions by NE (c). Prazosin (Praz) 1 μmol/L blocked responses of muscle strips to NE (d). (B) Summary of 4 parameters, AUC, amplitudes, tone, and frequency of SPCs for 10 minutes after applying NE 1 μmol/L and 10 μmol/L is shown by ratio to controls for 10 minutes before applying NE in the same recordings. Black asterisks (∗) indicate statistically significant differences between the values connected by black lines, and white asterisks indicate statistically significant differences of the values against controls. The numbers of asterisks indicate the following: ∗.05 > P ≥ .01; ∗∗.01 > P ≥ .001; ∗∗∗.001 > P ≥ .0001; ∗∗∗∗.0001 > P.
In the presence of propranolol (1 μmol/L), β AR antagonist, NE (1 μmol/L) accelerated SPCs and caused a larger increase in basal tone (n = 11; Figure 2Ac and green bars in Figure 2Bc and Bd), demonstrating that responses mediated by β ARs partially counteract the excitatory effects of NE (1 μmol/L). Prazosin (1 μmol/L), α1 AR antagonist, inhibited the excitatory effects of NE (1 μmol/L) on the colonic contractions (n = 5; Figure 2Ad and green and red bars in Figure 2Bc), suggesting that the excitatory effects of NE (1 μmol/L) were mediated by α1 ARs expressed in SIP syncytium.
A higher concentration of NE (10 μmol/L) suppressed SPCs (n = 4; Figure 2Aa and black bar in Figure 2Ba and Bb), and this effect was blocked by prazosin (1 μmol/L) (n = 5; Figure 2Ad and red bar in Figure 2Bb). Neither TTX (1 μmol/L) (n = 5) nor propranolol (1 μmol/L) (n = 11) affected the inhibitory effects of NE (10 μmol/L) (Figure 2Ab and Ac and blue and green bars in Figure 2Bb), suggesting these inhibitory effects were also mediated by α1 ARs in SIP cells, but not α2 ARs or β ARs. Summary for these experiments is shown in Figure 2B. Actual values of AUC, amplitude, tone, and frequency are in Supplementary Table 1.
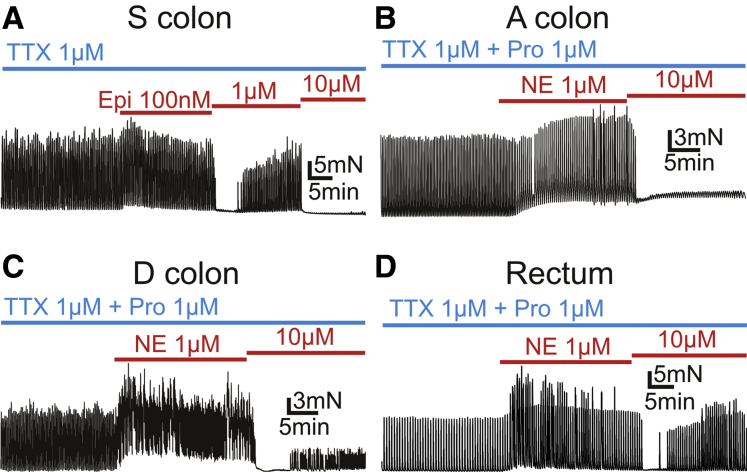
Epinephrine (Epi) exerted similar dual effects on SPCs as NE did (n = 3), and the similar effects of NE were also observed in ascending (A) (n = 1) and descending (D) (n = 2) colon and rectum (n = 1) (Figure 3), indicating that modulation of contractions by NE is consistent in various regions of the colon.
Figure 3.
Tension recordings of CM strips of sigmoid colon (S colon) (A), ascending colon (A colon) (B), descending colon (D colon) (C), and rectum (D). (A) Epinephrine (Epi) 100 nmol/L activated the spontaneous contractions of muscle strips, but Epi 1 μmol/L and 10 μmol/L inhibited them in dose-dependent manner under existence of TTX 1 μmol/L. (B–D) A and D colon and rectum also showed similar responses to NE to sigmoid colon, in which NE 1 μmol/L activated tonic contractions of muscle strips and NE 10 μmol/L inhibited amplitude of contractions under existence of TTX 1 μmol/L and propranolol (Pro) 10 μmol/L. In (B), A colon looked to have the tonic contraction increased in dose-dependent manner, while it had the amplitude of contractions inhibited by NE 10 μmol/L. In (D), rectum looked to have the amplitude of contractions increased as well as the tonic contractions by NE 1 μmol/L.
Roles of α1A and α1D Adrenoceptors in Adrenergic Responses
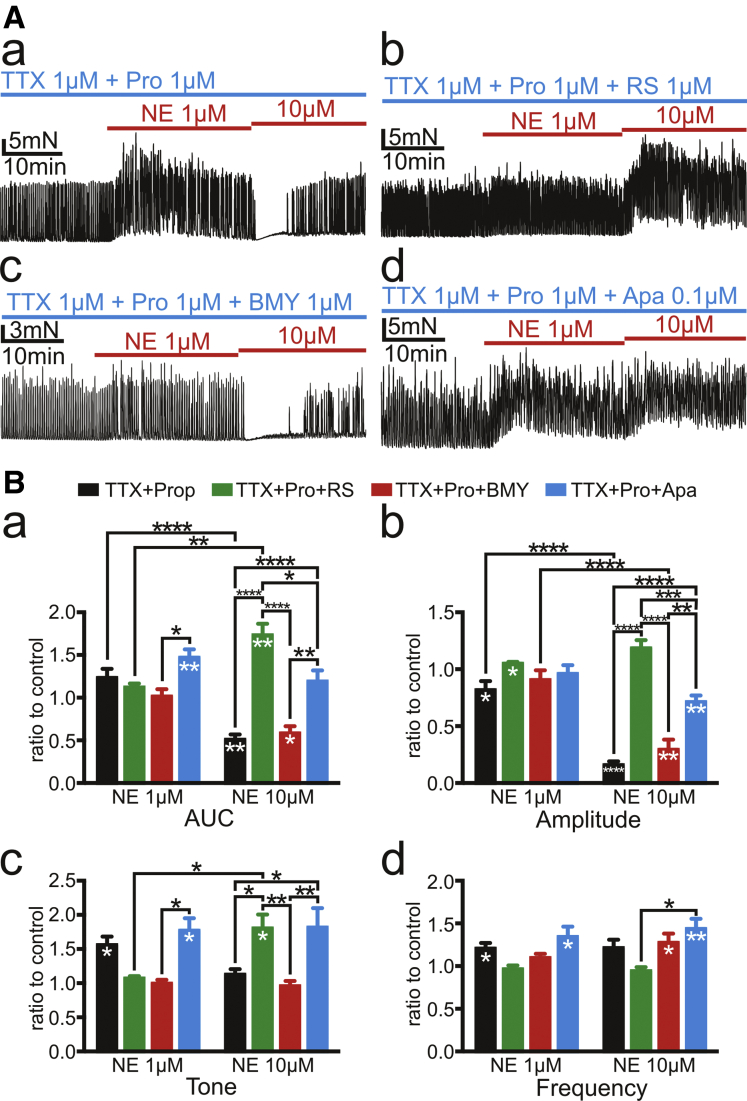
Two of α1 AR subtypes, α1A ARs and α1D ARs, were investigated in the presence of TTX and propranolol for possible roles in the dual effects of NE. We focused on these receptors because α1A ARs are exclusively expressed in mouse PDGFRα+ cells,14 and α1A ARs and α1D ARs have been reported to have important roles in lower urinary tract symptoms such as benign prostatic hyperplasia.29 RS100329 (1 μmol/L), an α1A AR antagonist (pKi of α1A, α1B, and α1D are 9.6 ± 0.1, 7.5 ± 0.1, and 7.9 ± 0.1, respectively),30 blocked the inhibitory effects of NE (10 μmol/L) on SPCs (n = 8; Figure 4Ab and black and green bars in Figure 4Ba and Bb). Under these conditions, basal tone increased in response to NE (10 μmol/L) (n = 8; black and green bars in Figure 4Bc). These findings suggest that the inhibitory actions of NE were mediated dominantly by α1A ARs. In the presence of BMY 7378 (1 μmol/L), an α1D AR antagonist (pKi of α1A, α1B, and α1D were 6.6 ± 0.20, 7.2 ± 0.05, and 9.4 ± 0.05, respectively),31 NE (1 μmol/L) failed to increase basal tone (n = 8; Figure 4Ac and red bars in Figure 4Bc), and NE (10 μmol/L) inhibited SPCs (n = 8; Figure 4Ac and red bars in Figure 4Ba and Bb). These findings suggest that the excitatory actions of NE were mediated predominantly by α1D ARs. Summary is shown in Figure 4B. Actual values of 4 parameters, AUC, amplitude, tone, and frequency are in Supplementary Table 1.
Figure 4.
Tension recordings of CM strips of sigmoid colon in the presence of TTX 1 μmol/L and propranolol (Pro) 1 μmol/L. (A) RS100329 (RS) 1 μmol/L and apamin (Apa) 0.1 μmol/L blocked inhibitory effects on amplitude of contractions by NE 10 μmol/L and revealed excitatory effects on tone by that (b and d). BMY7378 (BMY) 1 μmol/L did not block inhibitory effect on amplitude of contractions of NE 10 μmol/L but inhibited excitatory effects of NE 1 μmol/L in tone (c). (B) Summary of 4 parameters of responses of SPCs for 10 minutes after applying NE 1 μmol/L and 10 μmol/L is displayed using ratio to the controls as described in Figure 2. Black asterisks (∗) indicate statistically significant difference between the values connected by black line, and white asterisks indicate statistically significant difference of the values against controls. The numbers of asterisks indicate the following: ∗.05 > P ≥ .01; ∗∗.01 > P ≥ .001; ∗∗∗.001 > P ≥ .0001; ∗∗∗∗.0001 > P.
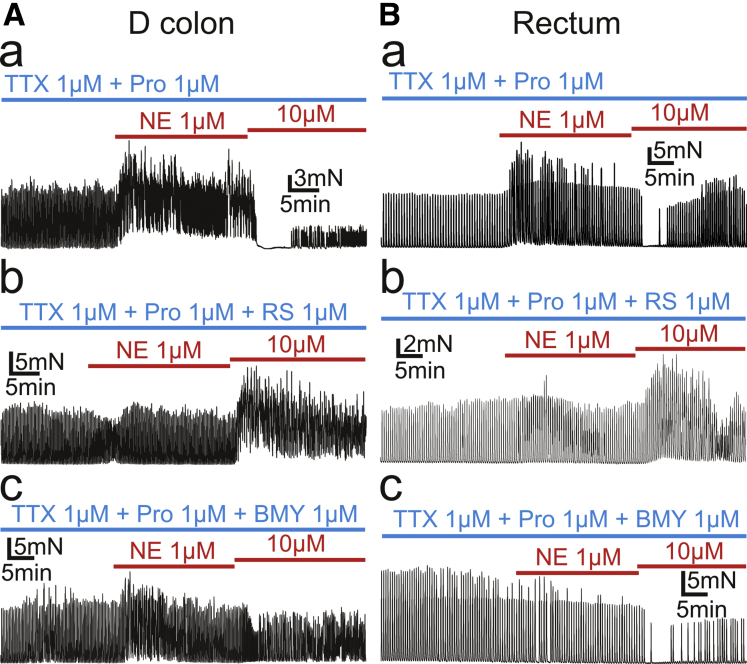
RS100329 and BMY7378 exerted similar actions on NE responses in descending (D) colon (n = 1) and rectum (n = 1) (TTX and propranolol present; Figure 5). Muscles from 11 of 12 patients (transverse colon, 1; descending colon, 1; sigmoid colon, 9; rectum, 1) displayed the same patterns of responses to RS100329 or BMY7378. However, RS100329 failed to block the NE (10 μmol/L)-induced suppression of SPCs in 1 patient.
Figure 5.
Tension recordings of CM muscle strips of D colon (A) and rectum (B) were performed. Both D colon and rectum showed similar responses to NE under the presence of RS100329 (RS) 1 μmol/L or BMY7378 (BMY) 1 μmol/L to S colon, in which RS inhibited inhibitory effects by NE 10 μmol/L and revealed excitatory effects by that (Ab and Bb), and BMY inhibited excitatory effects by NE 1 μmol/L (Ac and Bc).
Roles of Small Conductance Ca2+-Activated K+ Channels in Norepinephrine-Mediated Suppression of Spontaneous Phasic Contractions
SK3 channels are dominant among all SK channels in human colon (TPM of SK1, SK2, and SK3 are 0.1, 0.5 and 3.3, respectively) (www.proteinatras.org).27 SK3 channels are expressed exclusively in human PDGFRα+ cells.7 Therefore, suppression of SPCs by NE (10 μmol/L) is likely to be mediated via the α1A AR-SK channel signaling pathway in PDGFRα+ cells, which was observed in murine colon.14 The involvement of SK channels in NE responses was tested with apamin (0.1 μmol/L), SK channel specific antagonist, which suppressed the inhibitory effects of NE (10 μmol/L) on SPCs (n = 8; Figure 4Ad and black and blue bars in Figure 4Ba and Bb) and unmasked the excitatory effects of NE (10 μmol/L), similar to the effects of RS100329 (n = 8; Figure 4Ab and Ad and black, green, and blue bars in Figure 4Bc).
Mechanisms Involved in Sympathetic Nerve-Mediated Modulation of Spontaneous Phasic Contractions
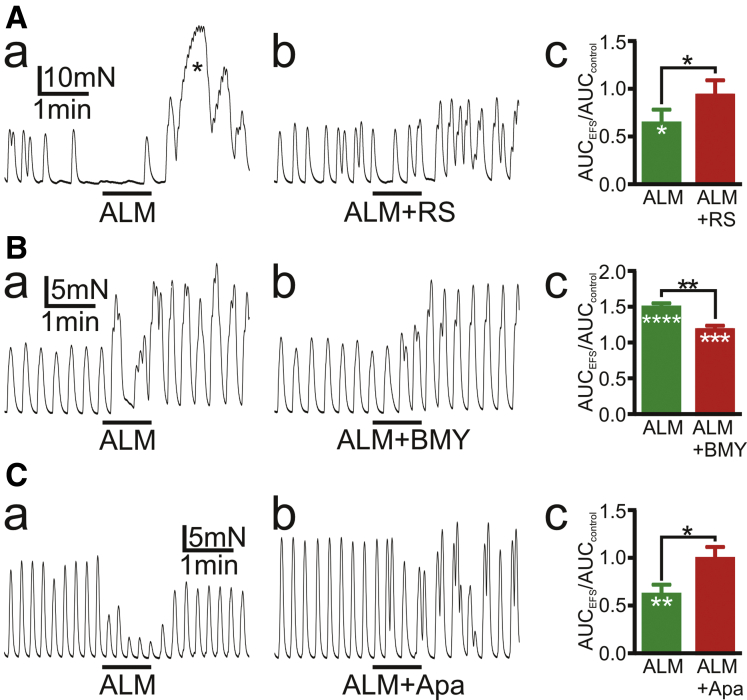
Electrical field stimulation (EFS) (100 V, 5 Hz, 50-microsecond pulse duration for 1 minute) was applied to determine whether endogenous NE, released from sympathetic nerves, modulates SPCs of CM strips of sigmoid colon. These experiments were performed in the presence of antagonists for major enteric neurotransmitters (atropine, 1 μmol/L; L-NNA [N-nitro-L-arginine methyl ester hydrochloride], 100 μmol/L; MRS2500, 500 nmol/L). This cocktail of antagonists is abbreviated as ALM in figures. Reagents used in Figure 4 were tested on responses induced by EFS (Figure 6). Experiments were performed on 49 muscle strips from 23 patients: EFS induced excitatory responses in 23 strips from 17 patients, inhibitory responses in 17 strips from 11 patients, no response in 8 strips from 6 patients, and a mixed response (initial excitatory followed by an inhibitory response) in 1 muscle strip. RS100329 (1 μmol/L; n = 3) or apamin (100 nmol/L; n = 5) attenuated inhibitory responses to EFS (Figure 6Aa and Ca) in 8 muscle strips from 7 patients (Figure 6Ab, Ac, Cb, and Cc). Interestingly, in the example in Figure 6A a robust rebound excitation occurred on cessation of EFS, and this response was blocked by RS100329 (Figure 6Aa and Ab), which suggested that α1A ARs hyperpolarized smooth muscles during EFS.32 BMY7378 (1 μmol/L) inhibited excitatory responses to EFS (n = 6; Figure 6Ba) in muscle strips from 6 patients (Figure 6Bb and Bc). BMY7378 (1 μmol/L) failed to abolish all excitatory responses to EFS, because this stimulus may also trigger release of excitatory peptides. EFS at frequencies higher than 5 Hz was not evaluated because release of excitatory peptides was likely to obscure responses to endogenous NE. These data suggest that NE released from sympathetic nerves inhibits SPCs through the α1A AR-SK channel signaling pathway in PDGFRα+ cells and enhances them through α1D ARs.
Figure 6.
Tension recordings of CM strips of sigmoid colon.Black bars represent EFS with 50-millisecond duration and 100 V at 5 Hz for 1 minute. Responses of SPCs to EFS in the presence of antagonists of main neurotransmitters, atropine 1 μmol/L, L-NNA 100 μmol/L, and MRS2500 500 nmol/L (ALM), were recorded. EFS induced inhibitory effects (Aa and Ca) or excitatory effects (Ba) on SPCs. Inhibitory effects of EFS on SPCs were attenuated by RS100329 (RS) 1 μmol/L (Ab) or apamin (Apa) 0.1 μmol/L (Cb). In (A), EFS evoked rebound excitation immediately after EFS as indicated by asterisk ∗ in (Aa), which were inhibited by RS. Excitatory effects of EFS on SPCs (Ba) were inhibited by BMY7378 (BMY) 1 μmol/L (Bb). Ac, Bc, and Cc depict the summary of AUC during EFS for 1 minute divided by AUC of control SPCs for 1 minute. Black asterisks (∗) indicate statistically significant difference between the values connected by black line, and white asterisks indicate statistically significant difference of the values against controls. AUC values (means ± standard error) (mN·min) were (Ac) ALM, 4.37 ± 0.37; ALM + RS, 5.96 ± 0.95; (Bc) ALM, 9.91 ± 0.31; ALM + BMY, 7.64 ± 0.29; and (Cc) ALM, 3.10 ± 0.56; ALM + apamin, 6.86 ± 1.04. The numbers of asterisks indicate the following: ∗.05 > P ≥ .01; ∗∗.01 > P ≥ .001; ∗∗∗.001 > P ≥ .0001; ∗∗∗∗.0001 > P.
Intracellular Ca2+ Responses in Platelet-Derived Growth Factor Receptor α+ Cells Are Mediated by α1 Adrenoceptors
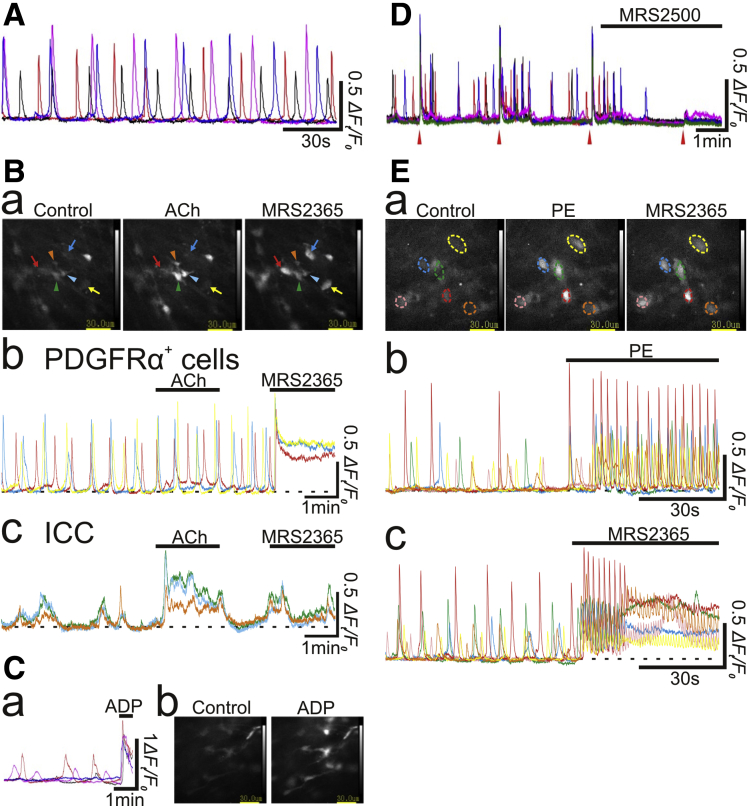
Ca2+ signaling in human colonic muscles was explored by using imaging studies of muscles loaded with Cal-520 AM (see Methods). Nifedipine (10 μmol/L) was used to suppress muscle contractions and stabilize fields of view during imaging. A population of cells was displaying spontaneous asynchronous Ca2+ transients in human colonic muscles. These cells had spindle or stellate morphologies and were distinct from SMCs (Figure 7A, Supplementary Video 1). Spontaneous Ca2+ transients occurred at the frequency of 2.1 ± 0.17 min-1, with a mean amplitude of 0.70 ± 0.06 ΔFt/F0 and half-width of 5.2 ± 1.0 s (n = 25, N = 18). Occasionally, another population of cells was observed that exhibited spontaneous synchronous Ca2+ transients. Basal Ca2+ levels in the “asynchronous” cells increased to 0.81 ± 0.08 ΔFt/F0 in response to MRS2365, a P2Y1 purinoceptor agonist (10 nmol/L; n = 9, N = 6; arrows in leftmost and rightmost panels of Figure 7Ba and Bb, Supplementary Video 2), and in some cases Ca2+ oscillations were superimposed (Figure 7Bb). This population of cells showed no response to acetylcholine (Ach) (10 μmol/L; n = 4, N = 3; arrows in leftmost and middle panels of Figure 7Ba and Bb, Supplementary Video 3). Adenosine diphosphate (ADP) (100 μmol/L) also increased Ca2+ levels by 1.7 ± 0.23 ΔFt/F0 of the asynchronous cells (n = 6, N = 3, n = 6, N = 3; Figure 7C, Supplementary Video 4). These characteristics of stellate morphology, spontaneous asynchronous Ca2+ transients, enhanced Ca2+ transients in response to P2Y1 agonists and ADP, and lack of response to ACh are signatures for PDGFRα+ cells, which are abundant in human colonic muscles.5, 6, 7, 8,33 In contrast, cells with synchronous Ca2+ transients responded to ACh (10 μmol/L) but not MRS2365 (100 nmol/L) (arrowheads in Figure 7Ba and Bc, Supplementary Videos 2 and 3), suggesting these cells were ICC.34,35
Figure 7.
Ca2+ imaging in human colonic muscles. (A) In a muscle preparation, 4 PDGFRα+ cells generated developed asynchronous spontaneous Ca2+ transients independently from each other. (B) In a preparation where PDGFRα+ cells generated asynchronous spontaneous Ca2+ transients (b), ICC exhibited synchronous spontaneous Ca2+ transients within their cluster (c). PDGFRα+ cells responded to MRS2365 100 nmol/L but not ACh 1 μmol/L (arrows in a and b), whereas ICC responded to ACh 1 μmol/L but not MRS2365 100 nmol/L (arrowheads in a and c). Graph of Ca2+ signals picked in each of cells pointed by color arrows or arrowheads in (a) were depicted in (b) and (c) in the same color as that of arrow or arrowhead. (C) In a preparation where PDGFRα+ cells generated asynchronous spontaneous Ca2+ transients, ADP 100 μmol/L evoked increases in basal Ca2+ level (a and b). (D) In a preparation where PDGFRα+ cells generated asynchronous spontaneous Ca2+ transients, EFS (20 Hz for 1 second) triggered synchronous increases in basal Ca2+ level (red arrowheads in D). MRS2500 500 nmol/L largely suppressed EFS-induced Ca2+ transients and also prevented generation of spontaneous Ca2+ transients. (E) In a preparation where PE 10 μmol/L caused increases in basal Ca2+ level associated with superimposed Ca2+ oscillations in several cells (middle panel in a and b), MRS2365 100 nmol/L evoked sustained increases in basal Ca2+ level in the same cells (right panel in a and c). Graph of Ca2+ signals picked in each of color circles in (a) were depicted in (b) and (c) in the same color as that of the circle.
Spontaneous asynchronous Ca2+ transients were enhanced and coordinated in response to EFS, indicating the cells were functionally innervated (Figure 7D, Supplementary Video 5). Ca2+ transients activated by EFS were suppressed or abolished by MRS2500, a P2Y1 purinoceptor antagonist (1 μmol/L; n =13, N = 11; Figure 7D, Supplementary Video 6) but unaffected by atropine (1 μmol/L) or L-NNA (10 μmol/L) (data not shown), suggesting that like PDGFRα+ cells in the mouse gastrointestinal tract,8 PDGFRα+ cells in human colon also receive and transduce purinergic neurotransmission.
Phenylephrine (PE) (10 μmol/L) induced sustained and/or oscillatory increases in Ca2+ transients in PDGFRα+ cells (leftmost and middle panels of Figure 7Ea and Eb, Supplementary Video 7). Cells responsive to PE (10 μmol/L) also responded to MRS 2365 (100 nmol/L) (Figure 7Ea and Ec, Supplementary Video 8), verifying that PDGFRα+ cells express functional α1 ARs. The increase in basal Ca2+ in response to PE had the amplitude of 0.41 ± 0.07 ΔFt/F0, n =13, N = 12).
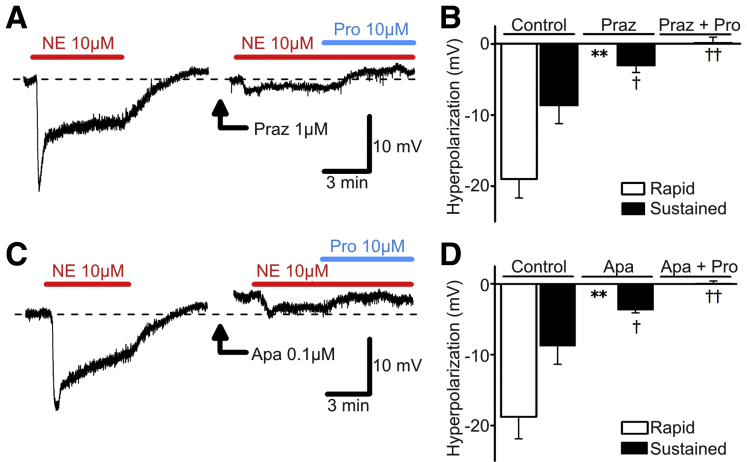
α1 Adrenoceptor Agonists Hyperpolarize Smooth Muscle Cells Through Small Conductance Ca2+-Activated K+ Channels
The α1 AR agonists mediate inhibitory contractile effects via the α1A AR-SK channel signaling pathway in PDGFRα+ cells, and P2Y1 agonists and PE enhance Ca2+ transients in cells identified as PDGFRα+ cells by functional criteria (see above). These observations suggest that sympathetic inhibitory effects via α1 AR would be caused by hyperpolarization of cells in the SIP syncytium. This hypothesis was tested by using intracellular electrical recording from human colonic muscles. Human sigmoid colon CM cells had resting membrane potentials averaging –47 ± 3.0 mV (n = 16). NE (10 μmol/L) evoked rapid and sustained components of hyperpolarization of CM cells (Figure 8). Prazosin (1 μmol/L) greatly reduced both components of hyperpolarization caused by NE (n = 4; Figure 8A and B). The residual hyperpolarization in response to NE was inhibited by propranolol (10 μmol/L) (n = 4; Figure 8A and B). Apamin (0.1 μmol/L) depolarized cells by 2.1 ± 0.7 mV (n = 4) and inhibited hyperpolarization responses to NE (n = 4; Figure 8C and D). Propranolol (10 μmol/L) inhibited the residual hyperpolarization (n = 4) in the presence of apamin (Figure 8C and D). These data confirmed that NE activated SK channels via α1 ARs in PDGFRα+ cells, leading to hyperpolarization of SMCs.
Figure 8.
Effects of NE on membrane potentials of human S colon circular SMCs. Application of NE 10 μmol/L induced a two-phase hyperpolarization, a rapid component followed by a sustained component. (A) NE-induced two-phase hyperpolarization was changed to small, sustained hyperpolarization by pretreatment of prazosin (Praz) 1 μmol/L. Residual hyperpolarization was inhibited by propranolol (Pro) 10 μmol/L. (B) Summarized bar graphs showing effects of Praz and Pro on NE-induced two-phase hyperpolarization. ∗∗P < .01, significant difference from control responses of rapid component. †P < .05, significant difference from control responses of sustained component. ††P < .01, significant difference from sustained responses in presence of Praz alone. (C) Apamin (Apa) 0.1 μmol/L inhibited two-phase hyperpolarization induced by NE, resulting in sustained hyperpolarization, which was inhibited by Pro 10 μmol/L. (D) Summary showing effects of Apa and Pro on NE-induced two-phase hyperpolarization. ∗∗P < .01, significant difference from control responses of rapid component. †P < .05, significant difference from control responses of sustained component. ††P < .01, significant difference from sustained responses in presence of Apa alone. Resting membrane potentials were A, −46 mV; C, −49 mV. A and C were recorded from different tissues. Each record in a given set of two was obtained from the same impalement.
Discussion
In this study we demonstrated motor regulation of human colonic contractions mediated by α1 ARs. Although the functional roles of α2 and β ARs in physiology and diseases of colonic motility have been extensively studied, less attention has been paid to α1 ARs.25,28,36 The lack of detailed information about α1 ARs in the neurogastroenterological research is due in part to the lack of specific antibodies against these receptors that can be used for immunohistochemistry.26 Additional confusing observations showed variability in responses in which some studies reported inhibitory effects mediated by α1 ARs,36 and others showed excitatory effects.37 Our study helps to clarify the role of α1 ARs in human colon by showing that the contrasting responses are mediated by different receptors expressed by different cells.
The α1 ARs are G protein-coupled receptor associated with Gq/11 or G12/13 subunit, which, when activated, lead to increased intracellular [Ca2+] or activation of the Rho-kinase pathway.38,39 Hence, the functional roles of α1 ARs in the SIP syncytium depend on the cell type expressing α1 ARs. The α1 ARs in SMCs would enhance colonic contractions either by increasing intracellular [Ca2+] or activating the Rho-kinase pathway.39 In ICC, α1 ARs would provide an excitatory signal by increasing [Ca2+], activation of Ca2+-activated Cl– channels (ANO1), and depolarize and contract SMCs.40 In contrast, α1 ARs expressed by PDGFRα+ cells would suppress colonic contractions via the α1A AR-SK channel signaling pathway and electrical coupling that convey hyperpolarization responses to SMC as shown in the mouse colon.14 In this study, functional expression of α1A ARs in human PDGFRα+ cells was scrutinized by tension recordings, Ca2+ imaging in situ, and intracellular electrical recordings. First, in tension recordings, NE 10 μmol/L showed inhibitory effects on SPCs via the α1A AR-SK channel signaling pathway (Figure 4). Second, Ca2+ imaging in situ validated that PDGFRα+ cells, identified by responses to P2Y1 agonists, developed Ca2+ transients in response to α1 AR agonists (Figure 7). Finally, intracellular electrical recordings confirmed that NE 10 μmol/L hyperpolarized SMC via α1 AR and SK channels (Figure 8). These data conclude that human PDGFRα+ cells express α1A ARs. On the other hand, because α1 AR agonists did not develop Ca2+ transients in ICC identified by responses to ACh in Ca2+ imaging in situ (Figure 7) and α1 ARs activation failed to depolarize SMCs even after antagonism of SK channels in intracellular electrical recordings (Figure 8), expression of α1 ARs in ICC is likely to be marginal. This finding suggests that the excitatory effects of NE via α1D ARs shown in tension recordings are generated by the activation of SMC, which means that SMC express α1D ARs. The α1 ARs on PDGFRα+ cells and SMCs can be activated by either neuronal or hormonal Epi and NE (Figure 9). In the presence of antagonists of both α1A and α1D ARs, NE (1 and 10 μmol/L) had no effect on SPCs (data not shown). Thus, expression of α1B ARs in SIP cells is not functionally significant.
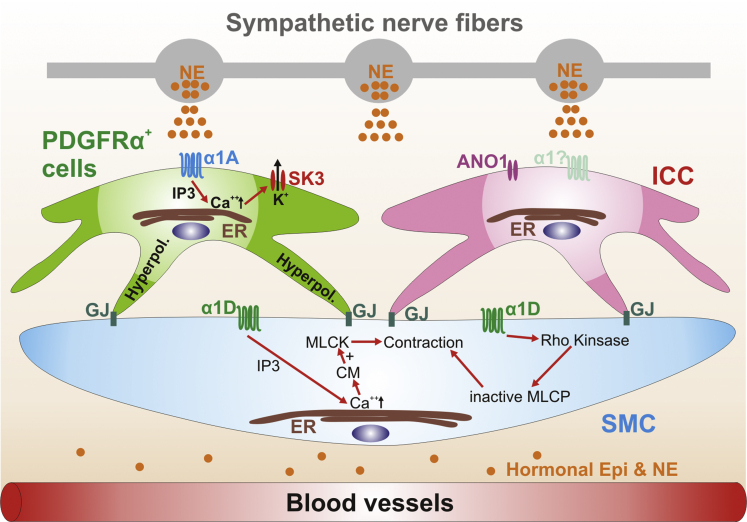
Figure 9.
Schematic diagram of the new concept based on this study. ARs are expressed on PDGFRα+ cells. Neuronal or hormonal NE or Epi, via binding to and activating α1A ARs in PDGFRα+ cells, opens SK3 channels through increasing intracellular [Ca2+] by inositol triphosphate (IP3) and hyperpolarize (Hyperpol) them. Hyperpolarization of PDGFRα+ cells is propagated to SMC via gap junctions (GJ) and inhibits contractions of them. α1D ARs are expressed by SMC. Neuronal or hormonal NE or Epi, via binding to and activating α1D ARs on SMC, can make myosin light chain kinase (MLCK) activated and SMC contract through activation of calmodulin (CM) via increase of intracellular [Ca2+] by IP3 or can activate Rho kinase pathway and inactivate myosin light chain phosphatase (MLCP), which leads to contractions of SMC. ICC might not express α1 ARs. ANO1, anoctamin-1, Ca2+ -activated Cl– channels. Altogether, neuronal or hormonal NE or Epi can inhibit human colonic contractions via α1A AR-SK channel signaling pathway in PDGFRα+ cells and excite them via α1D AR on SMC.
The inhibitory effects on SPCs mediated by α1A ARs were mainly due to effects on amplitude but not frequency. ICC or neural inputs transduced by ICC are responsible for the rhythm of SPCs.41 Therefore, hyperpolarization generated in PDGFRα+ cells by α1A AR-SK channel signaling pathway is likely to suppress the increase of intracellular [Ca2+] in SMCs and inhibit the amplitude of SPCs but might not affect ICC significantly. It should be noted that the potency of apamin in blocking the inhibitory effects of NE was significantly weaker than the effects of RS100329 (Figure 4Bb). This result is likely due to the fact that apamin does not quantitively block the human SK conductance,42 but RS100329 (1 μmol/L) results in strong block of α1A AR,30 which would prevent activation of SK channels in response to NE.
Excitatory effects of NE on colonic contractions were dominant at 1 μmol/L, whereas inhibitory effects dominated at 10 μmol/L. If equivalent to mouse expression profiles for α1 AR family (Figure 1), levels of α1A ARs expression on PDGFRα+ cells might be higher than α1D ARs on SMCs in human colon. However, PDGFRα+ cells are a minor population of cells relative to SMCs, which is based on our immunohistochemical studies of human colon.6 Therefore, the excitatory effects of NE mediated by SMCs may outcompete inhibitory effects developed in PDGFRα+ cells during lower levels of stimulation. However, higher levels of sympathetic stimulation may raise substantial levels of NE and recruit the powerful inhibitory responses via the α1A AR-SK channel signaling pathway in PDGFRα+ cells. Obviously, the integrated response to sympathetic input will depend on many factors, including accessibility of transmitter to populations of cells.
Sympathetic nerve fibers project to and form a complex network in the plane of the myenteric plexus and around arterioles but are sparse in muscle layer in human colon; thus, one might question whether NE reaches effective concentration amidst colonic muscle bundles in vivo.28 However, it should be noted that PDGFRα+ cells also form a dense network of cells in the plane of the myenteric plexus,6 where varicosities of sympathetic nerve fibers are plentiful. PDGFRα+ cells form close associations with nerve fibers; therefore during sympathetic activity, they could be exposed to high local concentrations of NE.1 In the present study, NE released by only 5 Hz EFS exerted both excitatory and inhibitory actions on SPCs of human colonic muscles. Therefore, because sympathetic nerve fibers are likely to be excited at more than 10 Hz in vivo,43 the colonic musculature should be exposed to NE enough to induce dual effects via α1 ARs in vivo.
In this study, the relative contributions of α1, α2, and β ARs effects to the sympathetic neural regulation of human colon were not investigated quantitatively. However, TTX did not affect NE effects on colonic SPCs significantly, although α2 ARs have been reported to inhibit excitatory motor neurons (Figure 2).28 Also, in the absence of β AR blocker, EFS inhibited or excited colonic SPCs, and α1A AR selective antagonist, RS100329, or α1D AR selective antagonist, BMY7378, significantly attenuated EFS induced inhibition or excitation, respectively (Figure 6). These findings suggest that in human colon NE effects mediated by α1 AR are dominant, and α2 and β AR effects are not sufficient to mask α1 AR effects, which is similar to the hierarchy of ARs in mouse colon.14 In addition, the effect of exogenous NE at the presence of TTX and the responses to endogenous NE released from sympathetic nerve fibers by EFS in the presence of enteric neurotransmitter antagonists were identical and inhibited by the same antagonists. These data argue against the possibility that the responses of colonic muscle strips in this study might be induced by other neurotransmitters released from nerve endings by presynaptic α1 ARs in a TTX-insensitive manner.
We demonstrated a novel mechanism by which stressful experiences might lead to either increased colonic contractions through α1D ARs or reduced contractions via α1A ARs. These dual effects of sympathetic stimulation may have relevance to the varied symptoms observed in patients with FBD. For example, some patients may have overexpression or overactivation of α1A ARs in PDGFRα+ cells and have constipation under stress. Others could have overexpression or overactivation of α1D ARs in SMC and have diarrhea or abdominal pain under stress. If so, then subtype-selective antagonism of α1A ARs or α1D ARs may have therapeutic potential in treating symptoms. Currently, one subtype selective antagonist of α1A ARs (silodosin) is available in the United States and used for the treatment of lower urinary tract symptoms associated with benign prostatic hypertrophy.44 Loose stool and diarrhea have been reported as adverse events of silodosin with probabilities of 9.1% and 6.9%, respectively.44 These data may result from changing colonic responses to sympathetic neural input by silodosin, whereby colonic motility is enhanced through blocking inhibitory effects mediated by the α1A AR-SK channel signaling pathway in PDGFRα+ cells. Thus, silodosin could be promising for treating stress-induced constipation.
In conclusion, we found functional expression of α1A ARs on PDGFRα+ cells and α1D ARs on SMCs of human colon. NE or Epi inhibits colonic contractions via the α1A AR-SK channel signaling pathway in PDGFRα+ cells or excites them via α1D ARs expressed in SMCs. These are novel pathways by which stressful occurrences could manifest as diverse bowel disorders.
Materials and Methods
Tissue
Human tissue samples were obtained from surgical waste of total of 58 patients (34 men aged 50–83 and 24 women aged 35–90) who underwent colorectomy for colorectal cancer at the Department of Gastroenterological Surgery, Nagoya City University from 2016 to 2017. All subjects gave written informed consent. The tumor-free parts of the human colorectum were used for experiments. The study design was approved by the Institutional Review Board of Nagoya City University. All samples were de-identified.
Human Muscle Strips Tension Recordings
Immediately after the colorectal resections, pieces of human colonic specimens were dissected out and kept in Krebs solution containing indomethacin 1 μmol/L cooled in ice to reduce inflammatory responses. Small muscle strips with 10 mm length and 2 mm width along the direction of CM fibers were prepared. Threads were tied around both ends of the strips, one thread was fixed at the bottom of an organ bath chamber, and the other was connected to an isometric force transducer with a bridge amplifier (ADInstruments Ltd, Hasting, UK). Tension was digitized with Digidata 1200 interface (Axon Instruments, Inc, San Jose, CA) and was analyzed with pCLAMP 10 software (Molecular Devices, LLC, San Jose, CA). The strips were perfused at a constant flow rate of 1 mL min-1 with oxygenized, warmed (36°C) Krebs solution for 1 hour, and then initial tension of 5–10 mN was applied. The experimental protocols were started when SPCs and basal tension became stable 1 hour or longer after applying the initial tension. EFS was applied to the strips by silver plates located at both sides of the strips on the organ bath chamber. To analyze the responses of SPCs to NE in the specific conditions, 4 parameters of SPCs (AUC, amplitude, tone, and frequency) were measured for 10 minutes after adding NE 1 and 10 μmol/L. The amplitude of SPCs was calculated as the average of the difference of tension from the bottom to the peak of the trace of SPCs, and the tone was calculated as the average of the tension at the bottom of the trace of SPCs.
Ca2+ Imaging
Circular muscle layer preparations of human colon, approximately 5 mm square, were prepared, pinned out on a Sylgard plate (silicone elastomer; Dow Corning Corporation, Midland, MI) at the bottom of the recording chamber (volume, approximately 1 mL), superfused with warmed (36°C) Krebs solution at a constant flow rate (2 mL min-1), and equilibrated for 60 minutes.
To visualize intracellular Ca2+ dynamics in PDGFRα+ cells, preparations were incubated in low Ca2+ Krebs ([Ca2+]o = 0.1 mmol/L) containing 1–3 μmol/L Cal-520 AM (AAT Bioquest Inc, Sunnyvale, CA) and Cremophor EL (0.01%; Sigma-Aldrich) for 20–30 minutes at 35°C and then 10–15 minutes at room temperature.
After incubation, the recording chamber was mounted on the stage of an upright epifluorescence microscope (BX51WI; Olympus, Tokyo, Japan) equipped with a back-thinned electron multiplying CCD camera (C9100-13; Hamamatsu Photonics, Hamamatsu, Japan). Preparations were superfused with dye-free Krebs containing 2.5 mmol/L Ca2+, viewed with a water immersion objective (UMPlanFL ×20 or LUMPlanFL ×40, ×60; Olympus), and illuminated at 495 nm. Fluorescence was captured through a barrier filter above 515 nm, and images were obtained every 47–100 milliseconds (frame interval), with an exposure time of 30–70 milliseconds using a micro-photoluminescence measurement system (AQUACOSMOS; Hamamatsu Photonics). Relative amplitudes of Ca2+ transients were expressed as ΔFt/F0 = (Ft - F0)/F0, where Ft is the fluorescence generated by an event, and baseline F0 is the basal fluorescence.
Intracellular Electrical Recordings
A tissue segment of human sigmoid colon CMs (1 × 3 mm) was pinned to the floor of a recording chamber. The tissue was superfused with warmed (35°C) and oxygenated Krebs solution at a constant flow rate of approximately 2 mL min−1. Experiments were carried out in the presence of 3 μmol/L nifedipine to minimize muscle movements. Conventional microelectrode techniques were used to record transmembrane potentials from human colonic muscle strips. Glass capillary microelectrodes (outer diameter 1.5 mm, inner diameter 0.86 mm; Hilgenberg, Malsfeld, Germany) were filled with KCl 2 M and had tip resistances ranging between 50 and 80 MΩ. Electrical responses were recorded via a high input impedance amplifier (Axoclamp-2B; Axon Instruments) and stored on a computer for subsequent analysis and display.
Solutions and Drugs
Composition of Krebs solution was (mmol/L) Na+ 137.5; K+ 5.9; Ca2+ 2.5; Mg2+ 1.2; HCO3– 15.5; H2PO4– 1.2; Cl– 134; and glucose 11.5. The solution was bubbled with 95% O2 and 5% CO2, and the pH of solution was maintained at 7.3–7.5. Reagents used in this study were RS100329, an α1A AR antagonist, and a P2Y1 purinoceptor antagonist from Tocris Bioscience (Ellisville, MO), TTX from Wako (Osaka, Japan), apamin from Peptide Institute (Osaka, Japan), and atropine, noradrenaline (NE), PE, L-NNA, ACh, ADP, propranolol, prazosin, BMY7378, an α1D ARs antagonist, from MilliporeSigma (Burlington, MA).
Statistical Analysis
Experimental values were represented with means ± standard error. All statistical analysis was performed with GraphPad Prism (La Jolla, CA). Statistical significance was tested with one-way analysis of variance or paired t test, and probabilities of less than 5% (P < .05) were considered significant.
Acknowledgments
The authors thank all the staff in the Department of Gastroenterological Surgery, Nagoya City University Graduate School of Medical Sciences for conducting acquirement of human colonic tissues in compliance with the regulations of the Institutional Review Board of Nagoya City University.
CRediT Authorship Contributions
Masaaki Kurahashi, MD, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Funding acquisition: Equal; Investigation: Lead; Methodology: Lead; Project administration: Equal; Resources: Equal; Supervision: Equal; Validation: Lead; Writing – original draft: Lead; Writing – review & editing: Equal)
Yoshihiko Kito, PhD (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Supporting; Investigation: Supporting; Methodology: Supporting; Supervision: Equal; Validation: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Masayasu Hara, MD, PhD (Data curation: Supporting; Project administration: Equal; Resources: Supporting; Writing – review & editing: Supporting)
Hiromitsu Takeyama, MD, PhD (Data curation: Supporting; Project administration: Supporting; Resources: Supporting; Supervision: Equal; Writing – review & editing: Supporting)
Kenton M. Sanders, PhD (Conceptualization: Supporting; Funding acquisition: Equal; Investigation: Supporting; Supervision: Equal; Writing – original draft: Supporting; Writing – review & editing: Equal)
Hikaru Hashitani, MD, PhD (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Supporting; Investigation: Supporting; Project administration: Supporting; Supervision: Equal; Writing – original draft: Supporting; Writing – review & editing: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01-DK-091336.
Supplementary Material
Supplementary Table 1.
Summary of Means ± Standard Error of 4 Parameters of Spontaneous Contractions of Circular Muscle Layers of Human Sigmoid Colon for 10 Minutes After Adding Norepinephrine 1 μmol/L and 10 μmol/L to Organ Baths
| AUC (mN·min) |
Amplitude (mN) |
Tone (mN) |
Freq (cont/min) |
|||||
|---|---|---|---|---|---|---|---|---|
| Nor 1 μmol/L | Nor 10 μmol/L | Nor 1 μmol/L | Nor 10 μmol/L | Nor 1μmol/L | Nor 10 μmol/L | Nor 1 μmol/L | Nor 10 μmol/L | |
| None (4)a | 69.65 ± 9.55 | 24.67 ± 4.94 | 9.54 ± 1.44 | 1.66 ± 0.66 | 2.95 ± 0.64 | 1.86 ± 0.54 | 3.6 ± 0.6 | 3.2 ± 0.3 |
| TTX (5) | 77.68 ± 15.19 | 28.80 ± 7.34 | 14.84 ± 2.82 | 3.26 ± 1.33 | 2.64 ± 0.61 | 1.95 ± 0.47 | 3.0 ± 0.2 | 2.8 ± 0.3 |
| TTX + Prop (11) | 84.70 ± 15.49 | 32.88 ± 5.26 | 12.08 ± 1.95 | 2.40 ± 0.58 | 3.57 ± 0.92 | 2.35 ± 0.40 | 4.3 ± 0.5 | 4.2 ± 0.3 |
| TTX + Prop + Praz (5) | 48.99 ± 7.64 | 36.96 ± 7.52 | 8.36 ± 1.97 | 6.44 ± 1.31 | 1.58 ± 0.54 | 1.40 ± 0.48 | 3.8 ± 0.7 | 3.9 ± 0.6 |
| TTX + Prop + RS (8) | 65.87 ± 8.95 | 101.30 ± 14.88 | 12.50 ± 1.17 | 13.98 ± 1.29 | 2.13 ± 0.37 | 3.74 ± 0.85 | 3.9 ± 0.4 | 3.8 ± 0.3 |
| TTX + Prop + BMY (8) | 49.53 ± 3.71 | 28.21 ± 4.07 | 9.59 ± 0.77 | 3.33 ±1 .18 | 1.89 ± 0.22 | 1.79 ± 0.17 | 3.8 ± 0.4 | 4.3 ± 0.5 |
| TTX + Prop + Apa (8) | 86.97 ± 10.30 | 71.94 ± 12.03 | 13.42 ± 1.87 | 9.88 ± 1.32 | 2.89 ± 0.71 | 3.08 ± 0.90 | 4.2 ± 0.3 | 4.5 ± 0.3 |
Apa, apamin 100 nmol/L; AUC, area under the curve; BMY, BMY7378 1 μmol/L; cont, contractions; Freq, frequency; Nor, noradrenaline; Praz, prazosin 1 μmol/L; Prop, propranolol 1 μmol/L; RS, RS100329 1 μmol/L; TTX, tetrodotoxin 1 μmol/L.
Numbers in parentheses represent number of patients in each of the protocols.
References
- 1.Komuro T., Seki K., Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- 2.Sanders K.M., Koh S.D., Ro S., Ward S.M. Regulation of gastrointestinal motility-insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–645. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders K.M., Kito Y., Hwang S.J., Ward S.M. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology. 2016;31:316–326. doi: 10.1152/physiol.00006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iino S., Horiguchi K., Horiguchi S., Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 5.Kurahashi M., Zheng H., Dwyer L., Ward S.M., Koh S.D., Sanders K.M. A functional role for the 'fibroblast-like cells' in gastrointestinal smooth muscle. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurahashi M., Nakano Y., Henning G.W., Ward S.M., Sanders K.M. Platelet-derived growth factor receptor α-positive cells in the tunica muscularis of human colon. J Cell Mol Med. 2012;16:1397–1404. doi: 10.1111/j.1582-4934.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurahashi M., Mutafova-Yambolieva V., Koh S.D., Sanders K.M. Platelet-derived growth factor receptor-α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol. 2014;307:C561–C570. doi: 10.1152/ajpcell.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker S.A., Hennig G.W., Ward S.M., Sanders K.M. Temporal sequence of activation of cells involved in purinergic neurotransmission in the colon. J Physiol. 2015;593:1945–1963. doi: 10.1113/jphysiol.2014.287599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang S.J., Blair P.J., Durnin L., Mutafova-Yambolieva V., Sanders K.M., Ward S.M. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peri L.E., Sanders K.M., Mutafova-Yambolieva V.N. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRα-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterol Motil. 2013;25:609–620. doi: 10.1111/nmo.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M.Y., Park C., Berent R.M., Park P.J., Fuchs R., Syn H., Chin A., Townsend J., Benson C.C., Redelman D., Shen T.W., Park J.K., Miano J.M., Sanders K.M., Ro S. Smooth muscle cell genome browser: enabling the identification of novel serum response factor target genes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M.Y., Ha S.E., Park C., Park P.J., Fuchs R., Wei L., Jorgensen B.G., Redelman D., Ward S.M., Sanders K.M., Ro S. Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha S.E., Lee M.Y., Kurahashi M., Wei L., Jorgensen B.G., Park C., Park P.J., Redelman D., Sasse K.C., Becker L.S., Sanders K.M., Ro S. Transcriptome analysis of PDGFRα+ cells identifies T-type Ca2+ channel CACNA1G as a new pathological marker for PDGFRα+ cell hyperplasia. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurahashi M., Kito Y., Baker S.A., Jennings L.K., Dowers J.G.R., Koh S.D., Sanders K.M. A novel postsynaptic signal pathway of sympathetic neural regulation of murine colonic motility. FASEB J. 2020;34:5563–5577. doi: 10.1096/fj.201903134R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norberg K.A., Sjoqvist F. New possibilities for adrenergic modulation of ganglionic transmission. Permacol Rev. 1966;18:743–751. [PubMed] [Google Scholar]
- 16.Burnstock G., Costa M. Inhibitory innervation of the gut. Gastroenterology. 1973;64:141–144. [PubMed] [Google Scholar]
- 17.Manber L., Gershon M.D. A reciprocal adrenergic -cholinergic axoaxonic synapse in the mammalian gut. Am J Physiol. 1979;236:738–745. doi: 10.1152/ajpendo.1979.236.6.E738. [DOI] [PubMed] [Google Scholar]
- 18.Furness J.B., Callaghan B.P., Rivera L.R., Cho H.J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 19.Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manabe N., Tanaka T., Hata J., Kusunoki H., Haruma K. Pathophysiology underlying irritable bowel syndrome-from the viewpoint of dysfunction of autonomic nervous system activity. J Smooth Muscle Res. 2009;45:15–23. doi: 10.1540/jsmr.45.15. [DOI] [PubMed] [Google Scholar]
- 21.Berman S., Suyenobu B., Naliboff B.D., Bueller J., Stains J., Wong H., Mandelkem M., Fitzgerald L., Ohning G., Gupta A., Labus J.S., Tillisch K., Mayer E.A. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage. 2012;63:1854–1863. doi: 10.1016/j.neuroimage.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drossman D.A., Camilleri M., Mayer E.A., Whitehead W.E. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 23.Drossman D.A. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150:1262–1279. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 24.Lacy B.E., Mearin F., Chang L., Chey W.D., Lembo A.J., Simren M., Spiller R. Bowel disorders. Gastroenterology. 2016;150:1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 25.Vanner S., Greenwool-Van Meerveld B., Mawe G., Shea-Donohue T., Verdu E.F., Wood J., Grundy D. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2016;150:1280–1291. doi: 10.1053/j.gastro.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pradidarcheep W., Stallen J., Labruyere W.T., Dabhoiwala N.F., Michel M.C., Lamers W.H. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:397–402. doi: 10.1007/s00210-009-0393-0. [DOI] [PubMed] [Google Scholar]
- 27.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A., Odeberg J., Djureinovic D., Takanen J.O., Hober S., Alm T., Edqvist P.H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J.M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Ponten F. Proteomics: tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 28.Lomax A.E., Sharkey K.A., Furness J.B. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 29.Nishino Y., Masue T., Miwa K., Takahashi Y., Ishihara S., Deguchi T. Comparison of two alpha 1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU Int. 2006;97:747–751. doi: 10.1111/j.1464-410X.2006.06030.x. [DOI] [PubMed] [Google Scholar]
- 30.Williams T.J., Blue D.R., Daniels D.V., Davis B., Elworthy T., Gever J.R., Kava M.S., Morgans D., Padila F., Tassa S., Vimont R.L., Chapple C.R., Chess-Williams R., Eglen R.M., Clarke D.E., Ford A.P. In vitro alpha1-adrenoceptor pharmacology of Ro 70-0004 and RS-100329, novel alpha1A-adrenoceptor selective antagonists. Br J Pharmacol. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetz A.S., King H.K., Ward S.D., True T.A., Rimele T.J., Saussy D.L., Jr. BMY 7378 is a selective antagonist of the D subtype of alpha 1-adreneceptors. Eur J Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- 32.Keef K.D., Du C., Ward S.M., McGregor B., Sanders K.M. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- 33.Gallego D., Hernandez P., Clave P., Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ward S.M., Bechett E.A., Wang X., Baker F., Khoyi M., Sanders K.M. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H.T., Henning G.W., Fleming N.W., Keef K.D., Spencer N.J., Ward S.M., Sanders K.M., Smith T.K. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology. 2007;132:1852–1865. doi: 10.1053/j.gastro.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 36.De Ponti F., Giaroni C., Cosentino M., Lecchini S., Frigo G. Adrenergic mechanisms in the control of gastrointestinal motility: from basic science to clinical applications. Pharmacol Ther. 1996;69:59–78. doi: 10.1016/0163-7258(95)02031-4. [DOI] [PubMed] [Google Scholar]
- 37.Gagnon D.J., Devroede G., Belisle S. Excitatory effects of adrenaline upon isolated preparations of human colon. Gut. 1972;13:654–657. doi: 10.1136/gut.13.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piascik M.T., Perez D.M. Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther. 2001;298:403–410. [PubMed] [Google Scholar]
- 39.Cotecchia S. The α1-adrenergic receptors: diversity of signaling networks and regulation. J Recept Signal Transduct Res. 2010;30:410–419. doi: 10.3109/10799893.2010.518152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu M.H., Kim T.W., Ro S., Yan W., Ward S.M., Koh S.D., Sanders K.M. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders K.M., Ordog T., Koh S.D., Ward S.M. A novel pacemaker mechanism drives gastrointestinal rhythmicity. News Physiol Sci. 2000;15:291–298. doi: 10.1152/physiologyonline.2000.15.6.291. [DOI] [PubMed] [Google Scholar]
- 42.Dale T.J., Cryan J.E., Chen M.X., Trezise D.J. Partial apamin sensitivity of human small conductance Ca2+-activated K+ channels stably expressed in Chinese hamster ovary cells. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:470–477. doi: 10.1007/s00210-002-0622-2. [DOI] [PubMed] [Google Scholar]
- 43.McAllen R.M., Malpas S.C. Sympathetic burst activity: characteristics and significance. Clin Exp Pharmacol Physiol. 1997;24:791–799. doi: 10.1111/j.1440-1681.1997.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 44.Kawabe K., Yoshida M., Homma Y. Silodosin, a new alpha1A-adenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006;98:1019–1024. doi: 10.1111/j.1464-410X.2006.06448.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.