Abstract
Two new benzoic acid derivatives: 1-p-hydroxy benzoyl-3-palmitoyl glycerol (1) and 6 -p-hydroxy benzoyl daucosterol (2), along with scutellarein-6-methyl ether (3), quercetin (4), and rutin (5) had been separated from Cassia italica (Fabaceae) aerial parts from EtOAc fraction. Their characterisation was accomplished by various spectroscopic techniques and by comparing with the published data. The Ethyl acetate (EtOAc) fraction and compounds 1–5 had been assessed for their antioxidant potential utilizing DPPH assay. They had significant antioxidant capacities with activity ranged from 19.7 to 95.8%, in comparison to butylated hydroxyanisole (BHA) (93.8%). These findings could provide a further evidence to support the traditional use of C. italica for the treatment of chronic or degenerative illnesses.
Keywords: Cassia italic, Fabaceae, Diglyceride derivative, Daucosterol derivative, Antioxidant
1. Introduction
Antioxidants possess a significant role in the defense system of the body towards deleterious reactive oxygen species (ROS) (Salah et al., 1995). Increase the intake of antioxidants could assist to maintain the physiological function of the body systems (Van Acker et al., 1996). They are classified into natural and synthetic antioxidants (Gupta and Sharma, 2006). In spite of the fact that antioxidants obtained from synthetic sources are vastly used but there are many published researches pointing out an obvious relation among the long-term use of these antioxidants and several health problems: skin allergies, GIT complications, and raised the cancer‘s risk (Lourenço et al., 2019). It was stated that natural antioxidants are more efficient, powerful, and safer than synthetic antioxidants (Tavasalkar et al., 2012). Thus, the studies have been intensified for discovering non-toxic and effective natural metabolites with antioxidant potential (Gupta and Sharma, 2006). Plants are of a remarkable value due to their bioactive metabolites which have nutritional and medicinal benefits (Hussain et al., 2012, Mansoor et al., 2016). Fabaceae plants range from perennial and annual herbs to vines, shrubs, trees and aquatic plants, which are distributed in temperate, tropical, and aquatic regions (Molares and Ladio, 2012). Astragalus; Acacia, Indigofera, Crotalaria, and Mimosa are largest genera (Ahmad et al., 2016). Many plants of these genera are known to possess antioxidant activities such as Cicer arietinum (Wagh et al., 2012); Cajanus cajan (Rao and Sresty, 2000); Pterocarpus marsupium (Tippani et al., 2010); Pseudopiptadenia contorta (Moreira et al., 2005); Delonix regia (Azab et al., 2013); Arachis hypogaea (Jiang et al., 2014), and Acacia arabica (Sundaram and Mitra, 2007). Cassia genus comprises 600 species (Dave and Ledwani, 2012). Cassia italic (Eshrinq) is abundantly growing in Saudi Arabia. In Saudi traditional medicine, it is used to treat skin infections, constipation, and oedema (Al-Yahya et al., 1990). Its leaves or plants decoction is utilized as expectorant and laxative as well as purgative (Al-Said, 1993). Additionally, it is used for various intestinal and rheumatic complains, gout, biliousness, ringworm, and urinary infections (Al-Said, 1993, Kamzi et al., 2014). It was reported to possess a wide array of bioactivities: antioxidant, antibacterial, CNS depressant, hepatoprotective, hypoglycaemic, antiviral, anti-inflammatory, and anticancer (Dabai et al., 2012, Masoko et al., 2012, Mohamed, 2014). This plant is considered to be a wealthy pool of various secondary metabolites (Kamzi et al., 2014, Dabai et al., 2012, Mohamed, 2014, Elsayed et al., 1992). Interestingly, Gololo et al. stated that the geographical location has an influence on the accumulation of phytochemicals in of C. italica (Gololo et al., 2018). Khalaf et al. reported the isolation of tinnevellin; emodin, physcion, 2-methoxy-emodin-6-O-β-D-glucopyranoside, 1,6,8-trihydroxy-3-methoxy-9,10-dioxo-9,10-dihydroanthracene, and rutin from C. italica growing in Egypt (Khalaf et al., 2019). 10,10‘-Chrysophanol bianthrone, chrysophanol, 1,1,8,8‘-tetrahydroxy-6′-methoxy-3,3‘-dimethyl-10,10‘-bianthracen-9,9‘-dione, physcion, and 1,1,8,8‘-tetrahydroxy-7‘-methoxy-3,3‘-dimethyl-10,10‘-bianthracen-9,9‘-dione were separated from C. italica pods growing in Sudan (Yagi et al., 2013). Moreover, (22E)-3-β-hydroxycycloart-22-en-24-one, uvaol, β-sitosterol, daucosterol, emodin, methyl 3,4-dihydroxybenzoate, aloin, 4-hydroxypheny-O-β-D-glucopyranoside, and rutin were repoted from aerial parts of Saudi C. italica (Mohamed, 2014). However, little available repots on phytoconstituents of C. italica growing in Saudi Arabia. In the present study, two new (1 and 2) and three known metabolites (3–5) were separated and characterized from C. italica aerial parts (Fig. 1). The isolated metabolites 1–5 and EtOAc fraction were assessed for their antioxidant capacities.
Fig. 1.
Chemical structures of isolated compounds 1–5 from Cassia italica.
2. Materials and methods
2.1. Experimental
Optical rotations were estimated with a Perkin-Elmer polarimeter (Model 341LC). Infrared-400 Shimadzu spectrophotometer was utilized to get IR (infrared) spectra. HRESIMS (high resolution electrospray ionization mass spectrometry) was recorded on a LTQ Orbitrap. GCMS Clarus 500 was used for GCMS (gas chromatography mass spectrometry) analysis (Perkin Elmer). NMR data were recorded on 600 and 850 BRUKER Unity INOVA. Chromatographic analysis was performed on RP-18 (reversed phase-18), sephadex LH-20, and SiO2 60. TLC SiO2 60 F254 plates were used for TLC (thin layer chromatography) analysis. Purification of compounds was achieved using a six mL extraction tube LiChrolut RP-18 solid phase. Detection of compounds was done using UV (ultraviolet) absorption (λmax 255 and 366 nm) and spray reagent (anisaldehyde/H2SO4).
2.2. Plant material
In April 2017, C. italica aerial parts were collected from Gabal Al-Ateeq, Al Madinah Al Munawwarah (24°24′37.5″N 39°32′34.0″E). The plant taxonomy was done based on its morphological characteristics and library database (Collenette, 1999) and confirmed by a taxonomist at the Department of pharmaceutical Chemistry, Taibah University. A specimen (CI-2017-1) was archived at the Pharmacognosy and Pharmaceutical Chemistry Department herbarium.
2.3. Extraction and isolation
The powdered aerial parts (300 g) were extracted with MeOH (2.5 L × 5) and the extracts were evaporated to give total extract (CI). The latter (CI, 27.0 g) was subjected to SiO2 VLC (silica gel vacuum liquid chromatography) using n-hexane, EtOAc, and MeOH to obtain 4 fractions; CI-1 (6.9 g), CI-2 (8.7 g), and CI-3 (10.3 g), respectively. The EtOAC (8.0 g) fraction was submitted to SiO2 CC (column chromatography) (n-hexane:EtOAc gradient) to give nine subfractions: CIE-1:CIE-9. SiO2 CC for CIE-4 eluting with n-hexane:EtOAc gradient afforded 1, which was purified on extraction tube (LiChrolut RP-18, H2O:acetonitrile) to give 1 (17.2 mg). Subfraction CIE-5 was separated on SiO2 CC (CHCl3:MeOH gradient) to get 2 and RP-18 CC (H2O:MeOH gradient) was utilized for its purification, giving 2 (23.8 mg). Based on TLC, fractions CIE-6 and CIE-7 were gathered and chromatographed on sephadex LH-20 using MeOH to give 3 and 4 which were further subjected to repeated SiO2 CC (CHCl3-MeOH gradient) to get 3 (7.9 mg) and 4 (15 mg). Compound 5 (26.9 mg) was obtained from CIE-8 using SiO2 CC (CHCl3:MeOH gradient).
2.4. Spectral data
1-p-Hydroxy benzoyl-3-palmitoyl glycerol (1): Yellow oil, [α]D + 35.2 (c 0.5, CHCI3); IR (KBr) γmax: 2956, 3354, 1605, 1726 cm−1; see NMR Table 1; HRESIMS m/z 451.3054 [M+H]+ (calcd for C26H43O6, 451.3060).
Table 1.
NMR spectral data of compound 1 (DMSO‑d6, 850 and 214 Hz).
| No. | δH [mult., J (Hz)] | δC (mult.) | HMBC |
|---|---|---|---|
| 1 | 4.22 dd (11.1, 4.3) 4.20 dd (11.1, 6.0) |
66.2 CH2 | 2, 3, 7‘ |
| 2 | 4.07 m | 69.3 CH | 1, 3 |
| 3 | 4.18 dd (11.3, 4.5) 4.16 dd (11.3, 5.8) |
65.4 CH2 | 1, 2, 1‘‘ |
| 1‘ | – | 123.8 C | – |
| 2‘, 6‘ | 7.32 d (8.5) | 128.4 CH | 3‘, 5‘, 4‘ |
| 4‘ | – | 158.5 C | |
| 3‘, 5‘ | 6.79 d (8.5) | 115.2 CH | 2‘, 6‘, 4‘ |
| 7‘ | – | 167.0 C | |
| 4‘–OH | 9.61 s | – | 3‘, 5‘ |
| 1‘‘ | – | 173.3 C | – |
| 2‘‘ | 2.29 t (6.8) | 33.5 CH2 | 3‘‘ |
| 3‘‘ | 1.51 m | 24.5 CH2 | 1‘‘, 2‘‘ |
| (CH2)10 | 1.25–1.22 m | 0.86 CH2 | |
| 14‘‘ | 1.23 m | 31.3 CH2 | 15‘‘, 16‘‘ |
| 15‘‘ | 1.26 m | 22.1 CH2 | 14‘‘, 16‘‘ |
| 16‘‘ | 0.86 t (6.8) | 14.0 CH3 | 14‘‘, 15‘‘ |
6‘-p-Hydroxy benzoyl daucosterol (2): White amorphous powder; [α]D + 64.3 (c 0.3, CH3OH); IR (KBr) γmax: 3425, 2964, 1716, 1662, 1085 cm−1; see NMR Table 2; HRESIMS m/z 697.4683[M+H]+ (calcd for C42H65O8, 697.4679).
Table 2.
NMR spectral data of compound 2 (DMSO‑d6, 850 and 214 MHz).
| No. | δH [mult., J (Hz)] | δC | HMBC |
|---|---|---|---|
| 1 | 2.36 m, 2.12 m | 36.9 CH2 | 2, 3, 5, 6 |
| 2 | 1.52 m, 1.26 m | 29.2 CH2 | 1, 3, 10 |
| 3 | 3.46 m | 77.0 CH | 1‘, 4 |
| 4 | 1.96 m, 1.12 m | 38.3 CH2 | 3, 5, 6 |
| 5 | – | 140.5 C | – |
| 6 | 5.33 t (2.6) | 121.3 CH | 4, 7, 8 |
| 7 | 1.92 m, 1.78 m | 31.3 CH2 | 5, 6, 9, 14 |
| 8 | 1.81 m, 1.46 m | 31.5 CH | 6, 13, 14 |
| 9 | 0.88 m | 49.7 CH | 7, 11, 19 |
| 10 | – | 31.4 C | – |
| 11 | 1.47 m, 1.38 m | 20.7 CH2 | 9, 12 |
| 12 | 1.76 m, 0.98 m | 36.3 CH2 | 13, 17 |
| 13 | – | 41.9 C | – |
| 14 | 0.97 m | 56.2 CH | 9, 16, 18 |
| 15 | 1.03 m | 23.9 CH2 | 14, 17 |
| 16 | 1.78 m | 27.9 CH2 | 14, 17 |
| 17 | 1.08 m | 55.5 CH | 14, 16 |
| 18 | 0.65 s | 11.7 CH3 | 12, 13, 14, 17 |
| 19 | 0.96 s | 19.2 CH3 | 1, 5, 9, 10 |
| 20 | 1.33 m | 35.5 CH | 17, 21 |
| 21 | 0.90 d (6.8) | 19.0 CH3 | 17, 20, 22 |
| 22 | 1.28 m, 0.99 m | 33.5 CH2 | 17, 21 |
| 23 | 1.13 m | 25.5 CH2 | 20, 28 |
| 24 | 0.91 m | 45.2 CH | 26, 27, 29 |
| 25 | 1.62 m | 28.8 CH | 24, 26, 27 |
| 26 | 0.80 d (6.8) | 18.7 CH3 | 24, 25, 27 |
| 27 | 0.82 d (6.8) | 19.8 CH3 | 24, 26, 27 |
| 28 | 1.18 m | 22.7 CH2 | 23, 24, 25, 29 |
| 29 | 0.83 t (7.2) | 11.8 CH3 | 24, 28 |
| 1‘ | 4.22 d (7.7) | 100.8 CH | 3, 2‘, 3‘ |
| 2‘ | 2.90 m | 73.5 CH | 1‘, 4‘ |
| 3‘ | 3.13 m | 76.8 CH | 1‘, 2‘, 5‘ |
| 4‘ | 3.02 m | 70.2 CH | 2‘ |
| 5‘ | 3.07 m | 76.8 CH | 3‘, 4‘ |
| 6‘ | 4.03 dd (11.2, 4.3) 3.89 dd (11.2, 6.3) |
65.5 CH2 | 4‘, 7‘‘ |
| 1‘‘ | – | 121.2 C | – |
| 2‘‘, 6‘‘ | 7.78 d (8.5) | 131.6 CH | 3‘‘, 5‘‘, 4‘‘, 7‘‘ |
| 3‘‘, 5‘‘ | 6.81 d (8.5) | 115.2 CH | 1‘‘, 2‘‘, 6‘‘, 4‘‘ |
| 4‘‘ | – | 161.2 C | – |
| 7‘‘ | – | 166.7 C | – |
2.5. Alkaline hydrolysis of compound 1
Compound 1 solution (5 mg, KOH/MeOH 3%, 4 mL) had been left at room temperature for 2 h then neutralized utilizing HCl/MeOH (1 N). The solution was extracted three times with CHCl3 (each 15 mL) and then concentrated. The obtained residue was chromatographed on a SiO2 CC using n-hexane:EtOAc (99:1–80:20) to furnish palmitic acid methyl ester, which was specified by GCMS (Khedr et al., 2018).
2.6. Acid hydrolysis of compound 2
To a solution of 2 (5 mg in 10 mL MeOH), 5 mL of H2SO4 (5%) was added and refluxed for 3 h on WB (water bath). The solution was then extracted with EtOAc and then concentrated. The obtained residue was subjected sephadex LH-20 (MeOH). The aglycone and p-hydroxy benzoic acid were specified using co-TLC along with authentic. The sugar in the aqueous layer was identified by co-PC with authentic material (Mohamed, 2014).
2.7. Antioxidant activity
2,2-Diphenylpicrylhydrazyl (DPPH) assay was utilized to estimate the antioxidant activity of the EtOAc fraction and isolated compounds using butylated hydroxyanisole (BHA) (standard) as previously outlined (Ahmed et al., 2019).
2.8. Statistical analysis
The results were demonstrated as mean ± SD. Significance was calculated using One-way ANOVA followed by Tukey Kramer test. p < 0.05 was assigned as statistically significant.
3. Results and discussion
Extensive chromatographic separation of the EtOAc fraction obtained from C. italica, using RP-18, sephadex LH-20, and SiO2 60 CC led to the separation of two new (1 and 2) and three known compounds (3–5) (Fig. 1).
Compound 1 was separated as yellow oil. Its HRESIMS revealed a pseudomolecular peak at m/z 451.3054 [M+H]+ (calcd for C26H42O6, 451.3060), correspondent to a formula C26H42O6, which required six double bond equivalent. It had absorptions at 2956 (C-H aliphatic), 3354 (OH), 1605 (C = C aromatic), and 1726 (C = O) cm−1 in the IR (Silverstein and Webster, 1998). The 13C and HSQC displayed signals for 26 carbons, containing 2 carbonyls [δC 167.0 (C-7‘) and 173.3 (C-1‘‘)], two oxymethylenes, oxymethine, five aromatic carbons, and one oxygen-bonded aromatic carbon (Table 1). The two doublet–doublet oxymethylene signals at δH 4.22 and 4.20 (H-1) and 4.18 and 4.16 (H-3) and a multiplet oxymethine at δH 4.07 (H-2) indicated that 1 was a 1,3-diglyceride derivative (Salvo et al., 2017, Nieva-Echevarría et al., 2014). These signals having cross peaks in HSQC to the carbons at δC 66.2, 65.4, and 69.3, respectively (Kumar et al., 2011). This assignment was assured by the COSY cross peak of H-3 and H-1/H-2 and cross peaks in HMBC of H-1/C-2 and C-3 and H-3/C-1 and C-2 (Fig. 2). The NMR spectra for 1 revealed signals at δH 6.79/δC 115.2 (C-5‘, 3‘), 7.32 (C-6‘, 2‘)/128.4 (C-6‘, 2‘), 9.61 (4‘–OH), 158.5 (C-4‘), 123.8 (C-1‘), and 167.0 (C-7‘) reflected the existence of p-hydroxy benzoyl moiety in 1 (Van et al., 2018). The HMBC cross peaks of H-5‘ and H-3‘/C-6‘, C-4‘, and C-2‘, H-6‘ and H-2‘/C-4‘, C-3‘, and C-5‘, and 4‘–OH/C-5‘ and C-3‘ proved this moiety (Fig. 2). The cross peak of H-1 to C-7‘ established the connection of the p-hydroxy benzoyl moiety to C-1 of glycerol (Table 1). The 13C and 1H NMR revealed signals at δH 2.29 (H-2‘‘)/δC 33.5 (C-2‘‘), 173.3 (C-1‘‘), 1.25–1.22 (CH2)n/29.1–28.5 (CH2)n, and 0.86 (H-16‘‘)/14.0 (C-16‘‘), characterizing a fatty acyl moiety in 1, which was secured by the HMBC and COSY correlations. The connectivity of this moiety at C-3 of glycerol was proved by the HMBC cross peak of H-3 to the carbon at δC 173.3 (C-1‘‘). This moiety was specified to be consisted of C16 on the basis of alkaline hydrolysis which afford methyl ester of palmitic acid that was established by GCMS (Machida et al., 1997). Therefore, 1 was assigned as 1-p-hydroxy benzoyl-3-palmitoyl glycerol and considered a new metabolite.
Fig. 2.
Some key 1H–1H COSY (-) and HMBC (→) correlations of compounds 1 and 2.
Compound 2 was separated as white amorphous powder with [α]D + 64.3 (c 0.3, CH3OH) and gave positive Lieberman Burchard test, suggesting a steroidal nature of 2 (Tieh and Chang, 1980, El-Shanawany et al., 2015). It had a molecular formula C42H64O8 based on the HRESIMS molecular peak at m/z 697.4683 [M+H]+ (calcd for C42H65O8, 697.4679). The IR spectrum displayed characteristic absorptions at 3425, 2964, 1716, 1662, and 1085 cm−1, indicating the existence of hydroxyl, C-H aliphatic, ester carbonyl, C C, and C—O—C functionalities, respectively (Silverstein and Webster, 1998). The HSQC and 13C spectra of 2 exhibited resonances for forty two carbons: six methyls, twelve methylenes, one of them for an oxymethylene (δC 65.5, C-6‘), eighteen methines, and six quaternary carbons, including an ester carbonyl (δC 166.7, C-7‘‘) and oxygenated aromatic carbon (δC 161.2, C-4‘‘) (Table 2). The extensive analysis of NMR spectra indicated that 2 was a daucosterol derivative (Peshin and Kar, 2017). This was established by the characteristic signals for a tri-substituted olefinic bond at δH 5.33 (H-6) and the methyl signals at δH 0.96 (H-19), 0.65 (s, H-18), 0.90 (H-21), 0.82 (H-27), 0.80 (d, J = 6.8 Hz, H-26), and 0.83 (H-29), correlating to the carbons at δC 121.3, 19.2, 11.7, 19.0, 19.8, 18.7, and 11.8, respectively in the HSQC (Table 2). This was confirmed by the observed HMBC cross peaks of H-19/ C-9, C-1, C-5, and C-10, H-18/C-14, C-12, C-13, and C-17, H-21/C-17, C-20, and C-22, H-27 and H-26/C-25 and C-24, and H-29/C-28 and C-24 (Fig. 2). In addition, the cross peaks of H-6/ C-7, C-4, and C-8 and H-4 and H-8/C-6 in HMBC established the C5-C-6 olefinic bond. Moreover, a doublet signal at δH 4.22 (d, J = 7.7 Hz, H-1‘), having HSQC cross peak to the carbon at δC 100.8 (C-1‘), characterized the β-glucose moiety in 2. H-1‘ had cross peak in HMBC to the carbon at δC 77.0 (C-3) established the connectivity of glucose moiety at C-3. Furthermore, signals relevant for p-hydroxy benzoyl moiety [δH 6.81 (H-5‘‘, 3‘‘)/δC 11.5.2, 7.78 (H-6‘‘, 2‘‘)/131.6, 121.2 (C-1‘‘), 161.2 (C-4‘‘), and 166.7 (C-7‘‘)] were observed (Salib et al., 2011). This was secured by the observed HMBC cross peaks of H-2‘‘ and H-6‘‘/C-3‘‘, C-5‘‘, C-4‘‘, and C-7‘‘ and H-3‘‘ and H-5‘‘/C-1‘‘, C-2‘‘, C-6‘‘, and C-4‘‘. This was assured by the observed fragment peak at m/z 576.8593 [M+H-C7H5O2]+. The HMBC correlation of H-6‘ to C-7‘‘ signified the attachment of the p-hydroxy benzoyl moiety at C-6‘ of the glucose moiety. On the basis of these findings, 2 was assigned as 6‘-p-hydroxy benzoyl daucosterol and found to be a new natural product.
The known metabolites; scutellarein-6-methyl ether (3), quercetin (4), and rutin (5) (Harborne, 1994) were specified by comparing their data (Table 3) to those previously reported as well as co-TLC along authentic samples.
Table 3.
NMR spectral data of compound 3–5 in DMSO‑d6.
|
3a |
4b |
5b |
||||
|---|---|---|---|---|---|---|
| No. | δH [mult., J (Hz)] | δC (mult.) | δH [mult., J (Hz)] | δC (mult.) | δH [mult., J (Hz)] | δC (mult.) |
| 2 | – | 163.8 C | – | 147.7 C | – | 156.6 C |
| 3 | 6.59 s | 102.4 CH | – | 135.7 C | – | 133.2 C |
| 4 | – | 182.1 C | – | 175.8 C | – | 177.3 C |
| 5 | – | 145.2 C | – | 160.7 C | – | 156.4 C |
| 6 | – | 131.4 C | 6.17 d (2.4) | 98.1 CH | 6.20 d (2.4) | 98.6 CH |
| 7 | – | 152.5 C | – | 163.8 C | – | 161.2 C |
| 8 | 6.78 s | 94.3 CH | 6.40 d (2.4) | 93.3 CH | 6.39 d (2.4) | 93.6 CH |
| 9 | – | 152.8 C | – | 156.1 C | – | 156.4 CH |
| 10 | – | 105.7 C | – | 103.0 C | – | 104.0 C |
| 1‘ | – | 121.3 C | – | 121.9 C | – | 121.5 C |
| 2‘ | 7.93 d (8.5) | 128.5 CH | 7.66 d (2.4) | 115.0 CH | 7.55 d (2.4) | 115.2 CH |
| 3‘ | 6.93 d (8.5) | 115.9 CH | – | 145.0 CH | – | 144.7 C |
| 4‘ | – | 161.2 C | – | 148.5C | – | 148.4 C |
| 5‘ | 6.93 d (8.5) | 115.9 CH | 6.87 d (8.4) | 115.6 CH | 6.84 d (8.4) | 116.2 CH |
| 6‘ | 7.93 d (8.5) | 128.5 CH | 7.53 dd (8.4, 2.4) | 119.9 CH | 7.54 dd (8.4, 2.4) | 121.1 CH |
| 1‘‘ | – | – | – | – | 5.34 d (7.6) | 101.1 CH |
| 2‘‘ | – | – | – | – | 3.05–5.33 (m) | 74.0 CH |
| 3‘‘ | – | – | – | – | 75.8 CH | |
| 4‘‘ | – | – | – | – | 70.0 CH | |
| 5‘‘ | – | – | – | – | 76.3 CH | |
| 6‘‘ | – | – | – | – | 68.2 CH2 | |
| 1‘‘‘ | – | – | – | – | 4.38 d (1.2) | 100.7 CH |
| 2‘‘‘ | – | – | – | – | 3.05–5.33 (m) | 70.5 CH |
| 3‘‘‘ | – | – | – | – | 70.5 CH | |
| 4‘‘‘ | – | – | – | – | 71.8 CH | |
| 5‘‘‘ | – | – | – | – | 68.2 CH | |
| 6‘‘‘ | – | – | – | – | 0.99 d (6.0) | 17.7 CH3 |
| 6-OCH3 | 3.75 s | 60.0 CH3 | – | – | – | – |
| 5-OH | 13.07 s | – | 12.45 s | – | 12.61 s | – |
Measured at 850 and 214 Hz.
Measured at 600 and 150 Hz.
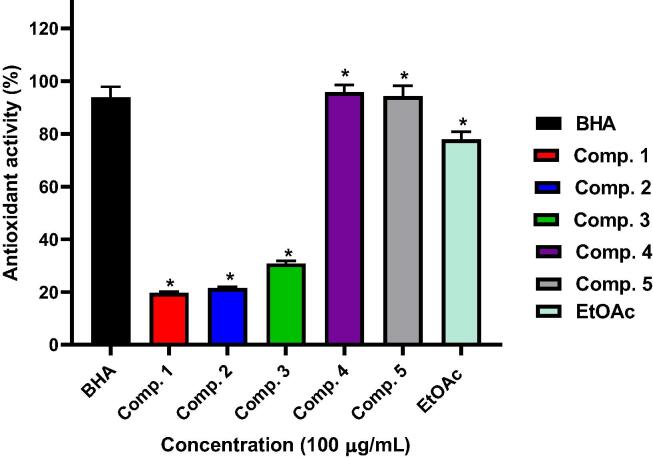
DPPH assay was utilized to assess the antioxidant capacities of phenolic metabolites. They are considered as safe natural antioxidants, as they delayed the progression many diseases by protecting the body from free radicals (El-Kashak et al., 2017). Thus, the antioxidant capacities of the EtOAc fraction and the isolated compounds were assessed using DPPH (Fig. 3). Compounds 1–5 and EtOAc fraction exhibited significant antioxidant potentials with antioxidant activities 19.7, 21.5, 30.7, 95.8, 94.2, and 77.9%, respectively at 100 µg/mL, compared to BHA (93.8%). Our results are in a good agreement with a number of publications that reported on the antioxidant activities of extracts of C. italica from different geographical locations which were attributed to the presence of various classes of phenolic metabolites. The ethyl acetate and n-butanol extracts of aerial parts of the Egyptian C. italica possessed antioxidant capacity using ABTS assay (Khalaf et al., 2019, Madkour et al., 2017). It is noteworthy that the extracts of the roots of the southern African C. italica exhibited antioxidant activity in the DPPH assay (Masoko et al., 2010, Mokgotho et al., 2013). Jothi et al reported that the different fractions of aerial parts of the Indian C. italica ssp micrantha displayed antioxidant potential using different assays such as DPPH, ABTS, superoxide, and reducing power assays (Jothi et al., 2015). It is noteworthy that these highest activity of quercetin (4) and rutin (5) are in a good agreement with the formerly stated results (Yang et al., 2008, Zheng et al., 2010).
Fig. 3.
Antioxidant activity of isolated compounds (1–5) and EtOAc extract of C. italica by DPPH method. * Compared to BHA (Positive control); (one-way ANOVA followed by Tukey Kramer). Data are the mean ± SE. (n = 3).
4. Conclusions
C. italica is a rich source of diverse natural compounds with variable pharmacological properties. Chromatographic fractionation of C. italica afforded two new (1 and 2) and three known compounds (3–5). Their structures elicitation was carried out by various spectral techniques. Compounds 1–5 had promising antioxidant activity in DPPH assay. The antioxidant capacities of isolated metabolites merit further concern for the use of this plant in various disorders related to oxidative stress. Also, this wok may also assist in validating the widely claimed ethnobotanical uses of the plant in folk and traditional medicine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad F., Anwar F., Hira S. Review on medicinal importance of Fabaceae family. Pharmacology Online. 2016;3:151–156. [Google Scholar]
- Ahmed S., Al-Rehaily A.J., Alam P., Alqahtani A.S., Hidayatullah S., Rehman M.T., Siddiqu N.A. Antidiabetic, antioxidant, molecular docking and HPTLC analysis of miquelianin isolated from Euphorbia schimperi C. Presl. Saudi Pharma. J. 2019;27:655–663. doi: 10.1016/j.jsps.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Said M.S. Traditional medicinal plants of Saudi Arabia. AMJ Chinese Med. 1993;2:1–12. doi: 10.1142/S0192415X93000340. [DOI] [PubMed] [Google Scholar]
- Al-Yahya M.A., Al-Meshal I.A., Mossa J.S., Al-Badr A.A., Tariq M. A phytochemical and biological approach. King Saud University; Riyadh: 1990. Saudi plants; p. 349. [Google Scholar]
- Azab S.S., Abdel-Daim M., Eldahshan O.A. Phytochemical, cytotoxic, hepatoprotective and antioxidant properties of Delonix regia leaves extract. Med. Chem. Res. 2013;22:4269–4277. [Google Scholar]
- Collenette, S., 1999. Wild flowers of Saudi Arabia. King of Saudi Arabia: National Commission for Wild life Conservation and Development (NCWCD) & Sheila Collenette, King Fahd National Library; p. 523.
- Dabai Y.U., Kawo A.H., Aliyu R.M. Phytochemical screening and antibacterial activity of the leaf and root extracts of S. italic. Afr. J. Pharm. Pharmacol. 2012;6:914–918. [Google Scholar]
- Dave H., Ledwani L. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour. 2012;3:291–319. [Google Scholar]
- El-Kashak W.A., Osman S.M., Gaara A.H., ElToumy S.A., Mohamed T.K., Brouard I. Phenolic metabolites, biological activities, and isolated compounds of Terminalia muelleri extract. Pharm. Biol. 2017;55:2277–2284. doi: 10.1080/13880209.2017.1406531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed N.H., Abu-Dooh A.M., Elkhrisy E.A.M., Mabry T.J. Flavonoids of Cassia italica. Phytochemistry. 1992;31:2187. [Google Scholar]
- El-Shanawany M.A., Sayed H.M., Ibrahim S.R.M., Fayed M.A.A. Stigmasterol tetracosanoate, a new stigmasterol ester from the Egyptian Blepharis ciliaris. Drug Res. 2015;65:347–353. doi: 10.1055/s-0034-1382064. [DOI] [PubMed] [Google Scholar]
- Gololo S.S., Mapfumari N.S., Mogale M.A. Comparative quantitative phytochemical analysis of the leaves of Senna italica collected from different areas in Limpopo province, South Africa. Int. J. Pharm. Pharm. Sci. 2018;10:67–71. [Google Scholar]
- Gupta V.K., Sharma S.K. Plants as natural antioxidants. Nat. Prod. Rad. 2006;5:326–334. [Google Scholar]
- Harborne J.B. Chapman and Hall; London: 1994. The Flavonoids Advances in Research Since 1986. [Google Scholar]
- Hussain M.S., Fareed S., Ansari S., Rahman M.A., Ahmad I.Z., Saeed M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied. Sci. 2012;4:10–20. doi: 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Ma Y., Yan D. Antioxidant and antimicrobial properties of water soluble polysaccharide from Arachis hypogaea seeds. J. Food Sci. Tech. 2014;51:2839–2844. doi: 10.1007/s13197-012-0786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothi R.S., Bharathy V., Uthayakumari F. Antioxidant potential of aerial part of Senna italic sub species micrantha Mill. J. Pharm. Sci. Res. 2015;7:621–625. [Google Scholar]
- Kamzi M.H., Abdul-Malik Saira-Hameed, Akhtar N., Ali S.N. An anthraquinone derivative from Cassia italica. Phytochemistry. 1994;36:761–763. [Google Scholar]
- Khalaf O.M., Ghareeb M.A., Saad A.M., Madkour H.M.F., El-Ziaty A.K., Abdel-Aziz M.S. Phenolic constituents, antimicrobial, antioxidant, and anticancer activities of ethyl acetate and n-butanol extracts of Senna italic. Acta Chromatographica. 2019;31:138–145. [Google Scholar]
- Khedr A.I.M., Ibrahimb S.R.M., Mohamed G.A., Ross S.A., Yamada K. Panduramides A-D, new ceramides from Ficus pandurata fruits. Phytochem. Lett. 2018;23:100–105. [Google Scholar]
- Kumar R., Bansal V., Tiwari A.K., Sharma M., Puri S.K., Patel M.B., Sarpal A.S. Estimation of glycerides and free fatty acid in oils extracted from various seeds from the Indian region by NMR spectroscopy. J. Am. Oil Chem. Soc. 2011;88:1675–1685. [Google Scholar]
- Lourenço S.C., Moldão-Martins M., Alves V.D. Antioxidants of natural plant origins: from sources to food industry applications. Molecules. 2019;24:4132. doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida K., Yamaguchi T., Kamiya Y., Kikuchi M. Acylated triterpenoids from Ligustrum ovalifolium. Phytochemistry. 1997;46:977–979. [Google Scholar]
- Madkour H.M.F., Ghareeb M.A., Abdel-Aziz M.S., Khalaf O.M., Saad A.M., ElZiaty A.K., Abdel-Mogib M. Gas chromatography-mass spectrometry analysis, antimicrobial, anticancer and antioxidant activities of n-hexane and methylene chloride extracts of Senna italica. J. Appl. Pharm. Sci. 2017;7:23–32. [Google Scholar]
- Mansoor S., Khan I., Fatima J., Saeed M., Mustafa H. Anti-bacterial, antioxidant and cytotoxicity of aqueous and organic extracts of Ricinus communis. African J. Microbiol. Res. 2016;10:260–270. [Google Scholar]
- Masoko P., Gololo S.S., Mokgotho M.P., Eloff J.N., Howard R.L., Mampuru L.J. Evaluation of the antioxidant, antibacterial, and antiproliferative activities of the acetone extract of the roots of Senna italic (Fabaceae) Afr. J. Trad. CAM. 2010;7:138–148. doi: 10.4314/ajtcam.v7i2.50873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoko P., Gololo S.S., Mokgotho M.P., Eloff J.N., Howard R.L., Mampuru L.J. Evaluation of the antioxidant, antibacterial and antiproliferative activities of the acetone extract of the roots of Senna italica (Fabaceae) Afr. J. Trad.CAM. 2012;7:138–148. doi: 10.4314/ajtcam.v7i2.50873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed G.A. New cytotoxic cycloartane triterpene from Cassia italica aerial parts. Nat. Prod. Res. 2014;28:976–983. doi: 10.1080/14786419.2014.902820. [DOI] [PubMed] [Google Scholar]
- Mokgotho M.P., Gololo S.S., Masoko P., Mdee L.K., Mbazima V., Shai L.J., Bagla V.P., Eloff J.N., Mampuru L. Isolation and chemical structural characterisation of a compound with antioxidant activity from the roots of Senna italica. Evid. Based Complement. Alternat. Med. 2013;2013 doi: 10.1155/2013/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molares S., Ladio A. The usefulness of edible and medicinal Fabaceae in Argentine and Chilean Patagonia: Environmental availability and other sources of supply. Evid. Based Complement. Alternat. Med. 2012;2012 doi: 10.1155/2012/901918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira D.L., Leitão S.G., Gonçalves J.L.S., Wigg M.D., Leitão G.G. Antioxidant and antiviral properties of Pseudopiptadenia contorta (Leguminosae) and of quebracho (Schinopsis sp.) extracts. Química Nova. 2005;28:421–425. [Google Scholar]
- Nieva-Echevarría B., Goicoechea E., Manzanos M.J., Guillén M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014;66:379–387. [Google Scholar]
- Peshin T., Kar H.K. Isolation and characterization of β-sitosterol-3-O-β-D-glucoside from the extract of the flowers of Viola odorata. British J. Pharma. Res. BJPR. 2017;16:1–8. [Google Scholar]
- Rao K.M., Sresty T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000;157:113–128. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Salah N., Miller N.J., Paganga G., Tijburg L., Bolwell G.P., Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- Salib J.Y., Daniel E.N., Hifnawy M.S., Azzam S.M., Shaheed I.B., Abdel-Latif S.M. Polyphenolic compounds from flowers of Hibiscus rosa-sinensis Linn. and their inhibitory effect on alkaline phosphatase enzyme activity in vitro. Z. Naturforsch. C. 2011;66:453–459. doi: 10.1515/znc-2011-9-1003. [DOI] [PubMed] [Google Scholar]
- Salvo A., Rotondo A., La Torre G.L., Cicero N., Dugo G. Determination of 1,2/1,3-diglycerides in Sicilian extra-virgin olive oils by 1H-NMR over a one-year storage period. Nat. Prod. Res. 2017;31:822–828. doi: 10.1080/14786419.2016.1247084. [DOI] [PubMed] [Google Scholar]
- Silverstein R.M., Webster F.X. 6th ed. Wiley; New York, NY: 1998. Spectrometric identification of organic compounds. [Google Scholar]
- Sundaram R., Mitra S. Antioxidant activity of ethyl acetate soluble fraction of Acacia arabica bark in rats. Indian J. Pharmacol. 2007;39:33–38. [Google Scholar]
- Tavasalkar S.U., Mishra H.N., Madhavan S. Evaluation of antioxidant efficacy of natural plant extracts against synthetic antioxidants in sunflower Oil. Open Access Sci. Rep. 2012;1:504. [Google Scholar]
- Tieh J.-H.-J., Chang T.-C. The chemical constituents of Nephrolepis Auriculata (L.) Trimen. J. Chinese Chem. Soc. 1980;27:113–117. [Google Scholar]
- Tippani R., Porika M., Allenki V., Anreddy R.N.R., Reddy Y.N., Devarakonda K., Thammidala C., Abbagani S. Antioxidant and analgesic activities of Pterocarpus marsupium Roxb. J. Herbs Spices Med, Plants. 2010;16:63–68. [Google Scholar]
- Van Acker S.A., van den Berg D.J., Tromp M.N., Griffioen D.H., van Bennekom W.P., van der Vijgh W.J., Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Boil. Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- Van Q.T.T., Vien L.T., Hanh T.T.H., Huong P.T.T., Thanh N.V., Cuong N.X., Nam N.H., Minh C.V. Structural elucidation of four flavonoid glycosides from Barringtonia acutangula. Vietnam J. Chem. 2018;56:187–190. [Google Scholar]
- Wagh S.S., Jain S.K., Patil A.V., Vadnere G.P. In vitro free radical scavenging and antioxidant activity of Cicer arietinum L. (Fabaceae). Int. J. PharmTech. Res. 2012;4:343–350. [Google Scholar]
- Yagi S., El-Tigani S., Ali M., Elkhidir I., Mohammed A.M.A. Chemical constituents and insecticidal activity of Senna italica Mill. from the Sudan. Int. Lett. Chem. Phys. Astron. 2013;9:146–151. [Google Scholar]
- Yang J., Guo J., Yuan J. In vitro antioxidant properties of rutin. LWT. 2008;41:1060–1066. [Google Scholar]
- Zheng C.-D., Li G., Li H.-Q., Xu X.-J., Gao J.-M., Zhang A.-L. DPPH-Scavenging activities and structure-activity relationships of phenolic compounds. Nat. Prod. Commun. 2010;5:1759–1765. [PubMed] [Google Scholar]