Abstract
The cardiovascular effects of testosterone (T) are controversial. Low T has been associated with accelerated vascular aging, characterized by large elastic artery stiffening (decreased compliance), intimal-medial thickening (IMT), and endothelial dysfunction. Endurance exercise improves vascular function, but resistance training may increase arterial stiffness. We sought to determine whether T supplementation improved markers of vascular aging in men with low–normal T and whether T supplementation prevented arterial stiffness with resistance exercise. We studied 160 community-dwelling older men (66 ± 5 yr) with low–normal baseline total T levels (200–350 ng/dl). Participants were randomized to transdermal T gel targeting either a lower (400–550 ng/dl) or higher (600–1,000 ng/dl) T range or to placebo gel and to either progressive resistance training (PRT) or to no exercise for 12 mo. Carotid artery stiffness (arterial compliance) and carotid IMT were measured at baseline, 6 mo, and 12 mo. Endothelial function (brachial artery flow-mediated dilation) was measured in a subset (n = 86). Changes in carotid artery compliance, IMT, and endothelial function with either the lower or higher range of T supplementation were not different from placebo at 6 or 12 mo. There were no differences between PRT and no PRT groups, alone or with T supplementation, in changes in any of the vascular measures at either time point. Supplementation of T and PRT in older men with low–normal levels do not appear to improve or harm vascular function.
NEW & NOTEWORTHY Increased promotion and prescription of testosterone (T) to aging men has raised concerns about potential adverse cardiovascular effects. We show that in older men with T levels in the low–normal range, 12 mo of T supplementation with or without resistance exercise did not improve or harm vascular function.
Keywords: arterial stiffness, endothelial function, resistance exercise, testosterone, vascular function
INTRODUCTION
Over the last decade, aggressive marketing of testosterone (T) has resulted in a 10-fold increase in T prescriptions in the United States, with sales of $2.2 billion in 2013 (19, 41). These increases have raised concerns about adverse effects of potentially inappropriate use of T. Whereas treatment is clearly indicated in men with symptomatic, unequivocally low T, health benefits in older men with T levels in the low–normal range have not been consistently demonstrated. Furthermore, potential adverse cardiovascular events with T treatment have generated additional controversy. Although population studies suggest an association between low T and cardiovascular and all-cause mortality (1), no randomized controlled trials of T therapy have specifically focused on cardiovascular events, and systematic reviews of the limited data from trials of T therapy have reached opposite conclusions (17, 42). Better understanding of the pathophysiology of T and cardiovascular health may help clarify potential risks and benefits of T treatment.
Aging is the major risk factor for the development of cardiovascular disease (CVD), specifically vascular aging (24). One hallmark of vascular aging is increased stiffness of the large elastic arteries within the cardiothoracic region. The mechanisms underlying age-related increases in arterial stiffness are not completely understood but appear to include both structural and functional changes.
Lower levels of T have been associated with arterial stiffness (40), and longitudinal declines in T predicted the increase in arterial stiffening in older men (22). T supplementation to normal levels also improved arterial stiffness in older men with hypogonadism (43). Although these studies suggest a beneficial effect of T on arterial stiffness, there have been no studies of older men with low–normal T levels, and it is not known whether T therapy is beneficial in this population.
Regular aerobic exercise attenuates age-related arterial stiffening (33) and partially restores arterial compliance in previously sedentary middle-aged and older men (37). In contrast, resistance training has been associated with increased arterial stiffness (26, 27). If T has favorable effects on arterial stiffness, supplementing T in older men during resistance training may mitigate increases in arterial stiffness. We previously reported the effects of T supplementation targeting a lower or higher range with or without progressive resistance training (PRT) on function, strength, and body composition in older men with low–normal serum T levels (200–350 ng/dl; 6.94–12.15 nmol/l) (21). In this secondary analysis, we report the results of vascular measures obtained to determine the effects of different levels of T supplementation, alone or with PRT, on arterial stiffness and potential mechanisms underlying these effects. We hypothesized that arterial stiffness would decrease with either level of T supplementation alone and that arterial stiffness would increase with PRT alone but not in combination with T supplementation.
MATERIALS AND METHODS
Study population and design.
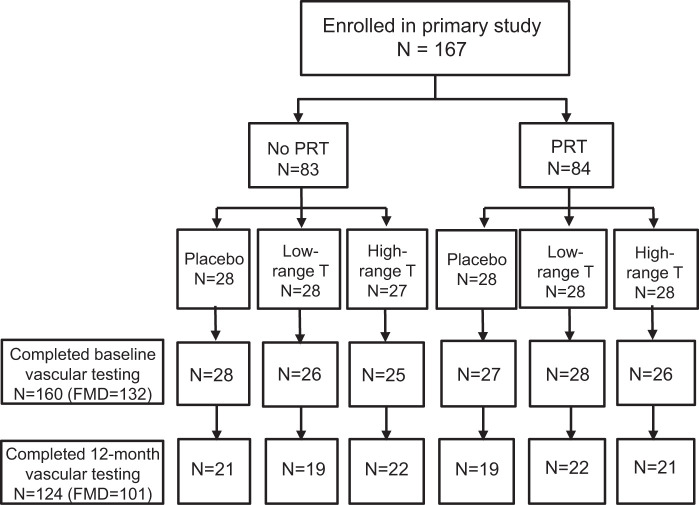
Details of the study population and experimental design of the primary trial were previously published (NCT 00112151) (21). Briefly, healthy community-dwelling men at least 60 yr of age with total serum T between 200 and 350 ng/dl (6.94–12.15 nmol/l) on 2 separate fasted morning samples were recruited from the Denver Metropolitan area. Participants were sedentary or recreationally active, weight stable (≥6 mo), with body mass index <35 kg/m2, no exercise-limiting conditions or evidence of active coronary artery disease on the screening graded exercise treadmill test, and on a stable medication regimen (≥3 mo). Exclusion criteria included an abnormal digital rectal exam or transrectal ultrasound, prostate-specific antigen above the age-adjusted normal level, an American Urological Association symptom score >20 (4), a history of prostate or breast cancer, uncontrolled hypertension, diabetes, untreated dyslipidemia, hematocrit >52%, and use of androgenic steroids or other drugs that could affect T levels. Nine-hundred thirty-four participants were screened for eligibility; 167 were enrolled. Vascular measures for the present secondary analysis were obtained in 160 participants (Fig. 1). The study was a 12-mo, randomized, placebo-controlled trial of 2 levels of T supplementation, targeting a lower- [400–550 ng/dl (13.88–19.09 nmol/l)] or higher-range [600–1,000 ng/dl (20.82–34.70 nmol/l)] of T versus placebo. T was supplied as 1% transdermal gel (AbbVie, Inc., Chicago, IL) or placebo gel. Starting doses were 2.5 and 5.0 g daily in the lower- and higher-range groups, respectively. Serum T levels were measured every 2 wk for the first 12 wk with dose titrations made in 2.5-g increments to achieve targeted levels. T treatment was crossed with thrice-weekly PRT consisting of four upper- and three lower-body exercises or no PRT. Training started with 2–3 wk of 3 sets of 10–12 repetitions at 50%–60% of baseline 1-repetition maximum, then progressed to 3 sets of 6–8 repetitions at 80% of the last 1-repetition maximum, measured monthly. Resistance was increased by 5%–10% when 3 full sets could be completed. Outcome measures were determined by assessors blinded to group assignment. The study took place at the University of Colorado Clinical and Translational Sciences Institute Clinical and Translational Research Center. The study was approved by the Colorado Multiple Institutional Review Board. All participants provided written informed consent.
Fig. 1.
Study enrollment. A total of 167 men were enrolled in the parent trial. The vascular cohort included 160 participants with baseline vascular testing. A total of 124 participants completed 12-mo testing with high-quality images and were included in the present analysis. FMD, flow-mediated dilation; PRT, progressive resistance training; T, testosterone.
Vascular measurements.
Vascular measurements were obtained at baseline, 6 mo, and 12 mo. Participants were studied in the supine position following an overnight fast with proper hydration (water only) and abstinence from caffeine. Follow-up studies were completed at the same time of day, at least 24 h after the last bout of exercise.
Carotid artery imaging.
Determination of carotid artery stiffness expressed as both arterial compliance and beta stiffness index was performed using high-resolution ultrasound imaging and measures of arterial pressure as previously described (28). Briefly, a longitudinal image of the cephalic portion of the carotid artery was acquired ~1–2 cm distal to the carotid bulb. Carotid images were analyzed for systolic and diastolic diameters and carotid artery intimal-medial thickness (IMT) using a computerized semiautomated edge-detection software, which allows accurate identification and measurements of carotid artery lumen diameter and IMT over a length of the artery (Vascular Analysis Tools v.5.5, Medical Imaging Applications, Coralville, IA). Peripheral artery blood pressures were measured with a semiautomated device (Dinamap, Johnson & Johnson, New Brunswick, NJ) over the brachial artery. To investigate possible structural determinants of arterial stiffness, carotid artery IMT was measured during end-diastole as previously described (28). All images were coded by number and analyzed by the same individual (K. L. M.) blinded to group assignment. The coefficient of variation (CV) and intraclass correlation coefficient for trial-to-trial reliability measured in nine individuals for carotid artery diameter and carotid arterial compliance were 2% and 0.88, and 4% and 0.94, respectively.
Endothelial function.
To investigate possible functional determinants of arterial stiffness, measures of endothelial function were subsequently added to the protocol and thus were only captured in a subpopulation of men (n = 132). Brachial artery flow-mediated dilation (FMD) was measured using duplex ultrasonography (GE Vivid I) with a multifrequency linear-array transducer as previously described (28). Briefly, a pediatric cuff was placed on the upper forearm, and brachial artery images were acquired ~3–6 cm above the antecubital fossa. The ultrasound probe was clamped to avoid involuntary movement. Probe placement was measured and landmarks were identified to ensure imaging of the same arterial segment with serial measurements. After obtaining concurrent measures of baseline brachial artery diameter and blood flow velocity, reactive hyperemia was produced by inflating the cuff to 250 mmHg for 5 min followed by rapid deflation. After cuff release, Doppler blood flow velocity was acquired for the first 10–15 beats, and B-mode ultrasound brachial artery diameter images were measured continuously for 2 min. Brachial artery diameter and blood flow velocity were analyzed using commercial software (Vascular Analysis Tools 5.5.1, Medical Imaging Applications, Coralville, IA). All images were coded by number and analyzed by the same individual (K. L. M.) blinded to group assignment. The CV and intraclass correlation coefficient for trial-to-trial reliability measured in 10 individuals for baseline brachial artery diameter, peak diameter, and FMD (%) were 2% and 0.97, 1.5% and 0.99, and 2.2% and 0.99, respectively.
Body composition, aerobic exercise capacity, physical activity, and blood sampling.
Body composition was measured using dual-energy X-ray absorptiometry (Hologic Discovery, Bedford, MA). Maximal oxygen consumption was determined by indirect calorimetry (TrueMax 2400; Parvo-Medics, Sandy, UT) during a standard, graded exercise treadmill test. Physical activity was assessed using the Yale Physical Activity Survey (9).
Total serum T, sex-hormone binding globulin (SHBG), and estradiol were measured by chemiluminescence using a Beckman Coulter Access II analyzer. Intra- and interday CVs were 2.1% and 5.1% for T, 3.6% and 5.7% for SHBG, and 4.3% and 8.2% for estradiol. Fasted plasma concentrations of glucose, insulin, total (Roche Diagnostic Systems, Indianapolis, IN), and high-density lipoprotein (Diagnostic Chemicals Ltd., Oxford, CT) cholesterol were determined using enzymatic/colorimetric methods. Low-density lipoprotein cholesterol was calculated using the Friedewald equation (15). All assays were performed by the Clinical Laboratory Improvement Amendments (CLIA)-certified Clinical and Translational Research Center Core laboratory at baseline, 6 mo, and 12 mo.
Statistical analysis.
Descriptive statistics and plots were produced for each vascular outcome measure (carotid arterial compliance, beta stiffness index, IMT, and FMD) at each time point for each of the six treatment groups (3 levels of T crossed with 2 levels of PRT). Extreme outliers were verified or corrected. Preliminary investigation plotted 6- and 12-mo changes in each outcome against baseline T for each treatment group to determine whether the degree of change was dependent on baseline T levels.
For each end point, the SAS software’s GENMOD procedure was used to fit a model to the correlated repeated measures using the generalized estimating equations method. For each model, treatment group (based on the PRT group and T treatment) and time were included in a cell means model to generate the estimated means and associated 95% confidence intervals at each time point (0, 6, and 12 mo) for each treatment.
General linear models were fit to investigate treatment effects on the change in each of the outcome measures at 6 and 12 mo. For each model, the T treatment group and PRT group were included as main effects. Covariates included age, baseline outcome measure, and use of antihypertensive or lipid-lowering medications. Interactions involving the T group, PRT group, and medication use were removed from the model as none was statistically significant.
Because the study was not powered to detect effects of T or PRT on secondary outcomes, exploratory analyses were conducted using paired t-tests on the change from baseline to 6 and 12 mo for each of the 6 treatment groups to evaluate within-group changes.
Pearson correlational analyses were used to test for linear bivariate relations between age, T, and estradiol levels with the vascular outcomes. Two-sided P values < 0.05 were used to define statistical significance. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Participants.
One hundred sixty-seven men were enrolled in the primary randomized controlled trial. The present secondary analysis includes only those participants who completed baseline vascular measures (n = 160 for carotid, n = 132 for FMD; Fig. 1). Of these, 26 participants were lost to follow up, and 10 had either poor quality or missing images, leaving 124 in the present analysis (101 for FMD). There were no differences in baseline characteristics or sex hormones, SHBG, lipids, glucose, or insulin levels among treatment groups (Tables 1 and 2). The subset of men in whom FMD was measured was not different from the larger group with respect to any of the baseline characteristics.
Table 1.
Baseline characteristics of participants
| No PRT |
PRT |
||||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 21) | Lower-range T (n = 19) | Higher-range T (n = 22) | Placebo (n = 19) | Lower-range T (n = 22) | Higher-range T (n = 21) |
P value |
|
| Characteristic | |||||||
| Age, yr | 67 ± 5 | 66 ± 5 | 67 ± 6 | 66 ± 5 | 69 ± 8 | 66 ± 5 | 0.69 |
| Race | 0.63 | ||||||
| White | 21 (100.0) | 19 (100.0) | 20 (90.9) | 18 (94.7) | 20 (90.9) | 20 (95.2) | |
| Black | 0 (0.0) | 0 (0.0) | 1 (4.5) | 1 (5.3) | 2 (9.1) | 1 (4.8) | |
| Other | 0 (0.0) | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Ethnicity | 0.69 | ||||||
| Hispanic | 1 (4.8) | 0 (0.0) | 1 (4.3) | 1 (4.2) | 0 (0.0) | 0 (0.0) | |
| Non-Hispanic | 20 (95.2) | 19 (100.0) | 21 (95.5) | 19 (100.0) | 22 (100.0) | 21 (100.0) | |
| Education, yr | 16 ± 2 | 16 ± 3 | 16 ± 2 | 16 ± 2 | 17 ± 2 | 15 ± 3 | 0.66 |
| Weight, kg | 84.3 ± 11.8 | 92.8 ± 12.1 | 87.6 ± 12.6 | 91.3 ± 13.0 | 89.5 ± 9.5 | 87.6 ± 11.0 | 0.26 |
| BMI, kg/m2 | 27.2 ± 3.0 | 28.9 ± 3.0 | 28.2 ± 3.3 | 28.9 ± 3.4 | 26.8 ± 2.0 | 27.9 ± 2.8 | 0.14 |
| Total body fat, % | 26.9 ± 5.3 | 29.4 ± 4.3 | 28.5 ± 4.8 | 29.1 ± 4.0 | 28.4 ± 4.3 | 30.3 ± 4.2 | 0.27 |
| Systolic BP, mmHg (seated) | 129 ± 19 | 133 ± 18 | 126 ± 13 | 129 ± 16 | 122 ± 11 | 125 ± 19 | 0.43 |
| Diastolic BP, mmHg (seated) | 75 ± 9 | 77 ± 8 | 75 ± 7 | 79 ± 9 | 75 ± 8 | 77 ± 8 | 0.45 |
| V̇o2max, ml·kg−1·min−1 | 26.1 ± 5.1 | 25.4 ± 4.9 | 25.7 ± 5.6 | 25.3 ± 3.4 | 24.1 ± 7.1 | 24.5 ± 4.6 | 0.83 |
| Antihypertensive medication | 13 (61.9) | 10 (52.6) | 11 (50.0) | 11 (57.9) | 10 (45.5) | 11 (52.4) | 0.92 |
| Lipid-lowering medication | 11 (57.9) | 12 (54.5) | 9 (42.9) | 8 (42.1) | 10 (45.5) | 12 (57.1) | 0.88 |
| Aspirin | 12 (57.1) | 8 (42.1) | 14 (63.6) | 11 (57.9) | 8 (36.4) | 13 (61.9) | 0.38 |
| NSAID | 5 (23.8) | 8 (42.1) | 3 (13.6) | 5 (26.3) | 3 (13.6) | 3 (14.3) | 0.19 |
Data are mean ± standard deviation or number (percent). BMI, body mass index; BP, blood pressure; NSAID, nonsteroidal anti-inflammatory drug; PRT, progressive resistance training; T, testosterone; V̇o2max, maximal aerobic power.
Table 2.
Sex hormones, lipids, and glucose
| No PRT |
PRT |
|||||
|---|---|---|---|---|---|---|
| Variable | Placebo | Lower-range T | Higher-range T | Placebo | Lower-range T | Higher-range T |
| Total T, ng/dl | ||||||
| Baseline | 297 (279, 315) | 297 (281, 314) | 297 (279, 315) | 288 (274, 302) | 282 (262, 302) | 296 (281, 311) |
| 6 mo | 317 (281, 352) | 508 (359, 656) | 517 (403, 630) | 295 (266, 323) | 527 (408, 647) | 536 (399, 672) |
| 12 mo | 280 (247, 312) | 509 (347, 670) | 594 (451, 736) | 297 (271, 323) | 454 (345, 562) | 546 (383, 709) |
| Estradiol, pmol/l | ||||||
| Baseline | 24 (19, 29) | 25 (21, 30) | 20 (17, 23) | 27 (22, 32) | 22 (17, 26) | 27 (22, 31) |
| 6 mo | 25 (21, 29) | 36 (25, 46) | 39 (29, 50) | 27 (20, 34) | 28 (23, 34) | 43 (33, 53) |
| 12 mo | 24 (19, 28) | 36 (26, 46) | 32 (25, 38) | 23 (19, 27) | 26 (20, 32) | 38 (27, 48) |
| SHBG, nmol/l | ||||||
| Baseline | 41 (32, 50) | 45 (37, 53) | 48 (37, 58) | 40 (33, 47) | 37 (32, 41) | 48 (38, 59) |
| 6 mo | 41 (34, 49) | 40 (33, 47) | 43 (37, 50) | 41 (34, 47) | 39 (33, 44) | 50 (38, 61) |
| 12 mo | 41 (31, 50) | 43 (36, 51) | 44 (36, 52) | 41 (34, 48) | 38 (34, 43) | 47 (40, 54) |
| Cholesterol, mg/dl | ||||||
| Baseline | 179 (161, 191) | 173 (158, 188) | 174 (161, 186) | 179 (161, 198) | 169 (154, 184) | 173 (162, 184) |
| 6 mo | 180 (163, 197) | 168 (155, 181) | 164 (152, 175) | 169 (152, 186) | 164 (148, 180) | 164 (148, 179) |
| 12 mo | 170 (155, 186) | 158 (143, 174) | 160 (151, 169) | 168 (149, 187) | 170 (155, 184) | 159 (147, 171) |
| HDL cholesterol, mg/dl | ||||||
| Baseline | 48 (43, 52) | 42 (39, 46) | 42 (37, 47) | 46 (43, 49) | 44 (39, 48) | 50 (43, 57) |
| 6 mo | 49 (44, 53) | 41 (37, 45) | 40 (35, 45) | 44 (40, 49) | 43 (38, 47) | 43 (39, 47) |
| 12 mo | 48 (44, 52) | 41 (37, 45) | 41 (37, 45) | 44 (40, 49) | 43 (38, 47) | 43 (40, 47) |
| LDL cholesterol, mg/dl | ||||||
| Baseline | 106 (94, 118) | 100 (88, 112) | 101 (89, 112) | 104 (89, 118) | 97 (84, 109) | 95 (85, 106) |
| 6 mo | 105 (90, 121) | 98 (87, 109) | 91 (80, 101) | 94 (81, 107) | 93 (81, 105) | 97 (84, 110) |
| 12 mo | 97 (83, 112) | 89 (78, 100) | 92 (81, 103) | 96 (82, 111) | 98 (88, 108) | 90 (81, 99) |
| Triglycerides, mg/dl | ||||||
| Baseline | 127 (104, 149) | 155 (123, 187) | 155 (120, 191) | 148 (117, 178) | 143 (117, 168) | 141 (102, 179) |
| 6 mo | 129 (99, 160) | 143 (105, 182) | 165 (123, 207) | 152 (100, 204) | 142 (112, 171) | 118 (95, 141) |
| 12 mo | 125 (104, 145) | 140 (104, 176) | 147 (112, 182) | 138 (100, 175) | 145 (104, 185) | 127 (99, 154) |
| Fasted glucose, mg/dl | ||||||
| Baseline | 96 (92, 100) | 95 (89, 100) | 95 (92, 99) | 93 (90, 97) | 96 (93, 100) | 95 (90, 100) |
| 6 mo | 93 (88, 98) | 100 (94, 107) | 95 (91, 99) | 96 (93, 100) | 98 (92, 103) | 98 (95, 101) |
| 12 mo | 99 (95, 103) | 97 (91, 102) | 96 (91, 102) | 94 (91, 98) | 103 (97, 109) | 97 (94, 101) |
| Fasted insulin, µIU/ml | ||||||
| Baseline | 14.0 (10.2, 17.9) | 14.6 (9.8, 19.4) | 13.6 (10.8, 16.4) | 13.9 (10.4, 17.4) | 12.3 (10.0, 14.6) | 14.1 (10.8, 17.4) |
| 6 mo | 10.6 (7.7, 13.6) | 18.2 (13.0, 23.5) | 11.9 (9.6, 14.2) | 11.3 (8.8, 13.8) | 11.5 (9.1, 14.0) | 13.6 (9.8, 17.4) |
| 12 mo | 16.1 (10.9, 21.3) | 16.8 (10.5, 23.1) | 13.6 (9.8, 17.5) | 15.0 (10.9, 19.1) | 13.0 (10.3, 15.6) | 14.8 (11.4, 18.2) |
Data are mean (95% confidence interval). SI conversion factors: total testosterone, 0.0347; total, HDL, and LDL cholesterol, 0.0259; triglycerides, 0.0113; glucose, 0.0555; and insulin, 6.945. HDL, high-density lipoprotein; LDL, low-density lipoprotein; PRT, progressive resistance training; SHBG, sex hormone binding globulin; T, testosterone.
As previously reported, expected increases in T and estradiol were observed in T-treated participants at 6 and 12 mo; there were no significant changes in SHBG, lipids, glucose, or insulin at either time point (21). The PRT intervention also produced expected increases in both upper- and lower-body strength compared with no exercise (21). There were no changes in maximal oxygen consumption or physical activity level in any group (21).
Main effects of T and resistance exercise on vascular outcomes.
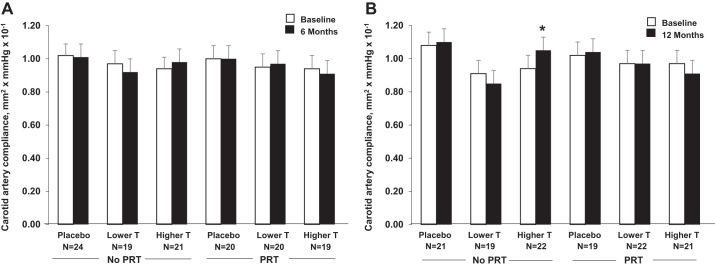
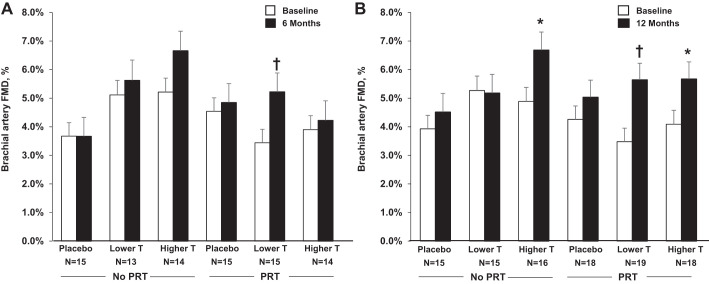
There were no main effects of either T or PRT on any of the vascular outcomes (Table 3, Figs. 2 and 3). Changes from baseline in carotid artery compliance, beta stiffness index, carotid IMT, and brachial artery FMD were not different among lower-range T supplementation, higher-range T supplementation, or placebo at either 6 or 12 mo. Likewise, changes in vascular outcomes were not different between PRT and no PRT, either alone or combined with T supplementation in the lower or higher range, at either time point. Results were unchanged after controlling for baseline values, medications, and age.
Table 3.
Hemodynamic and vascular parameters
| No PRT |
PRT |
|||||
|---|---|---|---|---|---|---|
| Variable | Placebo | Lower-range T | Higher-range T | Placebo | Lower-range T | Higher-range T |
| Supine systolic BP, mmHg | ||||||
| Baseline | 128 (121, 134) | 133 (124, 142) | 127 (122, 132) | 128 (120, 135) | 125 (120, 131) | 129 (123, 134) |
| 6 mo | 127 (121, 133) | 126 (119, 133) | 135 (128, 141) | 126 (119, 134) | 127 (122, 132) | 127 (121, 133) |
| 12 mo | 131 (122, 139) | 131 (123, 138) | 130 (124, 136) | 128 (122, 134) | 128 (122, 133) | 132 (125, 139) |
| Supine diastolic BP, mmHg | ||||||
| Baseline | 78 (74, 82) | 76 (71, 80) | 77 (75, 79) | 78 (74, 82) | 74 (71, 77) | 79 (76, 81) |
| 6 mo | 75 (72, 79) | 74 (71, 76) | 79 (76, 82) | 75 (70, 80) | 75 (72, 78) | 77 (74, 80) |
| 12 mo | 77 (74, 81) | 74 (71, 77) | 77 (73, 80) | 76 (73, 79) | 76 (73, 80) | 78 (74, 82) |
| Pulse pressure, mmHg | ||||||
| Baseline | 50 (45, 56) | 57 (51, 63) | 50 (45, 56) | 50 (44, 55) | 51 (46, 56) | 50 (45, 55) |
| 6 mo | 51 (47, 56) | 52 (47, 58) | 56 (50, 61) | 51 (47, 55) | 52 (47, 57) | 50 (45, 55) |
| 12 mo | 53 (46, 60) | 56 (51, 62) | 53 (48, 58) | 52 (48, 57) | 52 (46, 57) | 54 (48, 60) |
| Mean arterial pressure, mmHg | ||||||
| Baseline | 96 (92, 100) | 98 (92, 104) | 96 (93, 98) | 95 (90, 101) | 93 (89, 96) | 96 (93, 99) |
| 6 mo | 95 (90, 100) | 92 (88, 96) | 99 (93, 106) | 91 (84, 97) | 95 (91, 99) | 93 (90, 96) |
| 12 mo | 98 (91, 104) | 96 (91, 100) | 97 (92, 101) | 92 (88, 97) | 95 (91, 98) | 95 (91, 100) |
| Heart rate, beats/min | ||||||
| Baseline | 56 (52, 60) | 54 (51, 58) | 54 (51, 56) | 58 (54, 61) | 55 (52, 58) | 62 (57, 66) |
| 6 mo | 55 (51, 59) | 55 (52, 58) | 57 (53, 61) | 58 (56, 60) | 55 (52, 58) | 60 (58, 63) |
| 12 mo | 56 (52, 60) | 55 (52, 58) | 54 (52, 56) | 62 (58, 65) | 55 (52, 58) | 61 (57, 64) |
| Carotid diameter, mm | ||||||
| Baseline | 7.24 (6.92, 7.55) | 7.06 (6.76, 7.36) | 6.96 (6.60, 7.32) | 7.03 (6.62, 7.43) | 7.10 (6.38, 7.38) | 7.29 (6.97, 7.61) |
| 6 mo | 7.35 (7.02, 7.69) | 7.04 (6.73, 7.35) | 7.10 (6.82, 7.37) | 7.02 (6.78, 7.27) | 7.16 (6.86, 7.45) | 7.22 (6.90, 7.54) |
| 12 mo | 7.29 (6.93, 7.64) | 7.07 (6.79, 7.35) | 7.03 (6.70, 7.36) | 7.18 (6.92, 7.45) | 7.12 (6.84, 7.39) | 7.33 (6.98, 7.68) |
| Carotid distension, mm | ||||||
| Baseline | 0.46 (0.39, 0.53) | 0.46 (0.39, 0.53) | 0.43 (0.36, 0.49) | 0.44 (0.40, 0.47) | 0.43 (0.37, 0.48) | 0.41 (0.35, 0.46) |
| 6 mo | 0.49 (0.39, 0.58) | 0.43 (0.37, 0.49) | 0.46 (0.41, 0.51) | 0.44 (0.38, 0.49) | 0.44 (0.39, 0.50) | 0.40 (0.34, 0.45) |
| 12 mo | 0.50 (0.42, 0.57) | 0.43 (0.36, 0.49) | 0.47 (0.42, 0.52) | 0.47 (0.42, 0.52) | 0.44 (0.37, 0.50) | 0.41 (0.35, 0.47) |
| Carotid artery compliance, mm2/mmHg × 10−1 | ||||||
| Baseline | 1.08 (0.91, 1.26) | 0.91 (0.79, 1.03) | 0.94 (0.82, 1.06) | 1.02 (0.87, 1.18) | 0.97 (0.82, 1.12) | 0.97 (0.81, 1.13) |
| 6 mo | 1.09 (0.89, 1.28) | 0.94 (0.79, 1.09) | 0.97 (0.84, 1.10) | 0.96 (0.82, 1.10) | 1.00 (0.83, 1.16) | 0.94 (0.78, 1.10) |
| 12 mo | 1.10 (0.95, 1.25) | 0.85 (0.74, 0.95) | 1.05 (0.89, 1.21) | 1.04 (0.90, 1.19) | 0.97 (0.82, 1.13) | 0.91 (0.75, 1.07) |
| Beta stiffness index, AU | ||||||
| Baseline | 7.9 (6.7, 9.1) | 9.5 (6.6, 12.3) | 7.9 (6.4, 9.5) | 7.7 (6.9, 8.6) | 7.5 (5.7, 9.3) | 9.0 (7.5, 10.5) |
| 6 mo | 9.1 (6.5, 11.6) | 9.0 (7.2, 10.8) | 7.3 (6.1, 8.6) | 8.6 (7.3, 9.8) | 7.0 (5.8, 8.2) | 9.1 (7.4, 10.8) |
| 12 mo | 7.5 (6.6, 8.5) | 9.4 (7.8, 11.0) | 7.4 (5.9, 8.9) | 8.1 (7.0, 9.3) | 7.1 (5.9, 8.3) | 10.3 (8.1, 12.6) |
| Carotid IMT, mm | ||||||
| Baseline | 0.837 (0.764, 0.910) | 0.825 (0.749, 0.901) | 0.782 (0.695, 0.869) | 0.823 (0.755, 0.891) | 0.783 (0.727, 0.838) | 0.803 (0.730, 0.877) |
| 6 mo | 0.856 (0.764, 0.949) | 0.841 (0.751, 0.932) | 0.789 (0.700, 0.878) | 0.831 (0.759, 0.903) | 0.769 (0.713, 0.825) | 0.799 (0.727, 0.870) |
| 12 mo | 0.858 (0.770, 0.946) | 0.848 (0.773, 0.923) | 0.788 (0.688, 0.887) | 0.831 (0.760, 0.901) | 0.799 (0.731, 0.867) | 0.788 (0.715, 0.860) |
| Brachial diameter, mm | ||||||
| Baseline | 5.15 (4.91, 5.38) | 4.79 (4.57, 5.01) | 4.85 (4.59, 5.12) | 4.96 (4.70, 5.22) | 5.01 (4.76, 5.26) | 4.93 (4.70, 5.15) |
| 6 mo | 5.13 (4.89, 5.36) | 4.85 (4.66, 5.04) | 4.84 (4.54, 5.14) | 5.03 (4.81, 5.26) | 5.04 (4.75, 5.32) | 5.17 (4.86, 5.47) |
| 12 mo | 5.18 (4.97, 5.40) | 4.90 (4.65, 5.15) | 4.86 (4.61, 5.11) | 4.98 (4.73, 5.22) | 4.99 (4.69, 5.29) | 5.12 (4.88, 5.36) |
| FMD, % | ||||||
| Baseline | 3.9 (3.2, 4.7) | 5.1 (3.9, 6.3) | 5.2 (4.3, 6.0) | 4.3 (3.4, 5.1) | 3.5 (2.9, 4.1) | 4.1 (3.4, 4.7) |
| 6 mo | 3.5 (1.9, 5.1) | 4.9 (3.3, 6.4) | 6.4 (5.2, 7.6) | 5.0 (4.2, 5.9) | 5.3 (4.3, 6.3) | 4.4 (3.0, 5.7) |
| 12 mo | 4.5 (3.5, 5.5) | 4.9 (3.4, 6.5) | 6.8 (5.4, 8.2) | 5.0 (4.1, 6.0) | 5.7 (4.6, 6.7) | 5.7 (4.6, 6.8) |
Data are mean (95% confidence interval). BP, blood pressure; FMD, flow-mediated dilation; IMT, intima-medial thickness; PRT, progressive resistance training; T, testosterone.
Fig. 2.
Carotid artery compliance at baseline and at 6 mo (A) and 12 mo (B) by treatment group (mean ± SE). *P < 0.05, within-group effect. PRT, progressive resistance training; T, testosterone.
Fig. 3.
Brachial artery FMD at baseline and at 6 mo (A) and 12 mo (B) by treatment group (mean ± SE). *P < 0.05; †P < 0.01, within-group effect. FMD, flow-mediated dilation; PRT, progressive resistance training; T, testosterone.
Between-group analyses.
Hemodynamic and vascular parameters were similar among treatment groups at baseline, 6 mo, and 12 mo, with the exception of heart rate at baseline and 12 mo, beta stiffness index at 12 mo, and brachial artery FMD at baseline (Table 3, Fig. 3).
Exploratory analyses.
Exploratory analyses of within-group changes revealed a significant improvement in carotid artery compliance and brachial artery FMD at 12 mo (Figs. 2B and 3B; P < 0.05) in men assigned to higher-range T alone; brachial artery FMD tended to improve at 6 mo (Fig. 3A, P = 0.07). There were also significant improvements in brachial artery FMD at 6 and 12 mo in men assigned to PRT plus lower-range T supplementation (Fig. 3, A and B, both P < 0.01) and at 12 mo in men assigned to PRT plus higher-range T supplementation (Fig. 3B, P = 0.01).
There were no significant correlations between T or estradiol concentrations and any of the vascular outcome measures at any time point. The T-to-estradiol ratio was modestly correlated with FMD at baseline and with carotid artery compliance at 12 mo (both r = −0.19, P = 0.04). There were no significant correlations between changes in T, estradiol, or T-to-estradiol ratio and changes in any of the vascular outcomes at 6 or 12 mo.
DISCUSSION
In this secondary analysis, we investigated the effects of 12 mo of 2 different levels of T supplementation or placebo treatment with or without PRT on large elastic arterial stiffness and its structural and functional determinants in older men with low–normal T levels. Contrary to our hypothesis, the overall effects of T supplementation to either a lower or higher range on arterial stiffening were not different than placebo, nor were there overall effects of T on structural (IMT) or functional (endothelial function) outcomes. Moreover, we found no overall effects of PRT compared with no PRT, either alone or in combination with T, on arterial stiffening, IMT, or endothelial function. In exploratory analyses of within-group changes, we found a decrease in arterial stiffness after 12 mo in men treated to higher-range T and an improvement in endothelial function after 6 and 12 mo in men supplemented to lower-range T. Additionally, PRT improved endothelial function after 6 and 12 mo in men supplemented to lower-range T and after 12 mo in men supplemented to higher-range T.
T and arterial stiffness in men.
T decreases with advancing age in men, with 20% of 60-yr-old men and 50% of 80-yr-old men having serum levels of total T below the normal range for young men (20). Because men have a higher incidence of CVD than women of similar age, androgens have been implicated in the development of CVD in men. However, men with coronary artery disease have lower levels of T than age-matched controls, and low levels of T have been associated with a greater burden or atherosclerosis (18, 30). Although potential cardiovascular risks of T have recently received attention (16, 23), a wealth of existing data support an association between normal T levels and cardiovascular health (29). To date, there have been no large, long-term, randomized controlled trials of T therapy on cardiovascular outcomes to provide definitive conclusions about cardiovascular risk with T (29). Advancing understanding of the pathophysiology of T and cardiovascular health may help clarify potential risks and benefits.
Arterial compliance is the ability of an artery to expand and recoil with cardiac ejection and relaxation. Compliance of the large elastic arteries in the cardiothoracic circulation buffers the rise in systolic pressure by storing a portion of the ejected stroke volume during systole, maintaining steady blood flow across capillary beds. In sedentary humans, arterial compliance decreases (stiffness increases) with age, even in the absence of clinical CVD (37), and arterial stiffness is an independent risk factor for CVD (6). In the present study, baseline carotid artery compliance was consistent with previous observations in sedentary older men (37). The mechanisms underlying age-related arterial stiffening are not completely understood but appear to include both structural and functional changes. Structural changes include decreased elastin, increased collagen and connective tissue, and smooth muscle cell hypertrophy (32). Functional changes include increased sympathetic-adrenergic vasoconstrictor tone, with a corresponding change in vascular endothelium-dependent vasomotor tone toward reduced nitric oxide release, resulting in a chronically elevated state of vascular smooth muscle cell contraction (32). Although normal T levels appear to be associated with cardiovascular health in men, it is not clear how T may influence age-related arterial stiffening and its determinants. Cross-sectional studies have reported an association between low levels of T and increased pulse-wave velocity, an indirect measure of arterial stiffness representing the rate at which the aortic pressure wave propagates throughout the circulation (8, 40), brachial artery FMD, a biomarker of endothelial and nitric oxide vasodilatory function (11), and carotid IMT (36). Studies of rapid chemically induced gonadal suppression in men with prostate cancer have reported increased arterial stiffness (10, 35), which was reversed after cessation of therapy (35). In older men with untreated acquired hypogonadism, transdermal T treatment caused a rapid and sustained decrease in arterial stiffness (43). T treatment also increased brachial artery FMD in men with severe T deficiency (14). Collectively, these studies support the idea of a beneficial effect of T on arterial stiffness and endothelial function. To our knowledge, no previous studies have measured the effects of T supplementation on arterial stiffness, endothelial function, or IMT in healthy older men with low–normal T, a group to whom T is heavily marketed.
The lack of effect of either level of T supplementation on carotid artery compliance, brachial artery FMD, or IMT in our study may reflect the population enrolled. Specifically, participants were generally healthy and highly functional, with T levels in the low–normal range. It is possible there is a “threshold” level of T, above which additional increases provide no further cardiovascular benefit. Indeed, some data support such a threshold for the effects of T on erectile function (7). Although we found no correlation between T level and arterial stiffness or brachial artery FMD, it is possible that an association could be seen in men with unequivocally low T. Interestingly, we did show a within-group improvement in carotid artery compliance in men supplemented to higher-range T after 12 mo and improvement in brachial artery FMD in men supplemented to lower-range T after 6 and 12 mo; these findings suggest a beneficial effect of T on arterial compliance and endothelial function that warrants further investigation.
The degree of arterial remodeling needed to effect a change in carotid IMT may have made changes in this outcome unlikely during this study. One small study did report an increase in carotid IMT associated with 12 mo of T undecanoate treatment (3). In addition to a different form of T supplementation, the men in that study had lower T levels (240–250 ng/dl), symptoms of hypogonadism, and were less metabolically healthy than the men in the present study, meeting criteria for metabolic syndrome and/or type 2 diabetes mellitus at baseline (3).
Influence of resistance training with and without T on arterial stiffness in men.
In contrast to endurance exercise training, some studies have demonstrated an increase in arterial stiffness with resistance training. In middle-aged men, carotid artery compliance was 30% lower in resistance-trained compared with age-matched sedentary men (26). In young men, 4 mo of PRT was associated with a 19% decrease in carotid artery compliance compared with controls (27). We therefore hypothesized that PRT alone would increase carotid artery stiffness but that T supplementation would counter this effect. Contrary to our hypothesis, there was no increase in carotid artery stiffness with PRT compared with no PRT at 6 or 12 mo; this finding was consistent with a recent meta-analysis that found no effect of PRT on arterial stiffness measured by pulse-wave velocity (2). One possible reason for the lack of effect was the moderate intensity of the PRT intervention in the present study. Another meta-analysis found that increased arterial stiffness with PRT was limited to high-intensity training in young individuals with lower baseline arterial stiffness (25). Our exploratory analyses found improvements in brachial artery FMD with PRT in combination with T supplementation. Notably, the magnitude of increase in the PRT + lower-range T group was double that of either PRT or lower-range T alone. Whether T enhances the PRT response or vice versa needs further investigation. The absence of a change in carotid IMT with PRT was not unexpected in light of previous work showing no difference in this measure between resistance-trained and sedentary men (26) or in response to 4 mo of resistance training in young men (27).
Other considerations.
Other factors that influence arterial stiffness, endothelial function, and IMT were measured in this study, including body composition, blood pressure, lipid levels, glucose, and insulin. Although T treatment resulted in favorable changes in body composition (decreased fat mass, increased fat-free mass) (21), there were no changes in any of the other measures. Although participants were on average overweight, their baseline measures of cardiometabolic health were excellent, potentially leaving little room for improvement. Furthermore, variable metabolism and conversion from T to estradiol among participants may have influenced the results of this study, although T-to-estradiol ratios did not differ among the groups.
It is possible that the age and/or duration of low–normal T of the participants in this study may help explain the lack of effects of T on the vascular measures. In postmenopausal women, prolonged estrogen deficiency and aging is associated with a decrease in vascular endothelial responsiveness to estradiol treatment (31). Postmenopausal women aged 50 to 59 yr had a marked improvement in endothelial function 18 h after estradiol treatment, whereas women aged 60 to 79 yr showed no evidence of improvement (34). Additionally, the improvement in brachial artery FMD with acute and chronic estradiol treatment in postmenopausal women was reduced by a longer time since menopause (39). It is plausible that aging and prolonged T deficiency in men could likewise impair vascular responsiveness. However, adjustment for age did not modulate the effects of T with or without PRT on any vascular outcome.
Adverse effects.
Although large, long-term controlled trials evaluating the effects of T treatment on cardiovascular outcomes are lacking, several studies have reported increased adverse cardiovascular events associated with T treatment (5, 13, 38). We previously reported no increase in adverse cardiovascular events in men treated with T compared with placebo in this study (21). In the present analysis, there was no increase in carotid IMT nor was there a worsening of arterial stiffness or endothelial function associated with T treatment.
Strengths and limitations.
Strengths of this study included the rigorous randomized controlled design, large sample size, and study duration. Expected increases in T levels and strength with T supplementation and PRT, respectively, were achieved (21). This study provides new information on the effects of T supplementation on vascular function in healthy men with low–normal T, a group frequently prescribed T. Although generally healthy, approximately half of the participants were taking medications that positively affect vascular function, including antihypertensives, lipid-lowering medications, and aspirin. These medications may have limited further improvement in vascular measures with T and/or PRT; however, the sample size was not sufficient to compare medicated and nonmedicated participants. Use of these medications was stable during the study, with very few participants initiating or discontinuing any of these medications. As reported previously (21), absorption of the T gel was variable, and the absence of T levels measured after the initial titration period precludes assessment of actual T exposure. The duration of the study also does not permit conclusions about vascular effects of T with prolonged use. Finally, >90% of participants were non-Hispanic white; it is not known whether the findings would extend to other races and ethnic groups.
Significance.
T treatment has been aggressively marketed to aging men with nonspecific symptoms and T levels in the low–normal range. Lack of data supporting benefits of T in this population and reports of potential cardiovascular harms in some studies led the Food and Drug Administration to issue a safety announcement cautioning against the prescription of T for conditions other than hypogonadism. The announcement also requires that the approved uses and the possible increased risk of heart attack and stroke are clearly labeled on T products (12). We found no cardiovascular harm or benefit with T supplementation alone or with PRT, although within-group improvements in arterial stiffness and endothelial function need further investigation. This study therefore adds to the literature supporting T supplementation only for clearly hypogonadal men.
Conclusion.
In this secondary analysis of generally healthy older men with low–normal T levels, 12 mo of supplementation with T to either a lower or higher range with or without PRT had no detectable effect on arterial stiffness, endothelial function, or IMT compared with placebo. Supplementation of T in older men with mild, age-related declines in T does not appear to improve or to harm vascular function.
GRANTS
This work was supported by National Institutes of Health Award No. R01-AG-019339, Colorado Clinical and Translational Sciences Institute Grant No. UL1-TR001082, and Colorado Nutrition Obesity Research Center Grant No. P30-DK-048520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.S.S., J.V.G., W.M.K., and K.L.M. conceived and designed research; K.L.H., R.S.S., J.V.G., and K.L.M. performed experiments; K.L.H., R.S.S., P.J.B., and K.L.M. analyzed data; K.L.H., R.S.S., W.M.K., P.J.B., and K.L.M. interpreted results of experiments; K.L.H., P.J.B., and K.L.M. prepared figures; K.L.H., P.J.B., and K.L.M. drafted manuscript; K.L.H., R.S.S., J.V.G., W.M.K., P.J.B., and K.L.M. edited and revised manuscript; K.L.H., R.S.S., J.V.G., W.M.K., P.J.B., and K.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Andrew Hepler, Bethany Kelsey, and Tammie Nakamura for assistance.
REFERENCES
- 1.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 96: 3007–3019, 2011. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One 9: e110034, 2014. doi: 10.1371/journal.pone.0110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aversa A, Bruzziches R, Francomano D, Rosano G, Isidori AM, Lenzi A, Spera G. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 7: 3495–3503, 2010. doi: 10.1111/j.1743-6109.2010.01931.x. [DOI] [PubMed] [Google Scholar]
- 4.Barry MJ, Fowler FJ JR, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT; The Measurement Committee of the American Urological Association . The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 148: 1549–1557, 1992. doi: 10.1016/S0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 5.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med 363: 109–122, 2010. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buena F, Swerdloff RS, Steiner BS, Lutchmansingh P, Peterson MA, Pandian MR, Galmarini M, Bhasin S. Sexual function does not change when serum testosterone levels are pharmacologically varied within the normal male range. Fertil Steril 59: 1118–1123, 1993. doi: 10.1016/S0015-0282(16)55938-X. [DOI] [PubMed] [Google Scholar]
- 8.Corrigan FE III, Al Mheid I, Eapen DJ, Hayek SS, Sher S, Martin GS, Quyyumi AA. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int J Cardiol 194: 94–99, 2015. doi: 10.1016/j.ijcard.2015.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc 25: 628–642, 1993. doi: 10.1249/00005768-199305000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Dockery F, Bulpitt CJ, Agarwal S, Rajkumar C. Testosterone suppression in men with prostate cancer is associated with increased arterial stiffness. Aging Male 5: 216–222, 2002. doi: 10.1080/tam.5.4.216.222. [DOI] [PubMed] [Google Scholar]
- 11.Empen K, Lorbeer R, Dörr M, Haring R, Nauck M, Gläser S, Krebs A, Reffelmann T, Ewert R, Völzke H, Wallaschofski H, Felix SB. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol 32: 481–486, 2012. doi: 10.1161/ATVBAHA.111.232876. [DOI] [PubMed] [Google Scholar]
- 12.FDA FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. https://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. [15 July 2016]. [DOI] [PubMed]
- 13.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF JR, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One 9: e85805, 2014. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francomano D, Fattorini G, Gianfrilli D, Paoli D, Sgrò P, Radicioni A, Romanelli F, Di Luigi L, Gandini L, Lenzi A, Aversa A. Acute endothelial response to testosterone gel administration in men with severe hypogonadism and its relationship to androgen receptor polymorphism: a pilot study. J Endocrinol Invest 39: 265–271, 2016. doi: 10.1007/s40618-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972. [PubMed] [Google Scholar]
- 16.Goodman N, Guay A, Dandona P, Dhindsa S, Faiman C, Cunningham GR; AACE Reproductive Endocrinology Scientific Committee . American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract 21: 1066–1073, 2015. doi: 10.4158/EP14434.PS. [DOI] [PubMed] [Google Scholar]
- 17.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, Uraga MV, Erwin PJ, Montori VM. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc 82: 29–39, 2007. doi: 10.1016/S0025-6196(11)60964-6. [DOI] [PubMed] [Google Scholar]
- 18.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 87: 3632–3639, 2002. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 19.Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust 199: 548–551, 2013. doi: 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 20.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 86: 724–731, 2001. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 21.Hildreth KL, Barry DW, Moreau KL, Vande Griend J, Meacham RB, Nakamura T, Wolfe P, Kohrt WM, Ruscin JM, Kittelson J, Cress ME, Ballard R, Schwartz RS. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab 98: 1891–1900, 2013. doi: 10.1210/jc.2012-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hougaku H, Fleg JL, Najjar SS, Lakatta EG, Harman SM, Blackman MR, Metter EJ. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab 290: E234–E242, 2006. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kloner RA, Carson C III, Dobs A, Kopecky S, Mohler ER III. Testosterone and cardiovascular disease. J Am Coll Cardiol 67: 545–557, 2016. doi: 10.1016/j.jacc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 25.Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med 47: 393–396, 2013. doi: 10.1136/bjsports-2012-090488. [DOI] [PubMed] [Google Scholar]
- 26.Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, Tanaka H. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 41: 130–135, 2003. doi: 10.1161/01.HYP.0000047649.62181.88. [DOI] [PubMed] [Google Scholar]
- 27.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 110: 2858–2863, 2004. doi: 10.1161/01.CIR.0000146380.08401.99. [DOI] [PubMed] [Google Scholar]
- 28.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc 90: 224–251, 2015. doi: 10.1016/j.mayocp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb 14: 701–706, 1994. doi: 10.1161/01.ATV.14.5.701. [DOI] [PubMed] [Google Scholar]
- 31.Pinna C, Cignarella A, Sanvito P, Pelosi V, Bolego C. Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor alpha agonists. Hypertension 51: 1210–1217, 2008. doi: 10.1161/HYPERTENSIONAHA.107.106807. [DOI] [PubMed] [Google Scholar]
- 32.Quinn U, Tomlinson LA, Cockcroft JR. Arterial stiffness. JRSM Cardiovasc Dis 1: 1–8, 2012. doi: 10.1258/cvd.2012.012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seals DR. Habitual exercise and the age-associated decline in large artery compliance. Exerc Sport Sci Rev 31: 68–72, 2003. doi: 10.1097/00003677-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol 27: 1782–1787, 2007. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 35.Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, Cockcroft JR, Scanlon MF, Davies JS. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 86: 4261–4267, 2001. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 36.Svartberg J, von Mühlen D, Mathiesen E, Joakimsen O, Bønaa KH, Stensland-Bugge E. Low testosterone levels are associated with carotid atherosclerosis in men. J Intern Med 259: 576–582, 2006. doi: 10.1111/j.1365-2796.2006.01637.x. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 38.Vigen R, O’Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 310: 1829–1836, 2013. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 39.Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, Volterrani M, Fini M, Collins P, Rosano GM. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol 28: 348–352, 2008. doi: 10.1161/ATVBAHA.107.158634. [DOI] [PubMed] [Google Scholar]
- 40.Vlachopoulos C, Ioakeimidis N, Miner M, Aggelis A, Pietri P, Terentes-Printzios D, Tsekoura D, Stefanadis C. Testosterone deficiency: a determinant of aortic stiffness in men. Atherosclerosis 233: 278–283, 2014. doi: 10.1016/j.atherosclerosis.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Weintraub A. Safety concerns slow sales of testosterone therapy. Bloomberg Business Week, Nov 06, 2014. [Google Scholar]
- 42.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 11: 108, 2013. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaron M, Greenman Y, Rosenfeld JB, Izkhakov E, Limor R, Osher E, Shenkerman G, Tordjman K, Stern N. Effect of testosterone replacement therapy on arterial stiffness in older hypogonadal men. Eur J Endocrinol 160: 839–846, 2009. doi: 10.1530/EJE-09-0052. [DOI] [PubMed] [Google Scholar]