Abstract
Serum soluble Fas (sFas) levels are associated with erythropoietin (Epo) hyporesponsiveness in patients with chronic kidney disease (CKD). Whether sFas could predict the need for erythropoiesis-stimulating agent (ESA) usage and its influence in erythropoiesis remain unclear. We evaluated the relation between sFas and ESA therapy in patients with CKD with anemia and its effect on erythropoiesis in vitro. First, we performed a retrospective cohort study with 77 anemic patients with nondialysis CKD. We performed in vitro experiments to investigate whether sFas could interfere with the behavior of hematopoietic stem cells (HSCs). HSCs were isolated from umbilical cord blood and incubated with recombinant sFas protein in a dose-dependent manner. Serum sFas positively correlated with Epo levels (r = 0.30, P = 0.001) but negatively with hemoglobin (r = −0.55, P < 0.001) and glomerular filtration rate (r = −0.58, P < 0.001) in patients with CKD at baseline. Elevated sFas serum levels (4,316 ± 897 vs. 2,776 ± 749, P < 0.001) with lower estimated glomerular filtration rate (26.2 ± 10.1 vs. 33.5 ± 14.3, P = 0.01) and reduced hemoglobin concentration (11.1 ± 0.9 vs. 12.5 ± 1.2, P < 0.001) were identified in patients who required ESA therapy compared with patients with non-ESA. Afterward, we detected that the sFas level was slight correlated with a necessity of ESA therapy in patients with nondialysis CKD and anemia. In vitro assays demonstrated that the erythroid progenitor cell frequency negatively correlated with sFas concentration (r = −0.72, P < 0.001). There was decreased erythroid colony formation in vitro when CD34+ HSCs were incubated with a higher concentration of sFas protein (1.56 ± 0.29, 4.33 ± 0.53, P < 0.001). Our findings suggest that sFas is a potential predictor for ESA therapy in patients with nondialysis CKD and that elevated sFas could affect erythropoiesis in vitro.
Keywords: anemia and hematopoietic stem cells, chronic kidney disease, erythropoiesis-stimulating agents, soluble Fas
INTRODUCTION
Anemia is a frequent clinical complication that develops gradually during the progressive decline of renal function in patients with chronic kidney disease (CKD) (15, 18, 20). It is caused by several factors including a relative deficiency of erythropoietin (Epo) secretion reducing erythropoiesis (15, 18, 33). Anemia underlies many of symptoms associated with CKD with a reduction of the patient’s life quality and a wide range of clinically important consequences (2, 18, 31, 32).
Since the regulation of recombinant human Epo and other erythropoiesis-stimulating agents (ESAs) for the treatment of anemia in patients with CKD, there has been a considerable improvement in clinical symptoms including a reduction of red blood cell transfusions and consequently lower mortality (1, 14, 20, 26, 31).
The Fas (CD95/APO-1) molecule is a cell surface receptor that is a member of the TNF receptor superfamily. This receptor play a central role in the physiological regulation of programmed cell death and is expressed in various cells, including leukocytes and hematopoietic stem cells (HSCs); however, it can also stimulate nonapoptotic signals, thus promoting inflammation (3, 11, 17, 22, 24). However, the soluble form of the Fas receptor (sFas) is produced as a translation product of alternatively spliced mRNA or it can be cleaved by metalloproteases preventing target cell apoptosis via a Fas ligand (FasL; CD178) interaction (10, 15, 27).
We have previously reported a strong relationship between high levels of serum sFas and Epo hyporesponsiveness in patients with CKD (15). In another study (16), we also detected an interrelation between serum sFas levels and anemia in patients with acute kidney injury. In fact, others have reported that circulating levels of sFas are associated with nonresolving acute kidney injury (5).
In the present study, we investigated, in an outpatient setting, whether circulating levels of sFas could be related to the presence of anemia associated with ESA therapy necessity in patients with nondialysis CKD (ND-CKD). Furthermore, we performed colony-forming assays to determine whether recombinant sFas protein could directly influence during in vitro erythropoiesis.
MATERIALS AND METHODS
Patients and Data collection.
First, we performed a retrospective cohort study of all patients with ND-CKD, in an outpatient setting, who developed anemia up to 6 yr after enrollment in the Nephrology Service at Federal University of São Paulo between 2005 and 2010, considering that all these patients did not received ESA therapy at the time of Nephrology Service admission. We added to our data collection patients with ND-CKD from our internal database, and we collected additional data of 38 patients from other previously published studies (10, 15, 28).
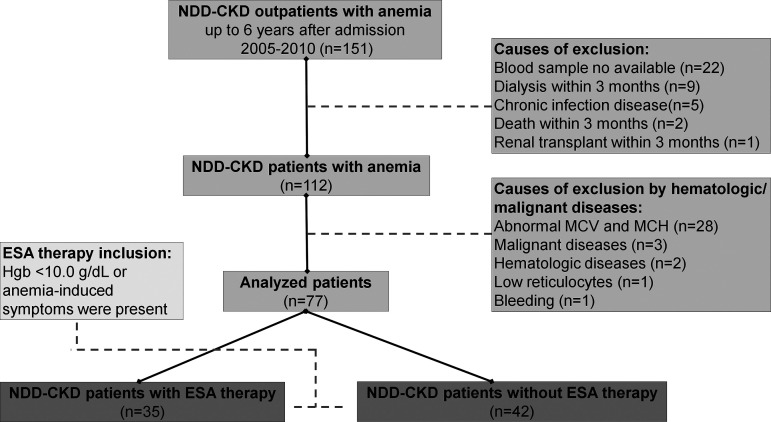
A total of 151 patients with ND-CKD and anemia met the following inclusion criteria: age > 18 yr old, CKD diagnosis for at least 3 mo, and normocytic and normochromic anemia within 6 yr of followup at our Nephrology service. The exclusion criteria were ESA requirement within 30 days of study enrollment, hematological diseases, bleeding, iron depletion, abnormality of mean corpuscular volume and mean corpuscular hemoglobin, chronic viral disease such as human immunodeficiency virus infection, hepatitis B and C infection, malignant diseases, and those commencing renal replacement therapy or who died within 3 mo after trial enrollment. On the basis of these criteria, 74 patients were excluded from the study. Thereafter, we divided the remaining patients with ND-CKD and anemia into two subgroups according to the need for ESA therapy within 6 yr after admission. A flowchart of all patient workflow is shown in Fig. 1.
Fig. 1.
Workflow diagram of the patient selection. A total of 151 patients with nondialysis chronic kidney disease (ND-CKD) with anemia were selected from Nephrology Service at the Federal University of São Paulo and from other previously published studies. After two exclusion criteria, the remaining patients (n = 77) were subdivided into two subgroups: 1) patients with ND-CKD with erythropoiesis-stimulating agent (ESA) therapy (n = 35) and 2) patients with ND-CKD without ESA therapy (n = 42). MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin (Hgb).
We primarily carried out a global correlation with all 77 patients included in this study to evaluate possible connections between several renal parameters with anemia parameters and both with sFas level. Second, when we verified a strong relationship among hemoglobin (Hb) concentration, iron status, and estimated glomerular filtration rate (eGFR) with sFas, we conducted a binary logistic regression to investigate if sFas could predict ESA therapy in patients with ND-CKD. All analyses performed in this study were run with data at baseline before ESA therapy implementation.
Measurements and laboratory parameters.
The baseline demographics and clinical data were collected from the patients’ medical records and included age, sex, smoking status, CKD etiology, body mass index, major comorbidities, medications, and ESA usage. In this study, we defined anemia as a Hb level of <13.0 g/dL in men and postmenopausal women or <12.0 g/dL in premenopausal women (1, 6, 20, 23, 26). ESA therapy was started when the Hb level decreased to <10.0 g/dL or when anemia-induced symptoms were present (20). The indications for ESA therapy and medications were determined by the patient's attending nephrologist. eGFR was calculated using the CKD Epidemiology Collaboration formula (21).
Levels of ferritin and intact parathyroid hormone (iPTH) were determined using a chemiluminescent microparticle immunoassay with an automatized method (Abbott Laboratories). Serum levels of sFas (BD PharMingen), IL-6 (BD OptEIA IL-6), IL-10 (BD OptEIA IL-10, BD Biosciences-PharMingen), and Epo (Quantikine Human Erythropoietin Immunoassay, R&D Systems, Minneapolis, MN) were measured using ELISA in accordance with the manufacturers’ instructions.
Routine tests were requested, when deemed appropriate, by the patient’s attending nephrologist. Thus, our study did not, in any way, interfere with the treatment, medical workup, or request for laboratory medical examinations in these patients.
Information in the patient records was anonymized and deidentified before the analysis of retrospectively collected variables. No individual personal data were included in the study. All patients provided necessary consent to participate in this study. The study was approved and reviewed by the local ethics committee on human research of the Federal University of São Paulo (São Paulo, Brazil, nos. 0108/2010 and 0175/2019). No external funding was used to support this work.
In vitro assessment of the effects of sFas on human hematopoiesis.
In parallel with the aforementioned clinical study, we performed in vitro experiments at Wake Forest Institute for Regenerative Medicine (Winston-Salem, NC) after obtaining local approval to investigate whether sFas could interfere with the growth and/or differentiation of multipotent HSCs. Human CD34+ HSCs were isolated from frozen umbilical cord blood mononuclear cells (UCB-MNCs; All Cells) using a MiniMACS magnetic separation system (CD34 Microbead Kit UltraPure Human, Miltenyi Biotec).
The UCB-MNCs and isolated CD34+ cells were assessed through quantitative fluorescence analysis with flow cytometry (BD Accuri C6 personal flow cytometer, BD Biosciences). All incubations were performed at 4°C in PBS-NaN3 and for 30 min with fluorochrome-conjugated monoclonal antibody to glycophorin A, CD34+, CD36+, CD38+, CD71+, and CD133+ (BD Biosciences). To enable two-color analysis, a combination of phycoerythrin, FITC, and peridinin chlorophyll protein complex conjugated to monoclonal antibody was used. Isotype controls were used in all experiments to account for any nonspecific binding.
Isolated CD34+ cells were seeded into Nunc four-well dishes (ThermoFisher Scientific) at a density of 1.0 × 105 cells/mL in 2 mL (per well) of methylcellulose-based Iscove’s modified DMEM containing FBS, BSA, human transferrin (iron-saturated), 2-mercaptoethanol, supplements, and the following recombinant human growth factors/cytokines: insulin, stem cell factor, IL-3, granulocyte-macrophage colony-stimulating factor, and Epo (MethoCult GF 4434 Classic, STEMCELL Technologies). Cells were divided into 18 wells in 6 plates for CD34+ cells and incubated for 14 days (13, 30) in the absence or presence of various levels of human recombinant sFas (FAS-FAS-281H, Creative BioMart). We tested the effects of both 1) high levels (sFas-Hc group: 2, 4, and 8 ng/mL) and 2) low levels (sFas-Lc group: 0, 0.5, and 1 ng/mL) of sFas on CD34+ HSCs. On day 14, we used an inverted light microscope (IX 73, Olympus) at ×40 magnification to enumerate erythroid (BFU-e/CFU-e), granulocyte/macrophage (CFU-GM), and granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM/CFU-Mix) colonies. This study was performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Statistical analyses.
The SPSS statistical software program (version 21.0, SPSS, Chicago, IL) and GraphPad Prism (version 7.0, La Jolla, CA) were used to perform all statistical analyses. Continuous variables are expressed as means ± SD; categorical clinical data are presented as percentages.
We used Pearson or Spearman coefficients for bivariate analysis when necessary. Qualitative variables were analyzed using Fisher’s exact test or χ2-tests. The compliance of data with a normal distribution was evaluated with a one-sample Kolmogorov-Smirnov test, and logarithmic conversion was used for non-normally distributed variables. Quantitative variables were compared using Student’s t test or nonparametric Mann-Whitney tests, as appropriate.
In the analysis of clinical outcomes, ESA therapy was the dependent variable in backward deletion binary logistic regression. All variables presenting a significance of at least 0.10 in the univariate analysis were included in regression analysis models. We divided the serum sFas levels by 1,000 to perform regression analysis and used Tukey and Bonferroni as confidence interval adjustment in the post hoc analysis. All differences were considered statistically significant when two-tailed tests yielded P ≤ 0.05.
RESULTS
Overall parameter description and data correlations.
In this study, a total of 77 patients with ND-CKD and anemia were enrolled in our analysis (with the workflow diagram shown in Fig. 1). It was detected that diabetes mellitus and hypertension were the predominant causes of CKD followed by chronic glomerulonephritis. We observed that anemia was diagnosed at 3.7 ± 1.3 yr after admission at the Nephrology Service in patients with CKD in the outpatient setting.
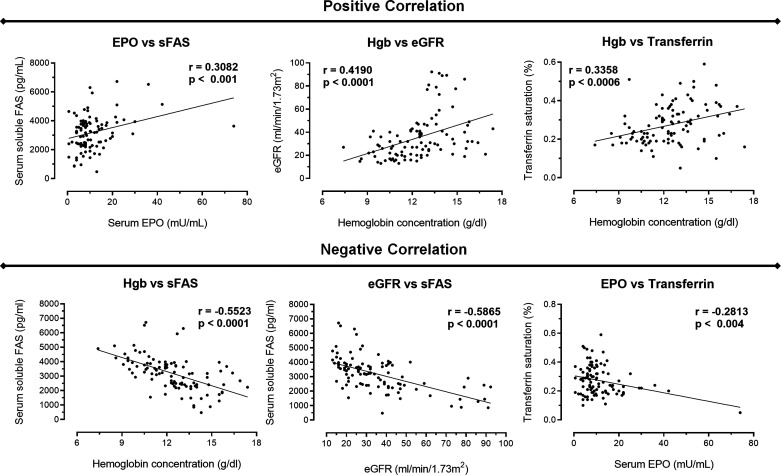
Furthermore, we pointed out several correlations between renal and hematological parameters and sFas levels. Figure 2 shows the correlations in all patients at baseline before they started ESA therapy, when required. There was a positive correlation between 1) serum Epo and sFas levels (r = 0.30, P = 0.001), 2) Hb level and eGFR (r = 0.41, P < 0.001), and 3) Hb level and transferrin saturation (r = 0.33, P < 0.001). In contrast, there was a negative correlation among 1) Hb and sFas levels (r = −0.55, P < 0.001), 2) eGFR and sFas level (r = −0.58, P < 0.001), and 3) serum Epo level and transferrin saturation (r = −0.28, P = 0.004). Thus, this correlation analysis highlighting an opposite relation between sFas levels with Hb and eGFR indexes.
Fig. 2.
Global correlations between the variables analyzed. Correlations were performed using our data collection comprising 77 patients with nondialysis chronic kidney disease patients with anemia and not submitted to erythropoiesis-stimulating agent (ESA) therapy at baseline. We observed a positive correlation between serum erythropoietin (EPO) versus soluble Fas (sFas), hemoglobin (Hgb) versus estimated glomerular filtration rate (eGFR) and Hgb versus transferrin. In contrast, we detected a negative correlation among Hgb versus sFas, eGFR versus sFas, and EPO versus transferrin. P < 0.05.
Direct comparison of renal and hematologic parameters between ESA and non-ESA groups.
Table 1 shows overall demographic characteristics such as renal function, hemogram findings, usage of medicines, iron status, serum levels of Epo, sFas, iPTH, IL-6, IL-10, and albumin, and left ventricular hypertrophy between patients who required ESA therapy for up to 6 yr of followup (ESA group; n = 35) and patients who did not need ESA usage since at their baseline (non-ESA group; n = 42). We observed that ESA was started at 2.2 ± 1.9 yr after anemia diagnosis and that the recombinant human Epo dosage used was 59.3 ± 17.4 U·kg−1·wk−1. However, we found 14 patients with CKD with diabetes that required ESA administration, whereas 16 patients with diabetes did not require ESA (P = 0.67).
Table 1.
General comparison of patients with nondialysis chronic kidney disease with and without ESA therapy at baseline
| ESA Group | Non-ESA Group | P Value | |
|---|---|---|---|
| n | 35 | 42 | |
| Sex, n (%) | 0.24 | ||
| Women | 18 (51) | 16 (38) | |
| Men | 17 (49) | 26 (62) | |
| Age, yr | 61 ± 12 | 56 ± 14 | 0.07 |
| Body mass index, kg/m2 | 34 ± 7 | 33 ± 8 | 0.89 |
| Estimated glomerular filtration rate, mL·min−1·1.73 m−2* | 26.2 ± 10.1 | 33.5 ± 14.3 | 0.01 |
| Creatinine, mg/dL | 3.1 ± 1.9 | 2.4 ± 0.9 | 0.08 |
| Chronic kidney disease etiology, n (%) | 0.45 | ||
| Diabetes mellitus | 14 (40) | 16 (38) | |
| Hypertension | 14 (40) | 19 (45) | |
| Chronic glomerulonephritis | 6 (17) | 4 (10) | |
| Chronic tubulointerstitial nephritis | 1 (3) | 1 (2) | |
| Autosomal dominant polycystic kidney disease | 2 (5) | ||
| Smoker, n (%) | 6 (17) | 5 (12) | 0.51 |
| Renin-angiotensin-aldosterone system blockers, n (%) | 29 (83) | 34 (81) | 0.83 |
| Iron therapy, n (%)* | 30 (86) | 27 (64) | 0.03 |
| Left ventricular hypertrophy, n (%) | 11 (31.5) | 13 (31) | 0.96 |
| Hemoglobin, g/dL* | 11.1 ± 0.9 | 12.5 ± 1.2 | <0.001 |
| Hematocrit, %* | 33 ± 5 | 36 ± 6 | 0.04 |
| Mean corpuscular volume, fL | 88.7 ± 5.2 | 88.1 ± 3.5 | 0.55 |
| Mean corpuscular hemoglobin, pg | 31.5 ± 1.4 | 31.9 ± 1.1 | 0.27 |
| Ferritin, µg/L | 137.1 ± 23.2 | 86.3 ± 11.4 | 0.08 |
| Transferrin saturation, % | 24.1 ± 1.8 | 26.9 ± 2.7 | 0.16 |
| Serum iron levels, µg/dL | 82.1 ± 4.5 | 79.2 ± 5.4 | 0.65 |
| Serum erythropoietin levels, mU/mL | 12.4 ± 6.8 | 10.7 ± 5.1 | 0.49 |
| Serum soluble Fas levels, pg/mL* | 4316 ± 897 | 2776 ± 749 | <0.001 |
| Intact parathyroid hormone levels, pg/mL | 128 ± 24 | 165 ± 30 | 0.36 |
| IL-6 levels, pg/mL | 6.4 ± 4.1 | 7.6 ± 5.4 | 0.38 |
| IL-10 levels, pg/mL | 13.1 ± 7.3 | 12.1 ± 6.4 | 0.52 |
| Albumin, g/dL | 3.6 ± 0.35 | 4.1 ± 0.77 | 0.06 |
Values are means ± SD; n, number of patients/group. The estimated glomerular filtration rate was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation. ESA, erythropoiesis-stimulating agent.
P < 0.05.
Additionally, we found that the requirement for ESA therapy in patients with CKD was more prevalent in older patients and with users of iron therapy. There were no significant differences in CKD etiology, smoking status, use of renin-angiotensin-aldosterone system blockers, red blood cell indexes, transferrin saturation, levels of serum Epo, iPTH, inflammatory cytokines, and iron, or left ventricular hypertrophy between these groups.
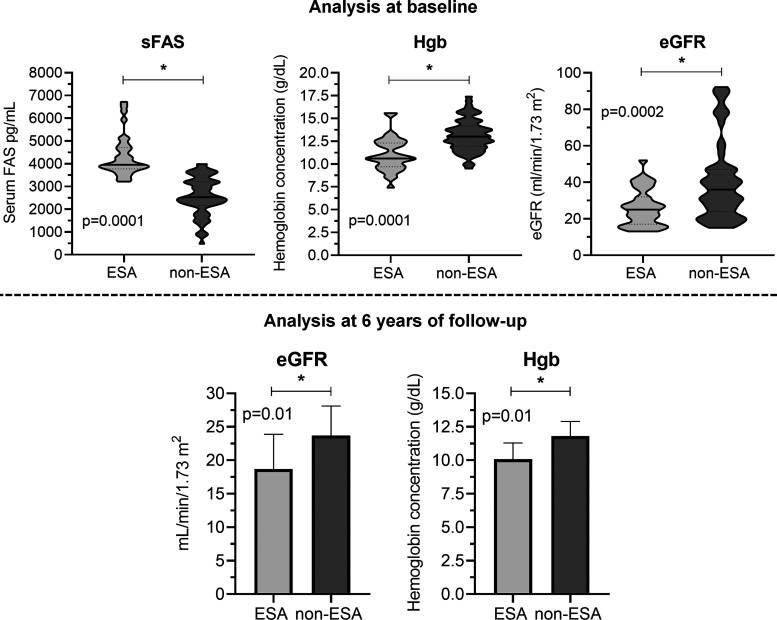
When we compared the two groups directly at baseline, we observed lower eGFR, hematocrit, and Hb levels in the group that required ESA treatment. More importantly, we also detected higher levels of ferritin and sFas in the ESA group (Fig. 3 and Table 1). Interestingly, we observed lower eGFR (18.7 ± 5.2 vs. 23.7 ± 4.4 mL·min−1·1.73 m−2, P = 0.01) and Hb levels (10.1 ± 1.2 vs. 11.8 ± 1.1 g/dL, P = 0.001) in the ESA group after 6 yr of followup (Fig. 3).
Fig. 3.
Direct comparison of soluble Fas (sFas), hemoglobin (Hgb), and estimated glomerular filtration rate (eGFR) levels in patients with erythropoiesis-stimulating agent (ESA) and non-ESA therapy. sFas, Hgb, and eGFR were compared between patients that needed ESA therapy and those who did not need ESA therapy at their baseline. The violin-plot chart shows clearly that patients who further needed ESA therapy in our followup presented higher levels of sFas and a lower index of Hgb and eGFR at baseline than patients who did not submitted to ESA therapy. After 6 yr of followup, we verified that eGFR and Hgb index remained decreased in patients who needed ESA treatment. P < 0.05.
Finally, we observed among several variables (i.e., age, eGFR, iron therapy usage, and Hb level) that serum sFas, ferritin, and albumin levels were the most significant independent predictors of ESA therapy at up to 6 yr of followup in the model of binary logistic regression analysis (Table 2).
Table 2.
Binary logistic regression of ESA therapy as response variable and its predictors at baseline
| 95% Confidence Intervals for the Odds Ratio |
||||
|---|---|---|---|---|
| ESA Therapy versus Non-ESA Therapy |
Odds Ratio | Lower | Upper | P Value |
| Soluble Fas, pg/mL* | 1.012 | 1.004 | 1.020 | 0.004 |
| Albumin, g/dL* | 0.07 | 0.002 | 0.28 | 0.009 |
| Ferritin, µg/L* | 1.026 | 1.005 | 1.047 | 0.01 |
| Age, yr | 1.072 | 0.981 | 1.212 | 0.12 |
| Iron therapy | 0.247 | 0.011 | 5.412 | 0.37 |
| Estimated glomerular filtration rate, mL·min−1·1.73 m−2 | 1.060 | 0.929 | 1.210 | 0.39 |
| Hemoglobin, g/dL | 0.864 | 0.420 | 1.778 | 0.69 |
R2 = 0.858; model (P = 0.002). The estimated glomerular filtration rate was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation. ESA, erythropoiesis-stimulating agent.
P < 0.05.
Effect of sFas on the colony-forming potential of UCB-derived hematopoietic cells.
To elucidate the mechanism underlying the interconnection between sFas and ESA treatment, we assessed recombinant sFas protein interference in hemopoiesis in vitro using a colony-forming assay with UCB-derived hematopoietic cells.
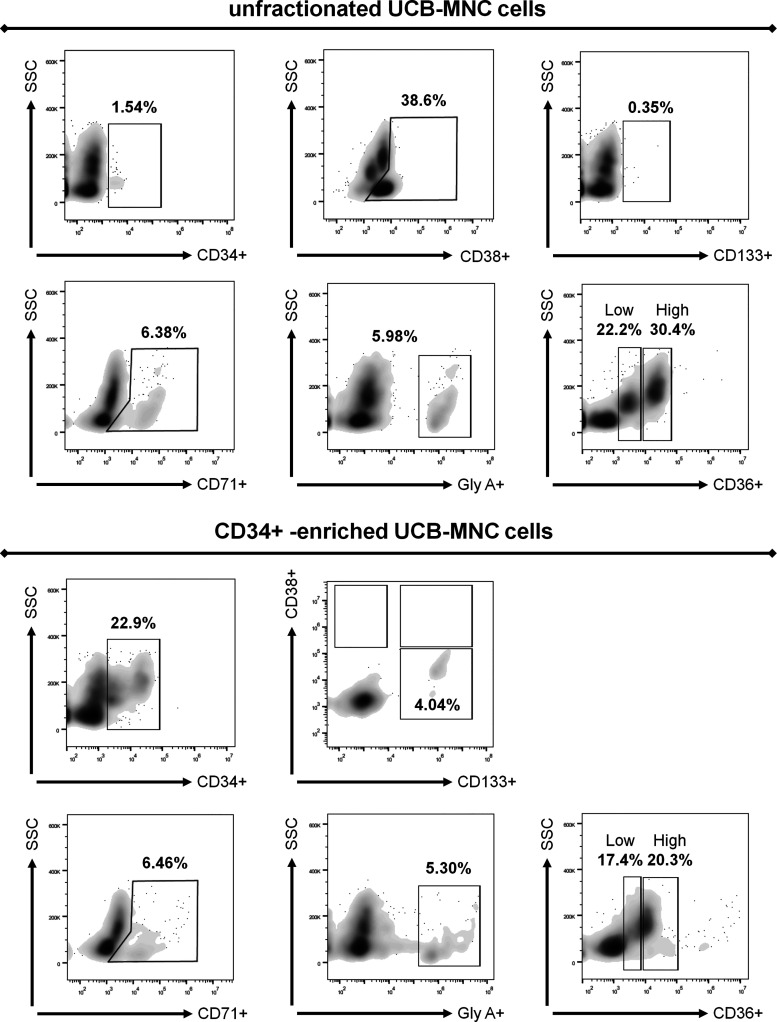
First, we performed flow cytometry on unfractionated UCB-MNCs and CD34+-enriched cells, obtained through magnetic selection. Within the unfractionated cell population, the fraction of CD34+ cells was very low at 1.54%, whereas CD38+ and CD133+ cells presented 38.6% and 0.35%, respectively. In this subpopulation, we found frequencies of 6.38% of CD71+ cells and 5.98% of glycophorin A+ cells. The CD36+ marker showed two subpopulations: 1) low with 22.2% (CD36+low) and 2) high with 30.4% (CD36+high). CD38+ and CD36+ were the markers with the highest levels of expression (Fig. 4). Furthermore, after magnetic selection, the cell population presented distinct phenotypes, including CD34+ (22.9%), CD34+/CD38−/CD133+ (4.04%), CD71+ (6.46%), glycophorin A+ (5.30%), CD36+low (17.4%), and CD36+high (20.3%), confirming the success of cellular enrichment of the classical progenitor HSCs in our UCB cell population (Fig. 4).
Fig. 4.
Flow cytometry characterization of enriched CD34+ human umbilical cord blood mononuclear cells (UCB-MNCs). First, we verified the general profile of unfractionated UCB-MNCs regarding the expression of CD34+, CD38+, CD133+, CD71+, glycophorin A (gly A), and CD36+. In addition, unfractionated cells presented higher levels of CD38+ (38.6%) compatible with a more differentiated phenotype. Afterward, we performed magnetic selection and enriched the UCB-MNC population with CD34+ cells (22.9%). This enriched population presented a typical hematopoietic progenitor stem cell phenotype, which was not detected in unfractionated cells (CD34+CD38−CD133+). The expression of CD133+, CD71+, glycophorin A, and CD36+ between both populations (CD34+ and unfractionated) was not statistically affected. SSC, side scatter.
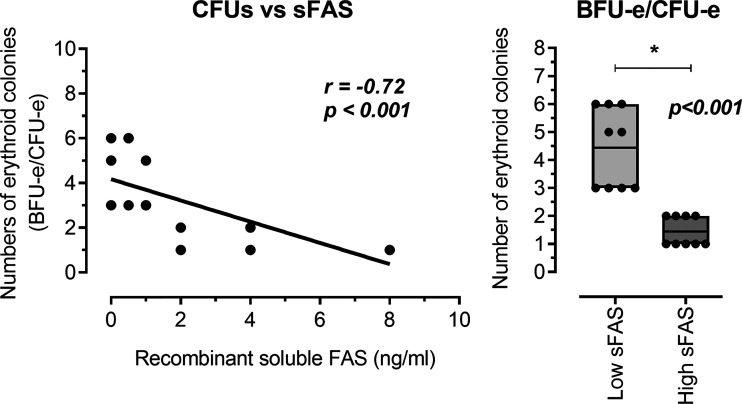
Next, we performed methylcellulose colony-forming assays on CD34+-enriched UCB cells in the absence or presence of either high levels (Hc group: 2, 4, and 8 ng/mL) and low levels (Lc group: 0, 0.5, and 1 ng/mL) of recombinant human sFas protein. In this analysis of CD34+-enriched cells, there was a significant negative correlation between the number of erythroid colonies (BFU-e/CFU-e) and sFas level in the medium (r = −0.72, P < 0.001; Fig. 5). Furthermore, we detected a decrement of total erythroid colonies with higher levels of sFas protein (Hc group: 1.56 ± 0.29 BFU-e/CFU-e vs. Lc group: 4.33 ± 0.53 BFU-e/CFU-e, P < 0.001; Fig. 5) in CD34+-enriched cells. All these analyses suggest that higher levels of sFas can affect global hemopoiesis in vitro.
Fig. 5.
Analyze of the human recombinant soluble Fas (sFas) influence on in vitro erythropoiesis. The concentration of sFas protein negatively correlated with the number of erythroid progenitor colonies (BFU-e/CFU-e). Also, it was observed that higher concentrations of recombinant human sFas protein presented smaller number of global erythroid colonies. BFU-e, burst forming unit-erythroid; CFU-e, colony forming unit-erythroid; Lc-sFas group, low concentrations of recombinant human sFas protein (0, 0.5, and 1 ng/mL); Hc-sFas group, high concentrations of recombinant human sFas protein (2, 4, and 8 ng/mL). P < 0.05.
DISCUSSION
The most important finding of this retrospective clinical study concerns the relationship among sFas, anemia, and need for ESA administration. Our study suggests that serum sFas seems a potential predictor of the need for ESA therapy in patients with CKD and anemia who do not require renal replacement therapy. In addition, we also observed that sFas protein can affects drastically in vitro erythropoiesis.
The main culprit of CKD-associated anemia is inadequate Epo production (1, 12, 18, 26, 32). Although generally normal or slightly increased in CKD-associated anemia, Epo levels are considered inappropriately low relative to the degree of anemia. This misinterpretation occurs because patients with anemia with regular kidney function normally present 10−100 times higher levels of Epo (1, 14, 15, 26). Interestingly, we found a positive correlation between serum Epo and sFas levels. In addition, we also observed a negative correlation between serum sFas levels and both eGFR and Hb levels. This indicates that renal function declines overtime (based on eGFR) with the reduction in Hb levels and Epo serum levels along with a conspicuous elevation of sFas indexes (15, 18). Although there was no significant difference in serum Epo levels between patient groups, we found higher sFas levels in patients with CKD who received ESA therapy. These findings are particularly interesting given our group has previously demonstrated an association between serum levels of sFas and hyporesponsiveness to Epo in patients with CKD (15).
In the present study, we reported that the serum sFas level is a potential independent predictor of ESA therapy and demonstrated that for each 1,000-unit of fold increase, there was a 12-fold increase in the likelihood of needing ESA administration. To gain further insights into the relation between sFas and renal anemia, we performed in vitro experiments to examine the dose-dependent effects of recombinant sFas protein during erythropoiesis (13). Our main findings evidenced that soluble sFas directly affects the number of erythroid colonies in the CD34+-enriched fraction of HSCs. The dose of sFas used in this in vitro assay was similar to lowest level of sFas observed in our patients with CKD and anemia, bringing more clinical relevance to our study.
To date, there are two different isoforms of Fas: membrane-anchored Fas or Fas receptor (FasR; CD95) and FasL (CD178). FasR is a 45-kDa cell surface protein, and its activation by FasL induces apoptosis in normal and tumor cells by a classical extrinsic program through caspase-8- and caspase-3-dependent signals. In contrast, sFas is generated when FasR lacks its transmembrane domain by either alternative splicing or a metalloproteinase cleavage process. Consequently, sFas binds to FasL in the extracellular space, interfering in the normal Fas signaling pathway, inhibiting apoptosis (9, 11, 17, 19, 24, 26, 29).
In this sense, early erythroid progenitors such as CD34+ cells express very low levels of FasR and are partially resistant to apoptosis induced by the Fas system (11, 22). However, upon cell differentiation, FasR expression increases and mature erythroid lineages become sensitive to Fas-induced apoptosis (22). FasL is functionally present only at the late stages of erythroblast differentiation, displaying Fas-based cytotoxicity against immature erythroblasts, which can be abrogated by high levels of Epo (11). In contrast, Carlile et al. (7) suggested that during the initial stage of human erythropoiesis, the FasR/FasL interaction triggers a positive important signal for erythroid maturation/differentiation without affecting cellular proliferation or inducing apoptosis. The reason for these distinct responses is unclear, but it may be associated with intrinsic differences in species, tissues, or pathological state (4, 7–9, 25, 29). Hence, we reported here, for the first time, that there is a negative correlation between human sFas protein level and the erythroid colony-forming potential of CD34+-enriched cells. Thus, based on our findings, higher concentrations of sFas could induce a retrograde signal in patients with CKD, reducing erythropoiesis.
Although intriguing, our study has some limitations, such as 1) the long duration of followup (6 yr); 2) the small number of sample; 3) the retrospective nature of the analysis, which precludes any investigational interventions in our patient groups; and 4) the necessity of future mechanistic studies to define the molecular basis behind the sFas influence in CKD-associated anemia, such as investigations as to whether sFas interferes with critical molecules of erythropoiesis. On the other hand, our findings have important implications in understanding novel mechanisms that contribute to the development and treatment of anemia in patients with CKD. In light of this evidence, recent discoveries, such as APG101, a fusion protein from the extracellular domain of CD95 and the Fc region of IgG1, can assists in the treatment of renal anemia. CD95/CD178 interaction negatively regulate erythrocyte production in the bone marrow; however, in myelodysplastic syndrome, this causes resistance to ESA therapy. A previous study (25) has demonstrated that APG101 increases the number of burst-forming unit-erythroid progenitors and improves their overall proliferation rate, inhibiting apoptosis. In addition, others have also evidenced that APG101 acts antiinvasively, resulting in an additive effect together with radiotherapy in glioblastoma. All these data suggests that APG101 and other new drugs could help us to understand the relationship between sFas and erythropoiesis in patients with CKD (4, 15, 18).
In conclusion, our study documented that a higher serum sFas level is closely associated with anemia in patients with CKD who do not require renal replacement therapy. Moreover, the serum sFas level seems a potential predictor for ESA therapy in patients with nondialysis CKD. Together, these findings provide a rationale for further investigations targeting alternative interventions. Thus, our study may highlight novel avenues for early investigations and could possibly represent an opportunity for monitoring the safety of newer therapeutic interventions for treating CKD-associated anemia.
GRANTS
This work was supported by the Brazilian Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Foundation (CNPq).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.M.C., M.E.F.C., N.E.-A., M.S.D., A.Z., G.A.-P., and M.A.G. conceived and designed research; D.C.d.A., M.A.D., S.K.G., A.M.M., N.E.-A., C.D.P., M.S.D., G.A.-P., and M.A.G. performed experiments; A.Z. and M.A.G. interpreted results of experiments; D.C.d.A., M.A.D., S.K.G., A.M.M., C.D.P., G.A.-P., and M.A.G. analyzed data; D.M.C. prepared figures; D.C.d.A., M.E.F.C., S.K.G., and M.SD drafted manuscript; A.M.M., A.Z., and M.A.G. edited and revised manuscript; D.M.C., D.C.d.A., M.E.F.C., N.E.-A., C.D.P., M.S.D., A.Z., G.A.-P., and M.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all professional and technicians from the Wake Forest Institute for Regenerative Medicine for providing support in the experimental design, assays, and manuscript review. Specially, we thank Prof. Nestor Schor (Nephrology Division, Federal University of São Paulo), in memoriam, for all assistance and consideration.
The results presented in this paper have not been previously published except in abstract form.
REFERENCES
- 1.Agarwal AK. Darbepoetin alfa for anemia in chronic kidney disease. Expert Rev Clin Pharmacol 1: 369–379, 2008. doi: 10.1586/17512433.1.3.369. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 38: 955–962, 2001. doi: 10.1016/S0735-1097(01)01470-X. [DOI] [PubMed] [Google Scholar]
- 3.Antoniani C, Romano O, Miccio A. Concise review: epigenetic regulation of hematopoiesis: biological insights and therapeutic applications. Stem Cells Transl Med 6: 2106–2114, 2017. doi: 10.1002/sctm.17-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaes J, Thomé CM, Pfenning PN, Rübmann P, Sahm F, Wick A, Bunse T, Schmenger T, Sykora J, von Deimling A, Wiestler B, Merz C, Jugold M, Haberkorn U, Abdollahi A, Debus J, Gieffers C, Kunz C, Bendszus M, Kluge M, Platten M, Fricke H, Wick W, Lemke D. Inhibition of CD95/CD95L (FAS/FASLG) signaling with APG101 prevents invasion and enhances radiation therapy for glioblastoma. Mol Cancer Res 16: 767–776, 2018. doi: 10.1158/1541-7786.MCR-17-0563. [DOI] [PubMed] [Google Scholar]
- 5.Bhatraju PK, Robinson-Cohen C, Mikacenic C, Harju-Baker S, Dmyterko V, Slivinski NSJ, Liles WC, Himmelfarb J, Heckbert SR, Wurfel MM. Circulating levels of soluble Fas (sCD95) are associated with risk for development of a nonresolving acute kidney injury subphenotype. Crit Care 21: 217, 2017. doi: 10.1186/s13054-017-1807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappellini MD, Motta I. Anemia in clinical practice-definition and classification: does hemoglobin change with aging? Semin Hematol 52: 261–269, 2015. doi: 10.1053/j.seminhematol.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Carlile GW, Smith DH, Wiedmann M. A non-apoptotic role for Fas/FasL in erythropoiesis. FEBS Lett 583: 848–854, 2009. doi: 10.1016/j.febslet.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol 154: 2706–2713, 1995. [PubMed] [Google Scholar]
- 9.Dai CH, Price JO, Brunner T, Krantz SB. Fas ligand is present in human erythroid colony-forming cells and interacts with Fas induced by interferon gamma to produce erythroid cell apoptosis. Blood 91: 1235–1242, 1998. doi: 10.1182/blood.V91.4.1235. [DOI] [PubMed] [Google Scholar]
- 10.Dalboni MA, Cenedeze MA, Manfredi SR, Cruz Andreoli MC, Pavao Dos Santos O, Canziani ME, Boim MA, Goes MA, Draibe SA, Balakrishnan V, Cendoroglo M. High serum levels of soluble Fas (sFas) in CKD patients: effects of renal clearance, reabsorption and synthesis. Int J Artif Organs 31: 405–410, 2008. doi: 10.1177/039139880803100505. [DOI] [PubMed] [Google Scholar]
- 11.De Maria R, Testa U, Luchetti L, Zeuner A, Stassi G, Pelosi E, Riccioni R, Felli N, Samoggia P, Peschle C. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood 93: 796–803, 1999. doi: 10.1182/blood.V93.3.796. [DOI] [PubMed] [Google Scholar]
- 12.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell 10: 120–136, 2012. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Duinhouwer LE, van Rossum BJ, van Tiel ST, van der Werf RM, Doeswijk GN, Haeck JC, Rombouts EW, Ter Borg MN, Kotek G, Braakman E, Cornelissen JJ, Bernsen MR. Magnetic resonance detection of CD34+ cells from umbilical cord blood using a 19F label. PLoS One 10: e0138572, 2015. doi: 10.1371/journal.pone.0138572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill KS, Muntner P, Lafayette RA, Petersen J, Fink JC, Gilbertson DT, Bradbury BD. Red blood cell transfusion use in patients with chronic kidney disease. Nephrol Dial Transplant 28: 1504–1515, 2013. doi: 10.1093/ndt/gfs580. [DOI] [PubMed] [Google Scholar]
- 15.Góes MA, Dalboni MA, Manfredi SR, Cendoroglo MS, Batista MC, Canziani ME, Balakrishnan VS, Pereira BJ, Draibe SA, Cendoroglo M. Serum-soluble Fas and serum levels of erythropoietin in chronic kidney disease. Clin Nephrol 73: 7–13, 2010. doi: 10.5414/CNP73007. [DOI] [PubMed] [Google Scholar]
- 16.Góes MA, Iizuka IJ, Quinto BM, Dalboni MA, Monte JC, Santos BC, , et al. Serum soluble-Fas, inflammation and anemia in acute kidney injury. Artif Organs, 2013. doi: 10.1111/aor.12019. [DOI] [PubMed] [Google Scholar]
- 17.Guégan JP, Legembre P. Nonapoptotic functions of Fas/CD95 in the immune response. FEBS J 285: 809–827, 2018. doi: 10.1111/febs.14292. [DOI] [PubMed] [Google Scholar]
- 18.Haase VH, Chertow GM, Block GA, Pergola PE, deGoma EM, Khawaja Z, Sharma A, Maroni BJ, McCullough PA. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant 34: 90–99, 2019. doi: 10.1093/ndt/gfy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holler N, Tardivel A, Kovacsovics-Bankowski M, Hertig S, Gaide O, Martinon F, Tinel A, Deperthes D, Calderara S, Schulthess T, Engel J, Schneider P, Tschopp J. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol 23: 1428–1440, 2003. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease Notice. Kidney Int Suppl 2: 279–335, 2012. doi: 10.1038/kisup.2012.37. [DOI] [Google Scholar]
- 21.Kumar BV, Mohan T. Retrospective comparison of estimated GFR using 2006 MDRD, 2009 CKD-EPI and Cockcroft-Gault with 24 hour urine creatinine clearance. J Clin Diagn Res 11: BC09–BC12, 2017. doi: 10.7860/JCDR/2017/25124.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Pop R, Sadegh C, Brugnara C, Haase VH, Socolovsky M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood 108: 123–133, 2006. doi: 10.1182/blood-2005-11-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr 12: 444–454, 2009. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 24.Nagata S, Golstein P. The Fas death factor. Science 267: 1449–1456, 1995. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 25.Raimbault A, Pierre-Eugene C, Rouquette A, Deudon C, Willems L, Chapuis N, Mathis S, Kunz C, Fricke H, Kosmider O, Bardet V, Fontenay M, Myélodysplasies GF; Groupe Francophone des Myélodysplasies . APG101 efficiently rescues erythropoiesis in lower risk myelodysplastic syndromes with severe impairment of hematopoiesis. Oncotarget 7: 14898–14911, 2016. doi: 10.18632/oncotarget.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato Y, Yanagita M. Renal anemia: from incurable to curable. Am J Physiol Renal Physiol 305: F1239–F1248, 2013. doi: 10.1152/ajprenal.00233.2013. [DOI] [PubMed] [Google Scholar]
- 27.Tejedor JR, Papasaikas P, Valcárcel J. Genome-wide identification of Fas/CD95 alternative splicing regulators reveals links with iron homeostasis. Mol Cell 57: 23–38, 2015. doi: 10.1016/j.molcel.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Tomiyama C, Higa A, Dalboni MA, Cendoroglo M, Draibe SA, Cuppari L, Carvalho AB, Neto EM, Canziani ME. The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol Dial Transplant 21: 2464–2471, 2006. doi: 10.1093/ndt/gfl291. [DOI] [PubMed] [Google Scholar]
- 29.Villamizar O, Chambers CB, Riberdy JM, Persons DA, Wilber A. Long noncoding RNA Saf and splicing factor 45 increase soluble Fas and resistance to apoptosis. Oncotarget 7: 13810–13826, 2016. doi: 10.18632/oncotarget.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Du Z, Cai H, Ye Z, Fan J, Tan WS. Low oxygen tension favored expansion and hematopoietic reconstitution of CD34+ CD38− cells expanded from human cord blood-derived CD34+ cells. Biotechnol J 11: 945–953, 2016. doi: 10.1002/biot.201500497. [DOI] [PubMed] [Google Scholar]
- 31.Wright DG, Wright EC, Narva AS, Noguchi CT, Eggers PW. Association of erythropoietin dose and route of administration with clinical outcomes for patients on hemodialysis in the United States. Clin J Am Soc Nephrol 10: 1822–1830, 2015. doi: 10.2215/CJN.01590215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoro M, Nakayama Y, Yamagishi SI, Ando R, Sugiyama M, Ito S, Yano J, Taguchi K, Kaida Y, Saigusa D, Kimoto M, Abe T, Ueda S, Fukami K. Asymmetric dimethylarginine contributes to the impaired response to erythropoietin in CKD-anemia. J Am Soc Nephrol 28: 2670–2680, 2017. doi: 10.1681/ASN.2016111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zachée P, Vermylen J, Boogaerts MA. Hematologic aspects of end-stage renal failure. Ann Hematol 69: 33–40, 1994. doi: 10.1007/BF01757345. [DOI] [PubMed] [Google Scholar]