Abstract
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease, which is accompanied by progressive joint damage and disability. The intolerability of conventional antirheumatic drugs by some patients necessitates the search for effective antirheumatic agents having better tolerability. In the current work, we aimed to investigate the efficacy of cinnamaldehyde, tadalafil, and aliskiren as potential antirheumatic candidates and to explore their modulatory effects on joint destruction, inflammatory response, and intracellular signaling. Arthritis was induced in female Wistar rats by complete Freund's adjuvant (CFA) 0.4 ml s.c. on days 1, 4, and 7. Treated groups received their respective drugs, starting from day 13, daily for 3 weeks. Methotrexate and prednisolone were the standard antirheumatic drugs, while cinnamaldehyde, tadalafil, and aliskiren were the test agents. Treatment with cinnamaldehyde, tadalafil, or aliskiren reduced serum levels of rheumatoid factor, and pro-inflammatory cytokines; tumor necrosis factor-alpha and interleukin-6 (IL-6), along with elevated level of IL-10 which is an anti-inflammatory cytokine. Besides, cartilage and bone destruction biomarkers; matrix metalloproteinase-3 (MMP-3) and receptor activator of nuclear factor-kappa B ligand (RANKL); were significantly reduced after treatment with the test agents, which was further confirmed by histopathological investigation. The elevated protein expressions of phosphorylated-Janus kinase 2 (p-JAK2), phosphorylated-signal transducer and activator of transcription 3 (p-STAT3), and inducible nitric oxide synthase (iNOS) in articular tissue were markedly attenuated after treatment with cinnamaldehyde, tadalafil, or aliskiren, while that of endothelial nitric oxide synthase (eNOS) was greatly enhanced. In addition, oxidative stress and inflammatory markers such as malondialdehyde, nitric oxide, and myeloperoxidase were reduced in joint tissue after treatment with the test agents, while glutathione content was elevated. Furthermore, the renin inhibitor aliskiren produced effects close to those of the normal and methotrexate, the gold standard antirheumatic drug, in most of the measured parameters.
Collectively, these findings led to the assumption that the downregulation of IL-6/JAK2/STAT3 signaling by cinnamaldehyde, tadalafil, and aliskiren could alleviate joint destruction by MMP-3 and RANKL, reduce iNOS, and enhance eNOS expressions. Moreover, aliskiren could be a promising therapeutic agent for RA, because of its ability to normalize most of the measured parameters after CFA-induced arthritis.
Keywords: CFA-induced arthritis, MMP-3, RANKL, IL-6/JAK2/STAT3 signaling, Aliskiren
Abbreviations: RA, rheumatoid arthritis; CFA, complete Freund's adjuvant; TNF-α, tumor necrosis factor-alpha; IL-6, interleukin-6; IL-10, interleukin-10; MMP-3, matrix metalloproteinase-3; RANKL, receptor activator of nuclear factor-kappa B ligand; JAK2, Janus kinase 2; STAT3, signal transducer and activator of transcription 3; MDA, malondialdehyde; GSH, reduced glutathione; MPO, myeloperoxidase; NO, nitric oxide; iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase; PDE, phosphodiesterase; RAS, renin angiotensin system; DMARD, disease-modifying antirheumatic drug; H&E, hematoxylin and eosin
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease having a systemic inflammatory pattern that affects mainly synovial joints, as well as other structures in the body (Saad et al., 2019). The disease occurs in 1% of the population with a high prevalence in females compared to males (McInnes and Schett, 2017). RA is accompanied by increased levels of autoantibodies like rheumatoid factor (RF) and anticitrullinated protein antibody (ACPA) which are characteristic markers of the disease, as well as the articular manifestations of inflammation, swelling, and erosion of cartilage and bone (McInnes and Schett, 2011).
The prevalence of the pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-1, and IL-17, largely contribute to the systemic inflammatory manifestations in RA. However, anti-inflammatory cytokines, such as IL-10 and IL-4 which retard the inflammatory response are greatly hampered in RA (Liu et al., 2016). The pro-inflammatory cytokines especially IL-6 can stimulate intracellular signaling through Janus kinase (JAK) that subsequently stimulates signal transducer and activator of transcription (STAT) (Malemud, 2018). Eventually, phosphorylation of STAT protein promotes sustained intracellular inflammatory response (Malemud, 2017), as well as the transcription of certain genes, such as matrix metalloproteinases MMPs which lead to articular tissue destruction (Ni et al., 2019). Additionally, STAT3 activation has been reported to enhance the expression of receptor activator of nuclear factor-kappa B ligand (RANKL), which interacts with RANK receptors on osteoclasts resulting in enhancement of their activity and thus bone erosion pattern of RA (Li et al., 2016).
Methotrexate is the cornerstone in RA therapy as a disease-modifying antirheumatic drug (DMARD) (Shinde et al., 2014), and corticosteroids are potent anti-inflammatory agents used in the early stages of the disease as a bridge or symptomatic therapy till DMARDs produce their therapeutic effect (Hoes et al., 2010). However, their disabling adverse effects (Buttgereit, 2020, Solomon et al., 2020) necessitate the search for new effective and safer therapeutic agents.
Cinnamaldehyde is a potent anti-inflammatory constituent of cinnamon essential oil (Gunawardena et al., 2015). It has been previously reported to alleviate arthritis induced by collagen through regulation of oxidative stress and inflammatory markers (Mateen et al., 2019a, Mateen et al., 2019b), and to suppress JAK/STAT signaling in synoviocytes (Cheng et al., 2020), but its effect on joint destruction biomarkers hasn't been investigated before.
Tadalafil is a phosphodiesterase-5 (PDE-5) inhibitor that has been reported to exhibit anti-inflammatory activity against different experimental models, including prostate inflammation (Okamoto et al., 2018), liver injury induced by thioacetamide (Mansour et al., 2018), allergic inflammatory airways (Mokry et al., 2017), and renal ischemia/reperfusion (Medeiros et al., 2017). In addition, PDE inhibitors have been documented to be effective in the management of autoimmune diseases, such as RA (Shenoy and Agarwal, 2010).
Aliskiren is a renin inhibitor that decreases angiotensin II levels with subsequent reduction of the inflammatory response evoked by renin angiotensin system (RAS) stimulation (Choi et al., 2011). Its efficacy has been proved previously in an experimental model of osteoarthritis through its inhibitory effect on RAS present in cartilage with subsequent reduction of erosion (Yan and Shen, 2017), while its effect on RA hasn't been investigated previously.
Therefore, the authors aimed from the current study to estimate the possible modulatory effects of cinnamaldehyde, tadalafil, and aliskiren on joint destruction biomarkers; MMP-3 and RANKL; against CFA-induced arthritis in rats. In addition, we aimed to investigate the effects of the test agents on IL-6/JAK2/STAT3 signaling pathway, iNOS, and eNOS expressions.
2. Materials and methods
2.1. Animals
This study was performed on healthy adult female Wistar rats, weighing 250 ± 20 g. Animals were obtained from Animal House of Faculty of Pharmacy, Nahda University, Beni-Suef, Egypt. All experimental rats were retained under stable temperature (23 ± 2 °C) and allowed free access to standard forage and tap water ad libitum. All experimental procedures performed on animals were in accordance with the National Institutes of Health guide for care and use of laboratory animals and also have been accepted by the “Research Ethical Committee” at the Faculty of Pharmacy, Beni-Suef University (REC-A-PhBSU-19003).
2.2. Drugs, chemicals, and kits
Methotrexate and prednisolone were obtained from (Mylan, France) and Egyptian Pharmaceutical Industries Co. (EIPICO, Egypt), respectively. The sources of tadalafil and aliskiren were Eli Lilly & Co. and Novartis Pharmaceuticals Corporation, respectively. Cinnamaldehyde (purity ≥ 98%) was purchased from LOBA Chemie (Mumbai, India) for laboratory reagents and fine chemicals. Complete Freund's adjuvant (CFA) was purchased from Sigma-Aldrich Co. (USA). The sources of RF, TNF-α, IL-6, IL-10, MMP-3, RANKL, and myeloperoxidase (MPO) ELISA kits were CUSABIO (Bio-Connect Diagnostics, The Netherlands) and MyBioSource (USA). The colorimetric kits of malondialdehyde (MDA), reduced glutathione (GSH), and nitric oxide (NO) measured as nitrite were purchased from Bio-Diagnostic Co. (Egypt). The primary antibodies for Western blot analysis, including p-JAK2, p-STAT3, iNOS, and eNOS were obtained from ThermoFisher Scientific (USA).
2.3. Experimental design
After one week of adaptation, 56 wt-matched healthy rats were divided into 7 groups, each of 8 rats. The distribution of animals in the groups was random. Doses were selected depending on pilot trials guided by published literature. Group 1 (normal) received vehicle only. Group 2 (arthritic control) received three s.c. doses of 0.4 ml CFA on days 1, 4, and 7 in the flank of different limbs to reduce the risk of ulceration (Hawkins et al., 2015, Wahba et al., 2015). Groups 3 and 4 were kept as reference treatment groups and received methotrexate (1 mg/kg/week; i.p.) (Bais et al., 2017) and prednisolone (10 mg/kg/day; p.o.) (El-Gaphar et al., 2015), respectively. Groups 5, 6, and 7 received the test agents; cinnamaldehyde (40 mg/kg/day; p.o.) (Abd El-Raouf et al., 2015), tadalafil (10 mg/kg/day; p.o.) (Bahadir et al., 2018), and aliskiren (20 mg/kg/day; p.o.) (Zhao et al., 2020), respectively. Groups from 3 to 7 received their respective treatments starting from day 13 after the first dose of CFA, and then continued for 3 weeks. Blood and knee joint samples were withdrawn at the end of the experiment, then stored at −80 °C till the estimation of biochemical and molecular parameters. Articular samples used for histopathological investigation were fixed in 10% buffered formalin.
Serum levels of RF, TNF-α, IL-6, IL-10, MMP-3, RANKL, and articular tissue contents of MDA, GSH, MPO, NO were measured according to kit manufacturer's instructions, while the protein expressions of p-JAK2, p-STAT3, iNOS, and eNOS were estimated in the knee joints by Western blot analysis.
2.4. Western blotting analysis
Knee joint samples were processed using RIPA lysis buffer (PL005; BIO BASIC INC., Canada), with supplementary protease and phosphatase inhibitors. Protein concentration was determined in the lysed samples using Bradford Protein Assay Kit (SK3041; BIO BASIC INC., Canada). Proteins were separated by gel electrophoresis (SDS-PAGE) using TGX Stain-Free™ FastCast™ Acrylamide Kit (Bio-Rad Laboratories, USA). After the transfer of proteins from the gel to PVDF membrane, the membrane was blocked by tris-buffered saline with Tween 20 (TBST) and 3% bovine serum albumin (BSA) at room temperature for 1 h. Overnight incubation with each primary antibody was carried out against the blotted target protein at 4 °C, followed by rinsing with TBST. Afterward, incubation with HRP-conjugated secondary antibody was carried out for 1 h at room temperature, followed by rinsing with TBST. Finally, detection of bands was performed via chemiluminescence technique and CCD camera-based imaging, followed by quantification using ImageJ software (USA).
2.5. Histopathological investigation
The fixed knee joint samples were decalcified by EDTA (10%, pH 7.4) (Allam et al., 2016). EDTA solutions were renewed twice/week for 7 weeks. Decalcification was checked using a surgical blade. Washing of the samples by PBS, dehydration by graded ethanol, and embedding in paraffin wax were carried out after confirming complete decalcification. Finally, sections (5 μm) were prepared and stained with hematoxylin and eosin (H&E).
Blind investigation of articular tissue sections was carried out by a histopathologist. Sections were graded for the histological changes (inflammation, cartilage erosion, bone destruction) as follows: (+) mild, (++) moderate, and (+++) severe alterations.
2.6. Statistical analysis
Data are expressed as the mean of 8 values ± standard error (SE). Statistical significance was tested using one-way analysis of variance (ANOVA) test, succeeded by Tukey's multiple comparisons test by the aid of Prism GraphPad software version 8 (USA), where p < 0.05 was regarded significant.
3. Results
3.1. Effect on rheumatoid factor
Rats injected with CFA revealed a remarkable increase in serum RF levels (3-fold, p < 0.0001) when compared with the normal rats. Treatment of rats with methotrexate, prednisolone, cinnamaldehyde, tadalafil, or aliskiren reduced serum levels of RF by about 89%, 85%, 62%, 56%, and 78% (p < 0.0001), respectively, as compared to the arthritic control rats. Serum levels of RF were returned to normal after treatment with aliskiren, showing response close to that of methotrexate and prednisolone (Fig. 1).
Fig. 1.
Effect on rheumatoid factor. Arthritic control group showed a marked elevation in serum level of RF, while treatment with methotrexate, prednisolone, cinnamaldehyde, tadalafil, or aliskiren significantly reduced it. Each column represents the mean of 8 rats ± SE. avs normal, bvs arthritic control, cvs methotrexate, and dvs prednisolone at p < 0.05.
3.2. Effect on pro-inflammatory/anti-inflammatory cytokine balance
Significant elevations in serum levels of the pro-inflammatory cytokines IL-6 (2-fold, p < 0.0001) and TNF-α (3-fold, p < 0.0001) were noticed after injection of CFA, while serum levels of the anti-inflammatory cytokine IL-10 were significantly reduced (1-fold, p < 0.0001) as compared to the normal group. Treatment with methotrexate, prednisolone, cinnamaldehyde, tadalafil, or aliskiren reduced serum levels of IL-6 by about 79%, 76%, 52%, 48%, 75% (p < 0.0001) and TNF-α by about 82%, 66%, 56%, 43%, 72% (p < 0.0001), respectively (Fig. 2A, B). Contrarily, there was a remarkable increase in serum IL-10 levels (85% p < 0.0001, 66% p = 0.001, 57% p = 0.006, 60% p = 0.003, 167% p < 0.0001), as compared to the arthritic control group (Fig. 2C). Moreover, treatment with aliskiren restored pro-/anti-inflammatory cytokine balance by normalizing the levels of IL-6, TNF-α, and IL-10.
Fig. 2.
Effect on pro-inflammatory/anti-inflammatory cytokine balance. Levels of the pro-inflammatory cytokines IL-6 (A) and TNF-α (B) were significantly elevated after induction of arthritis by CFA, but these elevations were reduced after treatment with the test agents. On the other hand, level of the anti-inflammatory cytokine IL-10 (C) was reduced in arthritic control but elevated in treated groups. Each column represents the mean of 8 rats ± SE. avs normal, bvs arthritic control, cvs methotrexate, and dvs prednisolone at p < 0.05.
3.3. Effect on biomarkers of joint destruction
The serum level of MMP-3 has been documented to be a credible marker for joint erosion and disease activity in RA patients (Lerner et al., 2018). RANKL has been proved to be an indicator of bone erosion both locally and systemically in experimentally-induced arthritis by either CFA or collagen (Stolina et al., 2005), as well as clinically in RA patients (van Tuyl et al., 2010). Our results revealed that CFA highly elevated serum levels of MMP-3 (4-fold, p < 0.0001) and RANKL (3-fold, p < 0.0001), compared to the normal. Treatment with methotrexate, prednisolone, cinnamaldehyde, tadalafil, or aliskiren markedly suppressed serum MMP-3 by about 87%, 67%, 61%, 47%, 79% (p < 0.0001), and RANKL by about 75%, 71%, 58%, 55%, 76% (p < 0.0001), respectively, in comparison to the arthritic control (Fig. 3A, B). The reduction of MMP-3 by methotrexate and aliskiren reached to the normal level. Besides, RANKL was reduced to the normal level by methotrexate, prednisolone, and aliskiren.
Fig. 3.
Effect on markers of joint destruction. The levels of MMP-3 (A) and RANKL (B) were markedly elevated after induction of arthritis by CFA, but these elevations were significantly reduced after treatment with the test agents. Each column represents the mean of 8 rats ± SE. avs normal, bvs arthritic control, cvs methotrexate, and dvs prednisolone at p < 0.05.
3.4. Effect on joint tissue oxidative stress and inflammatory markers
The arthritic control rats showed a remarkable increase in joint tissue MDA content (2-fold, p < 0.0001) as a lipid peroxidation marker, along with a decline in GSH content (1-fold, p < 0.0001) which indicates reduction in antioxidant defense. Treatment with methotrexate, prednisolone, cinnamaldehyde, tadalafil, or aliskiren suppressed elevated MDA content by about 56%, 40% 42%, 49%, 61% (p < 0.0001) and increased GSH content by about 137%, 113%, 104%, 133%, 120% (p < 0.0001), respectively, compared to the arthritic control group (Fig. 4A, B). The responses observed after treatment with tadalafil and aliskiren were comparable to those of methotrexate.
Fig. 4.
Effect on joint tissue oxidative stress and inflammatory markers. Articular tissue contents of MDA (A), GSH (B), MPO (C), and NO (D). MDA, MPO, and NO were significantly elevated in arthritic control group, while treatment with methotrexate, prednisolone, cinnamaldehyde, tadalafil, or aliskiren significantly reduced these parameters. On the other hand, the antioxidant GSH was reduced in arthritic control, but the reduction was ameliorated in treated groups. Each column represents the mean of 8 rats ± SE. avs normal, bvs arthritic control, cvs methotrexate, and dvs prednisolone at p < 0.05.
The neutrophil infiltration marker, MPO, that also participates in oxidative damage in RA (Stamp et al., 2012), and NO which propagates oxidative and inflammatory responses (Bala et al., 2017) were significantly elevated in joint tissues after injection of CFA by about 2-fold at p < 0.0001. Administration of methotrexate, prednisolone, cinnamaldehyde, tadalafil, or aliskiren retarded these elevations by about 63%, 62%, 67%, 50%, 70% for MPO (p < 0.0001) and 50%, 50%, 50%, 65%, 72% for NO (p < 0.0001) (Fig. 4C, D). Furthermore, the response of aliskiren treated group was like that of the normal group regarding both MPO and NO, while the effect of tadalafil reached to the normal level regarding NO content.
3.5. Effect on protein expressions of p-JAK2, p-STAT3, iNOS, and eNOS
The protein expressions of p-JAK2, p-STAT3, and iNOS were remarkably enhanced after induction of arthritis by CFA, thus confirming the provoked inflammatory signaling. On the other hand, the protein expression of eNOS was greatly reduced indicating endothelial dysfunction. Methotrexate, prednisolone, cinnamaldehyde, tadalafil, and aliskiren significantly hindered the elevations in protein expressions of p-JAK2 (61%, 66%, 64%, 68%, 70% at p < 0.05), p-STAT3 (57%, 57%, 75%, 68%, 75% at p < 0.001), and iNOS (76%, 68%, 67%, 74%, 69% at p < 0.0001), while greatly enhanced the expression of eNOS (150%, 155%, 179%, 152%, 214% at p < 0.05), compared to the arthritic control group (Fig. 5).
Fig. 5.
Effect on protein expressions of p-JAK2, p-STAT3, iNOS, and eNOS. Western blots demonstrating the changes in protein expressions of p-JAK2, p-STAT3, iNOS, and eNOS (A). Graphical presentation for the relative quantification of p-JAK2 (B), p-STAT3 (C), iNOS (D), and eNOS (E). Protein expressions of p-JAK2, p-STAT3, and iNOS were significantly upregulated in arthritic control group, while downregulated in methotrexate, prednisolone, cinnamaldehyde, tadalafil, and aliskiren treated groups. Contrarily, the protein expression of eNOS was reduced in arthritic control, while enhanced in treated groups. avs normal, bvs arthritic control, cvs methotrexate, and dvs prednisolone at p < 0.05.
3.6. Effect on histopathological alterations in articular tissue
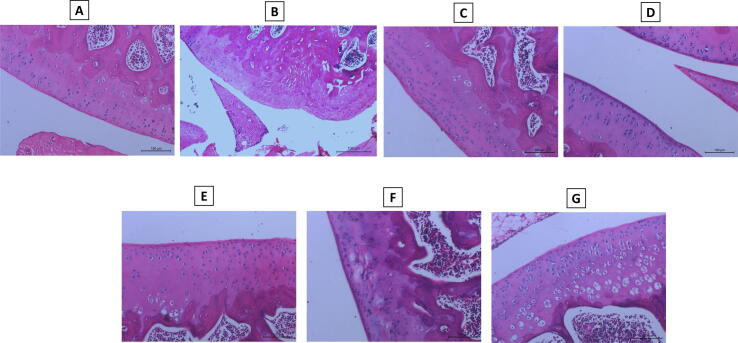
Knee joint tissue sections from the normal rats revealed no inflammation and normal histological structure of the joint (bone, cartilage and fibrous joint capsule) (Fig. 6A). However, sections from the arthritic control rats revealed marked histopathological changes (+++) in the form of synovial hyperplasia with inflammatory cells infiltration, pannus formation and erosion in cartilage and bone (Fig. 6B).
Fig. 6.
Effect on histopathological alterations. Hematoxylin and eosin stained articular tissue sections of: Normal group (A) showing no inflammation and normal histological structure of the joint (bone, cartilage and fibrous joint capsule). Arthritic control (B) showing marked histopathological changes (+++) in the form of synovial hyperplasia with inflammatory cells infiltration, pannus formation and erosion in cartilage and bone. The groups of the standard antirheumatic drugs, methotrexate and prednisolone (C, D), showing mild degree (+) of articular changes. Cinnamaldehyde, tadalafil, and aliskiren treated groups (E, F, G) showing mild (+) articular changes for cinnamaldehyde and aliskiren and moderate (++) changes for tadalafil.
On the other hand, rats treated with the standard antirheumatic drugs, methotrexate and prednisolone, showed mild degree (+) of articular changes (Fig. 6C, D). Cinnamaldehyde, tadalafil, and aliskiren treated groups (Fig. 6E, F, G) showed mild (+) articular changes for cinnamaldehyde and aliskiren, and moderate (++) changes for tadalafil.
Induction of arthritis by CFA produced synovitis, where proliferation of the synovial lining and underlying blood vessels, edema, and inflammatory cells infiltration were obvious. In many samples, the inflammatory cells encroached to the connective tissue and muscles. Synovial sloughing occurred in certain parts of the synovial membrane with mild proliferation due to fibroblast-like cells.
Pannus was formed as a granulation tissue with hyperplastic synoviocytes and inflammatory cells beside cartilage or cartilage-bone surface. The cartilage in some specimens showed irregular surface, where fibrillation along with dead or proliferated cells, extending sometimes deeper in the cartilage area. Additionally, bone destruction was accompanied by activated osteoclasts and fibroplasia. Whereas, mild to moderate degrees of the previously mentioned histopathological alterations were observed in the treated groups.
4. Discussion
Although there are different classes of antirheumatic drugs approved by international medicines regulatory agencies, conventional DMARDs and corticosteroids are the most commonly prescribed drugs especially in the early stages of the disease (Donahue et al., 2018). Because these treatments are intolerable by some patients, the search for new antirheumatic drugs possessing good efficacy and better tolerability is continuing. In our study, we examined the modulatory effects of some selected agents, with previously reported anti-inflammatory activities, against CFA-induced arthritis in rats. Besides, we compared the efficacy of these agents to that of methotrexate and prednisolone as the most commonly used drugs at the early stages of the disease.
The pro-inflammatory cytokines participate a substantial role in the inflammatory response in RA through the activation of B and T lymphocytes, where the levels of pro- greatly exceed those of anti-inflammatory cytokines (Mateen et al., 2016a, Mateen et al., 2016b). That was also evident from our results, where serum levels of IL-6 and TNF-α were greatly elevated, while the level of the anti-inflammatory cytokine IL-10 was reduced in the arthritic control group, as compared to the normal.
The prevalence of pro-inflammatory cytokines, such as IL-6 and TNF-α substantially contributes to the activation of intracellular JAK protein (Malemud, 2018). Not only IL-6, TNF-α also has been reported to increase the phosphorylation, and hence activation of STAT3 protein in chondrocytes (Malemud et al., 2012).
Janus kinases are a family of intracellular tyrosine kinase proteins, including JAK1-3 and TYK2, that have regulatory functions in different cells, including immune cells (Ghoreschi et al., 2009). That was confirmed by controlling the activities of T lymphocytes, natural killer cells, and dendritic cells, which are implicated in autoimmune diseases, upon inhibition of JAK proteins (McLornan et al., 2015). After binding of the pro-inflammatory cytokines to their receptors, especially IL-6, the cytoplasmic STAT proteins are stimulated to bind with the complex of cytokine and its JAK protein-linked receptor. Afterward, STAT protein is activated, where p-STAT dimers are formed and then translocated to the nucleus to stimulate the transcription of certain genes (Malemud, 2018). The importance of JAK/STAT signaling in the propagation of RA led to the development of new drugs targeting this pathway, such as tofacitinib (JAK3 inhibitor) which was the first JAK inhibitor approved by Food and Drug Administration (FDA) and European Medicines Agency (EMA) for treatment of RA (Kaur et al., 2014, Kawalec et al., 2018).
In accordance, our results revealed that the protein expressions of p-JAK2 and p-STAT3 were enhanced in the articular tissue of the arthritic control rats. Similarly, Bao et al. (2019) have demonstrated that CFA injection in rats could enhance both p-JAK2 and p-STAT3 expressions.
Our results also demonstrated that MMP-3 levels were elevated in the arthritic control. In parallel, different studies have explored the enhanced MMP-3 expression after induction of RA by CFA (Pandey et al., 2017; Purwaningsari et al., 2020). Besides, it has been reported that serum MMP-3 levels elevate early in RA patients, thus monitoring the subsequent articular erosion (Green et al., 2003), where MMP-3 participates a crucial role in degrading bone and cartilage via proteolysis of components of the extracellular matrix, such as proteoglycan, gelatin, and collagen (Galil et al., 2016). That could be attributed to the triggering effect of JAK2/STAT3 signaling, where Ni et al. (2019) have elucidated JAK/STAT3 as one of the pathways that contribute to MMP-1, 3, and 13 activation in RA. As well, the role of IL-6 in cartilage erosion in osteoarthritis has been explored previously through enhancement of STAT3 as the main signaling pathway, with subsequent activation of MMP-3. These findings have been confirmed through blocking the effect of IL-6 by a monoclonal antibody against IL-6 receptor, and also inhibiting the activation of STAT3 by its specific inhibitor, namely Stattic (Latourte et al., 2017).
An important contributor to bone destruction in RA is RANKL protein, which binds to its RANK receptor on osteoclasts resulting in their activation (Boman et al., 2017). RANKL can be released by different cells such as osteoblasts, synoviocytes, B lymphocytes, T lymphocytes, natural killer cells, neutrophils (Poubelle et al., 2019). The role of STAT3 in the upregulation of RANKL has been demonstrated experimentally on synovial fibroblast cells, where the chemokine CXCL16 couldn't increase the expression of RANKL upon inhibition of STAT3 (Li et al., 2016). Besides, it has been proved clinically that inhibition of RANKL by denosumab; a human monoclonal RANKL antibody used for treatment of osteoporosis; could alleviate bone erosion in RA patients (Tanaka et al., 2018, Tanaka and Ohira, 2018). In accordance, our results revealed that serum RANKL level was enhanced in arthritic control as compared to normal.
Accumulated evidence has demonstrated the role of oxidative stress and reduced antioxidant defense in RA, where RA patients showed increased reactive oxygen species generation, lipid peroxides formation, protein oxidation, DNA damage, and reduced endogenous antioxidants (Mateen et al., 2016a, Mateen et al., 2016b, Quiñonez-Flores et al., 2016, Mohammed et al., 2018). Our investigations also explored that CFA-induced arthritis elevated MDA, and reduced GSH contents in the articular tissue. Similarly, different studies have demonstrated oxidant/antioxidant imbalance after CFA-induced arthritis (Liu et al., 2017, Sun et al., 2017).
The elevated NO level in RA has been linked to the increase in inflammatory markers and endothelial dysfunction (Garg et al., 2017). That has been attributed to the increased activity of iNOS, which could be stimulated by cytokines or STAT1 (Dey et al., 2016). Besides, endothelial dysfunction in RA has been reported to reduce eNOS activity (Totoson et al., 2014), that could be attributed in part to the suppressor effect of IL-6 and its downstream regulator STAT3 on eNOS expression (Saura et al., 2006). That was also confirmed from our results, where the protein expression of iNOS was enhanced while eNOS expression was reduced in the arthritic control in comparison to the normal.
Myeloperoxidase enzyme, which is expressed by neutrophils, participates a crucial role in arthritis where MPO knockout mice showed alleviated severity of arthritis (Odobasic et al., 2014). Besides, MPO has been documented to increase endothelial permeability through hypochlorous acid generation resulting in an enhanced inflammatory response in autoimmune diseases (Strzepa et al., 2017). Additionally, MPO evokes oxidative response by the enhanced formation of hypochlorous acid, which has potent oxidizing properties (Stamp et al., 2012). From our investigation, MPO activity was elevated in the joint tissue of the arthritic control group in comparison to normal.
Treatment with cinnamaldehyde significantly reduced serum RF and the pro-inflammatory cytokines IL-6 and TNF-α, while elevated the anti-inflammatory cytokine IL-10. Our results were consistent with those of previous studies, where cinnamaldehyde has been reported to reduce IL-6 and TNF-α when administered to collagen treated rats (Mateen et al., 2019a, Mateen et al., 2019b), added to blood cell culture from RA patients (Mateen et al., 2019a, Mateen et al., 2019b), and J774A.1 cells stimulated by lipopolysaccharide resulting also in the enhancement of IL-10 production (Pannee et al., 2014). Additionally, treatment with cinnamaldehyde reduced serum RANKL and MMP-3, along with suppression of JAK2/STAT3 signaling that explored the molecular mechanism of cinnamaldehyde in reducing cartilage and bone erosion in RA. In parallel, the inhibitory effect of cinnamaldehyde on JAK/STAT signaling has been demonstrated previously on human synoviocytes (Cheng et al., 2020), as well as in different studies (Huang et al., 2015, Afify et al., 2020). Furthermore, different studies have explored the inhibitory effect of cinnamaldehyde on osteoclastogenesis induced by RANKL (Tsuji-Naito, 2008, Tsuji-Naito, 2010, Zhang et al., 2015), as well as the downregulation of MMP-3 in chondrocytes of osteoarthritis patients in vitro (Xia et al., 2019).
In our study, cinnamaldehyde treatment also improved oxidant/antioxidant balance after induction of arthritis, reduced MPO activity and iNOS expression, along with enhanced eNOS expression. In accordance, the ability of cinnamaldehyde to reduce iNOS protein expression and consequently NO production has been explored in vitro after addition of lipopolysaccharide, and also its ability to reduce iNOS expression and MPO activity in the edematous paws of mice after injection of carrageenan (Liao et al., 2012).
Rats treated with tadalafil showed significantly reduced serum RF, IL-6, TNF-α, MMP-3, RANKL, while serum levels of IL-10 were elevated compared to the arthritic control. Additionally, the joint tissue inflammatory and oxidative stress markers, p-JAK2, p-STAT3, iNOS, NO, MPO, and MDA, were alleviated along with enhanced protein expression of eNOS and GSH content.
The analgesic effect of tadalafil on zymosan-induced arthritis has been explored to be associated with reduced neutrophil infiltration and TNF-α (Rocha et al., 2011), which was in accordance with our results. Moreover, tadalafil has been demonstrated to partially protect against arthritis induced by CFA through the improvement of oxidant/antioxidant balance, but its effects on MMP-3, RANKL, and the inflammatory signaling haven't been investigated (Bahadir et al., 2018). The reduction in MMP-3 level could be attributed to the increased cGMP due to PDE-5 inhibition by tadalafil, where PDE-5 inhibition by sildenafil has been evidenced to inhibit the production of MMPs (Sun et al., 2010, Kuno et al., 2011). Comparable to our results, a previous study performed to test the efficacy of a PDE-4 inhibitor, apremilast, has explored its ability to reduce serum RANKL in ankylosing spondylitis patients (Pathan et al., 2013). Furthermore, it has been reported previously that activation of PDE enzyme is one of the mechanisms through which RANKL stimulates chemotaxis of monocytes and, subsequently, bone inflammation and loss by osteoclasts (Mosheimer et al., 2004).
Tadalafil has been demonstrated to enhance the healing of fractured bone through stimulation of osseous tissue formation (Toğral et al., 2015), which together with our results could be attributed to the enhanced expression of eNOS that has been documented to enhance bone formation by activating osteoblasts (Aguirre et al., 2001). It has been previously explored that cGMP could reduce TNF-α level and, subsequently, NO released by neutrophils through iNOS in inflamed joints (Bringel et al., 2020). Therefore, the regulation of nitric oxide production and the downregulation of inflammatory cytokines in our study could be attributed to the role of cGMP that accumulates upon treatment with a PDE-5 inhibitor, like tadalafil.
Treatment with aliskiren produced marked improvement in most of the measured parameters giving results close to that of the normal control. There is accumulated evidence regarding the participation of RAS locally in articular diseases such as RA (Cobankara et al., 2005, Sakuta et al., 2010, Wang et al., 2018). That has been explored earlier by the elevated plasma and synovial fluid levels of renin in RA and osteoarthritis patients (Boers et al., 1990, Izai et al., 1992), followed by several studies on different components of the RAS (Yigit et al., 2012, Wang et al., 2013, Chang and Wei, 2015).
The renin inhibitor aliskiren has been reported to alleviate bone turnover, which is a consequence of diabetes induction in experimental rats (Goto et al., 2017). Besides, the ability of aliskiren to alleviate articular cartilage erosion in an experimental rat model of osteoarthritis has been demonstrated (Yan and Shen, 2017). In addition, reduction of the inflammatory cytokines, TNF-α and IL-6, and oxidative stress biomarkers by aliskiren has been confirmed previously in a rat model of sepsis (Akpinar et al., 2014). Different studies have elucidated the relation between local RAS and the effect of RANKL on bone metabolism. Araújo et al. (2013) have concluded that telmisartan; an angiotensin 2 receptor blocker; could reduce the expression of RANKL and MMPs in periodontal tissues of rats. Likewise, Shuai et al. (2015) have suggested that the local RAS in trabecular bone could contribute to glucocorticoid-induced osteoporosis through an enhanced effect of RANKL.
The above-mentioned effects of aliskiren could be referred to suppression of JAK2/STAT3 intracellular signaling, where the upregulation of prorenin and its receptor has been documented to activate STAT3 (Chung et al., 2017). As well, angiotensin II has been elucidated to stimulate JAK2/STAT3 pathway by different studies (McWhinney et al., 1997, Kandalam and Clark, 2010, Ye et al., 2020).
Moreover, the modulatory effects of aliskiren on NOS enzymes, including reduced protein expression of iNOS and enhanced that of eNOS, are in harmony with those of previous studies (Zhang et al., 2014, Ziypak et al., 2015), giving further elucidation to the therapeutic effects of aliskiren against CFA-induced arthritis.
5. Conclusion
From our findings we can conclude that treatment with cinnamaldehyde, tadalafil, or aliskiren could alleviate CFA-induced arthritis by regulating inflammatory signaling and joint erosion markers. It can be emphasized also that suppression of IL-6/JAK2/STAT3 signaling pathway reduces joint destruction biomarkers, like MMP-3 and RANKL, along with reduced iNOS and enhanced eNOS expressions. Moreover, the renin inhibitor, aliskiren, could be a promising therapeutic agent for RA, owing to giving results close to those of the normal group and methotrexate, which is the gold standard antirheumatic drug.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are so grateful to Prof. Emad A. Mahdi (Department of Pathology, Faculty of Veterinary Medicine, Beni-Suef University, Egypt) for his assistance in histopathological investigation.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Raouf O.M., El-Sayed E.S.M., Manie M.F. Cinnamic acid and cinnamaldehyde ameliorate cisplatin-induced splenotoxicity in rats. J. Biochem. Mol. Toxicol. 2015;29:426–431. doi: 10.1002/jbt.21715. [DOI] [PubMed] [Google Scholar]
- Afify H., Abo-Youssef A.M., Abdel-Rahman H.M., Allam S., Azouz A.A. The modulatory effects of cinnamaldehyde on uric acid level and IL-6/JAK1/STAT3 signaling as a promising therapeutic strategy against benign prostatic hyperplasia. Toxicol. Appl. Pharmacol. 2020;402 doi: 10.1016/j.taap.2020.115122. [DOI] [PubMed] [Google Scholar]
- Aguirre J., Buttery L., O’Shaughnessy M., Afzal F., de Marticorena I.F., Hukkanen M., Huang P., MacIntyre I., Polak J. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am. J. Pathol. 2001;158:247–257. doi: 10.1016/S0002-9440(10)63963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpinar E., Halici Z., Cadirci E., Bayir Y., Karakus E., Calik M., Topcu A., Polat B. What is the role of renin inhibition during rat septic conditions: preventive effect of aliskiren on sepsis-induced lung injury. Naunyn-Schmiedeberg's Arch. Pharmacol. 2014;387:969–978. doi: 10.1007/s00210-014-1014-0. [DOI] [PubMed] [Google Scholar]
- Allam G., Mahdi E.A., Alzahrani A.M., Abuelsaad A.S. Ellagic acid alleviates adjuvant induced arthritis by modulation of pro-and anti-inflammatory cytokines. Cent. Eur. J. Immunol. 2016;41:339–349. doi: 10.5114/ceji.2016.65132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo A.A., Souza T.O., Moura L.M., Brito G.A., Aragão K.S., Araújo L.S., Medeiros C.A., Alves M.S. Araújo, Jr R.F. Effect of telmisartan on levels of IL-1, TNF-α, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J. Clin. Periodontol. 2013;40:1104–1111. doi: 10.1111/jcpe.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadir F.E., Köroğlu M.K., Yüksel M., Ercan F., Alican Y.İ. Effect of phosphodiesterase-5 inhibition on joint and muscle damage in rats with adjuvant arthritis. Turk. J. Med. Sci. 2018;48:635–643. doi: 10.3906/sag-1704-157. [DOI] [PubMed] [Google Scholar]
- Bais S., Abrol N., Prashar Y. Modulatory effect of standardised amentoflavone isolated from Juniperus communis L. agianst Freund’s adjuvant induced arthritis in rats (histopathological and X Ray anaysis) Biomed. Pharmacother. 2017;86:381–392. doi: 10.1016/j.biopha.2016.12.027. [DOI] [PubMed] [Google Scholar]
- Bala A., Mondal C., Haldar P.K., Khandelwal B. Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: clinical efficacy of dietary antioxidants. Inflammopharmacology. 2017;25:595–607. doi: 10.1007/s10787-017-0397-1. [DOI] [PubMed] [Google Scholar]
- Bao Y., Sun Y.-W., Ji J., Gan L., Zhang C.-F., Wang C.-Z., Yuan C.-S. Genkwanin ameliorates adjuvant-induced arthritis in rats through inhibiting JAK/STAT and NF-κB signaling pathways. Phytomedicine. 2019;63 doi: 10.1016/j.phymed.2019.153036. [DOI] [PubMed] [Google Scholar]
- Boers M., Breedveld F.C., Dijkmans B., Chang P., van Brummelen P., Derkx F., Cats A. Raised plasma renin and prorenin in rheumatoid vasculitis. Ann. Rheum. Dis. 1990;49:517–520. doi: 10.1136/ard.49.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman A., Kokkonen H., Ärlestig L., Berglin E., Rantapää-Dahlqvist S. Receptor activator of nuclear factor kappa-B ligand (RANKL) but not sclerostin or gene polymorphisms is related to joint destruction in early rheumatoid arthritis. Clin. Rheumatol. 2017;36:1005–1012. doi: 10.1007/s10067-017-3570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringel P.H.d.S.F., Marques G.F.O., de Queiroz Martins M.G., da Silva M.T.L., Nobre C.A.S., do Nascimento K.S., Cavada B.S., Castro R.R., Assreuy A.M.S. The lectin isolated from the alga hypnea cervicornis promotes antinociception in rats subjected to zymosan-induced arthritis: involvement of cGMP signalization and cytokine expression. Inflammation. 2020 doi: 10.1007/s10753-020-01222-z. [DOI] [PubMed] [Google Scholar]
- Buttgereit F. Views on glucocorticoid therapy in rheumatology: the age of convergence. Nat. Rev. Rheumatol. 2020;16:239–246. doi: 10.1038/s41584-020-0370-z. [DOI] [PubMed] [Google Scholar]
- Chang Y., Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin. Exp. Immunol. 2015;179:137–145. doi: 10.1111/cei.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W.-X., Zhong S., Meng X.-B., Zheng N.-Y., Zhang P., Wang Y., Qin L., Wang X.-L. Cinnamaldehyde inhibits inflammation of human synoviocyte cells through regulation of Jak/stat pathway and ameliorates collagen-induced arthritis in rats. J. Pharmacol. Exp. Ther. 2020;373:302–310. doi: 10.1124/jpet.119.262907. [DOI] [PubMed] [Google Scholar]
- Choi D.E., Jeong J.Y., Lim B.J., Chang Y.-K., Na K.-R., Shin Y.-T., Lee K.W. Aliskiren ameliorates renal inflammation and fibrosis induced by unilateral ureteral obstruction in mice. J. Urol. 2011;186:694–701. doi: 10.1016/j.juro.2011.03.122. [DOI] [PubMed] [Google Scholar]
- Chung S., Kim S., Kim M., Koh E.S., Shin S.J., Park C.W., Chang Y.S., Kim H.-S. Treatment combining aliskiren with paricalcitol is effective against progressive renal tubulointerstitial fibrosis via dual blockade of intrarenal renin. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobankara V., Öztürk M.A., Kiraz S., Ertenli I., Haznedaroglu I.C., Pay S., Çalgüneri M. Renin and angiotensin-converting enzyme (ACE) as active components of the local synovial renin-angiotensin system in rheumatoid arthritis. Rheumatol. Int. 2005;25:285–291. doi: 10.1007/s00296-004-0564-8. [DOI] [PubMed] [Google Scholar]
- Dey P., Panga V., Raghunathan S. A cytokine signalling network for the regulation of inducible nitric oxide synthase expression in rheumatoid arthritis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue K.E., Gartlehner G., Schulman E.R., Jonas B., Coker-Schwimmer E., Patel S.V., Weber R.P., Lohr K.N., Bann C., Viswanathan M. Drug therapy for early rheumatoid arthritis: a systematic review update [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US) 2018 [PubMed] [Google Scholar]
- El-Gaphar O.A.A., Abo-youssef A.M., Abo-saif A.A. Differential effects of atorvastatin and prednisolone on inflammation, oxidative stress and hematological biomarkers on freund's adjuvant induced-arthritis in rats. Int. J. Pharm. Sci. Rev. Res. 2015;33:235–241. [Google Scholar]
- Galil S.M.A., El-Shafey A.M., Hagrass H.A., Fawzy F., Sammak A.E. Baseline serum level of matrix metalloproteinase-3 as a biomarker of progressive joint damage in rheumatoid arthritis patients. Int. J. Rheum. Dis. 2016;19:377–384. doi: 10.1111/1756-185X.12434. [DOI] [PubMed] [Google Scholar]
- Garg N., Syngle A., Krishan P. Nitric oxide: link between inflammation and endothelial dysfunction in rheumatoid arthritis. Int. J. Angiol. 2017;26:165–169. doi: 10.1055/s-0036-1597577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K., Laurence A., O’Shea J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S., Fujii H., Awata R., Kono K., Nakai K., Shinohara M., Nishi S. Direct renin inhibitor aliskiren ameliorates low bone turnover in diabetic rat. Nephrol. Dial. Transplant. 2017;32 iii232-iii233. [Google Scholar]
- Green M., Gough A., Devlin J., Smith J., Astin P., Taylor D., Emery P. Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology. 2003;42:83–88. doi: 10.1093/rheumatology/keg037. [DOI] [PubMed] [Google Scholar]
- Gunawardena D., Karunaweera N., Lee S., van Der Kooy F., Harman D.G., Raju R., Bennett L., Gyengesi E., Sucher N.J., Münch G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts–identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015;6:910–919. doi: 10.1039/c4fo00680a. [DOI] [PubMed] [Google Scholar]
- Hawkins P., Armstrong R., Boden T., Garside P., Knight K., Lilley E., Seed M., Wilkinson M., Williams R.O. Applying refinement to the use of mice and rats in rheumatoid arthritis research. Inflammopharmacology. 2015;23:131–150. doi: 10.1007/s10787-015-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoes J.N., Jacobs J.W., Buttgereit F., Bijlsma J.W. Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat. Rev. Rheumatol. 2010;6:693–702. doi: 10.1038/nrrheum.2010.179. [DOI] [PubMed] [Google Scholar]
- Huang J.S., Lee Y.H., Chuang L.Y., Guh J.Y., Hwang J.Y. cinnamaldehyde and nitric oxide attenuate advanced glycation end products-induced the JAK/STAT signaling in human renal tubular cells. J. Cell. Biochem. 2015;116:1028–1038. doi: 10.1002/jcb.25058. [DOI] [PubMed] [Google Scholar]
- Izai M., Miyazaki S., Murai R., Morioka Y., Hayashi H., Nishiura M., Miura K. Prorenin-renin axis in synovial fluid in patients with rheumatoid arthritis and osteoarthritis. Endocrinol. Jpn. 1992;39:259–267. doi: 10.1507/endocrj1954.39.259. [DOI] [PubMed] [Google Scholar]
- Kandalam U., Clark M.A. Angiotensin II activates JAK2/STAT3 pathway and induces interleukin-6 production in cultured rat brainstem astrocytes. Regul. Pept. 2010;159:110–116. doi: 10.1016/j.regpep.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Kaur K., Kalra S., Kaushal S. Systematic review of tofacitinib: a new drug for the management of rheumatoid arthritis. Clin. Ther. 2014;36:1074–1086. doi: 10.1016/j.clinthera.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Kawalec P., Śladowska K., Malinowska-Lipień I., Brzostek T., Kózka M. European perspective on the management of rheumatoid arthritis: clinical utility of tofacitinib. Ther. Clin. Risk Manag. 2018;14:15–29. doi: 10.2147/TCRM.S138677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno Y., Iyoda M., Shibata T., Hirai Y., Akizawa T. Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in non-insulin-dependent Otsuka Long-Evans Tokushima Fatty rats. Br. J. Pharmacol. 2011;162:1389–1400. doi: 10.1111/j.1476-5381.2010.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latourte A., Cherifi C., Maillet J., Ea H.-K., Bouaziz W., Funck-Brentano T., Cohen-Solal M., Hay E., Richette P. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2017;76:748–755. doi: 10.1136/annrheumdis-2016-209757. [DOI] [PubMed] [Google Scholar]
- Lerner A., Neidhöfer S., Reuter S., Matthias T. MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2018;32:550–562. doi: 10.1016/j.berh.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Li C.-H., Xu L.-L., Zhao J.-X., Sun L., Yao Z.-Q., Deng X.-L., Liu R., Yang L., Xing R., Liu X.-Y. CXCL16 upregulates RANKL expression in rheumatoid arthritis synovial fibroblasts through the JAK2/STAT3 and p38/MAPK signaling pathway. Inflamm. Res. 2016;65:193–202. doi: 10.1007/s00011-015-0905-y. [DOI] [PubMed] [Google Scholar]
- Liao J.-C., Deng J.-S., Chiu C.-S., Hou W.-C., Huang S.-S., Shie P.-H., Huang G.-J. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2012;2012 doi: 10.1155/2012/429320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-Y., Hou Y.-L., Cao R., Qiu H.X., Cheng G.-H., Tu R., Wang L., Zhang J.-L., Liu D. Protodioscin ameliorates oxidative stress, inflammation and histology outcome in Complete Freund’s adjuvant induced arthritis rats. Apoptosis. 2017;22:1454–1460. doi: 10.1007/s10495-017-1420-0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li M., He Q., Yang X., Ruan F., Sun G. Periploca forrestii saponin ameliorates murine CFA-induced arthritis by suppressing cytokine production. Mediators Inflamm. 2016;2016:7941684. doi: 10.1155/2016/7941684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malemud C.J. Negative regulators of JAK/STAT signaling in rheumatoid arthritis and osteoarthritis. Int. J. Mol. Sci. 2017;18:E484. doi: 10.3390/ijms18030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malemud C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018;10:117–127. doi: 10.1177/1759720X18776224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malemud C.J., Sun Y., Pearlman E., Ginley N.M., Awadallah A., Wisler B.A., Dennis J.E. Monosodium urate and tumor necrosis factor-α increase apoptosis in human chondrocyte cultures. Rheumatology (Sunnyvale, Calif.) 2012;2:113. doi: 10.4172/2161-1149.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H.M., Salama A.A., Abdel-Salam R.M., Ahmed N.A., Yassen N.N., Zaki H.F. The anti-inflammatory and anti-fibrotic effects of tadalafil in thioacetamide-induced liver fibrosis in rats. Can. J. Physiol. Pharmacol. 2018;96:1308–1317. doi: 10.1139/cjpp-2018-0338. [DOI] [PubMed] [Google Scholar]
- Mateen S., Moin S., Khan A.Q., Zafar A., Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateen S., Rehman M.T., Shahzad S., Naeem S.S., Faizy A.F., Khan A.Q., Khan M.S., Husain F.M., Moin S. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur. J. Pharmacol. 2019;852:14–24. doi: 10.1016/j.ejphar.2019.02.031. [DOI] [PubMed] [Google Scholar]
- Mateen S., Shahzad S., Ahmad S., Naeem S.S., Khalid S., Akhtar K., Rizvi W., Moin S. Cinnamaldehyde and eugenol attenuates collagen induced arthritis via reduction of free radicals and pro-inflammatory cytokines. Phytomedicine. 2019;53:70–78. doi: 10.1016/j.phymed.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Mateen S., Zafar A., Moin S., Khan A.Q., Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta. 2016;455:161–171. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- McInnes I.B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet. 2017;389:2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- McLornan D.P., Khan A.A., Harrison C.N. Immunological consequences of JAK inhibition: friend or foe? Curr. Hematol. Malig. Rep. 2015;10:370–379. doi: 10.1007/s11899-015-0284-z. [DOI] [PubMed] [Google Scholar]
- McWhinney C.D., Hunt R.A., Conrad K.M., Dostal D.E., Baker K.M. The type I angiotensin II receptor couples to Stat1 and Stat3 activation through Jak2 kinase in neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 1997;29:2513–2524. doi: 10.1006/jmcc.1997.0489. [DOI] [PubMed] [Google Scholar]
- Medeiros V.d.F.L.P., Azevedo Í.M., Carvalho M.D.F., Oliveira C.N., Egito E.S.T.d., Medeiros A.C. The renoprotective effect of oral Tadalafil pretreatment on ischemia/reperfusion injury in rats. Acta Cir. Bras. 2017;32:90–97. doi: 10.1590/s0102-865020170201. [DOI] [PubMed] [Google Scholar]
- Mohammed A.H., Al Neaimy A., Salih K. Oxidative stress in patients with rheumatoid arthritis. Mosul J. Nurs. 2018;6:14–19. [Google Scholar]
- Mokry J., Urbanova A., Medvedova I., Kertys M., Mikolka P., Kosutova P., Mokra D. Effects of tadalafil (PDE5 inhibitor) and roflumilast (PDE4 inhibitor) on airway reactivity and markers of inflammation in ovalbumin-induced airway hyperresponsiveness in guinea pigs. J. Physiol. Pharmacol. 2017;68:721–730. [PubMed] [Google Scholar]
- Mosheimer B.A., Kaneider N.C., Feistritzer C., Sturn D.H., Wiedermann C.J. Expression and function of RANK in human monocyte chemotaxis. Arthritis Rheum. 2004;50:2309–2316. doi: 10.1002/art.20352. [DOI] [PubMed] [Google Scholar]
- Ni S., Li C., Xu N., Liu X., Wang W., Chen W., Wang Y., van Wijnen A.J. Follistatin-like protein 1 induction of matrix metalloproteinase 1, 3 and 13 gene expression in rheumatoid arthritis synoviocytes requires MAPK, JAK/STAT3 and NF-κB pathways. J. Cell. Physiol. 2019;234:454–463. doi: 10.1002/jcp.26580. [DOI] [PubMed] [Google Scholar]
- Odobasic D., Yang Y., Muljadi R.C., O'Sullivan K.M., Kao W., Smith M., Morand E.F., Holdsworth S.R. Endogenous myeloperoxidase is a mediator of joint inflammation and damage in experimental arthritis. Arthritis Rheumatol. 2014;66:907–917. doi: 10.1002/art.38299. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Kurita M., Yamaguchi H., Numakura Y., Oka M. Effect of tadalafil on chronic pelvic pain and prostatic inflammation in a rat model of experimental autoimmune prostatitis. The Prostate. 2018;78:707–713. doi: 10.1002/pros.23514. [DOI] [PubMed] [Google Scholar]
- Pandey P., Bhatt P., Kumar V. 75 Moringa oleifera lam ameliorates adjuvant induced arthritis via inhibition of inflammatory mediators and down-regulation of mmp3 and mmp-9 proteins. Lupus Sci. Med. 2017;4(Suppl 1) doi: 10.1136/lupus-2017-000215.75. [DOI] [Google Scholar]
- Pannee C., Chandhanee I., Wacharee L. Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A. 1 cells. J. Adv. Pharm. Technol. Res. 2014;5:164–170. doi: 10.4103/2231-4040.143034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan E., Abraham S., Van Rossen E., Withrington R., Keat A., Charles P.J., Paterson E., Chowdhury M., McClinton C., Taylor P.C. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in ankylosing spondylitis. Ann. Rheum. Dis. 2013;72:1475–1480. doi: 10.1136/annrheumdis-2012-201915. [DOI] [PubMed] [Google Scholar]
- Poubelle P.E., Rusu D., Chakravarti A., Allaeys I. Neutrophils present in synovial fluids and tissues of rheumatoid arthritis are a major source of RANKL. Ann. Rheum. Dis. 2019 doi: 10.1136/ard.2011.153312. [DOI] [Google Scholar]
- Purwaningsari D., Nugraha J., Wahyuningsih S.P.A., Hayaza S., Susilo R.J.K., Darmanto W. Effect of polysaccharide krestin on MMP3 expression and foot diameter in rheumatoid arthritis in rat. Indian Vet. J. 2020;97:24–26. [Google Scholar]
- Quiñonez-Flores C.M., González-Chávez S.A., Del Río Nájera D., Pacheco-Tena C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed. Res. Int. 2016;2016:6097417. doi: 10.1155/2016/6097417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, F.A.C.d., Silva Jr, F., Leite, A., Leite, A., Girão, V.C.C., Castro, R.Cunha, F.d.Q., 2011. Tadalafil analgesia in experimental arthritis involves suppression of intra‐articular TNF release. Br. J. Pharmacol. 164, 828–835. [DOI] [PMC free article] [PubMed]
- Saad M.A., El-Sahhar A.E., Arab H.H., Al-Shorbagy M.Y. Nicorandil abates arthritic perturbations induced by complete Freund's adjuvant in rats via conquering TLR4-MyD88-TRAF6 signaling pathway. Life Sci. 2019;218:284–291. doi: 10.1016/j.lfs.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Sakuta T., Morita Y., Satoh M., Fox D.A., Kashihara N. Involvement of the renin–angiotensin system in the development of vascular damage in a rat model of arthritis: effect of angiotensin receptor blockers. Arthritis Rheum. 2010;62:1319–1328. doi: 10.1002/art.27384. [DOI] [PubMed] [Google Scholar]
- Saura M., Zaragoza C., Bao C., Herranz B., Rodriguez-Puyol M., Lowenstein C.J. Stat3 mediates interelukin-6 inhibition of human endothelial nitric-oxide synthase expression. J. Biol. Chem. 2006;281:30057–30062. doi: 10.1074/jbc.M606279200. [DOI] [PubMed] [Google Scholar]
- Shenoy P., Agarwal V. Phosphodiesterase inhibitors in the management of autoimmune disease. Autoimmun. Rev. 2010;9:511–515. doi: 10.1016/j.autrev.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Shinde C.G., Venkatesh M., Kumar T.P., Shivakumar H. Methotrexate: a gold standard for treatment of rheumatoid arthritis. J. Pain Palliat. Care Pharmacother. 2014;28:351–358. doi: 10.3109/15360288.2014.959238. [DOI] [PubMed] [Google Scholar]
- Shuai B., Yang Y., Shen L., Zhu R., Xu X., Ma C., Lv L., Zhao J., Rong J. Local renin-angiotensin system is associated with bone mineral density of glucocorticoid-induced osteoporosis patients. Osteoporos. Int. 2015;26:1063–1071. doi: 10.1007/s00198-014-2992-y. [DOI] [PubMed] [Google Scholar]
- Solomon D.H., Glynn R.J., Karlson E.W., Lu F., Corrigan C., Colls J., Xu C., MacFadyen J., Barbhaiya M., Berliner N. Adverse effects of low-dose methotrexate: a randomized trial. Ann. Intern. Med. 2020 doi: 10.7326/M19-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp L.K., Khalilova I., Tarr J.M., Senthilmohan R., Turner R., Haigh R.C., Winyard P.G., Kettle A.J. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology. 2012;51:1796–1803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- Stolina M., Adamu S., Ominsky M., Dwyer D., Asuncion F., Geng Z., Middleton S., Brown H., Pretorius J., Schett G. RANKL is a marker and mediator of local and systemic bone loss in two rat models of inflammatory arthritis. J. Bone Miner. Res. 2005;20:1756–1765. doi: 10.1359/JBMR.050601. [DOI] [PubMed] [Google Scholar]
- Strzepa A., Pritchard K.A., Dittel B.N. Myeloperoxidase: a new player in autoimmunity. Cell. Immunol. 2017;317:1–8. doi: 10.1016/j.cellimm.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.-L., Wei J., Bi L.-Q. Rutin attenuates oxidative stress and proinflammatory cytokine level in adjuvant induced rheumatoid arthritis via inhibition of NF-κB. Pharmacology. 2017;100:40–49. doi: 10.1159/000451027. [DOI] [PubMed] [Google Scholar]
- Sun X.Z., Li Z.F., Liu Y., Fang P., Li M.X. Inhibition of cGMP phosphodiesterase 5 suppresses matrix metalloproteinase-2 production in pulmonary artery smooth muscles cells. Clin. Exp. Pharmacol. Physiol. 2010;37:362–367. doi: 10.1111/j.1440-1681.2009.05304.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Tanaka Y., Ishiguro N., Yamanaka H., Takeuchi T. RANKL: a therapeutic target for bone destruction in rheumatoid arthritis. Mod. Rheumatol. 2018;28:9–16. doi: 10.1080/14397595.2017.1369491. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Ohira T. Mechanisms and therapeutic targets for bone damage in rheumatoid arthritis, in particular the RANK-RANKL system. Curr. Opin. Pharmacol. 2018;40:110–119. doi: 10.1016/j.coph.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Toğral G., Arıkan Ş.M., Korkusuz P., Hesar R.H., Ekşioğlu M.F. Positive effect of tadalafil, a phosphodiesterase-5 inhibitor, on fracture healing in rat femur. Jt. Dis. Relat. Surg. 2015;26:137–144. doi: 10.5606/ehc.2015.29. [DOI] [PubMed] [Google Scholar]
- Totoson P., Maguin-Gaté K., Prati C., Wendling D., Demougeot C. Mechanisms of endothelial dysfunction in rheumatoid arthritis: lessons from animal studies. Arthritis Res. Ther. 2014;16:202. doi: 10.1186/ar4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji-Naito K. Aldehydic components of cinnamon bark extract suppresses RANKL-induced osteoclastogenesis through NFATc1 downregulation. Biorg. Med. Chem. 2008;16:9176–9183. doi: 10.1016/j.bmc.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Tsuji-Naito K. Additive inhibitory effects of α-lipoic acid with cinnamaldehyde against osteoclastogenesis. Food Sci. Technol. Res. 2010;16:353–358. [Google Scholar]
- van Tuyl L.H., Voskuyl A.E., Boers M., Geusens P., Landewé R.B., Dijkmans B.A., Lems W.F. Baseline RANKL: OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:1623–1628. doi: 10.1136/ard.2009.121764. [DOI] [PubMed] [Google Scholar]
- Wahba M.G.F., Messiha B.A.S., Abo-Saif A.A. Ramipril and haloperidol as promising approaches in managing rheumatoid arthritis in rats. Eur. J. Pharmacol. 2015;765:307–315. doi: 10.1016/j.ejphar.2015.08.026. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu S., Zhu J., Yuan J., Wu J., Zhou A., Wu Y., Zhao W., Huang Q., Chang Y. Angiotensin II type 2 receptor correlates with therapeutic effects of losartan in rats with adjuvant-induced arthritis. J. Cell. Mol. Med. 2013;17:1577–1587. doi: 10.1111/jcmm.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kou J., Zhang H., Wang C., Li H., Ren Y., Zhang Y. The renin-angiotensin system in the synovium promotes periarticular osteopenia in a rat model of collagen-induced arthritis. Int. Immunopharmacol. 2018;65:550–558. doi: 10.1016/j.intimp.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Xia T., Gao R., Zhou G., Liu J., Li J., Shen J. Trans-Cinnamaldehyde inhibits IL-1β-stimulated inflammation in chondrocytes by suppressing NF-κB and p38-JNK pathways and exerts chondrocyte protective effects in a rat model of osteoarthritis. Biomed. Res. Int. 2019;2019:4039472. doi: 10.1155/2019/4039472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K., Shen Y. Aliskiren has chondroprotective efficacy in a rat model of osteoarthritis through suppression of the local renin-angiotensin system. Mol. Med. Rep. 2017;16:3965–3973. doi: 10.3892/mmr.2017.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Luo W., Khan Z.A., Wu G., Xuan L., Shan P., Lin K., Chen T., Wang J., Hu X. Celastrol attenuates angiotensin II–induced cardiac remodeling by targeting STAT3. Circ. Res. 2020;126:1007–1023. doi: 10.1161/CIRCRESAHA.119.315861. [DOI] [PubMed] [Google Scholar]
- Yigit S., Inanir A., Tural S., Ates O. Association of angiotensin converting enzyme (ACE) gene I/D polymorphism and rheumatoid arthritis. Gene. 2012;511:106–108. doi: 10.1016/j.gene.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Zhang H., Guo Y., Xia L., Wang L., Zhou X., Zheng X., Lai N., Yao C. Protective effect of cinnamic aldehyde on hormone-induced osteoclasts differentiation and its molecular mechanisms. Chin. Pharmacol. Bull. 2015;2015(1):92–96. [Google Scholar]
- Zhang W., Han Y., Meng G., Bai W., Xie L., Lu H., Shao Y., Wei L., Pan S., Zhou S. Direct renin inhibition with aliskiren protects against myocardial ischemia/reperfusion injury by activating nitric oxide synthase signaling in spontaneously hypertensive rats. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Liu H., Guo D. Aliskiren attenuates cardiac dysfunction by modulation of the mTOR and apoptosis pathways. Braz. J. Med. Biol. Res. 2020;53 doi: 10.1590/1414-431X20198793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziypak T., Halici Z., Alkan E., Akpinar E., Polat B., Adanur S., Cadirci E., Ferah I., Bayir Y., Karakus E. Renoprotective effect of aliskiren on renal ischemia/reperfusion injury in rats: electron microscopy and molecular study. Ren. Fail. 2015;37:343–354. doi: 10.3109/0886022X.2014.991327. [DOI] [PubMed] [Google Scholar]